Introduction
Postoperative cognitive dysfunction (POCD) is a
common postoperative complication observed in elderly patients
following anesthesia and surgery (1,2). POCD
is often characterized by memory loss and reduced cognitive
dysfunction (3,4). Results of previous studies
demonstrated that hippocampal neuroinflammation induced by
anesthesia or surgery is a leading contributor to POCD (5-7).
Dexmedetomidine (Dex) is a highly selective α2-receptor agonist,
which is commonly used as an adjuvant in general anesthesia
(8,9). Notably, Dex possesses
anti-inflammatory and neuroprotective properties, thereby improving
POCD (10). Glumac et al
(11) reported that preoperative
administration of Dex was able to reduce the risk of POCD after
surgery by ameliorating the inflammatory response. Results of our
previous study reported that Dex attenuates neuroinflammation in
BV2 cells treated with LPS through targeting the microRNA
(miR)-340/NF-κB axis (12).
However, the molecular mechanisms by which Dex improves POCD remain
unclear.
Long non-coding RNAs (lncRNAs) are a class of
non-coding RNA molecules that are >200 nucleotides in length
(13). Results of a previous study
demonstrated that lncRNAs play critical roles in the development of
POCD (14). For example, Yu et
al (15) reported that lncRNA
Rian attenuates sevoflurane anesthesia-induced cognitive
dysfunction in mice by regulating the miR-143/LIMK1 axis. Wei et
al (16) indicated that lncRNA
NONMMUT055714 attenuates cognitive impairment in POCD mice by
targeting miR-7684. Moreover, Deng et al (17) demonstrated that Dex treatment
improves POCD in rats by modulating lncRNA LOC102546895, suggesting
that lncRNA may be involved in the protective effects of Dex
against POCD. Notably, lncRNA small nucleolar RNA host gene 14
(SNHG14) promotes neurological impairment and inflammatory
responses in rats following cerebral ischemia/reperfusion injury
(18). Zhang et al (19) revealed that SNHG14 downregulation
inhibits inflammatory responses in BV2 cells exposed to
oxygen-glucose deprivation. However, the role of SNHG14 in POCD has
not been widely studied. Data obtained using the StarBase
bioinformatics tool demonstrated that miR-340 exhibits a
complementary sequence to SNHG14(20). Moreover, Zhang et al
(20) revealed a potential
interaction between miR-340 and SNHG14.
Thus, the present study aimed to explore the role of
Dex in POCD in vitro and in vivo. To investigate
these aims, a mouse model of POCD was established to explore the
impact of Dex treatment on sevoflurane anesthesia-induced
neuroinflammation in aged mice. Meanwhile, LPS-stimulated BV2 cells
were used to mimic neuroinflammation to explore the impact of Dex
in LPS-induced neuroinflammation in vitro. Additionally, the
present study also aimed to determine the potential interaction
with the SNHG14/miR-340/NF-κB axis. These results may provide new
insights into the mechanisms underlying Dex in the treatment of
POCD.
Materials and methods
Cell culture and transfection
Mouse microglial BV2 cells were obtained from
Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd. BV2 cells were
cultured in Minimum Essential Medium (MEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin at 37˚C in 5%
CO2.
MiR-340 mimics (50 nM; 5'-UUAUAAAGCAAUGAGACUGAUU-3')
and mimics negative control (50 nM; NC;
5'-UUGUACUACACAAAAGUACUG-3'), miR-340 inhibitor, (100 nM;
5'-AAUCAGUCUCAUUGCUUUAUAA-3') and inhibitor NC (100 nM;
5'-UUCUCCGAACGUGUCACGUAA-3') were obtained from Guangzhou RiboBio
Co., Ltd. Small interfering (si)RNA SNHG14 (si-SNHG14) were
purchased from RiboBio Co., Ltd., and the sequences were as
follows: Forward, 5'-GCUGAUAUUUAAGGCACUATT-3', and reverse,
5'-UAGUGCCUUAAAUAUCAGCTT-3'. A total of 2x105 BV2 cells
were plated into six-well plates overnight at 37˚C, and then cells
were transfected with mimics NC (50 nM), miR-340 mimics (50 nM) or
si-SNHG14 (50 nM) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 6 h at 37˚C. Next, the culture
medium was replaced with fresh MEM containing FBS, cells were then
cultured for 24 h at 37˚C.
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was performed to assess SNHG14 and miR-340
expression levels in BV2 cells or in hippocampal tissues of mice.
Total RNA was extracted from cells using TRIzol® reagent
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. miRNA was reverse transcribed using the miScript II RT
kit (Qiagen GmbH) according to the manufacturer's instructions, and
qPCR experiments were carried out using a miScript SYBR Green PCR
kit (Qiagen GmbH). In addition, reverse transcription of other
genes was performed using the ReverTra Ace qPCR RT kit (Toyobo Life
Science), and qPCR experiments were carried out using the Power
SYBR Green PCR Master Mix (Vazyme Biotech Co., Ltd.). The cycling
parameters were as follows: Denaturation at 95˚C for 5 min,
followed by 45 cycles consisting for 15 sec at 94˚C, 30 sec at 55˚C
and 30 sec at 70˚C. Expression levels were quantified using the
2-ΔΔCq method (21) and normalized to the internal
reference gene U6 or β-actin. The following primer pairs were used
for qPCR: SNHG14 forward, 5'-CGTTGTCGAAAGCTAAAAGGA-3', and reverse,
5'-TGTTTCCATCTCACCAAATGC-3'; β-actin forward,
5'-CTGGAACGGTGAAGGTGACA-3', and reverse,
5'-CGGCCACATTGTGAACTTTG-3'; miR-340 forward,
5'-GTGGGGTTATAAAGCAATGAGA-3', and reverse, 5'-GTGCAGGGTCCGAGGT-3';
U6 forward, 5'-CTCGCTTCGGCAGCACAT-3', and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
Dual-luciferase reporter assay
StarBase bioinformatics tool (http://starbase.sysu.edu.cn) was used to predict the
potential targets of SNHG14. To determine the relationship between
SNHG14 and miR-340 in BV2 cells, dual-luciferase reporter assay was
conducted. The putative miR-340-3p binding sequences of wild-type
(WT) or mutant (MUT) SNHG14 were amplified and subcloned into
pGL6-miR-based luciferase reporter plasmids (Beyotime Institute of
Biotechnology). Subsequently, the aforementioned plasmids were
co-transfected into BV2 cells with miR-340 mimics or NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Relative luciferase activity was measured using
a Dual-Luciferase Reporter Assay System (Promega Corporation) at 48
h. Renilla luciferase activity was used as a control.
