Introduction
Acquired hemophilia A (AHA) is a rare disease, that
may result from factor VIII inhibitors causing abnormal
coagulation. Patients with AHA are characterized by a prolonged
activated partial thromboplastin time (APTT) despite a normal
prothrombin time (PT) (1). In
Japan, the incidence of AHA has been reported to be 0.8-1.83 per
million individuals per year (2,3).
Recently, the number of reported cases of AHA has increased
(4). There is no gender difference
in the incidence of AHA, but it tends to be more common in the
elderly (5). AHA presents suddenly
with subcutaneous or intramuscular hemorrhage in patients without a
history or family history of bleeding tendencies (6). The bleeding phenotype of AHA is
variable, ranging from life-threatening bleeds to mild or no
bleeding (5). Patients with AHA
also have a high early-onset mortality rate, most of which are
caused by severe hemorrhage and severe infections (7). AHA has been reported to be associated
with autoimmune diseases, malignant tumors, pregnancy, delivery and
drugs (8-10).
According to certain reports, AHA may develop after surgery
(11,12). The present study reported a case of
AHA that developed after subtotal stomach-preserving
pancreaticoduodenectomy (SSPPD) for distal cholangiocarcinoma.
Case report
A 68-year-old male presented to the emergency
department of Tokyo Metropolitan Tama Medical Center (Tokyo, Japan)
with upper abdominal pain in September 2019. The patient was a
sedentary worker on active duty and had no apparent history of
carcinogen exposure. He had no history of dementia, hepatitis B or
C, or diabetes. The patient was diagnosed with gallstone
pancreatitis at the first visit and underwent endoscopic retrograde
cholangiography the next day, during which a plastic stent was
placed at the department of gastroenterology. However, endoscopic
removal of a common bile duct stone revealed stenosis due to a
neoplastic lesion in the distal bile duct, and a biopsy confirmed
distal cholangiocarcinoma, so the patient was referred to our
department in November 2019. Preoperative blood tests indicated
that no blasts were present.
The patient underwent SSPPD as the first surgery.
Intraoperative rapid diagnosis was performed according to standard
procedures, and the result was positive for a bile duct stump, so
additional resection was performed. The bile duct stump was
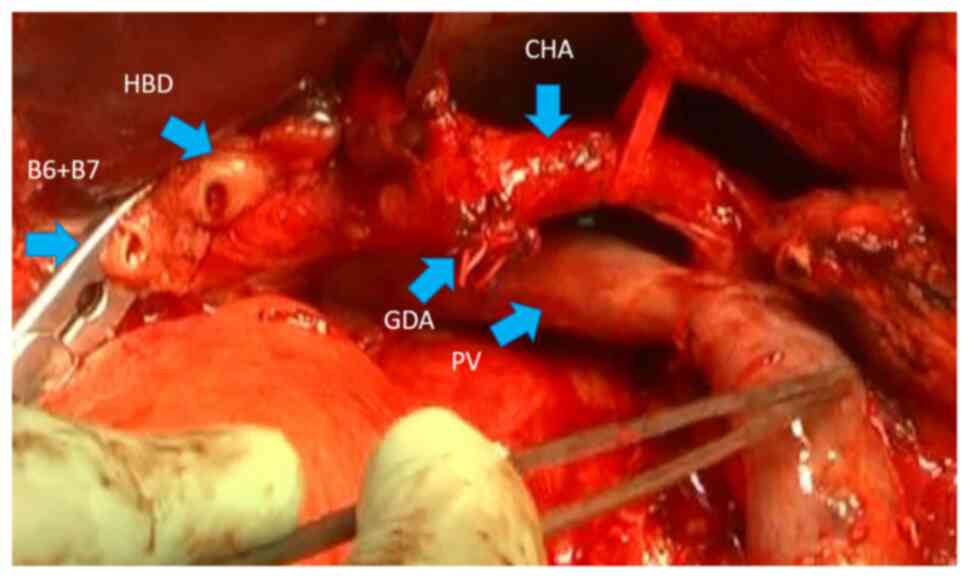
negative for cancer, and had two holes: The posterior segment
branch of the low confluence and the common hepatic duct (Fig. 1), which formed a hole for the
hepatocholangiojejunostomy. The operative time was 608 min, the
amount of bleeding was 1,055 ml and no blood transfusion was
required.
