Bone is a highly vascularized tissue whose vascular
supply strictly limits its development, remodeling and
regeneration. In specific pathologies/conditions, such as critical
bone defects due to trauma, osteonecrosis and tumor resection, the
limited ability of bone to heal itself requires external
regenerative bone procedures, where tissue engineering and
biomaterials come on stage. During bone regeneration, sufficient
vascular supply provides the bone tissue with essential nutrients,
oxygen, growth factors (GFs) and hormones (1). Therefore, while developing artificial
bone substitutes that provide temporary mechanical support and
boost bone regeneration, the necessary condition of
neovascularization must also be taken into account. Traditional
tissue engineering (TE) techniques treat bone defects by
introducing osteoblasts or osteogenic-differentiated mesenchymal
stem cells (MSCs) onto/into a scaffold and undergo a period of
in vitro culture followed by implantation. However, the
cell-loaded bone substitute is initially avascular. In
circumstances where the defect exceeds a thickness of 200 µm,
hypoxic conditions occur immediately after implantation, resulting
in the death of the seeded cells (2). To avoid necrosis, alternative
cell-free in situ TE (iTE) techniques were developed with
the fundamental recognition that mammals have self-regenerative
potential and may be manipulated by the provided microenvironmental
cues. In situ bone TE (iBTE) scaffolds may be engineered to
contain biologically instructive/microenvironmental cues that, when
implanted, may modulate the endogenous stem/progenitor cells'
behavior, such as angiogenesis-osteogenesis coupling and
inflammation, eventually leading to tissue repair (Fig. 1) (3,4).
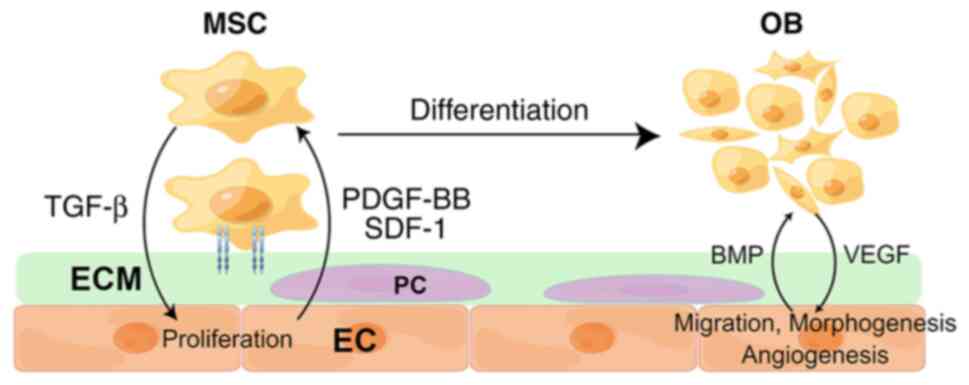
Studies at the cellular and molecular levels have revealed the
interaction between endothelial cells (ECs) and osteoblasts (OBs),
and by synchronously modulating the two, facilitated the
achievement of vascularized bone regeneration (VBR) (5,6). In
addition, a range of studies has found that promoting angiogenesis
alone was also able to enhance bone regeneration (Fig. 2) (7-9).
Based on the current understanding, the development of biomaterial
scaffolds for iBTE has been upgraded by combining proangiogenic
factors with osteoinductive/osteoinductive biomaterials. In the
present narrative review, iTE strategies, particularly those
targeting VBR, are summarized.
BTE has undergone significant advancements over the
years, transitioning from conventional methods to more
sophisticated approaches. One such development is the iBTE, which
has progressed from the traditional concept to the utilization of
cell-free scaffolds possessing microenvironmental cues.
TE traditionally emphasizes the importance of three
key components: Cells, scaffolds and signaling molecules (10). Cells generate the extracellular
matrix (ECM) and other factors crucial for tissue growth and
repair. Scaffolds offer a structural framework for cells to attach
and migrate, while signaling molecules modulate cell behavior and
differentiation. By utilizing endogenous cells, engineered
scaffolds and bioactive cues/signaling molecules, the iBTE method
remains consistent with the traditional concept while advancing its
application. It builds upon them by leveraging advanced
biomaterials and strategies to enhance the body's regenerative
capabilities.
In contrast to cartilage and nerve tissues, which
exhibit limited endogenous cellularity near defects and necessitate
the use of conventional cell-seeded TE scaffolds, bone tissue
presents a highly suitable target for iTE strategies. This
suitability arises from bone's innate characteristics, including
its abundant endogenous cell population, intrinsic structural
support, remarkable self-healing capacity and sensitivity to
microenvironmental cues (3).
The concept of iBTE originated from the observation
that the body's natural bone healing process may be harnessed and
enhanced by providing a suitable scaffold-microenvironment. Early
cell-free scaffolds were composed of natural or synthetic
biomaterials designed to mimic the structure and properties of
native bone tissue, such as calcium phosphate bioceramics,
collagen, hydroxyapatite and various biodegradable polymers
(11). Over time, researchers have
developed more advanced cell-free scaffolds, incorporating
bioactive materials and functional modifications to promote bone
regeneration (12). In the
subsequent sections, current perspectives on iTE approaches for VBR
will be explored.
The definition of VBR in the current literature
broadly consists of several terms: VBR, vascularized osteogenesis,
and angiogenesis and osteogenesis. Being familiar with the
terminology facilitates the search and summary of the relevant
literature. The cellular basis behind VBR is closely linked to the
coupling of angiogenesis and osteogenesis; therefore, to evaluate
the potential impact of iBTE scaffolds on the in situ VBR,
ECs and OBs or MSCs have been widely used (13,14).
However, it is suggested that numerous biological agents that may
promote angiogenesis also act on osteogenesis directly or
indirectly. Evolution has provided the physiological necessity that
the two processes are paired. Proliferation assays, such as the
Cell Counting Kit-8 and 5-bromo-2-deoxyuridine assay, are the
foremost modality for evaluating the cytotoxicity of different
bioactive agents (15). For
angiogenesis evaluation, at a cellular level, the effect of the
biomaterials on EC migration and morphogenesis is usually assessed
by scratch-healing assay and tube-formation assay (16). At the molecular level, biomarkers
related to angiogenesis, such as hypoxia-inducible factor (HIF)-1α,
VEGF, basic fibroblast GF (bFGF), platelet-derived GF (PDGF) and
angiopoietin 1, are usually detected by fluorescence quantitative
PCR and western blot analysis (16). As for bone regeneration, alkaline
phosphatase (ALP) and alizarin red are usually detected
qualitatively and quantitatively by co-incubating OBs or MSCs with
the biomaterials or supplemented with their extracts to the culture
medium. The expression of molecular markers related to bone
formation, such as ALP, bone morphogenetic protein (BMP), RUNX
family transcription factor 2 (Runx2) and collagen type I (Col1),
is further detected. With the advancement of high-throughput
technology, proteomics, transcriptome sequencing and enrichment
analysis have also been applied to evaluate the effect of scaffold
materials on endogenous cells and screen for their potential
mechanisms (17).
This did not hamper the development of the current
iBTE strategy, which emphasizes ‘biomimicry’, which describes the
designing of materials or structures that mimic the natural
properties of living organisms. In the context of iTE for VBR,
biomimicry involves creating a microenvironment at the implanted
site that closely resembles the sequence of events that occur
during natural bone healing (3).
This may involve using biomaterials that have similar mechanical
properties to bone, as well as incorporating GFs and other
signaling molecules that are known to have a role in bone
regeneration (28). By mimicking
the natural healing process, researchers hope to promote more
efficient and effective bone regeneration in vivo. Much
effort has been focused on two technical routes toward the common
goal: Directly endowing the scaffold with angiogenesis-osteogenesis
coupling factors or modulating the early inflammatory
microenvironment towards a proangiogenic and proosteogenic state
(29). The two routes share certain
commons by providing biophysical/biochemical cues through
extracellular or intracellular mechanisms.
Biophysical cues are physical properties of
biomaterials proven to have roles in directing cell function and
stem cell differentiation commitment (30). They are frequently regarded as
primary elements in biomaterial design. iBTE scaffolds are designed
for the common purpose of promoting in situ VBR. Their forms
and types may be broadly classified into e.g. mesoporous scaffolds,
hydrogel networks, nanoparticles, electrospun fiber and 3D printing
constructs (3).
Although different scaffolds are prepared in a
diversity of means, there is a consensus that scaffolds should have
a porous structure. Pores in the scaffold allow cells to penetrate,
attach, migrate and proliferate. At the same time, the infiltrating
neo-capillaries may deliver oxygen and nutrients and remove
metabolites (31). It has been
reported that the pore structure has important effects on cell
inoculation efficiency, viability, migration, morphology,
differentiation and angiogenesis (32-34).
The porous structure is characterized by pore size, geometry,
inter-pore connectivity and porosity. In terms of size,
hundred-micron, micron and nanoscale pore structures are referred
to as macropore, micropore and nanopore, respectively. Hayashi
et al (35) designed
honeycomb scaffolds (HCS) of different sizes (100, 200 and 300 µm)
to investigate the threshold of the most effective macropore size
of iBTE scaffolds. At four weeks after implantation into rabbit
femoral defects, it was observed that the HCS with 300 µm pore size
were extensively filled with new bone and vascular tissue,
demonstrating that scaffolds with a high degree of inter-pore
connectivity and homogeneity at 300 µm are more conducive to in
situ VBR. Studies further revealed that the progressive
hierarchy of pore size indicated that the multi-scaled pore
distribution is more advantageous than the single-scaled one. Wang
et al (36) fabricated an
apatite-collagen-polycaprolactone (PCL) scaffold by filling
crosslinked collagen into the pores of a 3D-printed PCL scaffold,
which was then mineralized in vitro by simulated body fluid
immersion. The scaffold possesses a macro-micro-nanoporous
hierarchy, which favors the host bone ingrowth and biomaterial
osseointegration. Another scaffold with a macro-medium-microporous
architecture was fabricated based on
poly(3-hydroxybutyrate-poly-hydroxyhexanoate) (37). The scaffold on which ECs were
cultured exhibited increased migration and metabolic activity,
suggesting proangiogenic potential. After being loaded with the
pro-osteogenic BMP, the multi-level porous scaffold achieved a
significant increase in bone regeneration and revascularization
after being implanted in a segmental bone defect model. Based on
the findings on porosity, the advancement of 3D printing technology
has further enabled the readiness of processing bone scaffolds with
gradient porosity, as well as customized architecture, shape and
mechanical strength. In addition, different bioactive molecules may
be loaded and precisely immobilized within specific regions
(38-40).
