Introduction
Wound repair is the result of multiple,
well-coordinated events, including hemostasis, inflammation,
proliferation and wound remodeling, which are associated with
various cellular and humoral mechanistic activities (1). It is well-documented that during the
proliferative phase of healing, fibroblast cell proliferation and
migration are crucial cellular mechanisms (2). Fibroblasts, which were first described
in the context of wound healing, have a role in the synthesis of
the extracellular matrix (ECM) and successful granulation tissue
formation (3). Collagen is a major
protein in the ECM, responsible for supporting cellular structure,
stability and elasticity (4). Type
I collagen, the most abundant type present in the human body,
promotes cell proliferation as well as tissue connection and
attachment (5). The synthesis of
collagen is regulated by several factors. The transforming growth
factor-β (TGF-β) signaling pathway contributes to increased
collagen biosynthesis through the phosphorylation within the Smad
signaling pathway. Upon phosphorylation of Smad2/3, it complexes
with phosphorylated (p)-Smad4. This complex subsequently
translocates to the nucleus, activating genes that govern collagen
production (6).
Wound treatment remains an important medical and
social issue. Numerous individuals worldwide suffer from injuries,
burns, ulcers and surgical wounds, the treatment of which requires
significant financial and healthcare resources. The search for
effective, affordable, easily applicable wound-healing drugs
continues to be a pressing issue in modern medicine.
Sulfated polysaccharides (SP) are highly regarded as
wound-healing compounds due to their low toxicity, excellent
degradability, biocompatibility and wide-ranging pharmaceutical
properties (7). For instance,
Eucheuma Cottonii SP, known for its antioxidant and
antibacterial activities, has proven beneficial in the
wound-healing process by preventing further tissue damage and
enhancing cell proliferation (8).
Gracilaria lemaneiformis SP demonstrated accelerated wound
healing in vitro human keratinocyte HaCaT cells due to
improved cell polarity and directed cell migration towards the
wound site (9). Furthermore,
Sanguisorba officinalis L. SP (SOSP) has been utilized for
burn wound treatment in mice. These outcomes suggest that SOSP
enhances wound healing, potentially through the promotion of
collagen synthesis (10).
Sulfated galactan (SG) cold-water extracts from Gracilaria
fisheri, an SP with a complex structure of galactose
monosaccharide residues coupled with sulfate ester, exhibit various
pharmaceutical properties, including immune regulation (11), antioxidant activity (12), antibacterial characteristics
(13) and promotion of
wound-healing (14). However, the
cellular uptake of many bioactive compounds, such as drugs, may be
limited by several factors, including their hydrophobicity and
size. Octanoyl esterification can address these limitations by
increasing the lipophilicity (fat-solubility) of the compound,
enabling improved penetration through the cell membrane (15). A previous study demonstrated that
galactose synthesized with octanoyl ester improved its accumulation
within the cell and its bioactivity (16). In addition, recent research by our
group indicated that a low molecular weight (mw) SG (LMSG) with
added octanoyl moiety (Oct-LMSG) exhibited enhanced wound healing
activity by activating cell proliferation and migration in both
in vitro scratched fibroblast studies and in vivo
excision wound rat models. The improved wound healing observed in
the scratched fibroblasts with Oct-LMSG was attributed to the
activation of proliferation and migration-related mRNA and proteins
(17). In the present study, the
hypothesis was tested that octanoyl esterification enhances the
membrane-penetrating efficacy of LMSG in fibroblast cells and
promotes collagen synthesis, which is an essential molecule in
tissue regeneration.
Materials and methods
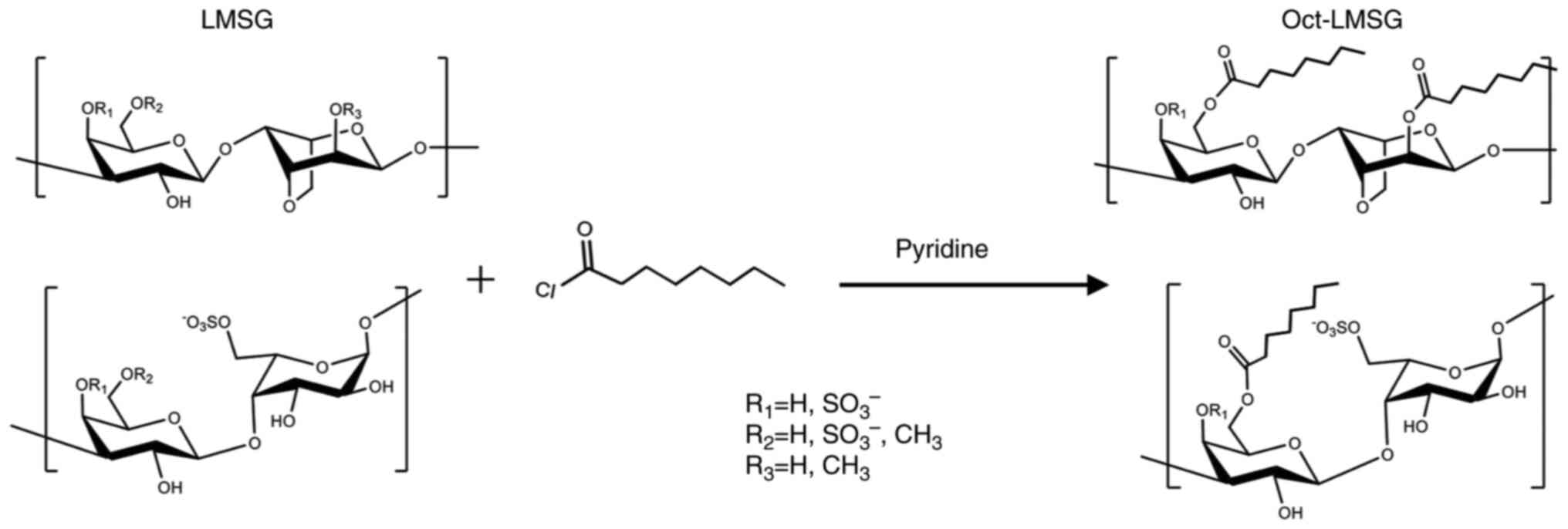
Oct-LMSG
An octanoyl moiety was added to LMSG (mw, 7.87 kDa)
to obtain Oct-LMSG following a previously described protocol
(17). The structure of Oct-LMSG,
analyzed by Fourier transform infrared and NMR spectroscopy
(Fig. S1), consists of the
alternative 3-linked β-D-galactopyranose and 4-linked
3,6-anhydro-α-L-galactopyranose or α-L-galactopyranose-6-sulfate
containing an octanoyl ester moiety (Fig. 1).
Fibroblast culture and cell
viability
L929 fibroblasts purchased from the American Type
Culture Collection were cultured in Gibco Minimum Essential Medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 2.2 g/l sodium
bicarbonate, 10% fetal bovine serum and 1% antibiotic-antimycotic
(Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator
containing 5% CO2 at 37˚C. To determine cell viability,
cells were seeded in a 96-well plate (3x104 cells/well)
and incubated with various concentrations of Oct-LMSG (10-1,000
µg/ml) for 24 and 48 h at 37˚C. Subsequently, cell viability was
measured with an MTT assay. In brief, the wells containing cells
were incubated with 10 µl of medium containing 5 mg/ml MTT (final
concentration in the well, 0.5 mg/ml) for 4 h at 37˚C in the dark.
The medium was removed and 100 µl dimethyl sulfoxide was added to
each well to solubilize formazan crystals in the cells. The
absorbance at 595 nm was measured using a Varioskan LUK multimode
microplate reader (Thermo Fisher Scientific, Inc.). The viability
of cells was expressed as a percentage of the control.
