Introduction
A cervical erosion (or cervical ectropion) can occur
for numerous reasons among women of childbearing age (1). It is considered a benign condition
caused by pathogens or induced by increased exposure of the
cervical epithelium to estrogen. Moreover, cervical erosions can be
of traumatic, mechanical etiology, such as intrauterine devices or
foreign bodies introduced into the vagina (2). Its prevalence ranges from 17 to 50%
(3,4). The presence of ectropion is detected
especially after the menarche, during pregnancy or with use of the
combined oral contraceptive pill and is very rare in postmenopausal
women (5).
Epithelialization of the vaginal mucus and cervix is
crucial in the management of cervical erosions. Acute inflammation
of the cervix as a result from direct infection or trauma
translates into several symptoms, such as white to yellow vaginal
discharge (predominant symptom caused by the mucus-secreting
glandular epithelium), post-coital or intermenstrual bleeding,
dysuria, pelvic pain, vulvovaginal irritation and dyspareunia
(6). Symptomatic women should be
screened for infective agents (7).
Cervical ectropion has been associated with both the combined oral
contraceptive pill and intrauterine contraceptive devices as
highlighted by the study conducted by Wright et al (4). Concurrently, a number of studies have
highlighted that the use of combined oral contraceptives is highly
associated with the development of cervical ectopy, edema and
erythema of the ectopic zone (7-9).
Pathogens such as streptococci, staphylococci, or
enterococci can be promoters of acute inflammation of the cervix
and cervical ectropion (10).
Cervicitis (inflammation of the cervix) is often asymptomatic and
can cause complications of the upper genital tract with ectopia and
cervical infection by Chlamidia trachomatis, Neisseria
gonorrhoeae, herpes simplex virus, and cytomegalovirus
(11). Some factors involved in the
pathogenesis of cervical ectopy involve the action of estrogen
(12). Estrogens influence immune
and inflammatory processes, by regulating chemokines and chemokine
receptors. In a previous study by Straub, the complex processes of
inflammation related to estrogen signaling toward immune cell
trafficking were studied (13). The
T helper 17 cells producing IL-17 are the main T cells responsible
for chronic inflammation. The cervical epithelium is highly
responsive to estrogen production. Mechanistically, it is
considered that estrogens induce apoptosis in cervical cells and
also increase gene expression of human papillomavirus-16 and -18,
the two genotypes frequently associated with cervical cancer
(14). Exposure to high levels of
estrogen is also linked to an increased risk of breast cancer
(15). Estrogen influences immune
and inflammatory processes by modulating the production of
pro-inflammatory cytokines, chemokines and other immune mediators.
Specifically, estrogen can activate pathways that lead to the
production of anti-inflammatory cytokines, such as interleukin-4
(IL-4), and chemokines, such as interferon-γ (IFN-γ) (16). Furthermore, estrogen can inhibit the
production of pro-inflammatory mediators such as tumor necrosis
factor (TNF) and IL-6. Estrogen can also regulate the expression of
chemokine receptors on immune cells, which in turn helps regulate
the migration of immune cells to sites of inflammation (17). For example, estrogen can
downregulate the expression of the CCR5 receptor, which is involved
in the migration of T cells to sites of inflammation. Estrogen can
also upregulate the expression of the CXCR4 receptor, which is
involved in the migration of macrophages and neutrophils to sites
of inflammation (18).
The presence of endocervical columnar epithelium on
the ectocervix favors an increased exposure to infections due to
low cell-mediated immunity. In these areas, the subpopulation of T
lymphocytes, namely, T helper cells, CD8 cells, and CD1 lymphocytes
are reduced in number (19).
Therefore, the columnar epithelium cells are more susceptible to
infections such as Chlamydia trachomatis or Neisseria
gonorrhoeae (20). Estrogens
are capable of markedly altering the responses of host cells to
microbes. In adolescence, pregnancy, during hormonal contraception,
or during the years of menstruation (mostly in the ovulatory
phase), the probability of developing cervical ectopy is very high,
and sometimes goes undetected (5).
Furthermore, cervical ectopy implies further risks of acquiring
sexually-transmitted diseases (gonorrhea, chlamydia and human
papilloma virus). In the study conducted by Sanchez et al a
causal relationship was found between cervical erosion and
bacterial vaginosis that alter the mucosal barrier and decrease
defense mechanisms of the cervix and vagina (21).
An ongoing debate remains of whether ectopy requires
a specific treatment. The association between squamous metaplasia
and induction of squamous cell carcinoma of the cervix is well
known. Moreover, the dysplastic cells are more susceptible to
carcinogens (22). Notwithstanding
this, according to a recent study conducted by Kleppa et al,
ectopy may be a biological risk factor for chlamydia infection and
for human immunodeficiency virus (HIV) in adolescents and in young
women (7).
Currently, cryotherapy (cryosurgery) of the cervix
is the standard treatment for symptomatic, benign cervical
ectropion (23). Cryosurgery
improves the cervical mucus characteristics and therefore it is
recommended in patients with hostile cervical mucus and ectropion
(24). Prior to and after surgery,
adjuvant treatments should be promoted with the support of local,
re-epithelizing treatments (25).
For example, Belfiore et al reported the effectiveness of a
topical treatment for cervical ectropion with 5 mg of
deoxyribonucleic acid (26).
Cerviron® has a substance content that
provides beneficial properties in non-infectious vulvovaginitis and
cervical erosion. The ovule melts in the vaginal mucosa forming a
cream that ensures dispersion of the substances contained and acst
as a protective barrier with an astringent effect, favouring the
reepithelization of damaged tissue and the restoration of the
initial colpoecosystem without affecting the Döderlein bacilli. The
main mechanism of action of the medical device is the dispersion of
the substances in the vagina and the formation of a protective
barrier that accelerates the natural healing process of the damaged
epithelium.
Performance and safety data on Cerviron®
vaginal ovules have been reported from multiple sources. Three
clinical investigations on Cerviron were reported (NCT04735705,
NCT04735718 and NCT05652959 available at https://clinicaltrials.gov/ct2/show/NCT04735705;
https://clinicaltrials.gov/ct2/show/NCT04735718; and
https://clinicaltrials.gov/ct2/show/NCT05652959,
respectively) and published articles on Cerviron®
include the characterization of its utility in the management of
cervical uterine fibroids and on vaginal atrophy after surgical
treatment and adjuvant radiation therapy for cervical cancer
(27,28). However, previous safety data was
limited and included only a restricted, homogenous population,
including 50 participants in study NCT04735705 and only 27
participants in study NCT04735718.