Lian et al (41) used a
low-temperature deposition model to prepare a spongy PCL scaffold
with the same hierarchical and interconnected pores, which was able
to promote the paracrine effects of MSCs via focal adherent kinase,
its downstream AKT and yes-associated protein (YAP) mechanical
signaling pathways, leading to a pro-regenerative macrophage
phenotype, neovascularization and eventually the VBR.
Other biophysical properties of iBTE scaffolds, such
as stiffness, surface geometry and mechanical stimulation, have
also been proven to alter the local tissue microenvironment through
intracellular and intercellular signaling (3,42). MSC
differentiation is influenced by the stiffness of the biomaterial,
in which rigid material induces the osteogenic differentiation of
MSCs and softer matrices promote their adipogenic differentiation
(43-45).
ECs also exhibit different morphology and transcriptomic profiles
when cultured on various substrates with varying stiffness. A shift
from round to elongated morphology was observed as the stiffness of
the culture surface increased from soft to hard (46,47).
For instance, Santos et al (48) incubated ECs onto collagen-coated
polyacrylamide (PAAm) hydrogels with different stiffnesses. They
observed that ECs on high-stiffness PAAm hydrogels had
downregulated expression of VEGF receptor-2 (VEGFR2) protein and an
upregulated expression of caveolin-1, wingless-type 2, BMP-2 and
bFGF, indicating that hydrogel rigidity has a particular effect to
promote both angiogenesis and bone formation (48).
The design of the surface geometry of the iBTE
scaffold has also been the focus of research in recent years. ECs
may sense the modification in the micro- and nano-texture of the
culture surface and regulate per se the actin polymerization
and migration via Rac family small GTPase 1 and cell division cycle
42(49). Abagnale et al
(50) compared the behavior of MSCs
on polyimide fabricated with different groove morphologies and
found that a 15-µm groove promoted adipogenic differentiation and
rendered cells with a rounded appearance, whereas a 2-µm groove
promoted osteogenic differentiation and led to elongated cell
morphology. Of note, MSCs were cultured on nanosheets with 600 nm
diameter, 650 nm spacing and 200 nm groove depth and exhibited an
elongated shape without any tendency to differentiate. MSCs were
able to express the corresponding genes for osteogenesis and
adipogenesis when treated with osteogenic and adipogenic media. It
has been postulated that the nanoscale surface structure resembles
cell receptors and the guidance by contact may influence and
regulate the fate of stem/progenitor cells (51). Although studies of material surface
morphology have focused on in vitro studies of EC, MSC or
macrophage behavior and in vivo studies are lacking, the
results provide a sound theoretical basis for designing
VBR-targeted iBTE scaffolds.
In addition to the surface topology, the mechanical
force generated by the scaffold is also a biophysical
microenvironmental cue affecting cells. The iBTE fibrous scaffolds
doped with magnetic nanoparticles undergo minor deformation when an
external magnetic field is applied and therefore, they were able to
produce bending and stretching effects on the cells to which they
are attached (52,53). Hao et al (54) discovered that their
superparamagnetic scaffolds inhibited the activation of macrophage
Toll-like receptor 2/4 and enhance VEGFR2 activity, inhibiting the
expression of downstream pro-inflammatory cytokines and
upregulating VEGF and PDGF expression. This discovery indicated the
possibility of mechanomodulation of macrophages to indirectly
achieve VBR.
Nanomaterials with piezoelectric properties have
also been investigated in iBTE scaffolds. Upon external stress, the
dipoles in crystallized poly(hydroxybutyrate-co-hydroxypentanoic
acid) (PHBHV) internally rotate and eventually generate electricity
or electrodeposition. It has been indicated that MSCs cultured on
PHBHV fibers improved the vascularization of engineered bone tissue
(55). Similarly, the GaN/AlGaN
scaffold developed by Zhang et al (56) was found to promote osteogenic
differentiation of MSCs and in vivo bone regeneration by
modulating the intensity and direction of the piezoelectric
polarization.
The biophysical cues, such as porosity and surface
geometry endowed by the iBTE scaffolds, acting on endogenous
stem/progenitor cells are under investigation (31). However, the explanation may also be
attributed to the rearrangement of cytoskeletal networks after
cell-receptor recognition and aggregation. For instance, integrin,
once bound to the surface of biomaterials, may, in turn, activate
the downstream Wnt, YAP and c-Jun N-terminal kinase signaling,
leading to changes in gene expression (57). The design of iBTE scaffolds aims to
create a bioinstructive microenvironment to regulate the behavior
of endogenous cells through materials, which requires a
comprehensive understanding of organismal physiopathology, cellular
function and material science. Previous studies have focused on the
effect of a single biochemical cue on cells. Still, as research
advances the understanding of biophysical signatures, the design of
iBTE scaffolds in the future will be able to integrate multiple
factors to achieve in situ VBR.
Compared to relatively recent times, when
researchers began to realize the role of physical factors in
biological processes, studies on biochemical molecules have a far
longer history. Biochemical cues refer to chemical signals that are
involved in regulating cellular behavior and communication
(30). Biochemical cues may be
broadly classified as GFs, bioactive protein molecules, metallic
ions, Traditional Chinese Medicine (TCM) and compound biologics,
such as decellularized ECM (dECM), platelet-rich plasma (PRP) and
exosomes (58), which act on other
cells in the extracellular environment. These cues may be
incorporated into/onto the iBTE scaffolds via various processing
methods and mechanisms, most of which have been examined previously
both in vitro and in vivo to elucidate a relatively
precise mechanism of action and therapeutic effects. Therefore, the
iBTE scaffolds are more likely to have a role not just as
structural support but also as carriers for cues. iBTE scaffolds
for in situ VBR were designed to deliver biochemical cues
via extracellular signaling mechanisms. This approach is considered
safer and more straightforward. This extracellular mechanism avoids
the need to directly manipulate the genetic material of cells,
which can be more complex and potentially riskier. Therefore, they
are more widely studied and gradually translated into clinical
practice.
The broad studies of GFs are an ideal arsenal for
bioengineering researchers to selectively choose their armors from.
The most widely studied GFs targeting VBR are the BMPs, members of
the TGF-β superfamily. BMP-2 and BMP-7 have been reported to have
dual functions in osteogenic differentiation and angiogenesis
(59-61).
Two products loaded with human recombinant BMP-2 and BMP-7, INFUSE™
and OP-1™, respectively, have completed clinical trials
and are approved for use (62-64).
BMP-2 promotes the osteogenic commitment of MSCs and
osteoprogenitors (OP) and indirectly enhances neovascularization
through the paracrine effects of Ops (65). Similarly, BMP-7 promotes
neovascularization by upregulating VEGF expression in ECs (66). However, applying BMPs has an
uncontrollable risk of ectopic bone formation (67). Due to these undesirable effects, the
OP-1™ was removed from the market globally. Therefore,
the latest BMP-based iBTE strategy focuses extensively on
developing novel biomaterials with optimal controlled and
spatiotemporal delivery properties (68).
Other GFs, such as VEGF, FGF and PDGF-BB, were also
found to be involved in the process of VBR. VEGF is the primary GF
controlling blood-vessel formation and osteogenesis (69). Various iBTE scaffolds delivering
VEGF have demonstrated a beneficial effect on the in situ
VBR (9,70-73).
As the spatial and temporal arrangement and the emergence of GFs
and their mechanism of action in the microenvironment of bone
regeneration were clarified, studies are more inclined to
investigate different fabrication modalities, such as 3D printing,
frozen microgels and nanomaterials, to achieve a precise spatial
and temporal delivery (74-77).
Lee et al (74) developed a
dual cryogel system consisting of gelatin/chitosan cryogel (GC) and
gelatin/heparin cryogel (GH) to achieve the sequential release of
two GFs: The outer GH releases VEGF to induce early angiogenesis to
provide blood supply in the defect area, while the inner GH
releases BMP-4 for the continuous osteogenic induction. In another
system, Zhou et al (76)
loaded bFGF in a gelatin methacrylate hydrogel to mimic the
angiogenic signal from soft callus during early bone healing, while
BMP-2 was incorporated into the mineral-coated microparticles to
simulate the osteogenic signal during hard callus formation and
bone remodeling. The biomimetic strategy has achieved an early bFGF
release accompanied by sustained release of BMP-2, mimicking the
typical GFs presentation in the natural bone healing process. Of
note, in vitro and in vivo studies indicated that
PDGF-BB, secreted by osteoclast (OC) precursors, was able to
promote bone marrow-derived MSC (BMSC)-based VBR by enhancing the
osteogenic and angiogenic capacity (78). On top of this, the scaffold
GEM21S™, loaded with human recombinant PDGF-BB, was
approved by the Food and Drug Administration for periodontal bone
regeneration procedures. In addition, PDGF-BB was indicated to
induce the formation of type H vessels that have recently been
identified as a critical process coupling angiogenesis and
osteogenesis (79-81).