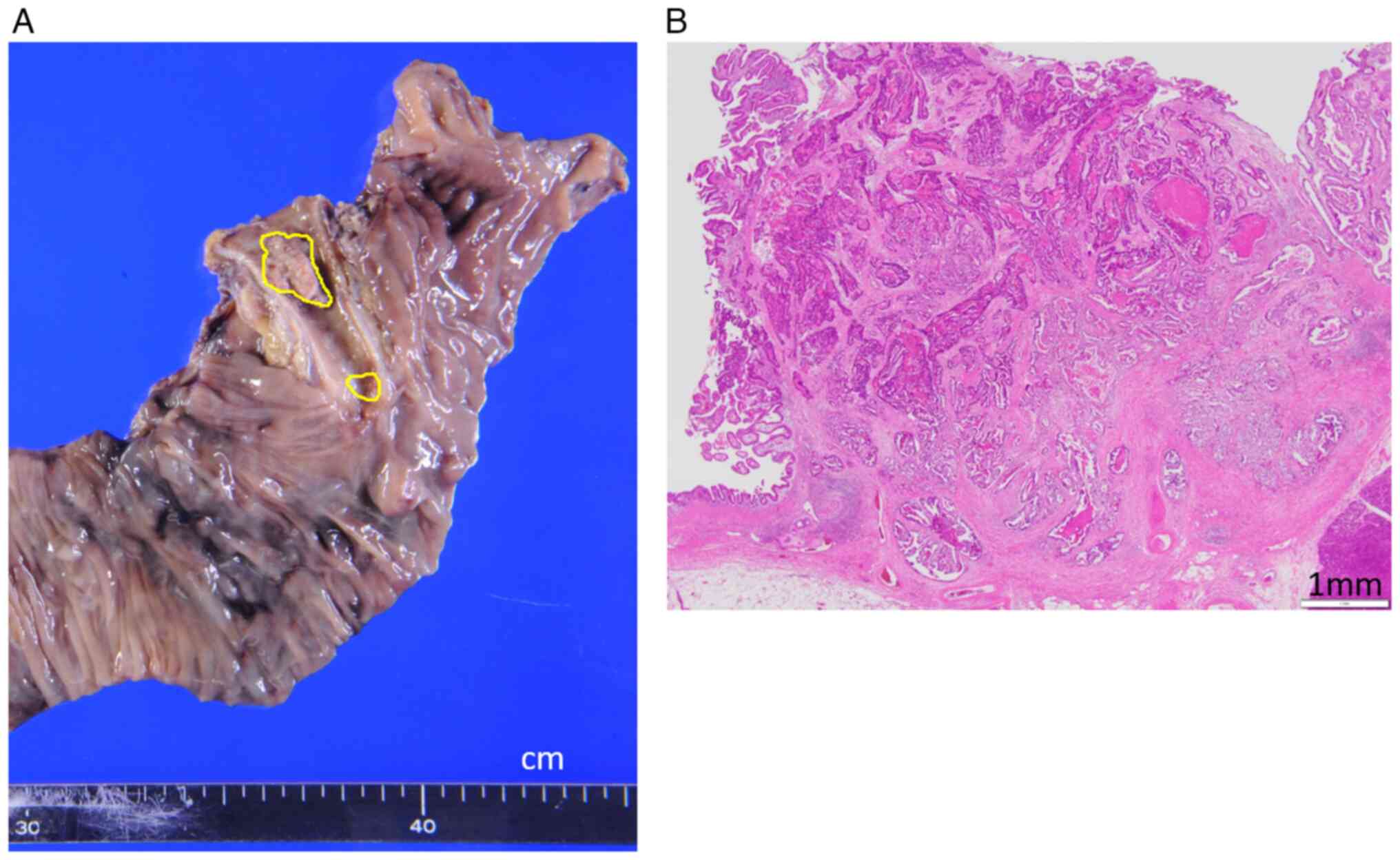
Pathological examination was performed by standard
procedures and revealed a well-to-moderately differentiated tubular
adenocarcinoma (pT1, pN1, pM0, pStage IIA) (Fig. 2A and B). On postoperative day (PD) 3, the
patient developed a surgical site infection, and on PD 5,
intestinal juice leaked from the wound, requiring a second surgery
to resuture the Braun's anastomotic rupture. On PD 6, there was
significant bleeding from the gastric tube and dynamic computed
tomography showed bleeding into the jejunal limb. Angiography was
performed, but hemostasis occurred spontaneously during the
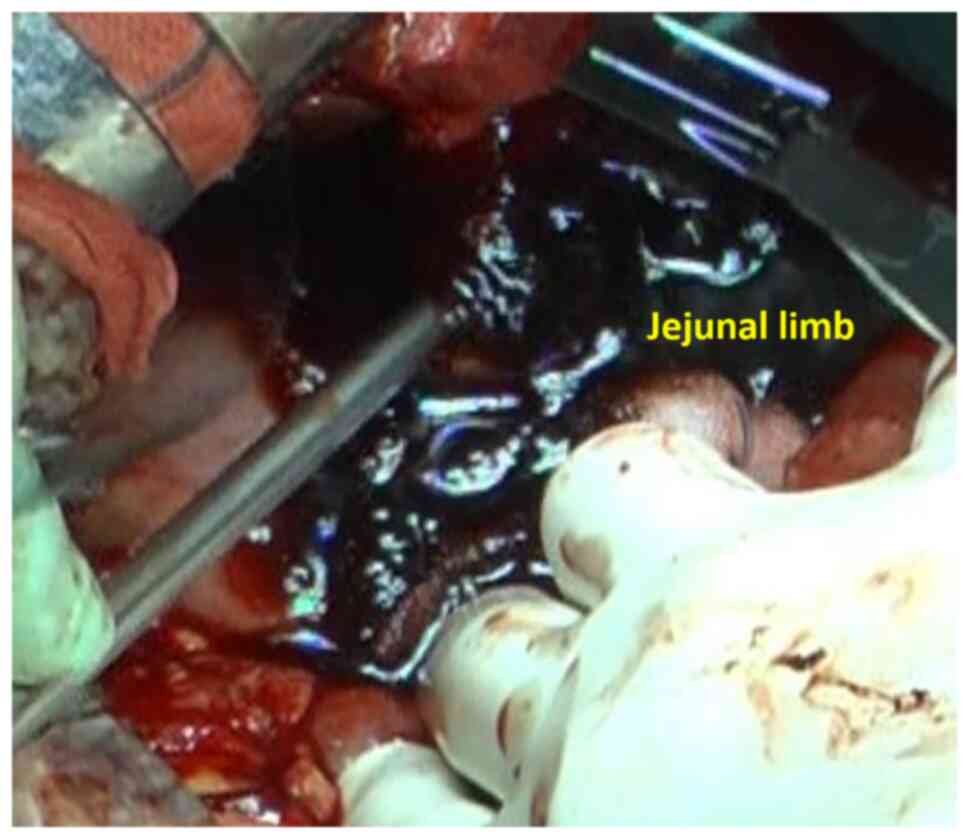
