Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an
aggressive malignancy with an extremely high mortality rate and
poor overall prognosis, largely due to delays in diagnosis, paucity
of specific biomarkers, early metastases, and resistance to
chemotherapy and other therapies (1). Therefore, its biology and genetics are
of interest to researchers and practitioners (2). Several solid tumors, including PDAC,
are characterized by the presence of regions of hypoxia, which is
defined as a state of insufficient oxygen levels for the
maintenance of normal cellular functions (3). Hypoxia, in this context, does not
necessarily refer to a certain concentration of oxygen since
several tissues can function physiologically at oxygen levels as
low as 1% (4,5). During hypoxia, the aberrant blood
vessels (poorly organized, elongated, dilated, twisted, and
blind-ended blood vessels) and the rapid proliferation of cells
cause marked heterogeneity in the perfusion of these tumors with
regions of hypoxia where the median PO2 could be ≤15
mmHg, while adjacent normal cells have a median PO2 of
≥35 mmHg (6). In such tumors, the
oxygen consumption is greater than oxygen supply, especially at the
boundaries where the distance from a functional blood vessel may be
>100 µm (7). Although several
tumor cells die under these hypoxic conditions, other cells may
survive in a dormant state (6), and
yet several other cells undergo genetic and adaptive changes that
permit them to survive and even proliferate in a hypoxic
environment. Therefore, as realized by Vaupel and Harrison in 2004,
hypoxia exerts a selection pressure that leads to the survival of a
subpopulation of cells that have the genetic machinery for
malignant progression (8). This
selection pressure includes proteomic and genomic changes within
tumor cells leading to cell cycle arrest, differentiation,
necrosis, apoptosis, and at a molecular level, accumulation of HIF
(9,10).
HIF is a heterodimeric transcription factor that
dissociates into HIF-1α and HIF-1β under normoxic conditions, but
accumulates during hypoxia to affect hypoxia-response elements of
target genes. It has been shown that HIF directly or indirectly
regulates >100 genes (11). Many
of those genes are implicated in tumor processes including
angiogenesis, invasion, metastasis, and metabolic adaptation. In
particular, HIF-1α is involved in the transcription of genes that
encode enzymes participating in glycolysis (12), glucose transporters, multidrug
resistance protein 1, and several growth factors (13).
Conversely, recent studies have shown the importance
of the crosstalk between tumor cells and their microenvironmental
factors through the release of exosomes from hypoxic tumor cells
(14). Exosomes are vesicles 30-100
nm in diameter, which contain various types of proteins, RNAs,
non-coding RNAs such as miRNAs, and DNA and they can act as
messengers for intercellular communication in local and distant
microenvironments and can regulate the expression of numerous genes
to promote tumor growth, local invasion, and create premetastatic
or metastatic niches (15-17).
For example, it was found that hypoxia-resistant multiple myeloma
cells produced more exosomes with a significantly higher expression
of miR-135b as compared to normoxic cells. Exosomal miR-135b
targets HIF-1 in endothelial cells in hypoxia-resistant myeloma
cells, thereby enhancing angiogenesis (18).
Although we now know many of the aspects of how
tumor-induced hypoxia leads to tumor-related phenomena such as
angiogenesis, tumor growth, invasion, and metastasis, the exact
mechanisms and the specific genes and enzymes involved in the
metabolic changes associated with cancer are far from completely
established. For example, during hypoxia, a shift towards anaerobic
glycolysis seems intuitive due to the deficiency of oxygen as the
ultimate electron acceptor. Due to the need for intermediates in
the synthesis of macromolecules, cancer cells, through HIF, modify
this process and regulates the expression of the pertinent enzymes
such as hexokinase, phosphofructokinase I, and phosphoglycerate
kinase 1 as well as the glucose transporters required for
internalization of glucose (19).
Moreover, the gluconeogenic enzyme fructose-1,6-bisphosphatase,
which opposes glycolytic flux and inhibits HIF function, was found
to be downregulated in clear cell renal carcinoma tumors (20). Such examples have been used to
demonstrate the complex regulation between HIF and its
transcriptional targets, especially those related to metabolism,
and to provide potential alternate therapeutic strategies in tumors
dependent on HIF signaling (21).
More recently, Jia et al (22) used mathematical modeling followed by
in vitro testing on triple-negative breast cancer cells
(TNBC) to demonstrate a direct association between the activities
of adenosine monophosphate-activated protein kinase (AMPK), a
regulator of oxidative phosphorylation, and HIF-1, a regulator of
glycolysis, with the activities of three major metabolic pathways:
Glucose oxidation, glycolysis, and fatty acid oxidation. The
maintenance of the hybrid metabolic phenotype by TNBC suggested
that targeting both glycolysis and oxidative phosphorylation is
necessary for the elimination of the ‘metabolic plasticity’ of
these cells (22). Therefore,
understanding such complex regulation of tumor metabolism is a
prerequisite for identifying efficient therapies for tumors.
The classification of hypoxia has been recently
reviewed by Saxina and Jolly (23).
They characterized 3 types of hypoxia: Chronic hypoxia or
diffusion-limited hypoxia due to over proliferation and extending
over 24 h; acute hypoxia or perfusion limited due to aberrant shut
down of small blood vessels and extending from a few mins to a few
h; and intermittent or cyclic hypoxia extending from a few mins to
days (23). The latter type results
from transient shut down of vasculature followed by reoxygenation
and reoxygenation injury. The overlapping time scale of the latter
2 categories makes it difficult to interpret the research data
obtained using varying time periods of hypoxia in experimental
approaches (24). The present study
was designed to mimic short-term and long-term cycling hypoxic
conditions in tumors, and to characterize metabolism-related gene
changes that may occur in pancreatic cancer cells in response to
cyclic acute or chronic hypoxia using the PANC1 cell line, which is
representative of PDAC. The primary aim of this study was to
uncover novel biomarkers present in tumor hypoxia that may assist
in the clinical decision regarding the use of chemotherapeutic
agents in cancer patients.
Materials and methods
Cell culture conditions
PANC1, a human pancreatic cancer cell line, was
purchased from the American Type Culture Collection. PANC1 cells
were cultured in DMEM high-glucose medium (EuroClone), supplemented
with 10% (v/v) heat-inactivated FBS, 2 mM L-glutamine, and
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin (all
from (HyClone; Cytiva). PANC1 cells were grown in 75 cm²
attached-type, filter-cap culture flasks (Membrane Solutions).
Cells were kept cultured at 37˚C in a humidified incubator supplied
with 5% CO2. All cell culture procedures were performed
under sterile conditions in a class II biological safety cabinet
(Heal-Force). All materials and disposables were disinfected with
76% ethanol before use, and subculturing was performed twice a week
when cells reached 80-90% confluence.
