Introduction
Fibrous dysplasia of bone (FDB) is a non-malignant
fibro-osseous lesion that accounts for 5-7% of all benign bone
tumors (1), with a low malignant
transformation or local aggressive form rate (2,3). FDB
may involve single bone (monostotic disease) or multiple bones
(polyostotic disease) (3,4). The main pathological changes of FDB
are that normal bone architecture and bone marrow are replaced by a
large amount of proliferative fibrous tissue, in which there are
ill-structured trabeculae (5). A
variety of causative factors, including repeated surgical
treatment, artificial limb implantation and radiotherapy, are
potential factors to stimulate the malignant transformation of FDB
(6,7). The age of the patient is another
factor that also correlates to sarcomatous transformation (8). Poorly defined margins, cortical
destruction and soft tissue involvement are features on imaging of
malignant transformation; however, these features overlap with
those of locally aggressive FDB (2,3).
Therefore, the diagnosis of malignant transformation of FDB is a
clinical challenge. However, the prognosis of patients with FDB
malignant transformation is poor as, even if they receive
preoperative and/or postoperative chemotherapy and subsequent
extensive resection, distant metastasis and death are inevitable
(9). Osteosarcoma accounts for more
than half of all the malignant transformations of FDB, followed by
fibrosarcoma and chondrosarcoma, secondary angiosarcomas and
malignant fibrous histiocytoma (4,10). The
present case study describes a rare case of FDB that was associated
with malignant sarcomatous transformation.
Case presentation
Presentation
A 23-year-old man was referred to the Department of
Orthopedics, The Third Affiliated Hospital of Guangzhou Medical
University in May 2018 with persistent pain in the left hip for 6
months that had been aggravated for 1 week.
History of present illness
The present patient presented with persistent pain
in the left hip without obvious inducement that started 6 months
before the hospital visit. The pain was obvious in squatting and
standing up. The patient was initially admitted to a local
hospital, where an X-ray and MRI investigation showed a central
intramedullary expansile lytic lesion with a wide zone of
transition at the proximal metadiaphysis of the left femur, but the
clinical diagnosis was inconclusive, and the patient did not
receive any special treatment. On 14 May 2018, the patient was
referred to the Department of Orthopedics, the Third Affiliated
Hospital of Guangzhou Medical University for further diagnostics
and treatment for aggravated pain in the left hip. After admission,
multimodal imaging including X-ray, CT, MRI and Technetium
99m-methyl diphosphonate (99mTc-MDP) three-phase bone
imaging were performed. It was agreed that these images were
consistent with FDB with malignant transformation after a
multidisciplinary team discussion. To make a definite diagnosis,
the patient underwent a left femur biopsy 4 days after referral to
hospital, followed by left femur surgical biopsy and a left tibia
percutaneous biopsy 10 days later. The pathological analysis showed
that the lesions were consistent with malignant mesenchymal tumors
of the left femur and it was concluded that they were malignant
fibrous histiocytoma of the bone and osteofibrosarcoma. The patient
underwent chemotherapy, left femur tumor segment resection and hip
joint replacement on 39 days after referral to our hospital. The
surgical and postoperative pathological findings confirmed
fibrosarcoma of the bone with extraosseous soft tissue
involvement.
History of past illness
The patient had no relevant previous medical
history, such as trauma, falls or tumors.
Personal and family history
The patient had no tumor-related family history. The
patient had no history of contact with carcinogenic chemical,
radioactive or toxic substances, and no history of drug abuse,
smoking and drinking.
Physical examination
There was no obvious deformity of the left thigh and
hip, and no obvious skin redness, swelling and ulceration. Local
skin temperature did not increase and the patient had good skin
sensation, but obvious local tenderness in the left hip, and
longitudinal percussion pain in the left lower limb.
Laboratory examinations
Laboratory tests revealed alkaline phosphatase 239
U/l (reference range, 45-125 U/l), erythrocyte sedimentation rate
68 mm/h (reference range, <15 mm/h), C-reactive protein 135.3
ng/l (reference range, <10 ng/l), total neutrophil count
7.74x109/l (reference range, 2.0-6.9x109/l)
and neutrophil ratio 82.2% (reference range, 37.0-80.0%).
