1. Introduction
The gut microbiota (GM) comprises all of the
bacteria, viruses, archaea and fungi that live in synergy in the
body. If the set of genes of these microorganisms is included, the
term is microbiome (1-3).
Microbial diversity may be described as the number of different
species in the same sample or in different samples, referred to as
alpha and beta diversity, respectively (4). As for bacteria, the GM of a healthy
person corresponds to three main phylae: Firmicutes (Gram
positive), 60%; Bacteroidetes (Gram negative), 10%; and
Actinobacteria (Gram positive), 10%. Firmicutes
represent the largest proportion and include around 200 genera, of
which the most important are Mycoplasma, Bacillus and
Clostridium. The remaining 20% belong to the phylae
Proteobacteria, Verrucomicrobia and
Euryarchaeota (5,6). Certain studies report that microbial
cells outnumber eukaryotic cells in the body, accounting for ~1 to
2 kg of the body weight, referring to the 10:1 ratio of
microbial/eukaryotic cells (1,7,8). The
large intestine is the part of the intestinal tract where there is
a higher number of bacteria, as the neutral pH conditions, decrease
in enzymes, decrease in secretions from other digestive organs,
less oxygen and slow transit consequently give a greater
possibility of survival and metabolic functions of microorganisms
derived from the fermentation of non-digestible dietary products
(1,8).
The functions of the GM are diverse and important
and beneficial for the body, as it has a role both locally and
systemically. At a local level, it participates in maintaining the
health of the intestinal mucosa, prevents intestinal permeability
and increases the absorption surface, among others. These
microorganisms also have a metabolic function. They intervene in
the digestion and absorption of dietary nutrients to obtain energy,
in the absorption of minerals such as calcium, phosphorus,
magnesium and iron, in the extraction of essential nutrients, and
synthesize vitamins (such as K, B5, B8, B12 and folic acid),
hormones and short-chain fatty acids (SCFAs). In addition, another
important function is immunomodulation. Intestinal bacteria
interact with the immune system, favor the maturation of the innate
and adaptive immune system, and promote the health and maturity of
the gut-associated lymphoid tissue (GALT) (1). Studies have demonstrated that the
immune system depends significantly on GM diversity. An
anti-inflammatory GM often demonstrates high taxonomic diversity
and microbiome variation (9). All
of this leads to an effective defense mechanism against pathogenic
microorganisms which, in turn, may change the GM composition
(1,10).
The GM is affected by several factors, such as
consumption of xenobiotics, infections, individual genetic and
immune response, diet and lifestyle. Diet is considered the main
modulator. Research has indicated that GM is related to the
maturation of cognitive and behavioral functions in the central
nervous system (CNS) (11-13).
The usual dynamics of the GM can be directed towards a state of
dysbiosis when stress conditions quickly decrease microbial
diversity and promote the spread of specific bacteria (6). The factors in intestinal dynamics,
their natural variations and stress mediate cascades of
destabilizing events of the intestinal microbiome. The underlying
mechanisms of intestinal dysbiosis remain to be fully elucidated.
However, in general, it has been observed that oxidative stress,
the induction of viruses that specifically affect bacteria
(bacteriophages) and the secretion of bacterial toxins can trigger
rapid changes between intestinal microbial groups, thus producing
dysbiosis (6). The human GM is
composed of native and transient microorganisms (6). In this context, only a relatively
small number of opportunistic (pathogenic) bacteria are considered
members of the GM, which reside unaltered within the GM and only
become a threat to the host's health when the ecosystem and
homeostasis of the GM are altered (state of dysbiosis). Throughout
an individual's life, the composition and function of the GM
diversity are affected and lead to CNS alterations (14). In gastrointestinal disorders, the
imbalance between pro-inflammatory and anti-inflammatory bacteria
causes systemic inflammation, which generates alterations in the
intestinal and blood-brain barrier (BBB) permeability. This, in
turn, accelerates the neuro-inflammatory processes that can trigger
neurological diseases, such as multiple sclerosis (MS) (14,15).
Evidence has confirmed that GM is associated with the development
of various disorders, including cardiovascular diseases (CVD),
cancer, diabetes, brain disorders and chronic kidney disease.
Therefore, its modulation has an important role in the prevention
and treatment of multiple diseases, from microbial fecal
transplantation to treat Clostridium difficile infection to
the use of probiotics in inflammatory bowel diseases and diet-based
modification for cancer. In October 2021, there were ~3,000
clinical trials in progress related to microbiota (16).
The Food and Agriculture Organization of the United
Nations and the World Health Organization define probiotics as
‘live microorganisms that, when administered in adequate amounts,
confer a health benefit on the host’. Probiotic administration is
used to improve GM homeostasis and maintain host health (17). As a result, the number of pathogenic
bacteria that cannot survive in an acidic environment decreases and
beneficial bacteria that grow well in an acidic state proliferate,
thus balancing the GM, and prebiotics are defined as ‘selectively
fermented ingredients that produce specific changes in the
composition and/or activity of the GM, thus conferring benefits to
the host's health’. Certain dietary fibers, such as carbohydrate
polymers that are not digested or absorbed, are subject to
bacterial fermentation in the gastrointestinal tract and,
therefore, affect the composition of the bacterial community and
metabolic activity. The combination of both is referred to as
symbiotic (17).
Prebiotics are partially digested in the upper
segments of the gastrointestinal tract. They then reach the colon
to be fermented by beneficial bacteria (e.g., from the genus
Bifidobacterium), achieving a state of synergy (symbiosis).
This stimulates the selective growth and/or activity of intestinal
bacteria potentially associated with health protection. Through
their main mechanisms, such as the production of antimicrobial
substances, competition for epithelial adhesion and nutrients,
increased production of SCFAs, increased fecal mass, reduced
colonic pH, reduced nitroso products and fecal enzymes, modulation
of mucin production and improved immune system (by increased IgA
secretion by GALT, which can stimulate the phagocytic function of
inflammatory macrophages), prebiotics confer beneficial effects for
human health. Some examples of prebiotics that are most commonly
used in human nutrition are fructooligosaccharides,
galactooligosaccharides, inulin, xylooligosaccharides and lactulose
(18).
Lactobacillus, members of the GM, are
producers of γ-aminobutyric acid (GABA) and serotonin, essential
neurological regulators. Likewise, several neurotransmitters and
neuromodulators, such as choline, tryptophan and SCFAs (acetate,
propionate and butyrate), are also produced by the GM (14). In this sense, a change in the GM
diversity or in certain bacteria may cause differential hormonal
responses, which influence brain activity and instigate
pathological processes (19).
Probiotic supplementation has shown crucial benefits in stress and
anxiety processes. Certain functions associated with the intake of
probiotics are as follows: Reducing oxidative stress and
inflammatory processes, facilitating modifications in hippocampal
synapse by increasing the expression of brain-derived neurotrophic
factor (BDNF) and increasing hypothalamic neuronal activity, which
translates into better cognitive and learning processes (20). The present review aimed to highlight
the role of the GM as an environmental risk factor in the
development and progression of MS, and how the modulation of the GM
may be part of the treatment for MS.
2. Methods
Articles published online in English from 2006 to
August 2023 were searched in the electronic databases of PubMed of
the National Library of Medicine (https://pubmed.ncbi.nlm.nih.gov). The search was
conducted from September 2021 to August 2023. The following medical
headers were used in various combinations: ‘multiple sclerosis’,
‘lipopolysaccharides’, ‘gastrointestinal microbiome’, ‘intestinal
mucosa’, ‘endotoxemia’, ‘intestinal dysbiosis’, ‘neurodegenerative
diseases’ and ‘neuroinflammation’. More than 200 studies were
assessed by title, abstract and study type regarding its potential
inclusion in the current review.
The inclusion criteria were as follows: i) Articles
written in English; ii) studies related to MS; iii) studies related
to the GM; iv) the relationship between intestinal dysbiosis and
impairment of CNS functions; v) studies on the existing evidence of
the association of intestinal dysbiosis with MS in patients from
different populations; vi) interventional studies including
randomized controlled trials (RCTs) and experimental studies that
evaluated the effect of probiotic administration on immune or
inflammatory markers in patients with MS and the animal model of MS
‘experimental autoimmune encephalomyelitis’ (EAE); vii) studies
that report the association between probiotic intake and immune and
inflammatory response in MS. In addition, the full text of
potentially eligible articles was analyzed independently.
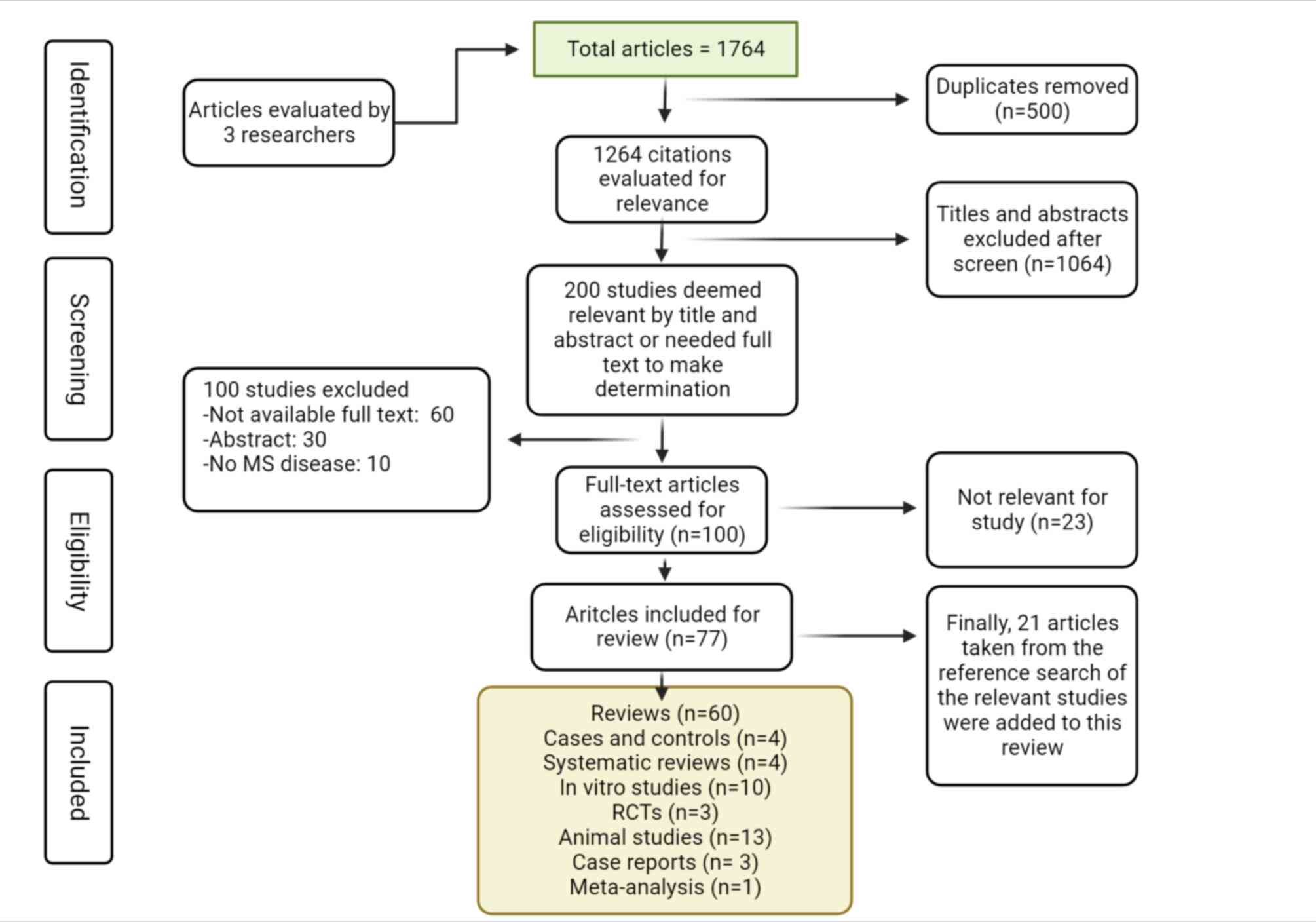
Initially, a total of 1,764 articles were retrieved,
as indicated in Fig. 1. After
excluding duplicate studies (n=500), 1,264 citations remained.