Hypoxic modeling
The hypoxic atmosphere was generated using a hypoxia
chamber apparatus (Stem Cell Technologies, Inc.). The chamber was
connected to a gas cylinder that provided a hypoxic gas mixture of
94% N2, 5% CO2, and 1% O2. To
expose the cells to the hypoxic atmosphere, PANC1 cells were placed
into the chamber and purged with the gas mixture for 5 min to
establish the hypoxic condition. The hypoxic chamber was then
placed into the CO2 incubator (NuAire).
For cycling acute hypoxia, PANC1 cells were exposed
to 7-h cycles of hypoxia, every other day for a total of 20 hypoxic
cycles. For cycling chronic hypoxia, PANC1 cells were exposed to
72-h cycles of hypoxia once a week for a period of 5 weeks. Each
chronic hypoxic cycle was separated from the other by 96 h of
incubation under the normoxic conditions. For comparison, a subset
of PANC1 cells was incubated under normoxic (95% O2, 5%
CO2) conditions (control PANC1 cells).
Cell proliferation assay
A non-radioactive cell proliferation assay
kit® (Promega Corporation) was used to assess the
cytotoxicity of doxorubicin (Ebewe) on PANC1 cells by measuring the
cell titer. An MTT proliferation assay was performed for the
control PANC1 cells and for the cells exposed to the hypoxic
conditions after 10 and 20 cycles of acute hypoxia, and after 5
cycles of chronic hypoxia.
The cytotoxicity of doxorubicin was determined using
an MTT assay. Briefly, cells were seeded at an initial density of
7-10x103 cells/well in 96-well culture plates (Costar)
in 100 µl complete culture medium and incubated in a humidified
incubator supplied with 5% CO2 at 37˚C for 24 h. Cells
were incubated in a stock solution of doxorubicin and dilutions
thereof (8x10-10 to 1x10-4 M) were prepared
in DMEM high glucose medium in a humidified incubator supplied with
5% CO2 at 37˚C for 72 h. The solutions were then removed
and replaced with 100 µl fresh DMEM to which 100 µl MTT solution
was added to each well, followed by incubation at 37˚C for 3 h.
MTT-media solution was then removed and 100 µl MTT stop solution
was added to dissolve the dark blue formazan crystals. Absorbance
was measured at 570 nm using a microplate reader
(Synergy™ HTX, BioTek Instruments Inc.), and the
IC50 values of doxorubicin when used to treat cells were
calculated.
RNA extraction
RNA was isolated from cells using an
RNeasy® Mini kit (Qiagen GmbH). Briefly, cells were
disrupted in RLT buffer (RNeasy lysis buffer:
guanidine-thiocyanate-containing buffer) and homogenized by
vortexing. A total of 1 ml 70% ethanol was then added to the
lysate, creating conditions that promote selective binding of RNA
to the RNeasy membrane. The sample was then applied to the RNeasy
Mini spin column where total RNA bound to the membrane, whilst
contaminants were efficiently washed away, and high-quality RNA was
eluted in RNase-free water. Binding, washing, and elution steps
were performed by centrifugation in a micro-centrifuge (Qiagen
GmbH). The purity of isolated RNA was determined by measuring the
ratio of the optical density of the samples at 260 and 280 nm. The
optical density ratio (OD260/OD280) ranged from 1.9-2.2 for all
samples. All RNA samples were stored at -80˚C until required for
cDNA synthesis.
Wound healing assay
In each 6-well plate, 2x104 control PANC1
cells, PANC1 cells after 10 and 20 cycles of acute hypoxia, and
PANC1 cells after 5 cycles of chronic hypoxia were seeded. Cells
were incubated in a humid atmosphere of 5% CO2 at 37˚C
until the cells formed confluent monolayers, after which they were
incubated in media supplemented with 10% FBS for 24 h. The
monolayer of cells was wounded using a 200 µl pipette tip to create
a 300-500 µm-wide scratch. Wounded monolayers were washed twice to
remove non-adherent cells. Images were then taken at 0, 24, and 48
h after the scratch was made using the Leica Application Suite
version 2.1.0 (Leica GmbH; magnification, x40). Wound healing was
quantified using ImageJ version 1.44 (National Institutes of
Health) as the mean percentage of the remaining cell-free area
compared with the area of the initial wound (25).
Reverse transcription-quantitative
(RT-q)PCR
The effect of hypoxia on gene expression in PANC1
cells was studied using a 96-well glucose metabolism RT2
profiler PCR array (cat. no. PAHS-006Z, Qiagen GmbH). In this
array, 96-well plates containing different primers for 84 genes
known to respond to hypoxia in addition to 12 genes for quality
control purposes (GEO accession no. GSE207065; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE207065).
Primers were supplied by the manufacturer as part of the array.
RNA was extracted using an RNeasy® Mini
kit (Qiagen GmbH) as mentioned above. Then, cDNA was synthesized by
converting 0.5 µg total RNA using the RT2 First Strand
Kit® (Qiagen GmbH) by genomic DNA elimination followed
by reverse transcription to produce cDNA. A diluted cDNA aliquot
was mixed with the RT2 SYBR® green MasterMix
(glucose metabolism RT2 profiler PCR array; cat. no.
PAHS-006Z; Qiagen GmbH) and loaded into the 96-well array plate.
qPCR reactions were performed using a CFX thermo-cycler (Bio-Rad
Laboratories, Inc.) with the following thermocycling conditions:
Initial denaturation of 95˚C for 10 min; followed by 40 cycles of
95˚C for 15 sec and 60˚C for 1 min. Data analysis was performed
using the 2-ΔΔCq method available from the Biosciences
company (Qiagen GmbH) web portal. Data were normalized across all
plates to the β-actin housekeeping gene. The threshold cycle values
of the control wells were all within the ranges recommended by the
PCR array user manual.
Fold change is the normalized gene expression in the
test sample divided by the normalized gene expression in the
control sample. Fold regulation represents fold change values in a
biologically meaningful way. Fold change values >1 indicate
upregulation, and fold regulation is equal to the fold change.
Western blotting analysis
Total protein was extracted from cells using a
Protein Extraction Kit (cat. no. ab270054, Abcam) according to
manufacturers' instructions. The protein concentration in cell
lysates from the control, acute, and chronic hypoxic PANC1 cells
were measured using an BCA Protein Quantification Kit (cat. no.
ab102536, Abcam). Protein samples were stored at -80˚C for further
use. A total of 20 µg of each protein sample was loaded onto 7.5%
mini-protein TGX precast gels (tris-glycine eXtended) (Bio-Rad
Laboratories, Inc.), and the resolved proteins were
electrophoretically transferred onto mini PVDF transfer packs
(Trans-blot® Turbo team, Bio-Rad Laboratories, Inc.)
using a Trans-Blot® Turbo™ blotting system.
