The proinflammatory cytokine interleukin-18 (IL-18)
is involved in host inflammation responses to infections or
injuries. It regulates both innate and adaptive immune responses. A
protein with a molecular weight of 38 kDa isolated and purified
from human urine was discovered to bind to IL-18. It was considered
to be a soluble receptor but was proved to have no transmembrane
region of a cytokine receptor (1).
Following this, it was identified in 1999 as a protein factor that
can specifically bind to IL-18 with high affinity and antagonize
the biological function of IL-18 and named IL-18 binding protein
(IL-18BP) (2). IL-18BP gene is
expressed in numerous types of tissues and cells of humans and
animals and its expression is regulated by interferon γ (IFN-γ)
(3). IL-18BP is a glycoprotein
belonging to the immune globulin superfamily. IL-18BP can
effectively inhibit action of IL-18 in vivo and in
vitro and is considered as a natural antagonist of IL-18
(4-10).
Studies have shown that there is no homology between IL-18BP and
two receptors of IL-18, IL-1 receptor-related protein (IL-1Rrp) and
accessory protein (AcPL) (11).
IL-1Rrp, a functional IL-18 receptor component (12,13),
leads to activation of signaling pathways similar to those used by
IL-1(14). IL-1Rrp1) and IL-1R
accessory protein-like (IL-1RAcPL) confer responsiveness to IL-18
in a highly specific and unique manner (no functional pairing with
other IL-1Rs and IL-1R-like molecules). Co-transfection with both
receptor components resulted in expression of both low and high
affinity binding sites for IL-18 [K:(d) of 11 and 0.4 nM,
respectively (15). Anti-IL-1RAcPL
mAb can effectively inhibit IL-18-induced activation of NF-κB
(15). IL-18 has weak affinity for
IL-1Rrp1. However, binding of murine recombinant IL-18 (rIL-18) is
not detected in T helper (Th)1-developing splenic CD4+ T
cells isolated from IL-1Rrp-deficient mice. This affects activation
of NF-κB or c-Jun N-terminal kinase in Th1 cells and cytolytic
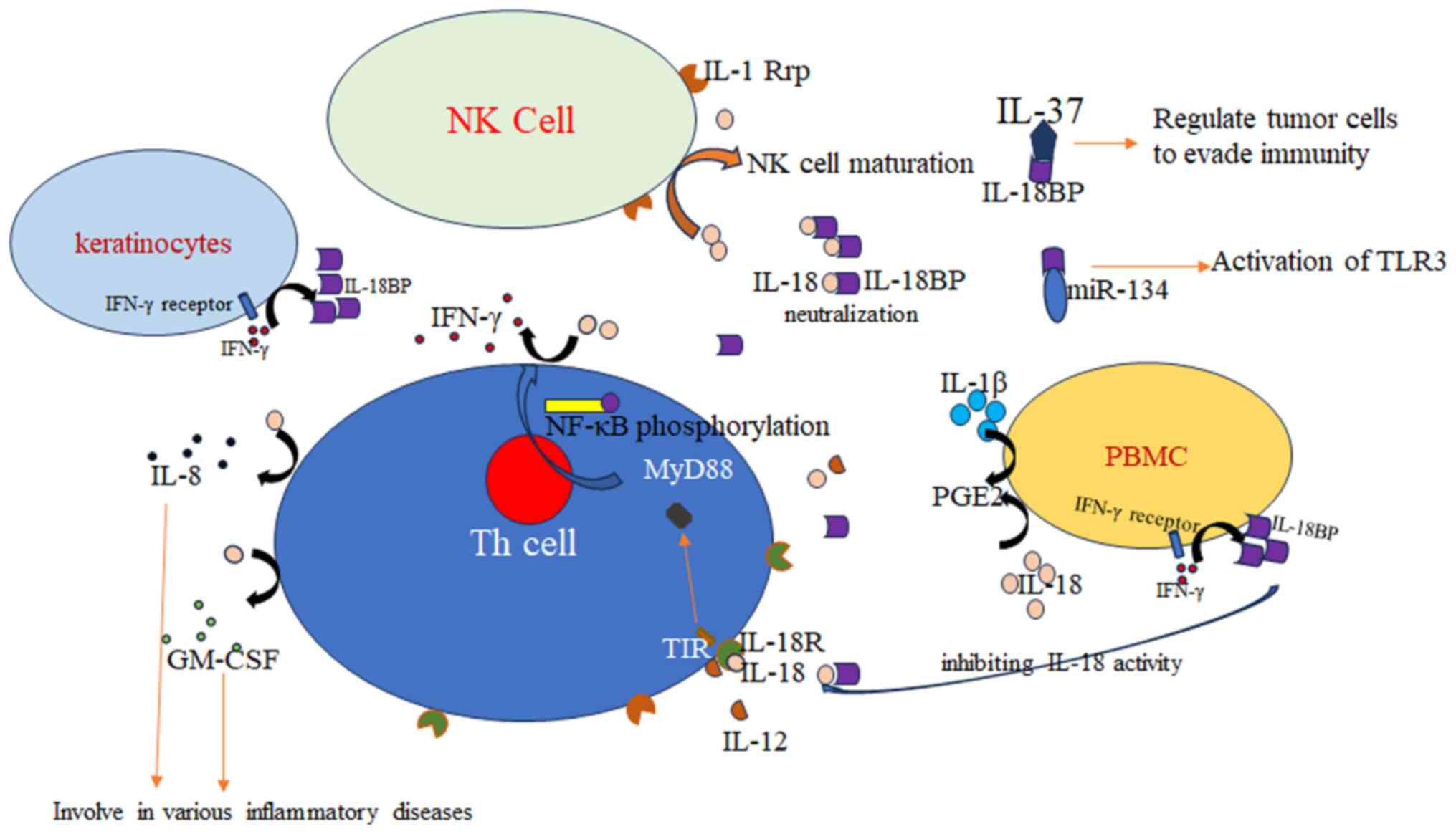
activity of natural killer (NK) cells as well as IFN-γ production
in response to IL-18. Expression of IL-18BP and IL-18 is balanced
in human and animals (16). Studies
on disease processes have found that there is a significant
positive correlation between expression levels of IL-18 and IL-18BP
in healthy people (3,11,17-21).
In the progression of certain types of diseases, such as secondary
hemophagocytic syndrome, sepsis, there is an imbalance in
expression levels of IL-18 and IL-18BP. The ratio of IL-18 to
IL-18BP increases, even though both proteins are present at higher
levels compared with control. This imbalance leads to an increased
disease severity (22). Although
expression levels of both IL-18 and IL-18BP are high in the serum
of patients with Wegener's granulomatosis, the amount of IL-18BP is
insufficient to neutralize IL-18. As a result, levels of free IL-18
in the serum are higher compared with those in healthy individuals,
leading to inflammatory reactions (4,23,24).
This phenomenon has been observed in patients with inflammatory
diseases such as sepsis (24,25),
systemic juvenile congenital arthritis (26) and macrophage activation syndrome
(27). Another feature of IL-18BP
is its ability to bind to anti-inflammatory factor IL-37, thereby
inhibiting its anti-inflammatory function (28). This dual role of IL-18BP helps
maintain balance in the host immune system. IL-18BP provides a
tipping point and once the amount of IL-18 exceeds this tipping
point, IL-18BP is unable to prevent IL-18-mediated Th1 immune
response. In a clinical setting, children with IL-18BP deficiency
and hepatitis A ultimately die from severe viral hepatitis
(29). A key factor is in this
outcome is lack of IL-18 inhibition due to the IL-18BP defect,
which leads to the progression of inflammation to a malignant
state. The return of the normal balance of IL-18 and IL-18BP
expression levels predicts the outcome of health. The role of
IL-18BP in immune regulation (30,31),
immunoprophylaxis (32-34)
and disease recovery (35-37)
in animals and humans provides broad application prospects in human
and veterinary medicine.