Subsequently, the titles and abstracts of the remaining articles
were reviewed and 200 studies were considered potentially eligible
after 1,064 were eliminated. The abstracts of the articles were
read and 100 were excluded. After reviewing the full texts of the
100 remaining candidate articles, only 77 were included (23 were
eliminated). Finally, 21 other studies were added due to being
relevant for the present review, totaling 98 articles (60 reviews,
4 cases and controls, 4 systematic reviews, 10 in vitro
studies, 3 RCTs, 13 animal studies, 3 case reports and 1
meta-analysis) (Fig. 1).
3. Multiple sclerosis
MS is a chronic, inflammatory, neurodegenerative
disease of the CNS in which demyelination and axonal degeneration
occur, making it highly disabling with a different disease
phenotype for each individual patient who suffers from it (21). Out of every 100,000 individuals, 40
live with MS, meaning that there are almost 3 million cases of MS
worldwide and numbers are increasing rapidly (22). MS occurs at the most formative stage
of life, the productive stage of work life, with an average age at
diagnosis of 32 years, and, as with other immunological diseases,
women are affected twice as frequently as men (22). The progression and severity of the
disease have a diverse pattern, where a small percentage of
individuals present with a benign phenotype characterized by
developing little or no disability over time, or an aggressive
course with frequent relapses without reaching a full recovery
between each, with rapid progression toward disability (23).
The vast majority of patients, ranging from 80 to
90%, present with relapsing-remitting variant MS (RRMS). They go
through relapse episodes (active MS) followed by remission or
partial or total recovery of symptoms (clinically silent periods).
Approximately 50% of these patients progress to secondary
progressive MS (SPMS), a form lacking clear periods of activity and
remission, since patients deteriorate clinically more quickly and
with serious sequelae that increase their disability. Another type
is the primary progressive variant, where patients present with
aggressive disease and constant progression, without the typical
cycle of relapse-remission from the time-point of diagnosis. The
prevalence of this type of MS is between 10 and 20% of patients
(24,25).
Clinically isolated syndrome (CIS) is a clinical
state where inflammation and demyelination may be observed in the
CNS when only one attack or relapse has occurred, and the patient
cannot yet be diagnosed as MS. MS begins as CIS in ~85% of patients
and becomes clinically defined MS when they develop a second attack
(26). In the most recent McDonald
2017 diagnostic criteria, CIS can be treated as MS when it is in
accordance with the nuclear magnetic resonance (NMR) data for
dissemination in space (involvement of different regions of the
CNS) and time (involvement at different times in the disease
course) (27), or oligoclonal bands
are present in the cerebrospinal fluid and spread in space.
Revisions to these criteria aim to diagnose most patients with less
active disease with MS (28). The
importance of these adjustments in the diagnostic criteria lies in
increasing the identification of previously undiagnosed cases of MS
and in not misdiagnosing individuals who do not have the disease
(29).
The etiology of MS remains largely elusive; however,
it is known to occur in genetically susceptible individuals, but
environmental factors are required to trigger the disease. The most
studied environmental factors are a western diet (high in saturated
fat, simple carbohydrates and sodium, and low in dietary fiber),
vitamin D deficiency, childhood obesity, a sedentary lifestyle,
tobacco smoking and certain infections, such as Epstein-Barr virus.
In addition, other risk factors found in a systematic review, such
as exposure to organic solvents, cytomegalovirus infection and
vaccination against diphtheria and tetanus, are associated with the
risk of MS (30). Environmental
factors have become more relevant due to their relationship with
autoimmune and neurodegenerative diseases, which had been
previously explained only by genetic factors. Other environmental
factors associated with autoimmune diseases are gastrointestinal
disease and dysbiosis of the GM, as well as intestinal barrier
permeability and consequent inflammation (31).
4. Intestinal dysbiosis and its relation
with neuroinflammation and neurodegeneration in MS
The GM is a dynamic entity and there are numerous
factors that predispose to its modification and, therefore, to its
function. In fact, each individual has their own GM, similar to
fingerprints. This is what prevents researchers from establishing a
single GM as a model to follow. However, there are characteristics
of these hosts that make them more or less desirable for the
gastrointestinal tract. It is established that GM dysfunction
depends on the type of bacterial strain and can trigger various
pathologies (32). There are a
number of studies on the relationship of dysbiosis with certain
diseases, such as gastrointestinal diseases, inflammatory bowel
disease and colon cancer, as well as obesity, diabetes, allergies,
arthritis and CVD. However, for some years now, it has been
suggested that the GM may have a role in CNS disorders (33). However, at present, only the tip of
the iceberg is known, and the role of the GM in neuroinflammation
and neurodegeneration has remained to be fully elucidated (32). For a long time, infections have been
investigated as a possible trigger for MS, although without
concrete evidence. Now, the concept of a pro-inflammatory GM as a
trigger for autoimmunity has emerged, with the possible implication
of dysbiosis, as has been demonstrated in MS (34).
In the present review, it is argued that the link
between the GM and MS is based on low-grade inflammation and that
the outcome of this low-grade inflammation depends, to a large
extent, on genetic factors, but above all on environmental factors,
such as eating habits and their effects on the GM and the
subsequent process of dysbiosis (32). It is thus possible that a trigger of
intestinal inflammation, through dysbiosis, e.g. diet, may alter
both the number and diversity of microbiota species. Studies have
indicated that a high-salt diet may be associated with an increased
risk of developing EAE and dementia, through the induction of T
helper 17 (Th17) cell differentiation; in particular, mice fed a
high-salt diet developed neurovascular and cognitive impairments
through the expansion of Th17 cells in the small intestine, leading
to an increase of interleukin 17 (IL-17) levels in plasma. This
increase of Th17 cells possibly results from a change in the
microbiome composition, is diet-dependent and may be induced by
activation of serum glucocorticoid kinase 1 that regulates sodium
intake (35). Studies in patients
with MS have shown that a high-salt diet induced an increase of
Th17 cells in the blood and a decrease in the intestinal strains of
the Lactobacillus genus, and is associated with the
worsening of clinical symptoms and increased activity on NMR
(17,36,37).
In addition, recent evidence suggests that the GM is one of the key
environmental factors in the development of MS (38). Clinical patient studies have
indicated the presence of a pro-inflammatory state originating in
the intestine (17), specifically
Th17 cells, which are involved in the pathogenesis of MS, and which
are present in abundance in the peripheral blood, cerebrospinal
fluid and brain lesions of patients with MS, and their counts and
inflammatory mediators increase even further during relapses
(39). Observations show that in
MS, there is an expansion of Th17 cells in the intestine, which is
associated with microbiota alterations and correlates with high
disease activity (17).
Furthermore, multiple studies in which the GM was
characterized by sequencing the 16S ribosomal RNA (rRNA) gene, have
shown that patients with autoimmune diseases such as MS have
alterations (they exhibit dysbiosis) in their microbiome (19). This dysbiosis is associated with
various pathologies, including those that affect organs of the host
that appear to have no connection to the intestine, e.g., the CNS
(40). It has been observed that
the modification of the GM composition, i.e., the state of
dysbiosis, produces a wide range of amyloid proteins that serve for
the crossing and propagation of pathological protein aggregates
from the intestine to the CNS, favoring the neurodegenerative
processes produced by the aberrant metabolism of these proteins
(41). Clinical evidence shows that
patients with neurodegenerative diseases present with symptoms of
gastrointestinal disease, even several years before the diagnosis
of their disease (42). Of note, a
specific change in the GM of patients with RRMS has been observed:
The presence of effector Th17 cells in the intestinal tissue was
found to be associated with a decrease in the Prevotella
genus and an increase in the Streptococcus genus (43). In another study, an association of
certain bacterial species that are normally rare in the intestine
of healthy humans was found. An increase in the
Acinetobacter genus and a lower presence of
Parabacteroides distasonis were found. It was also reported
that exposing the lymphocytes of healthy individuals to the
microbiota of patients with MS increased their differentiation into
Th1 cells and reduced the proportion of CD25+ forkhead
box protein 3 (FoxP3)+ regulatory T (Treg) cells. On the
other hand, exposure of these lymphocytes to Parabacteroides
distasonis led their T cells towards a regulatory phenotype
(44). In another study on patients
with MS who were given a mixture of the Lactobacillus,
Streptococcus and Bifidobacterium genera, this
probiotic mixture induced a change in the peripheral immune
response to anti-inflammatory drugs and restored the GM composition
of those patients (45). A similar
probiotic mixture (Lactobacillus and Bifidobacterium)
was evaluated in two randomized, double-blind, placebo-controlled
trials of three and four months' duration. Their results suggest
that daily supplementation with probiotics could improve the
clinical symptoms of MS (46).
In addition to probiotics, other techniques are
being used to modulate the composition and function of the
dysbiotic GM in MS. Although studies are scarce, encouraging
results have been obtained. To date, these studies have been
conducted to treat severe gastrointestinal symptoms caused by the
course of the disease, i.e., without the intention of observing
clinical benefits for the disease. This was the case with the
possible effect of fecal microbiota transplantation (FMT) in three
patients with MS treated with an average of 8 FMT infusions for
chronic severe constipation, where constipation resolution was
demonstrated, but they also achieved progressive neurological
improvement (47). Another
longitudinal proof-of-concept study with 12 months of follow-up,
also to evaluate the effect of FMT on RRMS, was carried out with a
single subject who received FMT infusions from five healthy donors
(48). Clinical progression, fecal
microbiota composition, fecal SCFA concentrations and serum levels
of inflammatory and neuroprotective biomarkers were evaluated. The
results were a microbiota with greater bacterial diversity, with a
higher relative abundance of butyrate-producing bacterial species
and a higher butyrate concentration. The microbiota modification
was associated with reduced levels of inflammatory cytokines and
elevated serum levels of BDNF. Clinically, the patient showed a
progressive improvement in gait and balance scores (disability
status) (48). In this context, a
case report indicated that following treatment with FMT for
Clostridium difficile enterocolitis, disease stability was
achieved in a patient with SPMS (34). All of these accidental findings and
with limited studies provide evidence that the GM of patients with
MS is in a state of dysbiosis and that, by restructuring it,
remarkable benefits are obtained. Above all, they give us the lead
to conduct research on FMT as a complementary approach to MS
treatment.
