Introduction
Effective management and treatment options for
late-stage cancers is limited. Early detection of cancer is crucial
for improving survival (1).
Molecular markers facilitate detection of cancer at the earliest
signs of carcinogenesis before it progresses to more
difficult-to-treat stages (2).
Despite recent growth in the genome-wide
characterization of abnormal patterns in many types of cancer,
there is a lack of sufficiently sensitive and specific cancer
biomarkers for early detection (3-6).
In addition, reliable DNA isolation and testing from available
clinical samples must be optimized for analytical validation.
Hence, molecular triage methods with biomarker panels are in active
development to predict risk of progression to cancer in the
point-of-care setting. For example, DNA methylation tests are being
actively examined to triage patients into colposcopy-driven
biopsies or ablative treatment across cervical cancer screening
clinics worldwide (7-11).
Cervical cancer (CC) is the fourth most frequently
diagnosed cancer and the fourth leading cause of cancer death in
female patients, with an estimated 604,000 new cases and 342,000
deaths worldwide in 2020(12); most
of these were in developing countries (13). In 2024, in the USA, ~14,000 new
cases of invasive cervical cancer are expected to be diagnosed, and
an estimated 4,360 patients will die of this disease (14).
Persistent infection by oncogenic human
papillomavirus (HPV) is an etiological factor for cervical squamous
cell carcinoma. HPV infection is associated with oncogenic
progression of cervical intraepithelial neoplasia lesions Grades 1,
2, and 3 (CIN1-3) to carcinoma in situ and cervical cancer
(5). However, more than 90% of HPV
infections resolve without clinical consequence over several years
(15,16), and ~30% of CIN3 progress to invasive
cancer within 30 years of initial diagnosis (17).
Screening with the Papanicolaou test (Pap test) and
the HPV tests has led to a reduction in cervical cancer mortality
rates worldwide, mostly in developed countries. However, the death
rate has not changed much over the past 10 years (18). Nevertheless, CC is preventable and
curable if detected early and managed effectively (19). Currently, there is only one
FDA-approved cytology triage test, CINtec PLUS Cytology (Roche),
which determines patients most at risk of developing cervical
cancer. This test helps identify those who would benefit from more
immediate follow-up and those at low risk who can be given more
time to clear the infection on their own. However, this is a new
test without much market traction yet. Consequently, clinicians do
not have a tool to triage HPV-positive patients into
colposcopy-driven biopsies, most of which are unnecessary (20,21).
Moreover, there is a great need for the development of
self-collection kits, especially in low- and middle-income
settings, to expedite the use of effective screening methods such
as DNA methylation analysis. This approach to detect and prevent
the progression of CC is emerging as a breakthrough (22).
Several studies show the feasibility of DNA
methylation signatures for effective risk stratification and
prognosis in cancer (21,23-25).
DNA methylation-based tests can discriminate between patients with
early lesions that will progress to become aggressive,
consequential disease, from patients with indolent lesions that
lead to inconsequential disease (26-28).
Moreover, relative accessibility of biological samples using
minimally invasive procedures (before, during and after cancer
treatment) demonstrates the viability of these liquid biopsy
approaches using epigenetic-based markers (29-32).
Several studies show the feasibility of DNA methylation signatures
for effective risk stratification and prognosis in various types of
cancer (21,23-25).
Liquid cytology is the standard of care for cervical epithelium
samples sent by clinicians to clinical laboratories for co-testing
with the Pap and HPV test in. Promoter methylation of ZNF516
and FKBP6 can discriminate between normal, premalignant and
cancer samples using precision DNA methylation detection by
quantitative methylation-specific PCR (qMSP) (33).
To the best of our knowledge, there is no
established workflow for early cancer detection in biofluids using
precision DNA methylation by qMSP. The gold standard workflow for
DNA methylation analysis requires DNA extraction and bisulfite
treatment prior to DNA methylation detection by qMSP,
pyrosequencing, methylation array or next-generation sequencing. A
new generation of kits performs bisulfite conversion in samples
without requiring prior DNA extraction. These kits provide workflow
improvements, both in terms of conversion efficiency and time to
results, while considering the limited amount of input samples
available for analysis in the clinical setting (34,35).
The objective of the present study was to evaluate
rapid spin-column and magnetic bead kits for precision DNA
methylation analysis in clinical and point-of-care settings using
qMSP. A comprehensive evaluation of commercially available kits was
used to develop an optimized workflow for precision DNA methylation
quantification of premalignant lesions in liquid cytology samples
from cervical epithelium. The performance of spin-column and
magnetic bead kits was compared in terms of turnaround time,
efficiency and cost. Additionally, we compared the pellet and
supernatant (cell-free DNA) parts of the samples to assess their
suitability for DNA methylation analysis. The results may enable
the use of affordable, PCR-based, precision DNA methylation
workflows for early detection of pre-malignant and malignant
lesions in biofluids. These workflows may be optimized to create
cost-effective diagnostic and screening tests for other tumor sites
where readily available non-invasive samples can be collected to
perform precision DNA methylation detection of pre-malignant
lesions, such as oral, oropharyngeal, and laryngeal epithelium
premalignant lesions in saliva, bladder epithelium lesions in
urine, vaginal and ovarian epithelium premalignant lesions in
self-collected vaginal samples and anal, sigmoid and colon cancer
epithelium premalignant lesions in self-collected anal swabs.
Materials and methods
Study design
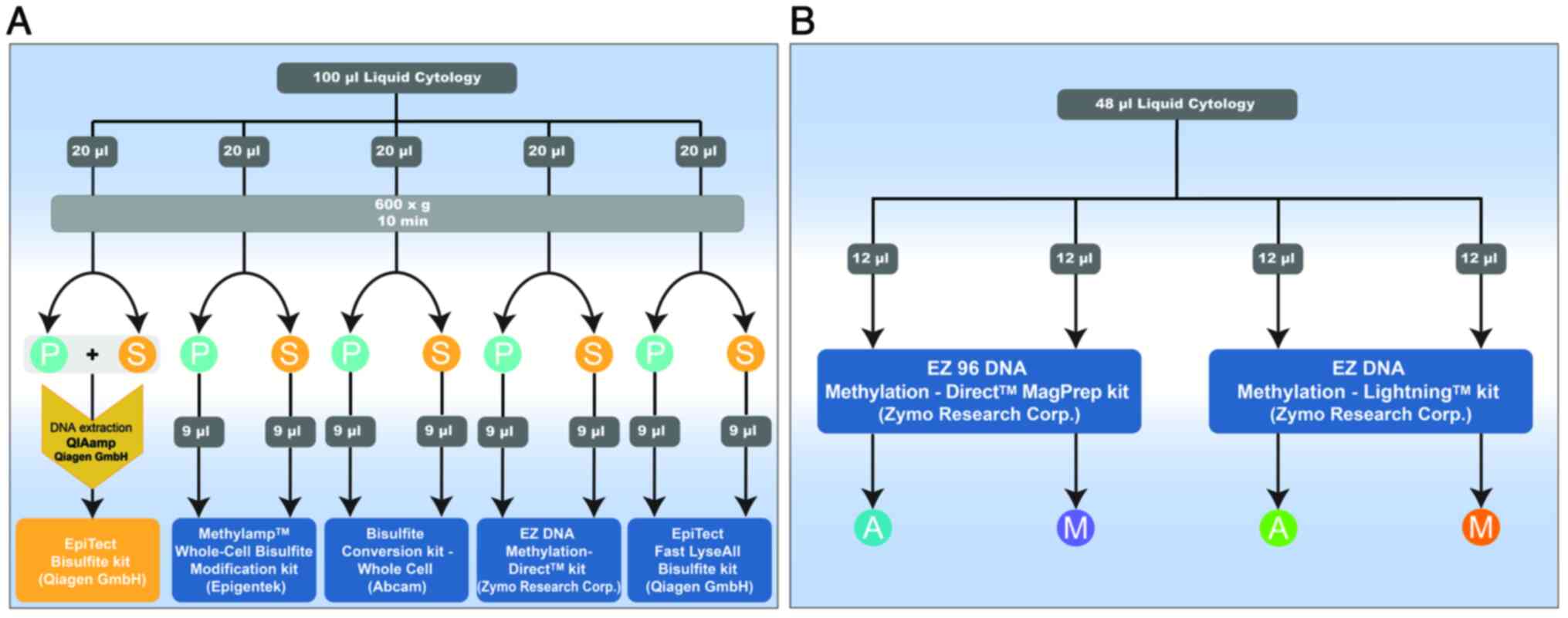
To evaluate silica spin-column kits, we utilized 100
µl of discarded cervical epithelium liquid cytology samples. These
samples were subdivided into five 20 µl aliquots. One aliquot
followed the standard protocol, involving traditional DNA
extraction using the QIAamp DNA Mini kit, followed by bisulfite
treatment with the EpiTecT Bisulfite kit. The remaining four
aliquots were designated for technical comparison. Each 20 µl
sample underwent centrifugation, separating it into pellet and
supernatant fractions, resulting in two distinct samples: ‘pellet’
and ‘supernatant’ (S). The supernatant part, considered to contain
cell-free DNA (cfDNA), was included to assess its suitability for
DNA methylation analysis alongside the cellular DNA in the pellet
fraction (Fig. 1A). For the
evaluation of magnetic beads kits, we used 48 µl of liquid cytology
samples, which were divided into four 12 µl parts. One part was
processed using the EZ 96 DNA Methylation Direct™ MagPrep
Kit-Automated (A), and another part using the EZ 96 DNA Methylation
Direct™ MagPrep Kit-Manual (M). The remaining 24 µl were split
equally, with 12 µl processed using the EZ DNA
Methylation-Lightning™ Kit Hybrid-Automated (A) and 12 µl processed
using the EZ DNA Methylation-Lightning™ Kit Hybrid-Manual (M)
(Fig. 1B).
