Introduction
mRNA is a single-stranded ribonucleic acid that
carries coding sequences for protein synthesis and undergoes
translation by ribosomes to produce proteins. mRNA-based
therapeutics have emerged as promising ways for promptly and
efficiently expressing therapeutic proteins in cells (1). The most advanced application of mRNA
therapeutics lies in vaccination against viral infections and
tumors (2,3). However, mRNA molecules are susceptible
to rapid degradation by extracellular RNases and cannot traverse
cellular membranes owing to their negative charge, hydrophilicity
and high molecular weight (4).
Therefore, the development of efficient and safe mRNA delivery
systems is imperative (5-7).
Previously, we demonstrated that cationic liposomes, comprising
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide (DC-1-16),
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and
poly(ethylene glycol) cholesteryl ether (PEG-Chol), induce high
protein expression in the lungs and spleen following intravenous
administration of mRNA/cationic liposome complexes (mRNA
lipoplexes) (8).
Histone deacetylases (HDACs) constitute a family of
enzymes recognized as a promising target to reverse aberrant
epigenetic states associated with tumors through the regulation of
histone acetylation levels (9).
HDAC inhibitors elevate acetylation levels within chromatin and
treating tumor cells with HDAC inhibitors induces growth arrest or
cell death (10). Among the HDAC
inhibitors, vorinostat [suberoylanilide hydroxamic acid (SAHA)]
stands as the U.S. first food and drug administration
(FDA)-approved drug to be used in the treatment of cutaneous T-cell
lymphoma (11,12). Additionally, HDAC inhibitors exhibit
the capability to enhance transgene expression from both plasmid
DNA transiently transfected into cells and transgenes stably
transfected into the cell genome via histone acetylation
(13,14). Following transfection into cells,
plasmid DNA binds to histones in the nucleus, thereby impeding
transgene expression through the limited accessibility of
transcription factors to the plasmid DNA (13). In stably transfected transgenes, a
relaxed chromatin structure becomes imperative for facilitating
transgene expression via enhanced transcription factor
accessibility (13). Therefore,
HDAC inhibitors augment transgene expression by relaxing chromatin
structure through histone acetylation.
However, to the to the best of the authors'
knowledge, there remains a paucity of reports regarding the effects
of HDAC inhibitors on protein expression from transfected mRNA. In
the present study, to examine the effect of HDAC inhibitors on
protein expression from transfected mRNA, mRNA lipoplexes were
prepared for transfection into both tumor cells and mouse tissues
and the effect of vorinostat on both in vitro and in
vivo protein expression following the administration of mRNA
lipoplexes was evaluated.
Materials and methods
Materials
DC-1-16 was obtained from Sogo Pharmaceutical Co.,
Ltd. DOPE and PEG-Chol (mean molecular weight of PEG: 1600) were
purchased from NOF Co., Ltd. Vorinostat (SAHA) was purchased from
Combi-Blocks, Inc. All the remaining chemicals were of the highest
available grade.
mRNAs
Firefly luciferase (FLuc) mRNA [1929 nt; cat. no.
L-7202; CleanCap FLuc mRNA (5 moU)] and enhanced green fluorescent
protein (EGFP) mRNA (996 nt; cat. no. L-7601; CleanCap EGFP mRNA)
were obtained from TriLink BioTechnologies. Cy5-labeled mRNA [1921
nt; cat. no. R1010; EZCap Cyanine 5 FLuc mRNA (5 moU)] was obtained
from ApexBio Technology LLC.
Cell culture
Human cervical carcinoma HeLa cells (cat. no.
93021013; Cellosaurus; CVCL_0030) and human liver cancer HepG2
cells (cat. no. 85011430; Cellosaurus; CVCL_0027) were obtained
from the European Collection of Authenticated Cell Cultures.
HeLa-FLuc cells, stably expressing pGL3 FLuc (Bioware Cell Line
HeLa-luc; Cellosaurus; CVCL_5J41), were obtained from Caliper Life
Sciences, Inc. HepG2-FLuc cells, stably expressing pGL3 FLuc (cat.
no. JCRB1592; Cellosaurus; CVCL_JG47) were purchased from the JCRB
Cell Bank (National Institutes of Biomedical Innovation, Health and
Nutrition).
HeLa cells and HeLa-FLuc cells were cultured in
Eagle's Minimum Essential Medium (EMEM; FUJIFILM Wako Pure Chemical
Corporation) supplemented with 10% heat-inactivated fetal bovine
serum (FBS, Thermo Fisher Scientific, Inc.) and 100 µg/ml
kanamycin, maintained in an incubator at 37˚C with a 5%
CO2 humidified atmosphere. HepG2 and HepG2-FLuc cells
were cultured in Dulbecco's Modified Eagle's Medium (DMEM; FUJIFILM
Wako Pure Chemical Corporation) supplemented with 10% FBS under the
same conditions.
Cytotoxicity of vorinostat on HeLa and
HepG2 cells
Vorinostat was initially dissolved in dimethyl
sulfoxide (DMSO) at a concentration of 1 mM. This stock solution
was diluted with culture medium supplemented with 10% FBS prior to
treatment. HeLa and HepG2 cells were then seeded into 96-well
culture plates (1x104 cells/well). Following a 24 h
incubation period, the cells were treated with 0.1, 0.5, 1, 5, 10,
or 20 µM vorinostat. Cell viability was assessed 24 and 48 h
post-treatment with vorinostat using a Cell Counting Kit-8 (CCK-8;
Dojindo Laboratories, Inc.), as previously described (15). WST-8 substrate was incubated with
cells at 37˚C for 60 min. The cell viability (%) was calculated
relative to that of untreated cells.