Native bacteria from fermented foods, such as curds,
sourdough and fermented milk, are another technique that may be
used to benefit various pathologies (49). For instance, Lactobacillus
fermentum from fermented milk was studied by Kumara et
al (50). The study evaluated
the ability of Lactobacillus fermentum to detoxify aflatoxin
B1 (AFB1) in vivo in albino mice. Lactobacillus
fermentum was administered orally to mice 24 h before AFB1
administration. The results showed that Lactobacillus
fermentum significantly reduced AFB1 levels in the liver and
kidneys of mice. In addition, Lactobacillus fermentum also
modulated the production of pro-inflammatory cytokines, suggesting
that it has immunomodulatory activity. In summary, the study found
that Lactobacillus fermentum has the potential to detoxify
AFB1 and modulate the immune response, which may be beneficial for
human health (50) and it would be
worth using it in patients with MS, taking into account that this
pathology has significant inflammatory potential. The same bacteria
were also studied in humans, but this time in the form of an
encapsulated probiotic (51).
Researchers evaluated the effects of supplementation with various
types of Lactobacillus, including Lactobacillus
fermentum, on gene expression related to inflammation, insulin
and lipids in patients with MS. The study was a randomized,
double-blind and placebo-controlled trial. The intervention group
(n=20) received a daily probiotic capsule containing a blend of
Lactobacillus acidophilus, Lactobacillus casei,
Bifidobacterium bifidum and Lactobacillus fermentum
for 12 weeks. Each capsule contained 2±109 colony-forming units/g.
Patients were assigned to receive 100 g of probiotics or placebo
per day for 12 weeks. The results showed that patients who received
probiotics had a significant decrease in the expression of genes
related to inflammation [IL-8 and tumor necrosis factor α], but did
not affect the expression of genes related to insulin PPAR-γ or
lipids/low-density lipoprotein (LDL) receptor. In addition,
patients who received probiotics also exhibited an improvement in
MS symptoms. In summary, the study found that supplementation with
Lactobacillus may be a potential therapy for MS (51). In addition, another study focused on
the molecular characterization of Bacillus sp. bacteria that
hydrolyze gluten and in their potential as a probiotic. The
bacteria were isolated from sourdough and curd samples and
characterized by their biochemical and molecular properties. The
results showed that the bacteria were able to hydrolyze gluten and
that they had antibacterial, anti-adhesive and pathogen exclusion
properties. Furthermore, the bacteria were also able to modulate
gene expression in Caco-2 cells (used in research as a model of the
intestinal epithelial barrier) (50).
In addition to probiotics, SCFA supplementation has
also shown good results as an adjuvant in the inflammatory response
in MS, as propionate has been indicated to be reduced in the serum
and stool of patients with MS at the same time that they have an
altered GM (34). In another study,
supplementation for 3 years achieved clinical improvement in
patients; these SCFAs were associated with a rebalancing of the
Th17/Treg cell ratio towards a more regulatory profile, in addition
to positive regulation of genes related to the induction of Treg
cells in the intestine (52).
Therefore, it appears that inflammation in the intestine may result
in the activation of encephalogenic T cells that may travel to the
CNS, where they can induce inflammatory damage with subsequent
demyelination and axonal loss. In addition, inflammation in the
intestine can also influence the diversity of the microbiota and
lead to dysbiosis, which in turn may enhance intestinal
inflammation (34). Chen et
al (38) also presented results
consistent with the hypothesis that patients with MS have GM
dysbiosis. They designed a study with patients with RRMS to
investigate whether their GM was altered. They compared their fecal
microbiota with that of healthy controls, revealing that patients
with MS had a distinct microbial profile compared to controls, with
the genera Pseudomonas, Mycoplana,
Haemophilus, Blautia and Dorea found in
patients with MS, while the control group had a higher abundance of
the genera Parabacteroides, Adlercreutzia and
Prevotella. Therefore, they made two important observations:
First, that certain gut microbes show decreased or increased
abundance in patients with MS compared to controls, and second, the
importance of the microbiota in this disease (38).
Possible mechanisms of the influence
of the GM in MS
It is important to clarify the mechanism by which
intestinal bacteria are related to MS. According to Wekerle
(53), the pathogenic mechanism of
MS requires at least 3 factors: A genetic predisposition, a
pro-inflammatory intestinal bacterial profile and the accumulation
of self-reactive T cells in GALT. In this bidirectional
communication between the gut and the brain, the mild inflammation
originating in the gut passes to the brain. In this path to the
brain, there are two barriers, the intestinal barrier and the BBB,
and it is easier for intestinal inflammation to cause a disruption
of the BBB than vice versa (34). However, to obtain an answer that
will lead to further clarification of the scope of intestinal
dysbiosis in MS, it would be necessary to clearly define the
sequence of events that lead to the dysfunction of both barriers
and ‘trace’ the moment at which they occur. This is easier to
demonstrate in EAE, but in MS, it is practically impossible.
However, research is underway that may lead to a good understanding
of this intricate relationship (34). It is thus that a large amount of
current evidence indicates that the gut-microbiota-brain axis
probably has a crucial role in the pathogenesis of neurological
diseases, such as MS. Both in MS and in its murine model,
gastrointestinal symptoms, altered GM and increased intestinal
permeability have been reported (34).
The gut-microbiota-brain axis acts as a link between
the external environment and the CNS. Its main components are the
microbiota, for its role in gastrointestinal homeostasis; the
intestinal barrier, which regulates the entry of food and microbial
metabolites into the body; and the sympathetic and parasympathetic
arms of the autonomic nervous system, specifically the enteric
nervous system (ENS) and the vagus nerve, which transmits signals
to the brain (34). In fact, the
possible implication of intestinal dysbiosis in MS was first
studied in EAE using germ-free mice and it was shown that the
microbiota has a crucial role in directing both pro-inflammatory
and anti-inflammatory immune responses in the CNS, possibly
increasing Treg- and Th2-cell responses (53,54).
EAE experiments with mice treated with specific antibiotics or GF
mice monocolonized with particular bacteria have clearly shown that
signals from an altered GM can induce inflammation in
extraintestinal tissues (54). This
concept has become even stronger through experiments in which
microbiota from patients with MS was administered to modulate EAE
(34). However, the dilemma of the
initial trigger for the dysfunction of the gut-microbiota-brain
axis in MS is still unknown within a chicken or egg causality
conundrum. Thus, innate immune activation in the brain could be
triggered by signals from the gut. On the one hand, metabolites
from the GM can affect the innate immune response in the CNS. This
was demonstrated in a study of mice fed high amounts of fiber,
where the diet modulated the GM and led to increased production of
SCFAs, particularly butyrate, and there was a decrease in the
expression of the inflammatory microglia gene (34).
The BBB is composed of tightly joined endothelial
cells with tight junctions along the blood vessels that vascularize
the brain. This barrier allows the CNS to be isolated from the
circulation and precisely regulates the passage of molecules, ions
and cells between the brain and the periphery (55). It also protects the brain from
pathogenic microorganisms and uncontrolled inflammatory reactions
that could damage brain neurons. Unfortunately, in response to a
state of dysbiosis, i.e., a high number of bacteria with
pro-inflammatory function, the intestinal barrier can become
permeable, consequently contributing to the chronic low-grade
bacterial translocation from the intestine to the circulation. This
overstimulates immune cells and cells of the CNS, leading to
alterations in the permeability of the BBB. This is how the GM
plays a fundamental role in the susceptibility to the development
of autoimmune diseases (55).
A study of mice with BBB permeability observed an
increase in the passage of molecules from the periphery, such as
lipopolysaccharides (LPS) and oxidative stress. Both molecules are
known to induce systemic inflammation and neuroinflammation. This
permeability was due to a decrease in bacterial species, which in
turn caused a 75% decrease in tight junction proteins, including
occludin and claudin-5 in the BBB (56,57).
Another study reported that this effect could be reversed with SCFA
supplementation, such as propionate and butyrate, which
demonstrates that SCFA production by the GM also has a beneficial
effect on the BBB (41). In
addition, bacterial products and metabolites may also cross the
permeable BBB (40) and contribute
to the development and progression of autoimmune diseases (57,58),
since they cause inflammation, demyelination of neuronal axons and
scarring (gliosis), which triggers abnormal neuronal signaling
function. In addition, studies in aged mice have indicated that
endotoxemia, i.e., the passage of LPS to the circulation (LPS
>200 pmol/ml), may alter the brain vasculature and promote a
neuroinflammatory phenotype, leading to memory problems and
cerebral amyloid angiopathy (59).
Thus, when MS patients do not have a known exogenous risk factor,
the question arises of whether MS originates in the periphery or in
the CNS. Therefore, there is an extrinsic CNS theory where
self-reactive T cells that are activated in the periphery, possibly
by molecular mimicry, activation and co-expression of T-cell
receptors with double specificity (60), travel to the CNS along with
activated B cells and monocytes. This theory is analogous to the
method used to induce EAE: These mice are administered emulsioned
CNS antigen along with immunostimulants, resulting in the
generation of pathogenic CD4+ Th1 and Th17 cells in the
draining lymph nodes; subsequently, these cells pass to the
circulation, cross the BBB and exert their effector functions
within the CNS (60).
In a healthy state, there is a regulatory center in
the thymus where numerous self-reactive T cells are eliminated,
maintaining central tolerance in order. However, despite this
regulation, certain self-reactive T cells escape to the periphery,
possibly because peripheral tolerance mechanisms are compromised by
reduced function of Treg cells or by increased resistance of
effector B cells and T cells to CNS-directed suppressor mechanisms.
Subsequently, self-reactive B cells and T cells are activated in
the periphery and become highly aggressive effector cells. This
activation of lymphocytes may be due to the recognition of
CNS-derived antigens sequestered in the periphery. Once lymphocytes
are activated and have differentiated into Th1 and Th17, they
infiltrate the CNS, along with B cells and innate immune cells,
causing inflammation and tissue damage there (61) (Fig.
2). In this sense, the health of the microbiota is a crucial
factor in maintaining the integrity of the intestinal barrier, as
the latter is also part of the correct maturation and response of T
and B cells, and of course, is involved in the tolerant
communication between the host and the microbial environment, i.e.,
that there is an adequate tolerant response during exposure in the
intestinal mucosa and an attack in the systemic circulation
(61). In addition, for triggering
an adequate immune response, it is necessary that there is adequate
colonization of the microbiota, since, in demyelinating lesions of
the CNS, it may be observed that the recruitment of self-reactive B
and T cells depends on the availability of target autoantigens and
commensal bacteria. In a study with germ-free EAE model mice, a
reduction in inflammation was observed, as the immune system cannot
mount an effective Th17-mediated response (61).