Cohort and samples
Anonymized discarded liquid cytology samples (n=106)
from clinical laboratories providing Pap and HPV testing
(PathAdvantage Laboratory, Dallas, Texas; and CorePlus and Lab Noy
laboratories in San Juan, Puerto Rico), were received and
processed, beginning in August 2020 until December 2021.
Clinicopathological data were not obtained as the objective was to
streamline the bisulfite conversion workflow needed to use
precision DNA methylation assays in Clinical Laboratory Improvement
Amendments-approved clinical laboratories. The present study (#
IRB00231112) was approved by the Johns Hopkins School of Medicine
Institutional Review Board Baltimore, Maryland, USA. The
requirement for informed consent was waived due to the anonymized
nature of samples.
Gold standard workflow for bisulfite
conversion
The gold standard workflow for bisulfite conversion
used the QIAamp DNA Mini kit for DNA extraction (cat. no. #51304),
prior to treating genomic DNA (gDNA) with sodium bisulfite using
the EpiTecT Bisulfite kit (both Qiagen GmbH; cat. no. #59104),
according to the manufacturer's protocols (Appendix S1). All kits are listed in
Table SI.
Silica spin-column technology for
bisulfite conversion
Paired pellet and supernatant samples were compared
with kits using spin-column technology, with minor modifications to
the manufacturer's instructions as described below (Fig. 1A).
Methylamp Whole Cell Bisulfite
Modification (kit #3)
The pellet and supernatant were resuspended in 9 µl
W3 solution. Next, 1 µl W1/W2 solution was added to PCR tubes
including samples and placed in a thermocycler at 65˚C for 45 min.
Post-incubation, 110 µl W4/W5/W6 solution was added to each tube
and subjected to thermocycling at 99˚C for 20 min, 65˚C for 90 min
and 99˚C for 10 min. Following PCR, 200 µl W7 was added to all the
spin-columns, which were placed in collection tubes. The sample and
100 µl 100% isopropanol were added to the column containing W7,
incubated at room temperature for 2 min and centrifuged at 13,000 x
g for 20 sec. The flowthrough was discarded and 200 µl 70% ethanol
was added and centrifuged at 13,000 x g for 25 sec. Then, 50 µl
W6/ethanol solution was added to columns, incubated at room
temperature for 10 min and centrifuged at 13,000 x g for 20 sec.
The flowthrough was discarded, and 200 µl 90% ethanol was added
twice and centrifuged at 13,000 x g for 20 and 40 sec,
respectively. The columns were transferred to 1.5 ml tubes and 18
µl W8 was added. Finally, the columns were centrifuged at 13,000 x
g for 1 min to elute the modified DNA.
Bisulfite Conversion kit-Whole Cell
(kit #4)
The pellet was resuspended in 9 µl Cell Collection
Buffer and combined with the supernatant (9 µl), then transferred
into a 0.2 ml PCR tube. To each PCR tube, 1 µl Final Digestion
Solution was added and placed in a thermocycler at 65˚C for 45 min,
followed by vortexing at 2,500 rpm. until the solution was clear or
saturated (about 2 min) at room temperature. Next, 110 µl DNA
Modification Solution was added, and thermocycling was performed at
99˚C for 20 min, 65˚C for 90 min and 99˚C for 10 min. Spin-columns
were loaded with 200 µl DNA Binding Solution and placed in
collection tubes. Samples were added to the columns, along with 100
µl 100% isopropanol. After a 2 min room temperature incubation,
centrifugation was performed at 13,000 x g for 20 sec and the
flowthrough was discarded. The columns were washed with 200 µl 70%
ethanol, centrifuged at 13,000 x g for 25 sec, 50 µl DNA Cleaning
solution was added, incubated at room temperature for 10 min and
centrifuged at 13,000 x g for 20 sec. This step was followed by two
washes with 200 µl 90% ethanol, each centrifuged at 13,000 x g for
20 and 40 sec, respectively. The columns were placed into new 1.5
ml tubes. Lastly, 18 µl Modified DNA Elution was added to columns
and centrifuged at 13,000 x g for 1 min to elute the modified
DNA.
EZ DNA Methylation-Direct kit (kit
#5)
The pellet was resuspended in 9 µl PBS, and 9 µl
supernatant was used. Samples were transferred to a 0.2 ml PCR tube
along with 10 µl 2X M-Digestion Buffer and 1 µl Proteinase K, then
incubated at 50˚C for 20 min. After centrifugation at 13,000 x g
for 5 min, 20 µl supernatant was combined with 130 µl CT Conversion
Reagent and subjected to thermocycling at 98˚C for 8 min, followed
by 64˚C for 3.5 h. Post-PCR, 600 µl M-Binding Buffer was added to
spin-columns placed into collection tubes. Samples were loaded into
columns containing M-Binding Buffer, centrifuged at 13,000 x g for
30 sec and the flow-through was discarded. Subsequently, 100 µl
M-Wash Buffer was added to the columns and centrifuged at 13,000 x
g for 30 sec. Columns were treated with 200 µl M-Desulfonation
Buffer at room temperature for 20 min and centrifuged at 13,000 x g
for 30 sec. Then, two washes with 200 µl M-Wash Buffer were
performed; each wash was centrifuged at 13,000 x g for 30 sec.
Columns were then placed into 1.5 ml tubes and eluted with 18 µl
M-Elution Buffer via centrifugation at 13,000 x g for 1 min to
obtain DNA.
EpiTect Fast LyseAll Bisulfite kit
(kit #6)
The pellet was resuspended in 9 µl distilled water,
and 9 µl supernatant was used. Samples were transferred to 0.2 ml
PCR tubes. Then, 15 µl Lysis Buffer FTB and 5 µl Proteinase K were
added. After centrifugation, samples were incubated at 56˚C for 30
min. Then, 85 µl Bisulfite Solution and 15 µl DNA Protect Buffer
were added and thermocycling was performed with denaturation at
95˚C for 5 min and incubation at 60˚C for 20 min. The denaturation
and incubation steps were repeated once. The samples were
transferred to microcentrifuge tubes with 310 µl Buffer BL
containing 10 µg/ml carrier RNA and 250 µl 100% ethanol.