Cerviron® is a medical device marketed by
Perfect Care Distribution in the following countries: Albania,
Latvia, Lithuania, Estonia, Kosovo, Montenegro, Romania, Kuwait,
and the United Arab Emirates. With a complex composition including
hexylresorcinol, collagen and bismuth subgallate, and four
phytotherapeutic extracts including Calendula officinalis,
Hydrastis canadensis, Thymus vulgaris extract and
Curcuma longa, it is intended as adjuvant treatment for the
management of cervical lesions and vulvovaginitis.
Materials and methods
Study objectives
The present study was designed as part of the
medical device post-marketing clinical follow-up, involving routine
care from a variety of clinical practices. The study includes an
open-label, multicentric, non-randomized, single-arm, real-world
evidence study design. The data were collected between the 20th of
May 2021 and 31st of July 2021.
Real-world evidence studies are post-marketing
studies bringing valuable information related to the medical
devices' safety and performance profiling and a broader
understanding of the practice pattern and the clinical outcomes.
The rationale of the study was aimed at capturing safety data in a
broader, more heterogenous population. Cerviron® vaginal
ovules have been used with success in the treatment of acute and
chronic vulvovaginitis of mechanical etiology, and in cervical
lesions of mechanical origin, but with a limited number of study
participants (NCT04735705 and NCT04735718). Real-world evidence
studies and clinical trials are complementary. The present study is
considered a real-world evidence study as it reflects actual
clinical aspects with data collected in the context of routine
delivery of care, as opposed to data collected within a clinical
trial, where study design controls variability in ways that are not
representative of real-world care and outcomes.
The primary objective was to evaluate the
tolerability of Cerviron® ovules in the treatment and
management of cervical erosions of various etiologies. The
secondary objective of this study was the assessment of performance
of the medical device by clinical exam and patients' degree of
satisfaction related to the use of the medical device.
Study population
The target population included women aged 20 to 70
years with symptomology associated with cervical ectropion of
various etiology such as cervix trauma, postpartum injuries,
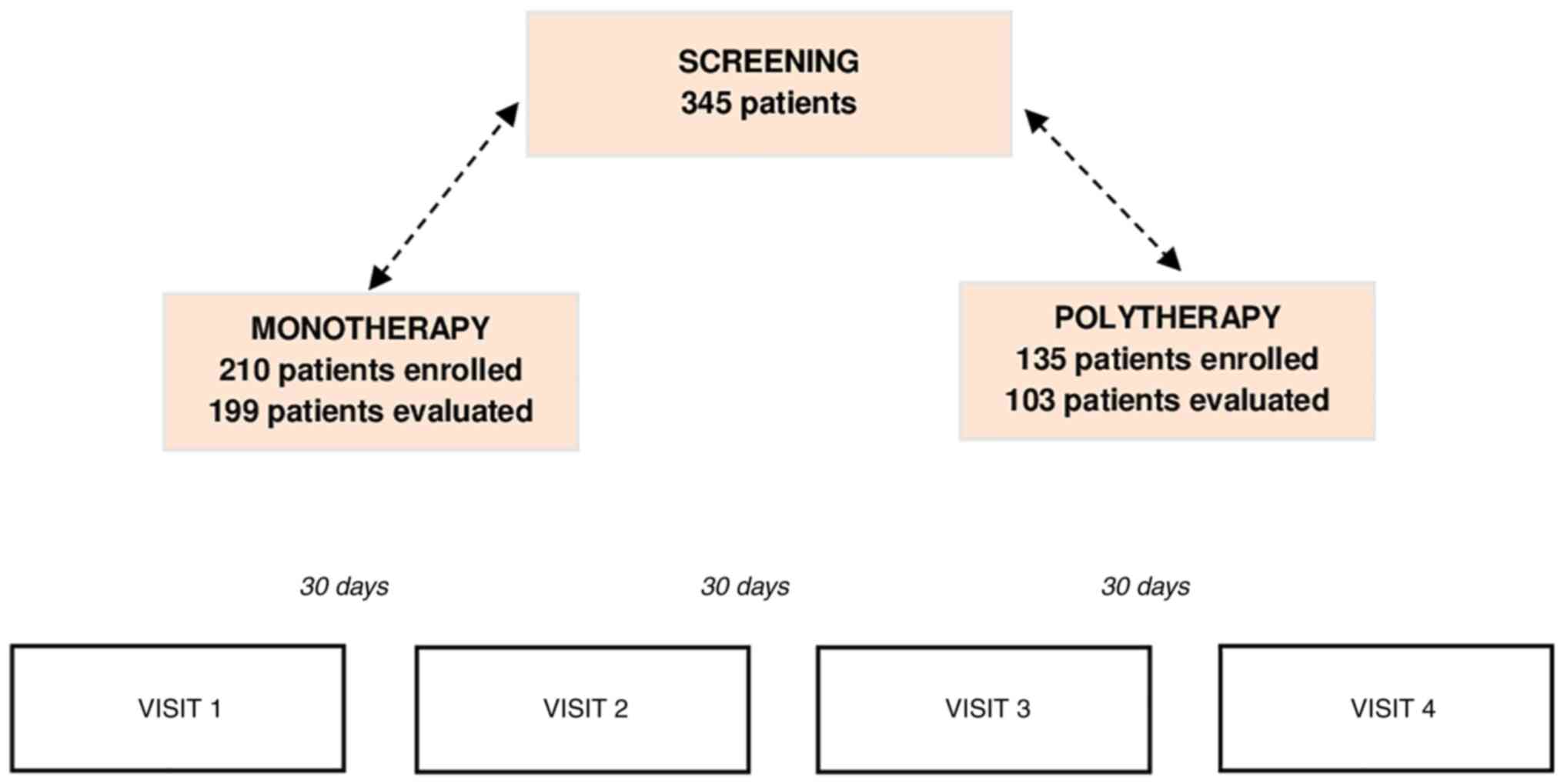
vaginal infections and cervicitis. A total of 345 women were
evaluated. A number of patients were treated with
Cerviron® ovules as monotherapy (n=210) and other
patients were prescribed Cerviron® ovules as an adjuvant
in therapeutic schemas containing antibiotics, antivirals and/or
anti-inflammatory drugs (n=135). Subjects with a previous history
of any malignancy, including subjects with vulvar, vaginal, or
cervical cancer or with undiagnosed abnormal genital bleeding were
excluded.
The study involved 30 Romanian specialist physicians
as investigators from 16 institutions each treating between 6 and
22 patients. The participating clinical practices and their
locations are listed as Table I.