Effect of vorinostat on Luc expression
in HeLa-FLuc and HepG2-FLuc cells
HeLa-FLuc and HepG2-FLuc cells were seeded in
12-well culture plates (1x105 cells/well). Following 24
h of incubation, 1 ml of culture medium supplemented with either 1
µM or 10 µM vorinostat was introduced to the HeLa-FLuc or
HepG2-FLuc cells. Luc activity [counts per sec (cps)] in the cells
was measured 24 h post-transfection with a chemoluminometer (ARVO
X2; PerkinElmer, Inc.), as previously described (15). Protein concentrations of the
supernatants were determined with bicinchoninic acid (BCA) reagent
(Pierce BCA Protein Assay Kit; Pierce; Thermo Fisher Scientific,
Inc.), using bovine serum albumin (Thermo Fisher Scientific, Inc.)
as a standard and the Luc activity (cps/µg protein) was
calculated.
Preparation of mRNA lipoplexes
DC-1-16, DOPE and PEG-Chol were dissolved in
chloroform at a molar ratio of 49.5:49.5:1. Chloroform was then
evaporated under vacuum using a rotary evaporator set at 60˚C to
obtain a thin film. The thin film was hydrated by adding sterilized
water at 60˚C, followed by vortexing and subsequent sonication for
10 min at 42 kHz, 100 W and at room temperature in a bath-type
sonicator (Bransonic 2510J-MTH; Branson Ultrasonics Corporation).
To prepare the mRNA lipoplexes, the cationic liposome suspension
was combined with the mRNA solution at a charge ratio (+:-) of 4:1,
as described in a previous study (8), thorough vortexing for 10 sec and
subsequently left undisturbed at room temperature for 15 min.
Particle size distribution and ζ-potential of the mRNA lipoplexes
were assessed using a light-scattering photometer (ELS-Z2; Otsuka
Electronics Co., Ltd.), as previously described (15).
Effect of vorinostat on Luc expression
in HeLa and HepG2 cells following transfection with FLuc mRNA
lipoplexes
HeLa and HepG2 cells were seeded into 12-well
culture plates (1x105 cells/well). Following incubation
at 37˚C for 24 h, FLuc mRNA lipoplexes (0.5 µg mRNA) were diluted
in 1 ml of culture medium (EMEM and DMEM for HeLa and HepG2 cells,
respectively) containing either 1 or 10 µM vorinostat (final mRNA
concentration: 0.5 µg/ml) and added to the cells. At 24 h
post-transfection, Luc activity (cps/µg protein) was quantified, as
described in the previous section.
Effect of vorinostat on EGFP
expression in HeLa and HepG2 cells following transfection with EGFP
mRNA lipoplexes
HeLa and HepG2 cells were seeded in 12-well culture
plates (1x105 cells/well). Following incubation at 37˚C
for 24 h, EGFP mRNA lipoplexes (0.5 µg mRNA) were diluted in 1 ml
of culture medium (EMEM and DMEM for HeLa and HepG2 cells,
respectively) supplemented with either 1 or 10 µM vorinostat (final
mRNA concentration: 0.5 µg/ml) and subsequently introduced to the
cells. At 24 h post-transfection, EGFP expression in the cells was
observed through fluorescence microscopy, as previously described
(8).
Animals
All animal experiments were conducted in accordance
with the Guidelines outlined in the Guide for the Care and Use
of Laboratory Animals (16),
adopted by the Institutional Animal Care and Use Committee of Hoshi
University (Tokyo, Japan). Ethical approval was obtained from the
Institutional Animal Care and Use Committee of Hoshi University
(approval number: P23-108).
A total of 15 female BALB/c mice (20 g, 8 weeks old;
Sankyo Labo Service Corporation) were housed under a 12-h
light/dark cycle, with lights on from 08:00 a.m. to 08:00 p.m. and
provided ad libitum access to food and water. The room
temperature was maintained at 24˚C with a relative humidity of
55%.
Effect of vorinostat on mRNA
biodistribution following mRNA lipoplex injection in mice
Vorinostat was initially dissolved in DMSO at a
concentration of 50 mg/ml. This stock solution was diluted with
saline before injection (final concentrations: 1 and 5 mg/ml for
doses of 5 and 25 mg/kg, respectively). Vorinostat was administered
intraperitoneally to female BALB/c mice at doses of 5 or 25 mg/kg,
followed by an intravenous injection of Cy5-labeled mRNA lipoplexes
(10 µg mRNA; n=1 per mRNA lipoplex). Following 1 h of intravenous
injection, the mice were sacrificed by cervical dislocation under
anesthesia induced by isoflurane inhalation (4% for induction and
1.5-2% for maintenance; FUJIFILM Wako Pure Chemical Corporation)
and mortality was confirmed by cessation of heartbeat. Cy5
fluorescence images of the lungs, heart, liver, spleen and kidneys
were acquired using the NightOWL LB981 NC100 System (Berthold
Technologies GmbH & Co. KG), as previously described (8,15).