Cellular uptake study
Fibroblasts (3x105 cells/well) were
cultured overnight on poly-L-lysine-coated round-glass coverslips
in a 24-well plate and treated with LMSG and Oct-LMSG at a
concentration of 100 µg/ml for 12 h at 37˚C. Cells with no
treatment served as the control. The coverslips were washed three
times (3x10 min) with 100 µl PBS, fixed with 100 µl 4%
paraformaldehyde in PBS for 20 min and blocked with 100 µl 1% BSA
in PBS for 30 min at room temperature (RT). After washing, the
coverslips were incubated with anti-LM5 primary antibody
(monoclonal-rat IgG, specific to β-D-galactan; dilution, 1:250; cat.
no. LM5-050; Plant Probes) overnight at 4˚C, followed by incubation
with the secondary antibody, FITC-conjugated goat anti-rat IgG
(dilution, 1:500; cat. no. 31629; Thermo Fisher Scientific, Inc.),
for 1 h at RT. The cell nuclei were specifically stained with DAPI.
The cytoplasmic and plasma membranes of the cells were stained with
CellMask™ Deep Red Plasma Membrane Stain (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. Cellular uptake of
LMSG and Oct-LMSG was observed under a confocal laser-scanning
microscope (Zeiss LSM800; Carl Zeiss AG). In addition, the
fluorescent label concentration was assessed by measuring the FITC
intensity using a Varioskan LUK multimode microplate reader (Thermo
Fisher Scientific, Inc.) to further investigate the cellular uptake
of Oct-LMSG in fibroblasts.
Cell-ultrastructure evaluation
After 12 h of treatment, fibroblasts were
immediately fixed in Karnovsky's fixative for 30 min at RT. Cells
were washed with PBS, centrifuged at 1,500 x g for 1 min at 25˚C
and then post-fixed with 1% OsO4 in 0.1 M phosphate
buffer for 1 h at RT. Fixed cells were washed again and centrifuged
at 1,500 x g for 1 min at 25˚C. The collected cell pellets were
placed in a small cavity in solidified 2% agarose and filled with
2% liquefied agarose. After solidification, the agarose containing
the embedded cells was cut into small cubes and dehydrated through
a series of ethyl alcohol gradients (at 50, 70, 80, 90 and 95% for
5 min each and 100% for 10 min). The agarose cubes were gently
infiltrated with propylene oxide, followed by a mixture of
propylene oxide and Epon 812 (1:1), and a mixture of propylene
oxide and Epon 812 (1:3) for 10 min each. They were finally
infiltrated with pure Epon 812 at RT overnight. The agarose cubes
were then embedded in fresh Epon 812 and polymerized at 60˚C for 48
h. Ultra-thin sections were obtained using an Ultracut N
ultramicrotome (Reichert-Nissei; Leica Microsystems, Inc.).
Sections were stained with 5% uranyl acetate and 2% lead citrate
prior to observation under a JEM1010 transmission electron
microscope (TEM; JEM-1010; Jeol, Co., Ltd.).
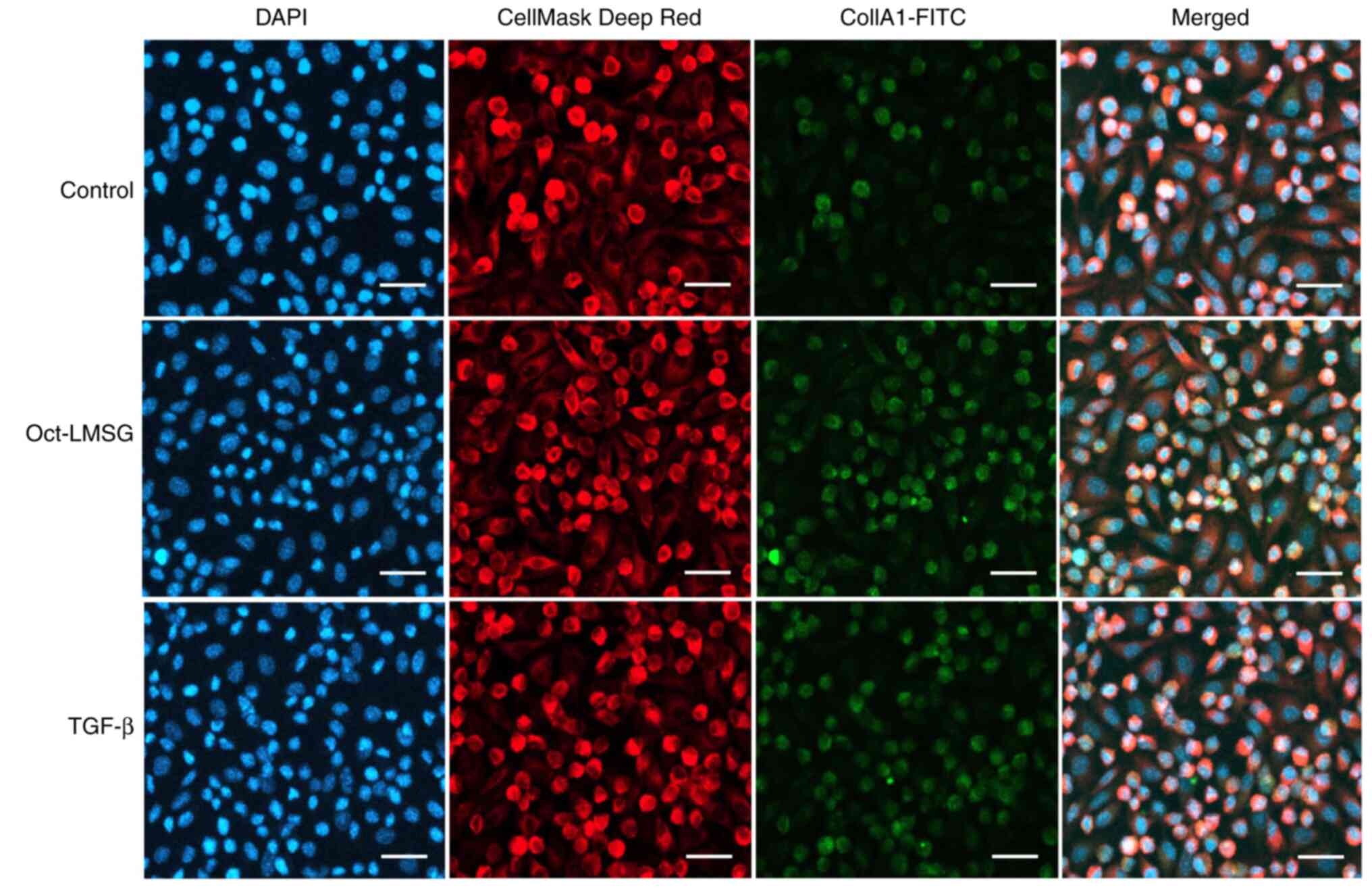
Immunofluorescence
TEM results indicated an increase in vesicles or
granules within the cytoplasm of fibroblasts. Therefore,
immunofluorescent staining for type I collagen was performed. Cells
cultured on coated round-glass coverslips were divided into three
groups: i) Normal control-cells without treatment; ii)
Oct-LMSG-cells treated with 100 µg/ml of Oct-LMSG; and iii)
TGF-β-cells treated with 10 ng/ml of TGF-β (Sigma-Aldrich; Merck
KGaA), a known pro-fibrogenic growth factor that binds with the
cell membrane receptors of fibroblasts and mediates type I collagen
biosynthesis (6). After 12 h of
treatment, cells were incubated with anti-collagen type I α1 chain
(Col1A1) antibody (dilution, 1:250; cat. no. A22089; ABclonal
Science, Inc.) overnight at 4˚C, followed by incubation with the
secondary antibody, FITC-conjugated goat anti-rabbit IgG (dilution,
1:500; cat. no. 701078; Thermo Fisher Scientific, Inc.), for 1 h at
RT. As a negative control for immunofluorescent staining, the
sample was prepared by omitting the secondary antibody. The cells
were counter-stained with DAPI (nuclei) and CellMask™ Deep Red
Plasma Membrane (cytoskeleton) according to the manufacturer's
protocols. Immunofluorescent images were observed under a
fluorescence microscope (Leica DFC 7000T; Leica Microsystems,
Inc.).