Upon study entry and at 1, 2 and 3 months after the initial visit,
participants were interviewed and received visual cervical
examinations by colposcopy. At each visit, participants received a
standardized pelvic exam with placement of a speculum and
visualization of the cervix.
 | Table IClinical practices and their
locations. |
Table I
Clinical practices and their
locations.
| Institution | City, Country
(Romania) |
|---|
| Clinical Hospital
‘Dr Ion Cantacuzino’ Bucharest | Bucharest |
| Hospital MedLife
Humanitas Cluj-Napoca | Cluj-Napoca |
| Medical office of
Dr Saleh K. Majed | Craiova |
| Medical office of
Dr Surpanelu Oana | Iasi |
| Natisan Medical
Center | Pitesti |
| Gynecological
office of Dr Rădulescu G. Mihaela Elena | Râmnicu Vâlcea |
| Medical office of
Dr. Popescu | Sibiu |
| MediBlue Medical
Clinic | Iasi |
| SC Pan Medical
SRL | Sibiu |
| Medical clinic of
Dr. Cioata Ionel Trifon | Timisoara |
| iMED Clinic | Sibiu |
| Tulcea County
Emergency Hospital | Tulcea |
| Bradmed SRL | Targu Jiu |
| Gynecological
office of Dr Ioana Trotea Targu Jiu | Targu Jiu |
| Medical office of
Obstetrics and Gynecology of Dr Sirbu Daniela SRL | Timisoara |
| Dr Todorut
Florina | Timisoara |
Prospective data were collected from each patient,
such as initial diagnosis, colposcopy examination results,
re-epithelialization degree, vaginal pH value, vaginal symptoms,
any worsening symptoms and adverse events.
Ethical and regulatory aspects of the
study
Written consent for study participation was
collected from all patients. General Data Protection Regulation
(GDPR) consent forms were collected from all patients. Due to legal
considerations (GDPR directive effective from 21 May 2018 in all
European Union countries), patients or their legal representatives
have an absolute right to request that their data be removed from
the study database. A Notified Body (Ente Certificazione Macchine
SRL) reviewed the post marketing clinical follow-up plan, including
ethical considerations. As this is a post-marketing clinical
follow-up study, an Ethics Committee approval was not required, as
per the regulations described below.
The study was conducted in accordance with the Guide
to medical devices: ‘Post-market clinical follow-up studies’
(https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-210325-wng65.pdf)
and the International Society for Pharmacoepidemiology (ISPE; 2015)
Guidelines for ‘Good pharmacoepidemiology practices (GPP)’
(https://www.pharmacoepi.org/resources/policies/guidelines-08027/).
The collected data and study procedures were
conducted in accordance with the ethical principles that have their
origin in the Declaration of Helsinki.
The study followed the definition of the
non-interventional (observational) study provided by the Guide to
Good Pharmacovigilance Practices (GVP; 2017): Module
VIII-Post-authorization safety studies (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-viii-post-authorisation-safety-studies-rev-3_en.pdf).
The study followed the nature of the non-interventional
(observational) studies mentioned in the ICH Harmonized Tripartite
Guide Pharmacovigilance Planning E2E (ICH, 2004; https://database.ich.org/sites/default/files/E2E_Guideline.pdf).
Revision risk analysis was carried out in accordance
with the medical device regulation, available from: https://www.anm.ro/en/dispozitive-medicale/regulamentele-europene-privind-dispozitivele-medicale.
Data were stored according to Annex E of ISO 14155:2020 (https://www.iso.org/standard/). The study and its
details are registered in www.clinicaltrials.gov under ID NCT05668806.
Statistical consideration
All statistical analyses were performed using the
Excel Analysis ToolPak, version 16.69.1, from Microsoft. P<0.05
was considered to indicate a statistically significant
difference.
The quality and completeness of the collected data
were preliminarily assessed in comparison with data analysis. No
study participant was involved in any violation of
inclusion/exclusion criteria. To examine the treatment significance
over time, Fisher's exact test was performed for categorical
variables, and Mann-Whitney U test was employed to perform
comparative analysis for variables non-normally distributed.
Results
Range of gynecological conditions and
medical history of selected patients
The selected patients presented with various
gynecological conditions consistent with the instructions for the
use of the medical device. Some patients presented with other
conditions with similar symptoms. As the evaluation of the
performance of the device was a secondary objective, other
conditions that would enable further investigations for expanding
the use of the medical device were also selected (Table II).
 | Table IIMedical history of selected
patients. |
Table II
Medical history of selected
patients.
| Gynecological
condition | Number of patients
(n=345) Age, 20-70 years |
|---|
| Anexitis | 4 |
| Atypical squamous
cells cannot exclude high grade squamous intraepithelial
lesion | 5 |
| Atypical squamous
cells of undetermined significance | 39 |
| Cervical
dysplasia | 7 |
| Cervical
ectropion | 47 |
| Cervicitis | 59 |
| Dyspareunia | 6 |
| Endocervicitis | 8 |
| Endocervicosis | 11 |
| Exocervicitis | 61 |
| Low-grade squamous
intraepithelial lesions | 6 |
| Metroanexitis | 2 |
| Negative for
intraepithelial lesion or malignancy | 2 |
| Ovarian cyst | 2 |
| Post
Conization | 10 |
| Uterine
fibroids | 1 |
| Uterus
filaments | 1 |
| Vaginal
atrophy | 2 |
| Vaginal polyps | 1 |
| Vaginal
prolapse | 1 |
| Vulvovaginitis | 70 |
Subjects were followed undergoing treatment with
Cerviron® vaginal ovules for ~3 months. Each enrolled
subject visited the clinic four times, at 30-day intervals each.
The study visit scheme is listed in Fig. 1. In the monotherapy group, 11
patients (5.23%) were considered dropouts due to the fact that they
did not attend all the study visits and could not be evaluated. In
the polytherapy group, 32 patients (23.7%) were considered dropouts
(Fig. 1).
Primary objectives
The study primary objective was to assess the
tolerability of the medical device by the number of possible
adverse reactions observed during the treatment. In one patient
treated exclusively with Cerviron® ovules, during the
routine gynecological examination one adverse reaction was noted,
which was erythema. The erythema was followed by itching and
abnormal vaginal discharge. The investigators determined that this
was not related to treatment with the medical device. At the
following visit, the reaction was absent.
Two other patients received combined treatment with
antibiotics and Cerviron® ovules. After 30 days, both
patients reported an adverse reaction of itching and erythema.
Investigators determined that the symptoms were consistent with the
initial diagnosis. After 60 days, these reactions were absent in
both patients. All the aforementioned incidents were linked to
preexisting conditions and the investigators decided that they must
not be regarded as adverse reactions to the medical device
Cerviron®.