Effect of vorinostat on Luc expression
following FLuc mRNA lipoplex injection in mice
Vorinostat was intraperitoneally administered to
female BALB/c mice (8 weeks old) at doses of 5 or 25 mg/kg,
followed by intravenous injection of FLuc mRNA lipoplexes (20 µg
mRNA; n=3 per group). As described in previous studies (8,17),
mice were sacrificed by cervical dislocation under anesthesia
induced by isoflurane inhalation at 4 h post-injection of mRNA
lipoplexes and the lungs, liver, spleen and kidneys were harvested
for analysis. Then 3 µl of ice-cold reporter lysis buffer (cat. no.
E3971; Promega Corporation) per 1 mg of tissue were added to each
tissue sample and immediately homogenized. Luc activity (cps/mg
protein) in the homogenized samples was measured as described in
the preceding section.
Statistical analysis
Statistical significance was assessed using the
unpaired Student's t-test or one-way analysis of variance, followed
by Tukey's post hoc test, employing GraphPad Prism (version 4.0;
Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxicity of vorinostat on HeLa and
HepG2 cells
Vorinostat, a potent inhibitor of HDAC activity,
exhibits antitumor effects on various tumor cells, including those
of prostate, ovarian and pancreatic carcinoma (18-20).
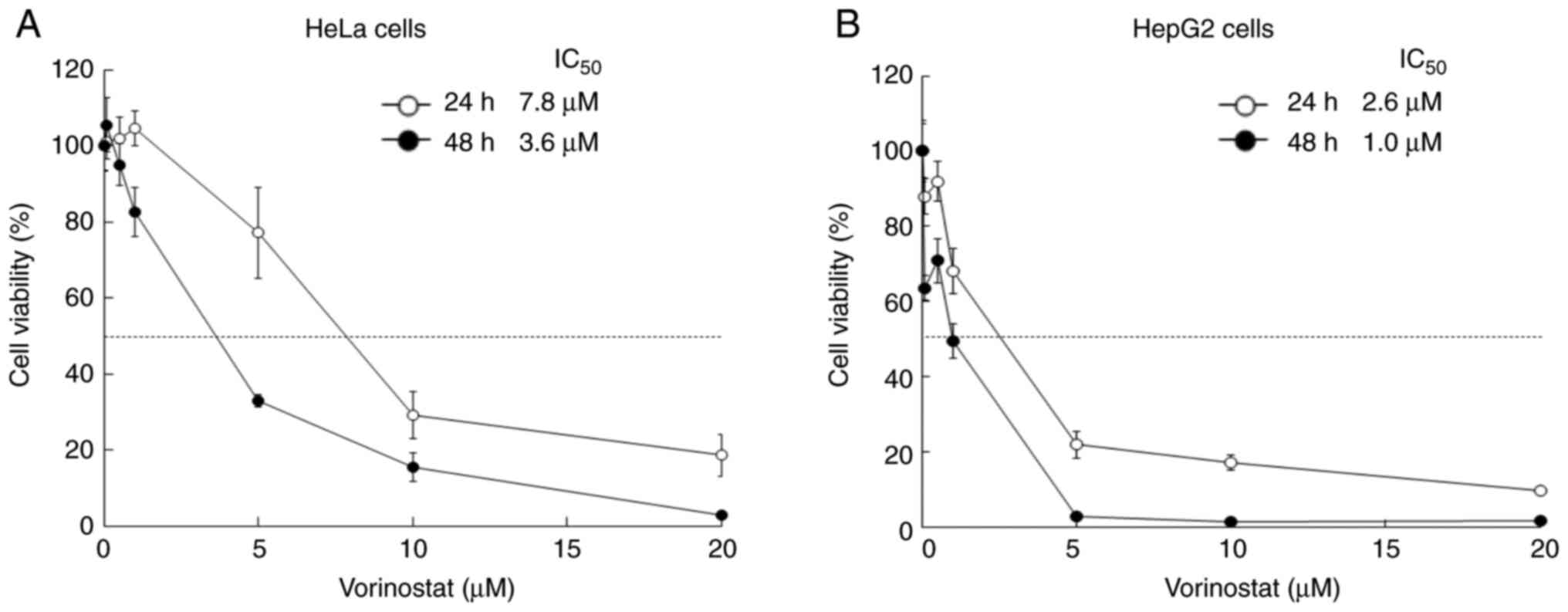
To investigate the cytotoxicity of vorinostat on HeLa and HepG2
cells, the cells were treated with vorinostat for 24 and 48 h
(Fig. 1). The IC50
values of vorinostat for HeLa and HepG2 cells were 7.8 and 2.6 µM,
respectively, following 24 h of incubation at 37˚C and 3.6 and 1.0
µM, respectively, following 48 h of incubation at 37˚C.