Therefore, it is reasonable to conjecture that the modulation of OC
precursors and PDGF-BB secretion to promote type-H vessel formation
may provide an additional path for building iBTE scaffolds.
However, there is also an alternative path to achieve in
situ VBR: To use the osteoimmune-related cytokines to modulate
the osteoimmune microenvironment. For instance, Zheng et al
(4) implanted demineralized bone
matrix scaffolds into bone defects, while providing interleukin-4
(IL-4), which shifted the macrophages from a pro-inflammatory M1 to
an anti-inflammatory M2 phenotype. Enhanced host bone ingrowth and
neovascular infiltration were overserved in this pro-reparative
inflammatory microenvironment.
As the indispensable component, the bioinorganic
ions are included in the spectrum of biochemical cues in the
extracellular environment (82),
most of which function as cofactors for enzymes or coenzymes in
different physiological activities and participate in signal
transduction indirectly and directly (83). For instance, numerous studies have
confirmed that magnesium (Mg2+), copper
(Cu2+), cobalt (Co2+), silicon
(Si4+) and also the ion-doped iBTE scaffolds promote the
angiogenesis-osteogenesis coupling or have an immunomodulatory
effect (38,84-92).
Mg2+ is a critical ion involved in bone metabolism, as
verified by OBs and OCs exhibiting functional abnormalities in the
absence of Mg2+ (93-95).
The Mg2+-rich microenvironment stimulates MSC osteogenic
differentiation and promotes neovascularization (96,97).
In vitro experiments have demonstrated that Mg2+
promotes the proliferation of OB and the expression of related
molecular markers. Furthermore, it also has immunomodulatory
effects, including the inhibition of the expression of
RANKL-induced cytokines, such as c-Src, MMP-9, and OC
activity-related genes such as tartrate-resistant acid phosphatase,
proteinase K and calcitonin receptor gene (92). Hu et al (98) found that Mg2+ reversed
the phenotype of M1-macrophages activated by
lipopolysaccharide/IFN-γ and upregulate the percentage of
M2-macrophages (98). Wang et
al (99) found that the
magnesium-containing calcium phosphate cement (MCPC) down-regulated
pro-inflammatory cytokines (TNF-α, IL-6) and upregulated
bone-repair cytokine (TGF-β1) (99). At the same time, it was indicated
that the osteogenic capacity of BMSCs and the angiogenic potential
of ECs were enhanced in the MCPC-induced immune microenvironment.
Another two ions, Cu2+ and Co2+, are elements
that may mimic hypoxia and stabilize HIF-1α, thereby promoting
downstream VEGF expression and angiogenesis (89,100-102).
The multifunctional Cu2+-doped bioactive glass-collagen
scaffold exerted osteogenic and angiogenic effects in vitro
(103). Similarly, it was found
that the addition of low doses of Co2+ (<5%) to
mesoporous bioactive glass scaffolds promoted the expression of
VEGF, HIF-1α and osteogenesis-related genes in BMSCs. In similar
studies, by doping Co2+ with β-tricalcium phosphate
(β-TCP), 45S5 bioglass scaffolds induced a coupling effect of
osteogenesis and angiogenesis. As a similar element to carbon in
the periodic table, silicon is a significant component of colloids
and bioceramics. Si4+ may promote osteogenesis of MSCs
and enhance angiogenesis of ECs, and it has also been widely used
in the preparation of iBTE scaffolds (104,105). Cell studies have reported that
Si4+ effectively promoted the proliferation, migration
and tube formation of ECs and upregulated the expression of
angiogenesis-related genes (VEGF, HIF1-α) (106-109).
The definite mechanism by which ions are pushed toward VBR is yet
to be defined at the moment but likely involves changes in various
signaling pathways and gene expression. However, based on these
preliminary results and their relatively safe profile, it is clear
that the above ions have become popular candidates for developing
iBTE scaffolds. However, studies have identified the appropriate
ion concentration ranges. Questions related to each bioinorganic
ion's possible mechanism of action and its dose-dependent and
time-dependent effects have not yet been fully answered. For
instance, a recent study has identified a bidirectional mode of
action of Mg2+ in bone repair (110). Mg2+ promotes the
upregulation of transient receptor potential cation channel member
7 during the early inflammatory phase, thus creating a favorable
osteoimmune microenvironment. By contrast, during the subsequent
bone remodeling phase, sustained high-dose exposure to
Mg2+ leads to excessive activation of NF-κB signaling in
macrophages and an increase in the number of OCs, which may have a
negative impact on osteogenesis that outweighs the initial
osteogenic effect. Although doping iBTE scaffolds with bioinorganic
ions is safer and more cost-effective than adding GFs, more
persuasive evidence is required.
In addition to ions, numerous biochemical molecules
were found to promote VBR. Due to the limitation in article length
and the diversity of molecules, only brief examples are provided.
Several studies have indicated that the activation or stabilization
of the HIF-1α transcriptional factor leads to the expression of
downstream genes, some of which couple angiogenesis and
osteogenesis (5,6,111).
Therefore, several trials targeting the HIF-1α were performed.
Deferoxamine (DFO), a medication approved for the treatment of iron
toxicity, was found to stabilize HIF-1α and maintain its activity
by inhibiting the prolyl hydroxylase (24). Yan et al (14) loaded the DFO into a 3D-printed PCL
scaffold using high-temperature melt-printing technology and
achieved in situ VBR by activating the HIF-1α signaling
pathway (112). Furthermore,
inspired by the structure of ‘lotus’, a 3D printed porous
bioceramic scaffold was used as the strut of the lotus, and the
DFO-releasing liposomes were combined with hydrogel microspheres as
‘lotus seeds’. The scaffold exhibited the potential to induce in
situ vascularization and MSC osteogenic differentiation in
vivo. Other molecules affecting the HIF-α were also studied. An
MBG (mesoporous bioactive glass)-poly(lactide-co-glycolide) (PLGA)
scaffold loaded with the bioactive lipid FTY720 achieved type H
vessel-related in situ VBR by upregulating HIF-1α expression
via the Erk1/2 pathway (113). Ha
et al (114) filled a
gelatin-silica nanofiber (GSN) network into a porous PCL scaffold,
followed by embedding the mesoporous silica nanoparticles (MSNs)
loaded with bone-forming peptide-1 within the GSN scaffold. The
outer surface of the scaffold was then anchored with MSNs loaded
with the angiogenic molecule dimethyl oxalyl glycine. The scaffold
achieved a spatial distribution and sequential release of the two
biochemical molecules targeting the respective angiogenesis and
osteogenesis processes. The following subcutaneous and cranial
defect implantation verified that the dual-drug delivery model with
hierarchical microstructure successfully facilitated
vascularization and bone regeneration. Another molecule, calcitonin
gene-related peptide (CGRP), is a neuropeptide worth mentioning, as
ongoing studies have revealed that factors secreted by peripheral
nerves in close proximity to the defect site take a role in
neovascularization and bone regeneration (115-119).
The physiological doses of CGRP coordinate the interaction of
osteoblasts with other cells and affect angiogenesis in addition to
osteogenesis, osteolysis and lipogenesis (120). In vitro experiments have
demonstrated that CGRP promotes osteogenesis in several cell types,
such as OB, MSC and periosteal-derived stem cells. Its osteogenic
effects are associated with the typical Wnt/β-catenin signaling
pathway and the cyclic AMP response element binding protein
signaling pathway (117,118,121). CGRP also activates adenylate
cyclase and the downstream protein kinase A upon binding to its
receptor, CGRPR, resulting in the efflux of nitric oxide from EC
and Ca2+ from smooth muscle to exert vasodilatory
effects (119). In vitro
experiments also revealed that CGRP promoted EC proliferation and
tubule formation by enhancing VEGF expression (115,116,120,122). CGRP was released in the fracture
site upon electrical stimulation applied in the dorsal ganglion
root and type H vessels were also found along with high expression
of CGRP (122). Similarly,
unpublished data by our research team also indicated that
upregulated CGRP expression colocalizes with the type H
vessel-related in situ VBR. These findings suggest that CGRP
is essential in coupling angiogenesis and osteogenesis. On top of
these findings, CGRP-loaded gelatin microspheres demonstrated
enhanced bone regeneration in osteoporotic rabbits, as indicated by
increased trabeculae and reduced trabecular separation (123). Continuous research on iBTE
scaffolds employing CGRP is being conducted (124).
Composite biologics such as PRP, dECM and exosomes
are also worth discussing. The therapeutic mechanisms of these
compounds are observed to be multifactorial and although the
effective molecule of these biologics is yet to be elucidated,
their efficacy in both preclinical and clinical settings has
attracted much attention. PRP is a mixed agent enriched with
multiple autologous GFs derived from the donors' blood. Numerous
studies on iBTE scaffolds incorporating PRP are being investigated
because of their inherent high safety and convenience. It was found
that PRP was also able to induce angiogenic-osteogenic coupling
(125-127),
which may be attributed to the various GFs, such as PDGF-BB, IGF
and FGF. However, varieties of PRP resulted from numerous factors,
including donor variability and preparation methods, leading to
relatively inconsistent effectiveness results. Another composite
biologics agent is the dECM, a low-immunogenic natural biomaterial
that retains multiple biochemical molecules simulating the
tissue-specific regenerative microenvironment. A periosteal
decellularized matrix (PEM) hydrogel was prepared using the
decellularized periosteal matrix by Qiu et al (128). The PEM hydrogels rapidly recruited
inflammatory cells and shifted macrophages from the M1
pro-inflammatory phenotype to the M2 reparative phenotype in the
early stage after implantation. In addition, the PEM hydrogels had
a positive role in promoting angiogenesis, osteogenesis and
subsequent mineralization in the later stage. He et al
(2) fabricated the human umbilical
vein endothelial cell-derived decellularized matrix/fibrin/PCL
scaffold, exhibiting accelerated VBR after implantation into rat
femoral defects, and revealed that the underlying mechanism may be
related to the formation of type H vessels. Other cell-derived
biologics, exosomes or extracellular vesicles (EV) are
membrane-like natural nanoparticles released by cells. Exosomes and
EVs may carry mRNA, micro (mi)RNA and bioactive proteins, and have
multiple potential biological functions, such as reducing the
inflammatory response, promoting angiogenesis and facilitating bone
formation (129-131).
