Introduction
Body temperature is one of the basic human vital
signs; therefore, temperature monitoring is essential during
anesthesia (1,2). Body temperature measurements include
peripheral compartment temperature and core temperature
measurements (3,4). The muscle or skin-surface temperatures
can reflect the peripheral compartment temperature (5), whereas the pulmonary artery blood
temperature is considered the gold standard for the core
temperature (6-10);
the ear, esophageal, nasopharyngeal and rectal temperatures are
considered approximated core temperatures (11,12).
However, when a temperature probe is placed in the aforementioned
positions, a risk of tissue or organ damage is possible (3). Several approaches have been developed
for the non-invasive estimation of core temperature; for example, a
method known as zero heat flux developed by Fox et al
(13) and an unobtrusive passive
heat flow sensor invented by Atallah et al (14). Flouris and Cheung (15) demonstrated that under spontaneous
breathing, a positive correlation was possible between the exhaled
breath temperature and the rectal temperature, which suggests a new
method of non-invasive temperature measurement. Logie et al
(16) reported that room
temperature and slow vital capacity (SVC) significantly influenced
exhaled breath temperature (EBT) variables in healthy children
under spontaneous breathing conditions. Under these conditions, it
is difficult to control certain respiratory parameters, such as
tidal volume, respiratory rate, and inspiratory and expiratory time
ratio (TI:TE). Compared with spontaneous breathing conditions, it
is easier to regulate breathing parameters under general
endotracheal anesthesia. However, to the best of our knowledge, EBT
measurements and the relationship between EBT and core temperature
under general endotracheal anesthesia, have not been reported to
date. Since exhaled breath comes from inside the body, its
temperature may be more representative of core temperature.
Therefore, the aim of the present study was to investigate the
breathing parameters that influence EBT and the feasibility of
using the EBT to monitor core temperature under general
endotracheal anesthesia. In the present study, it was hypothesized
that a correlation between the EBT and the nasopharyngeal
temperature (T nose) may provide a novel way of monitoring
temperature in patients undergoing general anesthesia.
Materials and methods
Patient and public involvement
In the present retrospective self-controlled trial,
patients who underwent a laparotomy under general anesthesia at the
First Affiliated Hospital of Harbin Medical University (Harbin,
China) between April 2011 and September 2012 were screened as study
subjects. The protocol of the present study was approved by the
Ethics Committee of Harbin Medical University (approval no.
201314), and written informed consent was obtained from the
patients prior to study inclusion.
Exclusion criteria
The following exclusion criteria were used: i) Past
history of asthma or chronic obstructive pulmonary disease (COPD);
ii) recent respiratory infection (within 2 weeks); iii)
space-occupying lesions of the lung (e.g. lung tumor or
tuberculosis); iv) pulmonary vascular disease (e.g. pulmonary
embolism or vasculitis); v) thoracic and pleural disease (e.g.
flail chest, pneumothorax or pleural effusion); vi) respiratory
failure; vii) acute lung injury or acute respiratory distress
syndrome; and viii) severe cardiac disease defined as New York
Heart Association class (17) III
or IV, acute coronary syndrome or persistent ventricular
tachyarrhythmia.
Standard procedures
An intraoperative colloid infusion of hydroxyethyl
starch (130/0.4) and a crystalloid infusion of acetated Ringer's
solution were infused. Up to 500 ml was administered to the patient
with induction of anesthesia and was subsequently continued at a
rate of 2-4 ml/kg/h. All solutions were pre-heated to 36˚C in an
incubator. Intraoperative liquid infusion and ion adjustment were
performed in accordance with perioperative fluid therapy
guidelines.
All patients were preoxygenated with a fraction of
inspired oxygen (FiO2) of 1.0 prior to tracheal
intubation and were maintained at an FiO2 of 0.4 during
the entire procedure of anesthesia. General anesthesia was induced
using 0.02 mg/kg midazolam, 0.4 µg/kg sufentanil, 1-2 mg/kg
propofol and 0.2 mg/kg cis-atracurium for endotracheal intubation.