Effect of vorinostat on Luc activity
in HeLa-FLuc and HepG2-FLuc cells
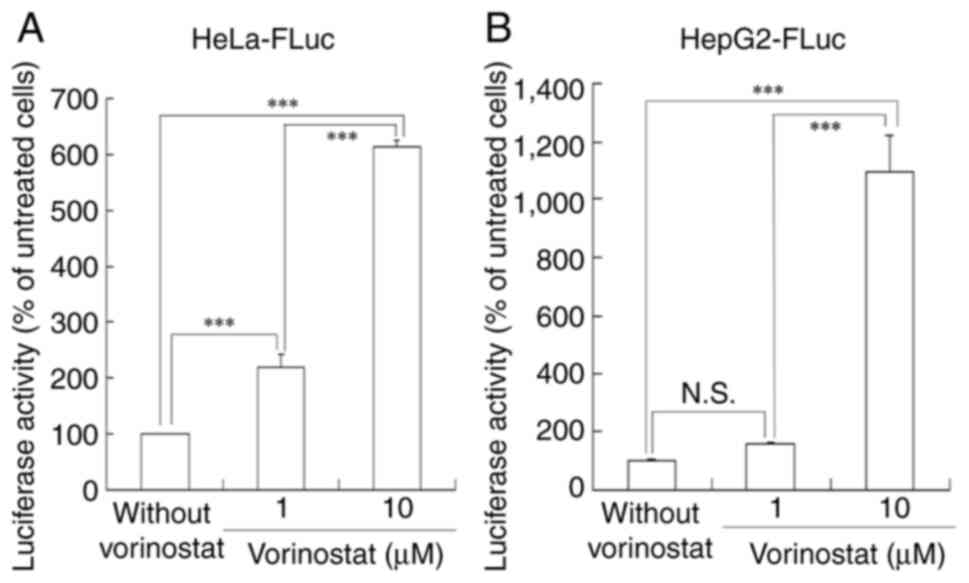
To investigate whether treatment with vorinostat
affects Luc expression from a Luc gene inserted into the
cellular genome, HeLa-FLuc and HepG2-FLuc cells were treated with
either 1 or 10 µM vorinostat, respectively, followed by Luc
activity measurement after 24 h of incubation (Fig. 2). Treatment of HeLa-FLuc cells with
1 and 10 µM vorinostat resulted in 2.2- and 6.1-fold higher Luc
expression, respectively, compared with untreated cells.
Furthermore, treatment of HepG2-FLuc cells with 1 and 10 µM
vorinostat led to 1.6- and 11-fold higher Luc expression,
respectively, compared with untreated cells. Treatment with 10 µM
vorinostat significantly increased Luc activity in both cell lines
compared with treatment with 1 µM vorinostat, indicating that
concentrations exceeding the IC50 of vorinostat might
effectively enhance protein expression from a foreign gene
integrated into the genome.
Effect of vorinostat on Luc activity
in HeLa and HepG2 cells following Luc mRNA lipoplex
transfection
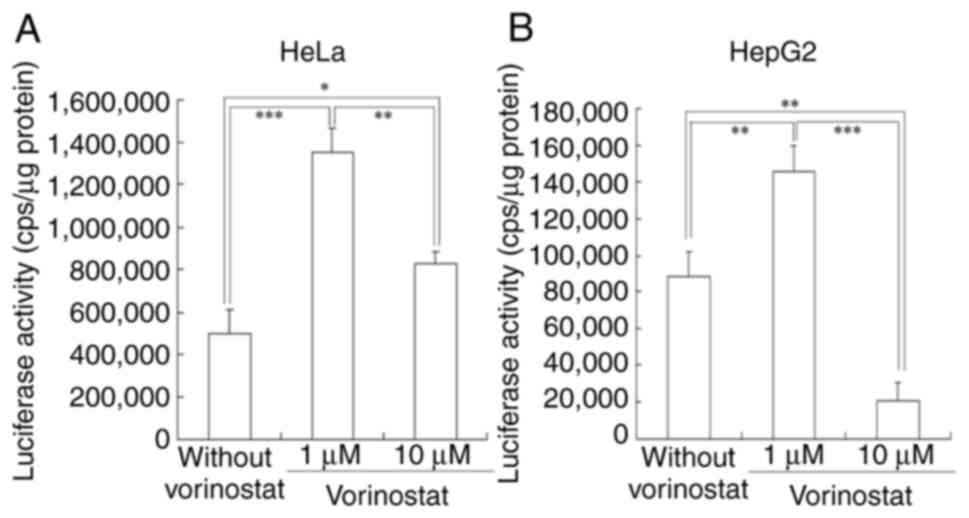
Next, the impact of vorinostat treatment on Luc
expression from transfected FLuc mRNA was investigated. For mRNA
transfection, cationic liposomes, comprising DC-1-16, DOPE and
PEG-Chol, were prepared, as previous research has indicated their
efficacy in achieving high protein expression both in vitro
and in vivo following mRNA lipoplex administration (8). The cationic liposomes measured 116.5
nm, while the FLuc mRNA lipoplexes measured 282 nm (data not
shown). Their respective ζ-potentials were 51 and 39.9 mV (data not
shown). FLuc mRNA lipoplexes were transfected into HeLa and HepG2
cells, respectively, in the presence or absence of 1 or 10 µM
vorinostat. Luc activity was then measured 24 h post-incubation
(Fig. 3). Treatment with 1 µM
vorinostat resulted in 2.7- and 1.6-fold higher Luc activities from
transfected FLuc mRNA in HeLa and HepG2 cells, respectively,
compared with untreated cells. However, treatment with 10 µM
vorinostat decreased Luc activity in both cell lines compared with
treatment with 1 µM vorinostat. As negative controls, transfection
with empty vector (only cationic liposomes) did not induce Luc
activities in HeLa and HepG2 cells in the presence or absence of
vorinostat (Fig. S1).