IL-18BP mRNA is highly expressed in human heart,
lung, placenta, spleen and colon tissue (38). IL-18BP is also strongly expressed in
the hypothalamus of Sprague-Dawley rats (39). Only low levels of IL-18BP mRNA are
found in unstimulated human keratinocytes and colon cancer and
glomerular mesangial cells (3).
Serum levels in healthy mice show a 20-fold
difference between IL-18BP and IL-18(8). Due to the one-to-one binding
characteristics of IL-18 and IL-18BP, the bound count vs. free
IL-18 in a mixture of both molecules can be calculated. This
balance and concentrations of free IL-18 predict whether subjects
are healthy. IL-18BP A is elevated (21.9±1.44 ng/ml) in the serum
and total IL-18 is elevated to 1.5±0.4 ng/ml in patients with
sepsis upon admission. At these levels, most IL-18 is bound to
IL-18BP A. However, the remaining free IL-18 levels are higher than
in healthy individuals (64±17 pg/ml). IL-18BPa inhibits circulating
IL-18 in sepsis and further decreases circulating IL-18 activity
(24).
IL-18BP has an immunoglobulin (Ig) region that
resembles the extracellular Ig structure of the cytokine receptor,
which differs from the IL-1 and IL-18 receptor families in its
three IgG domains (40). The Ig
region of IL-18BP is necessary for the protein function and its
binding and inhibition of IL-18 are associated with this region.
Sequence analysis confirms that the human and mouse IL-18BP genes
are 585 and 582 bp, respectively. Human IL-18BP consists of 164
amino acid residues. Its signal peptide contains 30 amino acid
residues. There are four N glycosylation sites in human IL-18BP.
The mature peptide contains 134 amino acids (42). Predictive analysis of hydrophilicity
and hydrophobicity shows that IL-18BP has no transmembrane region
(42). Mouse IL-18BP comprises 165
amino acid residues, including a signal peptide with 28 amino acid
residues and four N glycosylation sites. The amino acid sequences
are 60.8% homologous. IL-18BP gene in human is located in
chromosome 11Q13, encoded by exon without a transmembrane region
(43).
At least four IL-18BP isoforms are present in human
cDNA libraries due to tissue specificity and mRNA shearing. IL-18BP
sequences differ primarily in their carboxyl termini whereas the
N-terminal is identical (44). The
human IL-18BP A is abundant in cDNA libraries. IL-18BP B is found
in monocytes and Jurkat libraries, IL-18BP C is found in spleen and
Jurkat libraries, and IL-18BP D is only found in Jurkat cells.
Libraries have different IL-18BP isoforms which are key for immune
response. The amino acids of 1/3 to 2/3 of isoforms are the same.
The difference mainly lies in the C terminal. A total of six
IL-18BP isoforms have different functions due to different
conformation of their c-terminal amino acid residues and Ig region.
Human IL-18BP A and C differ only in the 29 amino acid residues at
the c-terminal. IL-18BP A has a high affinity with human IL-18 with
a dissociation constant of 399 nM. IL-18BP C can also bind to IL-18
with a dissociation constant of 2.94 nM. Since their Ig regions are
similar, human IL-18BP A and C can inhibit biological activity of
IL-18. However, human IL-18BP B and IL-18BP D lack a complete Ig
region so they cannot bind to IL-18 or inhibit its activity. A
total of two IL-18BP isoforms of mice are also found. Mouse IL-18BP
C and IL-18BP D have a complete Ig region and interact with mice
IL-18 to act as inhibitors. Moreover, mouse IL-18BP D and human
IL-18BP A share the same C-terminal, so mouse IL-18BP D can also
neutralize the effect of human IL-18, indicating the importance of
the Ig region of IL-18BP for its function (44,45).
The serum IL-18BP A levels have a significant
positive correlation with right ventricular systolic pressure
estimated by echocardiography. The signaling inhibition due to
interaction between IL-18 and IL-18BP A may be involved in
development of pulmonary vascular involvement leading to pulmonary
hypertension (46). It also
modulates systemic inflammation in systemic sclerosis. In human
skin cells, ultraviolet irradiation results in a dose-dependent
increase of melanogenesis following treatment with IL-18. However,
IFN-γ has the opposite effect (47). This is caused by IFN-γ markedly
upregulating IL-18BP production in normal human foreskin-derived
epidermal keratinocytes in a dose-dependent manner, indicating the
balance between IL-18BP, IL-18 and IFN-γ. Once external forces
disrupt this normal balance, the host inflammatory response and
occurrence of disease is triggered. There is an imbalance between
IL-18 and IL-18BP in the circulation of individuals with human
immunodeficiency virus (HIV), which may explain why HIV-infected
long-term non-progressors are able to delay autoimmune deficiency
syndrome (AIDS) progression (48).
Patients with autoinflammatory disorders also have high serum
levels of IL-18, without a corresponding increase in IL-18BP or
IL-1β (49). There are high serum
levels of total and free IL-18, IL-18BP and IL-37 in patients with
primary Sjögren's syndrome compared with healthy controls (50). To inhibit the inflammatory storm
caused by IL-18, small molecule inhibitors have been investigated
to block the IL-18-induced production of IFN-γ, which is associated
with inflammatory disease such as rheumatoid arthritis and Crohn's
disease (51). The small molecules
disrupt IL-18 binding to IL-18BP and its cognate receptors. In
patients with allergic asthma, expression of IL-18, IL-18BP and
IL-18R is increased (9). Similarly,
enhanced expression of IL-18 and IL-18BP is observed in the plasma
of patients with eczema (19).
IL-18 and IL-18BP are present in narrow ratio in patients with
non-allergic asthma and these cytokines exhibit a significant
association with each other. However, the molar concentration ratio
of plasma IL-18BP/IL-18 in skin mast cells of patients with eczema
is decreased. Additionally, the expressions of IL-18BP exhibits a
positive correlation in eosinophil-enriched cells (19). The concentrations of IL-18BP A and
IL-36 receptor antagonist (IL-36RA) increase following peripheral
blood mononuclear cell (PBMC) exposure to culture-derived hepatitis
C virus (52). There are
significant correlations between IL-18BP A and indices of liver
inflammation and fibrosis (52).
Furthermore, genetic variations in IL-18BP are linked to hepatitis
A severity (53). C-C Chemokine
receptor Type 2 (CCR2) antagonist RS504393 elevates the levels of
IL-18BP and decreases the mRNA and/or protein levels of
antinociceptive factors (54). CCR2
may be a promising target for decreasing neuropathic pain and
augmenting the effects of opioid analgesia and overexpression
IL-18BP can counteract the effects of IL-18 in asthma (9). Therapies that enhance IL-18BP activity
or block IL-18R may be beneficial for treating asthma. Systemic
juvenile idiopathic arthritis and adult-onset Still's disease
(AOSD) are associated with high serum IL-18 concentration and can
be treated with IL-18BP (55,56).
As a treatment option for AOSD, recombinant human IL-18BP,tadekinig
alfa, in patients receiving either 80 mg or 160 mg tadekinig alfa
showed good safety profiles and early signs of efficacy (56).
IL-18BP-/- mice display more
severe manifestations of macrophage activation syndrome (MAS) than
wild-type mice when persistently stimulated by toll-like receptor 9
(TLR9) with unmethylated cytosine guanine dinucleotide containing
single-stranded DNA (CpG) (57).