Western blotting
The protein was collected from cells and tissues
using the RIPA buffer (Sangon Biotech Co., Ltd.). Total protein
concentration was quantified using a BCA protein assay kit (Sangon
Biotech Co., Ltd.) and 20 µg/lane was separated via SDS-PAGE on a
10% gel. The separated proteins were subsequently transferred onto
a PVDF membrane, and membranes were blocked with 5% non-fat milk in
TBST (TBS with 0.1% Tween-20) for 1 h, and then incubated with
antibodies against NF-kB p65 (1:2,000; cat. no. ab32536; Abcam) and
GAPDH (1:2,000; cat. no. 60004-1-1; ProteinTech Group, Inc.) at 4˚C
overnight. Following primary incubation, membranes were incubated
with the HRP-conjugated goat anti-mouse secondary antibody
(1:1,000; cat. no. A0216; Beyotime Institute of Biotechnology).
Protein bands were visualized using the Pierce ECL Plus Western
Blotting Substrate (Teasen), and protein expression was quantified
using Image-Pro Plus software (v6.0; Media Cybernetics).
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was applied to analyze the levels of cytokines
in the supernatant of BV2 cells and in the hippocampus from mice.
Mouse TNF-α ELISA kit (cat. no. ab208348; Abcam), mouse IL-1β ELISA
kit (cat. no. ab197742; Abcam), mouse IL-6 ELISA kit (cat. no.
ab222503; Abcam), mouse IL-2 ELISA kit (cat. no. ELK1150; ELK
Biotechnology), mouse IL-8 ELISA kit (cat. no. EM30328S; Shanghai
Weiao Biotechnology Co., Ltd.) and mouse IL-12 ELISA kit (cat. no.
ELK9395; ELK Biotechnology) were used to detect the levels of
TNF-α, IL-1β, IL-6, IL-2, IL-8 and IL-12 in cell culture
supernatants, according to the manufacturers' protocols.
Animal experiments
The Ethics Committee of Jiading District Central
Hospital Affiliated Shanghai University of Medicine & Health
Sciences approved the animal experiments (approval no. 20190018). A
total of 50 healthy aged C57BL/6 male mice (age, 18 months; 28-32
g) were obtained from the SPF (Beijing) Biotechnology Co., Ltd.
(animal license no. SCXK (Beijing) 2019-0010), and housed in a
specific pathogen-free environment (23±2˚C and 55±5% humidified
atmosphere) under a 12/12 h light-dark cycle with free access to
food/water in accordance with the recommended procedures of The
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. Animals were randomly divided into 5 groups
(n=10/group) as follows: Control, POCD, POCD + Dex, POCD + miR-340
inhibitor and POCD + miR-340 inhibitor + Dex groups. In POCD
groups, mice were anesthetized using an anesthesia machine (R500;
RWD Life Technologies Inc.) with sevoflurane (induction dose, 3%;
maintenance dose, 2%). Subsequently, a 1.5-cm longitudinal incision
was created along the midline of the abdomen. The abdomen was
explored for 5 min, and the incision was closed using 5-0 and 4-0
sterile surgical sutures. Mice in the Dex treatment groups were
intravenously treated with Dex (25 µg/kg) 30 min prior to POCD
surgery. Mice in the miR-340 inhibitor groups were injected with a
miR-340 inhibitor in the hippocampus 48 h prior to the POCD
operation. Animals in the control group were not treated. After 48
h of operation, all mice were sacrificed using CO2 at a
displacement rate of 40% volume/min, and hippocampus tissues were
collected.
Hematoxylin & Eosin (H&E)
staining analysis
Hippocampus tissues were fixed in 4%
paraformaldehyde overnight at 4˚C and then embedded in paraffin and
sliced into 4-µm thick sections. Subsequently, sections were
stained with hematoxylin reagent for 8 min and then stained with
eosin reagent for 3 min at room temperature and examined under a
light microscope.
Morris water maze (MWM)
The MWM test was used to evaluate spatial learning
and memory. MWM equipment (Chengdu Techman Technology Co., Ltd.)
includes a round pool with a diameter of 150-cm that is divided
into four quadrants. A movable circular platform (diameter, 9-cm)
was placed 2-cm below the water surface in the fourth quadrant.
During the experiment, mice entered the water facing the wall at
each of the four starting locations and were trained to locate the
hidden platform. If mice were unable to board the platform in 120
sec, rats were guided to the platform and the latency period was
noted as 120 sec. A camera attached to a computer system (Chengdu
Techman Technology Co., Ltd.) was used to record the trajectory of
the mice. Following examination for 5 days, the platform was
removed on Day 6. Animals were released into the water and the
escape latency was recorded.