Clinical performance assessed by the
investigator by colposcopy
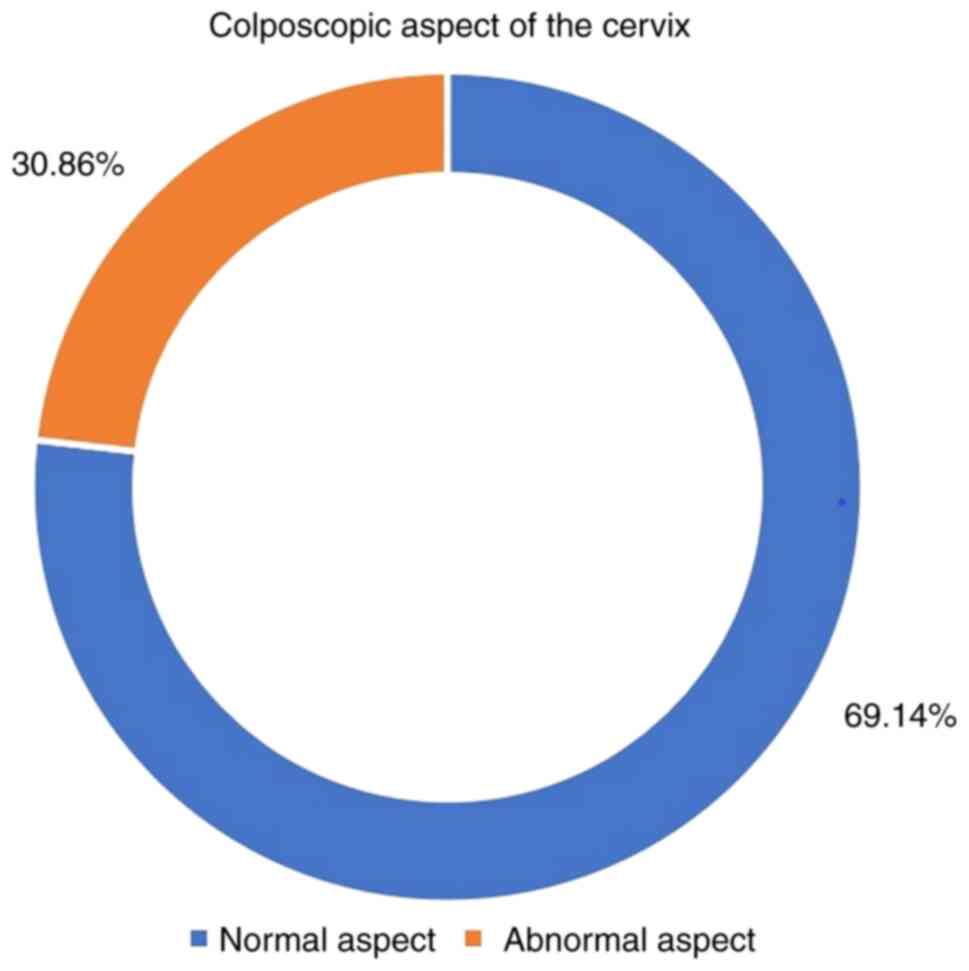
A significant proportion of 69.14% patients were
rated with the indicator ‘normal aspect’ during colposcopy
(Fig. 2).
Re-epithelialization degree of the
cervical mucosa by gynecological examination
For only 302 patients out of 345, medical records
evaluating the degree of epithelialization of the cervical mucosa
were available. At the initial visit, 302 participants were
assessed, of which 3.64% were rated with ‘complete epithelization’,
40.40% were rated with degree of ‘partial re-epithelialization’ and
55,96% were rated with ‘absent, ulcerations present’. At 30 days,
47.68% of the 302 women assessed, had a favorable degree of
re-epithelialization, with only 4.30% rated as ‘absent, ulcerations
present’. After 3 months, 73.17% of the 287 patients were rated
with ‘complete epithelization’ (Table
III).
 | Table IIIDegree of re-epithelization at
initial evaluation, 30 and 90 days. |
Table III
Degree of re-epithelization at
initial evaluation, 30 and 90 days.
| Degree of
re-epithelialization | Initial
evaluation | At 30 days | At 90 days |
|---|
| Absent | 169 (55.96%) | 13 (4.30%) | 6 (2.09%) |
| Partial | 122 (40.40%) | 145 (48.01%) | 71 (24.74%) |
| Complete | 11 (3.64%) | 144 (47.68%) | 210 (73.17%) |
| Total (N) | 302 | 302 | 287 |
Vaginal pH level evaluation
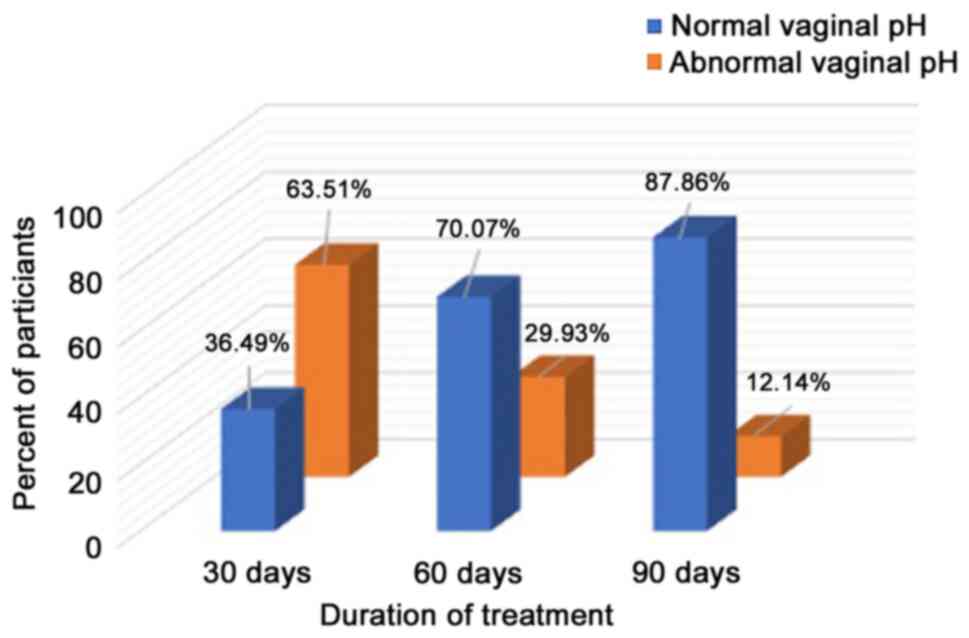
It was observed that following a 3-month treatment
with Cerviron®, 87.86% of the patients presented with a
normal pH (Fig. 3).
