Introduction
An accessory spleen (AS) is a relatively common
finding, with a prevalence of 10-30% in 3,000 autopsies of male
patients performed in the Veterans Administration Hospital
(Houston, USA), in the period from 1st October 1949 to
11th September 1958 (1-5).
The most common location of the AS is the splenic hilum (80%),
followed by in the pancreatic tail (17%) (1). The remaining 3% of ASs may be located
in the greater omentum, the splenic ligament, the small and large
bowel mesentery, the wall of the small bowel, the female adnexa,
and the scrotum (2).
Intrapancreatic AS (IPAS) is a benign congenital
anomaly of unknown aetiology, which usually does not present with
any symptoms and is revealed in a patient during unrelated
investigations (1-5).
To the best of our knowledge, there is not a described case of
symptomatic IPAS in the literature. IPAS occurs during embryologic
splenic development when a portion of the splenic tissue fails to
fuse with the main body of the spleen (1). There are no risk factors that are
associated with the occurrence of IPAS and, at present, there are
no reported mortalities from IPAS in the literature. Although IPAS
was found in ~17% of patients identified with ASs during the 3,000
autopsies in the Veterans Administration Hospital between 1949 and
1958(6), there are only 23 cases of
IPAS that have been histologically confirmed and reported in the
English literature (3). This is
because most IPASs are asymptomatic and too small to be detected by
routine diagnostic procedures (3).
Tumours such as IPAS were rarely revealed prior to the present era
of modern radiology, because they do not cause symptoms or clinical
consequences (3).
The more frequent detection of asymptomatic and
benign pancreatic tumours, such as IPAS, can be attributed to the
widespread use of various radiological imaging techniques, such as
ultrasound (US), computed tomography (CT) and magnetic resonance
imaging (MRI) (1). The proportion
of incidentally discovered tumours increased from 9 to 40% over the
two decades of data collection (7).
Although 60-75% of pancreatic incidentalomas are reported to be
malignant or premalignant lesions, the remainder, including IPAS,
are benign (3). Therefore, it is
important to accurately and differentially diagnose IPASs from
other potentially malignant tumours [such as pancreatic ductal
carcinoma, pancreatic neuroendocrine tumour (PNET), solid
pseudopapillary tumours and metastatic tumours] and prevent
unnecessary surgery (3-6).
Case study
A 60-year-old male patient presented to the
Department of Diagnostic and Interventional Radiology at Merkur
University Hospital (Zagreb, Croatia) in June 2016 with a suspected
pancreatic tumour. The patient was diagnosed with a mass in the
tail of the pancreas during a routine US examination of the abdomen
(data not shown). The remainder of the pancreas and abdomen were
normal. The patient had several diagnoses that conferred a risk for
cardiovascular disease, namely: Arterial hypertension, type II
diabetes, coronary artery disease with three stent implants and a
cerebrovascular accident in 2006, from which the patient fully
recovered. The patient had no history of malignant diseases.
A physical examination was unremarkable. Laboratory
findings of serum amylase (51 U/l; reference range, 23-91 U/l),
lipase (18 U/l; reference range, #x003C;67 U/l), carcinoembryonic
antigen (0.8 µg/l; reference range, #x003C;3.8 µg/l) and
carbohydrate antigen 19-9 (8.1 kIU/l; reference range, #x003C;34
kIU/l) were within the reference ranges. Additionally, the
laboratory data on inflammation, such as white blood cells
(8.81x109/l; reference range, 3.4-9.7x109/l)
and C-reactive protein (0.2 mg/l; reference range, #x003C;5 mg/l),
were within normal ranges and there were no signs of inflammation
from the laboratory results. The patient did not present with any
of the symptoms of a functioning PNET, such as tachycardia,
flushing, fainting, wheezing, fatigue, diarrhoea, skin rash,
constipation and weakness.