The membrane was then incubated with a β-actin antibody (cat. no.
ab8227, Abcam), anti-human HIF1-α rabbit polyclonal antibody (cat.
no. ab51608, Abcam), anti-human G6PI rabbit polyclonal antibody
(cat. no. ab76598, Abcam), or anti-human RBKS rabbit polyclonal
antibody (cat. no. ab228850, Abcam) all at a 1:1,000 dilution at
4˚C overnight. The membrane was then incubated with goat
anti-rabbit IgG heavy and light HRP (Abcam) at room temperature for
1 h, then with tetramethylbenzidine substrate (Thermo Fisher
Scientific, Inc.) for 2-3 min at room temperature in the dark. Then
membranes were incubated in skimmed-milk in TBST for 1 h at room
temperature. Finally, images were obtained using a
ChemiDoc™ XRS+ System (Bio-Rad Laboratories, Inc.).
Statistical analysis
Differences between the groups were compared using a
one-way ANOVA, followed by a Dunnett's post hoc test using GraphPad
Prism version 7 (GraphPad Software, Inc.). Data are presented as
the mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results and discussion
Effect of hypoxia on the morphology of
PANC1 cells
Cells exposed to cyclic acute and chronic hypoxia
exhibited an irregular, mostly elongated shape with abnormal
appendages and extensions in comparison to cells cultured under
normoxic conditions (Fig. 1). Cells
exposed to acute and chronic hypoxia extend their cell membranes to
give them support under the stress of hypoxia (25). Cells exposed to normoxia showed
regularity in size and distribution among the growth field. This
observation is consistent with that of Song et al (26) who showed that hypoxic culturing
altered cell morphology. Cell morphology changes include cell
flattening and acquisition of a fibroblast-like shape with several
cytoplasmic extensions and the absence of tight junctions observed
in an invasive phenotype (26).
Effect of hypoxia on the resistance of
PANC1 cells to doxorubicin
PANC1 cells exposed to hypoxia exhibited higher
resistance to doxorubicin compared to the control PANC1 cells
(Table I). The IC50 of
doxorubicin doubled when cells were exposed to chronic hypoxia,
tripled with 10 cycles of acute hypoxia, and increased by ~7x when
cells were exposed to 20 cycles of acute hypoxia. This is
consistent with several previous observations relating hypoxia to
drug resistance in tumor cells. For example, Minassian et al
(27) showed that incubation of
certain human and non-human tumor cell lines in hypoxic conditions
transiently increased their resistance to drugs such as etoposide
and doxorubicin. In addition, He et al (28) demonstrated that hypoxia-induced
chemoresistance to the pyrimidine analog gemcitabine in pancreatic
cancer cells and that was due to the regulation of ABCG2 through
the activation of ERK1/2/HIF-1α. Moreover, Shukla et al
(29) showed that
gemcitabine-resistant pancreatic cancer cells exhibited increased
HIF-1α expression, which was accompanied by the acquisition of a
glycolytic phenotype and dependence on glucose, and that cancer
cells increased their intracellular cytidine pools, which in turn,
rendered gemcitabine ineffective via molecular competition. They
further emphasized that inhibition of HIF-1α increased the
sensitivity of pancreatic cancer cells to gemcitabine.
 | Table IEffect of cyclic acute and chronic
hypoxia on the IC50 of doxorubicin on PANC1 cells. |
Table I
Effect of cyclic acute and chronic
hypoxia on the IC50 of doxorubicin on PANC1 cells.
| Treatment | IC50,
µM | IC50
fold increasea |
|---|
| Control cells | 0.44±0.2 | 1.0±0.2 |
| 10 cycles of acute
hypoxia | 1.32±0.3 | 3.0±0.2 |
| 20 cycles of acute
hypoxia | 3.01±0.5 | 6.8±0.7 |
| 5 cycles of chronic
hypoxia | 0.92±0.1 | 2.1±0.6 |
In addition, Kim and Lee (30) showed that tumor cells adapt to
chronic hypoxia by stimulating angiogenic factors, lowering
consumption of oxygen, and selecting for more invasive and
drug-resistant cancer types. The mechanisms by which hypoxia and
HIF signaling promote chemoresistance are now being revealed and
therefore, should be tackled for more effective therapies. In
hypoxia, which is a common feature of the microenvironment of
several solid tumors and even hematological malignancies, there are
multiple mechanisms including upregulation of drug efflux,
induction of autophagy, hypoxia-driven selection of tumor cells
with reduced apoptotic capacity, and inhibition of DNA damage,
metabolic reprogramming of epithelial to mesenchymal transition and
the cancer stem cell phenotype, and readjusting the
immunosuppressive tumor microenvironment (31,32).
Effect of hypoxia on wound
healing
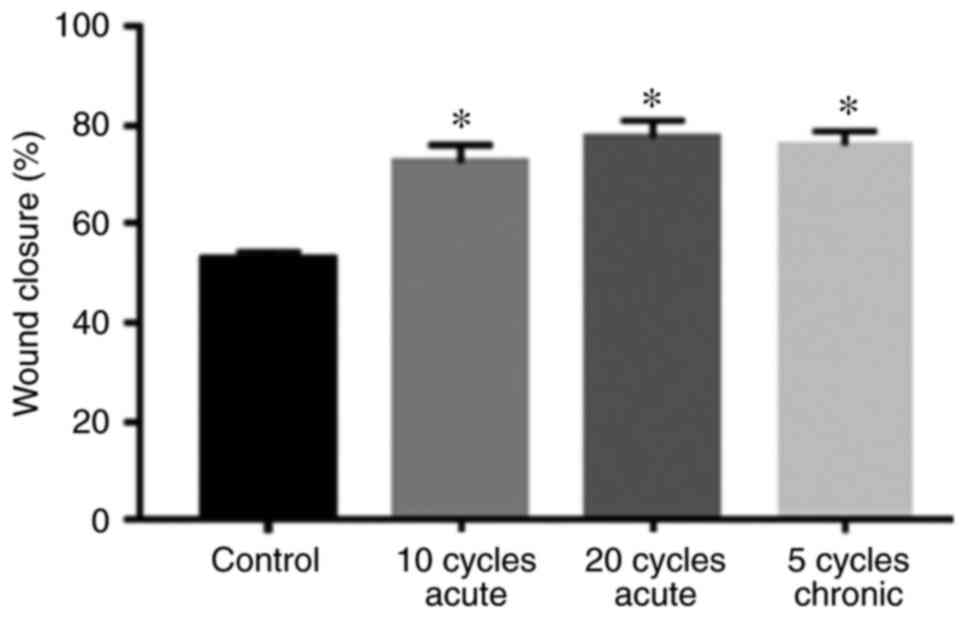
Fig. 2 shows that
the rate of wound closure of PANC1 cells exposed to acute and
chronic hypoxic cycles was significantly higher compared to
normoxic cells. Moreover, PANC1 cells exposed to 20 hypoxic cycles
exhibited a slightly higher wound closure rate compared to cells
exposed to 10 acute cycles and 5 chronic hypoxic cycles. Fig. 3 summarizes the percentages of wound
closure under normoxic conditions, 10 and 20 cycles of acute
hypoxia, and 5 chronic cycles of hypoxia after 48 h of exposure,
suggesting cell proliferation and migration.