Endogenous IL-18BP provides a protective role against MAS induced
by CpG. When exploring the effect of intrathecal administration of
bovine lactoferrin, in combination with signal transduction pathway
inhibition or an inflammatory cytokine production to
allodynia/hyperalgesia in the whisker pad area following mental
nerve transection (MNT) in rats, it was found that IL-18BP also
attenuates allodynia/hyperalgesia and IL-18 upregulation, similar
to bovine lactoferrin (58).
Dysregulated production of cytokines has a significant effect on
systemic lupus; higher levels of total IL-18, IL-18BP, IL-1Ra and
soluble receptor sIL-1R4 are observed in systemic lupus
erythematosus (SLE) (59),
suggesting that IL-18 and IL-18BP are upregulated in SLE. Treatment
of multiple sclerosis (MS) with IFN-β significantly downregulates
IL-18 and IL-18BP to normal levels, suggesting that its therapeutic
effect on MS may be, at least in part, due to its ability to slow
progression of disease on multiple levels (60). IL-18BP as an inhibitor to neutralize
IL-18 can inhibit the production of cytokines inducing injury such
as IL-6, IFN-γ, TNF-α, C-X3-C motif chemokine ligand 1 (CX3CL1) and
CXCL10 and improve allograft function (61). Therefore, IL-18BP may play an
important role in organ transplantation.
A number of experimental data has demonstrated the
positive role of IL-18BP in disease recovery (9,62-65).
It has been reported that remifentanil can protect the liver
against ischemia/reperfusion injury by upregulating hepatic
expression of IL-18BP (66). The
underlying mechanisms are hypothesized to be due to transcriptional
activation of the IL-18BP promoter, which can upregulate hepatic
IL-18BP expression (66). IL-18BP
pretreatment has been observed to suppress the infiltration of
inflammatory cells and release of inflammatory factors in acute
lung injury (ALI) mice in vivo and in primary macrophages
stimulated with lipopolysaccharide (LPS) in vitro (67-69).
Additionally, IL-18BP decreases activation of NF-κB and upregulates
Nrf2(70). This indicates IL-18BP
has potential pharmaceutical applications for ALI treatment
(70). IL-18BP is able to provide a
protective effect against renal fibrosis by neutralizing IL-18
biological activity (65).
Neutralization between IL-18 and IL-18Bp improves survival rate and
bleomycin (BLM)-induced pulmonary fibrosis (PF) in mice. IL-18BP
suppress the BLM-induced epithelial mesenchymal transition in
vivo (71). These findings
indicate IL-18BP as a potential option for PF therapy. IL-18BP as
an antagonist of IL-18 can neutralize the toxicity of human IL-18
in the liver. A 40-nucleotide deletion in IL18-BP results in loss
of function. In the absence of IL-18BP, excessive NK cell
activation by IL-18 leads to uncontrolled killing of human
hepatocytes in vitro (29).
Mouse (57,72) models have shown that IL-18BP has a
restorative effect on albuminuria and histopathological injury of
the kidney. It also restores induction of serum cytokines in mouse
model of minimal change disease induced by Adriamycin (63). The relative expression of IL-18 and
IL-18BP mRNA is significantly elevated in patients with active and
latent tuberculosis. The significant increase in IL-18 and IL-18BP,
as well as IFN-γ mRNA expression, is a manifestation of active
tuberculosis disease (20).
Administration of recombinant human IL-18BP enhances the survival
rate of CD2F1 mice compared with vector control-treated group.
Additionally, IL-18BP therapy inhibits expression of IFN-γ
targeting IL-18 downstream in mouse bone marrow. It also decreases
reactive oxygen species levels in irradiated mouse heart tissue,
weakens expression of stress responsive factor growth
differentiation factor-15 and improves the intestine protector
citrulline levels in serum of total body irradiated mice (73). This implies that IL-18BP may defend
multiple organs from radiation-induced inflammation and oxidative
stress. Gene expression analysis of patients with idiopathic
pulmonary fibrosis indicates that serum IL-18BP levels are
significantly higher than in healthy volunteers; independent
correlation between serum IL-18BP levels and idiopathic pulmonary
fibrosis suggests a novel prognostic biomarker for idiopathic
pulmonary fibrosis (74). Levels of
nine circulating cytokines, including IL-18 and IL-18BP, are
significantly higher in patients with proteinase 3-antineutrophil
cytoplasmic antibody compared with myeloperoxidase-associated
vasculitis (75). IL-18, IL-18BP
and resistin are considered to be circulating markers of
inflammation that explain seasonal variations in the morbidity and
severity of immune-mediated diseases (76).
IL-18BP as a tool for disease treatment and recovery
can be applied to patient care and potential drug development
(77-80).
By artificially injecting IL-18BP, the free IL-18 in the body can
be neutralized, tissue damage caused by inflammation can be reduced
and recovery accelerated (81-85).
The pro-inflammatory effects of IL-1 family
cytokines are determined by levels of transcription, expression of
decoy receptors, enzymatic processing of precursors and release of
soluble antagonists (95). IL-18BP
binding to IL-18 can competitively inhibit binding from the protein
products of the IL18R1 and IL18RAP genes (96). Additionally, IL-1R accessory
protein-like 2 and IL-1R8 show a similar amino acid sequence to
binding site A of human and viral IL-18BP (96). IL1R9 has similar structure to IL18BP
with conserved intron/exon boundaries, protein structure, and key
binding site amino acids by bioinformatics approaches. IL1R9,
IL18R1, IL18RAP or IL1R9 all bind IL-18. Human platelets contain
IL-18BP, which is present in pre-made form and is released
irrespective of platelet activation. Plasma and Platelet-Poor
Plasma (PPP) samples from healthy donors contains comparable
amounts of IL-18BP, while the PPP from HIV-infected people contains
notable amounts of IL-18. IL-18 and IL-18BP co-localize to alpha
granules inside platelets and are secreted out with different
kinetics (97).
IL-18 signaling is mediated by the inhibitory
effects of IL-18BP. The reduced abundance of splenic NK cells in
IL-18BP knockout mice, as well as, the increased abundance of
immature NK cells and the reduced mature population of NK cells
demonstrate that IL-18BP disrupts NK cell maturation and
contributes to sustaining steady-state levels of circulating
IL-18(69). IL-18BP appears to
function as a carrier protein, not just an inhibitor. The balance
of IL-18/IL-18BP/IL-18R expression in inflammatory cells, such as
monocytes, neutrophils and B cells, determines the role of IL-18 in
asthma (9). IL-18BP inducibility is
notable in human epithelial cells but weakened in monocytes.
Epigenetic silencing by single CpG methylation causes differential
IL18BP regulation in both types of cell. A specific CpG (coined
CpG2) adjacent to a γ-activated site is responsible for IL18BP
induction (98). In a mouse asthma
model, lower IL-18BP+ but increased IL-18R+
basophils in blood and IL-18BP+ mast cells in the
bronchoalveolar lavage fluid as well as enhanced IL-18R+
mast cells in the lung indicate that mast cells and basophils may
be associated with asthma pathogenesis by an IL-18-associated
mechanism (99). The persistent
IL-18 inhibition via IL-18BP leads to diminished cardiac fibrosis
and NF-κB phosphorylation, normalized electrical remodeling,
reformative diastolic function and attenuated IL-18-mediated
ventricular tachycardia (VT) in sickle cell disease mice. This
indicates that IL-18 is a mediator of VT and sickle cell
cardiomyopathy in mice, providing a novel therapeutic application
for patients at risk of sudden cardiac death (100). The mRNA levels of IL-18BP, IL-18
and IL-18R are notably increased in clinical pituitary tumors
compared with non-functional adenomas (101). This indicates that elevated
expression of IL-18BP follows increased expression of IL-18.