Centrifugation was performed at full speed (14.000 x g) for 1 min,
and the samples were loaded into columns placed in collection
tubes, followed by centrifugation at full speed (14.000 x g) for 1
min. After adding 500 µl Buffer BW, the samples were centrifuged at
full speed (14.000 x g) for 1 min. Next, 500 µl Buffer BD
(desulfonation buffer) was added to each spin column and incubated
for 15 min at room temperature (15-25˚C). After centrifugation at
14.000 x g, another wash with 500 µl Buffer BW was performed. The
columns were eluted with 250 µl 100% ethanol, and the residual
liquid was removed by centrifuging at full speed (14.000 x g) for 1
min. Following incubation at 60˚C for 5 min, the columns were
transferred to new microcentrifuge tubes and 18 µl Buffer EB was
added and centrifuged at 14.000 x g to elute DNA.
Magnetic beads technology for
bisulfite conversion
Supernatant samples were compared with kits using
magnetic beads technology (Fig.
1B).
EZ 96 DNA Methylation Direct™ MagPrep
kit (manual; kit #7)
Bisulfite conversion of DNA was performed using Zymo
Research EZ 96 DNA Methylation Direct™ MagPrep kit, as per
manufacturer's instructions. Briefly, 13 µl M-Digestion Buffer, 12
µl sample and 1 µl Proteinase K were used for sample digestion.
Samples were incubated for 20 min at 50˚C. A total of 130 µl CT
Bisulfite Conversion Reagent was added to 26 µl digested sample
solution. Samples were transferred to a thermocycler to perform
bisulfite conversion as follows: 98˚C for 8 min and 64˚C for 3.5 h.
Samples were placed on a heating element at 55˚C for 30 min to dry
the beads. Finally, 25 µl M-Elution Buffer was added for
elution.
EZ 96 DNA Methylation Direct™ MagPrep
kit (automated; kit #8)
Bisulfite conversion of DNA was performed using Zymo
Research EZ 96 DNA Methylation Direct™ MagPrep kit, as per
manufacturer's instructions. Briefly, 13 µl M-Digestion Buffer, 12
µl sample and 1 µl Proteinase K were added for sample digestion.
Samples were incubated for 20 min at 50˚C. A total of 130 µl CT
Conversion Reagent was added to 26 µl digested sample solution.
Samples were transferred to a thermocycler to perform bisulfite
conversion as follows: 98˚C for 8 min and 64˚C for 3.5 h. A total
of 600 µl M-Binding Buffer was added to a KingFisher 96 deep-well
plate with 10 µl MagBinding Beads. A total of three M-Wash plates
was prepared using 400 µl M-Wash Buffer twice. A total of 200 µl
M-Desulfonation Buffer was added into a new plate. A total of 50 µl
M-Elution Buffer was added to an elution plate.
EZ DNA Methylation-Lightning™ Kit
(manual) Hybrid (kit #9)
DNA was treated with bisulfite. The Zymo Research
EZ-96 DNA Methylation-Lightning™ MagPrep kit was used, but the
manufacturer's instructions were adjusted to include a digestion
step (D5044) prior to bisulfite conversion, instead of extracting
DNA beforehand. Briefly, 13 µl M-Digestion Buffer, 12 µl sample and
1 µl Proteinase K were used for sample digestion. Samples were
incubated for 20 min at 50˚C. A total of 130 µl Lightning Bisulfite
Conversion Reagent was added to 26 µl DNA sample. Samples were
transferred to a thermocycler for DNA denaturation and bisulfite
conversion as follows: 98˚C for 8 min and 54˚C for 60 min. Samples
were transferred to a new deep-well plate containing 600 µl
M-Binding Buffer and 10 µl MagBinding Beads (previously vortexed),
following a 5 min incubation step at room temperature. A total of
400 µl Wash Buffer was added, the plate was placed on magnetic
stand and supernatant was discarded after beads were pelleted
(supernatant was discarded after each wash). A 20 min incubation at
room temperature was performed using the magnetic stand for
desulfonation. A 30-min incubation at 55˚C was performed to dry the
beads. The beads were incubated with 25 µl Elution Buffer for 4 min
at 55˚C. Next, the plate was then placed on a magnetic stand for 1
min, allowing the beads to pellet. The supernatant was carefully
transferred to a clean elution plate.
EZ DNA Methylation-Lightning™ kit
(automated) Hybrid (kit #10)
DNA was treated with bisulfite according to a
modified protocol. The Zymo Research EZ-96 DNA
Methylation-Lightning™ MagPrep kit was used, but the manufacturer's
instructions were adjusted to include a digestion step (D5044)
prior to bisulfite conversion, instead of extracting DNA
beforehand. Briefly, 13 µl M-Digestion Buffer, 12 µl sample and 1
µl Proteinase K were used for sample digestion. Samples were
incubated for 20 min at 50˚C. A total of 130 µl Lightning
Conversion Reagent was added to 26 µl DNA sample. Samples were
transferred to a thermocycler for DNA denaturation and bisulfite
conversion with the following program: 98˚C for 8 min and 54˚C for
60 min. The desulfonation and washing steps) of the converted DNA
were performed while the DNA was bound to the MagBinding Beads,
using the KingFisher Flex instrument (Thermo Fisher Scientific,
Inc.).
DNA methylation analysis
DNA methylation was assessed by qMSP analysis of
bisulfite-modified genomic DNA, optimized for QuantStudio 6-Flex
Real-Time PCR (Thermo Fisher Scientific, Inc.). Primers and probes
were previously designed (33) to
amplify the CpG-rich regions located in the promoters of
ZNF516 (Zinc finger protein 516) and FKBP6 (FKBP
prolyl isomerase family member 6). β-actin, the reference
gene, was used to assess DNA input. Each reaction contained
methylated bisulfite-converted DNA as positive control and
unmethylated bisulfite-converted DNA as negative control. Standard
curves were created using the EpiTect PCR Control DNA Set (Qiagen
GmbH, cat. no. #59695) for quantification in silica-based column
kits, or the Zymo Methylated DNA Control kit (cat. no. #D5014) for
quantification in magnetic bead kits. Non-template controls were
added to guarantee the absence of contamination.
Bisulfite-converted DNA primers and probe sequences and annealing
temperatures are provided in Table
SII. Bisulfite treated DNA primers/probe sequences did not
overlap with genomic DNA sequences of the same genomic region using
NCBI primer blast check because genomic DNA sequences are altered
during bisulfite treatment (36).
Each plate included DNA samples, positive and
negative control and multiple water blanks as non-template
controls. Serial dilutions (0.001, 0.010, 0.1, 1, and 10 ng) of DNA
were used to generate a standard curve for each plate.
β-actin was used to measure sample cellularity and judge
sample adequacy. The relative levels of methylated DNA for each
gene in each sample were determined as a ratio of the amplified
gene quantity to the quantity of β-actin (mean value of
duplicates of gene of interest/mean value of the triplicates of
β-actin). The performance of each kit in amplifying the
β-actin gene was comparable since the same amount of sample
input was used. As the amount of DNA template decreases, cycle
threshold (Cq) value will increase (37). Therefore, the best performing kit
will amplify β-actin at lower Cq values.
Fluorogenic PCR reactions were performed in
duplicate for samples and triplicate for controls in a reaction
volume of 10 µl that contained 1 µl bisulfite-modified DNA, 600 nM
forward and reverse primer, 200 nM probe, 0.6 U platinum Taq
polymerase (Invitrogen; Thermo Fisher Scientific, Inc.), 200 µM
each dATP, dCTP, dGTP and dTTP and 2.5 mM MgCl2.
Amplifications were performed as follows: 95˚C for 5 min, followed
by 50 cycles at 95˚C for 15 sec and 58˚C for 1 min in a QuantStudio
6-Flex and were analyzed using QuantStudio™ Real-Time PCR Software
(Applied Biosystems; Thermo Fisher Scientific, Inc.; version
1.3).