Effect of vorinostat on EGFP
expression in HeLa and HepG2 cells following EGFP mRNA lipoplex
transfection
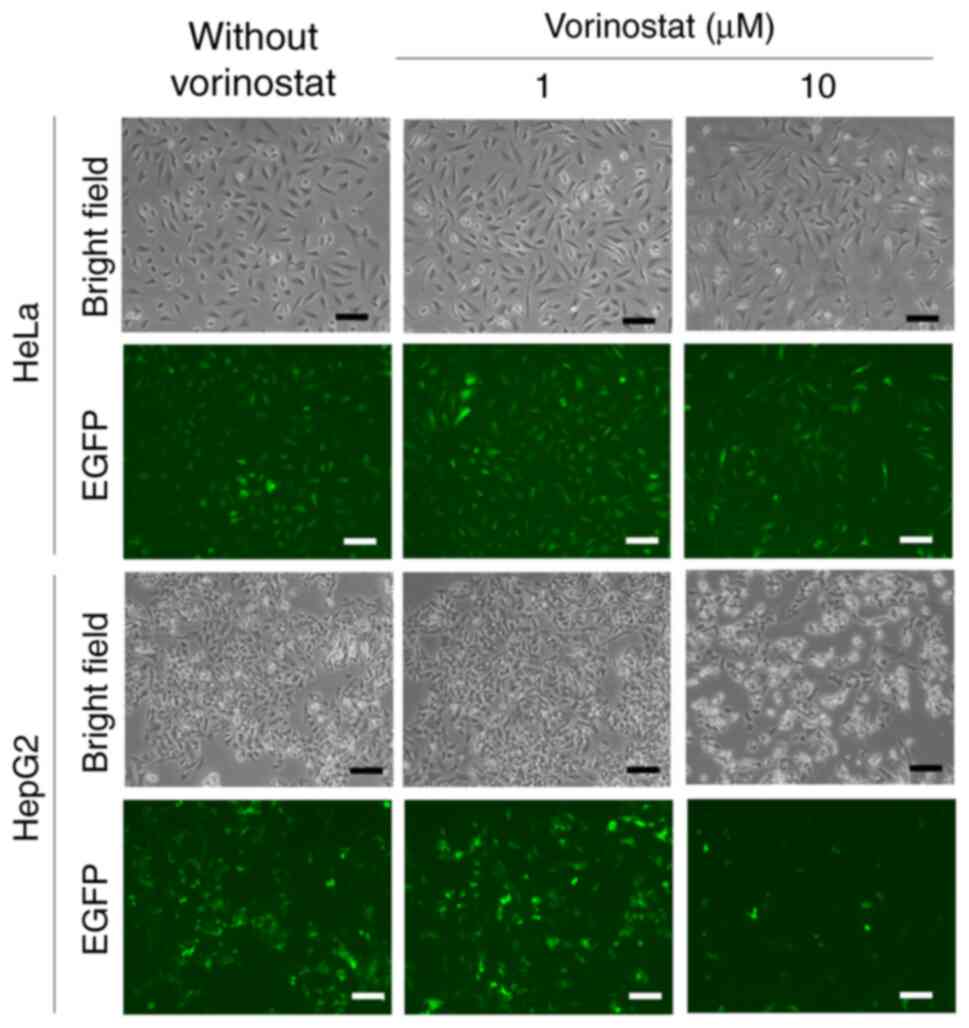
EGFP mRNA lipoplexes were transfected into HeLa and
HepG2 cells, respectively, in the presence or absence of 1 or 10 µM
vorinostat and EGFP expression was observed 24 h after incubation
(Fig. 4). Treatment with 1 µM
vorinostat increased EGFP expression from transfected EGFP mRNA in
both cell lines compared with untreated cells, while treatment with
10 µM vorinostat decreased EGFP expression in both cell lines
compared with treatment with 1 µM vorinostat. As negative controls,
transfection with empty vector (only cationic liposomes) did not
induce EGFP expression in HeLa and HepG2 cells in the presence or
absence of vorinostat (Fig. S2).
These results suggested that treatment with vorinostat at
concentrations lower than the IC50 might effectively
increase protein expression from transfected mRNA.