Evaluation of the level of pain
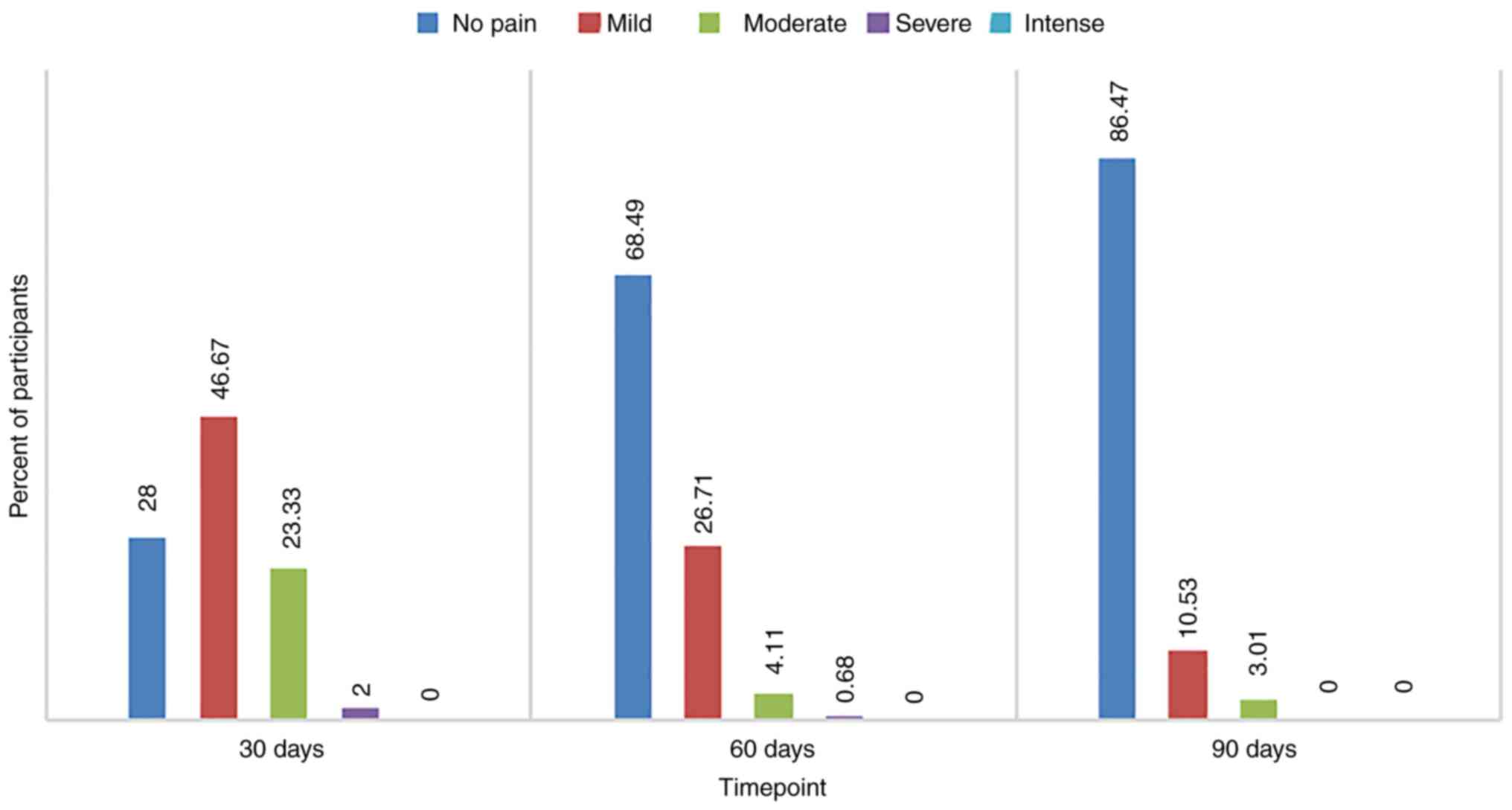
Of the total number (n=302) of patients examined at
visit 1, 46.67% reported mild pain levels and 28.00% had no pain at
all. At the second visit, 68.49% of the population (207) rated pain
severity as no pain or mild severity in 26.71%. A very high
proportion (86.47%) was pain free at the last visit after 3 months
of treatment with Cerviron® (Fig. 4).
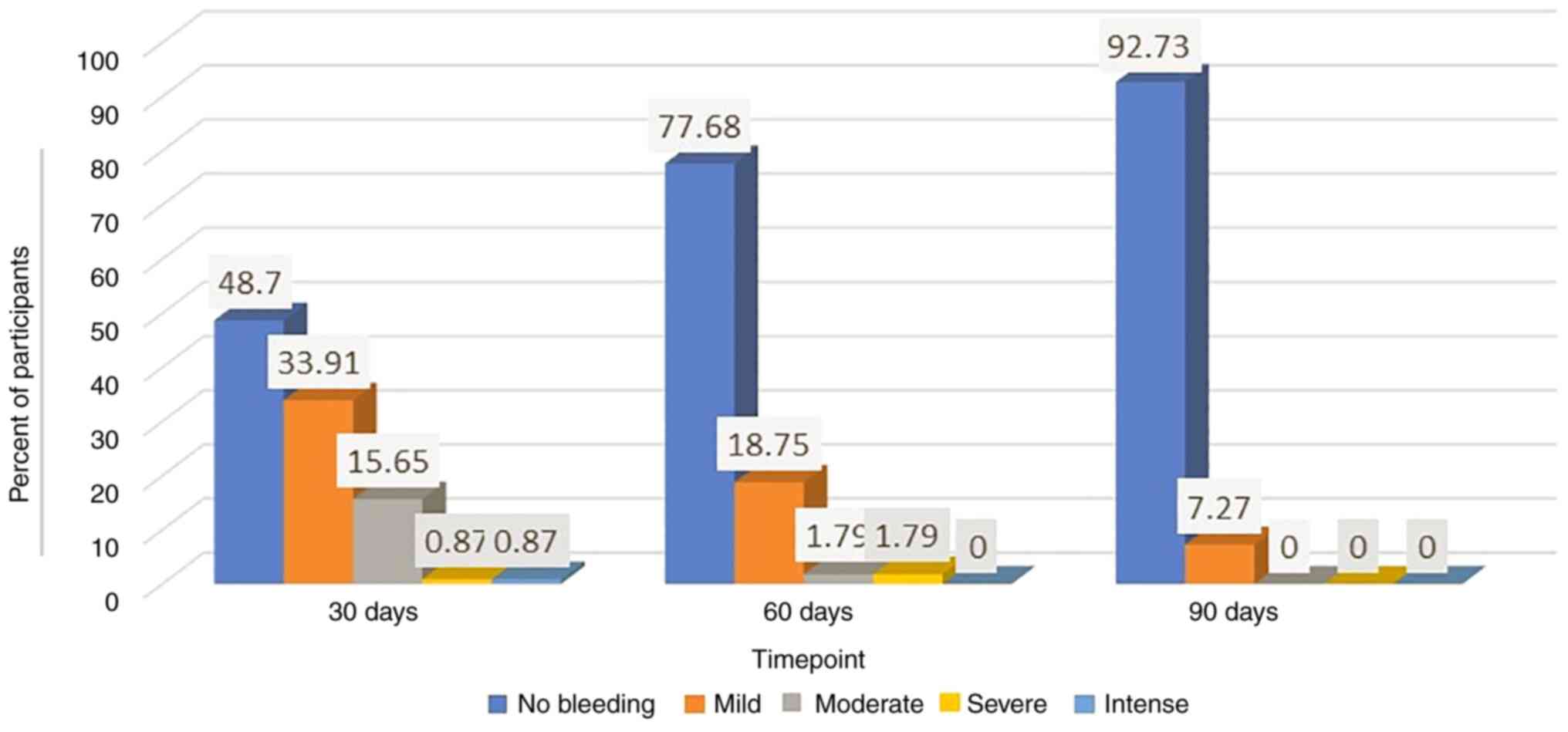
Evaluation of the level of vaginal
bleeding
At the first visit, 302 women included in the study
were evaluated, of whom 48.70% reported no bleeding observed after
treatment with Cerviron® and 33.91% reported low amounts
of vaginal bleeding. The efficacy of medical device was more
clearly observed at visit 2, where 77.68% of patients experienced
no bleeding at all and 1.79% experienced moderate or severe
bleeding. After 3 months of treatment, this improvement was
noteworthy when 92.73% of patients reported no bleeding and only
7.27% of patients reported a mild level of bleeding (Fig. 5).