Anesthesia was maintained by inhalation of sevoflurane (end tidal
concentration ≥0.7 minimum alveolar concentration) and the fresh
gas flow rate was controlled at 2 l/min; analgesia was induced with
continuous remifentanil infusion (0.05-0.3 µg/kg/min) or sufentanil
(0.1-0.3 µg/kg bolus) as required, and intermittent application of
0.05 mg/kg cis-atracurium every 40 min during surgery until 1 h
prior to the end of surgery. Intraoperative monitoring was
performed using a dedicated monitor (DatexOhmeda D-LCC15.03; Planar
Systems, Inc.) and included non-invasive blood pressure, pulse
oximetry, end-tidal fractions of carbon dioxide, electrocardiogram
and bispectral index measurements. A side stream spirometer
(DatexOhmeda S/5 Avance; Cytiva) and a D-lite transmitter were
connected to monitor peak airway pressure, plateau inspiratory
pressures, compliance and tidal volume.
Ventilation protocol
The basic ventilation consisted of volume-controlled
mechanical ventilation (DragerFabius GS premium; Drägerwerk AG
& Co. KGaA) at an FiO2 of 0.40, TI:TE of 1:2, a
respiratory rate of 12 breaths/min, positive end-expiratory
pressure (PEEP) of 0 cm H2O and tidal volume of 8 ml/kg
ideal body weight (IBW). IBW was calculated as follows: 50+0.91
[height (cm) -152.4] for men and 45.5+0.91 [height (cm) -152.4] for
women (18). The breathing loop
contained a heat moisture exchanger (HME). The T nose probe (GE
Healthcare) was placed according to the method published by Lee
et al (19); the temperature
of the operating room, the T nose, the inhaled air temperature and
the minimum EBT were recorded following 15 min of basic
ventilation. The temperature of the air was measured by
fast-response temperature probes (at 50-msec intervals). The probe
for measuring the EBT was placed between the HME and the tracheal
tube, and the probe for measuring the inhaled air temperature was
placed in the inspiratory limb outlet close to the Y-piece
(Fig 1).
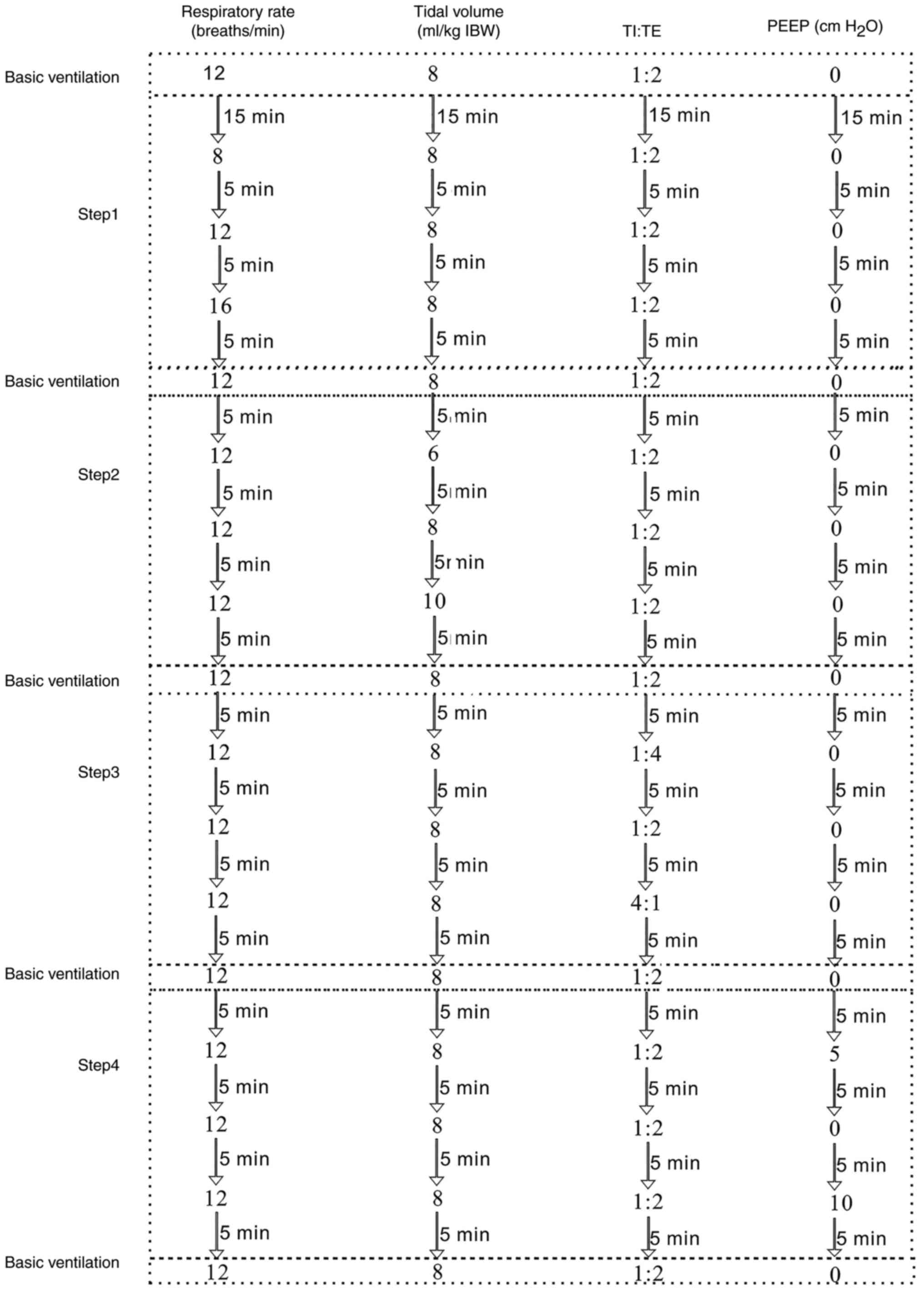
Following 15 min of basic ventilation, all patients
were ventilated in accordance with the following protocol (Fig 2): In step 1, the respiratory rate was
adjusted to 8 times/min and the other respiratory parameters were