Cost calculation and time assessment
for sample preparation and bisulfite treatment
The cost calculation for the sample preparation
process was conducted using the unit price of each item divided by
the number of samples per kit, excluding laboratory technician
costs. This calculation included all necessary consumables, such as
tubes, tips, and reagents. Detailed costs for sample preparation,
excluding the bisulfite modification kits, were outlined with each
cost item measured in terms of cost per sample and cost per plate
(83 samples). For PCR, the price was calculated for the
amplification of β-actin, ZNF516, and FKBP6 in
singleplex reactions. The time required to generate
bisulfite-treated DNA was recorded from the beginning of the
procedure to the preparation of ten samples. Hands-on time for the
spin-column kits was calculated by including the time for
centrifuging ten samples, dividing them into pellet and
supernatant, and all incubation steps. The total preparation time
for 10 samples was compared between direct kits and the gold
standard kit, with each process performed in triplicate.
Statistical analysis
PCR data were analyzed, interpreted, and exported as
.xlsx files using QuantStudio Software (Applied Biosystems; Thermo
Fisher Scientific, Inc.; version 1.7.1). The output files were
converted to .csv, and boxplots were prepared to visualize Cq
values data using R software (R Core Team; version 4.0.0). A paired
t-test with Bonferroni correction was used to compare Cq values of
pellet and supernatant, as proxy for cellularity. One-way ANOVA
with Tukey's HSD post hoc test was used for kit comparisons.
P#x003C;0.01 was considered to indicate a statistically significant
difference.
Results
Comparison between direct kits that
use spin-column technology
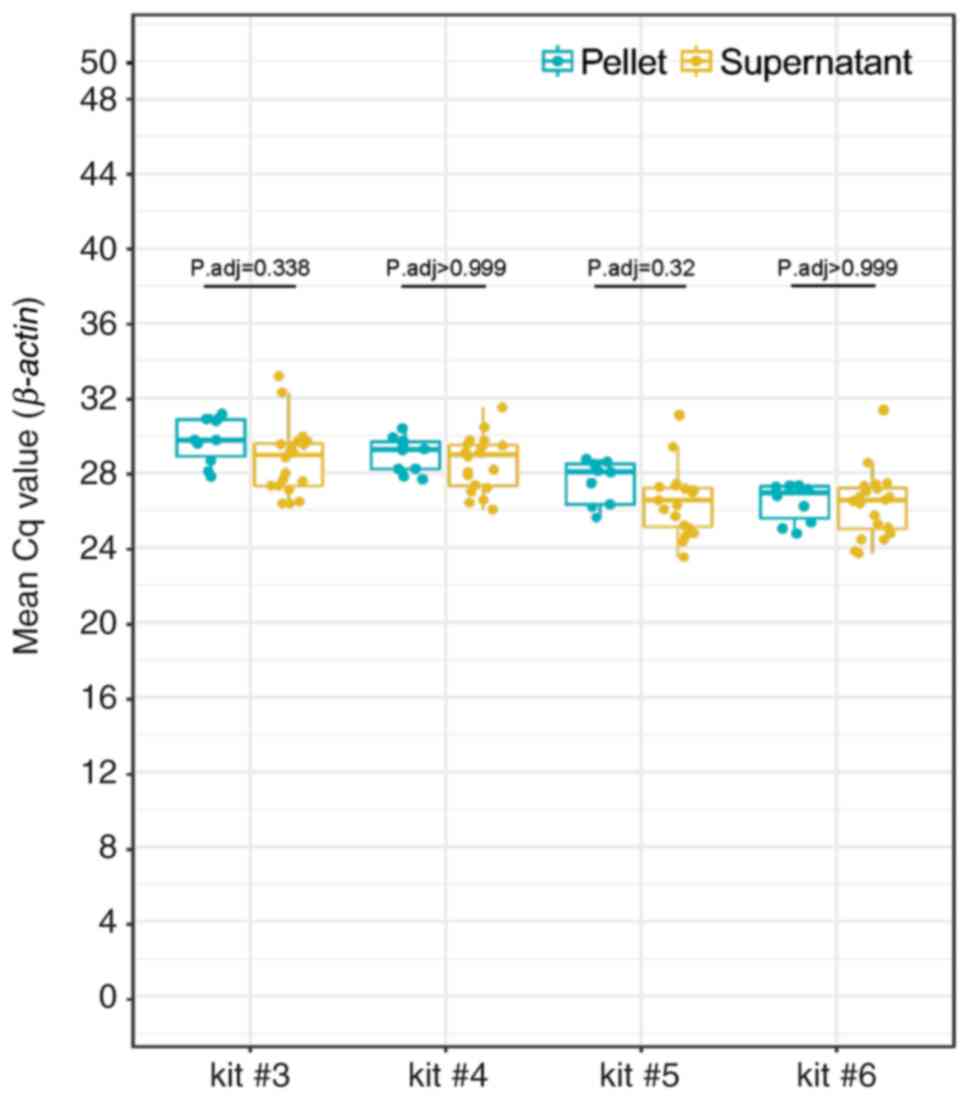
Summary statistics and interquartile range (IQR) of
β-actin Cq values are listed in Table SIII. There was no significant
difference in β-actin mean Cq values between pellet (n=10)
and supernatant (n=20; Fig. 2).
There was no significant difference in mean Cq values for pellets
when comparing kits #5 and #6. However, mean Cq values for pellets
were significantly higher for kits #4 and #3 compared with #6.
There was no statistical difference in mean Cq values for
supernatant samples between kits #5 and #6. Nevertheless, mean Cq
values for supernatant were significantly higher for kits #4 and #3
compared with #6 (Figs. S1 and
S2).
Comparison between direct kits that
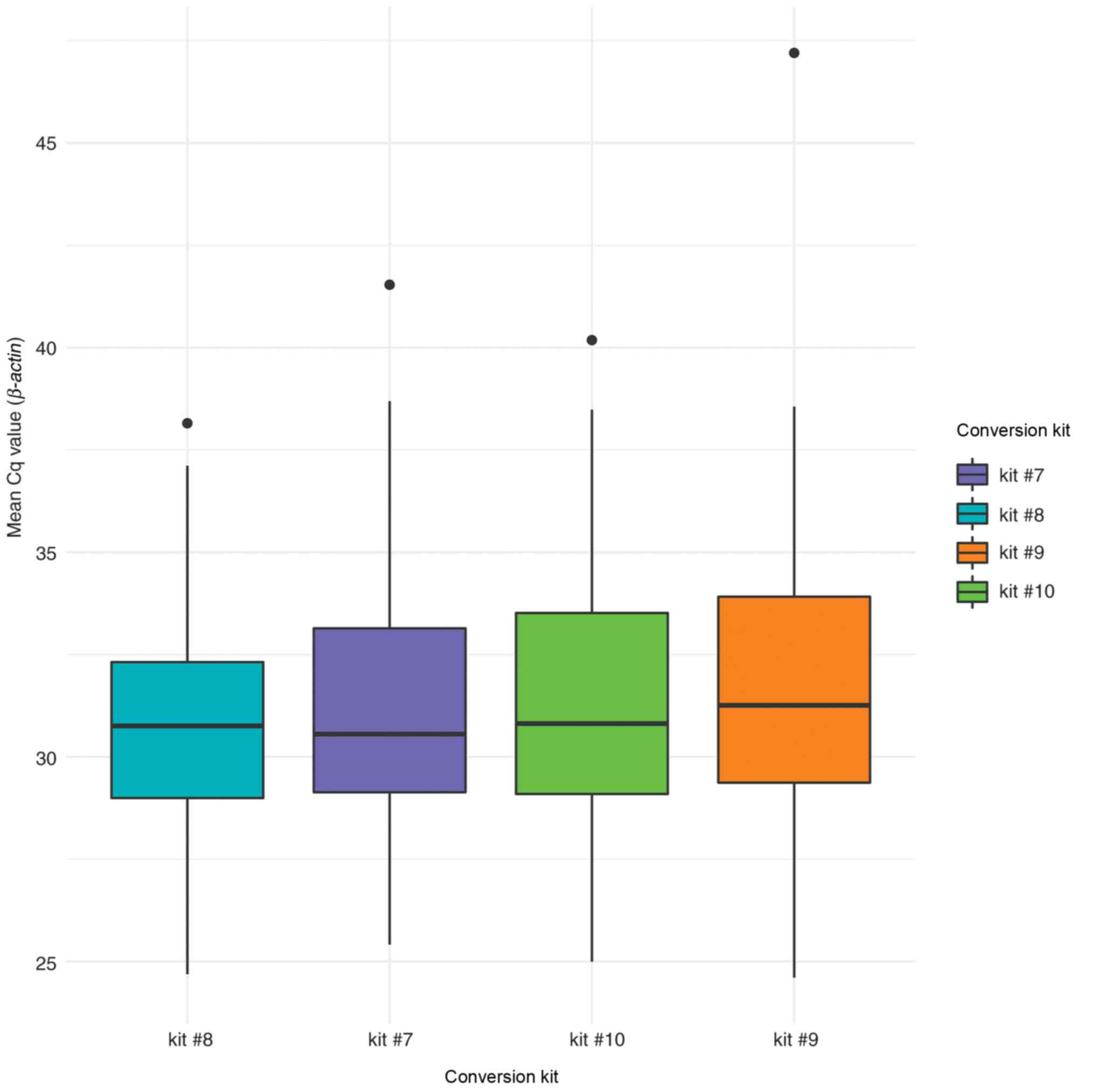
use magnetic beads technology
β-actin Cq values are listed in Table SIV. Kits #7 (manual) and #10
(automated) presented the same mean values, while kit #8 had
slightly higher values for automated sample processing. Kit #7
(manual) had the lowest median (29.7), and kit #9 (hybrid manual)
had the highest (32.3). There is a one-cycle difference in medians
for automated sample processing. Both automated kits had the lowest
IQRs, with kit #10 at 3.3 and kit #8 at 3.9, respectively. There
was no statistically significant difference when comparing mean Cq
values for manual and automated sample processing (Fig. 3).