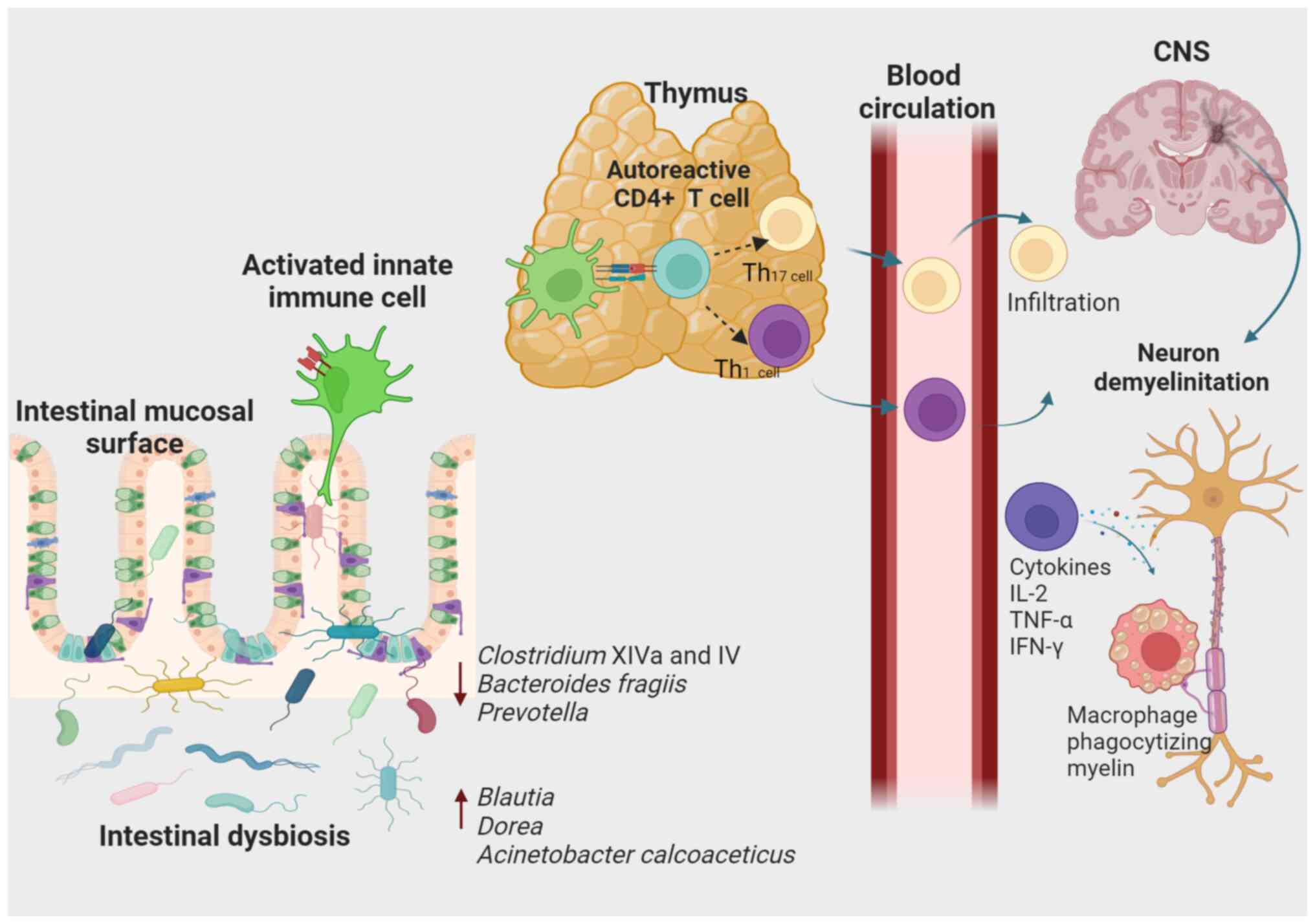 | Figure 2Association of intestinal dysbiosis
with susceptibility to the development of MS. According to an
extrinsic model of the CNS, autoreactive T cells in the periphery
are activated through molecular mimicry, some circulating cells or
the co-expression of T-cell receptors with different specificity.
Different genetic and environmental factors, such as modification
of gut microbiota composition, are thought to contribute to these
events. When activated, CD8+ T-lymphocytes,
CD4+ T-lymphocytes differentiated into CD4+
Th1 and Th17 cells, B cells and cells of the innate immune system
can infiltrate the CNS, causing inflammation and tissue damage. In
addition, the Th1-type response produces pro-inflammatory cytokines
such as IL-2, TNF-α and IFN-γ that activate antigen-presenting
cells. In turn, pro-inflammatory cytokines activate macrophages
responsible for phagocytizing myelin. CNS, central nervous system;
MS, multiple sclerosis; Th1, type 1 T-helper cell. |
Besides, the microbiota and its metabolites also
participate in the differentiation fate of T cells in the
intestine, thus modulating the immune response. For instance,
Bacteroides fragilis participates in the differentiation of
FoxP3+ Treg cells (62).
In addition, SCFAs increase the expression of Treg cells,
particularly butyrate, suppress the activation of Th17 cells, and
reduce the symptoms of EAE and axonal damage (63). Neurodegenerative diseases have shown
a compositional and functional modification of the GM with
resulting neuroinflammation. A diet high in simple sugars, high in
saturated fats and low in fiber (64), fructose and the heme component, are
the few factors that have also been reported to alter the GM
composition and the permeability of the intestinal epithelial
barrier in mouse models. In this way, GM dysbiosis in mice fed a
high-fructose diet was the main cause of neuroinflammation
(65), as a healthy microbiota can
modulate the neurological function of the mouse brain by being part
of the maturation and function of microglia (66). The pyrimidine domain of inflammasome
6 (NLRP6), part of the family of nucleotide-binding oligomerization
domain-like receptors (NLR), mediates innate cellular immunity to
defend the intestinal mucosa against bacterial pathogens. As
mentioned above, commensal bacteria and their metabolites are
essential for epithelial barrier integrity, perhaps by regulating
NLRP6 inflammasome signaling to maintain intestinal
microenvironment homeostasis (67).
As mentioned, commensal bacteria-derived metabolites are critical
in neurodegenerative diseases. They have shown a notable role in
immune-mediated neurodegeneration, as Treg cells promote the
differentiation and remyelination of oligodendrocytes (68). Certain commensal bacteria, such as
Streptococcus sp., and the most representative species of
the genus Lactobacillus, such as Lactobacillus casei,
Lactobacillus rhamnosus, Lactobacillus gasseri and
Lactobacillus delbrueckii (subspecies bulgaricus),
are able to increase levels of anti-inflammatory cytokines, such as
IL-10(69).
In a mouse study, direct binding of
Streptococcus sp. and Lactobacillus ligands initiates
a signaling cascade that induces increased expression of IL-10 by
C-type lectin-positive macrophages and macrophage galactose-type
lectin-1/CD301a, and downstream mediators such as spleen tyrosine
kinase, caspase recruitment domain-containing protein 9 and ERK are
also involved in the induction of IL-10 mRNA (69). The immunoregulatory effects of this
cytokine are critical for brain health, as indicated by another
study of oxygen and glucose deprivation-induced cerebral ischemia,
as IL-10 has an immunoregulatory role that attenuates neuronal
death and promotes neurite growth and synapse formation through the
JAK1/STAT3 pathway (70). Sun et
al (71) have demonstrated the
influence of the Bifidobacterium genus on Treg cells, which
increased the intracellular level of IL-10 and IL-10 receptor α
(IL-10Rα), suggesting that Bifidobacterium promotes the
IL-10/IL-10Rα autostimulatory circuit in intestinal Treg cells. On
the other hand, the metabolites of segmented filamentous bacteria
in the ileum activate C-X-C motif chemokine receptor 1+
macrophages that act as antigen-presenting cells that present
microbial antigens to naive T cells, which consequently
differentiate into RAR-related orphan receptor-γ+ T
cells and, ultimately, into Th17 cells. Therefore, these intestinal
bacteria modulate the CD4+ T-cell compartment in the
GALT and promote the differentiation and expansion of Th17 cells,
which, in turn, can promote neuroinflammation in the circulation.
As observed in a rat study using the EAE model, pro-inflammatory
Th17 cells promote neurodegeneration directly in neuronal cells;
IL-17 inhibits the maturation of oligodendrocyte cells in
vitro and reduces their survival. Oligodendrocyte lineage cells
are susceptible to IL-17-mediated toxicity, suggesting a direct
connection between intestinal bacteria, inflammation,
neuroinflammation and the pathogenesis of MS (72).
GM-derived neurotransmitters, such as histamine,
dopamine, norepinephrine, acetylcholine and serotonin, as well as
GABA produced by Lactobacillus brevis, have direct activity
in the CNS (73,74). Studies have shown a decrease in the
functionality of the serotonergic system in MS. Furthermore,
pro-inflammatory cytokines that are elevated in dysbiosis can
activate indoleamine 2,3-dioxygenase and tryptophan
2,3-dioxygenase, enzymes that activate the kynurenine pathway and
deplete the availability of tryptophan for serotonin synthesis.
Serotonin regulates the function of T cells and other immune
components (75). Approximately 90%
of serotonin is synthesized by gut enterochromaffin cells and is
mediated by the microbiome. In addition, the GM not only
independently generates serotonin, but it can also stimulate the
release of these molecules (76).
For instance, microbial metabolites such as SCFAs promote the
transcription of an enzyme that synthesizes serotonin from
tryptophan. In addition, the GM in animal models has been shown to
have an influence on the synthesis of serotonin receptors outside
the gut. Therefore, serotonin may be a critical mediator of the
gut-brain axis in MS. Similarly, a decrease in GABA brain
transporters is expressed in MS patients. GABA is the main
inhibitory mediator in the CNS and downregulates pro-inflammatory
T-cell mediators. The production of this neurotransmitter is
regulated by the GM, with Lactobacillus brevis being the
most effective producer. Furthermore, a decrease in dopamine levels
is linked to MS relapse. Dopamine controls locomotion, cognition,
endocrine regulation and immune regulation. It also suppresses
IL-17, suggesting an anti-inflammatory effect. Staphylococcus
aureus, Escherichia coli 157:H7 and other bacteria have
shown improved growth or mobility in vitro in the presence
of dopamine. However, there is no clinical evidence to confirm that
bacteria produce dopamine (77).
The mechanism behind this gut-microbiota-brain axis
remains to be fully elucidated. This axis may be regulated through
different pathways, and among them, neural pathways are most
important, mainly due to the stimulation of the vagus nerve, the
alteration of the activity of the hypothalamic-pituitary-adrenal
axis and the secretion of SCFAs that can activate microglial cells
and the systemic circulation (41),
which explains how the brain and the gut are bidirectionally
linked. The neurotransmitters produced by the GM bind to sensory
neurons in the ENS, sending afferent impulses through the vagus
nerve and sympathetic/parasympathetic pathways (77). On the other hand, intestinal
inflammation induced by bacterial pathogens causes activation of
the vagal sensory ganglia and the solitary tract nucleus in the
brainstem, providing an early warning pathway to the brain during
infections. In addition, the interaction between the GM and brain
also occurs indirectly, as the afferent vagal fibers do not cross
the epithelial layer of the intestine, so the passage of
metabolites produced by intestinal bacteria or their compounds is
the main way to activate the afferent fibers (78). Enteroendocrine cells present in the
intestinal epithelium are responsible for communicating with the
afferent fibers of the vagus nerve and release certain substances,
such as serotonin, ghrelin, cholecystokinin and peptide YY that
modulate motility, secretion and nutrient intake within the
gastrointestinal tract (79). As
mentioned above, SCFAs are the main metabolites produced by
bacteria and their production is affected during dysbiosis, leading
to an inflammatory phenotype (80).
SCFAs can induce transcription factors and modulate the expression
of critical enzymes through histone deacetylation. In addition,
SCFAs have vital roles in structural, metabolic and neurohormonal
regulation. In particular, the functions of SCFAs in MS, in
addition to those already described, are as follows: Promoting
immune cell tolerance, inducing immunoglobulin secretion,
inhibiting pathogens, stimulating mucus secretion and contributing
to intestinal barrier integrity (81).
