|
1
|
Wei C, Luo T, Zou S, Zhou X, Shen W, Ji X,
Li Q and Wu A: Differentially expressed lncRNAs and miRNAs with
associated ceRNA networks in aged mice with postoperative cognitive
dysfunction. Oncotarget. 8:55901–55914. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liebert AD, Chow RT, Bicknell BT and
Varigos E: Neuroprotective effects against POCD by
photobiomodulation: Evidence from assembly/disassembly of the
cytoskeleton. J Exp Neurosci. 10:1–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu J, Huang K, Zhu B, Zhou B, Ahmad Harb
AK, Liu L and Wu X: Neuropsychological tests in post-operative
cognitive dysfunction: Methods and applications. Front Psychol.
12(684307)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jeong H, Chung JY, Ko IG, Kim SH, Jin JJ,
Hwang L, Moon EJ, Lee BJ and Yi JW: Effect of
polydeoxyribonucleotide on lipopolysaccharide and
sevoflurane-induced postoperative cognitive dysfunction in human
neuronal SH-SY5Y Cells. Int Neurourol J. 23 (Suppl 2):S93–S101.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Safavynia SA and Goldstein PA: The role of
neuroinflammation in postoperative cognitive dysfunction: Moving
from hypothesis to treatment. Front Psychiatry.
9(752)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen L, Dong R, Lu Y, Zhou Y, Li K, Zhang
Z and Peng M: MicroRNA-146a protects against cognitive decline