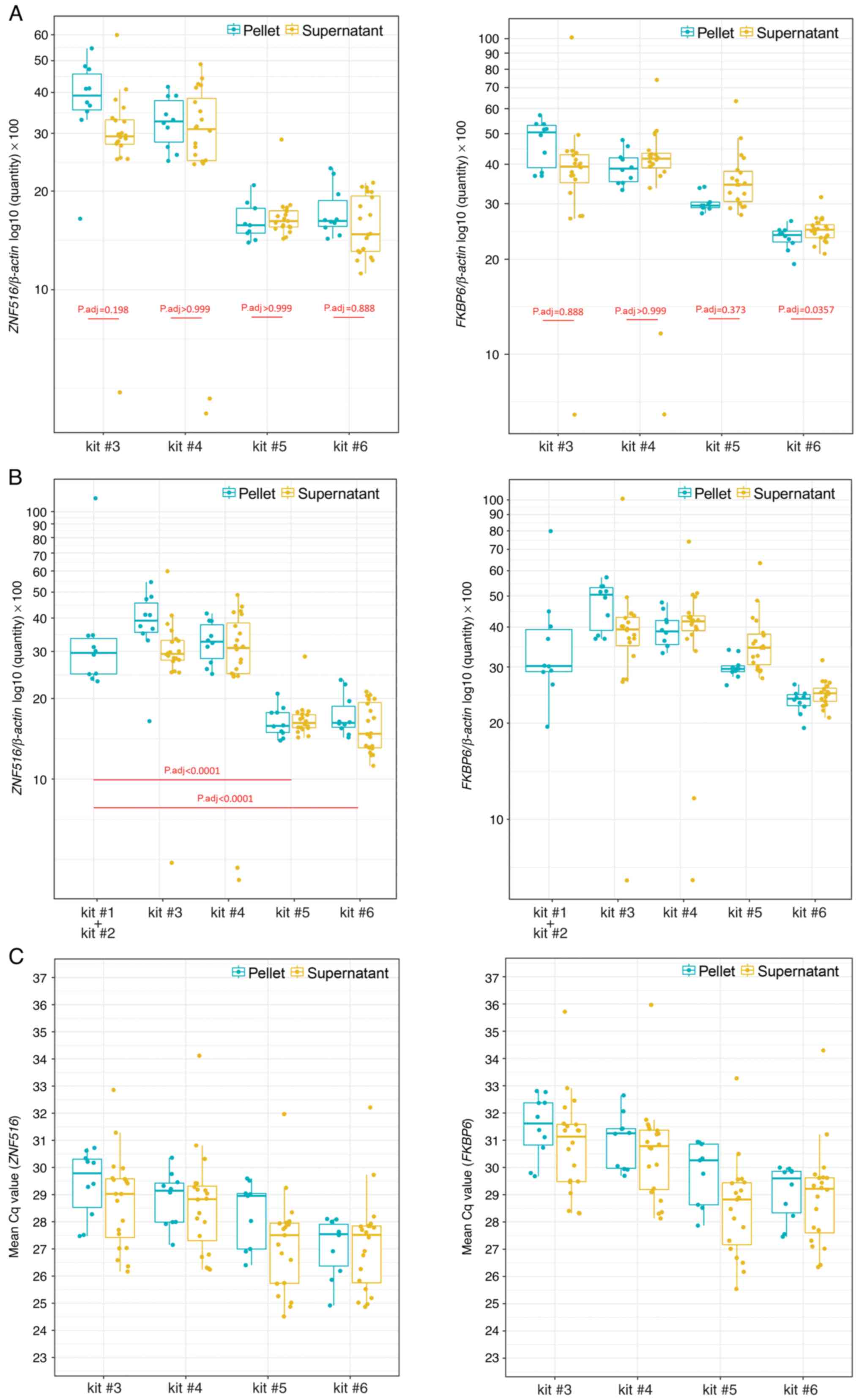
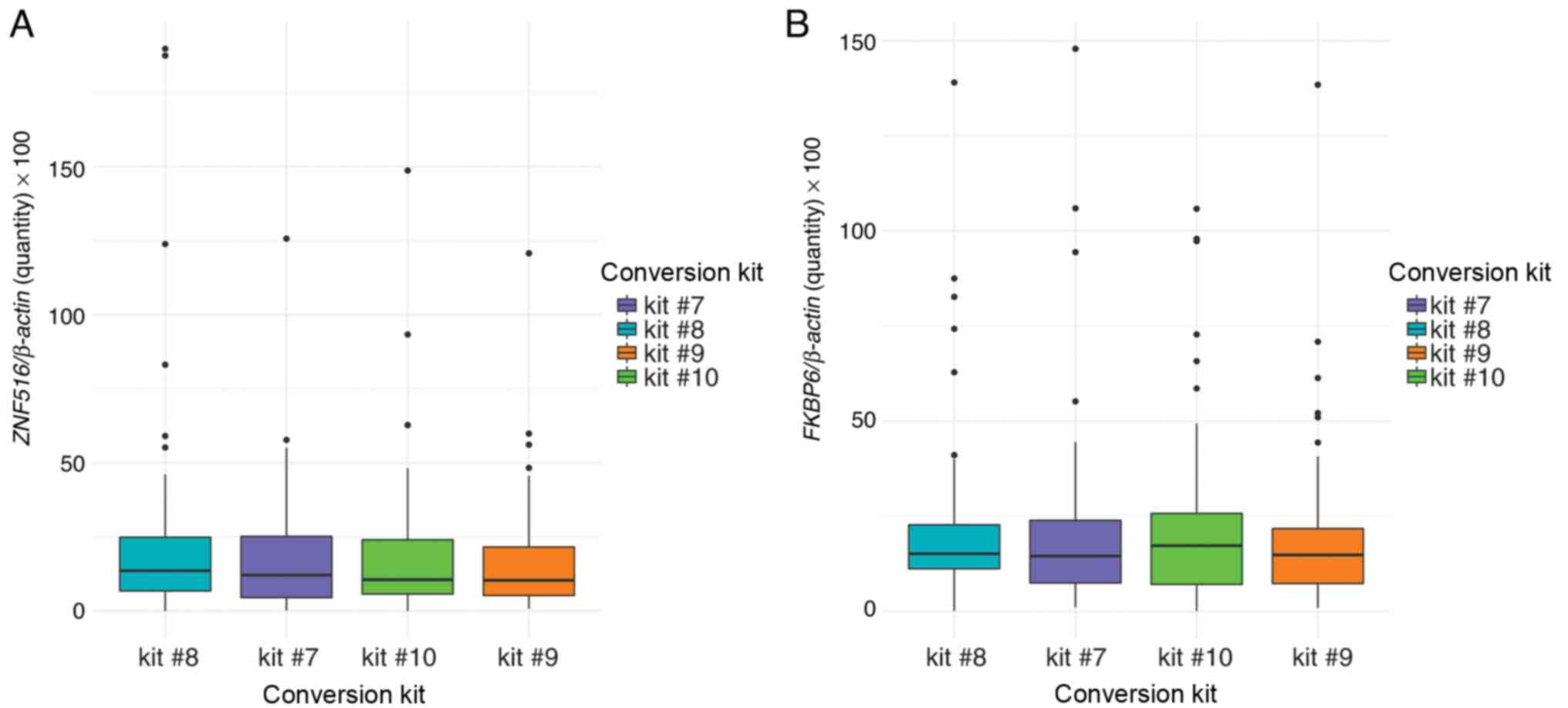
Amplification of CpG-rich regions
Amplification of ZNF516 and FKBP6
after bisulfite modification with spin-column or magnetic beads was
successful (Fig. 4). There were no
differences between pellet and supernatant (Fig. 4A), nor between the gold standard
protocol (kits #1 + #2) with kits #3 and #4. ZNF516
methylation levels were significantly different between the
standard protocol and kits #5 and #6 (Fig. 4B). There were no significant
differences in ZNF516 and FKBP6 methylation comparing
manual and automatic bisulfite conversion of samples with magnetic
bead technology in 96-well plates (Fig.