However, the mRNA and protein expression of IL-18BP, IL-18, IL-18Rα
and IL-18Rβ in epithelial cells from subjects with asthma is
different. IL-18 expression is decreased and the IL-18BP is absent.
However, IL-18Rα expression is not different between healthy and
asthmatic patients (21). A novel
tetramer with 2:2 stoichiometry is exhibited in the crystal
structure of the IL-18:IL-18BP complex. It has a higher-order
assembly between IL-18 and IL-18BP harmonized by a disulfide-bond
distal to the binding surface connecting two molecules (102).
IL-18BP binds cytokine IL-37 and serves as a sink
for the anti-inflammatory IL-18(103), potentially playing a crucial role
in tumor escape from immune surveillance. The concurrent increase
serum levels of IL-37 and IL-18BP and their positive correlation
may promote disease progression in low- and high-grade brain tumors
by suppressing antitumor immune responses (79).
IL-18BP, as the target of microRNA-134 (miR-134),
participates in the regulation of the activation of TLR3.
Expression of microRNA-134 (miR-134) has a negative correlation
with IL-18BP mRNA levels in peripheral blood cells following TLR3
ligand treatment (107). Shi et
al found that the activation of NLRP3 inflammasome induced by
Baicalin, a type of Chinese herbal medicine, could result in
increased hepatic expression of IL-18 and IL-1β, which alleviates
liver regeneration in acetaminophen-intoxicated mice. MCC950, a
NLRP3 inhibitor, and recombinant mouse IL-18BP can decrease this
promotion (108).
Homologs of IL18-BP are also encoded by many pox
viruses including MCV and orthopox viruses (113), such as variola virus (114), the causative agent of smallpox
(94). A previous report indicated
that IL-18BP of variola virus prevents IL-18 from binding to IL-18R
by interacting with three residues: Lys53, Ser55 and Leu5, which
are part of the binding site for hIL-18Rα (93). Yaba-like disease virus IL-18BP forms
a disulfide bonded homo-dimer that engages IL-18 in a 2:2
stoichiometry, with the absence of the key lysine-phenylalanine
interaction (115). A 14L protein
from Yaba monkey tumor virus, which is similar to orthopoxvirus
IL-18BPs, can bind both human and murine IL-18 with high affinity,
at 4.1 and 6.5 nM, respectively (116). It is also reported that the genes
of several poxviruses, including vaccinia (117), ectromelia and cowpox viruses
(118), encode proteins with
sequence similarity to IL-18BPs. The ectromelia virus protein
blocks NF-κB activation and induction of IFN-γ in response to
IL-18. An attenuated vaccinia virus, Ankara, encodes IL-18-binding
activity to improve the safety and immunogenicity of this promising
human vaccine candidate (118).
The dissociation constants of viral proteins for murine IL-18 are
12-50-fold lower than that for human IL-18. ectvIL-18BP adopts a
canonical Ig fold and interacts via one edge of its β-sandwich with
three cavities on the hIL-18 surface through extensive hydrophobic
and hydrogen bonding interactions. This blocks a putative
receptor-binding site on IL-18, preventing IL-18 from engaging its
receptor (94). Variola virus
protein D7L and ectromelia virus protein P13 both have a higher
affinity for murine than human IL-18. D7L interacts with
glycosaminoglycans (GAGs) via the C terminus, while P13 does not.
D7L interacts with both GAG and IL-18 simultaneously, indicating
that the binding sites are distinct (114). A total of three mutations (F49A,
E77A and E69A) significantly affect binding with both species of
IL-18, leading to the complete abrogation of binding affinity, to
different extents. However, mutant H70A shows reduced affinity for
human IL-18, while binding to murine IL-18 is not affected
(119). These proteins antagonize
the inflammatory response to infection, which is one of the
mechanisms by which pox viruses evade immune defense. IL-18BP is
predicted to have putative functions involving immune evasion in a
pathogenic fowlpox virus (120).
IL-18BP is a glycoprotein with an Ig region, a
member of the immunoglobulin superfamily. A total of six
isoproteins of IL-18BP have been found. IL-18BP can effectively
inhibit the action of IL-18 in vivo and in vitro and
is a natural antagonist of IL-18. The development of many types of
inflammatory disease is accompanied by changes in the expression of
IL-18BP and IL-18. In addition, proteins encoded by several pox
viruses have high homology with IL-18BP, and their viral products
attenuate the IL-18-induced Th1 response. Gene therapy utilizing
the antagonistic effect of IL-18BP against IL-18 may provide novel
treatment for certain types of autoimmune disease. IL-18BP has a
promising role in immune regulation and immunoprophylaxis and
potentially in disease recovery in animals and humans.
Not applicable.
Funding: The preset study was supported by the Inner Mongolia
Agricultural University Young Teachers Research Ability Promotion
Project (grant no. BR220113) and the Education Department of the
Inner Mongolia Autonomous Region ‘Young Scientific and
Technological Talents in Universities’ Project (grant no.
NJYT22043).
Not applicable.
FXW wrote and edited the manuscript. Data
authentication is not applicable. The author has read and approved
the final manuscript.
Not applicable.
Not applicable.
The author declares that he has no competing
interests.
|
1
|
Dinarello CA, Novick D, Rubinstein M and
Lonnemann G: Interleukin 18 and interleukin 18 binding protein:
Possible role in immunosuppression of chronic renal failure. Blood
Purif. 21:258–270. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim YM, Kang HS, Paik GS, Pyun GH,
Anderson KL, Torbett BE and Choi I: Roles of IFN consensus sequence
binding protein and PU.1 in regulating IL-18 gene expression. J
Immunol. 163:2000–2007. 1999.PubMed/NCBI
|
|
3
|
Muhl H, Kampfer H, Bosmann M, Frank S,
Radeke H and Pfeilschifter J: Interferon-gamma mediates gene
expression of IL-18 binding protein in nonleukocytic cells. Biochem
Biophys Res Commun. 267:960–963. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mazodier K, Marin V, Novick D, Farnarier
C, Robitail S, Schleinitz N, Veit V, Paul P, Rubinstein M,
Dinarello CA, et al: Severe imbalance of IL-18/IL-18BP in patients
with secondary hemophagocytic syndrome. Blood. 106:3483–3489.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chirathaworn C, Rianthavorn P,
Wuttirattanakowit N and Poovorawan Y: Serum IL-18 and IL-18BP
levels in patients with Chikungunya virus infection. Viral Immunol.
23:113–117. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Migliorini P, Anzilotti C, Pratesi F,
Quattroni P, Bargagna M, Dinarello CA and Boraschi D: Serum and
urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus
erythematosus. Eur Cytokine Netw. 21:264–271. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shan NN, Wang X, Zhu XJ, Peng J and Hou M:
Role of IL-18/IL-18BP balance in spleen of patients with primary
immune thrombocytopenia. Zhonghua Yi Xue Za Zhi. 91:239–242.