examination (first angiography). On PD 8, bleeding in the jejunal
limb reoccurred and direct surgical ligation was performed (third
operation) (Fig. 3). There was a
small hole in the Braun's enterostomy and gastrojejunostomy
sutures. The afferent leg was transected, bypassing to the anal
side, and the hole was closed on the efferent leg side. The
gastrostomy tube was inserted through the suture failure of the
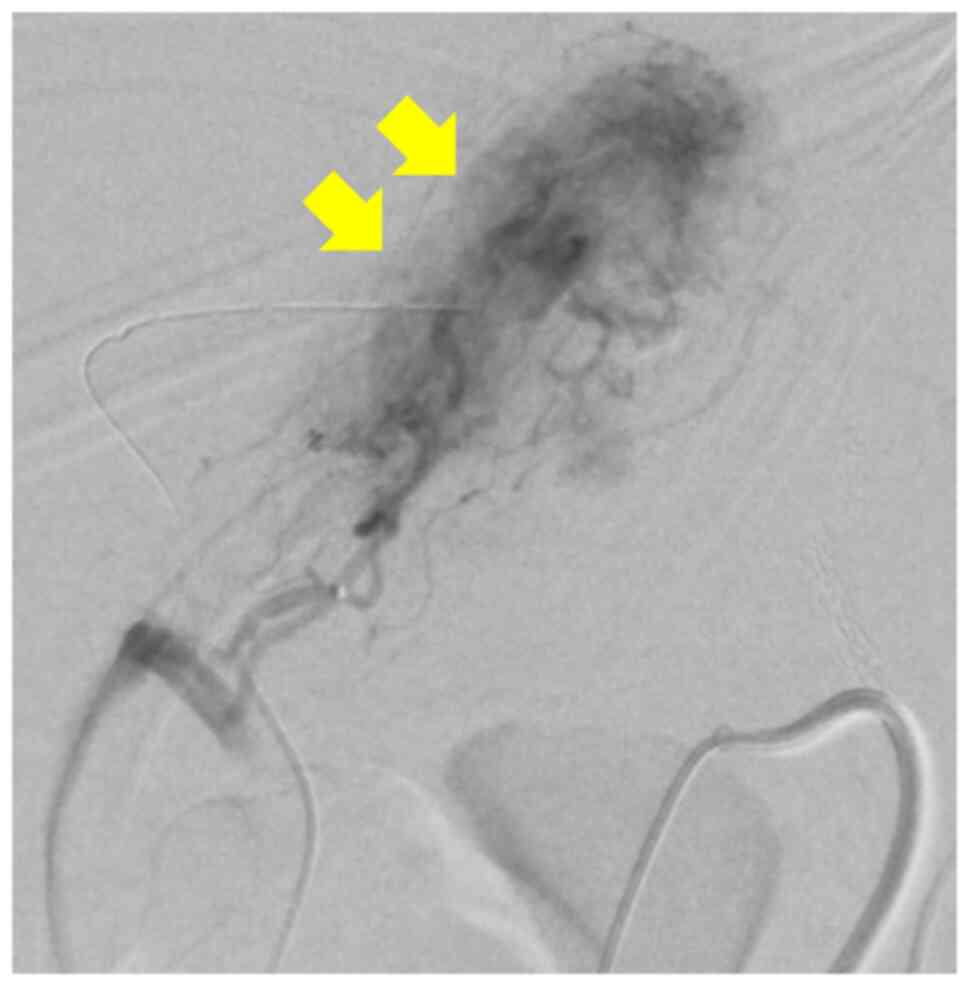
gastrojejunostomy. On PD 14, bleeding recurred in the jejunal limb
and angiography was performed to embolize the periphery of the
second jejunal artery (second angiography) (Fig. 4).