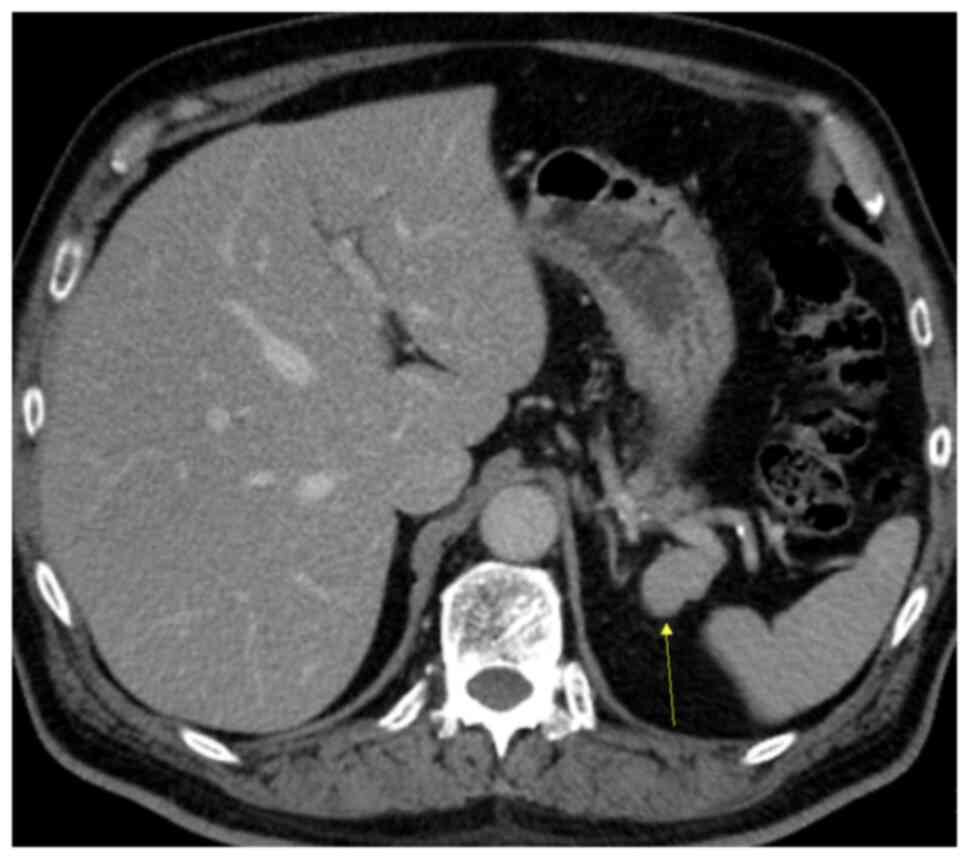
A triple-phased CT was used for further evaluation.
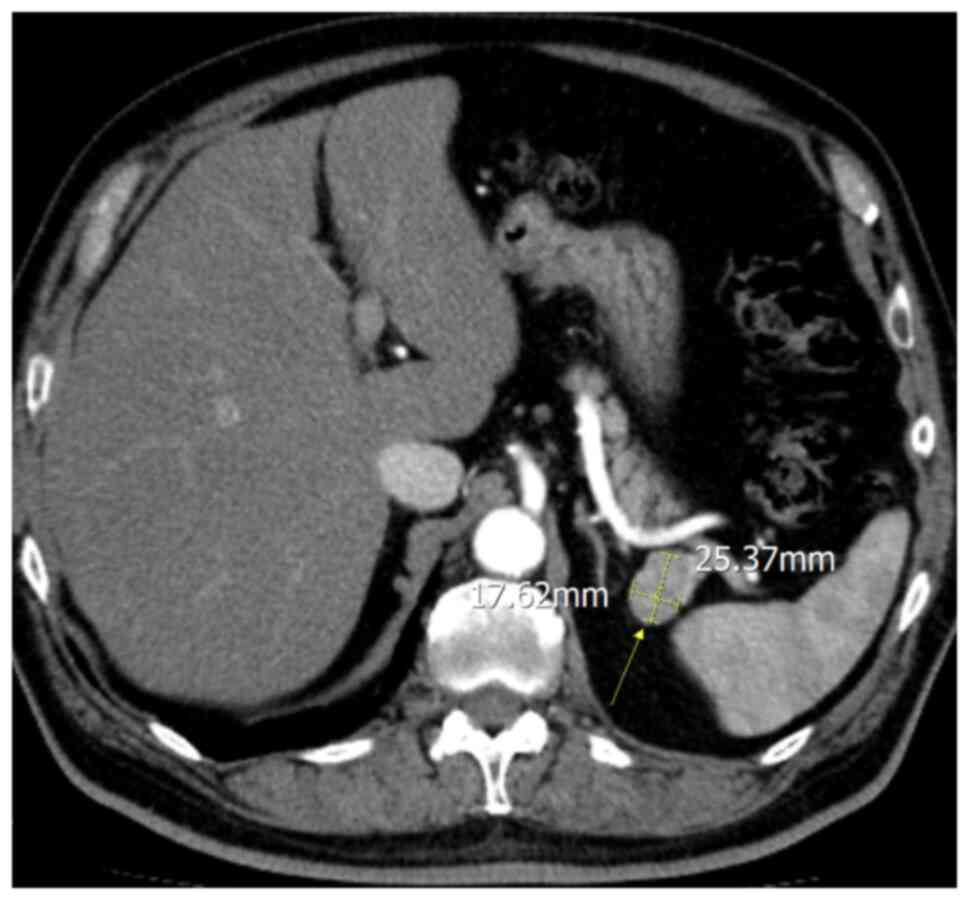
CT revealed an ovoid well-demarcated hypervascular pancreatic tail