This relatively fast pace of wound healing under
hypoxia was consistent with the reported fast invasion and
metastasis of pancreatic cancer. Several reports described the
impact of hypoxia on the proliferation and migration of PDAC. For
instance, it has been shown that HIF-1 active cancer cells locally
invaded, proliferated, and disseminated, creating a severely
hypoxic environment, and that selective eradication of HIF-1 active
cells by a pro-drug significantly suppressed the malignant
progression of advanced pancreatic cancer in animal experiments
(33). The role of hypoxia in
regulating tumor invasion through numerous molecular pathways is
widely accepted. For example, activation of multiple molecular
pathways such as PI3K/Akt, Wnt/ß-catenin, hedgehog, TGF-β, and
tyrosine kinase receptors are well accepted (34-37).
In general, hypoxia alters the expression of these genes through
HIF binding to promoters of genes containing hypoxia response
elements. Also, Chiou et al (37) found that intratumoral hypoxia in
advanced human and murine PDAC induced the expression of the
pro-metastatic transcription factor Blimp1 which serves as a key
transcriptional regulator of metastatic ability. In addition,
Velásquez et al (38) showed
that hypoxia upregulated ODZ1 gene expression and this upregulation
was correlated with a higher migratory capacity of glioblastoma
cells and when ODZ1 was knocked down, migration was drastically
reduced. The effect of hypoxia in the latter case was ascribed, in
part, to its control of the levels of hypomethylation of the ODZ1
gene promoter. Furthermore, Yu et al (39) showed that hypoxia promoted
colorectal cancer cell migration and invasion in a SIRT1-dependent
manner, and Li et al (34)
reported that hypoxia resulted in a notable increase in the
migration rate in PANC1 cells after incubation for 24 h, an effect
mediated by the hedgehog signaling pathway.
The increasing rate of wound closure (migration) in
response to hypoxia occurred as a consequence of promoting HIF1-α
and thus its effector genes. HIF1-α stimulation leads to increased
glycolysis by upregulating key genes such as HK, PKM2, and LDHA
among others (40), and by a shift
towards the non-oxidative arm of the pentose phosphate pathway
(PPP) by upregulating the expression of transketolases (TKT and
TKTL2) (41). The finding in the
present study that hypoxia upregulated HK2 (3.9 and 2.4-fold in
acute and chronic hypoxia, respectively) and TKT (2.7 and 1.5-fold,
respectively) expression are in agreement with the above
findings.
Effect of hypoxia on gene
expression
The coding genes of key glycolytic enzymes are
directly responsible for the regulation of the Warburg effect,
including GLUT1, HK2, GAPDH, PGK1, ENO1, PKM2, and LDHA (42).
Table II summarizes
the effects of 20 cycles of acute hypoxia and 5 cycles of chronic
hypoxia on the expression of selected genes. The metabolic pathways
that we focused on were: Glycolysis pathway, PPP, and the TCA
cycle. The upregulation of genes involved in these pathways showed
how hypoxia affected metabolic pathways in PANC1 cell lines. In the
glycolysis pathway, few enzymes were significantly upregulated when
PANC1 cell lines were exposed to hypoxia. These enzymes included HK
(3.9 and 2.4-fold for acute and chronic hypoxia, respectively),
G6PI (5.5 and 1.3-fold), PDHA (4.4 and 1.2-fold), and PDK (3.7 and
2.0-fold). Acute cyclic hypoxia resulted in a larger upregulation
in all of these enzymes compared with chronic hypoxia.
 | Table IIEffect of cyclic acute hypoxia and
chronic hypoxia on the mRNA expression levels of metabolism-related
genes in PANC1 cellsa. |
Table II
Effect of cyclic acute hypoxia and
chronic hypoxia on the mRNA expression levels of metabolism-related
genes in PANC1 cellsa.
| Gene symbol | Gene name | Acute hypoxia | Chronic
hypoxia | Gene function |
|---|
| G6PI | Glucose-6-Phosphate
Isomerase | 5.5±0.9 | 1.3±0.3 | Glycolysis |
| PDHA1 | Pyruvate
Dehydrogenase E1α | 2.8±0.3 | 2.1±0.2 | Catalyzes
conversion of pyruvate to acetyl-CoA and CO2. |
| PDHB | Pyruvate
Dehydrogenase E1β | 4.4±0.1 | 1.2±0.6 | Tricarboxylic acid
cycle |
| PDK1 | Pyruvate
Dehydrogenase Kinase 1 | 3.7±0.5 | 2.0±0.1 | Downregulates
mitochondrial pyruvate dehydrogenase |
| PDK2 | Pyruvate
Dehydrogenase Kinase 2 | 1.5±0.1 | 2.6±0.2 | Downregulates
mitochondrial pyruvate dehydrogenase |
| PDK3 | Pyruvate
Dehydrogenase Kinase 3 | 7.9±0.2 | 3.6±0.4 | Downregulates
mitochondrial pyruvate dehydrogenase |
| PDK4 | Pyruvate
Dehydrogenase Kinase 4 | 7.8±0.4 | 7.5±0.1 | Downregulates
mitochondrial pyruvate dehydrogenase |
| PGK1 | Phosphoglycerate
kinase 1 | 2.8±0.1 | -1.2±0.2 | Convert 1,3-DPG
into 3-PG |
| RBKS | Ribokinase | 6.8±0.6 | 4.3±0.7 | Pentose phosphate
pathway |
| HK2 | Hexokinase 2 | 3.9±0.5 | -2.4±0.8 | Glycolysis |
| TKT | Transketolase | 2.7±0.4 | 1.5±0.3 | Channeling excess
sugar phosphates to glycolysis in ppp |
| TaAldO1 | Transaldolase | 3.7±0.4 | 2.4±0.3 | Provides
ribose-5-phosphate for Nucleic acid synthesis and NADPHfor lipid
synthesis |
| FH | Fumarate
Hydratase | 4.7±0.1 | 3.8±0.2 | Tricarboxylic acid
cycle |
| PYGM | Glycogen
phosphorylase/muscle | 2.7±0.3 | 4.8±0.1 | Glycogen
degradation |
| MDH1B | Malate
Dehydrogenase 1B | 6.5±0.2 | 2.3±0.2 | Tricarboxylic acid
cycle |
HK, the first enzyme in the glycolysis pathway, has
4 isoforms: HKI, HKII, HKIII, and glucokinase. Several studies
indicated that HK is upregulated in PDAC (43,44).