Fan et al (132) developed
a bone marrow MSC-derived exosome-functionalized
polyetheretherketone implant (SPEEK). SPEEK promotes macrophage
polarization toward M2 by inhibiting the NF-κB signaling pathway,
enhancing the osteogenic differentiation of BMSCs. Also, SPEEK
exhibited superior osseointegration. Although angiogenesis was not
solely investigated in this study, the result demonstrated that the
proangiogenic role was ineligible. This study also suggested that
exosomes may be used as a surface-modified biochemical cue to
prepare iBTE scaffolds.
In addition, TCM has a deep historical background
and is being gradually used as an alternative therapy. Herbal
medicine has sparked the enthusiasm of numerous researchers due to
its diverse therapeutic effects and mechanisms of action. In-depth
research found that the active ingredients in various TCM
formulations promote osteogenesis and angiogenesis (133,134). Lin et al (133) used a low-temperature rapid
prototyping technique to prepare a PLGA/β-TCP composite scaffold
incorporating low, medium and high doses of salvianolic acid B. It
was found that salvianolic acid B promoted osteogenesis and
angiogenesis in a dose-dependent manner in vitro. Animal
experiments also confirmed the scaffold's dose-dependent effects on
new bone formation, mineralization and angiogenesis. It was
indicated that the PLGA/β-TCP composite scaffold doped with
salvianolic acid B increased the bony fusion of vertebral bodies by
contributing to bone and blood vessel formation. Wu et al
(134) developed novel
micro/nanostructured hydroxyapatite particles to construct a
delivery system for icariin. The scaffold exhibited enhanced
osteogenesis and angiogenesis in a rat femoral defect model. In
vitro experiments revealed that the delivery of icariin
promoted osteogenic differentiation and expression of
angiogenesis-related factors in MSCs via the Akt signaling pathway.
Although certain studies have proven the proangiogenic and
osteogenic activities of naringin and ginsenoside in vitro
(135-138),
their application in constructing iBTE scaffolds has rarely been
reported. A wide range of active components of TCM requires further
exploration to provide alternative solutions for the fabrication of
iBTE scaffolds.
The field strives to develop innovative strategies
to enhance VBR. A critical aspect of this process involves the
spatiotemporal delivery of GFs, which may be challenging to achieve
through conventional methods involving the use of biochemical
molecules. To overcome these limitations, researchers have turned
to genetic manipulation, exploring the potential of GF vectors,
gene activation matrix (GAM) and engineered exosomes as alternative
means to promote angiogenesis-osteogenesis coupling. By harnessing
the power of genetic manipulation, it is possible to create more
precise and cost-effective treatments that may mimic the natural
phases of VBR while minimizing unwanted side effects.
As previously mentioned, the delivery of biochemical
molecules through iBTE scaffolds, in most cases, cannot fulfil a
satisfying spatiotemporal release mimicking the natural phase of
VBR. For instance, excessive VEGF may lead to vascular leakage and
OC activation, and high concentrations of BMP result in ectopic
bone formation (139). In
addition, even with the appropriate dose and release kinetics, the
half-life of these biochemical molecules limits their effectiveness
within a short period. The GFs required in the regenerative process
may not be of therapeutic value if released too early. Fortunately,
genetic manipulation is more cost-effective than high-dose GF
delivery and with a more precise control (140). Furthermore, it is technically
achievable to deliver multiple customized genes (141). Researchers have verified the
strategies to maintain a sustained expression of target proteins
through direct gene delivery. As mentioned earlier, BMP and VEGF
are major GFs in angiogenesis-osteogenesis coupling and iBTE
strategies using genetic manipulation have been reported in several
pieces of literature (142-145).
Despite the fact that virus-based gene delivery is more effective
in certain animal studies, the safety issue remains a critical
question to be answered in human experiments (146,147). Non-viral vectors have lower
transfection efficiency than viral ones but are safer in consensus.
Therefore, the following section focuses on the intracellular gene
delivery iBTE strategies with non-viral vectors.
In addition to the naked plasmid described
previously, exosomes are among the ideal candidates for gene
delivery due to their excellent biocompatibility, low
immunogenicity and efficient cellular internalization.
Exosome-delivered mRNA and miRNA have also participated in VBR by
different mechanisms (130). For
instance, early healing of rat cranial defects was observed after
MSC-derived exosome administration, which may be associated with
the exosomal miRNA-196a that promotes osteoblast proliferation and
differentiation (151). Besides,
the miR-129, miR-136 and miR-17-92 clusters enriched in exosomes
were found to promote EC proliferation and angiogenesis (152). Exosomes have been explored as
biomimetic and safe cargo carriers, and exosome-based engineering
modifications have also been investigated. Zha et al
(153) constructed gene-activated
exosomes carrying the VEGF gene and then loaded them onto
3D-printed scaffolds with nanoparticles via the CP05. Subsequently,
the in vivo experiments verified that this gene-activated
exosome iBTE scaffold effectively induced a substantial amount of
neovascularization and new bone. Based on the above, the team
prepared a novel exosome analog (EM) encapsulated with VEGF165
plasmid DNA, aiming to improve the current shortcomings of
exosome-based therapeutics, such as low exosome yield and unstable
efficiency (154). Compared with
the traditional method of obtaining exosomes, the EM method has a
higher yield of exosomes with similar characteristics. The EM
encapsulated with VEGF165 plasmid DNA was attached to the GAM
composed of electrospun nanofiber membrane via the biotin-avidin
system and achieved the local release of the VEGF165 plasmid and
exhibited enhanced VBR in vitro and in vivo.
Upon examining the available evidence, it becomes
evident that the GAM approach constitutes a viable iBTE strategy
for accomplishing in situ VBR. Through the prolonged local
delivery of genes, endogenous cells undergo reprogramming and
persistently produce GFs. This process emulates the stepwise
presentation of GFs, thereby simulating the physiological process
of bone repair. While gene therapy has experienced significant
advancements across various fields, the current utilization of gene
editing and epigenetic modulation, particularly concerning VBR, has
not been thoroughly investigated. Consequently, further research is
necessary to ensure the safe and effective implementation of these
techniques in the context of VBR.
Evidence has indicated that the expeditious
establishment of vascular networks is crucial for successful bone
regeneration. In recent years, iTE strategies aimed at VBR have
garnered considerable interest due to their capacity to promote
angiogenesis and hasten the establishment of vascular supply. These
strategies encompass the employment of biophysical and biochemical
cues to facilitate the differentiation and proliferation of
bone-forming cells, stimulate angiogenesis and blood vessel
formation, and modulate the inflammatory response. Biophysical
cues, including mechanical forces, electrical and magnetic fields,
porosity and topography, may be utilized to direct the fate of
endogenous progenitor cells, a vital component in achieving
functional tissue regeneration. Furthermore, biochemical cues may
be delivered through extracellular signaling mechanisms or
regulation of intracellular genetic material. The former approach
offers a safer and more straightforward method than the latter,
which entails more intricate genetic manipulation. Both strategies
have exhibited promise in preclinical investigations and are
progressively being translated into clinical practice.
In light of the increasing diversity and
sophistication of biomaterials, drawing comparisons between
individual materials may be challenging. The advancement of
biomaterials research is intimately connected to the exploration of
host biology, as these two fields exhibit a reciprocal relationship
that fosters innovation and discovery in both areas. For instance,
recent developments in high-throughput sequencing have unveiled the
striking heterogeneity of host cells and their varied responses to
different biomaterials. This knowledge subsequently informs the
design and optimization of biomaterials, customizing them to elicit
specific biological responses and enhance their integration with
host tissues.
As increasingly sophisticated implanted
biomaterials are being developed and implemented, the understanding
of the complex biological reactions they induce within the body
deepens. This bidirectional relationship between biomaterials
research and host biology not only encourages the creation of
advanced materials with improved biocompatibility and functionality
but also clarifies the underlying mechanisms governing tissue
regeneration and repair.
As the field of iTE of VBR continues to progress,
several areas of potential growth and improvement emerge. First, as
the heterogeneity of host cells and biological responses becomes
apparent, it would be prudent to develop novel biomaterials with
tunable properties, enabling precise spatiotemporal control over
the biophysical and biochemical cues provided to cells in the
regenerative environment. In addition, future integrated strategies
combining biophysical and biochemical approaches may result in
synergistic effects that promote more efficient and robust VBR.
These advancements would not only be applicable to critical-sized
bone defects but may also extend to various bone diseases, such as
osteonecrosis and osteoporosis.
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 82072415), Panyu Key Medical and
Health Projects of Science and Technology Planning (grant no.
2022-Z04-101), Science and Technology Project of Foshan City (grant
no. 1920001000025), Project of The State Key Laboratory of
Respiratory Disease (grant no. SKLRD-Z-202105), Science Technology
Project of Guangzhou City (grant no. 2019ZD15) and the Fundamental
and Applied Basic Research Fund of Guangdong Province Regional
Joint Fund Project (Youth Fund Project; grant no.
2020A1515111046).