2011.PubMed/NCBI(In Chinese).
|
|
8
|
Ha CT, Li X, Fu D and Xiao M: Circulating
IL-18 Binding Protein (IL-18BP) and IL-18 as dual biomarkers of
total-body irradiation in mice. Radiat Res. 185:375–383.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang H, Wang J, Wang L, Xie H, Chen L and
He S: Role of IL-18 in atopic asthma is determined by balance of
IL-18/IL-18BP/IL-18R. J Cell Mol Med. 22:354–373. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mochol M, Tauboll E, Aukrust P, Ueland T,
Andreassen OA and Svalheim S: Interleukin 18 (IL-18) and its
binding protein (IL-18BP) are increased in patients with epilepsy
suggesting low-grade systemic inflammation. Seizure. 80:221–225.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yoshino O, Osuga Y, Koga K, Tsutsumi O,
Yano T, Fujii T, Kugu K, Momoeda M, Fujiwara T, Tomita K and
Taketani Y: Evidence for the expression of interleukin (IL)-18,
IL-18 receptor and IL-18 binding protein in the human endometrium.
Mol Hum Reprod. 7:649–654. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Torigoe K, Ushio S, Okura T, Kobayashi S,
Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, et
al: Purification and characterization of the human interleukin-18
receptor. J Biol Chem. 272:25737–25742. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hoshino K, Tsutsui H, Kawai T, Takeda K,
Nakanishi K, Takeda Y and Akira S: Cutting edge: generation of
IL-18 receptor-deficient mice: evidence for IL-1 receptor-related
protein as an essential IL-18 binding receptor. J Immunol.
162:5041–5044. 1999.PubMed/NCBI
|
|
14
|
Thomassen E, Bird TA, Renshaw BR, Kennedy
MK and Sims JE: Binding of interleukin-18 to the interleukin-1
receptor homologous receptor IL-1Rrp1 leads to activation of
signaling pathways similar to those used by interleukin-1. J
Interferon Cytokine Res. 18:1077–1088. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Debets R, Timans JC, Churakowa T, Zurawski
S, de Waal Malefyt R, Moore KW, Abrams JS, O'Garra A, Bazan JF and
Kastelein RA: IL-18 receptors, their role in ligand binding and
function: anti-IL-1RAcPL antibody, a potent antagonist of IL-18. J
Immunol. 165:4950–4956. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shao XT, Feng L, Gu LJ, Wu LJ, Feng TT,
Yang YM, Wu NP and Yao HP: Expression of interleukin-18, IL-18BP,
and IL-18R in serum, synovial fluid, and synovial tissue in
patients with rheumatoid arthritis. Clin Exp Med. 9:215–221.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Corbaz A, ten Hove T, Herren S, Graber P,
Schwartsburd B, Belzer I, Harrison J, Plitz T, Kosco-Vilbois MH,
Kim SH, et al: IL-18-binding protein expression by endothelial
cells and macrophages is up-regulated during active Crohn's
disease. J Immunol. 168:3608–3616. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Medina L, Rabinovich A, Piura B, Dyomin V,
Levy RS and Huleihel M: Expression of IL-18, IL-18 binding protein,
and IL-18 receptor by normal and cancerous human ovarian tissues:
Possible implication of IL-18 in the pathogenesis of ovarian
carcinoma. Mediators Inflamm. 2014(914954)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu Y, Wang J, Zhang H, Xie H, Song W,
Jiang Q, Zhao N and He S: Enhanced Expression of IL-18 and IL-18BP
in Plasma of Patients with Eczema: Altered Expression of IL-18BP
and IL-18 receptor on mast cells. Mediators Inflamm.
2017(3090782)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wawrocki S, Kielnierowski G, Rudnicka W,
Seweryn M and Druszczynska M: Interleukin-18, Functional IL-18
Receptor and IL-18 binding protein expression in active and latent
tuberculosis. Pathogens. 9(451)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kaur D, Chachi L, Gomez E, Sylvius N and
Brightling CE: Interleukin-18, IL-18 binding protein and IL-18
receptor expression in asthma: A hypothesis showing IL-18 promotes
epithelial cell differentiation. Clin Transl Immunology.
10(e1301)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prencipe G, Bracaglia C and De Benedetti
F: Interleukin-18 in pediatric rheumatic diseases. Curr Opin
Rheumatol. 31:421–427. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Novick D, Elbirt D, Dinarello CA,
Rubinstein M and Sthoeger ZM: Interleukin-18 binding protein in the
sera of patients with Wegener's granulomatosis. J Clin Immunol.
29:38–45. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Novick D, Schwartsburd B, Pinkus R, Suissa
D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G,
et al: A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis
and extensive decrease of free IL-18. Cytokine. 14:334–342.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tschoeke SK, Oberholzer A and Moldawer LL:
Interleukin-18: A novel prognostic cytokine in bacteria-induced
sepsis. Crit Care Med. 34:1225–1233. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen O, Shan N, Zhu X, Wang Y, Ren P, Wei
D and Sun R: The imbalance of IL-18/IL-18BP in patients with
systemic juvenile idiopathic arthritis. Acta Biochim Biophys Sin
(Shanghai). 45:339–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Harel M, Girard-Guyonvarc'h C, Rodriguez
E, Palmer G and Gabay C: Production of IL-18 binding protein by
radiosensitive and radioresistant cells in CpG-Induced macrophage
activation syndrome. J Immunol. 205:1167–1175. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McCurdy S, Yap J, Irei J, Lozano J and
Boisvert WA: IL-37-a putative therapeutic agent in cardiovascular
diseases. QJM. 115:719–725. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Belkaya S, Michailidis E, Korol CB,
Kabbani M, Cobat A, Bastard P, Lee YS, Hernandez N, Drutman S, de
Jong YP, et al: Inherited IL-18BP deficiency in human fulminant
viral hepatitis. J Exp Med. 216:1777–1790. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bufler P, Azam T, Gamboni-Robertson F,
Reznikov LL, Kumar S, Dinarello CA and Kim SH: A complex of the
IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18
activity. Proc Natl Acad Sci USA. 99:13723–13728. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu W, Liu G, Qin X and Wang G:
Recombinant cIL-18-binding protein as an antagonist to cIL-18
enhanced PBMCs secreting IFN-gamma. Wei Sheng Wu Xue Bao.
50:506–511. 2010.PubMed/NCBI(In Chinese).
|
|
32
|
Reznikov LL, Kim SH, Westcott JY, Frishman
J, Fantuzzi G, Novick D, Rubinstein M and Dinarello CA: IL-18
binding protein increases spontaneous and IL-1-induced
prostaglandin production via inhibition of IFN-gamma. Proc Natl
Acad Sci USA. 97:2174–2179. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Faggioni R, Cattley RC, Guo J, Flores S,
Brown H, Qi M, Yin S, Hill D, Scully S, Chen C, et al:
IL-18-binding protein protects against lipopolysaccharide-induced
lethality and prevents the development of Fas/Fas ligand-mediated
models of liver disease in mice. J Immunol. 167:5913–5920.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Plitz T, Saint-Mezard P, Satho M, Herren
S, Waltzinger C, de Carvalho Bittencourt M, Kosco-Vilbois MH and
Chvatchko Y: IL-18 binding protein protects against contact
hypersensitivity. J Immunol. 171:1164–1171. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lin XL, Zhu J, Wang LM, Yan F, Sha WP and
Yang HL: MiR-92b-5p inhibitor suppresses IL-18 mediated
inflammatory amplification after spinal cord injury via IL-18BP
up-regulation. Eur Rev Med Pharmacol Sci. 23:1891–1898.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yatsiv I, Morganti-Kossmann MC, Perez D,
Dinarello CA, Novick D, Rubinstein M, Otto VI, Rancan M, Kossmann
T, Redaelli CA, et al: Elevated intracranial IL-18 in humans and
mice after traumatic brain injury and evidence of neuroprotective
effects of IL-18-binding protein after experimental closed head
injury. J Cereb Blood Flow Metab. 22:971–978. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shan NN, Ji XB, Wang X, Li Y, Liu X, Zhu
XJ and Hou M: In vitro recovery of Th1/Th2 balance in PBMCs from
patients with immune thrombocytopenia through the actions of
IL-18BPa/Fc. Thromb Res. 128:e119–e124. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ushio S, Namba M, Okura T, Hattori K,
Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, et al:
Cloning of the cDNA for human IFN-gamma-inducing factor, expression
in Escherichia coli, and studies on the biologic activities of the
protein. J Immunol. 156:4274–4279. 1996.PubMed/NCBI
|
|
39
|
Alboni S, Benatti C, Montanari C, Tascedda
F and Brunello N: Chronic antidepressant treatments resulted in
altered expression of genes involved in inflammation in the rat
hypothalamus. Eur J Pharmacol. 721:158–167. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Novick D, Kim SH, Fantuzzi G, Reznikov LL,
Dinarello CA and Rubinstein M: Interleukin-18 binding protein: A
novel modulator of the Th1 cytokine response. Immunity. 10:127–136.