lesion, without contact with the pancreatic ducts, measuring 25x17
mm (Fig. 1). Pre-contrast CT
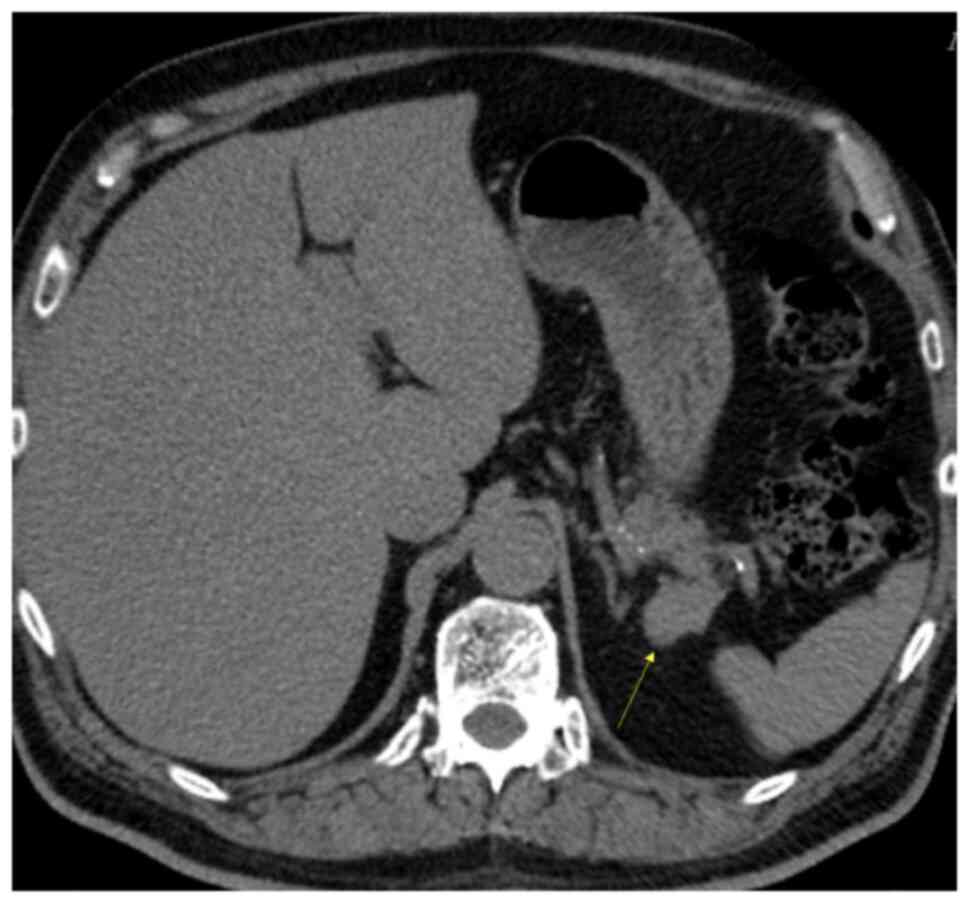
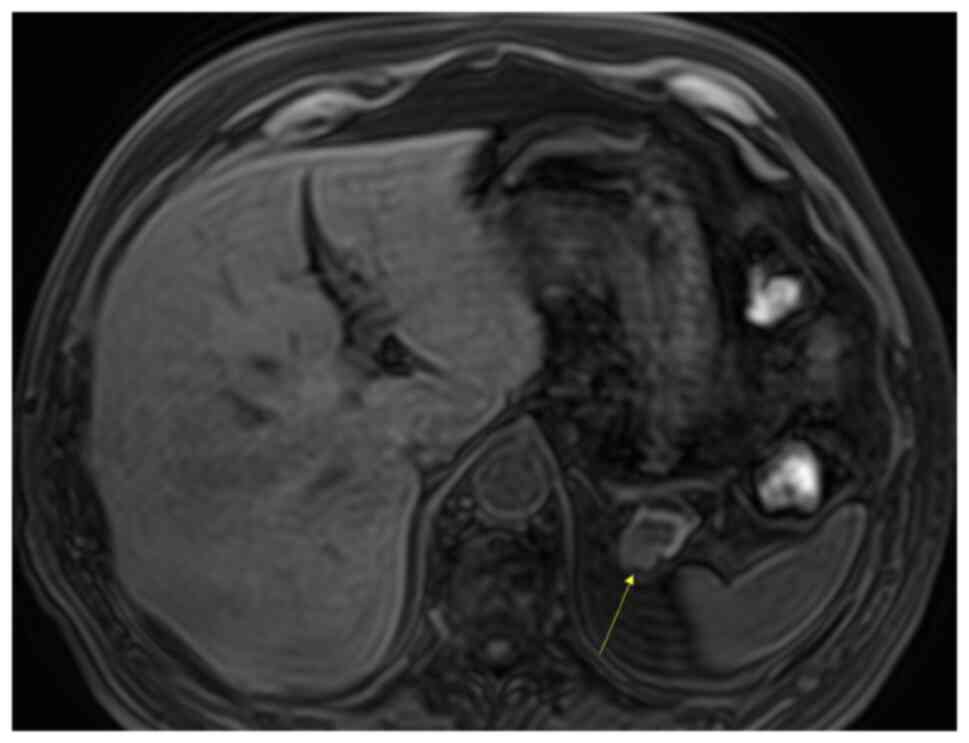
demonstrated a mass in the tail of the pancreas (Fig. 2), and the density of the lesion