In one study, a decrease in the Clostridium
genus was associated with the production of Treg cells and an
increase in the anti-inflammatory cytokine IL-10, and
administration of Clostridium tyrobutyricum or sodium
butyrate restored the BBB permeability in pathogen-free mice
(81). A fiber-rich diet and oral
butyrate supplements reduced symptoms and increased
CD4+FoxP3+ T cells in mouse EAE models
(82). A high-fat diet (such as the
Western diet) reduces butyrate production by the
Prevotellaceae family and Bacteroides genus in mouse
EAE models (82). On the other
hand, SCFA treatment mitigates the high-fat diet-induced change in
the GM and intestinal epithelial barrier integrity in C57BL/6 mice,
possibly by inhibiting systemic inflammation (83). SCFAs can modulate the maturation and
function of microglia in the brain, suggesting the potential
benefits of GM-derived SCFAs in protecting against the
neuroinflammatory process (65). In
a recent study, intestinal dysbiosis induced by a high-fat and
choline-deficient diet in C57BL/6N male mice occasionally increased
the Proteobacteria phylum and decreased the
Firmicutes phylum, and as a consequence, SCFA production
deteriorated, combined with a sudden increase in neurogenesis,
possibly depleting the pool of neural stem cells available for
long-term neurogenesis, neuroinflammation, oxidative stress,
synaptic loss and cell death in different brain regions (83). Other proposed mechanisms linking
dysbiosis with neuroinflammation and neurodegeneration in MS, as
the relationship between certain elevated bacteria in MS and mucin
degradation, bacteria metabolize bile acids with anti-inflammatory
and pro-inflammatory effects in patients with MS, and certain
vitamin and other compound deficiencies related to intestinal
barrier disruption and inflammation are presented in Table I. In any case, whether in the EAE
model or in MS, it is clear that the gut-microbiota-brain
interaction is crucial in the progression of the disease and
therapeutic approaches targeting intestinal dysbiosis and
intestinal barrier dysfunction should begin to be considered within
the MS drug regimen (34).
Therefore, in view of the great impact that dysbiosis may have on
MS, the use of probiotics has been proposed as a potential therapy
(34).
 | Table IOther proposed mechanisms link
dysbiosis to neuroinflammation and neurodegeneration in MS. |
Table I
Other proposed mechanisms link
dysbiosis to neuroinflammation and neurodegeneration in MS.
| Molecule or
biological compound | Definition or
function | Related
bacteria | Effect of bacteria
and their relationship with intestinal dysbiosis | (Refs.) |
|---|
| Mucin | A family of
glycosylated proteins secreted in the gut, forming the mucus
barrier gel that protects the epithelium | Akkermansia
muciniphila, Ruminococcus, Bifidobacterium and
Dorea | They are capable of
degrading mucin and they were found to increase in patients with
MS | (8) |
| Bile acids | Primary and
secondary bile acids modulate the immune response by increasing FXR
and the GPBAR1 expression. FXR, IL-1β and TNF-α mediate colon
inflammation by increasing LPS in dysbiosis | i)
Lactobacillus, Clostridium, Erysipelotrichaceae and
Parabacteroides ii) Acinetobacter, Bifidobacterium,
Pseudomonas and Bilophila | i) Bile acids
metabolizing bacteria with anti-inflammatory effects decreased in
patients with RRMS ii) Bile acids metabolizing bacteria with
pro-inflammatory effects increased in patients with MS | (84) |
| Vitamin D | Vitamin D has an
anti-inflammatory function by inhibiting the expression of NF-κB.
It does this by binding to VDR and RXR receptors, creating a
heterodimer that modulates gene expression |
Faecalibacterium | Vitamin D
deficiency has been linked to intestinal barrier disruption,
decreased butyrate-producing bacteria and dysbiosis | (32) |
| Phytoestrogens | There are four main
types of phytoestrogens (isoflavones, prenyl flavonoids, coumestans
and lignans), and some bacteria are responsible for metabolizing
isoflavones and lignans into equol and enterolactone, respectively.
In intestinal epithelial cells, isoflavones suppress
pro-inflammatory cytokines through estrogen receptors. They also
inhibit nitric oxide production induced by LPS (leading to
inflammation and neurodegenerative processes) and decrease the
expression of NF-κB and other pro-inflammatory cytokines | i) Prevotella,
Parabacteroides, Adlercreutzia, Slackia and Lactobacillus
ii) Lactobacillus plantarum and Lactobacillus
paracasei | i) These bacterial
genera are related to the conversion of phytoestrogens to their
metabolites; therefore, they possess an anti-inflammatory potential
and are decreased in patients with MS ii) Their administration was
effective in preventing EAE development in mice | (11,10,85,86) |
| Tryptophan | Tryptophan is an
essential amino acid metabolized from food to indole and indole
derivates by microbiota. These metabolites regulate immune cell
responses in the gut mucosa and increase mucin production. They
also activate the pregnane X receptor, essential in gut barrier
integrity |
Lactobacillus | Bacteria that
metabolize tryptophan to indole, which activates AhR with
anti-inflammatory functions | (8,82) |
| Cysteine | Cysteine is a
non-essential amino acid required in a rate-limiting step for
glutathione production. Glutathione is an antioxidant that protects
cells from ROS and peroxide damage, which plays a crucial role in
recruiting immune cells that start the myelin phagocytosis at CNS.
Superoxide increases disrupt the cell junction at the BBB |
Desulfovibrionaceae | They may induce a
deficiency of bioactive sulfur-containing antioxidant glutathione
by sequestering cysteine and are increased in patients with MS and
EAE | (82) |
| Choline | A water-soluble
nutrient grouped in complex B vitamins | Acinetobacter
baumannii | Metabolizes choline
to trimethylamine, which is metabolized in the liver to TMAO. An
increase in these bacteria has been observed in patients with
MS | (8,32) |
| MicroRNAs | In MS and EAE,
miRNAs mediate the upregulation and downregulation of different
miRNAs in cells like CD4+ T, Th17, Th1, Th2 and Treg.
miRNAs produced by host cells regulate GM | Lower levels of
butyrate-producing bacteria such as Clostridium | The GM may regulate
miRNA production in host cells. For example, butyrate triggers the
expression of miR-375, a biomarker related to MS progression. On
the other hand, certain exome miRNAs, such as let-7i, prevent Th1
and Th17 cell differentiation during MS onset | (87) |
| Steroid
hormones | Microbiota
dysbiosis may also relate to an increase in glucocorticoids,
mineralocorticoids or catecholamines in the CNS by the enteric
nervous system | Interaction between
the CNS and the enteric nervous system | This release may
increase gut permeability and immune responses | (87) |
5. Evidence of the association of intestinal
dysbiosis with MS in different human populations
Certain pathologies, such as MS, are related to
modifications of the GM composition, showing intestinal dysbiosis
compared with healthy controls. The relationship between MS and
intestinal dysbiosis has been scrutinized by different studies,
from in vitro analyses using mouse models to clinical trials
comparing MS patients with healthy subjects. Even though different
reviews regarding MS and the microbiome were found, only a few
studies (cohorts from the US, UK, Japan, Germany and Italy)
describe the cohorts in detail. Most were predominantly Caucasian,
although a few included Hispanic subjects from the US. In addition,
an increased intestinal permeability prevalence in MS patients is
related to this disease. However, it has not been related to brain
NMR lesion load, which is also true for EAE. The mice also
exhibited an increase of inflammatory T-cell migration to the
brain, linking intestinal permeability with BBB permeability
(88). Furthermore, an increase of
the LPS endotoxin in EAE compared with controls may imply bacterial
translocation due to an altered intestinal barrier. LPS can
stimulate immune microglial responses (88).
Although intestinal dysbiosis is a continuum seen
in different studies that include individuals with MS, there are
differences and similarities in the changes observed in each
phylum. In general, the GM in MS compared with healthy controls
shows a depletion of the phylum Bacteroidetes, particularly
the genera Prevotella and Parabacteroidetes (8), which is related to RRMS, expansion of
Th17 cells and disease activity (8). Prevotella depletion in MS has
been consistent in different geographical areas (8). By contrast, in a predominantly adult
caucasian cohort from the USA, a Prevotella increase was
observed after pharmacological treatment or disease remission in
fecal samples of patients with RRMS in comparison with untreated
patients with RRMS (89).
Changes around the Firmicutes phylum are
contradictory among studies, with both a decrease or increase of
the genera Clostridium and Faecalibacterium reported
(38). On the other hand, Chen
et al (38) found an
increase of the genera Dorea and Blautia (also of the
Firmicutes phylum) in fecal samples of an RRMS cohort from
the US (n=62). High levels of Dorea are also related to
other inflammatory diseases such as Crohn's disease. However, the
Dorea genus also shows an anti-inflammatory action, possibly
due to different species of this same genus or their proportion
with respect to another genus, since for example, gasses produced
by Dorea are utilized by Blautia (which can promote
mucin degradation) (38).
An increase of the Akkermansia genus
(Verrucomicrobia phylum) has been reported by different
studies comparing MS patients with healthy controls (90). The Akkermansia genus is
related to mucin degradation and increases of pro-inflammatory
cytokines. It has been shown that Akkermansia muciniphila
induces a pro-inflammatory T lymphocyte response in vitro;
however, in vivo studies using mouse models of MS have so
far failed to induce a similar response (90). Nevertheless, Akkermansia
muciniphila also may improve the intestinal barrier in
metabolic diseases, highlighting the entanglement of the microbiome
function (90).
Another genus consistently increased in patients
with MS compared with healthy controls is Methanobrevibacter
(Euryarchaeota phylum) (90). Methanobrevibacter and
Akkermansia muciniphila are methanogen bacteria associated
with constipation in patients with MS, a common condition in this
disease (90). Methane acts as a
neurotransmitter that slows bowel movement and may contribute to
microbiota changes and the pro-inflammatory local environment
(90). In vitro analyses and
mouse models linked the increase of both Methanobrevibacter
and Akkermansia genus, in addition to the decrease of
Butyricimonas genus, with the regulation of dendritic cell
maturation, interferon (IFN) signaling and nuclear factor κB
signaling (90). After medical
treatment with IFN-β and glatiramer acetate (GA), the genera
Prevotella and Sutterella increased in a
predominantly caucasian US cohort (91). This study comparing patients with MS
with healthy controls showed that GA treatment also decreased the
abundance of Bacteroidaceae and Lactobacillaceae families,
Ruminococcus and Clostridium genera and other clostridial
microbes (91).
Regarding the Proteobacteria phylum, human
cohorts from different countries observed enrichment of the
Mycoplana, Haemophilus, Sutterella,
Pseudomonas, Bilophila and Acinetobacter
genera (86). It should be noted
that, although they do not contradict each other, each research
group reports an enrichment in different Proteobacteria. A
Japanese cohort study comparing healthy controls with patients with
RRMS also showed a decrease of Clostridia XIVa and IV
clusters (both of the Clostridium genus), constituting up to
40% of healthy gut bacteria (92).