5).
Costs and time for sample
preparation
Costs of bisulfite conversion kits using spin-column
and magnetic bead technology were compared with gold standard
EpiTect kit (November 2023 prices). Gold standard cost/sample was
US$7.04, while direct kits were more affordable, ranging from $2.18
(kit #7 & #8) to $6.88 (kit #4; Table SV). For PCR, the price was
calculated for the amplification of β-actin, ZNF516 and
FKBP6 in singleplex reactions (Table SVI).
Next, time of sample preparation for 10 samples in
triplicate between direct kits and the gold standard kit was
compared (Table SVII). Kit #6
ranked better for spin-column kits in sample preparation (180 min).
Kit #10 had the lowest hands-on time (105 min) to prepare 83
samples in a 96-well plate format. The longest procedure was the
standard protocol (kits #1 + #2; 455 min).
Feasibility of using low volumes of
liquid cytology samples for DNA methylation studies
Kit #6 was the best option for assessing DNA in
liquid cytology samples because it provided the lowest
inter-variability between pellet and supernatant. Moreover, all
samples tested showed successful β-actin amplification with
favorable Cq values (median=28.48, IQR=1.92). These results were
comparable to those obtained from swab samples. Thus, it was
feasible to use small sample volumes for methylation studies, as 9
µl sample was sufficient for successful amplification.
Discussion
The present study demonstrated that small volumes of
liquid cytology samples can be used for efficient rapid bisulfite
conversion using magnetic beads prior to DNA methylation analysis
with qMSP. Automation increases throughput without compromising
amplification efficiency in open PCR systems, while providing swift
turnaround times (38). Efficiency
and cost analyses favored direct kits over the gold standard manual
protocols that require DNA extraction from cell pellets prior to
bisulfite conversion. These were obtained from real-world samples.
Therefore, these findings are relevant for future prospective
clinical trials of DNA methylation assays developed for use in
clinical and point-of-care settings.
Different kits have been assessed for quality of
converted DNA, primarily in terms of DNA fragmentation, degradation
and conversion efficiency, offering a valuable starting point in
selecting the most appropriate kit (39-42).
For example, Kint et al (2018) provided a comprehensive
workflow of 12 commercial bisulfite kits by comparing DNA
fragmentation, recovery or conversion efficiency using different
techniques, such as electrophoresis, quantitative and digital PCR,
DNA spectroscopic analysis and next-generation sequencing (39). The kits assessed included suppliers
such as Zymo Research Corp., Qiagen GmbH, and Promega. In the
aforementioned study, the DNA was extracted from peripheral blood
mononuclear cells and the results presented distinct performances
between kits. This variability suggests that the most appropriate
kit might differ depending on the specific goals of the study, such
as whether the priority is minimizing DNA fragmentation or
maximizing recovery efficiency.
Recently, Hong and Shin (43) tested six bisulfite conversion kits
using a multiplex quantitative real-time PCR system. A total of 20
peripheral blood samples with 50 ng gDNA as input was used and
three key features of bisulfite conversion were analyzed:
Efficiency, recovery and degradation levels (43). The aforementioned study found
>99% conversion efficiency in five kits and one performing near
94%. One kit presented a lower degradation level and recovery rates
of the kits ranged from 18 to 50%. All the kits used purified gDNA
followed by bisulfite treatment. Another study evaluated a DNA
hypermethylation marker panel of the six marker regions called
GynTect, in which liquid-based cytology media was directly treated
by sodium bisulfite with modifications (44). This demonstrates the feasibility and
effectiveness of directly treating liquid cytology samples with
sodium bisulfite for DNA methylation analysis, which aligns with
our approach of using small volumes of liquid cytology samples for
efficient rapid bisulfite conversion using magnetic beads prior to
DNA methylation analysis with qMSP. This supports the potential for
simplified and streamlined workflows in clinical settings.
Holmes et al (2014) evaluated nine kits:
EpiTect Fast FFPE Bisulfite, EpiTect Bisulfite, EpiTect Fast DNA
Bisulfite (Qiagen GmbH), EZ DNA Methylation-Gold, EZ DNA
Methylation-Direct, EZ DNA Methylation-Lightning (Zymo Research
Corp.), innuCONVERT Bisulfite All-In-One, innuCONVERT Bisulfite
Basic and innuCONVERT Bisulfite Body Fluids kit (Analytik Jena)
(45). High yields were obtained
using the EZ DNA Methylation-Gold and innuCONVERT Bisulfite kits.
All kits yielded high purity DNA suitable for PCR analyses and did
not exhibit PCR inhibition, meaning that the bisulfite-treated DNA
did not interfere with the PCR amplification process. The
innuCONVERT Bisulfite Body Fluids kit allowed the analysis of 3 ml
plasma, serum, ascites, pleural effusion and urine. However, to the
best of our knowledge, no study has compared methylation kits
released in recent years. Since liquid cytology is a valuable
sample source, the present study aimed to provide a comprehensive
comparison of commercial kits that generate DNA-bisulfite treated
sample in a few hours.
Understanding the pros and cons of kits is valuable
to pick the right option that fits the purpose of the markers to be
tested and the entire workflow. Here, supernatant could be used
directly in the bisulfite treatment process without centrifugation,
speeding up the entire process.
The costs for this streamlined process need to be
considered. Therefore, the present compared kits that perform
bisulfite conversion with spin-column and magnetic bead technology,
using manual and automatic processing. The price/sample was higher
for spin-column kits compared to 96-well format using magnetic
beads technology. Including PCR and all the reagents, test
cost/sample is $6.24 using kit #7, which is less costly than many
other FDA-approved kits currently available in different types of
cancer diagnostic.
The present study demonstrated the feasibility of
using as 9 µl methanol-based samples with good detection of
β-actin amplification, with no difference between pellet and
supernatant (cfDNA) parts using silica spin-columns. A
meta-analysis by Nanda et al (2000) on cytology samples
showed that despite relatively low sensitivity (51%), it is still
used for primary CC screening (46). CC screening program has recommended
high-risk HPV (hrHPV) DNA testing over the past decade (47). Moreover, due to a higher sensitivity
yet lower specificity than cytology (48), hrHPV test is usually used as a
co-test alongside cytology (49).