Expression of type I collagen in
fibroblasts
Fibroblasts (3x106 cells/well) were
cultured in a 6-well plate for 24 h and then divided into three
groups: i) Normal control-cells without treatment; ii)
Oct-LMSG-cells treated with 100 µg/ml Oct-LMSG; and iii)
TGF-β-cells treated with 10 ng/ml TGF-β as a positive control.
Cells were treated with an Oct-SG or TGF-β solution (2 ml) and
further incubated for 6, 12 and 24 h before being harvested for RNA
and protein extractions.
RNA was extracted from cultured cells using 200 µl
of TRI Reagent® RNA Isolation Reagent (Sigma-Aldrich;
Merck KGaA) according to the manufacturer's protocol. The
concentration and quality of RNA was measured by determining the
absorbance ratio at 260/280 nm using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA (1 µg) was
converted to cDNA using the Revert Aid First Strand cDNA Synthesis
Kit (Thermo Fisher Scientific, Inc.). Subsequently, mRNA
transcription of Col1A1, Col1A2 and GAPDH was amplified by
quantitative (q)PCR using 2 µl of cDNA with specific primers and
conditions. The qPCR conditions included initial denaturation at
95˚C for 10 min, followed by 40 cycles at 95˚C for 10 sec, 60˚C for
30 sec and 72˚C for 30 sec. Col1A1-specific primers were
5'-GAGAGGTGAACAAGGTCGCG-3' (forward) and 3'-AAACCTCTCTCGCCTCTTGC-5'
(reverse), Col1A2-specific primers were 5' CCCAGAGTGGAACAGCGATT-3'
(forward) and 3'-ATGAGTTCTTCGCTGGGGTG-5' (reverse), and
GAPDH-specific primers were 5'-GGTGAAGGTCGGTGTGAA-3' (forward) and
3'-CTCGCTCCTGGAAGATGGTG-5' (reverse) (14). The amplification and analysis were
performed on a QuantStudio™ 6 Flex Real-Time PCR System device
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The relative
gene expression in the different samples was calculated using the
2-ΔΔCq method (18). The relative mRNA transcription
levels were normalized to the GAPDH internal control and presented
as a fold-change relative to the control.
Proteins were extracted from the cells using a lysis
buffer containing 100X protease inhibitor solution (MedChemExpress,
LLC). The protein concentration was determined using a NanoDrop
2000 spectrophotometer (Thermo Fisher Scientific, Inc.). Proteins
(40 µg/lane) were then separated by 12%-SDS-PAGE and transferred to
a nitrocellulose membrane (Sigma-Aldrich; Merck KGaA). The membrane
was incubated with specific primary antibodies for Col1A1 (cat. no.
A22089; ABclonal Science, Inc.), Col1A2 (cat. no. A21059; ABclonal
Science, Inc.), Smad2/3 (cat. no. 8685; Cell Signaling Technology,
Inc.), Smad4 (cat. no. 46535; Cell Signaling Technology, Inc.),
p-Smad2/3 (cat. no. 8828; Cell Signaling Technology, Inc.) and
p-Smad4 (cat. no. AF8316; Affinity Biosciences, Inc.) at a dilution
of 1:1,000 at 4˚C overnight, followed by incubation with
HRP-conjugated secondary antibodies, anti-mouse IgG (cat. no.