The contradictions around the Firmicutes
phylum and the differences between genera of the
Proteobacteria phylum may be derived from the absence of
standardized sequencing methodologies and the bias represented by
using primers for the specific identification of the 16S rRNA gene
(8), which is a region of bacterial
DNA used to classify the sample, identifying the abundance and
variability of species. Although it is one of the most conserved
regions of bacterial DNA, it is formed by regions with high
variation, making it challenging to generate an accurate microbiome
profile. Other factors that limit GM studies are individual
variability and environmental influences. Genetics, diet,
geographical area and therapies may modify the microbiome (8).
Despite the discrepancy, the GM profile in patients
with MS is usually enriched with pro-inflammatory bacteria and
depleted from anti-inflammatory bacteria, which may influence the
severity of the disease. These changes in bacterial distribution
are related to the digestion/metabolism of several compounds,
resulting in either fewer nutrients reaching the host or an
increase of metabolites with detrimental effects. Thus, diet may be
a determining factor in GM.
6. Beneficial effects of GM on various
diseases
Most microbiota research focuses on the
relationship between changes in GM composition and various
pathological states. Environmental (external) factors affect the
balance of the microbial community, leading to the deregulation of
the organism's vital functions and diseases. Thus, there is
increasing evidence that the GM is associated with the development
of pathologies such as allergies, gastrointestinal disorders,
obesity, CVD, cancer and diabetes, among others (16). Also, in the scientific literature,
it has been indicated that the modulation of the GM by means of
probiotic administration brings important health benefits (93).
Allergies
The relationship between microbiota and diseases is
complex. For instance, infants born through cesarean section, who
acquire bacteria from the maternal epidermis, face an elevated risk
of developing allergies and asthma compared to those born through
the maternal vaginal canal. Although this distinction diminishes as
the infant develops, the microbiota has an important role in immune
system development. Certain pathogenic bacteria, such as
Haemophilus influenzae, have the potential to trigger
allergic reactions, whereas others like the Proteobacterium
Moraxella catarrhalis can exacerbate the allergic airway
reaction. Meanwhile, SCFAs may stimulate the generation of Treg
cells and contribute to reducing inflammation in allergic models
(16).
Possible mechanisms by which probiotics could
modulate the allergic response are as follows: Probiotics can
regulate the immune response, decreasing the production of IgE, Th2
cells and pro-inflammatory cytokines, restore the GM, which has
been shown to be altered in allergic patients and increase the
production of SCFAs, which have anti-inflammatory properties
(93). The results of clinical
trials evaluating the efficacy of probiotics for allergies are
mixed. Certain studies have found that probiotics are effective in
reducing allergic symptoms, such as allergic rhinitis and asthma.
However, other studies have found no benefit from probiotics. More
studies are needed to confirm the efficacy of probiotics for
allergies. Future studies should evaluate different types of
probiotics, doses and formulations, as well as different types of
allergies (93).
Irritable bowel syndrome (IBS)
A meta-analysis found an association of probiotics
with improvement of symptoms, specifically decreased pain in IBS
compared to placebo (94). Another
randomized, double-blind study of a probiotic containing
Lactobacillus plantarum and Bifidobacterium breve
also found improvement of pain in 38% of patients after 14 days and
52% after 28 days of consumption (95). Another clinical trial found that a
product containing 8 bacterial species significantly improved IBS
symptoms. In general, evidence suggests that probiotics may be a
useful therapy for certain patients with IBS. However, more studies
are needed to determine the efficacy of probiotics in the treatment
of different types of IBS and to identify the most effective types
and doses of probiotics (96).
Obesity
The idea that the GM may have a role in obesity has
been around for several years, based on studies that confirmed a
higher proportion of Firmicutes to Bacteroidetes in
obese mice compared to lean mice. There was also a higher
representation of genes involved in the extraction of energy from
food in the GM of the obese host compared to the microbes of the
lean host (97). In humans, one of
the first studies to link the GM with obesity compared the GM of
lean and obese individuals and revealed that obese subjects had a
reduced proportion of Bacteroidetes and higher levels of
Firmicutes. When a dietary intervention was given to these
individuals, the relative abundance of Bacteroidetes
increased, while that of Firmicutes decreased (97). It is possible that the GM has a role
in obesity, but more research is needed to better understand this
association (97).
In different studies where symbiotic
supplementation (mixture of probiotics and prebiotics) was
administered to obese subjects, different results were observed
depending on the type of bacteria supplemented. With
Lactobacillus gasseri SBT2055, a reduction in body mass
index, waist circumference, abdominal visceral fat area and hip
circumference was observed. With strains of Enterococcus
faecium and Streptococcus thermophilus, a reduction in
body weight, systolic blood pressure and LDL cholesterol (LDL-C),
and an increase in fibrinogen levels were obtained. With
Bifidobacterium, Lactobacillus and Streptococcus
thermophilus, there was an improvement in the lipid profile and
insulin sensitivity, and a decrease in C-reactive protein. With
Lactobacillus acidophilus La5 and Bifidobacterium
animalis subsp. lactis BB-12 (Bifidobacterium
lactis), there was a reduction in the fasting glucose
concentration and an increase in the homeostasis model assessment
of insulin resistance (18).
CVD
There is growing evidence that the GM has a role in
maintaining cardiovascular health and its dysregulation can
contribute to CVD. The gut and oral microbiota have an important
role in cardiovascular health. GM dysbiosis, or an imbalance in the
composition of gut bacteria, has been associated with an increased
risk of CVD, such as atherosclerosis, hypertension and heart
failure (16). The mechanisms by
which the GM can contribute to CVD include the following: i)
Inflammation-gut bacteria can release inflammatory mediators that
can damage endothelial cells, which line the blood vessels; ii)
oxidative stress-gut bacteria can produce free radicals that can
damage cells and tissues in the body; Iii) blood pressure
modulation: Metabolites produced by gut bacteria can affect blood
pressure (16).
Cancer
The microbiota, which is the collection of
microorganisms that live in the human body, may have a role in the
development of cancer (16).
Researchers have found that microbiota dysbiosis, or an imbalance
in the composition of the microorganisms, is associated with an
increased risk of cancer in several organs, including the colon,
lung and mouth (16). The
mechanisms by which the microbiota may contribute to cancer include
the following: i) Inflammation-chronic inflammation caused by
microbiota dysbiosis can damage DNA and promote the growth of
cancer cells; ii) invasion and metastasis-certain bacteria can
invade body tissues and help cancer cells to spread; iii) metabolic
alteration-metabolites produced by bacteria can affect cellular
metabolism, which can increase the risk of cancer (16).
In particular, studies have found that the bacteria
Fusobacterium nucleatum, Escherichia coli,
Streptococcus bovis, Bacteroides fragilis and
Helicobacter pylori are associated with an increased risk of
colon cancer. The genera Streptococcus, Prevotella
and Veillonella are associated with lung cancer, while
Porphyromonas gingivalis and Fusobacterium nucleatum
are associated with oral cancer (16). More research is needed to better
understand the mechanisms by which the microbiota may contribute to
cancer. However, emerging evidence suggests that the microbiota is
an important target for cancer prevention and treatment (16). In several studies, beneficial
effects have been identified with supplementation with
Lacticaseibacillus rhamnosus GG, Bifidobacterium
lactis and inulin. These include an increase in
Lacticaseibacillus rhamnosus and Bifidobacterium
lactis in the feces, reduction of Clostridium
perfringens, prevention of increased IL-2 secretion in
polypectomized patients and increased production of IFN-γ in cancer
patients (18).
Diabetes
The intestinal and oral microbiota may have a role
in the development of type 1 diabetes mellitus (T1DM) and T2DM. In
T1DM, the GM is characterized by a decrease in bacteria producing
butyrate, a fatty acid with anti-inflammatory properties. In
addition, an increase in pathogenic bacteria, such as the genus
Salmonella, has been observed. The mechanisms by which the
GM may contribute to T1DM are not fully elucidated, but it is
thought that GM dysbiosis may lead to chronic inflammation, which
may damage insulin-producing cells in the pancreas. In T2DM, the GM
is also characterized by a decrease in butyrate-producing bacteria
and an increase in pathogenic bacteria. In addition, an increase in
bacteria that produce primary bile acids, which can negatively
affect glucose metabolism, has been observed. It is thought that GM
dysbiosis may lead to chronic inflammation, abnormal intestinal
permeability and altered glucose metabolism, acting as a possible
mechanism that can contribute to T2DM (16).
The oral microbiota may also have a role in T2DM.
Oral bacteria can travel to the intestine, changing the GM
composition and potentially mediating the immune response (16). In general, research on the
intestinal and oral microbiota in T1DM and T2DM is ongoing.
However, emerging evidence suggests that the intestinal and oral
microbiota may be an important target for the development of new
therapies for these diseases (16).
Various combinations with probiotic strains are associated with a
reduction in fasting blood glucose, antioxidant status, hemoglobin
A1c, total cholesterol and LDL-C (Lactobacillus acidophilus
La5 and Bifidobacterium lactis) (18).
7. Concluding remarks
The present review deals with the relationship
between dysbiosis of the GM and neuroinflammatory processes in MS.
Although research is still ongoing, gut bacteria appear to be a
predisposing factor to the development of neurodegenerative
diseases such as MS. Studies carried out in different populations
around the world reported that patients with MS exhibit marked
changes in their GM composition, described by the decrease or
increase of determined bacterial phylae and species compared to
control subjects. Despite the controversy existing in the results
of microbiota studies in different populations, the difference in
composition and function between study groups remains clear. The
contradictions regarding the differences between species may be due
to the proportion between genera or to the fact that the products
of certain bacteria are used in the metabolic processes of other
bacteria. In addition, the medical treatment is different in each
patient; the clinical course of the disease and disease-modifying
treatment may also influence the bacterial response in the
host.
In this light, it appears that the factors that
interfere with the GM composition are multiple and diverse for each
population, from genetics to eating habits and lifestyle, the
complex and entangled relationship between the microbiota and the
host, and the relationship that exists between the same
microorganisms; therefore, further work is required on the
implementation of standardized techniques for the sequencing of the
16S rRNA gene and the protocols for studying the GM. It may be
argued that MS is triggered in the periphery, i.e., in the
intestine, where the inflammatory molecules produced by a dysbiotic
microbiota can enter the circulation as a result of an intestinal
barrier impaired by the same inflammatory process and reach the CNS
and initiate an immunological cascade that sensitizes T-lymphocytes
to myelin, also weakening the BBB and causing inflammation at the
local level and demyelination of neurons, and consequently the
nervous tissue. In its eagerness to repair the damage, it produces
remyelination by oligodendrocyte precursor cells causing scarring,
so axons with partial or complete loss of myelin do not transmit
full nerve impulses and classic MS symptoms begin to appear.
In conclusion, ongoing research should be performed
to answer the question of whether intestinal dysbiosis triggers MS,
or whether MS causes dysbiosis in patients. Studies indicate that
intestinal dysbiosis may be the most critical environmental factor
for developing the disease. Animal and in vitro studies
suggest that probiotic administration modifies the GM composition
towards an inflammation-mediating phenotype, which positively
affects CNS disorders and the demyelination process in EAE.
However, more research in humans is needed. For this reason, future
studies using large-scale RCTs are required to review effect-doses
in MS. Future therapies may target inflammation control via the
gut-CNS axis.