The overexpression of HK is, to some extent, the result of the
HIF1-α cascade in hypoxic states. HKII enhances tumor development
and spreading by controlling lactate production in pancreatic
cancer (45). In general, the
aforementioned studies stressed the fact that pancreatic cancer is
always correlated with elevated HK expression, which is consistent
with our findings.
The experiments performed in the present study also
showed that the G6PI gene was upregulated 5.5 fold under acute
hypoxia, but to a much lesser degree under chronic hypoxia
(Table II). Additionally, western
blotting analysis confirmed the high expression of G6PI in PANC1
cells exposed to acute hypoxia (Fig.
4). Its expression decreased gradually in PANC1 cells exposed
to 5 cycles of chronic hypoxia, and decreased further in normoxic
PANC1 (Fig. 5), indicating that the
more a cancer cell is stressed by hypoxia, the greater the
upregulation in G6PI to adapt metabolically to that stress.
Consistent with this finding, Das et al
(46) found that G6PI was
upregulated in mouse tumor tissues in association with pyruvate
kinase and GAPDH. Similarly, Chan et al (47) concluded that G6PI was overexpressed
together with genes encoding enzymes involved in the glycolysis
pathway to increase ATP production in PDAC cells that require
energy for fast growth and proliferation. In addition, Lucarelli
et al (48) showed that
G6PI, also known as autocrine motility factor, is overexpressed in
clear cell-renal cell carcinoma. The enzyme is not only
overexpressed but in fact secreted by the tumor cells to work as a
growth factor that plays key roles in cancer metastasis by
activating the MAPK/ERK or PI3K/AKT pathway (49). This enzyme is involved not only in
glycolysis but also in gluconeogenesis and the PPP, processes that
are required for tumor growth. This is consistent with the finding
of De Padua et al (50) who
showed that inhibition of G6PI resulted in cancer cells
becoming reliant on oxidative phosphorylation and complete
inhibition of the Warburg effect.
The present study also showed modest overexpression
of the pyruvate dehydrogenase complex PDHA1 (E1α) by 2.8 fold
(Table II) and PDHB (E1β) by 4.4
fold in PANC1 cells exposed to acute hypoxia but even more modest
expression in those under chronic hypoxia (2.1 and 1.2,
respectively) compared to those incubated under normoxic
conditions. PDH stimulates the conversion of pyruvate to acetyl-CoA
and CO2. It is composed of several copies of three
enzymatic constituents: Pyruvate dehydrogenase (E1),
dihydrolipoamide acetyltransferase (E2), and lipoamide
dehydrogenase (E3). In the mitochondria, the E1 enzyme is present
as a heterotetramer of two E1α subunits and two E1β subunits, with
thiamine pyrophosphate as a cofactor. Golias et al (51) recently showed that hypoxia inhibited
phosphorylation of pyruvate dehydrogenase E1α in turn promoting
tumor growth in three pancreatic carcinoma cell lines. More
importantly, they also demonstrated that regulation of PDHK1
activity by hypoxia can support tumor growth. They showed that
hypoxia not only regulates the expression of PDHK1, but its kinase
activity at serine 232 of pyruvate dehydrogenase E1α as well. For
example, it has been demonstrated that patients with high levels of
both PDHK1 and phosphoserine 232 E1α in head and neck cancers
tended to have poorer outcomes due to tumor growth. Although there
are 4 PDHK enzymes that are responsible for phosphorylation at
different sites of PDH, a unique relationship has been established
between hypoxia, PDHK1, phosphoserine 232 on E1α, and regulation of
mitochondrial function (51). In
the present study, PDHK1 was upregulated 3.7-fold under acute
hypoxia but only 2-fold under chronic hypoxia (Table II), indicating that acute hypoxia
resulted in the upregulation of PDHK1 to downregulate PDH. The
other three pyruvate dehydrogenase kinases were also overexpressed
by 1.5, 7.9, and 7.8-fold under acute hypoxia and by 2.6, 3.6, and
7.5-fold under chronic hypoxia in the present study, suggesting
that phosphorylation of PDH may occur at sites other than serine
232, and that acute hypoxia may have a larger effect on the
expression of these genes other than chronic hypoxia. In support of
this overexpression, especially of PDHK3, Prigione et al
(52) revealed that increased
HIF1-α expression in cancer cells reprogrammed metabolism and
resulted in the upregulation of several genes including PDHK3.
Moreover, it was demonstrated that increased PDHK3 expression due
to elevated HIF-1 expression in three cancer cell lines played a
critical role in the metabolic switch, resulting in increased
lactic acid accumulation and drug resistance during cancer
progression and inhibition of mitochondrial respiration (53). Furthermore, Kluza et al
(54) found that pharmacological or
genetic blockades of the HIF-1a pathway decreased glycolysis and
promoted mitochondrial respiration via the specific reduction in
the expression of PDHK3, and that inhibition of PDHK3 activity by
dichloroacetate or siRNA-mediated attenuation was sufficient to
increase pyruvate dehydrogenase activity, oxidative
phosphorylation, and mitochondrial reactive oxygen species
generation, thus potentiating the effects of antitumor drugs. In
the present study, PDHK3 was most notably upregulated (7.9 and
3.6-fold) along with PDHK4 (7.8 and 7.5-fold) in acute and chronic
hypoxia, respectively (Table II),
suggesting that they could be targeted by drugs to suppress
pancreatic cancer growth.
In the present study, acute hypoxia caused a modest
upregulation in the expression of PGK1 (2.8-fold) whereas chronic
hypoxia did not result in overexpression of this gene (-1.2-fold)
(Table II). It has been shown that
hypoxia stimulated the translocation of PGK1 to the mitochondria
where it phosphorylated PDHK1 and stimulated its function (50). This activation seems to be necessary
to inhibit the activity of PDH to promote tumor growth. Therefore,
although acute hypoxia modestly upregulated PDH, its activity may
be suppressed due to the increase in expression of PGK1 and the
stimulation of PDHK1 activity.