62-6520; AB_2533947) and anti-rabbit IgG (cat. no. 31460;
AB_228341; Thermo Fisher Scientific, Inc.) at a dilution of 1:2,000
for 1 h at RT. Immunoreactive protein bands were visualized using
the Clarity™ Western ECL substrate (Bio-Rad Laboratories, Inc.).
The relative protein expression was normalized to β-actin as the
internal control (cat. no. AF7018; Affinity Biosciences, Inc.) and
presented as a fold-change relative to the control. Protein
expression was quantified by ImageJ 1.46r analysis software
(National Institutes of Health).
Statistical analysis
Data are presented as the mean ± standard error of
the mean from three or more independent experiments. All treated
groups were compared with the control groups. Statistical
significance was analyzed using one-way ANOVA followed by Tukey's
multiple-comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased cellular uptake of
Oct-LMSG
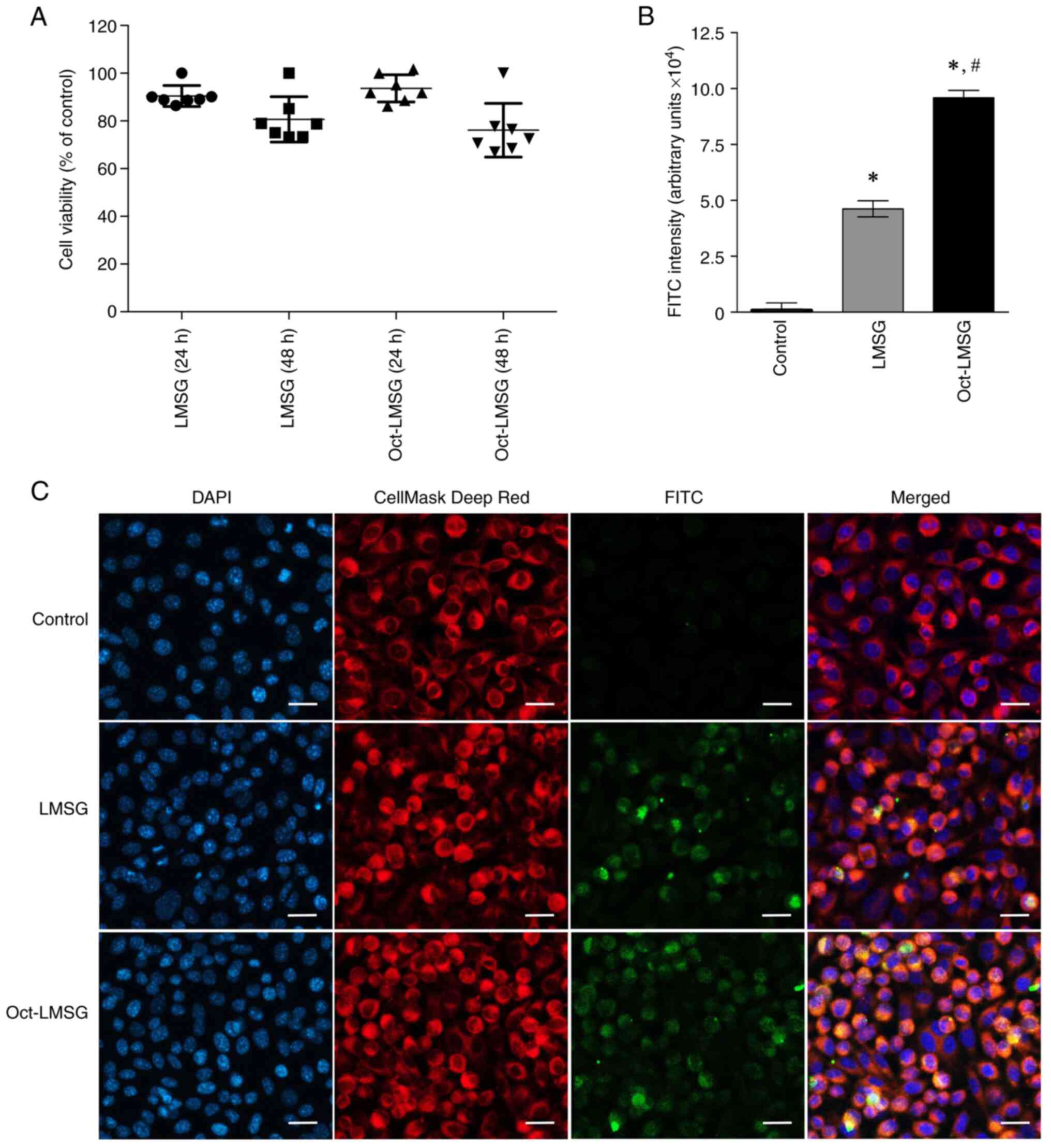
The cytotoxicity of LMSG and Oct-LMSG to
fibroblasts, as determined by the MTT assay, indicated that there
was no significant cytotoxicity, and no difference between groups
was observed after 24 and 48 h of incubation (Fig. 2A). To validate the hypothesis that
addition of the octanoyl moiety could mediate increases in LMSG
uptake into fibroblasts, an indirect immunofluorescent labelling
technique was performed and the FITC intensity was quantified. As
presented in Fig. 2C, the cells
treated with LMSG and Oct-LMSG followed by FITC labelling displayed
green fluorescence bound to the cells and localized within the
cytoplasm (orange color dispersed in the cytoplasm shown in merged
micrographs). No green fluorescence was detected in the control
cells. The FITC-LMSG fluorescence intensity of LMSG- and
Oct-LMSG-treated cells was 4.69±0.89x104 and
9.71±0.64x104 arbitrary units, respectively (Fig. 2B). Comparison between LMSG and
Oct-LMSG revealed that the introduction of the octanoyl moiety
increased the uptake of LMSG into fibroblasts, suggesting that the
cellular uptake of LMSG was enhanced by octanoyl
supplementation.
Ultrastructural changes of fibroblasts
by Oct-LMSG treatment
TEM analysis was performed to assess the ability of
Oct-LMSG to affect the ultrastructure of L929 fibroblasts. It was
noted that cultured fibroblasts in the control group exhibited a
small oval body occupied by the nucleus of a heterochromatin
structure and encircled by a small amount of cytoplasm (Fig. 3A). In addition, high-magnification
images of these controls showed that the cytoplasm was filled with
sparse vesicles containing pale particles, rough endoplasmic
reticulum (rER) and mitochondria (Fig.
3D). By contrast, fibroblasts cultured with Oct-LMSG and TGF-β
displayed primarily euchromatin nuclear features and were
surrounded by an extensive cytoplasmic medium containing abundant