maintained at a constant level. Following 2 min of stabilization,
the maximum temperature of exhaled air in each min (T exhale), the
inhaled gas temperature (T inhale) and the T nose at that time
point were recorded. The recording was repeated 3 times. The
respiratory rate was then adjusted to 12 times/min while
maintaining the other respiratory parameters; following 2 min of
stabilization, the aforementioned parameters were recorded. The
recording was repeated 3 times. Similarly, the respiratory rate was
subsequently adjusted to 16 times/min, and lastly, basic
ventilation was conducted for 5 min. In step 2, the tidal volume
was adjusted to the standard weight [(kg) x 6 ml] and the other
respiratory parameters were maintained at basic ventilation level.
Following 2 min of stabilization, the maximum T exhale, the T
inhale and the T nose were recorded. The recording was repeated 3
times. Similarly, the tidal volumes were sequentially adjusted to
the standard weight [(kg) x 8 ml and (kg) x10 ml, respectively] and
the T exhale, the T inhale and the T nose were recorded. The
recording was repeated 3 times. Subsequently, basic ventilation was
conducted for 5 min. In step 3, the TI:TE was altered to 1:4, while
the other respiratory parameters remained unaltered. Following 2
min of stabilization, the T exhale, the T inhale and the T nose
were recorded. The recording was repeated 3 times. Similarly, TI:TE
was adjusted sequentially to 1:2 and 4:1, and the T exhale, the T
inhale and the T nose were recorded. The recording was repeated 3
times. Subsequently, basic ventilation was conducted for 5 min. In
step 4, the PEEP was set to 5 cm H2O, while the other
respiratory parameters remained unchanged. Following 2 min of
stabilization, the T exhale, the T inhale and the T nose were
recorded. The recording was repeated 3 times. Similarly, the PEEP
was sequentially adjusted to 0 and 5 cm H2O,
respectively, and the T exhale, the T inhale and the T nose were
recorded. The recording was repeated 3 times. The respiratory
parameters were then maintained at basic ventilation level until
the end of surgery. The temperature in the operating room was
maintained at a range of 22-24˚C.