The PPP is the pathway that a cancer cell utilizes
to synthesize lipids, nucleotides, amino acids, and NADPH needed
for growth (54). PPP has two
phases: The oxidative phase, which produces NADPH, and the
non-oxidative phase which produces ribose-5-phosphate. In the
present study, the RBKS gene was upregulated 6.8 fold in PANC1
cells exposed to acute hypoxia, and by 4.3 fold in PANC1 cells
exposed to chronic hypoxia (Fig. 4;
Table II). RBKS is the enzyme that
phosphorylates ribose to form ribose-5-phosphate which then enters
the PPP, and it is important for the synthesis of certain amino
acids such as histidine and tryptophan. Since the two types of
hypoxia assessed in the present study significantly increased the
expression of RBKS, this indicated that cancer cells shift their
metabolism towards the PPP and thus may also shift the synthesis of
other building blocks that are needed for anabolic processes. Few
reports found a change in the levels of RBKS expression in tumor
cells. In 1982, Jin and Zhou (55)
found that in parental Novikoff hepatoma cells, RBKS expression was
cell cycle-dependent with peaks in activity seen during the S,
G2, and M phases. The increased expression in the S
phase is explained by the increased need for ribose-5-phosphate to
support phosphoribosyl pyrophosphate that is in demand at this
stage, whereas the high levels during the G2 phase are
needed to maintain the flow of ribose-5-phosphate to support
glycolysis. More recently, Chaika et al (56) examined RBKS expression in
tissues to which PDAC had migrated to and found that it was
overexpressed in metastatic liver tissues of PDAC, while Je et
al (57) indicated that
inhibition of the Src family kinases in pancreatic cancer halted
cancer propagation, spread, and invasion, indicating that kinases
are essential for cancer cell growth and development (56,57).
Moreover, Payen et al (58)
revealed that pancreatic cancer cells tended to undergo metabolic
changes, such as becoming highly dependent on the PPP which
involves RBKS. Thus, pancreatic cancer cells have higher levels of
RBKS than normal pancreatic cells. Oncogenic KRAS controls
the diversion of glycolytic intermediates into ribose biosynthesis
pathways via upregulation of the non-oxidative phase of the PPP, a
pathway that is fundamental to nucleic acid synthesis and thus
cancer cell proliferation (59).
In our experiments, we found that acute hypoxia
increased the expression of TKTs by 2.7-fold compared to a modest
1.5-fold increase under chronic hypoxia (Table II). TKT, the rate-limiting enzyme
of the non-oxidative part of the PPP, catalyzes the transfer of two
carbon units between ketose- and aldosephosphate, reversibly. In
contrast, TKTL1, with a different substrate affinity and a
different catalytic activity, produces ATP and either acetate or
acetyl-CoA for lipid biosynthesis, thus promoting tumor growth.
There is a positive correlation between the invasive capacity of
different cancer types, including urothelial and colon carcinoma,
and metastasis of renal cell, ovarian and papillary thyroid
carcinoma, with TKTL1 expression (58). The present study also found
increased expression of transaldolase 1 (TALDO1) by 3.7 and
2.4-fold during acute and chronic hypoxia, respectively (Table II). TALDO enzymes are important for
linking the PPP to glycolysis (60).
The present work also showed the overexpression of
fumarate hydratase (FH) by 4.7-fold in PANC1 cell lines exposed to
acute hypoxia (Table II). The FH
enzyme is part of the TCA cycle, which stimulates the formation of
L-malate from fumarate (61). In
support of this observation, Zhao and Jiang (62) reported that FH is essential in a
cell's response to nutrient stress which is also induced by hypoxia
whereas Wang et al (63)
indicated that FH is upregulated in conditions of glucose shortage
in human pancreatic cancer cells. In the present study, glucose
shortages occurred during chronic hypoxia in which cells were
cultured in the same medium for 72 h without replacement with fresh
medium. Under such hypoxic conditions, FH gene expression was
upregulated by 3.8-fold.
In the present study, PYGM was overexpressed by 2.7
and 4.8-fold in PANC1 cells exposed to 20 cycles of acute hypoxia
and to 5 cycles of chronic hypoxia, respectively (Table II). This is consistent with the
finding of Zois and Harris (64)
that the liver form of PYG (PYGL) was upregulated under hypoxic
conditions but also glycogen synthase and other components involved
in glycogen metabolism were upregulated, indicating that tumor
cells recruit all the available resources to secure their
proliferation and metastasis.
In the present study, MDH1B was overexpressed by 6.5
and 2.3-fold in PANC1 cells exposed to 20 cycles of acute hypoxia
and to 5 cycles of chronic hypoxia, respectively (Table II). This is consistent with the
findings of Zhang et al (65) who found that MDH1 and MDH2
expression levels were elevated in primary lung tumors compared
with the matched normal controls, indicating that the cancer cells
had developed a dependence on these enzymes, especially in
situations of stress, such as that experienced during hypoxia. In
future studies, HIF-1 knockdown experiments should be performed to
confirm the molecular changes regulated by this protein.
One limitation of this study is the fact that only
one pancreatic cancer cell line was used, and this does not reflect
all pancreatic cancer subtypes and their responses towards hypoxia.
Also, it is important to mention that this study was designed to
identify a molecular metabolic hypoxic biomarker rather than
finding all genomic hypoxic biomarkers.
In conclusion, this study showed that pancreatic
cancer cells adapt to hypoxic conditions at the genomic level. The
changes were more prominent with cyclic acute hypoxia compared with
chronic hypoxia. Genes encoding enzymes needed for glycolysis such
as glucose 6-phosphate isomerase, hexokinase, and phosphoglycerate
kinase 1 and those encoding enzymes for members of the pentose
phosphate pathway such as ribokinase, transketolase, and
transaldolase were significantly upregulated. These changes are
consistent with the concept that tumors cells shift their metabolic
machinery towards glycolysis and the PPP rather than to the TCA
cycle in order to obtain the maximum amount of energy from the
available nutrients, and to build up macromolecules such as
nucleotides, fatty acids, and proteins to achieve longer
sustainability and faster proliferation, a characteristic feature
of cancer cells. The study uncovered biomarkers in tumor hypoxia
that may assist in clinical decision-making regarding the use of
chemotherapeutic agents in cancer patients.
Acknowledgements
Not applicable.
Funding
Funding: This work is supported by the Graduate School of The
University of Jordan (grant no. 19/2016/2256).
Availability of data and materials
The raw data obtained during the present is
available in GEO (GEO accession no. GSE207065) repository at:
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE207065.
Authors' contributions
NMO curated the data. MAZ conceived the study. SSA,
DAA, and WA designed the study. DAA and WA performed the
experiments. AS helped in performing the biological assays. MA
analyzed the results. MAZ and SSA confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McDonald PC, Chafe SC, Brown WS, Saberi S,
Swayampakula M, Venkateswaran G, Nemirovsky O, Gillespie JA,
Karasinska JM, Kalloger SE, et al: Regulation of pH by carbonic
anhydrase 9 mediates survival of pancreatic cancer cells with
activated KRAS in response to hypoxia. Gastroenterology.
157:823–837. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dalla Pozza E, Dando I, Biondani G, Brandi
J, Costanzo C, Zoratti E, Fassan M, Boschi F, Melisi D, Cecconi D,
et al: Pancreatic ductal adenocarcinoma cell lines display a
plastic ability to bi-directionally convert into cancer stem cells.