induced by surgical trauma by suppressing hippocampal
neuroinflammation in mice. Brain Behav Immun. 78:188–201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ye JS, Chen L, Lu YY, Lei SQ, Peng M and
Xia ZY: SIRT3 activator honokiol ameliorates
surgery/anesthesia-induced cognitive decline in mice through
anti-oxidative stress and anti-inflammatory in hippocampus. CNS
Neurosci Ther. 25:355–366. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guler G, Akin A, Tosun Z, Eskitascoglu E,
Mizrak A and Boyaci A: Single-dose dexmedetomidine attenuates
airway and circulatory reflexes during extubation. Acta
Anaesthesiol Scand. 49:1088–1091. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee SH, Na S, Kim N, Ban MG, Shin SE and
Oh YJ: The effects of dexmedetomidine on myocardial function
assessed by tissue doppler echocardiography during general
anesthesia in patients with diastolic dysfunction: A
CONSORT-Prospective, Randomized, Controlled Trial. Medicine
(Baltimore). 95(e2805)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suo L and Wang M: Dexmedetomidine
facilitates the expression of nNOS in the hippocampus to alleviate
surgery-induced neuroinflammation and cognitive dysfunction in aged
rats. Exp Ther Med. 22(1038)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Glumac S, Kardum G, Sodic L, Supe-Domic D
and Karanovic N: Effects of dexamethasone on early cognitive