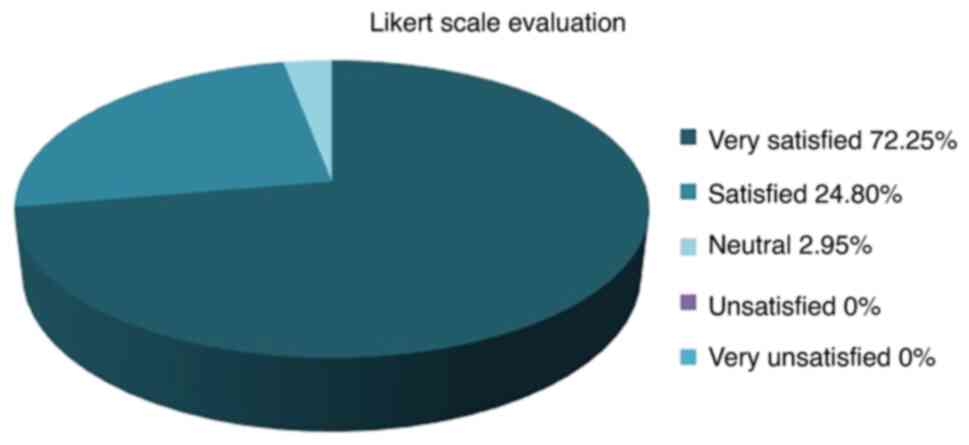
Degree of satisfaction after using
Cerviron® ovules
In terms of degree of satisfaction offered,
participants in the largest population (72.25%) were reported to be
‘very satisfied’ with the medical device. The degree of
satisfaction was measured by a 5-point Likert scale (29) (Fig.
6).
Discussion
A very important aspect that influences the
management of cervical ectopy is age. Cervical ectopy is
predominant in young adolescents, with a prevalence of up to 80% in
sexually active adolescents (30).
However, factors associated with the evolution and devolution of
cervical ectopy are diverse and mechanisms have yet to be
elucidated. Since ectopy is observed frequently in adolescent girls
and pregnant women, it may vary in response to hormonal
fluctuations observed in pregnancy and during contraception with
combined oral contraceptives (30).
Screening for Chlamydia trachomatis and Neisseria
gonorrhoeae can prevent a range of gynecological conditions,
such as urethritis, cervicitis, cervical erosions (31). Infection in the lower genital tract
can result in upper genital tract complications, such as pelvic
inflammatory diseases, ectopic pregnancy, chronic pelvic pain, and
infertility in asymptomatic women, and transmission of infection
during pregnancy and labor (32).
Cryotherapy (cryosurgery) of the cervix is an
effective treatment for symptomatic cervical ectropion (33). Other surgical techniques have been
applied for ablation of the squamocolumnar junction. Cauterization
has been applied for this purpose since 1920, but it involves
additional risks, such as burning lesions if the safeguard plate is
not located correctly (34). Some
specialists even consider the cervical ectropion as a normal
finding that does not require treatment despite the red and
inflamed appearance of the cervix. However, cervical ectropion is
considered as one of the most common types of chronic cervicitis
worldwide (35). Topical treatments
for cervical ectropion, including cervical painting with gentian
violet paint or microwave tissue coagulation, are still widely used
(4). Although the debate is still
ongoing, a harmonized treatment strategy would reduce complications
of cervical ectropion, such as developing abnormal metaplasia
and/or vaginal infection. Applying an intervention to treat
cervical ectopy is recommended, especially when considering taking
preventive action against cervical cancer. Ectopic cells are
modified over time by squamous metaplasia and epithelialization,
cervical infection with Neisseria gonorrhea or Chlamidia
trachomatis, low pH values or trauma (12). Cerviron® appears to have
a very potent action in rebalancing the vaginal pH. In a previous
clinical investigation, NCT04735705, vaginal pH values measured
over 90 days showed that Cerviron® restores altered
vaginal pH. The difference in vaginal pH values between baseline
vs. 90 days, at a 5% significance level was statistically
significant (P<0.05).
A literature review conducted by Machado et
al indicated that treatment can be used to relieve occasional
symptoms associated with ectopy, but does not support routine
treatment for ectopy (12).
By contrast, a study conducted by Soares et
al, showed a positive association between cervical ectopy and
human papillomavirus, HIV, bacterial vaginosis, cervical epithelial
atypia, postcoital bleeding, and desquamative inflammatory
vaginitis (36). Recognition of
cervical ectopy should alert the clinician to the possibility of a
genital chlamydia infection. Opportunistic screening for chlamydia
in young people should be offered to reduce the prevalence of
infection and its sequelae. Chlamydia trachomatis is the
most common bacterial sexually transmitted disease. In women,
chlamydial infection usually presents as cervicitis, which can lead
to up to 30 to 50% of pelvic inflammatory disease episodes. Pelvic
inflammatory disease has significant reproductive sequelae, such as
tubal infertility and ectopic pregnancy (37).
The main mechanism of action of the medical device
is the dispersion of the substances in the vagina and the formation
of a protective barrier that accelerates the natural healing
process of the damaged epithelium.
The effect of the medical device is obtained due to
the presence of the bismuth subgallate and vegetable collagen.
Bismuth subgallate is an insoluble solution, with a very low
bio-availability which will create a physical barrier over the
affected area of the vaginal mucosa and therefore will not allow
oxygen and pathogens to come into contact with it (38,39).