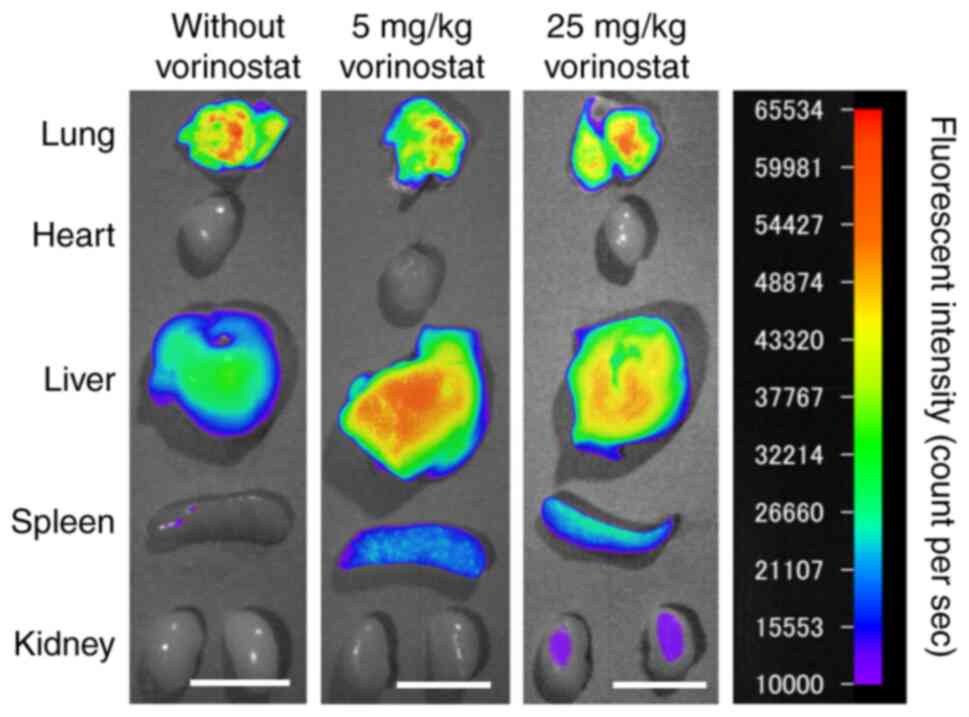
Effect of vorinostat on mRNA
biodistribution following intravenous injection of FLuc mRNA
lipoplexes
Vorinostat is often administered intraperitoneally
or orally to mice at doses ranging from 25 to 150 mg/kg/day for
cancer therapy (20,21). Therefore, the present study selected
5 mg/kg as a low dose and 25 mg/kg as a standard dose. To
investigate the effect of vorinostat on mRNA biodistribution
following intravenous injection of mRNA lipoplexes, mice were
intraperitoneally administered with either 5 or 25 mg/kg of
vorinostat, followed by intravenous injection of Cy5-labeled mRNA
lipoplexes. Injection of mRNA lipoplexes resulted in substantial
mRNA accumulation in the lungs; however, co-injection with either 5
or 25 mg/kg of vorinostat resulted in the accumulation of mRNA in
both the lungs and liver (Fig.
5).
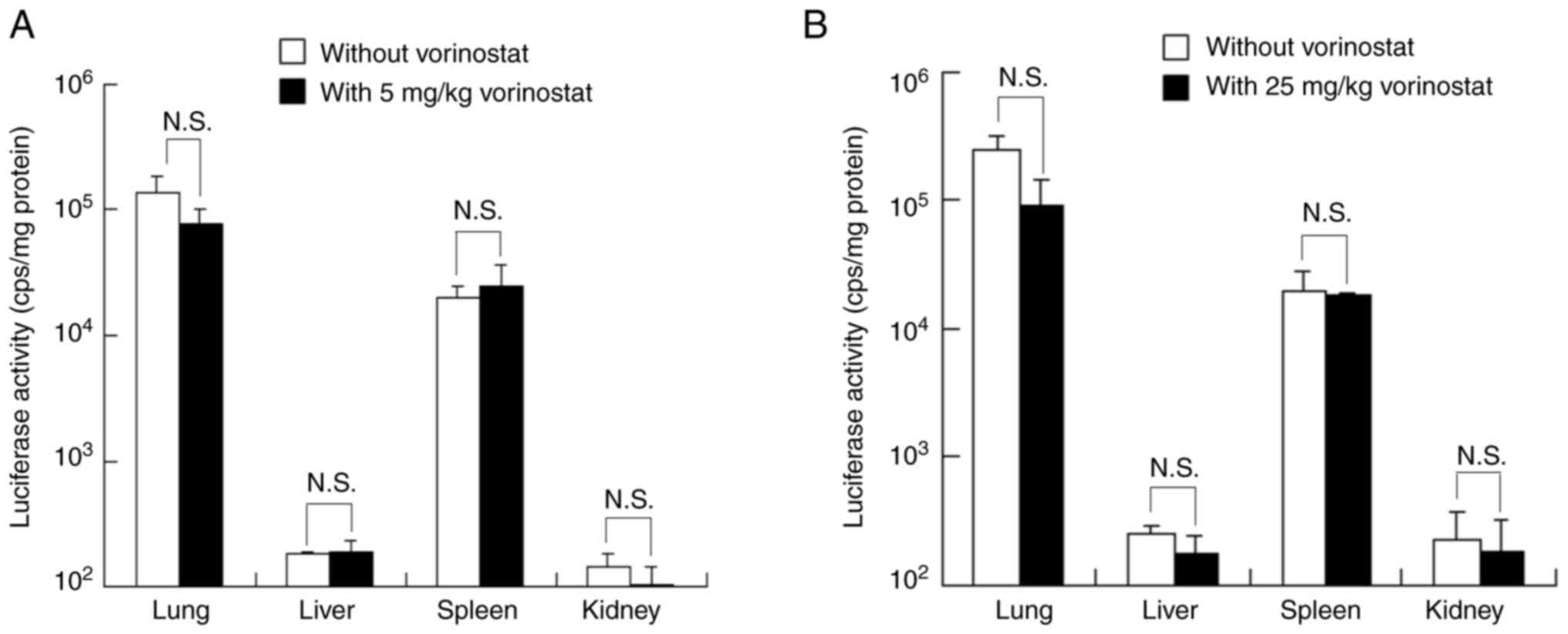
Effect of vorinostat on Luc expression
in tissues following intravenous injection of FLuc mRNA
lipoplexes
To investigate the effect of vorinostat on in
vivo protein expression from transfected mRNA, mice were
intraperitoneally administered with vorinostat at doses of 5 or 25
mg/kg, followed by intravenous injection of FLuc mRNA lipoplexes.