Imaging and histological
examinations
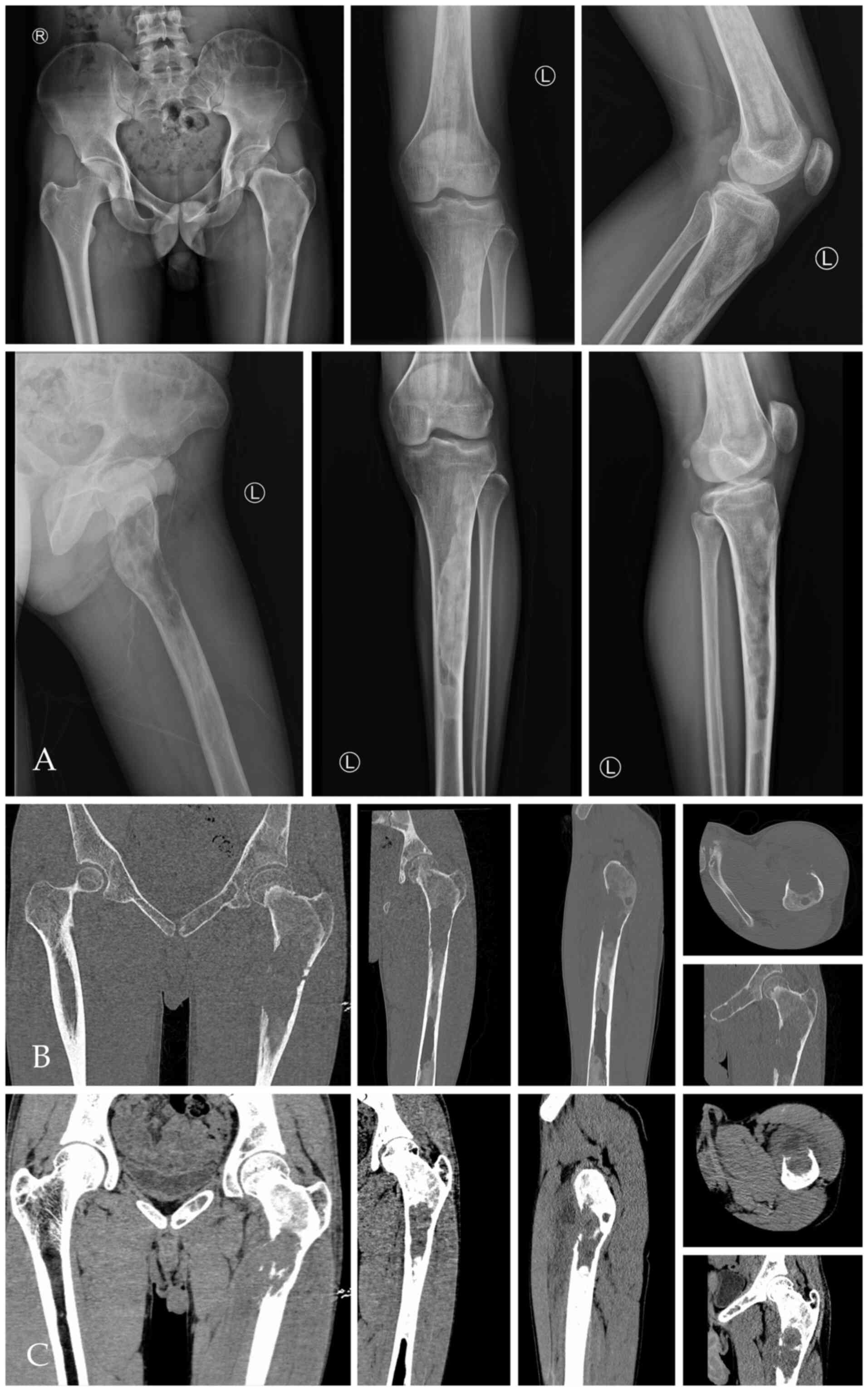
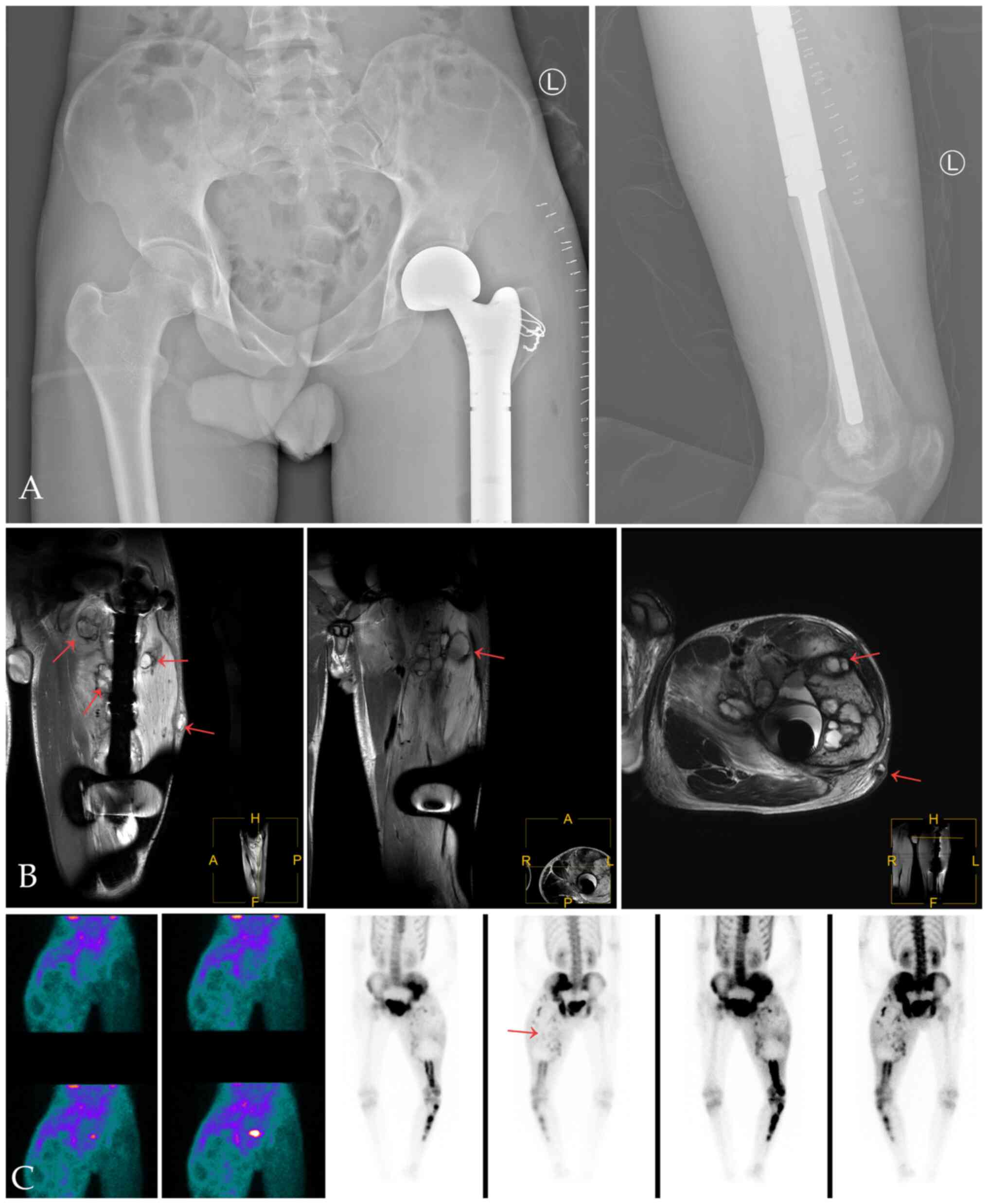
X-ray and CT of the left femur showed abnormal
density of the left femur and the bone marrow cavity of the left
hip, upper tibia and sacrum, and bone destruction of the
anteromedial cortex of the left femur and formation of surrounding
soft tissue masses (Fig. 1A-C). The