Statistical analysis
All experiments were repeated at least three times
independently. Data are presented as the mean ± standard deviation
(SD). Data were analyzed using GraphPad Prism software (version
7.0; GraphPad Software, Inc.; Dotmatics). One-way analysis of
variance (ANOVA) followed by Tukey's post hoc tests were used to
determine the differences between three or more groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Dex attenuates neuroinflammation in
LPS-treated BV2 cells by regulating the SNHG14/miR-340/NF-κB
axis
To investigate whether the SNHG14/miR-340/NF-κB axis
is involved in the development of POCD, the StarBase bioinformatics
tool was used to predict whether miR-340 is a potential target of
SNHG14. The data indicated that miR-340 had a complementary
sequence to SNHG14 (Fig. 1A). In
addition, results of the present study demonstrated that
transfection with miR-340 mimics significantly reduced the
luciferase activity of the SNHG14-WT vector in BV2 cells compared
with the SNHG14-WT + mimics NC group (Fig. 1B). Collectively, these results
demonstrated that SNHG14 exhibits potential as a target of
miR-340.
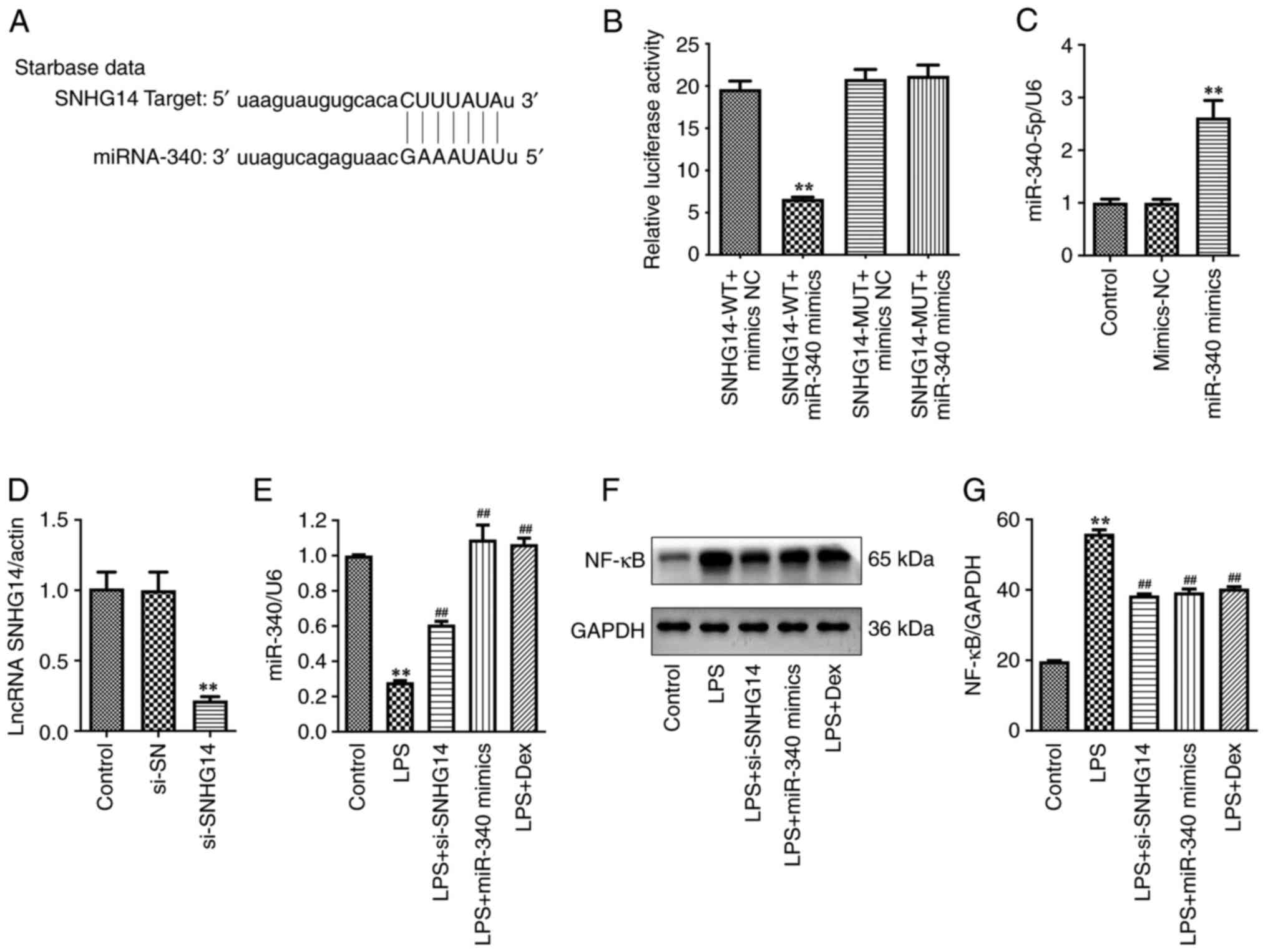 | Figure 1MiR-340 is a direct target of SNHG14
in BV2 cells. (A) Putative binding sites of miR-340 on SNHG14. (B)
Luciferase assay of BV2 cells transfected with SNHG14-WT or
SNHG14-MUT reporter together with miR-340. (C) RT-qPCR was applied
to measure miR-340 level in BV2 cells transfected with miR-340
mimics. (D) SNHG14 level in si-SHNG14-transfected BV2 cells was
detected using RT-qPCR. (E) BV2 cells were transfected with miR-340
mimics and si-SHNG14 for 24 h, and then treated with Dex for 30
min, followed by stimulation with 10 µg/ml LPS treatment for 24 h.