The criteria for protocol discontinuation were as
follows: i) Hemodynamic instability (mean arterial blood pressure
<60 mmHg); ii) end-tidal carbon dioxide partial pressure <30
or >40 mmHg; iii) oxygen saturation <90%; and iv) peak airway
pressure >40 cm H2O.
Statistical analysis
One-way ANOVA with Bonferroni's post-hoc test was
used to assess whether the T inhale of the patients varied with the
alterations in time. To assess whether the four different
parameters affected the T exhale, a repeated-measures ANOVA with
Bonferroni's post-hoc test was used for the four respiratory
parameters. Pearson's correlation coefficients were used to assess
the agreement between EBT and the T nose. The results are expressed
as the mean ± SD. All statistical analyses were performed with the
SPSS (version 19; IBM Corp.) statistical software package.
P<0.05 was considered to indicate a statistically significant
difference.
Results
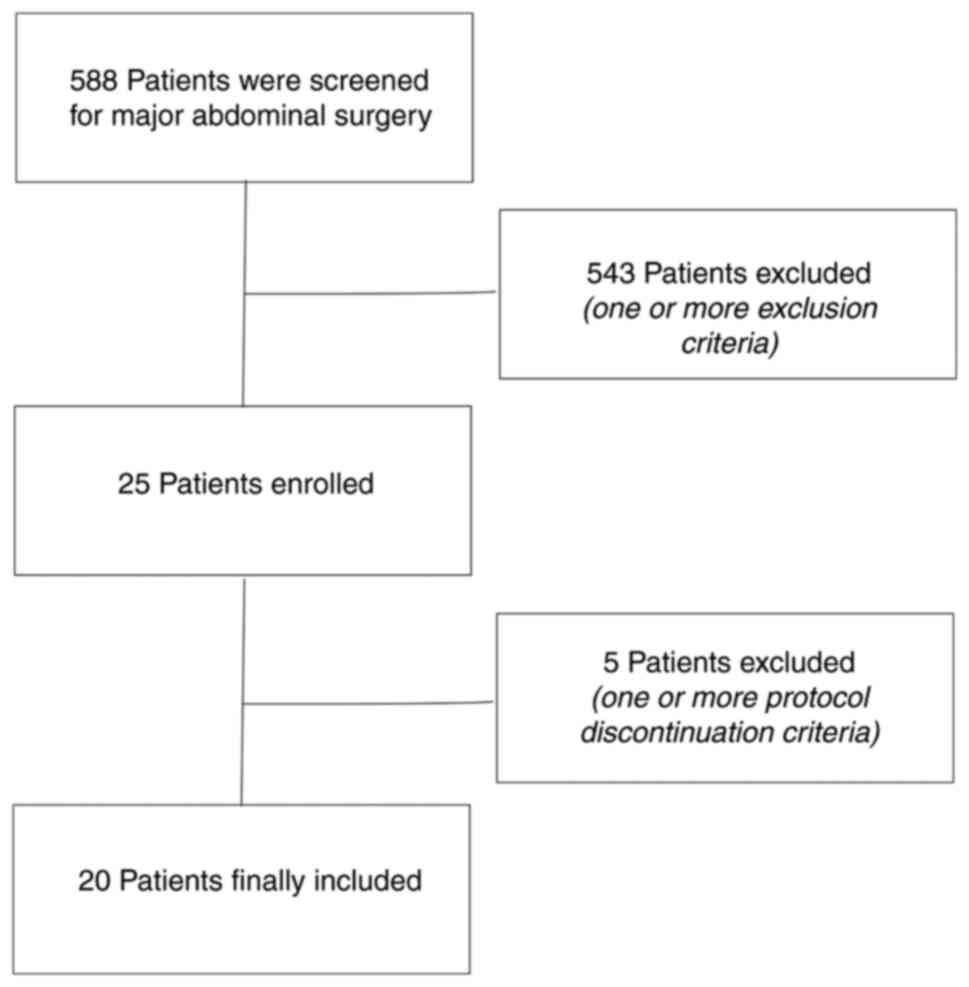
A total of 568 consecutive patients who were
scheduled to undergo major abdominal surgery were screened, of whom
543 were excluded due to one or more of the aforementioned
exclusion criteria, leading to a final inclusion of 25 patients in
the study. A total of 5 patients experienced one or more protocol
discontinuation criteria; 2 cases involved end-tidal carbon dioxide
partial pressure <30 mmHg, 2 cases had a mean arterial blood
pressure <60 mmHg and 1 case had an oxygen saturation level of
<90%. A total of 20 patients (age range, 45-65 years) therefore
participated in the final study, including 5 men and 15 women
(Fig 3). The precision of the
temperature probe was determined by the relative standard deviation
(RSD). The intraday RSD values of the exhaled and inhaled air
probes were 0.58 and 0.70%, respectively, and the interday RSD
values for the exhaled and inhaled air probes were 0.96 and 0.85%,
respectively. The demographics, EBT, operating room temperature,
operation time, intraoperative blood loss, liquid infusion and
baseline T nose at the beginning of the experiment are presented in
Table I.