decline after cardiac surgery: A randomised controlled trial. Eur J
Anaesthesiol. 34:776–784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
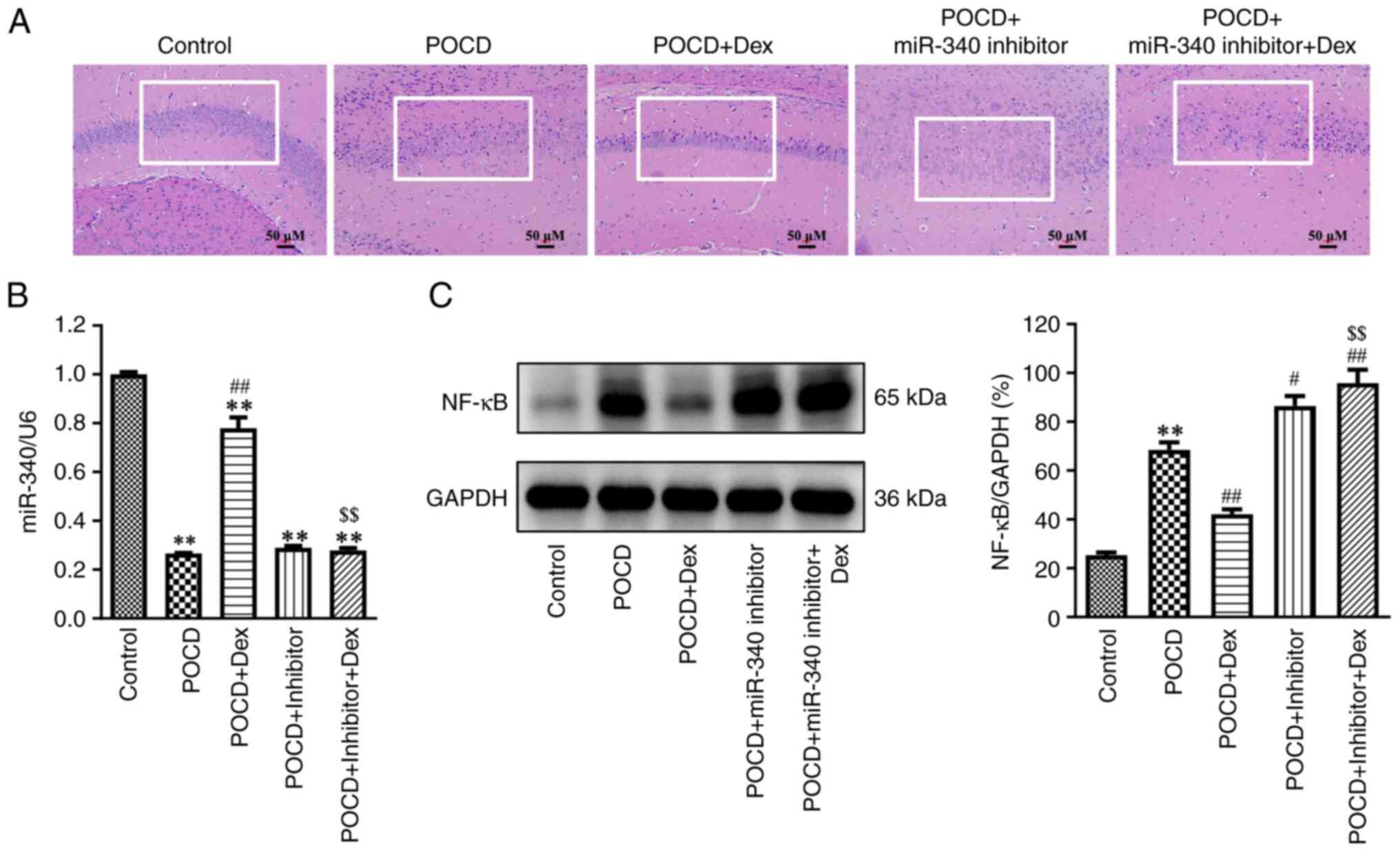
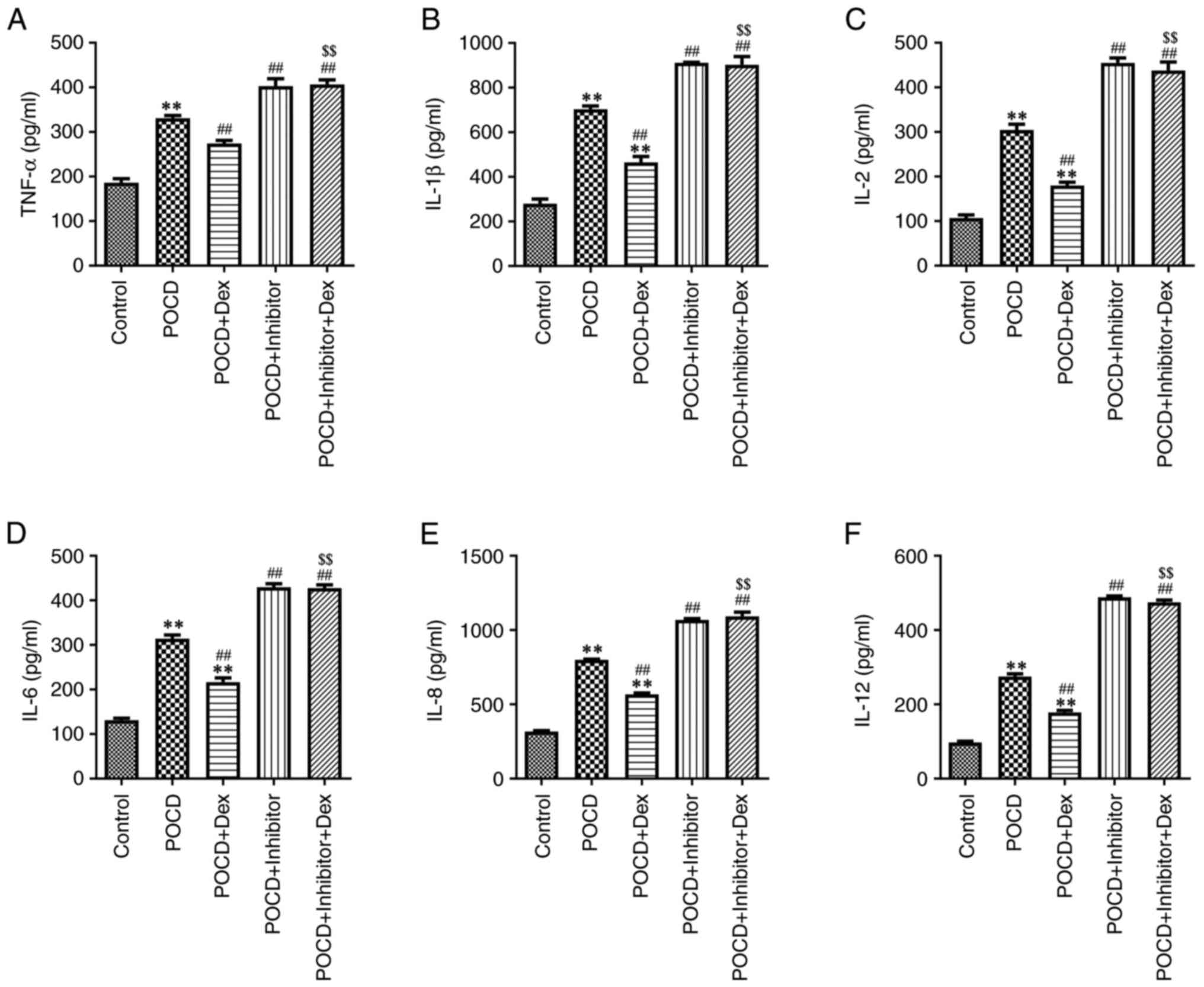
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation In
LPS-Stimulated BV2 microglia cells through upregulation Of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18(206)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Y, Zhang Y, Ye G, Sheng C, Kong L and
Yuan L: Knockdown of lncRNA PCAI protects against cognitive decline
induced by hippocampal neuroinflammation via regulating SUZ12. Life
Sci. 253(117626)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Y, Zhang W, Zhu D, Wang H, Shao H and
Zhang Y: LncRNA Rian ameliorates sevoflurane anesthesia-induced
cognitive dysfunction through regulation of miR-143-3p/LIMK1 axis.
Hum Cell. 34:808–818. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wei C, Sun Y, Wang J, Lin D, Cui V, Shi H
and Wu A: LncRNA NONMMUT055714 acts as the sponge of
microRNA-7684-5p to protect against postoperative cognitive
dysfunction. Aging (Albany NY). 3:12552–12564. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Deng F, Cai L, Zhou B, Zhou Z and Xu G:
Whole transcriptome sequencing reveals dexmedetomidine-improves
postoperative cognitive dysfunction in rats via modulating lncRNA.