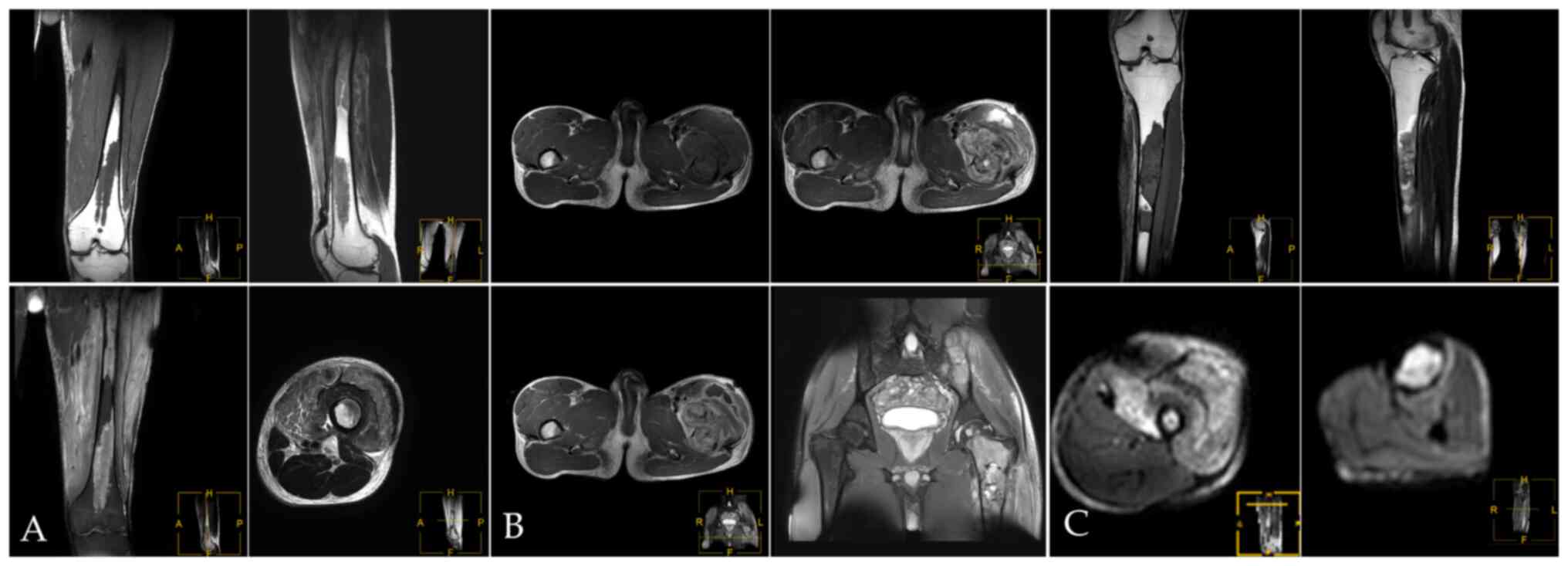
possibility of malignancy was considered. MRI showed multiple bone
destruction areas occupying most of the space of the marrow cavity
with hypointensity on T1-weight imaging (T1WI), heterogeneous
enhancement on contrast-enhanced T1WI, heterogeneous iso- and
hyper-intensity on T2WI, and heterogeneous signal intensity on
T2-weight spectral attenuated inversion recovery (T2W-SPAIR).