1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dinarello CA: Novel targets for
interleukin 18 binding protein. Ann Rheum Dis. 60 (Suppl
3):iii18–iii24. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aizawa Y, Akita K, Taniai M, Torigoe K,
Mori T, Nishida Y, Ushio S, Nukada Y, Tanimoto T, Ikegami H, et al:
Cloning and expression of interleukin-18 binding protein. FEBS
Lett. 445:338–342. 1999.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Maiti PK, Im SH, Souroujon MC and Fuchs S:
A monoclonal antibody specific for rat IL-18BP and its application
in determining serum IL-18BP. Immunol Lett. 85:65–70.
2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim SH, Eisenstein M, Reznikov L, Fantuzzi
G, Novick D, Rubinstein M and Dinarello CA: Structural requirements
of six naturally occurring isoforms of the IL-18 binding protein to
inhibit IL-18. Proc Natl Acad Sci USA. 97:1190–1195.
2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee S, Kim S, Bae S, Choi J, Hong J, Ryoo
S, Jhun H, Hong K, Kim E, Jo S, et al: Development of
isoform-specific monoclonal antibodies against human IL-18 binding
protein. Hybridoma (Larchmt). 29:517–524. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nakamura K, Asano Y, Taniguchi T,
Minatsuki S, Inaba T, Maki H, Hatano M, Yamashita T, Saigusa R,
Ichimura Y, et al: Serum levels of interleukin-18-binding protein
isoform a: Clinical association with inflammation and pulmonary
hypertension in systemic sclerosis. J Dermatol. 43:912–918.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhou J, Ling J, Wang Y, Shang J and Ping
F: Cross-talk between interferon-gamma and interleukin-18 in
melanogenesis. J Photochem Photobiol B. 163:133–143.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Iannello A, Samarani S, Allam O, Jenabian
MA, Mehraj V, Amre D, Routy JP, Tremblay C and Ahmad A: A
potentially protective role of IL-18 Binding Protein in
HIV-infected Long-Term Non-Progressors. Cytokine. 90:96–99.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Standing AS, Malinova D, Hong Y, Record J,
Moulding D, Blundell MP, Nowak K, Jones H, Omoyinmi E, Gilmour KC,
et al: Autoinflammatory periodic fever, immunodeficiency, and
thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene
WDR1. J Exp Med. 214:59–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liuqing W, Liping X, Hui S and Jing L:
Elevated IL-37, IL-18 and IL-18BP serum concentrations in patients
with primary Sjogren's syndrome. J Investig Med. 65:717–721.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Krumm B, Meng X, Xiang Y and Deng J:
Identification of small molecule inhibitors of Interleukin-18. Sci
Rep. 7(483)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mele D, Mantovani S, Oliviero B, Grossi G,
Lombardi A, Mondelli MU and Varchetta S: Monocytes inhibit
hepatitis C virus-induced TRAIL expression on CD56bright
NK cells. J Hepatol. 67:1148–1156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang M and Feng Z: Mechanisms of
hepatocellular injury in hepatitis A. Viruses.
13(861)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kwiatkowski K, Piotrowska A, Rojewska E,
Makuch W and Mika J: The RS504393 influences the level of
nociceptive factors and enhances opioid analgesic potency in
neuropathic rats. J Neuroimmune Pharmacol. 12:402–419.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yasin S, Solomon K, Canna SW,
Girard-Guyonvarc'h C, Gabay C, Schiffrin E, Sleight A, Grom AA and
Schulert GS: IL-18 as therapeutic target in a patient with
resistant systemic juvenile idiopathic arthritis and recurrent
macrophage activation syndrome. Rheumatology (Oxford). 59:442–445.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gabay C, Fautrel B, Rech J, Spertini F,
Feist E, Kotter I, Hachulla E, Morel J, Schaeverbeke T, Hamidou MA,
et al: Open-label, multicentre, dose-escalating phase II clinical
trial on the safety and efficacy of tadekinig alfa (IL-18BP) in
adult-onset Still's disease. Ann Rheum Dis. 77:840–847.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Girard-Guyonvarc'h C, Palomo J, Martin P,
Rodriguez E, Troccaz S, Palmer G and Gabay C: Unopposed IL-18
signaling leads to severe TLR9-induced macrophage activation
syndrome in mice. Blood. 131:1430–1441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Horie K, Watanabe M, Chanbora C, Awada T,
Kunimatsu R, Uchida T, Takata T and Tanimoto K: Bovine lactoferrin
reduces extra-territorial facial allodynia/hyperalgesia following a
trigeminal nerve injury in the rat. Brain Res. 1669:89–96.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Italiani P, Manca ML, Angelotti F, Melillo
D, Pratesi F, Puxeddu I, Boraschi D and Migliorini P: IL-1 family
cytokines and soluble receptors in systemic lupus erythematosus.
Arthritis Res Ther. 20(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
D'Angelo C, Reale M, Costantini E, Di
Nicola M, Porfilio I, de Andres C, Fernandez-Paredes L,
Sanchez-Ramon S and Pasquali L: Profiling of Canonical and
Non-Traditional Cytokine Levels in Interferon-β-Treated
relapsing-remitting-multiple sclerosis patients. Front Immunol.
9(1240)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu C, Chen J, Liu B, Yuan S, Shou D, Wen
L, Wu X and Gong W: Role of IL-18 in transplant biology. Eur
Cytokine Netw. 29:48–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Weiss ES, Girard-Guyonvarc'h C, Holzinger
D, de Jesus AA, Tariq Z, Picarsic J, Schiffrin EJ, Foell D, Grom
AA, Ammann S, et al: Interleukin-18 diagnostically distinguishes
and pathogenically promotes human and murine macrophage activation
syndrome. Blood. 131:1442–1455. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Dong M, Zhao M, Cui M, Sun J, Meng X, Sun
W, Wang L and Du P: Interleukin-18 binding protein attenuates renal
injury of adriamycin-induced mouse nephropathy. Int J Clin Exp
Pathol. 12:3005–3012. 2019.PubMed/NCBI
|
|
64
|
Kim HS, Kim K, Lee H, Yang EA, Chun YH,
Kim HH and Kim JT: Level of interleukin-18 binding protein is
significantly different in patients with anaphylaxis than
urticaria. Asian Pac J Allergy Immunol. 40:368–373. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang LM, Zhang Y, Fei C, Zhang J, Wang L,
Yi ZW and Gao G: Neutralization of IL-18 by IL-18 binding protein
ameliorates bleomycin-induced pulmonary fibrosis via inhibition of
epithelial-mesenchymal transition. Biochem Biophys Res Commun.