 | Table IBaseline characteristics of the
patients. |
Table I
Baseline characteristics of the
patients.
| Characteristic | Patients (n=20) |
|---|
| Mean age (±SD),
years | 55.15 (11.71) |
| Mean height (±SD),
cm | 167.2 (8.02) |
| Mean weight (±SD),
kg | |
|
Actual | 62.78 (12.43) |
|
Predicted | 56.80 (5.39) |
| Smoking history, n
(%) | 11(55) |
| Type of surgery, n
(%) | |
|
Partial
hepatectomy | 4(20) |
|
Colorectal
resection | 4(20) |
|
Gastrectomy | 3(15) |
|
Exploratory
surgery | 3(15) |
|
Pancreaticoduodenectomy | 2(10) |
|
Other
procedure | 4(20) |
| Mean operation time
(±SD), min | 123.15 (3.35) |
| Mean intraoperative
blood loss (±SD), ml | 152.75 (16.61) |
| Mean intraoperative
liquid infusion (±SD), ml | 1505.25
(18.75) |
| Mean inspired
temperature during basic ventilation (±SD), ˚C | 25.41 (1.47) |
| Mean expired
temperature during basic ventilation (±SD), ˚C | 35.64 (0.70) |
| Mean operating room
temperature (±SD), ˚C | 23.34 (0.19) |
| Mean nasopharyngeal
temperature during basic ventilation (±SD), ˚C | 36.15 (0.38) |
During the experiment, the T inhale of the patients
did not vary significantly over time (Fig 4). During the first stage, no
significant difference was noted in the EBT at different levels of
respiratory rate (Fig 5). At the
second stage, no significant differences were noted in the EBT at
different levels of tidal volume (Fig
6). At the third stage, no significant differences were
observed in the EBT at different levels of TI:TE (Fig 7). At the last stage, no significant
difference was noted in the EBT at the different levels of PEEP
(Fig 8). Moreover, the data
indicated that the EBT was significantly correlated with the T nose
(Pearson's correlation r2=0.119, P<0.001; Fig 9).
Discussion
Various factors can cause hypothermia during an
operation. For example, anesthetics may impair thermoregulation,
whereas other factors include the high airflows and cold
temperatures in the operation rooms, as well as the exposure to and
use of cold fluids or blood for infusion (1). Intraoperative hypothermia is a serious
complication, which contributes to higher mortality rates and
increases surgical wound infection incidence, blood loss and
duration of post-anesthetic recovery (2). The increase in body temperature during
general anesthesia is rare; however, it is often associated with
serious adverse events, such as malignant hyperthermia or sepsis
(20,21). Therefore, temperature monitoring is
of great significance during an operation.
The present study demonstrated that the EBT of
patients undergoing abdominal surgery under general endotracheal
anesthesia was not affected by specific respiratory parameters
(tidal volume, respiratory rate, TI:TE and PEEP) when they were
within a certain range (tidal volume, 6, 8 and 10 ml/kg IBW;
respiratory rate, 8, 12 and 16 breaths/min; TI:TE, 1:2, 1:4 and
4:1; PEEP, 0, 5 and 10 cm H2O). To the best of our
knowledge, these findings represent the first report on the
relationship between EBT and respiratory parameters in patients
undergoing general anesthesia.