Currently, most samples are methanol-based, and after the screening
test, they are discarded. Therefore, the availability of these
samples opens opportunity to be further used for molecular testing
such as methylation detection since it requires only a few
microliters of the discarded sample. In addition, it was not
necessary to pellet the samples to obtain methylation profiles
comparable to the standard extraction protocol used in the present
study.
The Liquid Biopsy Working Group recently emphasized
the importance of clinical assay validation of next-generation
sequencing assays that use circulating tumor DNA (50). While DNA methylation sequencing is
beyond the scope of the present study, the present assay also uses
cfDNA. Consequently, technical challenges are similar, including
processing time from clinical sample to bisulfite-converted DNA and
the clinical assay standardization processes. Next-generation
sequencing and qPCR DNA methylation based-tests offer non-invasive
insight into tumor genetics and epigenetics, aiding treatment
decisions and disease monitoring.
The primary limitation of the present study is that
selection of kits was constrained by market availability. Cost and
throughput are two primary entry barriers to DNA methylation PCR
tests in US clinical laboratories. Consequently, the aim of the
present study was not to perform a clinical study but to evaluate
differences in performing DNA methylation screening tests with
silica columns and magnetic beads on discarded samples from
clinical laboratories.
Overall, the present study provided better
understanding of the direct methylation kits available in the
market using the panel of methylated genes we previously developed
to distinguish normal and CC liquid pap smear samples from patients
(33). Kits will achieve the
highest use if they are commercially available at low cost and
easy-to-use format. These kits should provide the best performance
and require simple equipment that is readily available at most
point-of-care facilities.
Supplementary Material
Supplementary material and
methods
Mean β-actin Cq values using
direct silica-based kits comparing pellet and supernatant
fractions.
Mean β-actin Cq values using
direct silica-based kits comparing pellet and supernatant
samples.
Spin-column and magnetic beads
technology kits.
Primers and probe sequences of genes
for quantitative methylation-specific PCR.
β-actin cycle threshold
values.
β-actin cycle threshold values
using M and A workflows in PreservCyt samples (n=83)
Cost/sample.
Costs for sample preparation excluding
the price of the bisulfite modification kits.
Time to generate bisulfite-treated
DNA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by the National
Institute of Minority Health and Health Disparities (grant no.
R44MD014911), National Cancer Institute (grant no. R44CA254690) and
Small Business Innovation Research/Small Business Technology
Transfer Matching Grant Program of the Puerto Rico Science,
Technology and Research Trust.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FTZ and RGP conceived and supervised the study,
designed the experiments, visualized data and wrote the manuscript.
FTZ and RGP confirm the authenticity of all the raw data. FTZ, EW,
ARL, AGN, APW, ACO, ARC and RGP performed experiments and analyzed
data. KG performed experiments. FTZ, EW and RGP wrote and revised
the manuscript. FTZ, ARL, AGN, APW, ACO, ARC, KG, DS and RGP
interpreted data and revised the manuscript. DS and RGP supervised
the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical standards as formulated in the Helsinki Declaration (1964)
and approved by the Johns Hopkins School of Medicine (approval no.
#IRB00231112) and Ponce School of Medicine Institutional Review
Board (approval no. #2301130571). The requirement for informed
consent was waived due to the anonymized nature of samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pashayan N and Pharoah PDP: The challenge
of early detection in cancer. Science. 368:589–590. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pepe MS, Etzioni R, Feng Z, Potter JD,
Thompson ML, Thornquist M, Winget M and Yasui Y: Phases of
biomarker development for early detection of cancer. J Natl Cancer
Inst. 93:1054–1061. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rubinstein WS, Patriotis C, Dickherber A,
Han PKJ, Katki HA, LeeVan E, Pinsky PF, Prorok PC, Skarlupka AL,
Temkin SM, et al: Cancer screening with multicancer detection
tests: A translational science review. CA Cancer J Clin: Mar 22,
2024 (Epub ahead of print).
|
|
4
|
Baran E, Lee M, Aviv S, Weiss J,
Pettengell C, Karam I, Bayley A, Poon I, Chan KKW, Parmar A, et al:
Oropharyngeal cancer staging health record extraction using
artificial intelligence. JAMA Otolaryngol Head Neck Surg: May 16,
2024 (Epub ahead of print).
|
|
5
|
Virdee PS, Collins KK, Friedemann Smith C,
Yang X, Zhu S, Roberts SE, Roberts N, Oke JL, Bankhead C, Perera R,
et al: The association between blood test trends and undiagnosed
cancer: A systematic review and critical appraisal. Cancers
(Basel). 16(1692)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Menon U, Gentry-Maharaj A, Burnell M,
Singh N, Ryan A, Karpinskyj C, Carlino G, Taylor J, Massingham SK,
Raikou M, et al: Ovarian cancer population screening and mortality
after long-term follow-up in the UK Collaborative Trial of Ovarian
Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet.
397:2182–2193. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Adcock R, Nedjai B, Lorincz AT,
Scibior-Bentkowska D, Banwait R, Torrez-Martinez N, Robertson M,
Cuzick J and Wheeler CM: New Mexico HPV Pap Registry Steering
Committee. DNA methylation testing with S5 for triage of high-risk
HPV positive women. Int J Cancer. 151:993–1004. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hernandez-Lopez R, Lorincz AT,
Torres-Ibarra L, Reuter C, Scibior-Bentkowska D, Warman R, Nedjai
B, Mendiola-Pastrana I, León-Maldonado L, Rivera-Paredez B, et al:
Methylation estimates the risk of precancer in HPV-infected women
with discrepant results between cytology and HPV16/18 genotyping.
Clin Epigenetics. 11(140)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gilham C, Nedjai B, Scibior-Bentkowska D,
Reuter C, Banwait R, Brentnall AR, Cuzick J, Peto J and Lorincz AT:
Long-term prediction by DNA methylation of high-grade cervical
intraepithelial neoplasia: Results of the ARTISTIC cohort. Int J
Cancer. 155:81–92. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ramirez AT, Sanchez GI, Nedjai B, Agudelo
MC, Brentnall AR, Cuschieri K, Castañeda KM, Cuzick J and Lorincz
AT: ASC-US-COL Trial Group. Effective methylation triage of HPV
positive women with abnormal cytology in a middle-income country.
Int J Cancer. 148:1383–1393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kremer WW, Steenbergen R, Heideman D,
Kenter GG and Meijer C: The use of host cell DNA methylation
analysis in the detection and management of women with advanced
cervical intraepithelial neoplasia: A review. BJOG. 128:504–514.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wright TC Jr and Kuhn L: Alternative
approaches to cervical cancer screening for developing countries.
Best Pract Res Clin Obstet Gynaecol. 26:197–208. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen L, Qiu X, Zhang N, Wang Y, Wang M, Li
D, Wang L and Du Y: APOBEC-mediated genomic alterations link
immunity and viral infection during human papillomavirus-driven
cervical carcinogenesis. Biosci Trends. 11:383–388. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sen P, Ganguly P and Ganguly N: Modulation
of DNA methylation by human papillomavirus E6 and E7 oncoproteins
in cervical cancer. Oncol Lett. 15:11–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
McCredie MR, Sharples KJ, Paul C, Baranyai
J, Medley G, Jones RW and Skegg DC: Natural history of cervical
neoplasia and risk of invasive cancer in women with cervical
intraepithelial neoplasia 3: A retrospective cohort study. Lancet
Oncol. 9:425–434. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
U.S. USCSWG: Cancer Statistics Data
Visualizations Tool, based on 2020 submission data (1999-2018):
U.S. Department of Health and Human Services, Centers for Disease
Control and Prevention and National Cancer Institute; Available
from: www.cdc.gov/cancer/dataviz. Released in
June 2021.
|
|
19
|
Nene B, Jayant K, Arrossi S, Shastri S,
Budukh A, Hingmire S, Muwonge R, Malvi S, Dinshaw K and
Sankaranarayanan R: Determinants of womens participation in
cervical cancer screening trial, Maharashtra, India. Bull World
Health Organ. 85:264–272. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bhatia R, Kavanagh K, Cubie HA, Serrano I,
Wennington H, Hopkins M, Pan J, Pollock KG, Palmer TJ and Cuschieri
K: Use of HPV testing for cervical screening in vaccinated
women-Insights from the SHEVa (Scottish HPV Prevalence in
Vaccinated Women) study. Int J Cancer. 138:2922–2931.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bonde J, Floore A, Ejegod D, Vink FJ,
Hesselink A, van de Ven PM, Valenčak AO, Pedersen H, Doorn S, Quint
WG, et al: Methylation markers FAM19A4 and miR124-2 as triage
strategy for primary human papillomavirus screen positive women: A
large European multicenter study. Int J Cancer. 148:396–405.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cuschieri K, Ronco G, Lorincz A, Smith L,
Ogilvie G, Mirabello L, Carozzi F, Cubie H, Wentzensen N, Snijders
P, et al: Eurogin roadmap 2017: Triage strategies for the
management of HPV-positive women in cervical screening programs.