The miR-340 level in BV2 cells was detected using RT-qPCR. (F and
G) Western blotting was used to determine NF-κB expression in BV2
cells. **P<0.01 vs. control group;
##P<0.01 vs. LPS group. MiR, microRNA; SNHG14, small
nucleolar RNA host gene 14; WT, wild-type; MUT, mutated; RT-qPCR,
reverse transcription-quantitative PCR; si, small interfering; Dex,
dexmedetomidine; LPS, lipopolysaccharide; lncRNA, long non-coding
RNA. |
Moreover, transfection with the miR-340 mimics
notably elevated the expression levels of miR-340 in BV2 cells
compared with the control group, which was confirmed using an
RT-qPCR assay (Fig. 1C). In
addition, transfection with si-SNHG14 significantly reduced SNHG14
expression levels in BV2 cells compared with the control group
(Fig. 1D). LPS treatment
significantly decreased miR-340 expression levels in BV2 cells
compared with the control; however, this was significantly reversed
following transfection with si-SNHG14, miR-340 mimics or Dex
treatment (Fig. 1E). Notably,
miR-340 expression levels were increased following si-SNHG14
transfection in LPS-treated BV2 cells (Fig. 1E). These results demonstrated that
SNHG14 negatively regulated the expression of miR-340.
Results of previous studies demonstrate that surgery
or anesthesia-induced neuroinflammation play critical roles in the
progression of POCD (5,22). Thus, the levels of NF-κB protein and
pro-inflammatory cytokines (TNF-α, IL-1β, IL-2, IL-6, IL-8 and
IL-12) in LPS-treated BV2 cells were detected in the present study.
As presented in Fig. 1F-G and
2A-F, LPS treatment significantly
upregulated the levels of NF-κB, TNF-α, IL-1β, IL-2, IL-6, IL-8 and
IL-12 in BV2 cells. However, si-SNHG14, miR-340 mimics or Dex
treatment significantly decreased the levels of NF-κB and the
aforementioned pro-inflammatory cytokines in LPS-treated BV2 cells
compared with the LPS group (Figs.
1F-G and 2A-F). Collectively,
results of the present study demonstrated that Dex treatment
attenuated neuroinflammation in LPS-treated BV2 cells by regulating
the SNHG14/miR-340/NF-κB axis.
 | Figure 2Dex attenuates neuroinflammation in
LPS-stimulated BV2 cells by regulating the SNHG14/miR-340/NF-κB
axis. BV2 cells were transfected with miR-340 mimics and si-SHNG14
for 24 h, and then treated with Dex for 30 min, followed by
stimulation with 10 µg/ml LPS treatment for 24 h. ELISA assay was
conducted to detect the level of (A) TNF-α, (B) IL-1β, (C) IL-2,
(D) IL-6, (E) IL-8 and (F) IL-12 in the culture supernatant of BV2
cells. **P<0.01 vs. control group;
##P<0.01 vs. LPS group. MiR, microRNA; SNHG14, small
nucleolar RNA host gene 14; si, small interfering; Dex,
dexmedetomidine; LPS, lipopolysaccharide. |
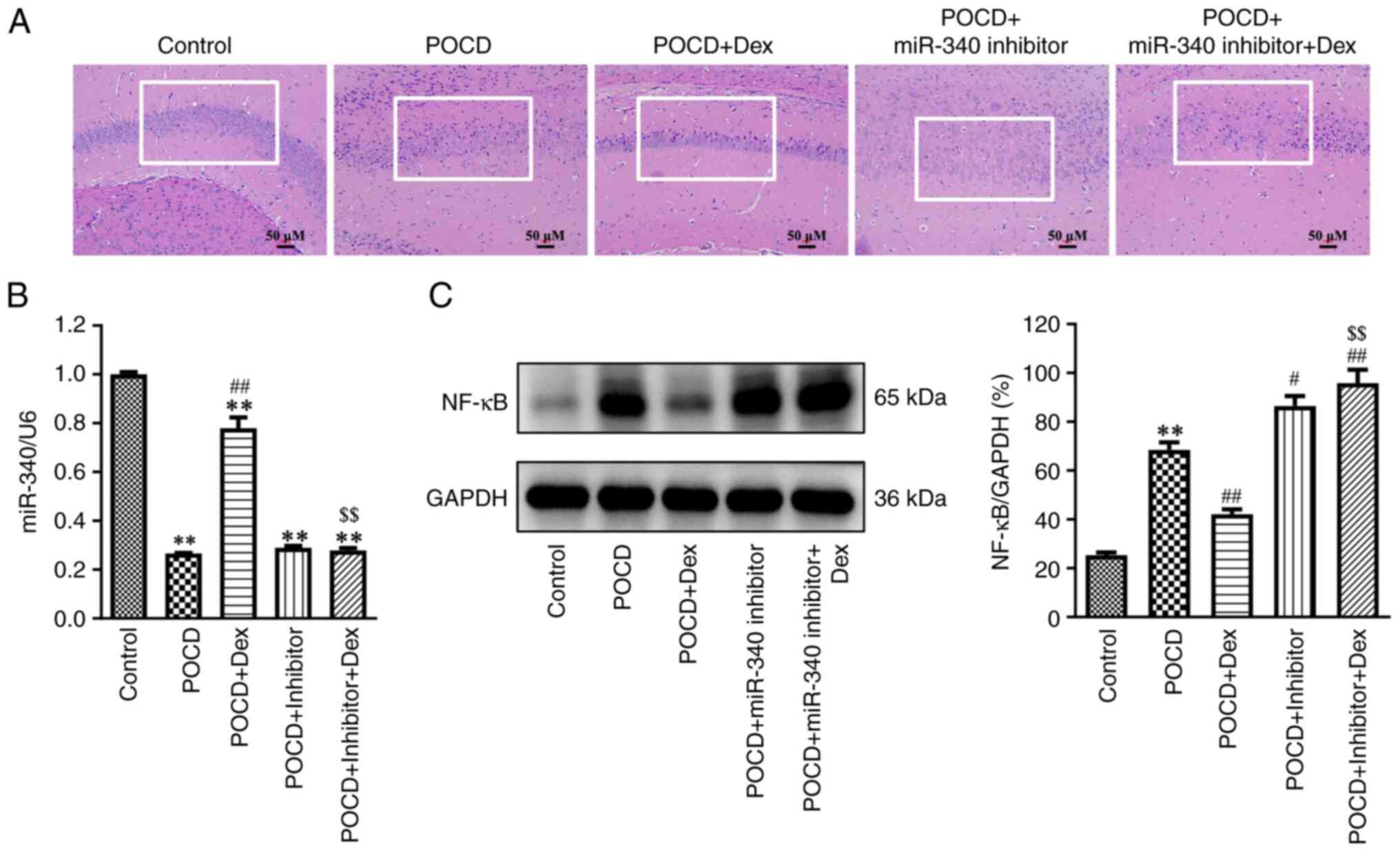