Int J Oncol. 46:1099–1108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nakazawa MS, Keith B and Simon MC: Oxygen
availability and metabolic adaptations. Nat Rev Cancer. 16:663–673.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Keeley TP and Mann GE: Defining
physiological normoxia for improved translation of cell physiology
to animal models and humans. Physiol Rev. 99:161–234.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Silverman HS, Wei S, Haigney MC, Ocampo CJ
and Stern MD: Myocyte adaptation to chronic hypoxia and development
of tolerance to subsequent acute severe hypoxia. Circ Res.
80:699–707. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Butturini E, Carcereri de Prati A, Boriero
D and Mariotto S: Tumor Dormancy and interplay with hypoxic tumor
microenvironment. Int J Mol Sci. 20(4305)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Al Tameemi W, Dale TP, Al-Jumaily RMK and
Forsyth NR: Hypoxia-modified cancer cell metabolism. Front Cell Dev
Biol. 7(4)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9 (Suppl 5):S4–S9. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schito L and Rey S: Hypoxic pathobiology
of breast cancer metastasis. Biochim Biophys Acta Rev Cancer.
1868:239–245. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wolff M, Kosyna FK, Dunst J, Jelkmann W
and Depping R: Impact of hypoxia inducible factors on estrogen
receptor expression in breast cancer cells. Arch Biochem Biophys.
613:23–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Goda N and Kanai M: Hypoxia-inducible
factors and their roles in energy metabolism. Int J Hematol.
95:457–463. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu CJ, Wang LY, Chodosh LA, Keith B and
Simon MC: Differential roles of hypoxia-inducible factor 1alpha
(HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell
Biol. 23:9361–9374. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shao C, Yang F, Miao S, Liu W, Wang C, Shu
Y and Shen H: Role of hypoxia-induced exosomes in tumor biology.
Mol Cancer. 17(120)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hannafon BN and Ding WQ: Intercellular
communication by exosome-derived microRNAs in cancer. Int J Mol
Sci. 14:14240–14269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li B, Qiu B, Lee DS, Walton ZE, Ochocki
JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I and Simon MC:
Fructose-1, 6-bisphosphatase opposes renal carcinoma progression.
Nature. 513:251–255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xie C, Yagai T, Luo Y, Liang X, Chen T,
Wang Q, Sun D, Zhao J, Ramakrishnan SK, Sun L, et al: Activation of
intestinal hypoxia-inducible factor 2α during obesity contributes
to hepatic steatosis. Nat Med. 23:1298–1308. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jia D, Lu M, Jung KH, Park JH, Yu L,
Onuchic JN, Kaipparettu BA and Levine H: Elucidating cancer
metabolic plasticity by coupling gene regulation with metabolic
pathways. Proc Natl Acad Sci USA. 116:3909–3918. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Saxena K and Jolly MK: Acute vs chronic vs
cyclic hypoxia: Their differential dynamics, molecular mechanisms,
and effects on tumor progression. Biomolecules.
9(339)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rofstad EK, Galappathi K, Mathiesen B and
Ruud EB: Fluctuating and diffusion-limited hypoxia in
hypoxia-induced metastasis. Clin Cancer Res. 13:1971–1978.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. FASEB J. 20:1892–1894. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Song J, Miermont A, Lim CT and Kamm RD: A
3D microvascular network model to study the impact of hypoxia on
the extravasation potential of breast cell lines. Sci Rep.
8(17949)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Minassian LM, Cotechini T, Huitema E and
Graham CH: Hypoxia-induced resistance to chemotherapy in cancer.
Adv Exp Med Biol. 1136:123–139. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He X, Wang J, Wei W, Shi M, Xin B, Zhang T
and Shen X: Hypoxia regulates ABCG2 activity through the
activivation of ERK1/2/HIF-1α and contributes to chemoresistance in
pancreatic cancer cells. Cancer Biol Ther. 17:188–198.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shukla SK, Purohit V, Mehla K, Gunda V,
Chaika NV, Vernucci E, King RJ, Abrego J, Goode GD, Dasgupta A, et
al: MUC1 and HIF-1alpha signaling crosstalk induces anabolic
glucose metabolism to impart gemcitabine resistance to pancreatic
cancer. Cancer Cell. 32:71–87.e7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim JY and Lee JY: Targeting tumor
adaption to chronic hypoxia: Implications for drug resistance, and
how it can be overcome. Int J Mol Sci. 18(1854)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qian J and Rankin EB: Hypoxia-induced
phenotypes that mediate tumor heterogeneity. In: Gilkes D (ed).
Hypoxia and Cancer Metastasis. Advances in Experimental Medicine
and Biology. Vol. 1136. Springer, Cham, pp43-55, 2019.
|
|
32
|
Kizaka-Kondoh S, Itasaka S, Zeng L, Tanaka
S, Zhao T, Takahashi Y, Shibuya K, Hirota K, Semenza GL and Hiraoka
M: Selective killing of hypoxia-inducible factor-1-active cells
improves survival in a mouse model of invasive and metastatic
pancreatic cancer. Clin Cancer Res. 15:3433–3441. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Joseph JV, Conroy S, Pavlov K, Sontakke P,
Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den
Dunnen WF and Kruyt FA: Hypoxia enhances migration and invasion in
glioblastoma by promoting a mesenchymal shift mediated by the
HIF1α-ZEB1 axis. Cancer Lett. 359:107–116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li W, Cao L, Chen X, Lei J and Ma Q:
Resveratrol inhibits hypoxia-driven ROS-induced invasive and
migratory ability of pancreatic cancer cells via suppression of the
Hedgehog signaling pathway. Oncol Rep. 35:1718–1726.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang W, Ding X, Ye H, Wang J, Shao J and
Huang T: Hypoxia enhances the migration and invasion of human
glioblastoma U87 cells through PI3K/Akt/mTOR/HIF-1α pathway.
Neuroreport. 29:1578–1585. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Y, Liu T, Yang N, Xu S, Li X and Wang
D: Hypoxia and macrophages promote glioblastoma invasion by the
CCL4-CCR5 axis. Oncol Rep. 36:3522–3528. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chiou SH, Risca VI, Wang GX, Yang D,
Grüner BM, Kathiria AS, Ma RK, Vaka D, Chu P, Kozak M, et al:
BLIMP1 induces transient metastatic heterogeneity in pancreatic
cancer. Cancer Discov. 7:1184–1199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Velásquez C, Mansouri S, Gutiérrez O,
Mamatjan Y, Mollinedo P, Karimi S, Singh O, Terán N, Martino J,
Zadeh G and Fernández-Luna JL: Hypoxia can induce migration of
glioblastoma cells through a methylation-dependent control of ODZ1
gene expression. Front Oncol. 9(1036)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yu S, Zhou R, Yang T, Liu S, Cui Z, Qiao Q
and Zhang J: Hypoxia promotes colorectal cancer cell migration and
invasion in a SIRT1-dependent manner. Cancer Cell Int.