vesicles, vacuoles and free ribosomes (Fig. 3B and C). Under high magnification, these
vesicles, which appeared to be dilated cisternae of the rER,
exhibited morphological differences from those of the control
group, containing dense and dark materials (Fig. 3E and F). The presence of euchromatic nuclear
features, increased rER-dilated cisternae and free ribosomes in the
cytoplasm of fibroblasts treated with Oct-LMSG indicated that
Oct-LMSG could activate fibroblasts in a similar manner to
TGF-β.
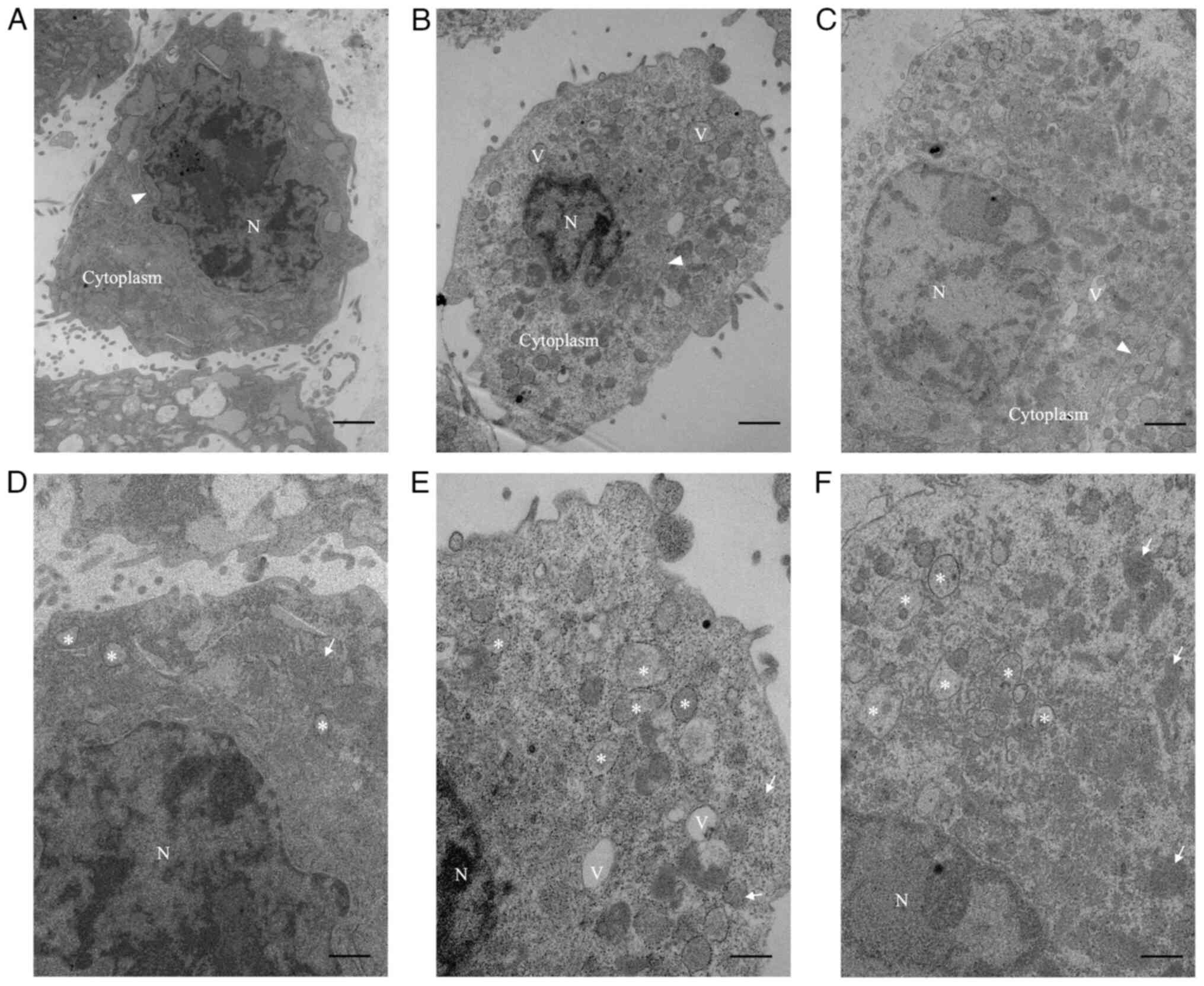 | Figure 3TEM images showing the cultured L929
fibroblasts after Oct-LMSG and TGF-β treatment. Low-magnification
TEM of (A) control fibroblasts, (B) Oct-LMSG-treated fibroblasts
and (C) TGF-β-treated fibroblasts. High-magnification TEM of (D)
control fibroblasts, (E) Oct-LMSG treated fibroblasts and (F) TGF-β
treated fibroblasts (scale bars: Low magnification, 1 µm; high
magnification, 500 nm). N, nucleus; V, vacuole; arrowhead, rough
endoplasmic reticulum; arrow, mitochondria; asterisk, vesicle;
Oct-LMSG, low molecular weight sulfated galactan added with
octanoyl moiety; TEM, transmission electron microscopy. |
Oct-LMSG enhances the synthesis of
type I collagen
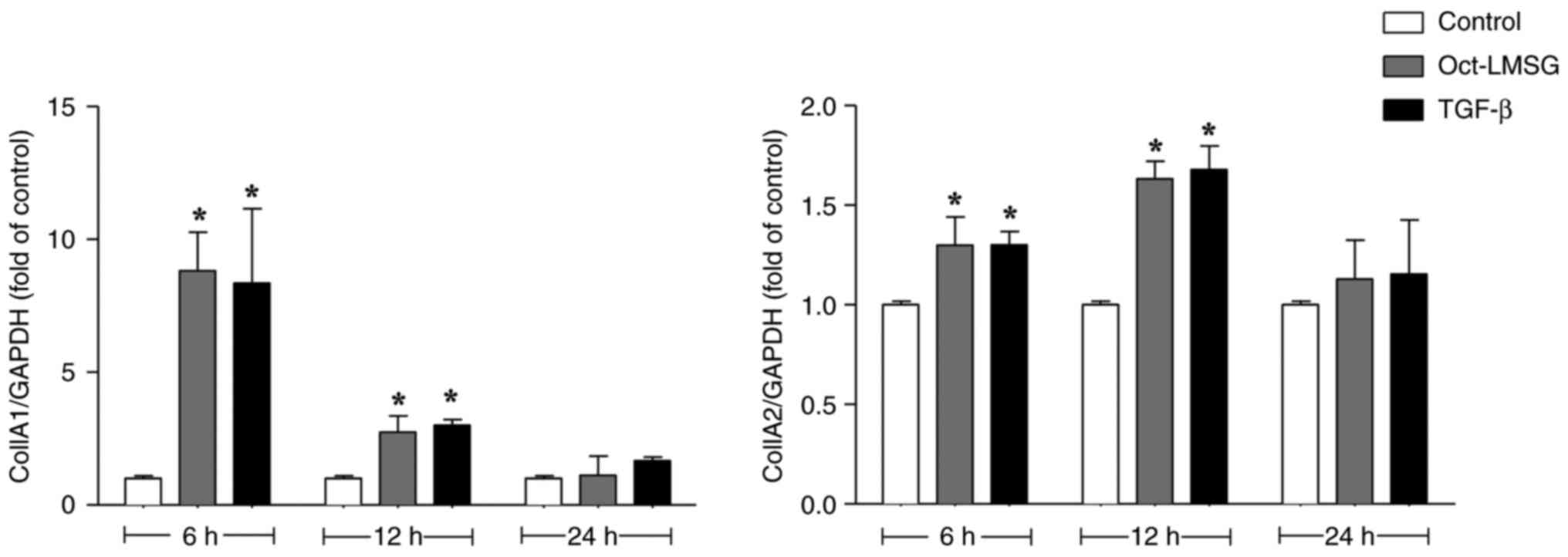
To investigate the effect of Oct-LMSG on the
synthesis of type I collagen in L929 fibroblasts, cells were
initially exposed to Oct-LMSG (100 µg/ml) for 6, 12 and 24 h. Cells
were also treated with TGF-β (10 ng/ml), a growth factor that
induces the synthesis of collagen in fibroblasts (19). Col1A1 and Col1A2 mRNA transcription
following treatment were measured via reverse transcription-qPCR
analysis. As presented in Fig. 4,
Oct-LMSG and TGF-β significantly elevated mRNA transcription levels
of Col1A1 and Col1A2. The stimulatory effect of Oct-LMSG on
collagen mRNA transcription was more pronounced at 6-12 h of
incubation and tapered off to control levels at the 24-h mark.
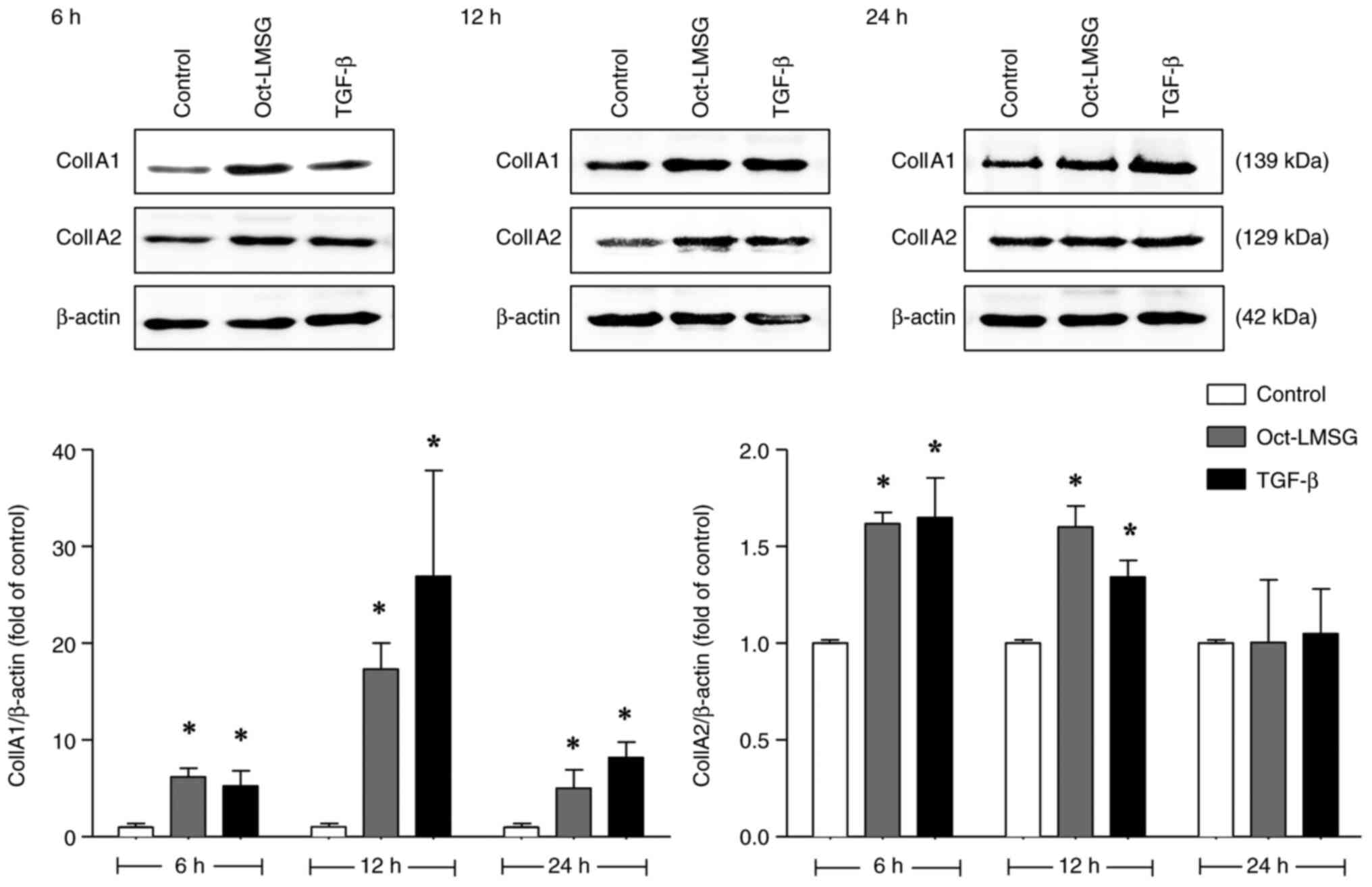
Following this, the effect of the Oct-LMSG on protein expression
was determined by western blot analysis. As with mRNA transcription
levels, exposure to Oct-LMSG increased expression of type I
collagen proteins (Col1A1 and Col1A2; Fig. 5), particularly Col1A1 protein
expression, which demonstrated a higher expression level that was
sustained throughout the first 24 h of incubation compared to the
control. In addition, Col1A1-immunofluorescent staining was
performed to confirm collagen synthesis in the fibroblasts. The
result showed heightened fluorescein activity in Oct-LMSG and
TGF-β-treated fibroblasts compared to the control (Fig. 6), indicating that increased collagen
synthesis was induced by Oct-LMSG. Taken together, these results
suggest that Oct-LMSG enhances the expression of type I collagen
in vitro.