Dex attenuates hippocampal damage in
aged mice with POCD by regulating the SNHG14/miR-340/NF-κB
axis
To explore whether Dex attenuated hippocampal damage
in aged mice with POCD by regulating the SNHG14/miR-340/NF-κB axis,
the expression levels of miR-340, SNHG14 and NF-κB were detected.
As presented in Fig. 3A-C, miR-340
expression levels were significantly decreased and SNHG14 and NF-κB
expression levels were notably increased in the hippocampus of aged
mice with POCD compared with the control. In addition, this was
partially reversed following treatment with Dex. In addition,
results of the H&E staining assay demonstrated that the
hippocampal neurons in the control group were neatly arranged and
the cell structure was complete (Fig.
4A). By contrast, the hippocampal neurons in the POCD group
demonstrated inflammatory cell infiltration and nuclear
condensation, whereas the opposite results were observed following
Dex treatment (Fig. 4A). Meanwhile,
compared with the inhibitor NC group, miR-340 inhibitor
significantly reduced miR-340 level in the hippocampus of aged mice
(Fig. S1). Dex treatment
significantly elevated miR-340 expression levels and reduced NF-κB
expressions levels in the hippocampus of aged mice with POCD
compared with the POCD group. Notably, the opposite results were
observed following transfection with the miR-340 inhibitor
(Fig. 4B and C). Collectively, results of the present
study demonstrated that treatment with Dex attenuated hippocampal
damage in aged mice with POCD by regulating the
SNHG14/miR-340/NF-κB axis.
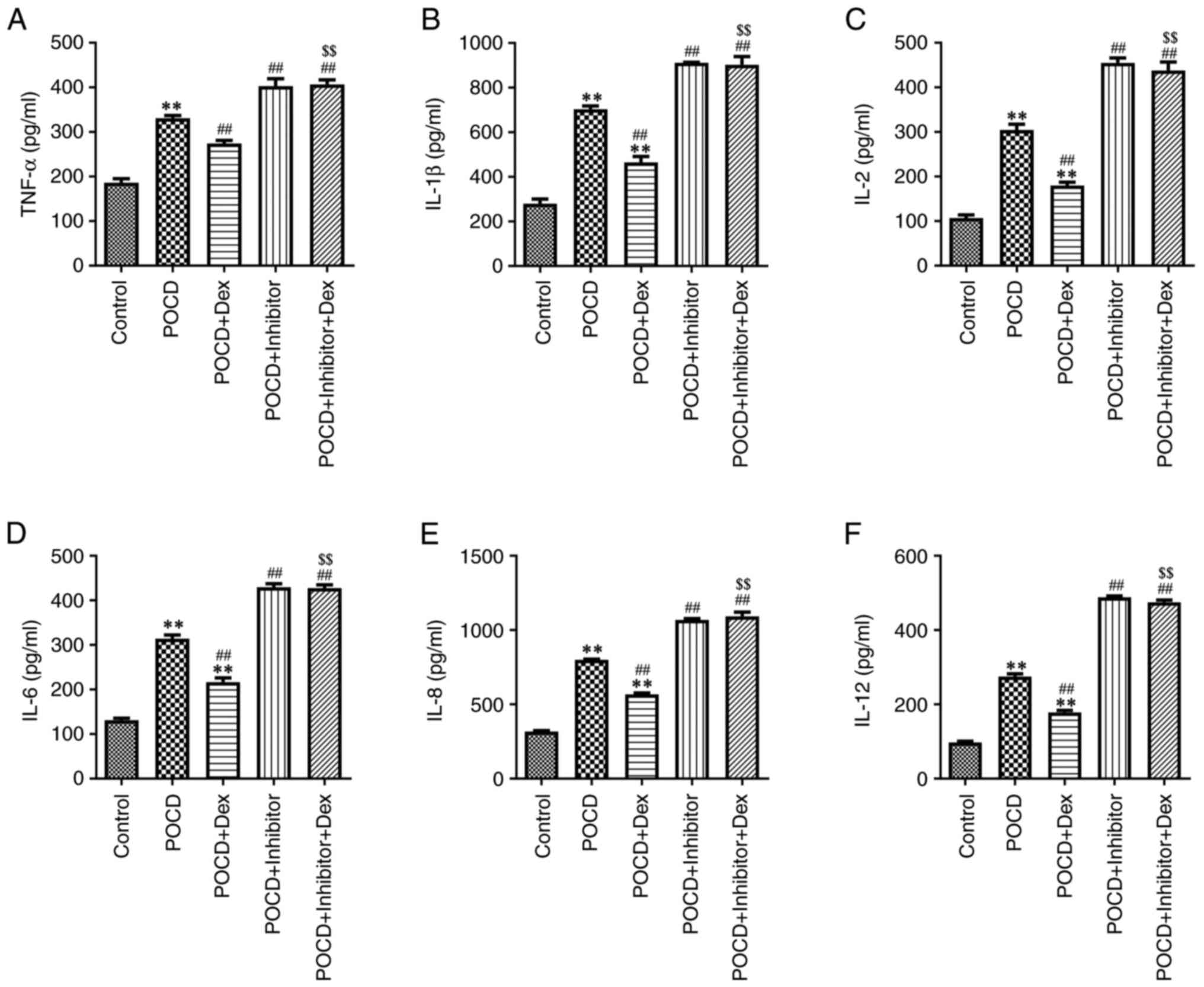
Dex attenuates hippocampal
neuroinflammation and improves spatial memory impairments in aged
mice with POCD by regulating the SNHG14/miR-340/NF-κB axis
To evaluate the effects of Dex on hippocampal
inflammation in a mouse model of POCD, the levels of
pro-inflammatory cytokines were measured using ELISA. As displayed
in Fig. 5A-F, miR-340 inhibitor
obviously increased, but Dex treatment significantly decreased the
levels of TNF-α, IL-1β, IL-2, IL-6, IL-8 and IL-12 in the
hippocampus of aged mice with POCD compared with the POCD group.
However, compared with the POCD + Dex group, the inhibitory effects
of Dex on the levels of these pro-inflammatory cytokines in the
hippocampus of aged mice with POCD were reversed by miR-340
inhibitor (Fig. 5A-F). In addition,
results of the MWM analysis demonstrated that Dex treatment
significantly decreased latency to escape in aged mice with POCD,
and these results were reversed following transfection with the
miR-340 inhibitor (Fig. 6A and
B). Collectively, results of the
present study demonstrated that Dex attenuated hippocampal