These features indicated local bone destruction,
swelling of surrounding soft tissue and soft tissue invasion
(Fig. 2A and B). In the upper left femur, an irregular
mass was seen in front of the upper left femur, which broke through
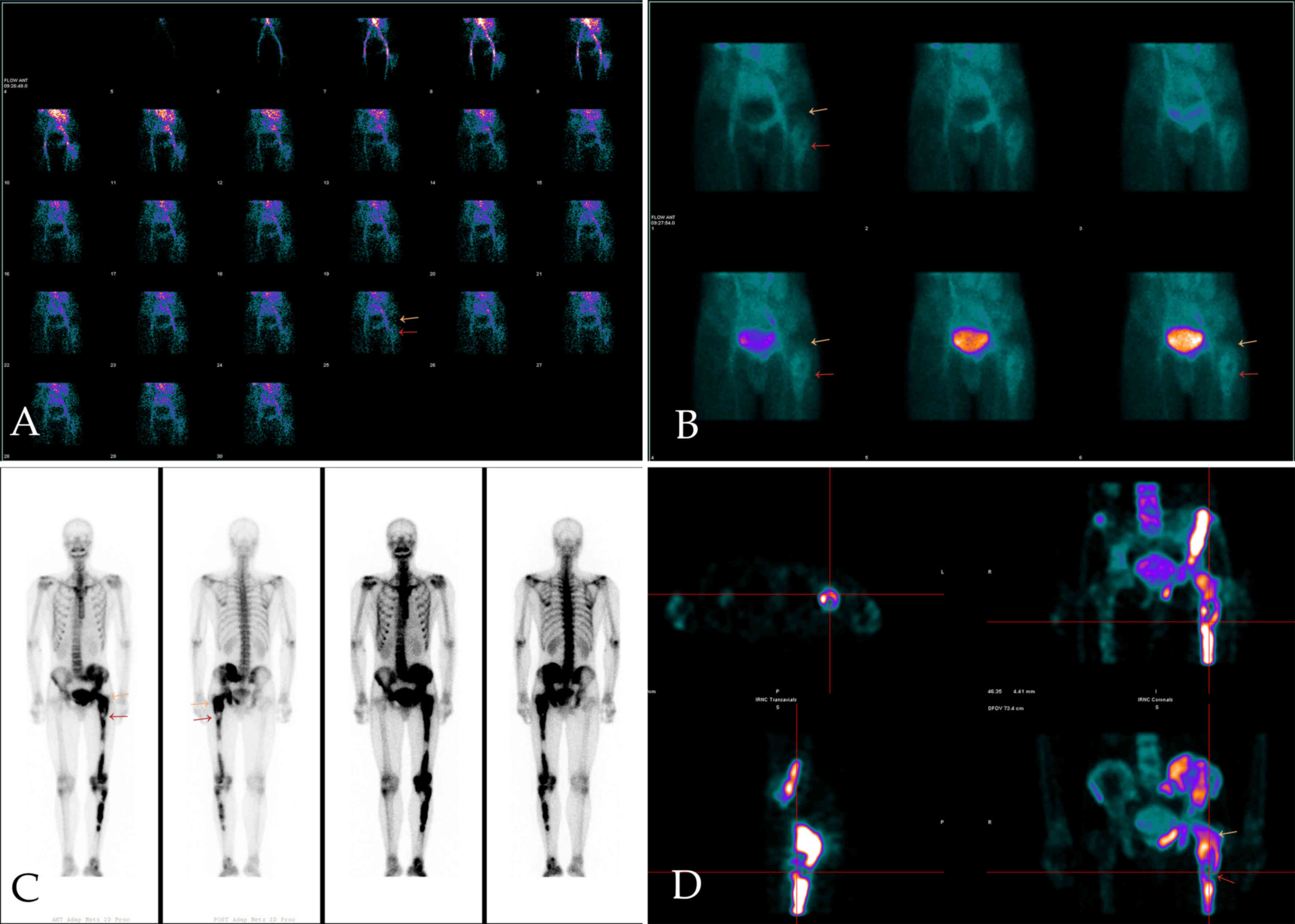
the cortex and protruded into the surrounding soft tissue (Fig. 2C). 99mTc-MDP three-phase
bone imaging was recommended to confirm the blood supply and
abnormal uptake of 99mTc-MDP. The areas of bone and
marrow surrounding the greater trochanter and the neck of the left
femur showed increased blood perfusion on the perfusion and blood
pool phase, while bone marrow destruction areas in MRI showed
higher amounts of blood perfusion in the upper femur (below the
greater trochanter) (Fig. 3A and
B). However, the levels of tracer
uptake of the two areas were reversed in the delayed phase and were
clearer on tomography. The other tracer uptake lesions in the left
pelvic and lower limb bone corresponded to an ill-defined
radiolucent lesion on X-ray (Fig.
3C and D).