19(116)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Semenza GL: HIF-1 mediates metabolic
responses to intratumoral hypoxia and oncogenic mutations. J Clin
Invest. 123:3664–3671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao F, Mancuso A, Bui TV, Tong X, Gruber
JJ, Swider CR, Sanchez PV, Lum JJ, Sayed N, Melo JV, et al:
Imatinib resistance associated with BCR-ABL upregulation is
dependent on HIF-1alpha-induced metabolic reprograming. Oncogene.
29:2962–2972. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Natsuizaka M, Ozasa M, Darmanin S,
Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W,
Koide H, et al: Synergistic up-regulation of Hexokinase-2, glucose
transporters and angiogenic factors in pancreatic cancer cells by
glucose deprivation and hypoxia. Exp Cell Res. 313:3337–3348.
2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
von Forstner C, Egberts JH, Ammerpohl O,
Niedzielska D, Buchert R, Mikecz P, Schumacher U, Peldschus K, Adam
G, Pilarsky C, et al: Gene expression patterns and tumor uptake of
18F-FDG, 18F-FLT, and 18F-FEC in PET/MRI of an orthotopic mouse
xenotransplantation model of pancreatic cancer. J Nucl Med.
49:1362–1370. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Anderson M, Marayati R, Moffitt R and Yeh
JJ: Hexokinase 2 promotes tumor growth and metastasis by regulating
lactate production in pancreatic cancer. Oncotarget. 8:56081–56094.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Das MR, Bag AK, Saha S, Ghosh A, Dey SK,
Das P, Mandal C, Ray S, Chakrabarti S, Ray M, et al: Molecular
association of glucose-6-phosphate isomerase and pyruvate kinase M2
with glyceraldehyde-3-phosphate dehydrogenase in cancer cells. BMC
Cancer. 16(152)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chan AK, Bruce JI and Siriwardena AK:
Glucose metabolic phenotype of pancreatic cancer. World J
Gastroenterol. 22:3471–3485. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lucarelli G, Rutigliano M, Sanguedolce F,
Galleggiante V, Giglio A, Cagiano S, Bufo P, Maiorano E, Ribatti D,
Ranieri E, et al: Increased expression of the autocrine motility
factor is associated with poor prognosis in patients with clear
cell-renal cell carcinoma. Medicine (Baltimore).
94(e2117)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kho DH, Nangia-Makker P, Balan V, Hogan V,
Tait L, Wang Y and Raz A: Autocrine motility factor promotes HER2
cleavage and signaling in breast cancer cells. Cancer Res.
73:1411–1419. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
de Padua MC, Delodi G, Vučetić M,
Durivault J, Vial V, Bayer P, Noleto GR, Mazure NM, Ždralević M and
Pouysségur J: Disrupting glucose-6-phosphate isomerase fully
suppresses the ‘Warburg effect’ and activates OXPHOS with minimal
impact on tumor growth except in hypoxia. Oncotarget.
8:87623–87637. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Golias T, Papandreou I, Sun R, Kumar B,
Brown NV, Swanson BJ, Pai R, Jaitin D, Le QT, Teknos TN and Denko
NC: Hypoxic repression of pyruvate dehydrogenase activity is
necessary for metabolic reprogramming and growth of model tumours.
Sci Rep. 6(31146)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Prigione A, Rohwer N, Hoffmann S, Mlody B,
Drews K, Bukowiecki R, Blümlein K, Wanker EE, Ralser M, Cramer T
and Adjaye J: HIF1α modulates cell fate reprogramming through early
glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells.
32:364–376. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lu CW, Lin SC, Chen KF, Lai YY and Tsai
SJ: Induction of pyruvate dehydrogenase kinase-3 by
hypoxia-inducible factor-1 promotes metabolic switch and drug
resistance. J Biol Chem. 283:28106–28114. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kluza J, Corazao-Rozas P, Touil Y,
Jendoubi M, Maire C, Guerreschi P, Jonneaux A, Ballot C, Balayssac
S, Valable S, et al: Inactivation of the HIF-1α/PDK3 signaling axis
drives melanoma toward mitochondrial oxidative metabolism and
potentiates the therapeutic activity of pro-oxidants. Cancer Res.
72:5035–5047. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jin L and Zhou Y: Crucial role of the
pentose phosphate pathway in malignant tumors. Oncol Lett.
17:4213–4221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chaika NV, Yu F, Purohit V, Mehla K,
Lazenby AJ, DiMaio D, Anderson JM, Yeh JJ, Johnson KR,
Hollingsworth MA and Singh PK: Differential expression of metabolic
genes in tumor and stromal components of primary and metastatic
loci in pancreatic adenocarcinoma. PLoS One.
7(e32996)2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Je DW, O YM, Ji YG, Cho Y and Lee DH: The
inhibition of SRC family kinase suppresses pancreatic cancer cell
proliferation, migration, and invasion. Pancreas. 43:768–776.
2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Payen VL, Porporato PE, Baselet B and
Sonveaux P: Metabolic changes associated with tumor metastasis,
part 1: Tumor pH, glycolysis and the pentose phosphate pathway.
Cell Mol Life Sci. 73:1333–1348. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Camelo F and Le A: The intricate
metabolism of pancreatic cancers. In: Le A (ed): The Heterogeneity
of Cancer Metabolism. Advances in Experimental Medicine and
Biology. Vol. 1063. Springer, Cham, pp73-81, 2018.
|
|
60
|
Best SA, De Souza DP, Kersbergen A,
Policheni AN, Dayalan S, Tull D, Rathi V, Gray DH, Ritchie ME,
McConville MJ and Sutherland KD: Synergy between the KEAP1/NRF2 and
PI3K pathways drives non-small-cell lung cancer with an altered
immune microenvironment. Cell Metab. 27:935–943.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Stewart L, Glenn GM, Stratton P, Goldstein
AM, Merino MJ, Tucker MA, Linehan WM and Toro JR: Association of
germline mutations in the fumarate hydratase gene and uterine
fibroids in women with hereditary leiomyomatosis and renal cell
cancer. Arch Dermatol. 144:1584–1592. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhao Q and Jiang Y: Fumarase mediates
transcriptional response to nutrient stress. Cell Stress. 1:68–69.
2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang Z, Wang C, Wu Z, Xue J, Shen B, Zuo
W, Wang Z and Wang SL: Artesunate suppresses the growth of
prostatic cancer cells through inhibiting androgen receptor. Biol
Pharm Bull. 40:479–485. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zois CE and Harris AL: Glycogen metabolism
has a key role in the cancer microenvironment and provides new
targets for cancer therapy. J Mol Med (Berl). 94:137–154.
2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang B, Tornmalm J, Widengren J,
Vakifahmetoglu-Norberg H and Norberg E: Characterization of the
role of the malate dehydrogenases to lung tumor cell survival. J
Cancer. 8:2088–2096. 2017.PubMed/NCBI View Article : Google Scholar
|