3 Biotech. 10(202)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
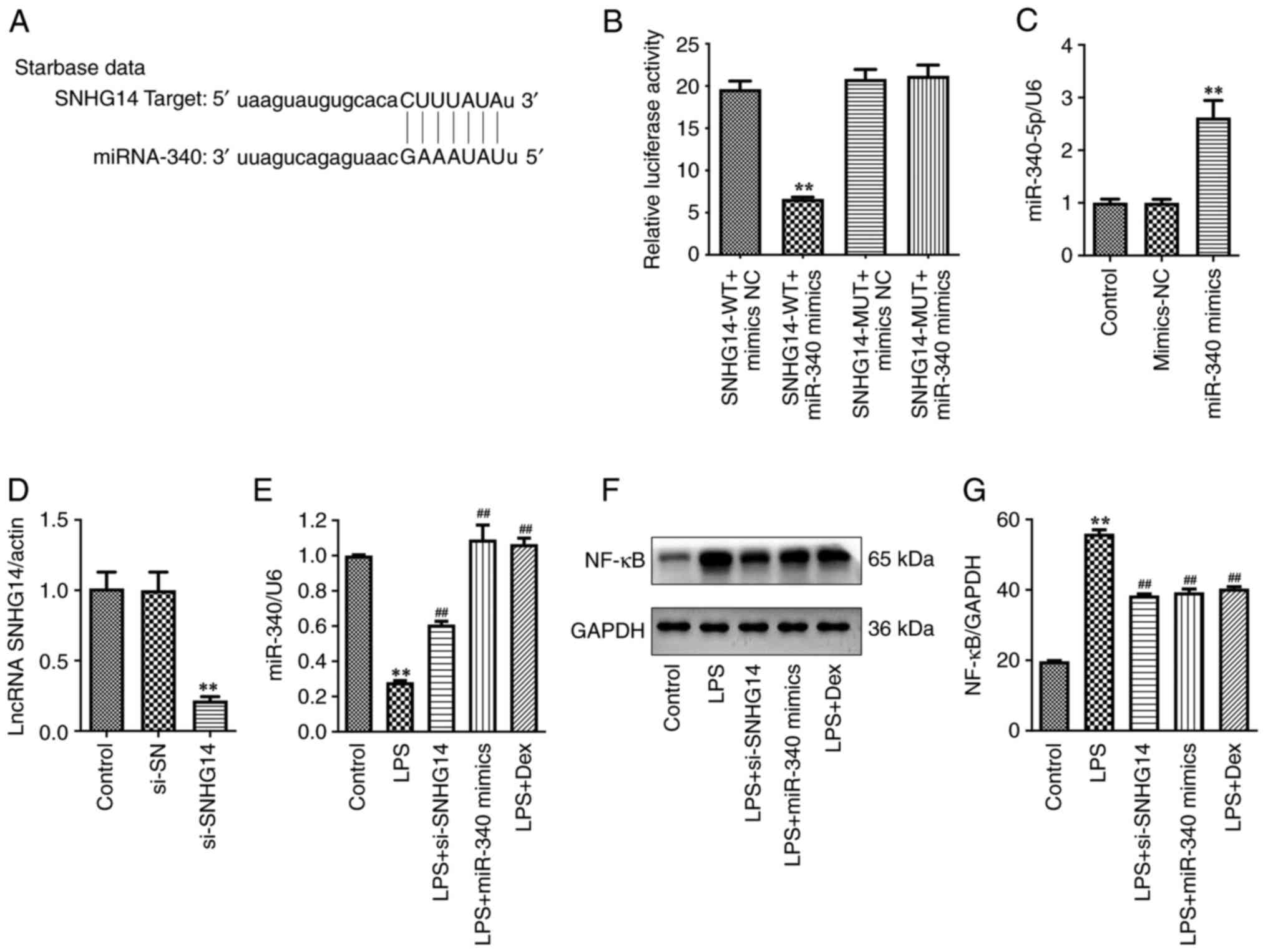
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p/ROCK1.
Cancer Gene Ther. 26:234–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang G, Li T, Chang X and Xing J: Long
Noncoding RNA SNHG14 Promotes ischemic brain injury via regulating
miR-199b/AQP4 Axis. Neurochem Res. 46:1280–1290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Z, Wang Y, Zhang W, Li J, Liu W and
Lu W: Long non-coding RNA SNHG14 exerts oncogenic functions in
non-small cell lung cancer through acting as an miR-340 sponge.
Biosci Rep. 39(BSR20180941)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li N, Zhang X, Dong H, Hu Y and Qian Y:
Bidirectional relationship of mast cells-neurovascular unit
communication in neuroinflammation and its involvement in POCD.