Not applicable.
YH drafted the manuscript and performed critical
analyses of the literature. LL and CL collected the raw data for
analysis. JH and ZZ organized the framework of this paper,
supervised the work and revised the manuscript. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hankenson KD, Dishowitz M, Gray C and
Schenker M: Angiogenesis in bone regeneration. Injury. 42:556–561.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He Y, Wang W, Lin S, Yang Y, Song L, Jing
Y, Chen L, He Z, Li W, Xiong A, et al: Fabrication of a
bio-instructive scaffold conferred with a favorable
microenvironment allowing for superior implant osseointegration and
accelerated in situ vascularized bone regeneration via type H
vessel formation. Bioact Mater. 9:491–507. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gaharwar AK, Singh I and Khademhosseini A:
Engineered biomaterials for in situ tissue regeneration. Nat Rev
Mater. 5:686–705. 2020.
|
|
4
|
Zheng ZW, Chen YH, Wu DY, Wang JB, Lv MM,
Wang XS, Sun J and Zhang ZY: Development of an accurate and
proactive immunomodulatory strategy to improve bone substitute
material-mediated osteogenesis and angiogenesis. Theranostics.
8:5482–5500. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng J and Ye L: Coupling between
osteogenesis and angiogenesis. FASEB J. 22(233.2)2008.
|
|
6
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ramasamy SK, Kusumbe AP, Wang L and Adams
RH: Endothelial Notch activity promotes angiogenesis and
osteogenesis in bone. Nature. 507:376–380. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rather HA, Jhala D and Vasita R: Dual
functional approaches for osteogenesis coupled angiogenesis in bone
tissue engineering. Mater Sci Eng C Mater Biol Appl.
103(109761)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu H, Du Y, Yang G, Hu X, Wang L, Liu B,
Wang J and Zhang S: Delivering proangiogenic factors from
3D-printed polycaprolactone scaffolds for vascularized bone
regeneration. Adv Healthc Mater. 9(2000727)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lanza R, Langer R, Vacanti J and Atala A
(eds): Principles of tissue engineering. 5th edition. xli,
2020.
|
|
11
|
De Pieri A, Rochev Y and Zeugolis DI:
Scaffold-free cell-based tissue engineering therapies: Advances,
shortfalls and forecast. NPJ Regen Med. 6(18)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li L, Lu H, Zhao Y, Luo J, Yang L, Liu W
and He Q: Functionalized cell-free scaffolds for bone defect repair
inspired by self-healing of bone fractures: A review and new
perspectives. Mater Sci Eng C Mater Biol Appl. 98:1241–1251.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang C, Ma H, Wang Z, Younis MR, Liu C, Wu
C, Luo Y and Huang P: 3D printed wesselsite nanosheets
functionalized scaffold facilitates NIR-II photothermal therapy and
vascularized bone regeneration. Adv Sci (Weinh).
8(2100894)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan Y, Chen H, Zhang H, Guo C, Yang K,
Chen K, Cheng R, Qian N, Sandler N, Zhang YS, et al: Vascularized
3D printed scaffolds for promoting bone regeneration. Biomaterials.
190-191:97–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Komeri R, Kasoju N and Kumar PRA: In vitro
cytotoxicity and cytocompatibility assays for biomaterial testing
under regulatory platform. Biomedical Product and Materials
Evaluation, pp329-353, 2022.
|
|
16
|
Liu WC, Chen S, Zheng L and Qin L:
Angiogenesis assays for the evaluation of angiogenic properties of
orthopaedic biomaterials-a general review. Adv Healthc Mater.
6(1600434)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ji C, Qiu M, Ruan H, Li C, Cheng L, Wang
J, Li C, Qi J, Cui W and Deng L: Transcriptome analysis revealed
the symbiosis niche of 3D scaffolds to accelerate bone defect
healing. Adv Sci (Weinh). 9(e2105194)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song W, Fhu CW, Ang KH, Liu CH, Johari NA,
Lio D, Abraham S, Hong W, Moss SE, Greenwood J and Wang X: The
fetal mouse metatarsal bone explant as a model of angiogenesis. Nat
Protoc. 10:1459–1473. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bellacen K and Lewis EC: Aortic ring
assay. J Vis Exp. 24(1564)2009.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Diomede F, Marconi GD, Fonticoli L,
Pizzicanella J, Merciaro I, Bramanti P, Mazzon E and Trubiani O:
Functional relationship between osteogenesis and angiogenesis in
tissue regeneration. Int J Mol Sci. 21(3242)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schott NG, Friend NE and Stegemann JP:
Coupling osteogenesis and vasculogenesis in engineered orthopedic
tissues. Tissue Eng Part B Rev. 27:199–214. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang T, Zhai Y, Nuzzo M, Yang X, Yang Y
and Zhang X: Layer-by-layer nanofiber-enabled engineering of
biomimetic periosteum for bone repair and reconstruction.
Biomaterials. 182:279–288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tang Y, Luo K, Tan J, Zhou R, Chen Y, Chen
C, Rong Z, Deng M, Yu X, Zhang C, et al: Laminin alpha 4 promotes
bone regeneration by facilitating cell adhesion and
vascularization. Acta Biomater. 126:183–198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peng Y, Wu S, Li Y and Crane JL: Type H
blood vessels in bone modeling and remodeling. Theranostics.
10:426–436. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mangir N, Dikici S, Claeyssens F and
MacNeil S: Using ex ovo chick chorioallantoic membrane (CAM) assay
to evaluate the biocompatibility and angiogenic response to
biomaterials. Acs Biomater Sci Eng. 5:3190–3200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Duan R, Zhang Y, van Dijk L, Barbieri D,
van den Beucken J, Yuan H and de Bruijn J: Coupling between
macrophage phenotype, angiogenesis and bone formation by calcium
phosphates. Mater Sci Eng C Mater Biol Appl.
122(111948)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang YH, Zhao CZ, Wang RY, Du QX, Liu JY
and Pan J: The crosstalk between macrophages and bone marrow
mesenchymal stem cells in bone healing. Stem Cell Res Ther.
13(511)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fernandez-Yague MA, Abbah SA, McNamara L,
Zeugolis DI, Pandit A and Biggs MJ: Biomimetic approaches in bone
tissue engineering: Integrating biological and physicomechanical
strategies. Adv Drug Deliver Rev. 84:1–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Niu Y, Wang Z, Shi Y, Dong L and Wang C:
Modulating macrophage activities to promote endogenous bone
regeneration: Biological mechanisms and engineering approaches.
Bioact Mater. 6:244–261. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li J, Liu Y, Zhang Y, Yao B, Enhejirigala
Li Z, Song W, Wang Y, Duan X, Yuan X, et al: Biophysical and
biochemical cues of biomaterials guide mesenchymal stem cell
behaviors. Front Cell Dev Biol. 9(640388)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bobbert FSL and Zadpoor AA: Effects of
bone substitute architecture and surface properties on cell
response, angiogenesis, and structure of new bone. J Mater Chem B.
5:6175–6192. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Amini AR, Adams DJ, Laurencin CT and
Nukavarapu SP: Optimally porous and biomechanically compatible
scaffolds for large-area bone regeneration. Tissue Eng Part A.
18:1376–1388. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Reinwald Y, Johal RK, Ghaemmaghami AM,
Rose FRAJ, Howdle SM and Shakesheff KM: Interconnectivity and
permeability of supercritical fluid-foamed scaffolds and the effect
of their structural properties on cell distribution. Polymer.
55:435–444. 2014.
|
|
34
|
Murphy CM, Haugh MG and O'Brien FJ: The
effect of mean pore size on cell attachment, proliferation and
migration in collagen-glycosaminoglycan scaffolds for bone tissue
engineering. Biomaterials. 31:461–466. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hayashi K, Munar ML and Ishikawa K:
Effects of macropore size in carbonate apatite honeycomb scaffolds
on bone regeneration. Mater Sci Eng C Mater Biol Appl.
111(110848)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Wu D, Zhang Z, Li J, Shen Y, Wang
Z, Li Y, Zhang ZY and Sun J: Biomimetically ornamented rapid
prototyping fabrication of an apatite-collagen-polycaprolactone
composite construct with nano-micro-macro hierarchical structure
for large bone defect treatment. ACS Appl Mater Interfaces.
7:26244–26256. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu Y, Yang S, Cao L, Zhang X, Wang J and
Liu C: Facilitated vascularization and enhanced bone regeneration
by manipulation hierarchical pore structure of scaffolds. Mater Sci
Eng C Mater Biol Appl. 110(110622)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shen J, Wang W, Zhai X, Chen B, Qiao W, Li
W, Li P, Zhao Y, Meng Y, Qian S, et al: 3D-printed nanocomposite
scaffolds with tunable magnesium ionic microenvironment induce in
situ bone tissue regeneration. Appl Mater Today. 16:493–507.
2019.
|
|
39
|
Zhang ZZ, Zhang HZ and Zhang ZY: 3D
printed poly(ε-caprolactone) scaffolds function with
simvastatin-loaded poly(lactic-co-glycolic acid) microspheres to
repair load-bearing segmental bone defects. Exp Ther Med. 17:79–90.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang W, Shi W, Wu S, Kuss M, Jiang X,
Untrauer JB, Reid SP and Duan B: 3D printed composite scaffolds
with dual small molecule delivery for mandibular bone regeneration.
Biofabrication. 12(035020)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lian M, Sun B, Han Y, Yu B, Xin W, Xu R,
Ni B, Jiang W, Hao Y, Zhang X, et al: A low-temperature-printed
hierarchical porous sponge-like scaffold that promotes
cell-material interaction and modulates paracrine activity of MSCs
for vascularized bone regeneration. Biomaterials.