After discussion, it was agreed that this was
consistent with malignant mesenchymal tumors. To obtain a definite
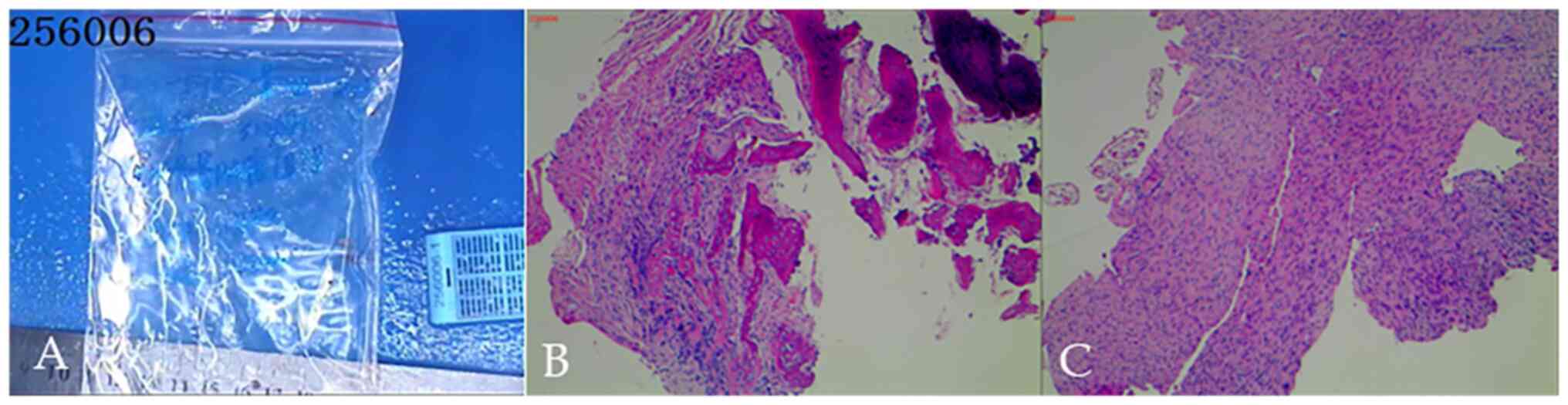
diagnosis, the patient underwent left femoral and tibial
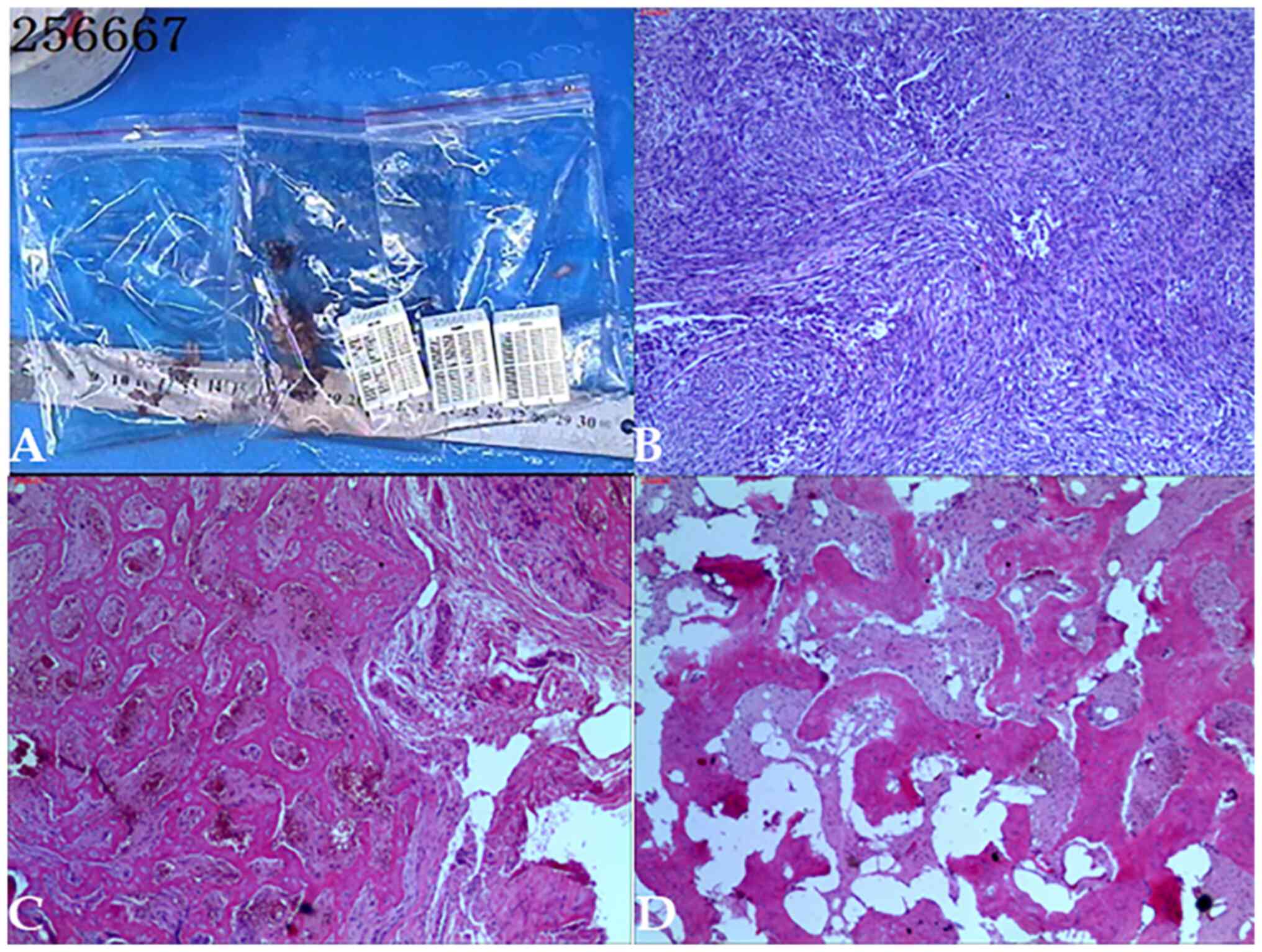
percutaneous biopsy (Fig. 4) and
left femoral surgical biopsy (Fig.
5). The pathological analysis (Fig.
5) showed that the lesions were consistent with malignant
mesenchymal tumors of the left femur and concluded that they were
malignant fibrous histiocytoma of the bone and osteofibrosarcoma.