As the patient continued to exhibit hemorrhagic
tendencies, a prolonged activated partial thromboplastin time
(APTT) (44.5 sec) was observed from PD 2, despite the preoperative
APTT (22.9 sec) being within the almost normal range (24.3-36.0
sec). Although the prothrombin time (PT) was within the normal
range postoperatively, the APTT reached 91.5 sec on PD 12. On PD
14, acquired hemophilia A (AHA) was suspected and activated
prothrombin complex concentrate (APCC; FEIBA®; 100 U/kg,
q12h, four times) was administered while performing a coagulation
factor activity test. Prednisolone (PSL) was also started on PD 15
at 1 mg/kg/day. However, the gastrojejunostomy site was perforated,
resulting in a labial fistula. On PD 18, the diagnosis of AHA was
confirmed by a decrease in factor VIII activity (<1%; normal
range, 60-150%), the appearance of a factor VIII inhibitor (18
BU/ml; normal value, undetectable) and the absence of a lupus
anticoagulant. Other results of coagulation factor activity tests
were as follows: Factor IX activity, 73% (normal range, 70-130%),
factor IX inhibitor, 0 BU/ml (normal value, undetectable); factor
XIII activity, 37% (normal range, 70-140%), von Willebrand factor,
294% (normal range, 60-170%); anticardiolipin
antibody-anti-β2-glycoprotein I, <1.2 U/ml (upper limit of
normal, 3.5 U/ml). Loss due to hemorrhage was thought to be the
reason for the decreased factor XIII clotting activity.
On PD 21, blood transfusion was no longer required
and hemostasis was observed, allowing us to initiate the
administration of glutamine, fiber and oligosaccharide (GFO)
through the intestinal fistula. However, on PD 22, the patient
experienced melena and anemia progressed, prompting us to halt the
administration of GFO and administer APCC for two days.
However, on PD 26, melena resumed and APCC was
administered again for two days. The administration of GFO was
resumed on PD 29 through an intestinal fistula, but it was required
to stop again on PD 31 due to the recurrence of melena, leading to
the administration of APCC for three days. The patient also
exhibited decreased factor XIII activity, and hence, freeze-dried
blood-coagulation factor XIII derived from human plasma
(Fibrogammin P®; CSL Behring; 1,440 U/day) was
administered for three days. By PD 36, the patient was stable and
was discharged from the intensive care unit (ICU) (Fig. 5A), after receiving APCC for 10 days
and 102 units of red blood cells. Hemostasis was achieved by PD 41
and enteral nutrition was resumed.
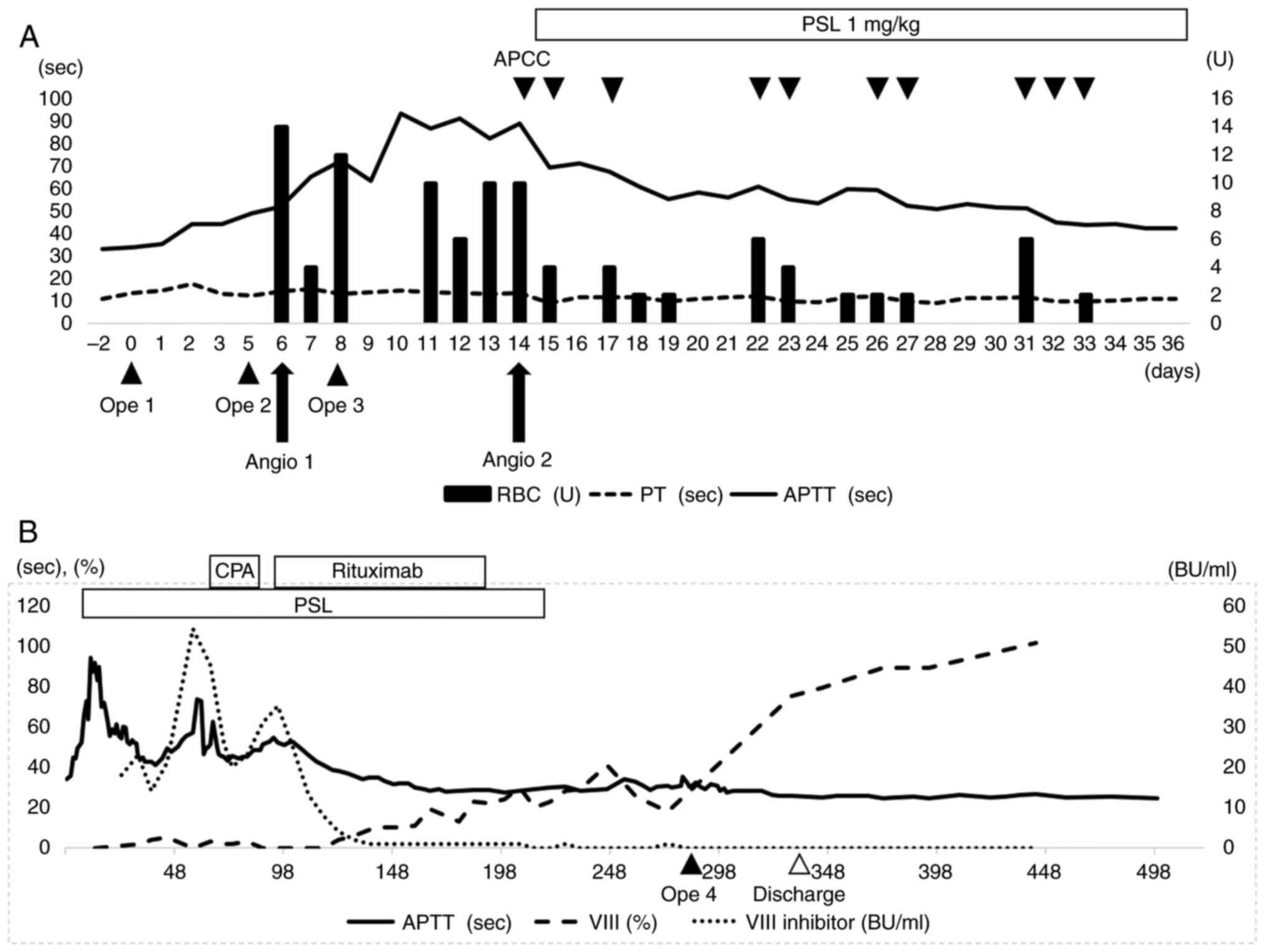 | Figure 5(A) Course of treatment at the ICU. PT
was consistently within the normal range before and after surgery,
but the APTT began to prolong after surgery and reached 91.5 secs
on PD 12. Acquired hemophilia A was suspected on PD 14 and APCC was
administered. The administration of steroids started on PD 15.