For instance, the oral administration of myelin
antigens suppressing the immune response has proven effective in
EAE animal models. A number of case reports have suggested a
potential benefit of FMT on patients with MS. Despite FMT,
antibiotic treatment is more widely used; however, it significantly
alters the GM composition. Meanwhile, probiotic, prebiotic and
diet-based microbiota treatments may be a promising approach for
MS, since accessibility is wider, enabling GM modification,
competitive adherence to the mucosa and epithelium, strengthening
the gut epithelium and BBB, preventing bacterial translocation and
modulating immune response (98).
Likewise, molecular characterization of bacteria indigenous in
fermented foods, such as sourdough or curd, may have a broader
biotherapeutic potential (17).
Acknowledgements
Figs. 1 and
2 were created with BioRender.com (accessed in September 2023).
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization: METC, NMTC and NTC. Search
methodology and data curation: METC, NMTC, AVML, SGR and NTC.
Writing-original draft preparation: METC, NMTC, AVML, SGR and NTC.
Writing-review and editing: METC, NMTC, AVML, SGR, SM, IAGH, JRBR,
ESP and NTC. Data authentication is not applicable. All authors
have read and agreed to the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tinahones FJ: The importance of the
microbiota in obesity. Rev Esp Endocrinol Pediatr. 8 (Suppl
1):16–20. 2017.
|
|
2
|
Bibbò S, Dore MP, Pes GM, Delitala G and
Delitala AP: Is there a role for gut microbiota in type 1 diabetes
pathogenesis? Ann Med. 49:11–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim S and Jazwinski SM: The gut microbiota
and healthy aging: A mini-review. Gerontology. 64:513–520.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ordoñez-Rodriguez A, Roman P, Rueda-Ruzafa
L, Campos-Rios A and Cardona D: Changes in gut microbiota and
multiple sclerosis: A systematic review. Int J Environ Res Public
Health. 20(4624)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cornejo-Pareja I, Muñoz-Garach A,
Clemente-Postigo M and Tinahones FJ: Importance of gut microbiota
in obesity. Eur J Clin Nutr. 72 (Suppl 1):S26–S37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Passos MDCF and Moraes-Filho JP:
Intestinal microbiota in digestive diseases. Arq Gastroenterol.
54:255–262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mörkl S, Butler MI, Holl A, Cryan JF and
Dinan TG: Probiotics and the microbiota-gut-brain axis: Focus on
psychiatry. Curr Nutr Rep. 9:171–182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Freedman SN, Shahi SK and Mangalam AK: The
‘Gut Feeling’: Breaking down the role of gut microbiome in multiple
sclerosis. Neurotherapeutics. 15:109–125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Altieri C, Speranza B, Corbo MR,
Sinigaglia M and Bevilacqua A: Gut-Microbiota, and multiple
sclerosis: Background, evidence, and perspectives. Nutrients.
15(942)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang HX and Wang YP: Gut microbiota-brain
axis. Chin Med J (Engl). 129:2373–2380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
De Luca F and Shoenfeld Y: The microbiome
in autoimmune diseases. Clin Exp Immunol. 195:74–85.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Forssberg H: Microbiome programming of
brain development: Implications for neurodevelopmental disorders.
Dev Med Child Neurol. 61:744–749. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharon G, Sampson TR, Geschwind DH and
Mazmanian SK: The central nervous system and the gut microbiome.
Cell. 167:915–932. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kesika P, Suganthy N, Sivamaruthi BS and
Chaiyasut C: Role of gut-brain axis, gut microbial composition, and
probiotic intervention in Alzheimer's disease. Life Sci.
264(118627)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gotkine M, Kviatcovsky D and Elinav E:
Amyotrophic lateral sclerosis and intestinal microbiota-toward
establishing cause and effect. Gut Microbes. 11:1833–1841.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D,
Xiao C, Zhu D, Koya JB, Wei L, Li J and Chen ZS: Microbiota in
health and diseases. Signal Transduct Target Ther.
7(135)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vasudha M, Prashantkumar CS, Bellurkar M,
Kaveeshwar V and Gayathri D: Probiotic potential of
β-galactosidase-producing lactic acid bacteria from fermented milk
and their molecular characterization. Biomed Rep.
18(23)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Markowiak P and Śliżewska K: Effects of
probiotics, prebiotics, and synbiotics on human health. Nutrients.
9(1021)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Marietta E, Mangalam AK, Taneja V and
Murray JA: Intestinal dysbiosis in, and enteral bacterial therapies
for, systemic autoimmune diseases. Front Immunol.
11(573079)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu
KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling
C, Golubeva AV, et al: The microbiota-gut-brain axis. Physiol Rev.
99:1877–2013. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Klineova S and Lublin FD: Clinical course
of multiple sclerosis. Cold Spring Harb Perspect Med.
8(a028928)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Walton C, King R, Rechtman L, Kaye W,
Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der
Mei I, et al: Rising prevalence of multiple sclerosis worldwide:
Insights from the Atlas of MS, third edition. Mult Scler.
26:1816–1821. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iacobaeus E, Arrambide G, Amato MP,
Derfuss T, Vukusic S, Hemmer B, Tintore M and Brundin L: 2018
ECTRIMS Focused Workshop Group. Aggressive multiple sclerosis (1):
Towards a definition of the phenotype. Mult Scler.
26(1352458520925369)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Olsson T, Barcellos LF and Alfredsson L:
Interactions between genetic, lifestyle and environmental risk
factors for multiple sclerosis. Nat Rev Neurol. 13:25–36.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cotsapas C and Mitrovic M: Genome-wide
association studies of multiple sclerosis. Clin Transl Immunology.
7(e1018)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
López-Gómez J, Sacristán-Enciso B,
Caro-Miró MA and Querol Pascual MR: Clinically isolated syndrome:
Diagnosis and risk of developing clinically definite multiple
sclerosis. Neurologia (Engl Ed).
S0213-4853(21)(00028-1)2021.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
27
|
Thompson AJ, Baranzini SE, Geurts J,
Hemmer B and Ciccarelli O: Multiple sclerosis. Lancet.
391:1622–1636. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
van der Vuurst de Vries RM, Wong YYM,
Mescheriakova JY, van Pelt ED, Runia TF, Jafari N, Siepman TA,
Melief MJ, Wierenga-Wolf AF, van Luijn MM, et al: High
neurofilament levels are associated with clinically definite
multiple sclerosis in children and adults with clinically isolated
syndrome. Mult Scler. 25:958–967. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sorensen PS, Sellebjerg F, Hartung HP,
Montalban X, Comi G and Tintoré M: The apparently milder course of
multiple sclerosis: Changes in the diagnostic criteria, therapy and
natural history. Brain. 143:2637–2652. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuan S, Xiong Y and Larsson SC: An atlas
on risk factors for multiple sclerosis: A Mendelian randomization
study. J Neurol. 268:114–124. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ilchmann-Diounou H and Menard S:
Psychological stress, intestinal barrier dysfunctions, and
autoimmune disorders: An overview. Front Immunol.
11(1823)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Riccio P and Rossano R: Diet, Gut
microbiota, and vitamins D + A in multiple sclerosis.
Neurotherapeutics. 15:75–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Scheperjans F: Can microbiota research
change our understanding of neurodegenerative diseases?
Neurodegener Dis Manag. 6:81–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Parodi B and Kerlero de Rosbo N: The
gut-brain axis in multiple sclerosis. is its dysfunction a
pathological trigger or a consequence of the disease? Front
Immunol. 12(718220)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S,
Kishi Y, Regev A and Kuchroo VK: Induction of Pathogenic TH17 Cells
by Inducible Salt-Sensing Kinase SGK1. Nature. 496:513–517.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wilck N, Matus MG, Kearney SM, Olesen SW,
Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L,
et al: Salt-Responsive Gut Commensal modulates TH17 axis
and disease. Nature. 551:585–589. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Farez MF, Fiol MP, Gaitán MI, Quintana FJ
and Correale J: Sodium intake is associated with increased disease
activity in multiple sclerosis. J Neurol Neurosurg Psychiatry.
86:26–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen J, Chia N, Kalari KR, Yao JZ, Novotna
M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, et
al: Multiple sclerosis patients have a distinct gut microbiota
compared to healthy controls. Sci Rep. 6(28484)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Moser T, Akgün K, Proschmann U, Sellner J
and Ziemssen T: The role of TH17 cells in multiple sclerosis:
Therapeutic implications. Autoimmun Rev. 19(102647)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rutsch A, Kantsjö JB and Ronchi F: The
gut-brain axis: How microbiota and host inflammasome influence
brain physiology and pathology. Front Immunol.
11(604179)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gentile F, Doneddu PE, Riva N,
Nobile-Orazio E and Quattrini A: Diet, microbiota and brain health:
Unraveling the network intersecting metabolism and
neurodegeneration. Int J Mol Sci. 21(7471)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chidambaram SB, Essa MM, Rathipriya AG,
Bishir M, Ray B, Mahalakshmi AM, Tousif AH, Sakharkar MK, Kashyap
RS, Friedland RP and Monaghan TM: Gut dysbiosis, defective
autophagy and altered immune responses in neurodegenerative
diseases: Tales of a vicious cycle. Pharmacol Ther.
231(107988)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cosorich I, Dalla-Costa G, Sorini C,
Ferrarese R, Messina MJ, Dolpady J, Radice E, Mariani A, Testoni
PA, Canducci F, et al: High frequency of intestinal TH17 cells
correlates with microbiota alterations and disease activity in
multiple sclerosis. Sci Adv. 3(e1700492)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vijayakumar TM, Kumar RM, Agrawal A, Dubey
GP and Ilango K: Comparative inhibitory potential of selected
dietary bioactive polyphenols, phytosterols on CYP3A4 and CYP2D6
with fluorometric high-throughput screening. J Food Sci Technol.
52:4537–4543. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tankou SK, Regev K, Healy BC, Cox LM, Tjon
E, Kivisakk P, Vanande IP, Cook S, Ghandi R, Glanz B, et al:
Investigation of probiotics in multiple sclerosis. Mult Scler.
24:58–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kouchaki E, Tamtaji OR, Salami M, Bahmani
F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P and
Asemi Z: Clinical and metabolic response to probiotic
supplementation in patients with multiple sclerosis: A randomized,
double-blind, placebo-controlled trial. Clin Nutr. 36:1245–1249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Borody T, Leis S, Campbell J, Torres M and
Nowak A: Fecal Microbiota Transplantation (FMT) in Multiple
Sclerosis (Ms). Am J Gast. 106 (Suppl 2)(S352)2011.
|
|
48
|
Engen PA, Zaferiou A, Rasmussen H, Naqib
A, Green SJ, Fogg LF, Forsyth CB, Raeisi S, Hamaker B and
Keshavarzian A: Single-Arm, non-randomized, time series,
single-subject study of fecal microbiota transplantation in
multiple sclerosis. Front Neurol. 11(978)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rashmi BS and Gayathri D: Molecular
characterization of gluten hydrolysing Bacillus sp. and their
efficacy and biotherapeutic potential as probiotics using Caco-2
cell line. J Appl Microbiol. 123:759–772. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kumara SS, Gayathri D, Hariprasad P,
Venkateswaran G and Swamy CT: In vivo AFB1 detoxification by
Lactobacillus fermentum LC5/a with chlorophyll and
immunopotentiating activity in albino mice. Toxicon. 187:214–222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tamtaji OR, Kouchaki E, Salami M,
Aghadavod E, Akbari E, Tajabadi-Ebrahimi M and Asemi Z: The effects
of probiotic supplementation on gene expression related to
inflammation, insulin, and lipids in patients with multiple
sclerosis: A randomized, double-blind, placebo-controlled trial. J
Am Coll Nutr. 36:660–665. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Duscha A, Gisevius B, Hirschberg S,
Yissachar N, Stangl GI, Eilers E, Dawin E, Bader V, Haase S,
Kaisler J, et al: Proprionic acid shapes the multiple sclerosis
disease course by an immunomodulatory mechanism. Cell.