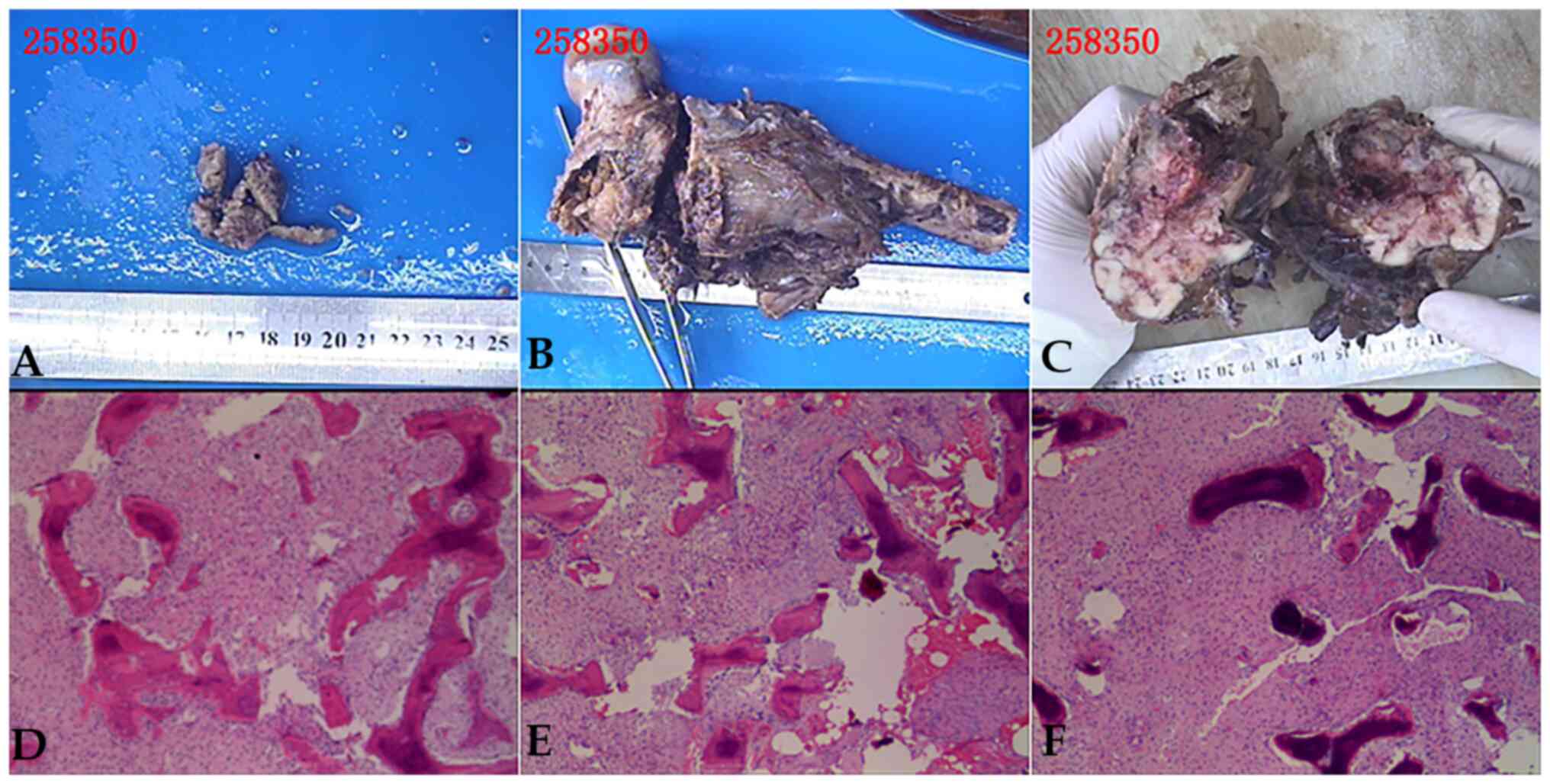
Postoperative histological examination (Fig. 6) confirmed bone tumor tissue and a
distal femoral medullary cavity consistent with fibrosarcoma.
Final diagnosis
The final diagnosis was made following biopsy and
segmental resection, based on a histopathological examination of
the resected tumor. The left femur tumor, which destroyed the
diaphysis and invaded the soft tissue outside the bone, was
confirmed to be osteofibrosarcoma (Figs. 5B and 6). The tumor tissue in the left
upper-middle tibia was consistent with fibrous dysplasia without
malignant transformation (Fig. 5C
and D).
Treatment and follow-up
After the final diagnosis, the patient received
chemotherapy with epirubicin 50 mg + vincristine 2 mg +
methotrexate 10 g, and the process was successful. Subsequently, 2
weeks later (22 June 2018), left femur tumor segment resection and
hip replacement were performed (Fig.
7A), and postoperative histological examination confirmed that
the bone tumor tissue and distal femoral medullary cavity were
consistent with fibrosarcoma. In September 2018, ~3 months after
the operation, a surgical biopsy was performed on a mass resected
from the surface of the left thigh because of suspicion of local
tumor recurrence (Fig. 7B and
C). Pathological examination showed
recurrence of the left femur fibrosarcoma, and surgical resection
and symptomatic treatment were performed again. In March 2019,
another operation of the left hip disarticulation was scheduled for
tumor resection following further local recurrence. The operation
was successful without obvious postoperative acute
complications.
Outcome
The last operation was performed in March 2019.
Subsequently, 1 month later, the condition of the patient
deteriorated and the patient died of cachexia caused by extensive
metastasis in April 2019.
Discussion
FDB is commonly found in long bones (proximal femur
and tibia), and can be divided into single or multiple bone types
(11). It is mostly confined to one
limb, but other anatomical sites, such as the jaws, craniofacial
bones and sacrum can be also involved (12-14).
In the early stage of FDB, patients present with no symptoms, but
local aggression to the periosteum or increased bone marrow
pressure can cause discomfort, bone pain and movement disorders.