Previous studies have reported that the EBT of
patients with asthma is higher than that of healthy subjects
(22,23). These studies reported a correlation
between EBT and the level of exhaled nitric oxide, which is an
inflammatory marker present in asthmatics (24). Vascularization increases in the
airway mucosal layer of asthmatics, which also increases the heat
exchange of the gas during exhalation and leads to increased EBT.
The latter has been suggested to be a novel biomarker for patients
with asthma (25,26).
Previous studies that examined the effect of EBT in
various subject types were performed under spontaneous breathing
conditions. Recently, Logie et al (16) reported that room temperature and SVC
significantly influenced EBT variables in healthy children. Flouris
and Cheung (15) reported that EBT
was not influenced by the breathing patterns. However, under
spontaneous breathing, it is impossible to precisely control
ventilator parameters, such as respiratory rate, tidal volume,
inspiratory time and expiratory time. In the present study, all
patients were under a controlled respiratory status during general
anesthesia, and it was possible to more easily regulate breathing
parameters as well as measure the relationship between respiratory
parameters and EBT. Logie et al (16) demonstrated that the inhaled air
temperature could affect the EBT. In the present study, the inhaled
air temperature of the patients did not change with time, which is
consistent with the results of the study by de Castro Jr et
al (27).
It has previously been hypothesized that the level
of EBT is related to the degree of airway inflammation (22,23,28,29).
Previous studies have shown that EBT is increased in asthmatic
children (30-33)
and in patients with non-small cell lung cancer (34), as well as in patients exhibiting
exacerbated COPD (35); therefore,
patients who presented with the aforementioned diseases were
excluded in the present study.
During the conduct of the study, the T nose of each
patient was monitored. Although extraordinary caution was taken
during the installation of the probe, which was used for the
detection of the T nose according to the method by Lee et al
(19), epistaxis occurred in 4
patients. Similarly, placement of the temperature probe in the ear
canal, esophagus, rectum or other body parts represents a risk for
tissue damage. Relative to the temperature measurement of the
aforementioned body parts, the measurement of EBT is non-invasive
and may potentially represent the optimal means of measuring the
core temperature. Flouris and Cheung (15) confirmed that under spontaneous
breathing, an optimal correlation was present between the EBT and
the rectal temperature. In the present study, a significant
correlation between the T nose and the EBT was also found, which
led to the postulation that under controlled breathing conditions,
an optimal correlation may exist between the EBT and the core
temperature. In future experiments, the relationship between the
EBT and the blood temperature of the pulmonary artery (the gold
standard of core temperature) under controlled breathing conditions
will be investigated to validate the present hypothesis. The
present study highlights a novel outline for the application of the
EBT as a marker for monitoring the core temperature of patients
undergoing general endotracheal anesthesia.
The low number of subjects was a limitation of the
present study. Larger trials can provide a better correlation
between the EBT and the T nose. Another limitation of the present
study was the sole assessment of well-prepared inpatients who
underwent laparotomy under general anesthesia. The addition of
other types of patients and surgery types may increase the validity
of the findings. In addition, a limitation of this study is that
the nasopharyngeal temperature was used to represent core
temperature, rather than the gold standard of pulmonary artery
blood temperature, which will be investigated in the following
study.
The present study demonstrated that the EBT of
patients undergoing abdominal surgery under general endotracheal
anesthesia was not affected by specific respiratory parameters when
they were within a certain range and that the EBT represented a
viable method to monitor the core temperature.
Acknowledgements
Not applicable.
Funding
Funding: Financial support was provided by the National Natural
Science Foundation of China (grant no. 81402462) and the Foundation
of Heilongjiang Educational Committee (grant no. 12531245).
Availability of data and materials
The data and materials used and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
All authors participated in the collection of
samples and the observation/analysis of data and results. EL and CW
provided the conception and design of the study. DL, YW, HT, YL, PY
and YF performed sample collection. LG and JS analyzed the samples.