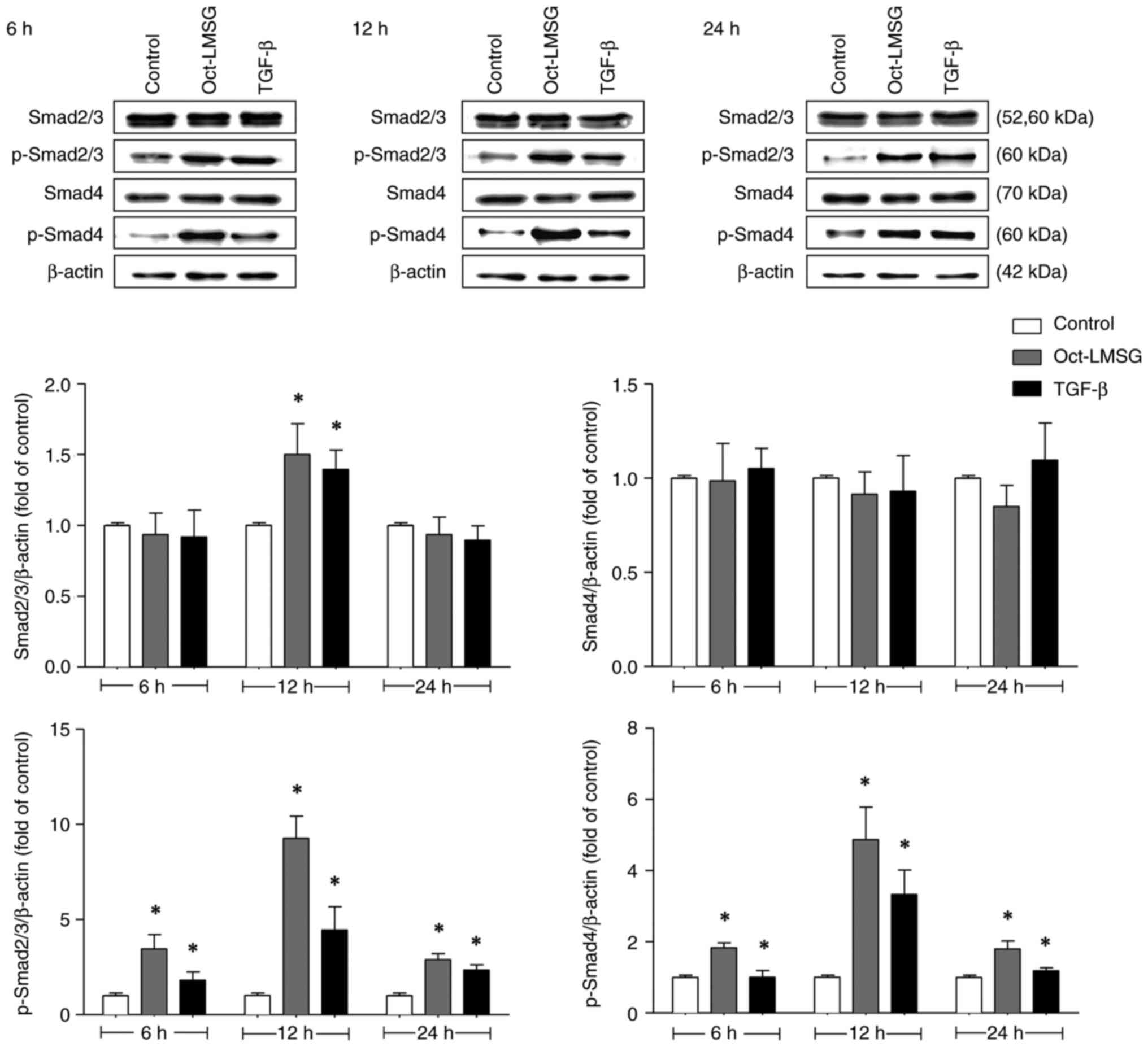
Oct-LMSG activates the phosphorylation
of Smad2/3/4 proteins in fibroblasts
Given that the TGF-β/Smad signaling pathway is
crucial in regulating Col1A1 synthesis (6), the phosphorylation status of Smad
proteins after Oct-LMSG treatment was analyzed. The results
indicated that p-Smad2/3 and p-Smad4 were upregulated after
Oct-LMSG treatment relative to controls and that the upregulated
phosphorylation had no effect on total protein levels, except for
Smad2/3 at 12 h of incubation (Fig.
7). These findings were consistent with those of fibroblasts
treated with TGF-β. Oct-LMSG significantly upregulated p-Smad2/3
levels to 3.5±0.7-fold of the control at 6 h, 9.3±1.2-fold of
control at 12 h and 2.9±0.3-fold of the control at 24 h of
incubation. Concurrently, p-Smad4 levels also increased
significantly with Oct-LMSG treatment to 1.9±0.1-fold of the
control at 6 h, 4.9±0.9-fold of the control at 12 h and
1.8±0.2-fold of the control at 24 h of incubation. These results
indicated that Oct-LMSG enhances type I collagen synthesis, at
least in part, mediated by the phosphorylation of the Smad
signaling pathway in fibroblasts.
Discussion
Wound healing is a complex process that involves the
interaction of cells, the extracellular matrix (ECM) and numerous
growth factors. Collagen fibers have an important role in wound
repair by recruiting fibroblasts and promoting the deposition of
new collagen in the injured tissues (20). Increased collagen expression is
known to significantly enhance tissue recovery and appearance
(21). Several studies have shown
that SPs derived from algae, such as Hizikia fusiforme
(22), Fucus vesiculosus
(23) and Gracilaria fisheri
(14), promote type I collagen
synthesis and facilitate tissue healing. Furthermore, it has been
observed that galactose conjugated with octanoyl ester markedly
enhances cellular uptake and bioactivity (16). A recent study by our group reported
that Oct-LMSG was able to enhance wound healing activity both in
vitro and in vivo by stimulating the proliferation and
migration of fibroblasts (17).
However, it has remained elusive whether Oct-LMSG can penetrate the
plasma membrane and has a potential role in promoting type I
collagen synthesis in fibroblasts. Therefore, the aim of the
present study was to investigate the ability of Oct-LMSG to
permeate the plasma membrane and regulate the expression of type I
collagen mRNA and protein in L929 fibroblast cultures. The
underlying signaling pathway of Oct-LMSG in promoting collagen
synthesis was also unraveled.