Serious cases can lead to stress fracture or pathological fracture
(15). When localized osteolytic
destruction, soft tissue extension, sudden aggravation of pain or
pathological fractures are found, the possibility of malignant
transformation should be suspected (15,16).
However, the treatment and prognosis of malignant transformation
are markedly different from locally aggressive FDB. In the present
case, the lesions in the left limb were polyostotic and extensive,
including the tibia and femur, and involved the ipsilateral pelvis,
with further extraosseous tissue invasion. The present case study
aimed to find the features of malignant transformation of FDB in
multimodal imaging.
FDB is most often discovered based on radiographic
evidence (1), and radiographic
classification for fibrous dysplasia is beneficial to guide
treatment planning and evaluation of the effects of surgery
(17). It is recommended that
patients with FDB should undergo radiological examinations before
pathological biopsy and clinical treatment because malignant
transformation and local aggression can usually be distinguished
from non-aggressive FDB based on imaging findings (18).
The specific findings of X-ray and CT depend on the
degree of fibrous tissue hyperplasia in the lesion and the content
of new and mature bone trabeculae. When the lesion is mainly
fibrous tissue, it appears as a cystic clear area. When the lesions
are new bone trabeculae and the fibrous tissue of hyperplasia is
woven into the bone, it shows a ground glass appearance (2,19),
whilst when the lesion area is mature bone tissue, it shows a
high-density strip and patchy ossification shadowing. The
radiographic features of malignant transformation or local
aggression of FDB include poorly defined margins, mineralized
osteolytic lesions, cortical destruction and extension into soft
tissue (2,15). MRI findings are related to the
amount of fibrous tissue, whether there is hemorrhage,
cartilaginous island or residual bone marrow fat. FDB is usually
featured with homogeneous T1WI hypo-intensity and a surrounding
sclerotic rim T1WI and T2WI hypo-intensity (13). T1WI and T2WI hyperintensity is
observed in the corresponding areas when cystic degeneration,
hemorrhage, cartilage island or residual bone marrow fat are
present in the lesion. If the lesion capsule is completely
depleted, T1WI hypo-intensity and T2WI hyperintensity are observed
(20).
MRI can show cortical destruction and soft tissue
extension for locally aggressive FDB which overlaps with malignant
transformation (15). A prominent
hyper-vascularized soft tissue mass extending from bone may be
helpful in the differential diagnosis. 99mTc-MDP single
photon emission computed tomography (SPECT) directly reflects the
calcium/phosphate metabolism of bone tissue and indicates the
functionality and number of osteoblasts. The three-phase bone
imaging can be used to observe the blood supply and the active
state of bone metabolism, which is helpful for the differential
diagnosis of benign and malignant bone diseases (21). FDB has distinctive characteristics
on 99mTc-MDP SPECT/CT; 85.7% of cases show moderate or
high radiotracer uptake on delayed whole-body bone scintigraphy
(WBS) (21). This helps identify
FDB as the SPECT/CT shows features of ground-glass opacity and
expansion in the areas of high radiotracer uptake while without
soft tissue occupying lesions in bone marrow. However, a high
tracer uptake can be seen on locally aggressive FDB because its
tissue type is composed of trabeculae of immature bone and fibers
stroma (11). Comparatively low
tracer uptake in the delayed phase but high blood perfusion in the
perfusion phase and the blood pool phase is considered to have a
malignant transformation diagnosis (21,22).
In the present case study, the three-phase bone imaging showed that
the blood perfusion and blood pool were increased, and the abundant
blood perfusion was indicative of malignancy. Delayed phase
findings indicated that there were fewer osteoblasts and
calcium/phosphate but more interstitial tissue. The corresponding
lesions were confirmed through finer anatomic proximity on MRI.
All imaging examinations have their unique
functions. The degree of X-ray radiographic density is directly
proportional to the degree of mineralization and brighter areas
reflect predominantly fibrous zones, which can help with earlier
FDB detection. SPECT/CT is important for assessing local function
and microscopic components. MRI is an excellent method for
assessing cases of complex fibrous dysplasia, reflecting the
variable tissue components. CT complements and enhances the
interpretation of MRI of bone lesions, especially in cases where
MRI shows enhancement that is suspicious of a malignant neoplasm
(19,23). However, the presentation of those
features is often non-specific, and it is recommended to use
multimodal imaging that can show the anatomy, blood supply,
molecular components and metabolism of the lesion at the same time
to improve the diagnostic accuracy of FDB and its malignant
transformation. In actual clinical practice, other clinical data
need to be considered to make a comprehensive judgment.
Consideration of potential factors such as age and history of
surgery is important. It is necessary to combine relevant
laboratory indicators, including serum alkaline phosphatase. Wang
et al showed that FDB with high serum alkaline phosphatase
level has a tendency to progress to severe disease (24). In the present case study, alkaline
phosphatase was ~2-fold the upper limit of the normal range, which
was significantly higher compared with the normal level. A
limitation of the present case study is the lack of imaging data
before the malignant transformation.
Although biopsy is often the gold standard for
diagnosis, multimodal imaging is useful to define the scope of the
lesion and help to guide treatment planning. Comprehensive analysis
of multimodal images may improve the detection rate and diagnostic
accuracy of local FDB aggressiveness and its malignant
transformation, thus guiding clinical diagnosis and treatment
decision-making.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, MK, XY and JZ confirm the authenticity of all
the raw data. JL, MK and JZ contributed to the conception of the
study. JL and MK were responsible for writing the original draft,
reviewing and editing the manuscript. XY, JL, MK were responsible
for acquisition of clinical data. XY and JZ were responsible for
critical revision of the manuscript and the analysis and
interpretation of the data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study passed ethical review by the Ethics
Committee of the Third Affiliated Hospital of Guangzhou Medical
University. All the data used were collected from the Third
Affiliated Hospital of Guangzhou Medical University with the
consent of the patient.