Int J Cancer. 143:735–745. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cai S, Yu X, Gu Z, Yang Q, Wen B, Sheng J
and Guan R: A 10-gene prognostic methylation signature for stage
I-III cervical cancer. Arch Gynecol Obstet. 301:1275–1287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dick S, Kremer WW, De Strooper LMA,
Lissenberg-Witte BI, Steenbergen RDM, Meijer CJLM, Berkhof J and
Heideman DAM: Long-term CIN3+ risk of HPV positive women after
triage with FAM19A4/miR124-2 methylation analysis. Gynecol Oncol.
154:368–373. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu W, Xu M, Wang L, Zhou W, Xiang R, Shi
Y, Zhang Y and Piao Y: Integrative analysis of DNA methylation and
gene expression identified cervical cancer-specific diagnostic
biomarkers. Signal Transduct Target Ther. 4(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Locke WJ, Guanzon D, Ma C, Liew YJ,
Duesing KR, Fung KYC and Ross JP: DNA methylation cancer
biomarkers: Translation to the clinic. Front Genet.
10(1150)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sacdalan DB, Ul Haq S and Lok BH: Plasma
cell-free tumor methylome as a biomarker in solid tumors: Biology
and applications. Curr Oncol. 31:482–500. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Thomas ML and Marcato P: Epigenetic
modifications as biomarkers of tumor development, therapy response,
and recurrence across the cancer care continuum. Cancers (Basel).
10(101)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leal A, Sidransky D and Brait M: Tissue
and cell-free DNA-Based epigenomic approaches for cancer detection.
Clin Chem. 66:105–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Patel KB, Padhya TA, Huang J,
Hernandez-Prera JC, Li T, Chung CH, Wang L and Wang X: Plasma
cell-free DNA methylome profiling in pre- and post-surgery oral
cavity squamous cell carcinoma. Mol Carcinog. 62:493–502.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gai W and Sun K: Epigenetic biomarkers in
cell-free DNA and applications in liquid biopsy. Genes (Basel).
10(32)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zeng H, He B, Yi C and Peng J: Liquid
biopsies: DNA methylation analyses in circulating cell-free DNA. J
Genet Genomics. 45:185–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brebi P, Maldonado L, Noordhuis MG, Ili C,
Leal P, Garcia P, Brait M, Ribas J, Michailidi C, Perez J, et al:
Genome-wide methylation profiling reveals Zinc finger protein 516
(ZNF516) and FK-506-binding protein 6 (FKBP6) promoters frequently
methylated in cervical neoplasia, associated with HPV status and
ethnicity in a Chilean population. Epigenetics. 9:308–317.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rausch S, Hasinger O, Konig T, Schlegel A
and Weiss G: An automated high throughput solution for DNA
extraction and bisulfite-conversion from high volume liquid biopsy
specimens: Sample preparation for epigenetic analysis. BMC Res
Notes. 12(551)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Stark A, Pisanic TR II, Herman JG and Wang
TH: High-throughput sample processing for methylation analysis in
an automated, enclosed environment. SLAS Technol. 27:172–179.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Carr IM, Valleley EM, Cordery SF, Markham
AF and Bonthron DT: Sequence analysis and editing for bisulphite
genomic sequencing projects. Nucleic Acids Res.
35(e79)2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ruiz-Villalba A, Ruijter JM and van den
Hoff MJB: Use and Misuse of C(q) in qPCR data analysis and
reporting. Life (Basel). 11(496)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Petralia S and Conoci S: PCR technologies
for point of care testing: Progress and perspectives. ACS Sens.
2:876–891. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kint S, De Spiegelaere W, De Kesel J,
Vandekerckhove L and Van Criekinge W: Evaluation of bisulfite kits
for DNA methylation profiling in terms of DNA fragmentation and DNA
recovery using digital PCR. PLoS One. 13(e0199091)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Leontiou CA, Hadjidaniel MD, Mina P,
Antoniou P, Ioannides M and Patsalis PC: Bisulfite Conversion of
DNA: Performance comparison of different kits and methylation
quantitation of epigenetic biomarkers that have the potential to be
used in non-invasive prenatal testing. PLoS One.
10(e0135058)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tierling S, Schmitt B and Walter J:
Comprehensive evaluation of commercial bisulfite-based DNA
methylation kits and development of an alternative protocol with
improved conversion performance. Genet Epigenet.
10(1179237X18766097)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Worm Orntoft MB, Jensen SO, Hansen TB,
Bramsen JB and Andersen CL: Comparative analysis of 12 different
kits for bisulfite conversion of circulating cell-free DNA.
Epigenetics. 12:626–636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hong SR and Shin KJ: Bisulfite-Converted
DNA quantity evaluation: A multiplex quantitative real-time PCR
system for evaluation of bisulfite conversion. Front Genet.
12(618955)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Schmitz M, Eichelkraut K, Schmidt D,
Zeiser I, Hilal Z, Tettenborn Z, Hansel A and Ikenberg H:
Performance of a DNA methylation marker panel using liquid-based
cervical scrapes to detect cervical cancer and its precancerous
stages. BMC Cancer. 18(1197)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Holmes EE, Jung M, Meller S, Leisse A,
Sailer V, Zech J, Mengdehl M, Garbe LA, Uhl B, Kristiansen G and
Dietrich D: Performance evaluation of kits for bisulfite-conversion
of DNA from tissues, cell lines, FFPE tissues, aspirates, lavages,
effusions, plasma, serum, and urine. PLoS One.
9(e93933)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nanda K, McCrory DC, Myers ER, Bastian LA,
Hasselblad V, Hickey JD and Matchar DB: Accuracy of the
Papanicolaou test in screening for and follow-up of cervical
cytologic abnormalities: A systematic review. Ann Intern Med.
132:810–819. 2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wei B, Mei P, Huang S, Yu X, Zhi T, Wang
G, Xu X, Xiao L, Dong X and Cui W: Evaluation of the SureX HPV
genotyping test for the detection of high-risk HPV in cervical
cancer screening. Virol J. 17(171)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Agorastos T, Chatzistamatiou K,
Katsamagkas T, Koliopoulos G, Daponte A, Constantinidis T and
Constantinidis TC: HERMES study group. Primary screening for
cervical cancer based on high-risk human papillomavirus (HPV)
detection and HPV 16 and HPV 18 genotyping, in comparison to
cytology. PLoS One. 10(e0119755)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shibata T, Nakagawa M, Coleman HN, Owens
SM, Greenfield WW, Sasagawa T and Robeson MS II: Evaluation of DNA
extraction protocols from liquid-based cytology specimens for
studying cervical microbiota. PLoS One. 16(e0237556)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lockwood CM, Borsu L, Cankovic M, Earle
JSL, Gocke CD, Hameed M, Jordan D, Lopategui JR, Pullambhatla M,
Reuther J, et al: Recommendations for cell-free DNA assay
validations: A joint consensus recommendation of the association
for molecular pathology and college of american pathologists. J Mol
Diagn. 25:876–897. 2023.PubMed/NCBI View Article : Google Scholar
|