Conjugation of polysaccharides with delivery agents
has been shown to enhance their pharmacological properties by
increasing cellular uptake. For instance, in Hela cells, enhanced
cellular uptake was observed when SPs from Codium fragile
were conjugated with a selective drug delivery agent, folic acid
(24). Similarly, the cellular
uptake of hyaluronic acid (HA) was significantly increased in lung
cancer cells when HA oligosaccharides were conjugated to a
polyethylene glycol-phospholipid (25). The impact of medium-chain fatty acid
conjugated polysaccharides on cellular uptake has also been
demonstrated. Modification of the coix seed component microemulsion
(C-ME) with Oct-galactose ester significantly increased C-ME uptake
in HepG2 cells, suggesting that the galactose ester with medium
chain fatty acids provided a significant advantage in cellular
uptake (16). This finding aligns
with the present study, which demonstrated that Oct-LMSG increases
cellular uptake into L929 fibroblasts. Furthermore, Oct-LMSG
exhibited green fluorescence on the cell surface, suggesting that
LMSG probably interacted or bound to protein receptors on the cell
membrane (26). However, the
interaction between LMSG and protein receptors on the fibroblast
cell membrane warrants further investigation.
In the present study, TEM was performed to
investigate the impact of Oct-LMSG on the ultrastructure of
fibroblasts. The findings demonstrated that fibroblasts cultured
with Oct-LMSG exhibited changes in cytoplasmic organelles. These
changes included a prominent euchromatic nucleus, dilated cisternae
of the rER and increased presence of free ribosomes. These
alterations are typically associated with active fibroblasts.
Previous studies have documented that active fibroblasts can be
identified under TEM by their abundant rER, free ribosomes and
prominent Golgi apparatus, all indicative of cells involved in
protein synthesis (27). They also
exhibited large and irregular nuclei with abundant euchromatin
(28). These characteristics align
with earlier research (29)
indicating that the calcium hydroxylapatite filler affected the
cytoplasmic organelles of fibroblasts, particularly the rER
profiles, which consist of ribosome-bound membranes that often form
dilated cisternae filled with electron-dense, finely filamentous
material. These alterations in the cytoplasm suggest fibroblast
activation, leading to the production of molecular precursors of
fibrillar components, primarily collagen, for the ECM (29). The specific changes in the
ultrastructural features in the present study support the
hypothesis that Oct-LMSG activates fibroblasts. Furthermore, TEM
images of cultured fibroblasts treated with Oct-LMSG revealed the
presence of cytoplasmic vacuoles, suggesting that the compound may
be internalized through either endocytosis or macropinocytosis
(19).
Collagen, a major protein in the ECM produced by
fibroblasts, has a crucial role in providing structural support,
strength and elasticity to tissues (4). Among the different types of collagens,
type I collagen is the most abundant in the human body and is
composed of the Col1A1 and Col1A2 proteins (30). A previous study indicated that
Col1A1 is predominantly expressed in fibroblasts, with a higher
production ratio compared to Col1A2 (ratio, 2:1) (31). In the present study, the stimulatory
effect of Oct-LMSG on the mRNA and protein levels of both Col1A1
and Col1A2 was confirmed, particularly on the significant increase
in Col1A1 at 12 h compared to the control. This finding indicates
that Oct-LMSG sustains the stimulation of type I collagen
expression. In a previous study by our group, it was demonstrated
that increased collagen production by SG was a result of elevated
Col1A1 mRNA expression (14). The
findings from our group are consistent with other studies, which
have shown enhanced Col1A1 mRNA levels and collagen type I protein
synthesis in Hs27 human dermal fibroblasts treated with emodin for
12 h (32). In addition, treatment
of SPs from Hizikia fusiforme improved collagen synthesis
and protected against UVB-induced collagen degradation (22). However, these findings suggest that
collagen biosynthesis may vary depending on the cell type and
culture conditions.
The TGF-β/Smad signaling pathway has a role in
increasing collagen production by inducing the phosphorylation of
Smad2 and Smad3. When Smad2/3 is phosphorylated, it forms a complex
with p-Smad4, which then moves into the nucleus to activate target
genes responsible for producing procollagen, such as Col1A1 and
Col1A2(6). In the present study,
the effects of Oct-LMSG on type I collagen through the TGF-β/Smad
signaling pathway were compared with those of exogenous TGF-β
protein and it was found that Oct-LMSG can penetrate fibroblast
cells and activate the TGF-β/Smad signaling pathway by
phosphorylation. Several studies have indicated that various
compounds enhance type I collagen expression through the TGF-β/Smad
signaling pathway, such as Pyropia yezoensis, a marine
seaweed (33), and poly-L-lactic
acid (34). Furthermore, the
present data indicate that Oct-LMSG specifically activates Smad2/3
and Smad4 by causing their phosphorylation, leading to increased
expression of Col1A1 and Col1A2. As a result, Oct-LMSG shows
potential as a candidate for promoting type I collagen synthesis in
the pharmaceutical and cosmetic industries.
In conclusion, in the present study, it was
demonstrated that Oct-LMSG had significantly enhanced cellular
uptake efficacy as compared with LMSG in L929 fibroblasts.
Furthermore, Oct-LMSG was found to increase the expression of type
I collagen mRNA and protein by activating the phosphorylation of
the Smad signaling pathway. These findings suggest that Oct-LMSG
can be used to stimulate collagen synthesis. However, wound healing
is a complex process related to numerous systemic cellular and
humoral actions. The effects of Oct-LMSG on different cell types
require further investigation.
Supplementary Material
(A) Fourier-transform infrared
spectral analysis of Oct-LMSG, (B) 1H-NMR spectral
analysis of Oct-LMSG and (C) 13C-NMR spectral analysis
of Oct-LMSG. Oct-LMSG, low molecular weight sulfated galactan added
with octanoyl moiety.
Acknowledgements
The authors would like to acknowledge Dr Dylan
Southard from the KKU Publication Clinic (Thailand) for editing the
manuscript.
Funding
Funding: This work was financially supported by the Office of
the Permanent Secretary at the Ministry of Higher Education,
Science, Research and Innovation and the Thailand Science Research
and Innovation (grant no. RGNS 64-044), the National Research
Council of Thailand (NRCT; grant no. N42A650206) and the Khon Kaen
University Faculty of Medicine Invitation Research fund (grant no.
IN66028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, KJ, KW, JK and TR conceived and designed the
study. WS, KJ and TR performed the experiments. WS, KJ, JEA, SS,
KW, JK and TR were responsible for the analysis of data. WS, KJ,
JEA, SS and TR participated in the drafting of the manuscript. KW,
JK and TR edited and finalized the draft of the manuscript. KW and
JK supervised the study. TR provided funding and project
administration. WS, KJ and TR confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilkinson HN and Hardman MJ: Wound
healing: cellular mechanisms and pathological outcomes. Open Biol.
10(200223)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Addis R, Cruciani S, Santaniello S, Bellu
E, Sarais G, Ventura C, Maioli M and Pintore G: Fibroblast
proliferation and migration in wound healing by phytochemicals:
Evidence for a novel synergic outcome. Int J Med Sci. 17:1030–1042.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diller RB and Tabor AJ: The role of the
extracellular matrix (ECM) in wound healing: A review. Biomimetics.