Patient consent for publication
All the test results, imaging images, and their
publication were obtained with written consent from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hakim DN, Pelly T, Kulendran M and Caris
JA: Benign tumours of the bone: A review. J Bone Oncol. 4:37–41.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qu N, Yao WW, Cui X and Zhang H: Malignant
transformation in monostotic fibrous dysplasia: Clinical features,
imaging features, outcomes in 10 patients, and review. Medicine
(Baltimore). 94(e369)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Martini M, Klausing A, Heim N, Fischer HP,
Sommer A and Reich RH: Fibrous dysplasia imitating malignancy. J
Craniomaxillofac Surg. 46:1313–1319. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Riddle ND and Bui MM: Fibrous Dysplasia.
Arch Pathol Lab Med. 137:134–138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zoccali C, Attala D, Rossi B, Zoccali G
and Ferraresi V: Fibrous dysplasia: An unusual case of a very
aggressive form with costo-vertebral joint destruction and invasion
of the contralateral D7 vertebral body. Skeletal Radiol.
47:1571–1576. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ruggieri P, Sim FH, Bond JR and Unni KK:
Malignancies in fibrous dysplasia. Cancer. 73:1411–1424.
1994.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hoshi M, Matsumoto S, Manabe J, Tanizawa
T, Shigemitsu T, Izawa N, Takeuchi K and Kawaguchi N: Malignant
change secondary to fibrous dysplasia. Int J Clin Oncol.
11:229–235. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim HG, Baek JH and Na K: Osteosarcoma
arising in fibrous dysplasia of the long bone: Characteristic
images and molecular profiles. Diagnostics (Basel).
12(1622)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Su XY, Sun WP, Yuan JQ, Li LX, Jiang ZM
and Zhang HZ: Sarcoma arising in fibrous dysplasia: A
clinicopathological analysis. Zhonghua Bing Li Xue Za Zhi.
51:733–737. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
DiCaprio MR and Enneking WF: Fibrous
dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint
Surg Am. 87:1848–1864. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Singh V, Gupta K and Salunke P: Monostotic
craniofacial fibrous dysplasia: Report of two cases with
interesting histology. Autops Case Rep. 9(e2018092)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu XX, Xin X, Yan YH and Ma XW: Imaging
characteristics of a rare case of monostotic fibrous dysplasia of
the sacrum: A case report. World J Clin Cases. 9:1111–1118.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Davidova LA, Bhattacharyya I, Islam MN,
Cohen DM and Fitzpatrick SG: An analysis of clinical and
histopathologic features of fibrous dysplasia of the jaws: A series
of 40 cases and review of literature. Head Neck Patho. 14:353–361.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Muthusamy S, Subhawong T, Conway SA and
Temple HT: Locally aggressive fibrous dysplasia mimicking
malignancy: A report of four cases and review of the literature.
Clin Orthop Relat Res. 473:742–750. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ogul H and Keskin E: Locally aggressive
fibrous dysplasia mimicking malign calvarial lesion. J Craniofac
Surg. 29:e318–e319. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Y, Luo Y, Min L, Zhou Y, Wang J,
Zhang Y, Lu M, Duan H and Tu C: The West China Hospital
radiographic classification for fibrous dysplasia in femur and
adjacent bones: A retrospective analysis of 205 patients. Orthop
Surg. 14:2096–2108. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Pozzessere C, Cicone F, Barberio P, Papa
A, Coppolino G, Biagini R and Cascini GL: Cross-sectional
evaluation of FGD-avid polyostotic fibrous dysplasia: MRI, CT and
PET/MRI findings. Eur J Hybrid Imaging. 6(19)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Atalar MH, Salk I, Savas R, Uysal IO and
Egilmez H: CT and MR imaging in a large series of patients with
craniofacial fibrous dysplasia. Pol J Radiol. 80:232–240.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kinnunen AR, Sironen R and Sipola P:
Magnetic resonance imaging characteristics in patients with
histopathologically proven fibrous dysplasia-a systematic review.
Skeletal Radiol. 49:837–845. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang LQ, He Q, Li W and Zhang RS: The
value of 99mTc-methylene diphosphonate single photon
emission computed tomography/computed tomography in diagnosis of
fibrous dysplasia. BMC Med Imaging. 17(46)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei WJ, Sun ZK, Shen CT, Zhang XY, Tang J,
Song HJ, Qiu ZL and Luo QY: Value of 99mTc-MDP SPECT/CT
and 18F-FDG PET/CT scanning in the evaluation of
malignantly transformed fibrous dysplasia. Am J Nucl Med Mol
Imaging. 7:92–104. 2017.PubMed/NCBI
|
|
23
|
Gokce E and Beyhan M: Radiological imaging
findings of craniofacial fibrous dysplasia. Turk Neurosurg.
30:799–807. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang J, Du Z, Li D, Yang R, Tang X, Yan T
and Guo W: Increasing serum alkaline phosphatase is associated with
bone deformity progression for patients with polyostotic fibrous
dysplasia. J Orthop Surg Res. 15(583)2020.PubMed/NCBI View Article : Google Scholar
|