JS and DL confirm the authenticity of all the raw data. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
The protocol in this study was approved by the
Ethics Committee of Harbin Medical University (Harbin, China;
approval no. 201314), and written informed consent was obtained
from the patients prior to study involvement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frank SM, Beattie C, Christopherson R,
Norris EJ, Rock P, Parker S and Kimball AW Jr: Epidural versus
general anesthesia, ambient operating room temperature, and patient
age as predictors of inadvertent hypothermia. Anesthesiology.
77:252–257. 1992.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Morettini E, Turchini F, Tofani L, Villa
G, Ricci Z and Romagnoli S: Intraoperative core temperature
monitoring: Accuracy and precision of zero-heat flux heated
controlled servo sensor compared with esophageal temperature during
major surgery; the ESOSPOT study. J Clin Monit Comput.
34:1111–1119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hymczak H, Gołąb A, Mendrala K, Plicner D,
Darocha T, Podsiadło P, Hudziak D, Gocoł R and Kosiński S: Core
temperature measurement-principles of correct measurement,
problems, and complications. Int J Environ Res Public Health.
18(10606)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lenhardt R and Sessler DI: Estimation of
mean body temperature from mean skin and core temperature.
Anesthesiology. 105:1117–1121. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sessler DI: Temperature monitoring and
perioperative thermoregulation. Anesthesiology. 109:318–338.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Krizanac D, Stratil P, Hoerburger D,
Testori C, Wallmueller C, Schober A, Haugk M, Haller M, Behringer
W, Herkner H, et al: Femoro-iliacal artery versus pulmonaryartery
core temperature measurement during therapeutic hypothermia: An
observational study. Resuscitation. 84:805–809. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Uleberg O, Eidstuen SC, Vangberg G and
Skogvoll E: Temperature measurements in trauma patients: Is the ear
the key to the core? Scand J Trauma Resusc Emerg Med.
23(101)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Launey Y, Larmet R, Nesseler N, Malledant
Y, Palpacuer C and Seguin P: The accuracy of temperature
measurements provided by the edwards lifesciences pulmonary artery
catheter. Anesth Analg. 122:1480–1483. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Verheyden C, Neyrinck A, Laenen A, Rex S
and Van Gerven E: Clinical evaluation of a cutaneous zero-heat-flux
thermometer during cardiac surgery. J Clin Monit Comput.
36:1279–1287. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iden T, Horn EP, Bein B, Böhm R, Beese J
and Höcker J: Intraoperative temperature monitoring with zero heat
flux technology (3M SpotOn sensor) in comparison with sublingual
and nasopharyngeal temperature: An observational study. Eur J
Anaesthesiol. 32:387–391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wagner M, Lim-Hing K, Bautista MA, Blaber
B, Ryder T, Haymore J and Badjatia N: Comparison of a continuous
noninvasive temperature to monitor core temperature measures during
targeted temperature management. Neurocrit Care. 34:449–455.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cereda M and Maccioli GA: Intraoperative
temperature monitoring. Int Anesthesiol Clin. 42:41–54.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fox RH, Solman AJ, Isaacs R, Fry AJ and
MacDonald IC: A new method for monitoring deep body temperature
from the skin surface. Clin Sci. 44:81–86. 1973.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Atallah L, Ciuhu C, Paulussen I, Bongers
E, Blom AHM, Idrissi A and Noordergraaf G: Perioperative
measurement of core body temperature using an unobtrusive passive
heat flow sensor. J Clin Monit Comput. 34:1351–1359.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Flouris AD and Cheung SS: The validity of
tympanic and exhaled breath temperatures for core temperature
measurement. Physiol Meas. 31:N35–N42. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Logie KM, Kusel MM, Sly PD and Hall GL:
Exhaled breath temperature in healthy children is influenced by
room temperature and lung volume. Pediatr Pulmonol. 46:1062–1068.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Caraballo C, Desai NR, Mulder H, Alhanti
B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O'Connor CM, Lindenfeld
J, et al: Clinical Implications of the New York Heart Association
Classification. J Am Heart Assoc. 8(e014240)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Parsons PE, Eisner MD, Thompson BT,
Matthay MA, Ancukiewicz M, Bernard GR and Wheeler AP: NHLBI Acute
Respiratory Distress Syndrome Clinical Trials Network. Lower tidal
volume ventilation and plasma cytokine markers of inflammation in
patients with acute lung injury. Crit Care Med. 33:1–6; discussion
230-2. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee J, Lim H, Son KG and Ko S: Optimal
nasopharyngeal temperature probe placement. Anesth Analg.