Injection of FLuc mRNA lipoplexes resulted in substantial Luc
expression in both the lungs and spleen. However, co-injection with
vorinostat at doses of 5 or 25 mg/kg slightly decreased Luc
expression in the lungs compared with untreated mice, with no
effect on the spleen (Fig. 6A and
B).
Discussion
HDAC inhibitors induce histone acetylation,
resulting in chromatin structure relaxation; therefore, they
possess the capacity to enhance protein expression from both
plasmid DNA transiently transfected into cells and transgenes
stably transfected into the cellular genome (13,14).
The present study evaluated the effect of HDAC inhibitors on in
vitro and in vivo protein expression from mRNA
transfected via cationic liposomes. Treatment with
vorinostat augmented in vitro protein expression from
transfected mRNA at concentrations lower than the IC50;
however, treatment with higher concentrations was necessary to
boost protein expression from stably transfected transgenes. These
findings indicated a differential mechanism underlying protein
expression enhancement between transfected mRNA and stably
transfected transgenes. Unlike stably transfected transgenes,
transfected mRNA does not necessitate nuclear entry for protein
translation. Therefore, it was hypothesized that vorinostat's
promotion of protein expression from transfected mRNA might have
resulted from increased translation of transfected mRNA and/or mRNA
stabilization of transfected mRNA. Previous research has
demonstrated that the stability of ATP binding cassette subfamily B
member 1 mRNA is markedly enhanced upon treatment with 0.6 µM
vorinostat, resulting in P-glycoprotein (P-gp) upregulation
(22). Therefore, one plausible
mechanism for increased protein expression may involve
vorinostat-mediated stabilization of transfected mRNA.
Intravenous injection of mRNA lipoplexes resulted in
substantial mRNA accumulation in the lungs. By contrast,
co-injection with vorinostat resulted in mRNA accumulation not only
in the lungs but also in the liver. However, the mechanism by which
vorinostat affects mRNA biodistribution subsequent to mRNA lipoplex
injection remains unclear. Positively charged lipoplexes interact
electrostatically with negatively charged erythrocytes, inducing
agglutination (23). These
agglutinates become trapped in lung capillaries (24). Therefore, vorinostat might affect
the interaction between mRNA lipoplexes and erythrocytes in the
blood circulation.
In the present study, intravenous injection of FLuc
mRNA lipoplexes resulted in high Luc expression in both the lungs
and spleen, whereas co-injection with vorinostat slightly
diminished Luc expression in the lungs while showing no effect on
Luc expression in spleen. Previous research has indicated the
presence of acetylated histones in the spleen at 2 h
post-subcutaneous administration of vorinostat (25), suggesting that acetylation induced
by vorinostat does not markedly affect protein expression from
transfected mRNA in the spleen. Moreover, the reduction in Luc
expression in the lungs might be attributed to decreased mRNA
accumulation in the lungs following vorinostat injection. These
findings suggested that vorinostat did not markedly affect in
vivo protein expression from transfected mRNA. Numerous
clinical trials of mRNA tumor therapeutics have been initiated and
exhibit considerable performance (1). In combination therapy with mRNA
therapeutics and vorinostat for patients with cutaneous T-cell
lymphoma, at least, vorinostat might not reduce the effectiveness
of mRNA therapeutics. Further investigations are necessary to
elucidate why vorinostat affects protein expression in vitro
but not in vivo. In addition, the toxicity in the tissues
after co-injection of mRNA lipoplexes and vorinostat will have to
be evaluated.
In conclusion, the present study is the first
report, to the best of the authors' knowledge, to examine the
effect of protein expression from transfected mRNA by treatment
with HDAC inhibitor. Vorinostat enhanced in vitro protein
expression from transfected mRNA after treatment at a lower
IC50 concentration. However, its effect on in
vivo protein expression from transfected mRNA in tissues was
not significant. The present study provided insights into the
effects of vorinostat on protein expression from transfected
mRNA.
Supplementary Material
Effect of vorinostat on Luc activity
in HeLa and HepG2 cells following transfection of cationic liposome
(empty vector). HeLa (A) and HepG2 cells (B) were transfected with
only cationic liposomes in the presence or absence of 1 or 10
μM vorinostat and then incubated for 24 h. Each value
represents the mean ± SD (n=3). FLuc, firefly luciferase.
Effect of vorinostat on EGFP
expression in HeLa and HepG2 cells following transfection of
cationic liposomes (empty vector). HeLa and HepG2 cells were
transfected with only cationic liposomes in the presence or absence
of 1 or 10 μM vorinostat and then incubated for 24 h. EGFP
expression in the cells was observed using a fluorescence
microscope. Scale bar, 100 μm. EGFP, enhanced green
fluorescent protein.
Acknowledgements
The authors thank Ms. Kumi Kawano (Department of
Molecular Pharmaceutics, Hoshi University, Tokyo, Japan) for
assistance with the animal experiments.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YH conceived and designed the present study. YH and
MT conducted the experiments. YH wrote the manuscript. Both authors
have read and approved the final manuscript. YH and MT confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Hoshi University (approval no.
P23-108).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sahin U, Karikó K and Türeci Ö: mRNA-based
therapeutics-developing a new class of drugs. Nat Rev Drug Discov.