While at the ICU, APCC was administered for 10 days and a total of
102 units of red blood cells were administered. (B) The long-term
therapeutic course is indicated. On PD 58, CPA was administered in
addition to steroids, but since factor VIII inhibitors increased,
administration of rituximab was started on PD 98. After 12 courses
of rituximab, the factor VIII inhibitor finally became negative on
PD 219. ICU, intensive care unit; PT, prothrombin time; APTT,
activated partial thromboplastin time; APCC, activated prothrombin
complex concentrate; CPA, cyclophosphamide; PD, postoperative day;
PSL, prednisolone; OPE, operation; RBC, red blood cells; Angio,
angiography. |
On PD 58, the factor VIII inhibitor levels increased
(54 BU/ml), prompting the initiation of combination therapy with
cyclophosphamide (CPA; 15 mg/kg, every three weeks) and PSL. On PD
79, negative pressure wound therapy was started at a different
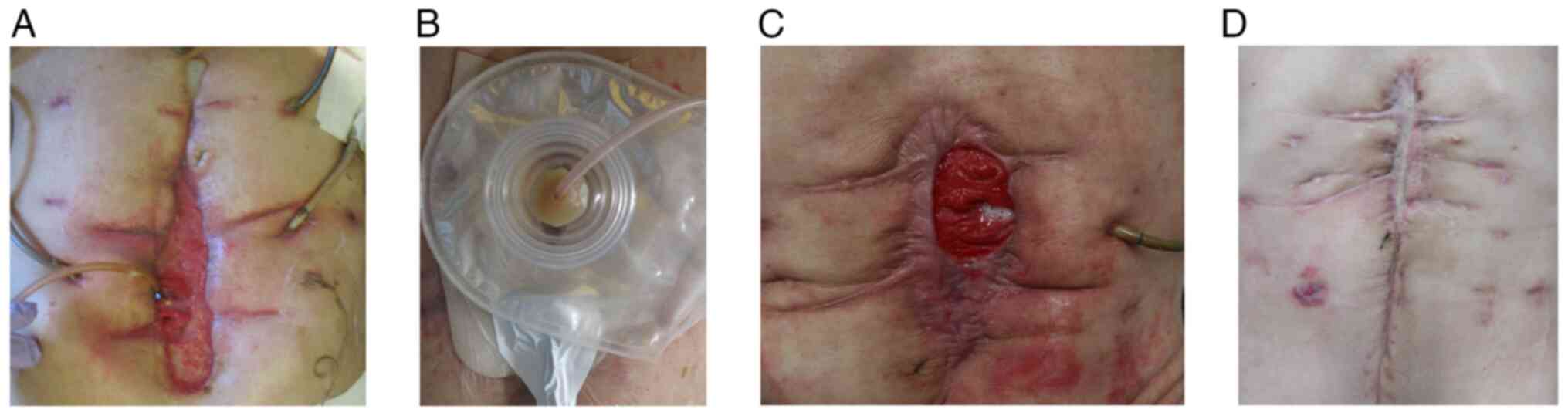
wound location from the labial fistula of the wound (Fig. 6A and B). On PD 98, the factor VIII inhibitor
levels increased (35 BU/ml), prompting the initiation of
combination therapy with rituximab (375 mg/m2, weekly)
and PSL. With treatment, the factor VIII inhibitor levels gradually
decreased, factor VIII coagulation activity increased and APTT
prolongation improved. The PSL dose was subsequently reduced
gradually.
After 12 courses of rituximab, the factor VIII
inhibitors became negative on PD 219, but factor VIII activity
remained low at 20-30% (Fig. 5B).
The patient was temporarily discharged on PD 245, with a labial
fistula and enteral nutrition management (Fig. 6C). Surgery under general anesthesia
was planned to close the labial fistula and it was decided to
perform the surgery while supplementing factor VIII. On PD 268, a
pharmacokinetic test was conducted by intravenous injection of a
recombinant factor VIII preparation (rFVIII) (ADVATE®).