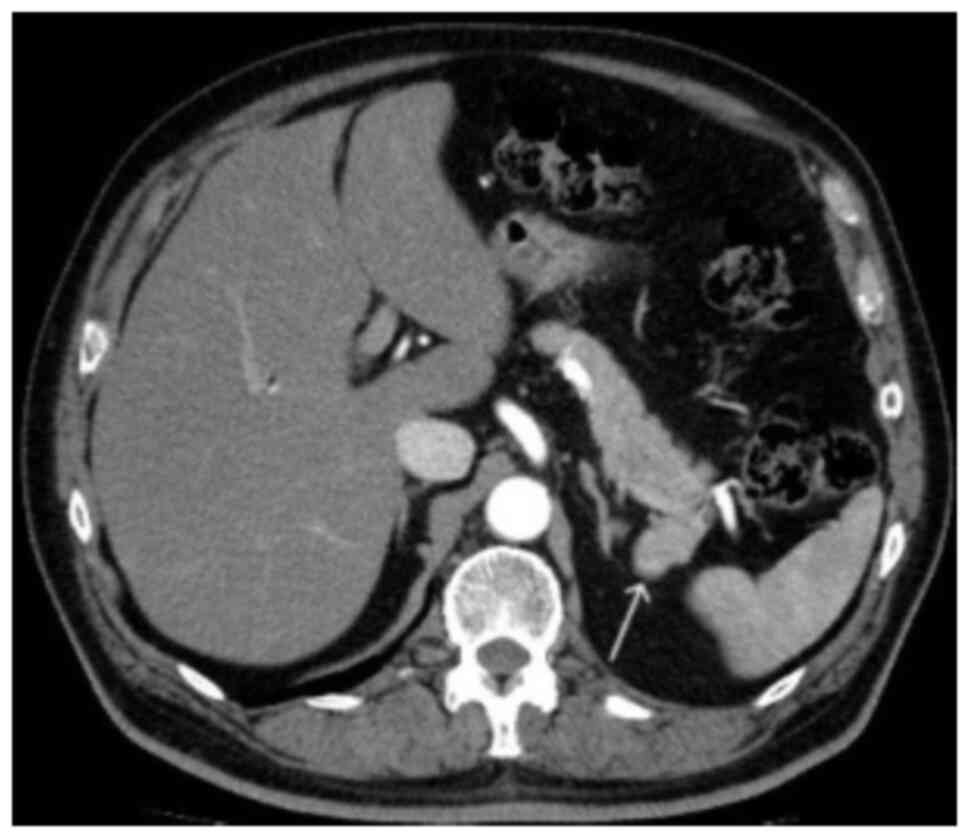
corresponded with that of the spleen. In the arterial phase, the
mass showed an inhomogeneous zebra-patterned enhancement, such as
that observed in the spleen (Fig.