274(120841)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Musumeci G: The effect of mechanical
loading on articular cartilage. J Funct Morphol Kinesiol.
1:154–161. 2016.
|
|
43
|
Lee J, Abdeen AA, Tang X, Saif TA and
Kilian KA: Matrix directed adipogenesis and neurogenesis of
mesenchymal stem cells derived from adipose tissue and bone marrow.
Acta Biomater. 42:46–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guo M, Pegoraro AF, Mao A, Zhou EH, Arany
PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, et al: Cell
volume change through water efflux impacts cell stiffness and stem
cell fate. Proc Natl Acad Sci USA. 114:E8618–E8627. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Meng Z, Qiu Y, Lin KC, Kumar A, Placone
JK, Fang C, Wang KC, Lu S, Pan M, Hong AW, et al: RAP2 mediates
mechanoresponses of the Hippo pathway. Nature. 560:655–660.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bastounis EE, Yeh YT and Theriot JA:
Subendothelial stiffness alters endothelial cell traction force
generation while exerting a minimal effect on the transcriptome.
Sci Rep. 9(18209)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yeh YT, Hur SS, Chang J, Wang KC, Chiu JJ,
Li YS and Chien S: Matrix stiffness regulates endothelial cell
proliferation through septin 9. PLoS One. 7(e46889)2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Santos L, Fuhrmann G, Juenet M, Amdursky
N, Horejs CM, Campagnolo P and Stevens MM: Extracellular stiffness
modulates the expression of functional proteins and growth factors
in endothelial cells. Adv Healthc Mater. 4:2056–2063.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang Y, Wang X, Zhang Y, Liu Y, Wang D,
Yu X, Wang H, Bai Z, Jiang YC, Li X, et al: Endothelial cell
migration regulated by surface topography of poly(ε-caprolactone)
nanofibers. ACS Biomater Sci Eng. 7:4959–4970. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abagnale G, Steger M, Nguyen VH, Hersch N,
Sechi A, Joussen S, Denecke B, Merkel R, Hoffmann B, Dreser A, et
al: Surface topography enhances differentiation of mesenchymal stem
cells towards osteogenic and adipogenic lineages. Biomaterials.
61:316–326. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang C, Zhao C, Wang X, Shi M, Zhu Y, Jing
L, Wu C and Chang J: Stimulation of osteogenesis and angiogenesis
by micro/nano hierarchical hydroxyapatite via macrophage
immunomodulation. Nanoscale. 11:17699–17708. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sapir Y, Cohen S, Friedman G and Polyak B:
The promotion of in vitro vessel-like organization of endothelial
cells in magnetically responsive alginate scaffolds. Biomaterials.
33:4100–4109. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yun HM, Ahn SJ, Park KR, Kim MJ, Kim JJ,
Jin GZ, Kim HW and Kim EC: Magnetic nanocomposite scaffolds
combined with static magnetic field in the stimulation of
osteoblastic differentiation and bone formation. Biomaterials.
85:88–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hao S, Meng J, Zhang Y, Liu J, Nie X, Wu
F, Yang Y, Wang C, Gu N and Xu H: Macrophage phenotypic
mechanomodulation of enhancing bone regeneration by
superparamagnetic scaffold upon magnetization. Biomaterials.
140:16–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zonari A, Novikoff S, Electo NRP, Breyner
NM, Gomes DA, Martins A, Neves NM, Reis RL and Goes AM: Endothelial
differentiation of human stem cells seeded onto electrospun
polyhydroxybutyrate/polyhydroxybutyrate-co-hydroxyvalerate fiber
mesh. PLoS One. 7(e35422)2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang C, Wang W, Hao X, Peng Y, Zheng Y,
Liu J, Kang Y, Zhao F, Luo Z, Guo J, et al: A novel approach to
enhance bone regeneration by controlling the polarity of GaN/AlGaN
heterostructures. Adv Funct Mater. 31(2007487)2021.
|
|
57
|
Safina I and Embree MC: Biomaterials for
recruiting and activating endogenous stem cells in situ tissue
regeneration. Acta Biomater. 143:26–38. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Vermeulen S, Tahmasebi Birgani Z and
Habibovic P: Biomaterial-induced pathway modulation for bone
regeneration. Biomaterials. 283(121431)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pan Y, Chen J, Yu Y, Dai K, Wang J and Liu
C: Enhancement of BMP-2-mediated angiogenesis and osteogenesis by
2-N,6-O-sulfated chitosan in bone regeneration. Biomater Sci.
6:431–439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang W and Yeung KWK: Bone grafts and
biomaterials substitutes for bone defect repair: A review. Bioact
Mater. 2:224–247. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kanakaris NK, Calori GM, Verdonk R,
Burssens P, De Biase P, Capanna R, Vangosa LB, Cherubino P, Baldo
F, Ristiniemi J, et al: Application of BMP-7 to tibial non-unions:
A 3-year multicenter experience. Injury. 39 (Suppl 2):S83–S90.
2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jones AL, Bucholz RW, Bosse MJ, Mirza SK,
Lyon TR, Webb LX, Pollak AN, Golden JD and Valentin-Opran A: BMP-2
Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study
Group. Recombinant human BMP-2 and allograft compared with
autogenous bone graft for reconstruction of diaphyseal tibial
fractures with cortical defects. A randomized, controlled trial. J
Bone Joint Surg Am. 88:1431–1441. 2006.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gillman CE and Jayasuriya AC: FDA-approved
bone grafts and bone graft substitute devices in bone regeneration.
Mater Sci Eng C Mater Biol Appl. 130(112466)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Pearson HB, Mason DE, Kegelman CD, Zhao L,
Dawahare JH, Kacena MA and Boerckel JD: Effects of bone
morphogenetic protein-2 on neovascularization during large bone
defect regeneration. Tissue Eng Part A. 25:1623–1634.
2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Akiyama I, Yoshino O, Osuga Y, Shi J,
Harada M, Koga K, Hirota Y, Hirata T, Fujii T, Saito S and Kozuma
S: Bone morphogenetic protein 7 increased vascular endothelial
growth factor (VEGF)-a expression in human granulosa cells and VEGF
receptor expression in endothelial cells. Reprod Sci. 21:477–482.
2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Boraiah S, Paul O, Hawkes D, Wickham M and
Lorich DG: Complications of recombinant human BMP-2 for treating
complex tibial plateau fractures: A preliminary report. Clin Orthop
Relat Res. 467:3257–3262. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chen J, Zhou X, Sun W, Zhang Z, Teng W,
Wang F, Sun H, Zhang W, Wang J, Yu X, et al: Vascular derived ECM
improves therapeutic index of BMP-2 and drives vascularized bone
regeneration. Small. 18(e2107991)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Keramaris NC, Calori GM, Nikolaou VS,
Schemitsch EH and Giannoudis PV: Fracture vascularity and bone
healing: A systematic review of the role of VEGF. Injury. 39 (Suppl
2):S45–S57. 2008.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Eckardt H, Bundgaard KG, Christensen KS,
Lind M, Hansen ES and Hvid I: Effects of locally applied vascular
endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit
tibia during distraction osteogenesis. J Orthop Res. 21:335–340.
2003.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Leach JK, Kaigler D, Wang Z, Krebsbach PH
and Mooney DJ: Coating of VEGF-releasing scaffolds with bioactive
glass for angiogenesis and bone regeneration. Biomaterials.
27:3249–3255. 2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kaigler D, Wang Z, Horger K, Mooney DJ and
Krebsbach PH: VEGF scaffolds enhance angiogenesis and bone
regeneration in irradiated osseous defects. J Bone Miner Res.
21:735–744. 2006.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Gu J, Zhang Q, Geng M, Wang W, Yang J,
Khan AUR, Du H, Sha Z, Zhou X and He C: Construction of nanofibrous
scaffolds with interconnected perfusable microchannel networks for
engineering of vascularized bone tissue. Bioact Mater. 6:3254–3268.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lee SS, Kim JH, Jeong J, Kim SHL, Koh RH,
Kim I, Bae S, Lee H and Hwang NS: Sequential growth factor
releasing double cryogel system for enhanced bone regeneration.
Biomaterials. 257(120223)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Subbiah R, Hwang MP, Van SY, Do SH, Park
H, Lee K, Kim SH, Yun K and Park K: Osteogenic/angiogenic dual
growth factor delivery microcapsules for regeneration of
vascularized bone tissue. Adv Healthc Mater. 4:1982–1992.
2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhou X, Chen J, Sun H, Wang F, Wang Y,
Zhang Z, Teng W, Ye Y, Huang D, Zhang W, et al: Spatiotemporal
regulation of angiogenesis/osteogenesis emulating natural bone
healing cascade for vascularized bone formation. J
Nanobiotechnology. 19(420)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang C, Lai J, Li K, Zhu S, Lu B, Liu J,
Tang Y and Wei Y: Cryogenic 3D printing of dual-delivery scaffolds
for improved bone regeneration with enhanced vascularization.
Bioact Mater. 6:137–145. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zhang M, Yu W, Niibe K, Zhang W, Egusa H,
Tang T and Jiang X: The effects of platelet-derived growth
factor-BB on bone marrow stromal cell-mediated vascularized bone
regeneration. Stem Cells Int. 2018(3272098)2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Han Y, You X, Xing W, Zhang Z and Zou W:
Paracrine and endocrine actions of bone-the functions of secretory
proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res.