In this way, the substances create the premise that allows the
damaged tissue to heal naturally. The role of exogenous collagen is
to be a "sacrificial substrate" in order to reduce excess
metalloproteins that delay wound healing and to regulate the
healing of damaged tissue (14,40-43).
As such, Cerviron® supportive therapy may be prescribed
in sexually active patients for at least 1-3 months, 10-15
consecutive days, to prevent these infections.
The findings in the present study revealed that a
90-day treatment (3 treatment sessions of 30 days each) with
Cerviron vaginal ovules was beneficial in providing a complete
degree of cervical epithelialization and reduced the multitude of
the vaginal symptoms, including bleeding, inflammation, malodor,
dysuria, dyspareunia, pain and leukorrhea. This result is
consistent with previous clinical studies that included the same
medical device, NCT04735705 and NCT04735718(44).
In conclusion, the findings of the present study
revealed that administration of Cerviron® vaginal ovules
provided a complete degree of epithelization in majority of
patients attending a 90-days tretatment. Moreover, subjects
presenting a high grade of ulceration at the baseline visit,
following treatment with Cerviron® presented a complete
degree of epithelization. Cerviron® ovules are an
exceptional adjuvant for acceleration of healing of cervical
erosions. They favor the re-epithelialization of the damaged tissue
and restoration of the initial colpo-ecosystem. Their topical
application was observed to be effective in reducing unpleasant
symptoms such as vaginal discharge, pelvic pain, and vaginal
bleeding. In terms of adverse events, the medical device is
considered safe. The only contraindication to Cerviron®
ovules has been determined as hypersensitivity to the active
substance or to any of the excipients within the medical
device.
Acknowledgements
We would like to thank Mrs Florentina Liliana
Calancea (MDX Research, Timisoara, Romania) for medical writing and
Mr Alexandru Pinta (Labcorp Austria GmbH, Vienna Austria) for
support in data management and statistical analysis.
Funding
Funding: Perfect Care Distribution SRL
(https://www.perfectcare.ro/), the study sponsor, offered the
tested medical devices and a partial grant support.
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable request.
The study and its details are registered in www.clinicaltrials.gov under ID NCT05668806.
Authors' contributions
IP, RP and ADT were involved in the study design,
methodology and original draft preparation. IP and DTS performed
the data collection and data analysis. RP, ADT and IP were involved
in analysis and interpretation of data. EP and FD-P were involved
in manuscript review and editing. IP, DTS and ADT participated in
the interpretation of the study results. FD-P and EP confirm the
authenticity of all the raw data. All authors approved the final
version of the manuscript to be published.
Ethics approval and consent to
participate
Only participants that voluntarily provided their
written consent to the collection of their study data were
included. The collected data and study procedures were conducted in
accordance with the ethical principles that have their origin in
the Declaration of Helsinki. As this is a post-marketing clinical
follow-up study, an Ethics Committee approval was not required, but
the study protocol was approved by a Notified Body (Ente
Certificazione Macchine SRL, Bologna Italy).
Patient consent for publication
Not applicable.
Competing interests
FD-P and EP are employed at Perfect Care
Distribution SRL. All the other authors declare no competing
interests. The funder Perfect Care Distribution SRL had no role in
the design of the study, or in the collection, analyses, or
interpretation of data, or in the writing of the manuscript, or in
the decision to publish the results.
References
|
1
|
Wildenberg JC, Yam BL, Langer JE and Jones
LP: US of the nongravid cervix with multimodality imaging
correlation: Normal appearance, pathologic conditions, and
diagnostic pitfalls. Radiographics. 36:596–617. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferenczy A: Benign Lesions of the Cervix.
In: Pathology of the Female Genital Tract [Internet]. Blaustein A
(ed). Springer, New York, NY, 1977.
|
|
3
|
Kuhn L, Denny L, Pollack AE and Wright TC:
Prevalence of visible disruption of cervical epithelium and
cervical ectopy in African women using Depo-Provera. Contraception.
59:363–367. 1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wright KO, Mohammed AS, Salisu-Olatunji O
and Kuyinu YA: Cervical Ectropion and Intra-Uterine Contraceptive
Device (IUCD): A five-year retrospective study of family planning
clients of a tertiary health institution in Lagos Nigeria. BMC Res
Notes. 7(946)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aggarwal P and Ben Amor A: Cervical
Ectropion. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2023.
|
|
6
|
Mitchell L, King M, Brillhart H and
Goldstein A: Cervical Ectropion May Be a cause of desquamative
inflammatory vaginitis. Sex Med. 5:e212–e214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kleppa E, Holmen SD, Lillebø K, Kjetland
EF, Gundersen SG, Taylor M, Moodley P and Onsrud M: Cervical
ectopy: Associations with sexually transmitted infections and HIV.
A cross-sectional study of high school students in rural South
Africa. Sex Transm Infect. 91:124–129. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldacre MJ, Loudon N, Watt B, Grant G,
Loudon JD, McPherson K and Vessey MP: Epidemiology and clinical
significance of cervical erosion in women attending a family
planning clinic. Br Med J. 1:748–750. 1978.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dugas LR, Lie L, Plange-Rhule J, Bedu-Addo
K, Bovet P, Lambert EV, Forrester TE, Luke A, Gilbert JA and Layden
BT: Gut microbiota, short chain fatty acids, and obesity across the
epidemiologic transition: The METS-Microbiome study protocol. BMC
Public Health. 18(978)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Critchlow CW, Wölner-Hanssen P, Eschenbach
DA, Kiviat NB, Koutsky LA, Stevens CE and Holmes KK: Determinants
of cervical ectopia and of cervicitis: Age, oral contraception,
specific cervical infection, smoking, and douching. Am J Obstet
Gynecol. 173:534–543. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ortiz-de la Tabla V and Gutiérrez F:
Cervicitis: Etiology, diagnosis and treatment. Enferm Infecc
Microbiol Clin (Engl Ed). 37:661–667. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Machado Junior LC, Dalmaso AS and Carvalho
HB: Evidence for benefits from treating cervical ectopy: Literature
review. Sao Paulo Med J. 126:132–139. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Straub RH: The Complex Role of Estrogens
in Inflammation. Endocr Rev. 28:521–574. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Singh M and Singh N: Curcumin counteracts
the proliferative effect of estradiol and induces apoptosis in
cervical cancer cells. Mol Cell Biochem. 347:1–11. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang
N, Conaway M, Wang H, Korach KS, Bocchinfuso W and Santen R:
Effects of estrogen on breast cancer development: Role of estrogen
receptor independent mechanisms. Int J Cancer. 127:1748–1757.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kassi E and Moutsatsou P: Estrogen
receptor signaling and its relationship to cytokines in systemic
lupus erythematosus. J Biomed Biotechnol.