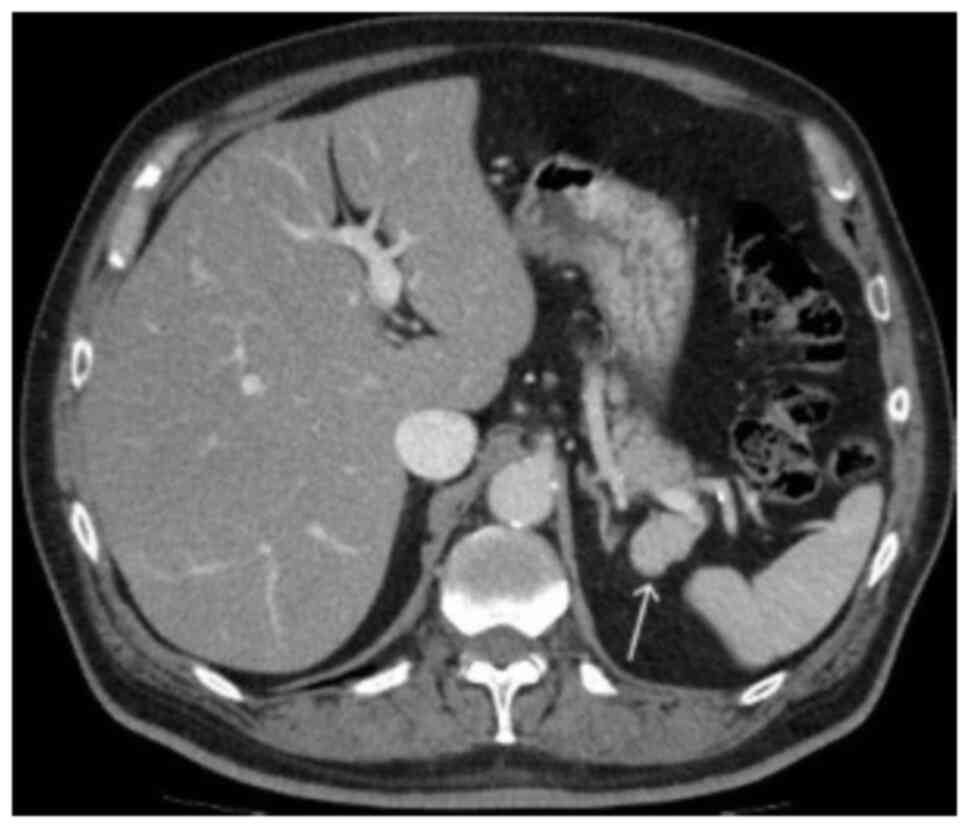
3). In the portal venous phase, the mass revealed a homogeneous
enhancement, similar to that in the spleen (Fig. 4). Differential diagnoses included
IPAS and non-functioning PNET. The density of the lesion
corresponded to that of the spleen on all imaging phases, which was
suggestive of an IPAS.