180:1067–1080. e16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wekerle H: The gut-brain connection:
Triggering of brain autoimmune disease by commensal gut bacteria.
Rheumatology (Oxford). 55 (suppl 2):ii68–ii75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Miyauchi E, Kim SW, Suda W, Kawasumi M,
Onawa S, Taguchi-Atarashi N, Morita H, Taylor TD, Hattori M and
Ohno H: Gut microorganisms act together to exacerbate inflammation
in spinal cords. Nature. 585:102–106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bernardo-Castro S, Sousa JA, Brás A,
Cecília C, Rodrigues B, Almendra L, Machado C, Santo G, Silva F,
Ferreira L, et al: Pathophysiology of blood-brain barrier
permeability throughout the different stages of ischemic stroke and
its implication on hemorrhagic transformation and recovery. Front
Neurol. 11(594672)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Buscarinu MC, Romano S, Mechelli R,
Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, Reniè R, Cerasoli
B, Morena E, Romano C, et al: Intestinal permeability in
relapsing-remitting multiple sclerosis. Neurotherapeutics.
15:68–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Braniste V, Al-Asmakh M, Kowal C, Anuar F,
Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al:
The gut microbiota influences blood-brain barrier permeability in
mice. Sci Transl Med. 6(263ra158)2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bermúdez-Morales VH, Fierros G, Lopez RL,
Martínez-Nava G, Flores-Aldana M, Flores-Rivera J and
Hernández-Girón C: Vitamin D receptor gene polymorphisms are
associated with multiple sclerosis in Mexican adults. J
Neuroimmunol. 306:20–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Püntener U, Booth SG, Perry VH and Teeling
JL: Long-term impact of systemic bacterial infection on the
cerebral vasculature and microglia. J Neuroinflammation.
9(146)2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yang L, Jama B, Wang H, Labarta-Bajo L,
Zúñiga EI and Morris GP: TCRα reporter mice reveal contribution of
dual TCRα expression to T cell repertoire and function. Proc Natl
Acad Sci USA. 117:32574–32583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li H, Limenitakis JP, Greiff V, Yilmaz B,
Schären O, Urbaniak C, Zünd M, Lawson MAE, Young ID, Rupp S, et al:
Mucosal or systemic microbiota exposures shape the B cell
repertoire. Nature. 584:274–278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Round JL and Mazmanian SK: Inducible
Foxp3+ regulatory T-cell development by a commensal bacterium of
the intestinal microbiota. Proc Natl Acad Sci USA. 107:12204–12209.
2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Haghikia A, Jörg S, Duscha A, Berg J,
Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, et al:
Dietary fatty acids directly impact central nervous system
autoimmunity via the small intestine. Immunity. 43:817–829.
2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
López-Taboada I, González-Pardo H and
Conejo NM: Western Diet: Implications for brain function and
behavior. Front Psychol. 11(564413)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Li JM, Yu R, Zhang LP, Wen SY, Wang SJ,
Zhang XY, Xu Q and Kong LD: Dietary fructose-induced gut dysbiosis
promotes mouse hippocampal neuroinflammation: A benefit of
short-chain fatty acids. Microbiome. 7(98)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Erny D, Hrabě de Angelis AL, Jaitin D,
Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T,
Jakobshagen K, Buch T, et al: Host microbiota constantly control
maturation and function of microglia in the CNS. Nat Neurosci.
18:965–977. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ghimire L, Paudel S, Jin L and Jeyaseelan
S: The NLRP6 inflammasome in health and disease. Mucosal Immunol.
13:388–398. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dombrowski Y, O'Hagan T, Dittmer M,
Penalva R, Mayoral SR, Bankhead P, Fleville S, Eleftheriadis G,
Zhao C, Naughton M, et al: Regulatory T cells promote myelin
regeneration in the central nervous system. Nat Neurosci.
20:674–680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kurashina R, Denda-Nagai K, Saba K, Hisai
T, Hara H and Irimura T: Intestinal lamina propria macrophages
upregulate interleukin-10 mRNA in response to signals from
commensal bacteria recognized by MGL1/CD301a. Glycobiology.
31:827–837. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chen H, Lin W, Zhang Y, Lin L, Chen J,
Zeng Y, Zheng M, Zhuang Z, Du H, Chen R and Liu N: IL-10 promotes
neurite outgrowth and synapse formation in cultured cortical
neurons after the oxygen-glucose deprivation via JAK1/STAT3
pathway. Sci Rep. 6(30459)2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sun S, Luo L, Liang W, Yin Q, Guo J, Rush
AM, Lv Z, Liang Q, Fischbach MA, Sonnenburg JL, et al:
Bifidobacterium alters the gut microbiota and modulates the
functional metabolism of T regulatory cells in the context of
immune checkpoint blockade. Proc Natl Acad Sci USA.
117:27509–27515. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao
J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM and Li
X: Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+
glial cells. Nat Neurosci. 16:1401–1408. 2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Averina OV, Zorkina YA, Yunes RA, Kovtun
AS, Ushakova VM, Morozova AY, Kostyuk GP, Danilenko VN and
Chekhonin VP: Bacterial metabolites of human gut microbiota
correlating with depression. Int J Mol Sci. 21(9234)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Cui Y, Miao K, Niyaphorn S and Qu X:
Production of gamma-aminobutyric acid from lactic acid bacteria: A
systematic review. Int J Mol Sci. 21(995)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Anderson G, Rodriguez M and Reiter RJ:
Multiple sclerosis: Melatonin, orexin, and ceramide interact with
platelet activation coagulation factors and gut-microbiome-derived
butyrate in the circadian dysregulation of mitochondria in glia and
immune cells. Int J Mol Sci. 20(5500)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Malinova TS, Dijkstra CD and de Vries HE:
Serotonin: A mediator of the gut-brain axis in multiple sclerosis.
Mult Scler. 24:1144–1150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Strandwitz P: Neurotransmitter modulation
by the gut microbiota. Brain Res. 1693(Pt B):128–133.
2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bonaz B, Bazin T and Pellissier S: The
vagus nerve at the interface of the microbiota-gut-brain axis.
Front Neurosci. 12(49)2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wu T, Rayner CK, Young RL and Horowitz M:
Gut motility and enteroendocrine secretion. Curr Opin Pharmacol.
13:928–934. 2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hirschberg S, Gisevius B, Duscha A and
Haghikia A: Implications of diet and the gut microbiome in
neuroinflammatory and neurodegenerative diseases. Int J Mol Sci.
20(3109)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Jhangi S, Gandhi R, Glanz B, Cook S, Nejad
P, Ward D, Li N, Gerber G, Bry L and Weiner H: Increased Archaea
species and changes with therapy in gut microbiome of multiple
sclerosis subjects (S24.001). Neurology. 82 (Suppl
10)(S24.001)2014.
|
|
82
|
Dopkins N, Nagarkatti PS and Nagarkatti M:
The role of gut microbiome and associated metabolome in the
regulation of neuroinflammation in multiple sclerosis and its
implications in attenuating chronic inflammation in other
inflammatory and autoimmune disorders. Immunology. 154:178–185.
2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ribeiro MF, Santos AA, Afonso MB,
Rodrigues PM, Sa Santos S, Castro RE, Rodrigues CMP and Solá S:
Diet-dependent gut microbiota impacts on adult neurogenesis through
mitochondrial stress modulation. Brain Commun.
2(fcaa165)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Schepici G, Silvestro S, Bramanti P and
Mazzon E: The gut microbiota in multiple sclerosis: An overview of
clinical trials. Cell Transplant. 28:1507–1527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
González-Sanmiguel J, Schuh CMAP,
Muñoz-Montesino C, Contreras-Kallens P, Aguayo LG and Aguayo S:
Complex interaction between resident microbiota and misfolded
proteins: Role in neuroinflammation and neurodegeneration. Cells.
9(2476)2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Castillo X, Castro-Obregón S,
Gutiérrez-Becker B, Gutiérrez-Ospina G, Karalis N, Khalil AA,
Lopez-Noguerola JS, Rodríguez LL, Martínez-Martínez E, Perez-Cruz
C, et al: Re-thinking the etiological framework of
neurodegeneration. Front Neurosci. 13(728)2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Fan Y and Zhang J: Dietary modulation of
intestinal microbiota: Future opportunities in experimental
autoimmune encephalomyelitis and multiple sclerosis. Front
Microbiol. 10(740)2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Camara-Lemarroy CR, Metz L, Meddings JB,
Sharkey KA and Wee Yong V: The intestinal barrier in multiple
sclerosis: Implications for pathophysiology and therapeutics.
Brain. 141:1900–1916. 2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Shahi SK, Freedman SN and Mangalam AK: Gut
microbiome in multiple sclerosis: The players involved and the
roles they play. Gut Microbes. 8:607–615. 2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mirza A, Forbes JD, Zhu F, Bernstein CN,
Van Domselaar G, Graham M, Waubant E and Tremlett H: The multiple
sclerosis gut microbiota: A systematic review. Mult Scler Relat
Disord. 37(101427)2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chu F, Shi M, Lang Y, Shen D, Jin T, Zhu J
and Cui L: Gut microbiota in multiple sclerosis and experimental
autoimmune encephalomyelitis: Current applications and future
perspectives. Mediators Inflamm. 2018(8168717)2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Miyake S, Kim S, Suda W, Oshima K,
Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, et al:
Dysbiosis in the Gut microbiota of patients with multiple
sclerosis, with a striking depletion of species belonging to
clostridia XIVa and IV clusters. PLoS One.
10(e0137429)2015.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Madonini ER: Probiotics and allergies:
Myth or reality? Eur Ann Allergy Clin Immunol. 46:196–200.
2014.PubMed/NCBI
|
|
94
|
Mcfarland LV and Dublin S: Meta-analysis
of probiotics for the treatment of irritable bowel syndrome. World
J Gastroenterol. 14:2650–2661. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Saggioro A: Probiotics in the treatment of
irritable bowel syndrome. J Clin Gastroenterol. 38 (6
Suppl):S104–S106. 2004.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kim HJ, Camilleri M, McKinzie S, Lempke
MB, Burton DD, Thomforde GM and Zinsmeister AR: A randomized
controlled trial of a probiotic, VSL#3, on gut transit and symptoms
in diarrhoea-predominant irritable bowel syndrome. Aliment
Pharmacol Ther. 17:895–904. 2003.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Abenavoli L, Scarpellini E, Colica C,
Boccuto L, Salehi B, Sharifi-Rad J, Aiello V, Romano B, De Lorenzo
A, Izzo AA and Capasso R: Gut microbiota and obesity: A role for
probiotics. Nutrient. 11(2690)2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang X, Liang Z, Wang S, Ma D, Zhu M and
Feng J: Role of gut microbiota in multiple sclerosis and potential
therapeutic implications. Curr Neuropharmacol. 20:1413–1426.
2022.PubMed/NCBI View Article : Google Scholar
|