neuroinflammation and improved spatial memory impairments in aged
mice with POCD by regulating the SNHG14/miR-340/NF-κB axis.
Discussion
The present study revealed that Dex could protect
microglia from LPS-induced neuroinflammation in vitro and
attenuate hippocampal neuroinflammation in aged mice with POCD
in vivo. Mechanistically, Dex could ameliorate inflammatory
injury of hippocampal microglia cells through modulating the
SNHG14/miR-340/NF-κB axis.
The inflammatory activation of hippocampal microglia
plays a key role in the progression of POCD (23-25).
In addition, microglia are resident immune cells in the central
nervous system, and are key players in age-related
neurodegenerative and neuroinflammatory diseases (26,27).
It has been shown that NF-κB signaling exhibits a key role in POCD
(28). Activation of NF-κB
signaling in microglial cells is critical during neuroinflammation
in neurodegenerative diseases (29,30).
Zheng et al (31) revealed
that NF-κB expression levels are upregulated in the hippocampal
zone in rats following sevoflurane anesthesia (31). In addition, inactivating NF-κB
signaling attenuates neuronal apoptosis and inflammatory responses
in the hippocampus of aged rats with POCD (32). Moreover, Ding et al (33) found that andrographolide an
alleviate POCD progression by modulating NF-κB/MAPK signaling.
These data demonstrate that inhibiting neuroinflammation via the
NF-κB signaling pathway may exhibit potential in alleviating
POCD.
Zhou et al (34) indicated that Dex attenuates
inflammatory responses in the hippocampus of aged mice with POCD by
suppressing the TLR4-NF-κB signaling pathway. In addition, results
of our previous study demonstrated that Dex reduces NF-κB
expression levels in LPS-treated BV2 cells by upregulating miR-340,
thereby attenuating microglial-mediated neuroinflammation (12). Thus, the present study aimed to
explore whether Dex could attenuate neuroinflammation and cognitive
impairment in aged mice with POCD in vivo. Results of the
present study demonstrated that Dex treatment upregulated miR-340
expression levels and reduced NF-κB expression levels in the
hippocampus of aged mice with POCD. In addition, Dex treatment
decreased the levels of pro-inflammatory cytokines in the
hippocampus of aged mice with POCD by upregulating miR-340.
Moreover, Dex notably improved spatial memory impairments in aged
mice with POCD via upregulation of miR-340. Collectively, these
results revealed that Dex attenuated neuroinflammation and improved
spatial memory impairments in aged mice with POCD by regulating the
miR-340/NF-κB axis.
However, the molecular mechanisms by which Dex
improves POCD remain unclear. The lncRNA-miRNA-mRNA axis plays key
roles in POCD development (15,16).