2010(317452)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chakraborty B, Byemerwa J, Krebs T, Lim F,
Chang CY and McDonnell DP: Estrogen receptor signaling in the
immune system. Endocr Rev. 44:117–141. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rodriguez-Lara V, Peña-Mirabal E,
Baez-Saldaña R, Esparza-Silva AL, García-Zepeda E, Cerbon Cervantes
MA, Diaz D and Fortoul TI: Estrogen receptor beta and CXCR4/CXCL12
expression: Differences by sex and hormonal status in lung
adenocarcinoma. Arch Med Res. 45:158–169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
De Luca Brunori I, Facchini V, Filippeschi
M, Battini L, Giusti G, Romani L, Scida P and Urbano M:
Cell-mediated immunity in the course of cervical ectropion. Clin
Exp Obstet Gynecol. 21:105–107. 1994.PubMed/NCBI
|
|
20
|
Wang LC, Yu Q, Edwards V, Lin B, Qiu J,
Turner JR, Stein DC and Song W: Neisseria gonorrhoeae infects the
human endocervix by activating non-muscle myosin II-mediated
epithelial exfoliation. PLoS Pathog. 13(e1006269)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sánchez A, Rivera A, Castillo F and Ortiz
S: Cervical erosion as result of infectious vaginitis. Euro J Exp
Bio. 2:1659–1663. 2012.
|
|
22
|
Çekmez Y, Şanlıkan F, Göçmen A, Vural A
and Türkmen SB: Is cryotherapy friend or foe for symptomatic
cervical ectopy? Med Princ Pract. 25:8–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yildiz S, Alay I, Eren E, Karaca I,
Gultekin G, Kaya C and Cengiz H: The impact of cryotherapy for
symptomatic cervical ectropion on female sexual function and
quality of life. J Obstet Gynaecol. 41:815–820. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baram A, Paz GF, Peyser MR, Schachter A
and Homonnai ZT: Treatment of cervical ectropion by cryosurgery:
Effect on cervical mucus characteristics. Fertil Steril. 43:86–89.
1985.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu T, Chen Z, Xia Q, Jiang S, Jin Q,
Farahani MR and Cai L: A suppository for treating cervical erosion
and its preparation method. Clin Exp Obstet Gynecol. 40:361–366.
2013.PubMed/NCBI
|
|
26
|
Belfiore P, Costa E, De Cantis S, Vassallo
R and Marino A: Effectiveness and persistence of a topical
treatment for cervical ectropion with deoxyribonucleic acid.
Minerva Ginecol. 57:461–466. 2005.PubMed/NCBI
|
|
27
|
Bacalbasa N and Balescu IC:
2022-RA-462-ESGO The effects of Cerviron on vaginal atrophy after
surgically treated and adjuvant radiation therapy for cervical
cancer. Int J Gynecol Cancer. 32(Suppl 2):A1–A504. 2022.
|
|
28
|
Bacalbasa N, Balescu I, Aloul AA, Bohiltea
R, Socea B, Ursut B and Filipescu GA: The role of topic vaginal
products in posthysterectomy vaginal atrophy. Ro Med J. 68:344–346.
2021.
|
|
29
|
Robinson J: Likert Scale. In: Encyclopedia
of Quality of Life and Well-Being Research [Internet]. Michalos AC
(ed). : Springer Netherlands, Dordrecht, 2014.
|
|
30
|
Bright PL, Norris Turner A, Morrison CS,
Wong EL, Kwok C, Yacobson I, Royce RA, Tucker HO and Blumenthal PD:
Hormonal contraception and area of cervical ectopy: A longitudinal
assessment. Contraception. 84:512–519. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pillay J, Wingert A, MacGregor T, Gates M,
Vandermeer B and Hartling L: Screening for chlamydia and/or
gonorrhea in primary health care: Systematic reviews on
effectiveness and patient preferences. Syst Rev.
10(118)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Senn T: Sexually Transmitted Diseases
(STDs). In: Encyclopedia of Behavioral Medicine [Internet]. Gellman
MD and Turner JR (eds). Springer, New York, NY, 2013.
|
|
33
|
Agah J, Sharifzadeh M and Hosseinzadeh A:
Cryotherapy as a method for relieving symptoms of cervical ectopy:
A Randomized clinical trial. Oman Med J. 34:322–326.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen J, Zhou D, Liu Y, Peng J, Li C, Chen
W and Wang Z: A comparison between ultrasound therapy and laser
therapy for symptomatic cervical ectopy. Ultrasound Med Biol.
34:1770–1774. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang K, Li J, Liu Y, Ma B, Roberts H, Tan
J, Tian J, Wu T and Zhang P: Microwave therapy for cervical
ectropion. Cochrane Database Syst Rev.
2007(CD006227)2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Soares LC, Braz FLTA, Araújo AR and
Oliveira MAP: Association of sexually transmitted diseases with
cervical ectopy: A systematic review. Sex Transm Dis. 46:452–457.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jacobson DL, Peralta L, Graham NM and
Zenilman J: Histologic development of cervical ectopy: Relationship
to reproductive hormones. Sex Transm Dis. 27:252–258.
2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tramontina VA, Machado MA, Nogueira Filho
Gda R, Kim SH, Vizzioli MR and Toledo Sd: Effect of bismuth
subgallate (local hemostatic agent) on wound healing in rats.
Histological and histometric findings. Braz Dent J. 13:11–16.
2002.PubMed/NCBI
|
|
39
|
Thorisdottir H, Ratnoff OD and Maniglia
AJ: Activation of Hageman factor (factor XII) by bismuth
subgallate, a hemostatic agent. J Lab Clin Med. 112:481–486.
1988.PubMed/NCBI
|
|
40
|
Jafari B, Jafari-Sales A, Khaneshpour H,
Fatemi S, Pashazadeh M, Al-Snafi AE and Shariat A: Antibacterial
effects of Thymus vulgaris, Mentha pulegium,
Crocus sativus and Salvia officinalis on pathogenic
bacteria: A brief review study based on gram-positive and
gram-negative bacteria. Jorjani Biomed J. 8:58–74. 2020.
|
|
41
|
Jafari B and Ahmadizadeh C: The In Vitro
Study of Antimicrobial Effect of Marigold (Calendula officinalis)
Extract on Infectious Microorganisms. Electronic J Biol.
13:348–352. 2017.
|
|
42
|
Dai C, Lin J, Li H, Shen Z, Wang Y, Velkov
T and Shen J: The natural product curcumin as an antibacterial
agent: Current achievements and problems. Antioxidants (Basel).
11(459)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Petrita R: Real-World Performance and
Safety of Cerviron® Medical Device in the Treatment of Various
Types of Vaginitis. Biomed J Sci & Tech Res. 47:38364–38373.
2022.
|