13:759–780. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Liu T, Liang Y and Huang L: Development
and delivery systems of mRNA vaccines. Front Bioeng Biotechnol.
9(718753)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramachandran S, Satapathy SR and Dutta T:
Delivery strategies for mRNA vaccines. Pharm Med. 36:11–20.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yen A, Cheng Y, Sylvestre M, Gustafson HH,
Puri S and Pun SH: Serum nuclease susceptibility of mRNA cargo in
condensed polyplexes. Mol Pharm. 15:2268–2276. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yan Y, Liu XY, Lu A, Wang XY, Jiang LX and
Wang JC: Non-viral vectors for RNA delivery. J Control Release.
342:241–279. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou X, Zaks T, Langer R and Dong Y: Lipid
nanoparticles for mRNA delivery. Nat Rev Mater. 6:1078–1094.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Buschmann MD, Carrasco MJ, Alishetty S,
Paige M, Alameh MG and Weissman D: Nanomaterial delivery systems
for mRNA vaccines. Vaccines (Basel). 9(65)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang M, Sagawa A, Inoue N, Torii S, Tomita
K and Hattori Y: Efficient mRNA delivery with mRNA lipoplexes
prepared using a modified ethanol injection method. Pharmaceutics.
15(1141)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gallinari P, Di Marco S, Jones P, Pallaoro
M and Steinkühler C: HDACs, histone deacetylation and gene
transcription: From molecular biology to cancer therapeutics. Cell
Res. 17:195–211. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sakajiri S, Kumagai T, Kawamata N, Saitoh
T, Said JW and Koeffler HP: Histone deacetylase inhibitors
profoundly decrease proliferation of human lymphoid cancer cell
lines. Exp Hematol. 33:53–61. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng Y, Cheng Q and He J: HDAC inhibitors:
Promising agents for leukemia treatment. Biochem Biophys Res
Commun. 680:61–72. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Duvic M: Histone deacetylase inhibitors
for cutaneous T-cell lymphoma. Dermatol Clin. 33:757–764.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ishiguro K and Sartorelli AC: Activation
of transiently transfected reporter genes in 3T3 Swiss cells by the
inducers of differentiation/apoptosis-dimethylsulfoxide,
hexamethylene bisacetamide and trichostatin A. Eur J Biochem.
271:2379–2390. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamano T, Ura K, Morishita R, Nakajima H,
Monden M and Kaneda Y: Amplification of transgene expression in
vitro and in vivo using a novel inhibitor of histone deacetylase.
Mol Ther. 1:574–580. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in pegylated siRNA lipoplexes
on in vitro gene-silencing effects and siRNA biodistribution in
mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guide for the Care and Use of Laboratory
Animals: National Research Council (US). 8th Edition. The National
Academies Press, Washington, DC, 2011.
|
|
17
|
Di J, Du Z, Wu K, Jin S, Wang X, Li T and
Xu Y: Biodistribution and non-linear gene expression of mRNA LNPs
affected by delivery route and particle size. Pharm Res.
39:105–114. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kumagai T, Wakimoto N, Yin D, Gery S,
Kawamata N, Takai N, Komatsu N, Chumakov A, Imai Y and Koeffler HP:
Histone deacetylase inhibitor, suberoylanilide hydroxamic acid
(vorinostat, SAHA) profoundly inhibits the growth of human
pancreatic cancer cells. Int J Cancer. 121:656–665. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takai N, Kawamata N, Gui D, Said JW,
Miyakawa I and Koeffler HP: Human ovarian carcinoma cells: Histone
deacetylase inhibitors exhibit antiproliferative activity and
potently induce apoptosis. Cancer. 101:2760–2770. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Butler LM, Agus DB, Scher HI, Higgins B,
Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA and Richon
VM: Suberoylanilide hydroxamic acid, an inhibitor of histone
deacetylase, suppresses the growth of prostate cancer cells in
vitro and in vivo. Cancer Res. 60:5165–5170. 2000.PubMed/NCBI
|
|
21
|
Hrzenjak A, Moinfar F, Kremser ML,
Strohmeier B, Petru E, Zatloukal K and Denk H: Histone deacetylase
inhibitor vorinostat suppresses the growth of uterine sarcomas in
vitro and in vivo. Mol Cancer. 9(49)2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang H, Huang C, Zhao L, Zhang H, Yang JM,
Luo P, Zhan BX, Pan Q, Li J and Wang BL: Histone deacetylase
inhibitors regulate P-gp expression in colorectal cancer via
transcriptional activation and mRNA stabilization. Oncotarget.
7:49848–49858. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eliyahu H, Servel N, Domb AJ and Barenholz
Y: Lipoplex-induced hemagglutination: Potential involvement in
intravenous gene delivery. Gene Ther. 9:850–858. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Simberg D, Weisman S, Talmon Y, Faerman A,
Shoshani T and Barenholz Y: The role of organ vascularization and
lipoplex-serum initial contact in intravenous murine lipofection. J
Biol Chem. 278:39858–39865. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hockly E, Richon VM, Woodman B, Smith DL,
Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, et
al: Suberoylanilide hydroxamic acid, a histone deacetylase
inhibitor, ameliorates motor deficits in a mouse model of
Huntington's disease. Proc Natl Acad Sci USA. 100:2041–2046.
2003.PubMed/NCBI View Article : Google Scholar
|