6(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L,
Li C, Xie L, Crane J, Wan M, et al: PDGF-BB secreted by
preosteoclasts induces angiogenesis during coupling with
osteogenesis. Nat Med. 20:1270–1278. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Xu R, Yallowitz A, Qin A, Wu Z, Shin DY,
Kim JM, Debnath S, Ji G, Bostrom MP, Yang X, et al: Targeting
skeletal endothelium to ameliorate bone loss. Nat Med. 24:823–833.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lin Z, Shen D, Zhou W, Zheng Y, Kong T,
Liu X, Wu S, Chu PK, Zhao Y, Wu J, et al: Regulation of
extracellular bioactive cations in bone tissue microenvironment
induces favorable osteoimmune conditions to accelerate in situ bone
regeneration. Bioact Mater. 6:2315–2330. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Habibovic P and Barralet JE: Bioinorganics
and biomaterials: Bone repair. Acta Biomater. 7:3013–3026.
2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Zhai W, Lu H, Wu C, Chen L, Lin X, Naoki
K, Chen G and Chang J: Stimulatory effects of the ionic products
from Ca-Mg-Si bioceramics on both osteogenesis and angiogenesis in
vitro. Acta Biomater. 9:8004–8014. 2013.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Du Z, Leng H, Guo L, Huang Y, Zheng T,
Zhao Z, Liu X, Zhang X, Cai Q and Yang X: Calcium silicate
scaffolds promoting bone regeneration via the doping of Mg2+ or
Mn2+ ion. Compos Part B Eng. 190(107937)2020.
|
|
86
|
Dashnyam K, Buitrago JO, Bold T,
Mandakhbayar N, Perez RA, Knowles JC, Lee JH and Kim HW:
Angiogenesis-promoted bone repair with silicate-shelled hydrogel
fiber scaffolds. Biomater Sci. 7:5221–5231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Lin Z, Wu J, Qiao W, Zhao Y, Wong KHM, Chu
PK, Bian L, Wu S, Zheng Y, Cheung KMC, et al: Precisely controlled
delivery of magnesium ions thru sponge-like monodisperse
PLGA/nano-MgO-alginate core-shell microsphere device to enable
in-situ bone regeneration. Biomaterials. 174:1–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Valerio P, Pereira MM, Goes AM and Leite
MF: The effect of ionic products from bioactive glass dissolution
on osteoblast proliferation and collagen production. Biomaterials.
25:2941–2948. 2004.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Feng W, Ye F, Xue W, Zhou Z and Kang YJ:
Copper regulation of hypoxia-inducible factor-1 activity. Mol
Pharmacol. 75:174–182. 2009.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lin Z, Cao Y, Zou J, Zhu F, Gao Y, Zheng
X, Wang H, Zhang T and Wu T: Improved osteogenesis and angiogenesis
of a novel copper ions doped calcium phosphate cement. Mater Sci
Eng C Mater Biol Appl. 114(111032)2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Bose S, Fielding G, Tarafder S and
Bandyopadhyay A: Understanding of dopant-induced osteogenesis and
angiogenesis in calcium phosphate ceramics. Trends Biotechnol.
31:594–605. 2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhai Z, Qu X, Li H, Yang K, Wan P, Tan L,
Ouyang Z, Liu X, Tian B, Xiao F, et al: The effect of metallic
magnesium degradation products on osteoclast-induced osteolysis and
attenuation of NF-κB and NFATc1 signaling. Biomaterials.
35:6299–6310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Wallach S: Effects of magnesium on
skeletal metabolism. Magnes Trace Elem. 9:1–14. 1990.PubMed/NCBI
|
|
94
|
Sojka JE and Weaver CM: Magnesium
supplementation and osteoporosis. Nutr Rev. 53:71–74.
1995.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Pichler K, Kraus T, Martinelli E, Sadoghi
P, Musumeci G, Uggowitzer PJ and Weinberg AM: Cellular reactions to
biodegradable magnesium alloys on human growth plate chondrocytes
and osteoblasts. Int Orthop. 38:881–889. 2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lin S, Yang G, Jiang F, Zhou M, Yin S,
Tang Y, Tang T, Zhang Z, Zhang W and Jiang X: A magnesium-enriched
3D culture system that mimics the bone development microenvironment
for vascularized bone regeneration. Adv Sci (Weinh).
6(1900209)2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zhang X, Huang P, Jiang G, Zhang M, Yu F,
Dong X, Wang L, Chen Y, Zhang W, Qi Y, et al: A novel magnesium
ion-incorporating dual-crosslinked hydrogel to improve bone
scaffold-mediated osteogenesis and angiogenesis. Mater Sci Eng C
Mater Biol Appl. 121(111868)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hu T, Xu H, Wang C, Qin H and An Z:
Magnesium enhances the chondrogenic differentiation of mesenchymal
stem cells by inhibiting activated macrophage-induced inflammation.
Sci Rep. 8(3406)2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wang M, Yu Y, Dai K, Ma Z, Liu Y, Wang J
and Liu C: Improved osteogenesis and angiogenesis of
magnesium-doped calcium phosphate cement via macrophage
immunomodulation. Biomater Sci. 4:1574–1583. 2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Minchenko A and Caro J: Regulation of
endothelin-1 gene expression in human microvascular endothelial
cells by hypoxia and cobalt: Role of hypoxia responsive element.
Mol Cell Biochem. 208:53–62. 2000.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Tanaka T, Kojima I, Ohse T, Ingelfinger
JR, Adler S, Fujita T and Nangaku M: Cobalt promotes angiogenesis
via hypoxia-inducible factor and protects tubulointerstitium in the
remnant kidney model. Lab Invest. 85:1292–1307. 2005.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Ryan EJ, Ryan AJ, González-Vázquez A,
Philippart A, Ciraldo FE, Hobbs C, Nicolosi V, Boccaccini AR,
Kearney CJ and O'Brien FJ: Collagen scaffolds functionalised with
copper-eluting bioactive glass reduce infection and enhance
osteogenesis and angiogenesis both in vitro and in vivo.
Biomaterials. 197:405–416. 2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Hoppe A, Güldal NS and Boccaccini AR: A
review of the biological response to ionic dissolution products
from bioactive glasses and glass-ceramics. Biomaterials.
32:2757–2774. 2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Saghiri MA, Asatourian A, Orangi J,
Sorenson CM and Sheibani N: Functional role of inorganic trace
elements in angiogenesis-Part II: Cr, Si, Zn, Cu, and S. Crit Rev
Oncol Hematol. 96:143–155. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Dashnyam K, Jin GZ, Kim JH, Perez R, Jang
JH and Kim HW: Promoting angiogenesis with mesoporous microcarriers
through a synergistic action of delivered silicon ion and VEGF.
Biomaterials. 116:145–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
A A, Menon D, T B S, Koyakutty M, Mohan
CC, Nair SV and Nair MB: Bioinspired composite matrix containing
hydroxyapatite-silica core-shell nanorods for bone tissue
engineering. ACS Appl Mater Interfaces. 9:26707–26718.
2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Kim JJ, El-Fiqi A and Kim HW: Synergetic
cues of bioactive nanoparticles and nanofibrous structure in bone
scaffolds to stimulate osteogenesis and angiogenesis. ACS Appl
Mater Interfaces. 9:2059–2073. 2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Šalandová M, van Hengel IAJ, Apachitei I,
Zadpoor AA, van der Eerden BCJ and Fratila-Apachitei LE: Inorganic
agents for enhanced angiogenesis of orthopedic biomaterials. Adv
Healthc Mater. 10(e2002254)2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Qiao W, Wong KHM, Shen J, Wang W, Wu J, Li
J, Lin Z, Chen Z, Matinlinna JP, Zheng Y, et al: TRPM7
kinase-mediated immunomodulation in macrophage plays a central role
in magnesium ion-induced bone regeneration. Nat Commun.
12(2885)2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1alpha in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495.
2004.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Han X, Sun M, Chen B, Saiding Q, Zhang J,
Song H, Deng L, Wang P, Gong W and Cui W: Lotus seedpod-inspired
internal vascularized 3D printed scaffold for bone tissue repair.
Bioact Mater. 6:1639–1652. 2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Li S, Song C, Yang S, Yu W, Zhang W, Zhang
G, Xi Z and Lu E: Supercritical CO2 foamed composite
scaffolds incorporating bioactive lipids promote vascularized bone
regeneration via Hif-1α upregulation and enhanced type H vessel
formation. Acta Biomater. 94:253–267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Ha Y, Ma X, Li S, Li T, Li Z, Qian Y,
Shafiq M, Wang J, Zhou X and He C: Bone microenvironment-mimetic
scaffolds with hierarchical microstructure for enhanced
vascularization and bone regeneration. Adv Funct Mater.
32(2200011)2022.
|
|
115
|
Mapp PI, McWilliams DF, Turley MJ, Hargin
E and Walsh DA: A role for the sensory neuropeptide calcitonin
gene-related peptide in endothelial cell proliferation in vivo. Br
J Pharmacol. 166:1261–1271. 2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Zheng S, Li W, Xu M, Bai X, Zhou Z, Han J,
Shyy JY and Wang X: Calcitonin gene-related peptide promotes
angiogenesis via AMP-activated protein kinase. Am J Physiol Cell
Physiol. 299:C1485–C1492. 2010.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Wang L, Shi X, Zhao R, Halloran BP, Clark
DJ, Jacobs CR and Kingery WS: Calcitonin-gene-related peptide
stimulates stromal cell osteogenic differentiation and inhibits
RANKL induced NF-kappaB activation, osteoclastogenesis and bone
resorption. Bone. 46:1369–1379. 2010.PubMed/NCBI View Article : Google Scholar
|
|
118
|
He H, Chai J, Zhang S, Ding L, Yan P, Du W
and Yang Z: CGRP may regulate bone metabolism through stimulating
osteoblast differentiation and inhibiting osteoclast formation. Mol
Med Rep. 13:3977–3984. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Brain SD and Grant AD: Vascular actions of
calcitonin gene-related peptide and adrenomedullin. Physiol Rev.