508:660–666. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Liu X, Yang H, Liu Y, Jiao Y, Yang L, Wang
X, Yu W, Su D and Tian J: Remifentanil upregulates hepatic IL-18
binding protein (IL-18BP) expression through transcriptional
control. Lab Invest. 98:1588–1599. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang J, Long Q, Zhang W and Chen N:
Protective effects of exogenous interleukin 18-binding protein in a
rat model of acute renal ischemia-reperfusion injury. Shock.
37:333–340. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
O'Brien LC, Mezzaroma E, Van Tassell BW,
Marchetti C, Carbone S, Abbate A and Toldo S: Interleukin-18 as a
therapeutic target in acute myocardial infarction and heart
failure. Mol Med. 20:221–229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Harms RZ, Creer AJ, Lorenzo-Arteaga KM,
Ostlund KR and Sarvetnick NE: Interleukin (IL)-18 binding protein
deficiency disrupts natural killer cell maturation and diminishes
circulating IL-18. Front Immunol. 8(1020)2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhang LM, Zhang J, Zhang Y, Wang L, Fei C,
Yi ZW and Dong L: Interleukin-18 binding protein attenuates
lipopolysaccharide-induced acute lung injury in mice via
suppression NF-κB and activation Nrf2 pathway. Biochem Biophys Res
Commun. 505:837–842. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Li YH, Wei X, Ji S, Gui SY and Zhang SM:
In vivo effects of the NLRP1/NLRP3 inflammasome pathway on latent
respiratory virus infection. Int J Mol Med. 41:3620–3628.
2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Doi K, Katagiri D, Negishi K, Hasegawa S,
Hamasaki Y, Fujita T, Matsubara T, Ishii T, Yahagi N, Sugaya T and
Noiri E: Mild elevation of urinary biomarkers in prerenal acute
kidney injury. Kidney Int. 82:1114–1120. 2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Li X, Cui W, Hull L, Wang L, Yu T and Xiao
M: IL-18 binding protein (IL-18BP) as a novel radiation
countermeasure after radiation exposure in mice. Sci Rep.
10(18674)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Nakanishi Y, Horimasu Y, Yamaguchi K,
Sakamoto S, Masuda T, Nakashima T, Miyamoto S, Iwamoto H, Ohshimo
S, Fujitaka K, et al: IL-18 binding protein can be a prognostic
biomarker for idiopathic pulmonary fibrosis. PLoS One.
16(e0252594)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Berti A, Warner R, Johnson K, Cornec D,
Schroeder D, Kabat B, Langford CA, Hoffman GS, Fervenza FC,
Kallenberg CGM, et al: Brief Report: Circulating cytokine profiles
and antineutrophil cytoplasmic antibody specificity in patients
with antineutrophil cytoplasmic antibody-associated vasculitis.
Arthritis Rheumatol. 70:1114–1121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ter Horst R, Jaeger M, van de Wijer L, van
der Heijden WA, Janssen AMW, Smeekens SP, Brouwer MAE, van
Cranenbroek B, Aguirre-Gamboa R, Netea-Maier RT, et al: Seasonal
and nonseasonal longitudinal variation of immune function. J
Immunol. 207:696–708. 2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Min HK, Kim S, Lee JY, Kim KW, Lee SH and
Kim HR: IL-18 binding protein suppresses IL-17-induced
osteoclastogenesis and rectifies type 17 helper T cell/regulatory T
cell imbalance in rheumatoid arthritis. J Transl Med.
19(392)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Otterdal K, Berg A, Michelsen AE, Yndestad
A, Patel S, Gregersen I, Halvorsen B, Ueland T, Langeland N and
Aukrust P: IL-18 and IL-18 binding protein are related to disease
severity and parasitemia during falciparum malaria. BMC Infect Dis.
21(1073)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Haghshenas MR, Saffarian A,
Khademolhosseini A, Dehghanian A, Ghaderi A and Sotoodeh Jahromi A:
Simultaneous Increase in Serum Levels of IL-37 and IL-18 binding
protein in low-grade and high-grade brain tumors. Asian Pac J
Cancer Prev. 23:2851–2856. 2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Yang YC, Chen SN, Gan Z, Huang L, Li N,
Wang KL and Nie P: Functional characterization of IL-18 receptor
subunits, IL-18Rα and IL-18Rβ, and its natural inhibitor, IL-18
binding protein (IL-18BP) in rainbow trout Oncorhynchus mykiss. Dev
Comp Immunol. 140(104610)2023.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Zaccone P, Phillips J, Conget I, Cooke A
and Nicoletti F: IL-18 binding protein fusion construct delays the
development of diabetes in adoptive transfer and
cyclophosphamide-induced diabetes in NOD mouse. Clin Immunol.
115:74–79. 2005.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Thompson SR, Novick D, Stock CJ, Sanders
J, Brull D, Cooper J, Woo P, Miller G, Rubinstein M and Humphries
SE: Free Interleukin (IL)-18 levels, and the impact of IL18 and
IL18BP genetic variation, in CHD patients and healthy men.
Arterioscler Thromb Vasc Biol. 27:2743–2749. 2007.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Cao Q, Cai W, Niu G, He L and Chen X:
Multimodality imaging of IL-18-binding protein-Fc therapy of
experimental lung metastasis. Clin Cancer Res. 14:6137–6145.
2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kimura T, Kato Z, Ohnishi H, Tochio H,
Shirakawa M and Kondo N: Expression, purification and structural
analysis of human IL-18 binding protein: A potent therapeutic
molecule for allergy. Allergol Int. 57:367–376. 2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Leach ST, Messina I, Lemberg DA, Novick D,
Rubenstein M and Day AS: Local and systemic interleukin-18 and
interleukin-18-binding protein in children with inflammatory bowel
disease. Inflamm Bowel Dis. 14:68–74. 2008.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Dao T, Ohashi K, Kayano T, Kurimoto M and
Okamura H: Interferon-gamma-inducing factor, a novel cytokine,
enhances Fas ligand-mediated cytotoxicity of murine T helper 1
cells. Cell Immunol. 173:230–235. 1996.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Favilli F, Anzilotti C, Martinelli L,
Quattroni P, De Martino S, Pratesi F, Neumann D, Beermann S, Novick
D, Dinarello CA, et al: IL-18 activity in systemic lupus
erythematosus. Ann N Y Acad Sci. 1173:301–309. 2009.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Shimizu C, Fujita T, Fuke Y, Ito K,
Satomura A, Matsumoto K and Soma M: High circulating levels of
interleukin-18 binding protein indicate the severity of glomerular
involvement in systemic lupus erythematosus. Mod Rheumatol.
22:73–79. 2012.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Yamamura M, Kawashima M, Taniai M,
Yamauchi H, Tanimoto T, Kurimoto M, Morita Y, Ohmoto Y and Makino
H: Interferon-gamma-inducing activity of interleukin-18 in the
joint with rheumatoid arthritis. Arthritis Rheum. 44:275–285.
2001.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Dinarello CA: Interleukin-18 and the
treatment of rheumatoid arthritis. Rheum Dis Clin North Am.
30:417–434, ix. 2004.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Woldbaek PR, Tonnessen T, Henriksen UL,
Florholmen G, Lunde PK, Lyberg T and Christensen G: Increased
cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial
infarction in the mouse; a potential role in cardiac dysfunction.