As a result, in vivo recovery, half-life time and clearance
using rFVIII was 1.79 (IU/dl)/(IU/kg), 17.6 h and 2.5 ml/kg/h,
respectively. Suzuki et al (13) analyzed 34 patients and determined
that the in vivo recovery, half-life time and clearance
using rFVIII was 1.42±0.36 (IU/dl)/(IU/kg), 12 h and 4.25±2.25
ml/kg/h, respectively.
On PD 289, the fourth surgery was performed,
trimming and closing the perforated site of the gastrojejunostomy
with an Albert-Lembert suture. Before the surgery, a bolus dose of
58 IU/kg rFVIII was administered, and during the surgery, rFVIII
was continuously infused at 2.35 IU/kg/h. Factor VIII coagulation
activity was monitored during and after surgery, and the rFVIII
dose was gradually reduced while maintaining ≥100%. On the third
day after the fourth surgery (PD 292), continuous intravenous
infusion of rFVIII was discontinued.
The patient was able to resume oral intake on PD 322
and was discharged from the hospital on PD 342 (Fig. 6D). However, on PD 765, the patient
passed away due to the recurrence of liver metastasis from distal
cholangiocarcinoma.
Discussion
AHA is a rare bleeding disorder caused by
neutralizing autoantibodies known as inhibitors of coagulation
factor VIII. It is associated with underlying conditions such as
pregnancy, delivery, autoimmune diseases, malignant diseases and
drug reactions (5). Napolitano
et al (14) conducted a
study involving 105 patients with AHA with underlying malignant
tumors, including 60 solid tumors and 45 hematological
malignancies. Prostate (25.3%) and lung (15.8%) cancers had the
highest frequency, followed by colon cancer (9.5%). Of the 105
cases, only two (1.9%) had distal bile duct cancer. It is worth
noting that the patient of the present study and the two
aforementioned patients had no coagulation abnormalities prior to
pancreaticoduodenectomy and they developed AHA after tumor
resection.
In certain cases, AHA may develop following major
surgery, including pancreatoduodenectomy (11,12,15).
APCC (16) and recombinant
activated factor VII (NovoSeven®) (17) preparations are typically used in the
acute phase of AHA for hemostasis (5). Clinical trial data have indicated that
recombinant porcine factor VII is also effective in treating AHA
(18).
In the case of the present study, the bleeding was
finally stopped after repeated use of APCC. For the chronic phase
of AHA, international recommendations from 2020(5) suggest first-line treatment with PSL
alone for three to four weeks for patients with factor VIII ≥1
IU/dl and inhibitor titer ≤20 BU at baseline (19), while those with factor VIII <1
IU/dl or inhibitor titer >20 BU receive a combination of PSL
with rituximab or a cytotoxic agent (CPA or mycophenolate mofetil)
as first-line treatment (20). As a
second-line treatment, rituximab or a cytotoxic agent that was not
used during first-line treatment has been suggested (19).
In the present case, factor VIII activity was <1%
and factor VIII inhibitor was 18 BU. As the present case was
encountered before the 2020 guidelines were published, PSL was
first administered, followed by combination therapy of CPA and PSL
as the second-line treatment and a combination of rituximab and PSL
as the third-line treatment (1,21).
In the case of the present study, factor VIII
inhibitor disappeared after 31 weeks of treatment. Reports of
surgery in patients with AHA are scarce. As in the second and third
surgeries in the present case, emergency surgery has been reported
in the presence of a bleeding tendency (22-24),
and while a small number of studies reported that surgery was
performed after confirming negative factor VIII inhibitor status,
as in the fourth surgery in the present case. Ichikawa et al
(25) reported that a patient with
AHA was treated with rituximab and underwent surgery for sigmoid
colon cancer after the disappearance of factor VIII inhibitors with
no adverse events. Jena et al (26) performed a pancreatoduodenectomy on a
patient with periampullary carcinoma and AHA after treating
AHA.
Upon scheduling intestinal fistula closure, it was
observed that while factor VIII inhibitor had resolved, factor VIII
activity remained low. In such cases, it becomes challenging to
assess both hemostatic and thrombotic tendencies while using bypass
preparations due to the incomplete resolution of AHA. Kruse-Jarres
et al (27) reported that
rFVIII replacement therapy is also a treatment option when the
factor VIII inhibitor level is <5 BU/ml. There have been certain
reported cases where hemostatic management and surgery were
performed while administering rFVIII (28-31),
and in the present case, hemostatic management with rFVIII under
adequate monitoring was chosen. As a result, there were no
perioperative bleeding or thrombotic complications and AHA did not
recur.
The pathogenesis of AHA remains to be fully
elucidated. Although in the present study, it was considered to
measure autoantibodies and complement, they were not examined
because the effects of surgical invasion and early initiation of
immunosuppressive therapy with steroids may make the interpretation
of the results difficult. In addition to malignant tumors and
autoimmune diseases that have been reported so far, there have been
an increasing number of reports of onset triggered by infectious
diseases, such as reports related to COVID-19 (32-34).