119:875–879. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ellinas H and Albrecht MA: Malignant
hyperthermia update. Anesthesiol Clin. 38:165–181. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bindu B, Bindra A and Rath G: Temperature
management under general anesthesia: Compulsion or option. J
Anaesthesiol Clin Pharmacol. 33:306–316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tufvesson E, Nilsson E, Popov TA,
Hesselstrand R and Bjermer L: Fractional exhaled breath temperature
in patients with asthma, chronic obstructive pulmonary disease, or
systemic sclerosis compared to healthy controls. Eur Clin Respir J.
7(1747014)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sol IS, Kim YH, Kim SY, Choi SH, Kim HR,
Kim KW and Sohn MH: Exhaled breath temperature as a tool for
monitoring asthma control after an attack in children. Pediatr
Pulmonol. 54:230–236. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Crespo Lessmann A, Giner J, Torrego A,
Mateus E, Torrejón M, Belda A and Plaza V: Usefulness of the
exhaled breath temperature plateau in asthma patients. Respiration.
90:111–117. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Melo RE, Popov TA and Sole D: Exhaled
breath temperature, a new biomarker in asthma control: A pilot
study. J Bras Pneumol. 36:693–699. 2010.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
26
|
Piacentini GL, Peroni DG, Bodini A,
Corradi M and Boner AL: Exhaled breath temperature as a marker of
airway remodelling in asthma: A preliminary study. Allergy.
63:484–485. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
de Castro J Jr, Bolfi F, de Carvalho LR
and Braz JR: The temperature and humidity in alow-flow anesthesia
workstation with and without a heat and moisture exchanger. Anesth
Analg. 113:534–538. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Piacentini GL, Bodini A, Peroni D, Ress M,
Costella S and Boner AL: Exhaled air temperature and eosinophil
airway inflammation in allergic asthmatic children. J Allergy Clin
Immunol. 114:202–204. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Paredi P, Kharitonov SA and Barnes PJ:
Correlation of exhaled breath temperature with bronchial blood flow
in asthma. Respir Res. 6(15)2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Piacentini GL, Peroni D, Crestani E,
Zardini F, Bodini A, Costella S and Boner AL: Exhaled air
temperature in asthma: Methods and relationship with markers of
disease. Clin Exp Allergy. 37:415–419. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pifferi M, Ragazzo V, Previti A, Pioggia
G, Ferro M, Macchia P, Piacentini GL and Boner AL: Exhaled air
temperature in asthmatic children: A mathematical evaluation.
Pediatr Allergy Immunol. 20:164–171. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
García G, Bergna M, Uribe E, Yañez A and
Soriano JB: Increased exhaled breath temperature in subjects with
uncontrolled asthma. Int J Tuberc Lung Dis. 17:969–972.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Svensson H, Nilsson D, Bjermer L and
Tufvesson E: Exhaled breath temperature increases after exercise in
asthmatics and controls. Respiration. 84:283–290. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Carpagnano GE, Lacedonia D, Spanevello A,
Martinelli D, Saliani V, Ruggieri C and Foschino-Barbaro MP:
Exhaled breath temperature in NSCLC: Could be a new non-invasive
marker? Med Oncol. 31(952)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lázár Z, Bikov A, Martinovszky F, Gálffy
G, Losonczy G and Horváth I: Exhaled breath temperature in patients
with stable and exacerbated COPD. J Breath Res.
8(046002)2014.PubMed/NCBI View Article : Google Scholar
|