84:903–934. 2004.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Xu J, Wang J, Chen X, Li Y, Mi J and Qin
L: The effects of calcitonin gene-related peptide on bone
homeostasis and regeneration. Curr Osteoporos Rep. 18:621–632.
2020.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Zhang Y, Xu J, Ruan YC, Yu MK, O'Laughlin
M, Wise H, Chen D, Tian L, Shi D, Wang J, et al: Implant-derived
magnesium induces local neuronal production of CGRP to improve
bone-fracture healing in rats. Nat Med. 22:1160–1169.
2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Mi J, Xu JK, Yao Z, Yao H, Li Y, He X, Dai
BY, Zou L, Tong WX, Zhang XT, et al: Implantable electrical
stimulation at dorsal root ganglions accelerates osteoporotic
fracture healing via calcitonin gene-related peptide. Adv Sci
(Weinh). 9(e2103005)2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Chen J, Liu W, Zhao J, Sun C, Chen J, Hu
K, Zhang L and Ding Y: Gelatin microspheres containing calcitonin
gene-related peptide or substance P repair bone defects in
osteoporotic rabbits. Biotechnol Lett. 39:465–472. 2017.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Li Y, Yang L, Zheng Z, Li Z, Deng T, Ren
W, Wu C and Guo L: Bio-Oss® modified by calcitonin
gene-related peptide promotes osteogenesis in vitro. Exp
Ther Med. 14:4001–4008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Moreira DC, Sá CN, Andrade MG, Bório dos
Santos Calmon de Bittencourt TC, de Almeida Reis SR, Pithon MM and
Sadigursky M: Angiogenesis and osteogenesis at incorporation
process of onlay bone graft. J Oral Maxillofac Surg. 71:2048–2057.
2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Jeon YR, Kim MJ, Kim YO, Roh TS, Lee WJ,
Kang EH and Yun IS: Scaffold free bone regeneration using
platelet-rich fibrin in calvarial defect model. J Craniofac Surg.
29:251–254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Kim YH, Furuya H and Tabata Y: Enhancement
of bone regeneration by dual release of a macrophage recruitment
agent and platelet-rich plasma from gelatin hydrogels.
Biomaterials. 35:214–224. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Qiu P, Li M, Chen K, Fang B, Chen P, Tang
Z, Lin X and Fan S: Periosteal matrix-derived hydrogel promotes
bone repair through an early immune regulation coupled with
enhanced angio- and osteogenesis. Biomaterials.
227(119552)2020.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Narayanan R, Huang CC and Ravindran S:
Hijacking the cellular mail: exosome mediated differentiation of
mesenchymal stem cells. Stem Cells Int.
2016(3808674)2016.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Qin Y, Sun R, Wu C, Wang L and Zhang C:
Exosome: A novel approach to stimulate bone regeneration through
regulation of osteogenesis and angiogenesis. Int J Mol Sci.
17(712)2016.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu
W, Wang H, Liu H, Zhou H and Chen Y: Exosomes from bone marrow
mesenchymal stem cells enhance fracture healing through the
promotion of osteogenesis and angiogenesis in a rat model of
nonunion. Stem Cell Res Ther. 11(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Fan L, Guan P, Xiao C, Wen H, Wang Q, Liu
C, Luo Y, Ma L, Tan G, Yu P, et al: Exosome-functionalized
polyetheretherketone-based implant with immunomodulatory property
for enhancing osseointegration. Bioact Mater. 6:2754–2766.
2021.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Lin S, Cui L, Chen G, Huang J, Yang Y, Zou
K, Lai Y, Wang X, Zou L, Wu T, et al: PLGA/β-TCP composite scaffold
incorporating salvianolic acid B promotes bone fusion by
angiogenesis and osteogenesis in a rat spinal fusion model.
Biomaterials. 196:109–121. 2019.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Wu Y, Xia L, Zhou Y, Ma W, Zhang N, Chang
J, Lin K, Xu Y and Jiang X: Evaluation of osteogenesis and
angiogenesis of icariin loaded on micro/nano hybrid structured
hydroxyapatite granules as a local drug delivery system for femoral
defect repair. J Mater Chem B. 3:4871–4883. 2015.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Pang WY, Wang XL, Mok SK, Lai WP, Chow HK,
Leung PC, Yao XS and Wong MS: Naringin improves bone properties in
ovariectomized mice and exerts oestrogen-like activities in rat
osteoblast-like (UMR-106) cells. Br J Pharmacol. 159:1693–1703.
2010.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Shangguan WJ, Zhang YH, Li ZC, Tang LM,
Shao J and Li H: Naringin inhibits vascular endothelial cell
apoptosis via endoplasmic reticulum stress- and
mitochondrial-mediated pathways and promotes intraosseous
angiogenesis in ovariectomized rats. Int J Mol Med. 40:1741–1749.
2017.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Wang Z, Jiang R, Wang L, Chen X, Xiang Y,
Chen L, Xiao M, Ling L and Wang Y: Ginsenoside Rg1 improves
differentiation by inhibiting senescence of human bone marrow
mesenchymal stem cell via GSK-3β and β-catenin. Stem Cells Int.
2020(2365814)2020.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Salarian M, Samimi R, Xu WZ, Wang Z, Sham
TK, Lui EMK and Charpentier PA: Microfluidic synthesis and
angiogenic activity of ginsenoside Rg1-loaded PPF
microspheres. Acs Biomater Sci Eng. 2:1872–1882. 2016.PubMed/NCBI View Article : Google Scholar
|
|
139
|
García JR and García AJ:
Biomaterial-mediated strategies targeting vascularization for bone
repair. Drug Deliv Transl Re. 6:77–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Kessler PD, Podsakoff GM, Chen X,
McQuiston SA, Colosi PC, Matelis LA, Kurtzman GJ and Byrne BJ: Gene
delivery to skeletal muscle results in sustained expression and
systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA.
93:14082–14087. 1996.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Atluri K, Seabold D, Hong L, Elangovan S
and Salem AK: Nanoplex-mediated codelivery of fibroblast growth
factor and bone morphogenetic protein genes promotes osteogenesis
in human adipocyte-derived mesenchymal stem cells. Mol Pharm.
12:3032–3042. 2015.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Sun K, Lin H, Tang Y, Xiang S, Xue J, Yin
W, Tan J, Peng H, Alexander PG, Tuan RS and Wang B: Injectable
BMP-2 gene-activated scaffold for the repair of cranial bone defect
in mice. Stem Cell Transl Med. 9:1631–1642. 2020.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Raftery RM, Mencía-Castaño I, Sperger S,
Chen G, Cavanagh B, Feichtinger GA, Redl H, Hacobian A and O'Brien
FJ: Delivery of the improved BMP-2-advanced plasmid DNA within a
gene-activated scaffold accelerates mesenchymal stem cell
osteogenesis and critical size defect repair. J Control Release.
283:20–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Geiger F, Bertram H, Berger I, Lorenz H,
Wall O, Eckhardt C, Simank HG and Richter W: Vascular endothelial
growth factor gene-activated matrix (VEGF165-GAM) enhances
osteogenesis and angiogenesis in large segmental bone defects. J
Bone Miner Res. 20:2028–2035. 2005.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Curtin CM, Tierney EG, McSorley K, Cryan
SA, Duffy GP and O'Brien FJ: Combinatorial gene therapy accelerates
bone regeneration: Non-viral dual delivery of VEGF and BMP2 in a
collagen-nanohydroxyapatite Scaffold. Adv Healthc Mater. 4:223–227.
2015.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Zu H and Gao D: Non-viral vectors in gene
therapy: Recent development, challenges, and prospects. AAPS J.
23(78)2021.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Kalidasan V, Ng WH, Ishola OA, Ravichantar
N, Tan JJ and Das KT: A guide in lentiviral vector production for
hard-to-transfect cells, using cardiac-derived c-kit expressing
cells as a model system. Sci Rep. 11(19265)2021.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Bonadio J, Smiley E, Patil P and Goldstein
S: Localized, direct plasmid gene delivery in vivo: Prolonged
therapy results in reproducible tissue regeneration. Nat Med.
5:753–759. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
149
|
Bonadio J: Review: Local gene delivery for
tissue regeneration. E-Biomed J Regen Med. 1:25–29. 2000.
|
|
150
|
Bozo IY, Drobyshev AY, Redko NA, Komlev
VS, Isaev AA and Deev RV: Bringing a gene-activated bone substitute
into clinical practice: From bench to bedside. Front Bioeng
Biotechnol. 9(599300)2021.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Qin Y, Wang L, Gao Z, Chen G and Zhang C:
Bone marrow stromal/stem cell-derived extracellular vesicles
regulate osteoblast activity and differentiation in vitro and
promote bone regeneration in vivo. Sci Rep. 6(21961)2016.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Salomon C, Ryan J, Sobrevia L, Kobayashi
M, Ashman K, Mitchell M and Rice GE: Exosomal signaling during
hypoxia mediates microvascular endothelial cell migration and
vasculogenesis. PLoS One. 8(e68451)2013.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Zha Y, Li Y, Lin T, Chen J, Zhang S and
Wang J: Progenitor cell-derived exosomes endowed with VEGF plasmids
enhance osteogenic induction and vascular remodeling in large
segmental bone defects. Theranostics. 11:397–409. 2021.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Zha Y, Lin T, Li Y, Zhang X, Wang Z, Li Z,
Ye Y, Wang B, Zhang S and Wang J: Exosome-mimetics as an engineered
gene-activated matrix induces in-situ vascularized osteogenesis.
Biomaterials. 247(119985)2020.PubMed/NCBI View Article : Google Scholar
|