In addition, lncRNAs, as important regulators in biological
processes, are involved in the pathogenesis of nervous system
disease (15,35). Mechanistically, lncRNAs regulate
gene expression by sponging miRNAs (36). Tian et al (37) demonstrated that lncRNA SNHG8 may
inhibit microglial inflammatory responses in mice subjected to
ischemic injury through targeting the miR-425/SIRT1/NF-κB axis. Cao
et al (38) reported that
downregulation of lncRNA HOXA11-AS attenuates neuroinflammation and
neuronal apoptosis in mice with Parkinson's disease via the
miR-124/FSTL1/NF-κB axis. These data indicate that lncRNAs may
modulate NF-κB expression by targeting miRNAs. Results of the
present study demonstrated that SNHG14 exhibited potential as a
target of miR-340. In addition, SNHG14 knockdown increased miR-340
levels and reduced NF-κB levels in LPS-treated BV2 cells in
vitro. Moreover, Dex treatment reduced SNHG14 levels in the
hippocampus of aged mice with POCD in vivo. Collectively,
results of the present study demonstrated that Dex ameliorated
neuroinflammation and cognitive dysfunction in aged mice with POCD
through targeting the SNHG14/miR-340/NF-κB axis. However, except
for the SNHG14/miR-340/NF-κB axis, other signaling pathways
involved in the neuroprotective effects of Dex in POCD are needed
to be further investigated in the future.
Results of the present study demonstrated that Dex
may protect microglia from LPS-induced neuroinflammation in
vitro and attenuate hippocampal neuroinflammation in aged mice
with POCD in vivo via the SNHG14/miR-340/NF-κB axis. The
present study may provide further insights into the mechanisms
underlying Dex in the treatment of POCD.
Supplementary Material
MiR-340 inhibitor reduces miR-340
level in the hippocampus in aged mice. MiR-340 level in the
hippocampus was measured with reverse transcription-quantitative
PCR. **P<0.01 vs. inhibitor NC group. miR,
microRNA.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Project of Science and
Technology Commission of Jiading District, Shanghai (grant no.
JDKW-2020-0012), the Medical Key Discipline Construction Project of
Jiading District, Shanghai (grant no. 2020-jdyxzdxk-03) and the
Project of Science and Technology Commission of Jiading District,
Shanghai (grant no. JDKW-2021-0036).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GH, HN, KW and HG were responsible for data
investigation, data acquisition, data analysis, data interpretation
and manuscript revision. YL, JG, XN, ZW and YB made substantial
contributions to conception and design of the study and revised the
manuscript. All authors agreed to be accountable for all aspects of
the work. All authors confirmed the authenticity of all the raw
data and read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Jiading District Central
Hospital Affiliated Shanghai University of Medicine & Health
Sciences approved the protocol of animal experiments (approval no.
20190018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei C, Luo T, Zou S, Zhou X, Shen W, Ji X,
Li Q and Wu A: Differentially expressed lncRNAs and miRNAs with
associated ceRNA networks in aged mice with postoperative cognitive
dysfunction. Oncotarget. 8:55901–55914. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liebert AD, Chow RT, Bicknell BT and
Varigos E: Neuroprotective effects against POCD by
photobiomodulation: Evidence from assembly/disassembly of the
cytoskeleton. J Exp Neurosci. 10:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu J, Huang K, Zhu B, Zhou B, Ahmad Harb
AK, Liu L and Wu X: Neuropsychological tests in post-operative
cognitive dysfunction: Methods and applications. Front Psychol.
12(684307)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jeong H, Chung JY, Ko IG, Kim SH, Jin JJ,
Hwang L, Moon EJ, Lee BJ and Yi JW: Effect of
polydeoxyribonucleotide on lipopolysaccharide and
sevoflurane-induced postoperative cognitive dysfunction in human
neuronal SH-SY5Y Cells. Int Neurourol J. 23 (Suppl 2):S93–S101.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Safavynia SA and Goldstein PA: The role of
neuroinflammation in postoperative cognitive dysfunction: Moving
from hypothesis to treatment. Front Psychiatry.
9(752)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen L, Dong R, Lu Y, Zhou Y, Li K, Zhang
Z and Peng M: MicroRNA-146a protects against cognitive decline
induced by surgical trauma by suppressing hippocampal
neuroinflammation in mice. Brain Behav Immun. 78:188–201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ye JS, Chen L, Lu YY, Lei SQ, Peng M and
Xia ZY: SIRT3 activator honokiol ameliorates
surgery/anesthesia-induced cognitive decline in mice through
anti-oxidative stress and anti-inflammatory in hippocampus. CNS
Neurosci Ther. 25:355–366. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guler G, Akin A, Tosun Z, Eskitascoglu E,
Mizrak A and Boyaci A: Single-dose dexmedetomidine attenuates
airway and circulatory reflexes during extubation. Acta
Anaesthesiol Scand. 49:1088–1091. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee SH, Na S, Kim N, Ban MG, Shin SE and
Oh YJ: The effects of dexmedetomidine on myocardial function
assessed by tissue doppler echocardiography during general
anesthesia in patients with diastolic dysfunction: A
CONSORT-Prospective, Randomized, Controlled Trial. Medicine
(Baltimore). 95(e2805)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suo L and Wang M: Dexmedetomidine
facilitates the expression of nNOS in the hippocampus to alleviate
surgery-induced neuroinflammation and cognitive dysfunction in aged
rats. Exp Ther Med. 22(1038)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Glumac S, Kardum G, Sodic L, Supe-Domic D
and Karanovic N: Effects of dexamethasone on early cognitive
decline after cardiac surgery: A randomised controlled trial. Eur J
Anaesthesiol. 34:776–784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation In
LPS-Stimulated BV2 microglia cells through upregulation Of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18(206)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Y, Zhang Y, Ye G, Sheng C, Kong L and
Yuan L: Knockdown of lncRNA PCAI protects against cognitive decline
induced by hippocampal neuroinflammation via regulating SUZ12. Life
Sci. 253(117626)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Y, Zhang W, Zhu D, Wang H, Shao H and
Zhang Y: LncRNA Rian ameliorates sevoflurane anesthesia-induced
cognitive dysfunction through regulation of miR-143-3p/LIMK1 axis.