Behav Brain Res. 322(Pt A):60–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu B, Yuan H, Zhai X, Li X, Qin J, Chen J
and Meng B: High-Pressure Pneumoperitoneum aggravates
surgery-induced neuroinflammation and cognitive dysfunction in aged
mice. Mediators Inflamm. 2020(6983193)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Duan S, Wang X, Chen G, Quan C, Qu S and
Tong J: Inhibiting RIPK1 limits neuroinflammation and alleviates
postoperative cognitive impairments in D-Galactose-Induced Aged
Mice. Front Behav Neurosci. 12(138)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Z, Yuan H, Zhao H, Qi B, Li F and An
L: PPARγ activation ameliorates postoperative cognitive decline
probably through suppressing hippocampal neuroinflammation in aged
mice. Int Immunopharmacol. 43:53–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Damani MR, Zhao L, Fontainhas AM, Amaral
J, Fariss RN and Wong WT: Age-related alterations in the dynamic
behavior of microglia. Aging Cell. 10:263–276. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Orihuela R, McPherson CA and Harry GJ:
Microglial M1/M2 polarization and metabolic states. Br J Pharmacol.
173:649–665. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yuan N, Wang X, Zhang Y, Kong L, Yuan L
and Ge Y: Intervention of NF-Κb Signaling pathway and preventing
post-operative cognitive dysfunction as well as neuronal apoptosis.
Iran J Public Health. 51:124–132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shabab T, Khanabdali R, Moghadamtousi SZ,
Kadir HA and Mohan G: Neuroinflammation pathways: A general review.
Int J Neurosci. 127:624–633. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mockenhaupt K, Gonsiewski A and Kordula T:
RelB and Neuroinflammation. Cells. 10(1609)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zheng JW, Meng B, Li XY, Lu B, Wu GR and
Chen JP: NF-κB/P65 signaling pathway: A potential therapeutic
target in postoperative cognitive dysfunction after sevoflurane
anesthesia. Eur Rev Med Pharmacol Sci. 21:394–407. 2017.PubMed/NCBI
|
|
32
|
Wei P, Zheng Q, Liu H, Wan T, Zhou J, Li
D, Zhou H and Li J, Ji F, Tang W and Li J: Nicotine-Induced
neuroprotection against cognitive dysfunction after partial
hepatectomy involves activation of BDNF/TrkB signaling pathway and
inhibition of NF-κB signaling pathway in aged rats. Nicotine Tob
Res. 20:515–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ding Y, Shi C, Chen L, Ma P, Li K, Jin J,
Zhang Q and Li A: Effects of andrographolide on postoperative
cognitive dysfunction and the association with NF-κB/MAPK pathway.
Oncol Lett. 14:7367–7373. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou XY, Liu J, Xu ZP, Fu Q, Wang PQ, Wang
JH and Zhang H: Dexmedetomidine ameliorates postoperative cognitive
dysfunction by inhibiting Toll-like receptor 4 signaling in aged
mice. Kaohsiung J Med Sci. 36:721–731. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Riva P, Ratti A and Venturin M: The Long
Non-Coding RNAs in neurodegenerative diseases: Novel mechanisms of
pathogenesis. Curr Alzheimer Res. 13:1219–1231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Karagkouni D, Karavangeli A,
Paraskevopoulou MD and Hatzigeorgiou AG: Characterizing
miRNA-lncRNA Interplay. Methods Mol Biol. 2372:243–262.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tian J, Liu Y, Wang Z, Zhang S, Yang Y,
Zhu Y and Yang C: LncRNA Snhg8 attenuates microglial inflammation
response and blood-brain barrier damage in ischemic stroke through
regulating miR-425-5p mediated SIRT1/NF-κB signaling. J Biochem Mol
Toxicol. 35(e22724)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cao H, Han X, Jia Y and Zhang B:
Inhibition of long non-coding RNA HOXA11-AS against
neuroinflammation in Parkinson's disease model via targeting
miR-124-3p mediated FSTL1/NF-κB axis. Aging (Albany NY).
13:11455–11469. 2021.PubMed/NCBI View Article : Google Scholar
|