7(87)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Araújo R, Lôbo M, Trindade K, Silva DF
and Pereira N: Fibroblast growth factors: A controlling mechanism
of skin aging. Skin Pharmacol Physiol. 32:275–282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Irawan V, Sung TC, Higuchi A and Ikoma T:
Collagen scaffolds in cartilage tissue engineering and relevant
approaches for future development. Tissue Eng Regen Med.
15:673–697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xue N, Liu Y, Jin J, Ji M and Chen X:
Chlorogenic acid prevents UVA-induced skin photoaging through
regulating collagen metabolism and apoptosis in human dermal
fibroblasts. Int J Mol Sci. 23(6941)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kang J, Jia X, Wang N, Xiao M, Song S, Wu
S, Li Z, Wang S, Cui SW and Guo Q: Insights into the
structure-bioactivity relationships of marine sulfated
polysaccharides: A review. Food Hydrocoll. 123(107049)2022.
|
|
8
|
Teo BSX, Gan RY, Abdul Aziz S, Sirirak T,
Mohd Asmani MF and Yusuf E: In vitro evaluation of antioxidant and
antibacterial activities of Eucheuma Cottonii extract and its in
vivo evaluation of the wound-healing activity in mice. J Cosmet
Dermatol. 20:993–1001. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Veeraperumal S, Qiu HM, Zeng SS, Yao WZ,
Wang BP, Liu Y and Cheong KL: Polysaccharides from Gracilaria
lemaneiformis promote the HaCaT keratinocytes wound healing by
polarized and directional cell migration. Carbohydr Polym.
241(116310)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang H, Chen J and Cen Y: Burn wound
healing potential of a polysaccharide from Sanguisorba officinalis
L. in mice. Int J Biol Macromol. 112:862–867. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wongprasert K, Rudtanatip T and Praiboon
J: Immunostimulatory activity of sulfated galactans isolated from
the red seaweed Gracilaria fisheri and development of resistance
against white spot syndrome virus (WSSV) in shrimp. Fish Shellfish
Immunol. 36:52–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rudtanatip T, Pariwatthanakun C, Somintara
S, Sakaew W and Wongprasert K: Structural characterization,
antioxidant activity, and protective effect against hydrogen
peroxide-induced oxidative stress of chemically degraded Gracilaria
fisheri sulfated galactans. Int J Biol Macromol. 206:51–63.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rudtanatip T, Boonsri N, Asuvapongpatana
S, Withyachumnarnkul B and Wongprasert K: A sulfated galactans
supplemented diet enhances the expression of immune genes and
protects against Vibrio parahaemolyticus infection in shrimp. Fish
Shellfish Immunol. 65:186–197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pariwatthanakun C, Rudtanatip T, Boonsri
B, Pratoomthai B and Wongprasert K: In vitro evaluation of wound
healing potential of sulfated galactans from red alga Gracilaria
fisheri in fibroblast cells. Songklanakarin J Sci Technol.
43:1374–1381. 2021.
|
|
15
|
Layek B and Singh J: N-hexanoyl,
N-octanoyl and N-decanoyl chitosans: Binding affinity, cell uptake,
and transfection. Carbohydr Polym. 89:403–410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qu D, Liu M, Huang M, Wang L, Chen Y, Liu
C and Liu Y: Octanoyl galactose ester-modified microemulsion system
self-assembled by coix seed components to enhance tumor targeting
and hepatoma therapy. Int J Nanomed. 12:2045–2059. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rudtanatip T, Somintara S, Sakaew W,
El-Abid J, Cano ME, Jongsomchai K, Wongprasert K and Kovensky J:
Sulfated galactans from Gracilaria fisheri with supplementation of
octanoyl promote wound healing activity in vitro and in vivo.
Macromol Biosci. 22(e2200172)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rakshit M, Gautam A, Toh LZ, Lee YS, Lai
HY, Wong TT and Ng KW: Hydroxyapatite particles induced modulation
of collagen expression and secretion in primary human dermal
fibroblasts. Int J Nanomed. 15:4943–4956. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chattopadhyay S and Raines RT: Review
collagen-based biomaterials for wound healing. Biopolymers.
101:821–833. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ganceviciene R, Liakou AI, Theodoridis A,
Makrantonaki E and Zouboulis CC: Skin-anti-aging strategies.
Dermatoendocrinol. 4:308–319. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang L, Lee W, Oh JY, Cui YR, Ryu B and
Jeon YJ: Protective effect of sulfated polysaccharides from
celluclast-assisted extract of Hizikia fusiforme against
ultraviolet B-induced skin damage by regulating NF-kB, AP-1, and
MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar
Drugs. 16(239)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Song YS, Li H, Balcos MC, Yun HY, Baek KJ,
Kwon NS, Choi HR, Park KC and Kim DS: Fucoidan promotes the
reconstruction of skin equivalents. Korean J Physiol Pharmacol.
18:327–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li CS, Palanisamy S, Talapphet N, Cho M
and You SG: Preparation and characterization of folic acid
conjugated sulfated polysaccharides on NK cell activation and
cellular uptake in Hela cells. Carbohydr Polym.
254(117250)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cano ME, Lesur D, Bincoletto V, Gazzano E,
Stella B, Riganti C, Arpicco S and Kovensky J: Synthesis of defined
oligohyaluronates-decorated liposomes and interaction with lung
cancer cells. Carbohydr Polym. 248(116798)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rudtanatip T, Withyachumnarnkul B and
Wongprasert K: Sulfated galactans from Gracilaria fisheri bind to
shrimp haemocyte membrane proteins and stimulate the expression of
immune genes. Fish Shellfish Immunol. 47:231–238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
McAnulty RJ: Fibroblasts and
myofibroblasts: Their source, function and role in disease. Int J
Biochem Cell Biol. 39:666–671. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Repiskà V, Varga I, Lehocký I, Böhmer D,
Blaško M, Polák Š, Adamkov M and Danišovič Ľ: Biological and
morphological characterization of human neonatal fibroblast cell
culture B-HNF-1. Biologia. 65:919–924. 2010.
|
|
29
|
Zerbinati N, D'Este E, Parodi PC and
Calligaro A: Microscopic and ultrastructural evidences in human
skin following calcium hydroxylapatite filler treatment. Arch
Dermato Res. 309:389–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Martinez-Lozano E, Beeram I, Yeritsyan D,
Grinstaff MW, Snyder BD, Nazarian A and Rodriguez EK: Management of
arthrofibrosis in neuromuscular disorders: A review. BMC
Musculoskelet Disord. 23(725)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sawamura S, Makino K, Ide M, Shimada S,
Kajihara I, Makino T, Jinnin M and Fukushima S: Elevated alpha 1(I)
to alpha 2(I) collagen ratio in dermal fibroblasts possibly
contributes to fibrosis in systemic sclerosis. Int J Mol Sci.
23(6811)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Song P, Jo HS, Shim WS, Kwon YW, Bae S,
Kwon Y, Azamov B, Hur J, Lee D, Ryu SH and Yoon JH: Emodin induces
collagen type I synthesis in Hs27 human dermal fibroblasts. Exp
Ther Med. 21(420)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim CR, Kim YM, Lee MK, Kim IH, Choi YH
and Nam TJ: Pyropia yezoensis peptide promotes collagen synthesis
by activating the TGF-β/Smad signaling pathway in the human dermal
fibroblast cell line Hs27. Int J Mol Med. 39:31–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu W and Dong C: Poly-L-Lactic acid
increases collagen gene expression and synthesis in cultured dermal
fibroblast (Hs68) through the TGF-β/Smad pathway. J Cosmet
Dermatol. 22:1213–1219. 2023.PubMed/NCBI View Article : Google Scholar
|