Cardiovasc Res. 59:122–131. 2003.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Reznikov LL, Kim SH, Zhou L, Bufler P,
Goncharov I, Tsang M and Dinarello CA: The combination of soluble
IL-18Ralpha and IL-18Rbeta chains inhibits IL-18-induced IFN-gamma.
J Interferon Cytokine Res. 22:593–601. 2002.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Meng X, Leman M and Xiang Y: Variola virus
IL-18 binding protein interacts with three human IL-18 residues
that are part of a binding site for human IL-18 receptor alpha
subunit. Virology. 358:211–220. 2007.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Krumm B, Meng X, Li Y, Xiang Y and Deng J:
Structural basis for antagonism of human interleukin 18 by poxvirus
interleukin 18-binding protein. Proc Natl Acad Sci USA.
105:20711–20715. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Palomo J, Dietrich D, Martin P, Palmer G
and Gabay C: The interleukin (IL)-1 cytokine family-Balance between
agonists and antagonists in inflammatory diseases. Cytokine.
76:25–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Booker CS and Grattan DR: IL1R9 Is
Evolutionarily Related to IL18BP and May Function as an IL-18
Receptor. J Immunol. 198:270–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Allam O, Samarani S, Jenabian MA, Routy
JP, Tremblay C, Amre D and Ahmad A: Differential synthesis and
release of IL-18 and IL-18 Binding Protein from human platelets and
their implications for HIV infection. Cytokine. 90:144–154.
2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Bachmann M, Pfeilschifter J and Muhl H:
Epigenetic regulation by CpG methylation splits strong from
retarded IFNү-induced IL-18BP in epithelial versus monocytic cells.
Biochim Biophys Acta Gene Regul Mech. 1861:191–199. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wang Z, Liu Z, Wang L, Wang J, Chen L, Xie
H, Zhang H and He S: Altered expression of IL-18 binding protein
and IL-18 receptor in basophils and mast cells of asthma patients.
Scand J Immunol. 87(e12658)2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Gupta A, Fei YD, Kim TY, Xie A, Batai K,
Greener I, Tang H, Ciftci-Yilmaz S, Juneman E, Indik JH, et al:
IL-18 mediates sickle cell cardiomyopathy and ventricular
arrhythmias. Blood. 137:1208–1218. 2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Shao Q, Liu N, Li GF, Meng QC, Yao JH and
Wang N: IL-18 expression in clinical human pituitary adenoma.
Technol Health Care. 30:11–16. 2022.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Detry S, Andries J, Bloch Y, Gabay C,
Clancy DM and Savvides SN: Structural basis of human IL-18
sequestration by the decoy receptor IL-18 binding protein in
inflammation and tumor immunity. J Biol Chem.
298(101908)2022.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Yang Y, Zhang ZX, Lian D, Haig A,
Bhattacharjee RN and Jevnikar AM: IL-37 inhibits IL-18-induced
tubular epithelial cell expression of pro-inflammatory cytokines
and renal ischemia-reperfusion injury. Kidney Int. 87:396–408.
2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Tsuji-Takayama K, Aizawa Y, Okamoto I,
Kojima H, Koide K, Takeuchi M, Ikegami H, Ohta T and Kurimoto M:
Interleukin-18 induces interferon-gamma production through
NF-kappaB and NFAT activation in murine T helper type 1 cells. Cell
Immunol. 196:41–50. 1999.PubMed/NCBI View Article : Google Scholar
|
|
105
|
McIlroy A, Caron G, Blanchard S, Fremaux
I, Duluc D, Delneste Y, Chevailler A and Jeannin P: Histamine and
prostaglandin E up-regulate the production of Th2-attracting
chemokines (CCL17 and CCL22) and down-regulate IFN-gamma-induced
CXCL10 production by immature human dendritic cells. Immunology.
117:507–516. 2006.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Walker W and Rotondo D: Prostaglandin E2
is a potent regulator of interleukin-12- and interleukin-18-induced
natural killer cell interferon-gamma synthesis. Immunology.
111:298–305. 2004.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Liao TL, Chen YM, Hsieh CW, Chen HH, Lee
HC, Hung WT, Tang KT and Chen DY: Upregulation of circulating
microRNA-134 in adult-onset Still's disease and its use as
potential biomarker. Sci Rep. 7(4214)2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Shi L, Zhang S, Huang Z, Hu F, Zhang T,
Wei M, Bai Q, Lu B and Ji L: Baicalin promotes liver regeneration
after acetaminophen-induced liver injury by inducing NLRP3
inflammasome activation. Free Radic Biol Med. 160:163–177.
2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Xiang Y and Moss B: Identification of
human and mouse homologs of the MC51L-53L-54L family of secreted
glycoproteins encoded by the Molluscum contagiosum poxvirus.
Virology. 257:297–302. 1999.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Xiang Y and Moss B: Molluscum contagiosum
virus interleukin-18 (IL-18) binding protein is secreted as a
full-length form that binds cell surface glycosaminoglycans through
the C-terminal tail and a furin-cleaved form with only the IL-18
binding domain. J Virol. 77:2623–2630. 2003.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Xiang Y and Moss B: IL-18 binding and
inhibition of interferon gamma induction by human poxvirus-encoded
proteins. Proc Natl Acad Sci USA. 96:11537–11542. 1999.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Xiang Y and Moss B: Correspondence of the
functional epitopes of poxvirus and human interleukin-18-binding
proteins. J Virol. 75:9947–9954. 2001.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Calderara S, Xiang Y and Moss B:
Orthopoxvirus IL-18 binding proteins: Affinities and antagonist
activities. Virology. 279:22–26. 2001.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Esteban DJ, Nuara AA and Buller RML:
Interleukin-18 and glycosaminoglycan binding by a protein encoded
by Variola virus. J Gen Virol. 85(Pt 5):1291–1299. 2004.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Krumm B, Meng X, Wang Z, Xiang Y and Deng
J: A unique bivalent binding and inhibition mechanism by the
yatapoxvirus interleukin 18 binding protein. PLoS Pathog.
8(e1002876)2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Nazarian SH, Rahman MM, Werden SJ,
Villeneuve D, Meng X, Brunetti C, Valeriano C, Wong C, Singh R,
Barrett JW, et al: Yaba monkey tumor virus encodes a functional
inhibitor of interleukin-18. J Virol. 82:522–528. 2008.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Symons JA, Adams E, Tscharke DC, Reading
PC, Waldmann H and Smith GL: The vaccinia virus C12L protein
inhibits mouse IL-18 and promotes virus virulence in the murine
intranasal model. J Gen Virol. 83(Pt 11):2833–2844. 2002.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Smith VP, Bryant NA and Alcami A:
Ectromelia, vaccinia and cowpox viruses encode secreted
interleukin-18-binding proteins. J Gen Virol. 81(Pt 5):1223–1230.
2000.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Esteban DJ and Buller RM: Identification
of residues in an orthopoxvirus interleukin-18 binding protein
involved in ligand binding and species specificity. Virology.
323:197–207. 2004.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Afonso CL, Tulman ER, Lu Z, Zsak L, Kutish
GF and Rock DL: The genome of fowlpox virus. J Virol. 74:3815–3831.
2000.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Yan Y, Deng J, Niu L, Wang Q, Yu J, Shao
H, Cao Q, Zhang Y and Tan X: Cloning and characterization of giant
panda (Ailuropoda melanoleuca) IL-18 binding protein. Res Vet Sci.
106:170–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Gibson MS, Steyn A, Kealy D, Kaspers B and
Fife MS: Molecular cloning and characterisation of chicken IL-18
binding protein. Dev Comp Immunol. 114(103850)2021.PubMed/NCBI View Article : Google Scholar
|