Furthermore, Alzheimer's dementia, hepatitis B and diabetes may
also be important risk factors for AHA (35). In the case of the present study, AHA
developed just after surgery for a malignant tumor, so it was
difficult to collect information that may be related to the
pathogenesis, but at least there was no background of Alzheimer's
dementia, hepatitis B or diabetes. Further cases need to be
accumulated to study the pathogenesis and establish a suitable
management strategy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MT drafted the manuscript. YM contributed to the
preoperative checks and diagnoses. MT, THa, YM and ZD performed the
initial surgery. MT, THa and ZD performed the second surgery. YM
and MT performed the third surgery. YM, NI and MT performed the
fourth surgery. KS directed the management of perioperative care
for the patient in the care unit. YK and YC supervised hemostatic
therapy and immunotherapy for the patient. NI and NS managed the
patient's labial fistulas. FH contributed to the nutritional
management of the patient. YM followed up the patient. THi, KK,
SSat, SY, SSas, KF, TS, AI, HOh and YI provided postoperative
management for the patient. HOk made the pathological diagnosis. MT
and YK checked and approved the authenticity of the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huth-Kühne A, Baudo F, Collins P,
Ingerslev J, Kessler CM, Lévesque H, Castellano ME, Shima M and
St-Louis J: International recommendations on the diagnosis and
treatment of patients with acquired hemophilia A. Haematologica.
94:566–575. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ogawa Y, Yanagisawa K, Uchiumi H, Ishizaki
T, Mitsui T, Gouda F, Ieko M, Ichinose A, Nojima Y and Handa H:
Clinical characteristics and outcomes of acquired hemophilia A:
experience at a single center in Japan. Int J Hematol. 106:82–89.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ogawa Y, Amano K, Matsuo-Tezuka Y, Okada
N, Murakami Y, Nakamura T, Yamaguchi-Suita H and Nogami K: ORIHIME
study: Real-world treatment patterns and clinical outcomes of 338
patients with acquired hemophilia A from a Japanese administrative
database. Int J Hematol. 117:44–55. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tiede A, Werwitzke S and Scharf RE:
Laboratory diagnosis of acquired hemophilia A: Limitations,
consequences, and challenges. Semin Thromb Hemost. 40:803–811.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tiede A, Collins P, Knoebl P, Teitel J,
Kessler C, Shima M, Di Minno G, d'Oiron R, Salaj P, Jiménez-Yuste
V, et al: International recommendations on the diagnosis and
treatment of acquired hemophilia A. Haematologica. 105:1791–1801.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Delgado J, Jimenez-Yuste V,
Hernandez-Navarro F and Villar A: Acquired haemophilia: Review and
meta-analysis focused on therapy and prognostic factors. Br J
Haematol. 121:21–35. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Collins PW, Hirsch S, Baglin TP, Dolan G,
Hanley J, Makris M, Keeling DM, Liesner R, Brown SA and Hay CR: UK
Haemophilia Centre Doctors' Organisation. Acquired hemophilia A in
the United Kingdom: A 2-year national surveillance study by the
United Kingdom Haemophilia Centre Doctors' Organisation. Blood.
109:1870–1877. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bossi P, Cabane J, Ninet J, Dhote R,
Hanslik T, Chosidow O, Jouan-Flahault C, Horellou MH, Leynadier F,
Liozon E, et al: Acquired hemophilia due to factor VIII inhibitors
in 34 patients. Am J Med. 105:400–408. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morrison AE, Ludlam CA and Kessler C: Use
of porcine factor VIII in the treatment of patients with acquired
hemophilia. Blood. 81:1513–1520. 1993.PubMed/NCBI
|
|
10
|
Green D and Lechner K: A survey of 215
non-hemophilic patients with inhibitors to Factor VIII. Thromb
Haemost. 45:200–203. 1981.PubMed/NCBI
|
|
11
|
Miura T, Ban D, Koyama T, Kudo A, Ochiai
T, Irie T, Nakamura N, Tanaka S and Arii S: Severe postoperative
hemorrhage caused by antibody-mediated coagulation factor
deficiencies: Report of two cases. Surg Today. 44:976–981.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mekenkamp LJ, Beishuizen A, Slomp J,
Legdeur MC, Klaase JM and Trof RJ: Successful treatment of
fulminant postoperative bleeding due to acquired haemophilia. Neth
J Med. 73:182–186. 2015.PubMed/NCBI
|
|
13
|
Suzuki N, Hirakawa A, Kishimoto M,
Kanematsu T, Ogawa M, Kiyoi H and Matsushita T: Retrospective
analysis of in vivo recovery and clearance during continuous
infusion of recombinant factor VIII products: A single-institution
study. Haemophilia. 23:215–221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Napolitano M, Siragusa S, Mancuso S and
Kessler CM: Acquired haemophilia in cancer: A systematic and
critical literature review. Haemophilia. 24:43–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Geethakumari PR, Sama A, Caro JG, Yeo CJ
and Nagalla S: ‘The Immune Conundrum’: Acquired Hemophilia A,
immune thrombocytopenia, and neutropenia in a patient with
pancreatic cancer. Case Rep Pancreat Cancer. 2:14–18.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sallah S: Treatment of acquired
haemophilia with factor eight inhibitor bypassing activity.