Hum Cell. 34:808–818. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wei C, Sun Y, Wang J, Lin D, Cui V, Shi H
and Wu A: LncRNA NONMMUT055714 acts as the sponge of
microRNA-7684-5p to protect against postoperative cognitive
dysfunction. Aging (Albany NY). 3:12552–12564. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Deng F, Cai L, Zhou B, Zhou Z and Xu G:
Whole transcriptome sequencing reveals dexmedetomidine-improves
postoperative cognitive dysfunction in rats via modulating lncRNA.
3 Biotech. 10(202)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p/ROCK1.
Cancer Gene Ther. 26:234–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang G, Li T, Chang X and Xing J: Long
Noncoding RNA SNHG14 Promotes ischemic brain injury via regulating
miR-199b/AQP4 Axis. Neurochem Res. 46:1280–1290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Z, Wang Y, Zhang W, Li J, Liu W and
Lu W: Long non-coding RNA SNHG14 exerts oncogenic functions in
non-small cell lung cancer through acting as an miR-340 sponge.
Biosci Rep. 39(BSR20180941)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li N, Zhang X, Dong H, Hu Y and Qian Y:
Bidirectional relationship of mast cells-neurovascular unit
communication in neuroinflammation and its involvement in POCD.
Behav Brain Res. 322(Pt A):60–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu B, Yuan H, Zhai X, Li X, Qin J, Chen J
and Meng B: High-Pressure Pneumoperitoneum aggravates
surgery-induced neuroinflammation and cognitive dysfunction in aged
mice. Mediators Inflamm. 2020(6983193)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Duan S, Wang X, Chen G, Quan C, Qu S and
Tong J: Inhibiting RIPK1 limits neuroinflammation and alleviates
postoperative cognitive impairments in D-Galactose-Induced Aged
Mice. Front Behav Neurosci. 12(138)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Z, Yuan H, Zhao H, Qi B, Li F and An
L: PPARγ activation ameliorates postoperative cognitive decline
probably through suppressing hippocampal neuroinflammation in aged
mice. Int Immunopharmacol. 43:53–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Damani MR, Zhao L, Fontainhas AM, Amaral
J, Fariss RN and Wong WT: Age-related alterations in the dynamic
behavior of microglia. Aging Cell. 10:263–276. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Orihuela R, McPherson CA and Harry GJ:
Microglial M1/M2 polarization and metabolic states. Br J Pharmacol.
173:649–665. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yuan N, Wang X, Zhang Y, Kong L, Yuan L
and Ge Y: Intervention of NF-Κb Signaling pathway and preventing
post-operative cognitive dysfunction as well as neuronal apoptosis.
Iran J Public Health. 51:124–132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shabab T, Khanabdali R, Moghadamtousi SZ,
Kadir HA and Mohan G: Neuroinflammation pathways: A general review.
Int J Neurosci. 127:624–633. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mockenhaupt K, Gonsiewski A and Kordula T:
RelB and Neuroinflammation. Cells. 10(1609)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng JW, Meng B, Li XY, Lu B, Wu GR and
Chen JP: NF-κB/P65 signaling pathway: A potential therapeutic
target in postoperative cognitive dysfunction after sevoflurane
anesthesia. Eur Rev Med Pharmacol Sci. 21:394–407. 2017.PubMed/NCBI
|
|
32
|
Wei P, Zheng Q, Liu H, Wan T, Zhou J, Li
D, Zhou H and Li J, Ji F, Tang W and Li J: Nicotine-Induced
neuroprotection against cognitive dysfunction after partial
hepatectomy involves activation of BDNF/TrkB signaling pathway and
inhibition of NF-κB signaling pathway in aged rats. Nicotine Tob
Res. 20:515–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ding Y, Shi C, Chen L, Ma P, Li K, Jin J,
Zhang Q and Li A: Effects of andrographolide on postoperative
cognitive dysfunction and the association with NF-κB/MAPK pathway.
Oncol Lett. 14:7367–7373. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou XY, Liu J, Xu ZP, Fu Q, Wang PQ, Wang
JH and Zhang H: Dexmedetomidine ameliorates postoperative cognitive
dysfunction by inhibiting Toll-like receptor 4 signaling in aged
mice. Kaohsiung J Med Sci. 36:721–731. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Riva P, Ratti A and Venturin M: The Long
Non-Coding RNAs in neurodegenerative diseases: Novel mechanisms of
pathogenesis. Curr Alzheimer Res. 13:1219–1231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Karagkouni D, Karavangeli A,
Paraskevopoulou MD and Hatzigeorgiou AG: Characterizing
miRNA-lncRNA Interplay. Methods Mol Biol. 2372:243–262.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tian J, Liu Y, Wang Z, Zhang S, Yang Y,
Zhu Y and Yang C: LncRNA Snhg8 attenuates microglial inflammation
response and blood-brain barrier damage in ischemic stroke through
regulating miR-425-5p mediated SIRT1/NF-κB signaling. J Biochem Mol
Toxicol. 35(e22724)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cao H, Han X, Jia Y and Zhang B:
Inhibition of long non-coding RNA HOXA11-AS against
neuroinflammation in Parkinson's disease model via targeting
miR-124-3p mediated FSTL1/NF-κB axis. Aging (Albany NY).
13:11455–11469. 2021.PubMed/NCBI View Article : Google Scholar
|