Haemophilia. 10:169–173. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tiede A and Worster A: Lessons from a
systematic literature review of the effectiveness of recombinant
factor VIIa in acquired haemophilia. Ann Hematol. 97:1889–1901.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kruse-Jarres R, St-Louis J, Greist A,
Shapiro A, Smith H, Chowdary P, Drebes A, Gomperts E, Bourgeois C,
Mo M, et al: Efficacy and safety of OBI-1, an antihaemophilic
factor VIII (recombinant), porcine sequence, in subjects with
acquired haemophilia A. Haemophilia. 21:162–170. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tiede A, Klamroth R, Scharf RE, Trappe RU,
Holstein K, Huth-Kühne A, Gottstein S, Geisen U, Schenk J, Scholz
U, et al: Prognostic factors for remission of and survival in
acquired hemophilia A (AHA): Results from the GTH-AH 01/2010 study.
Blood. 125:1091–1097. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Collins P, Baudo F, Knoebl P, Lévesque H,
Nemes L, Pellegrini F, Marco P, Tengborn L and Huth-Kühne A:
Immunosuppression for acquired hemophilia A: Results from the
European Acquired Haemophilia Registry (EACH2). Blood. 120:47–55.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Franchini M: Acquired hemophilia A.
Hematology. 11:119–125. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gok E, Akay MH, de Armas IS, Klein K, Tint
H, Allison PM, Chen AJ, Akkanti B, Kar B and Gregoric ID: Aortic
root repair in a patient with acquired hemophilia A: case report.
Surg Case Rep. 7(176)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tjønnfjord GE, Brinch L, Gedde-Dahl T and
Brosstad FR: Activated prothrombin complex concentrate (FEIBA)
treatment during surgery in patients with inhibitors to FVIII/IX.
Haemophilia. 10:174–178. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hay CR, Negrier C and Ludlam CA: The
treatment of bleeding in acquired haemophilia with recombinant
factor VIIa: A multicentre study. Thromb Haemost. 78:1463–1467.
1997.PubMed/NCBI
|
|
25
|
Ichikawa S, Kohata K, Okitsu Y, Suzuki M,
Nakajima S, Yamada MF, Onishi Y, Yamamoto J, Suzuki S, Ishizawa K,
et al: Acquired hemophilia A with sigmoid colon cancer: Successful
treatment with rituximab followed by sigmoidectomy. Int J Hematol.
90:33–36. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jena SS, Meher D and Dhankar N: Unforeseen
encounter of acquired hemophilia A in a preoperative case of
periampullary carcinoma: A case report. Int J Surg Case Rep.
79:146–149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kruse-Jarres R, Kempton CL, Baudo F,
Collins PW, Knoebl P, Leissinger CA, Tiede A and Kessler CM:
Acquired hemophilia A: Updated review of evidence and treatment
guidance. Am J Hematol. 92:695–705. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shen M, Wang S, Sessa J, Hanna A, Axelrad
A and Ali F: Acquired hemophilia A: A case report. J Pharm Pract.
33:562–566. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Prencipe MA, D'Errico M, Di Giorgio G,
Gatta G, Gesuete A, Nobile M and Stallone C: Macrohaematuria
post-partum: An unusual case of acquired haemophilia after
pregnancy. G Ital Nefrol. 19:204–208. 2002.PubMed/NCBI(In Italian).
|
|
30
|
Baudo F, Collins P, Huth-Kühne A, Lévesque
H, Marco P, Nemes L, Pellegrini F, Tengborn L and Knoebl P: EACH2
registry contributors. Management of bleeding in acquired
hemophilia A: results from the European Acquired Haemophilia
(EACH2) Registry. Blood. 120:39–46. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lindahl R, Nummi V, Lehtinen AE, Szanto T,
Hiltunen L, Olsson A, Glenthoej A, Chaireti R, Vaide I, Funding E
and Zetterberg E: Acquired Haemophilia A in four north European
countries: Survey of 181 patients. Br J Haematol. 201:326–333.
2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guerra JD, Gowarty J, Buess J, Mason J and
Halka K: A case of acquired hemophilia a in a patient with exposure
to COVID-19. Case Rep Hematol. 2022(9494249)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Murali A, Wong P, Gilbar PJ and Mangos HM:
Acquired Hemophilia A following Pfizer-BioNTech SARS CoV-2 mRNA
vaccine, successfully treated with prednisolone and rituximab. J
Oncol Pharm Pract. 28:1450–1453. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Franchini M, Cappello E, Valdiserra G,
Bonaso M, Moretti U, Focosi D and Tuccori M: Investigating a signal
of acquired hemophilia associated with COVID-19 Vaccination: A
systematic case review. Semin Thromb Hemost. 49:15–26.
2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shoji-Asahina A, Nakatani E, Imaichi Y,
Ohata E, Oshima M, Miyakoshi A, Miyake H, Ichikawa Y, Dote H,
Ubukata N, et al: Risk factors, treatment and survival rates of
late-onset acquired haemophilia A: A cohort study from the Shizuoka
Kokuho Database. Haemophilia. 29:799–808. 2023.PubMed/NCBI View Article : Google Scholar
|