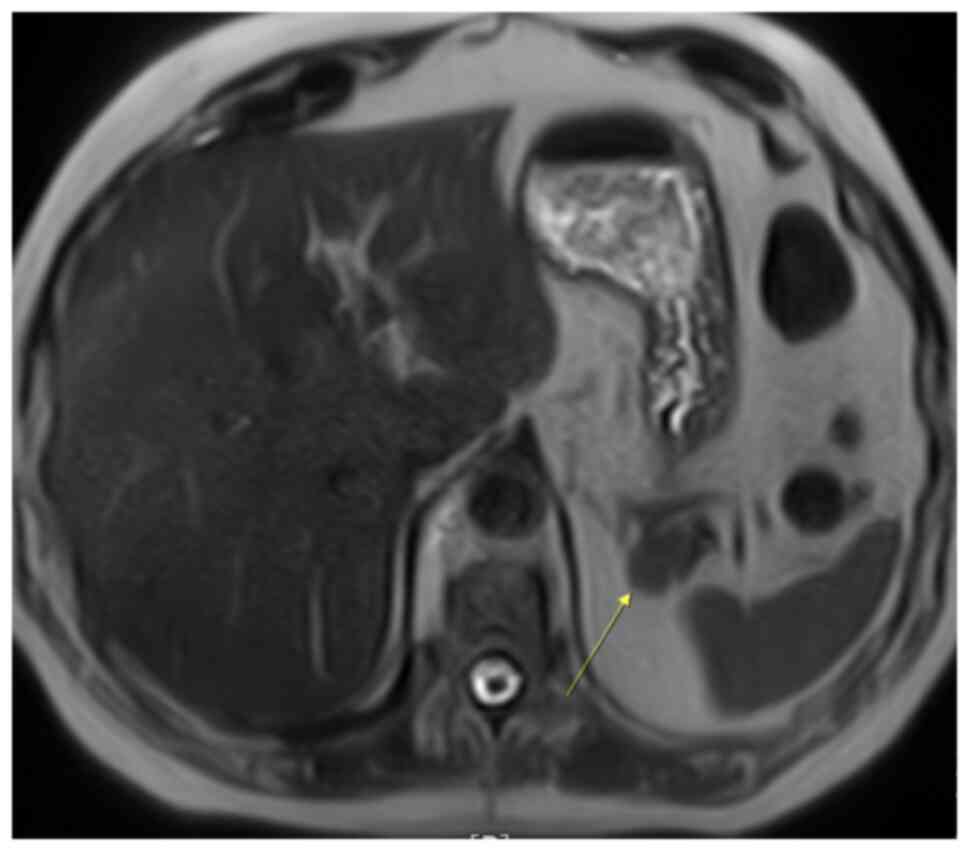
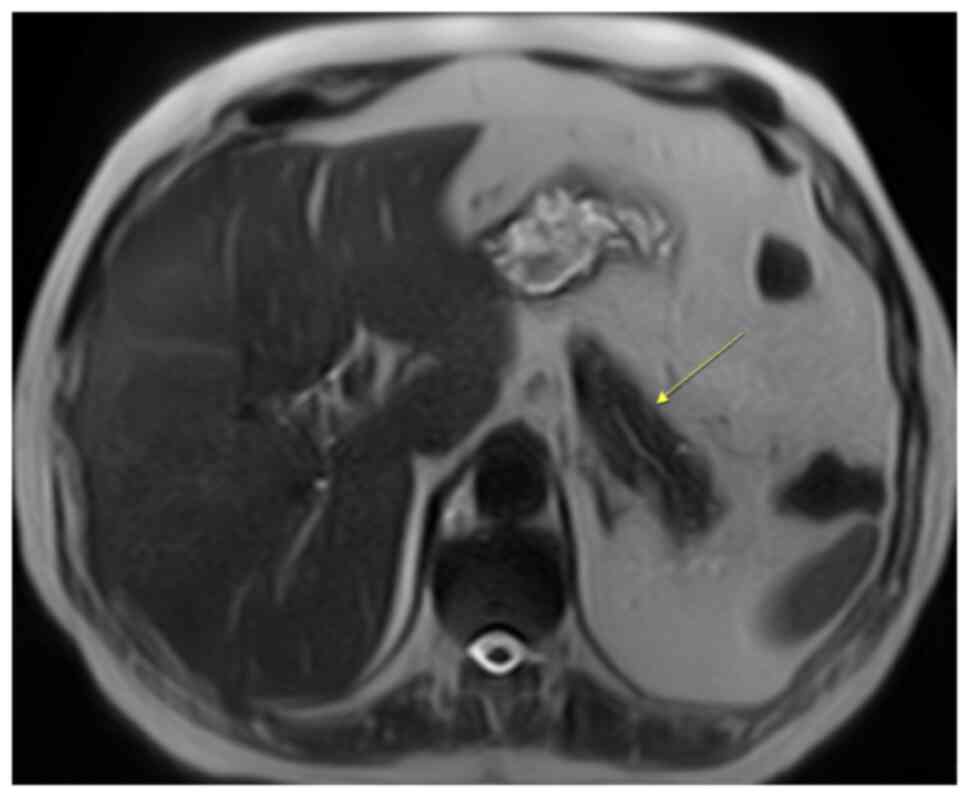
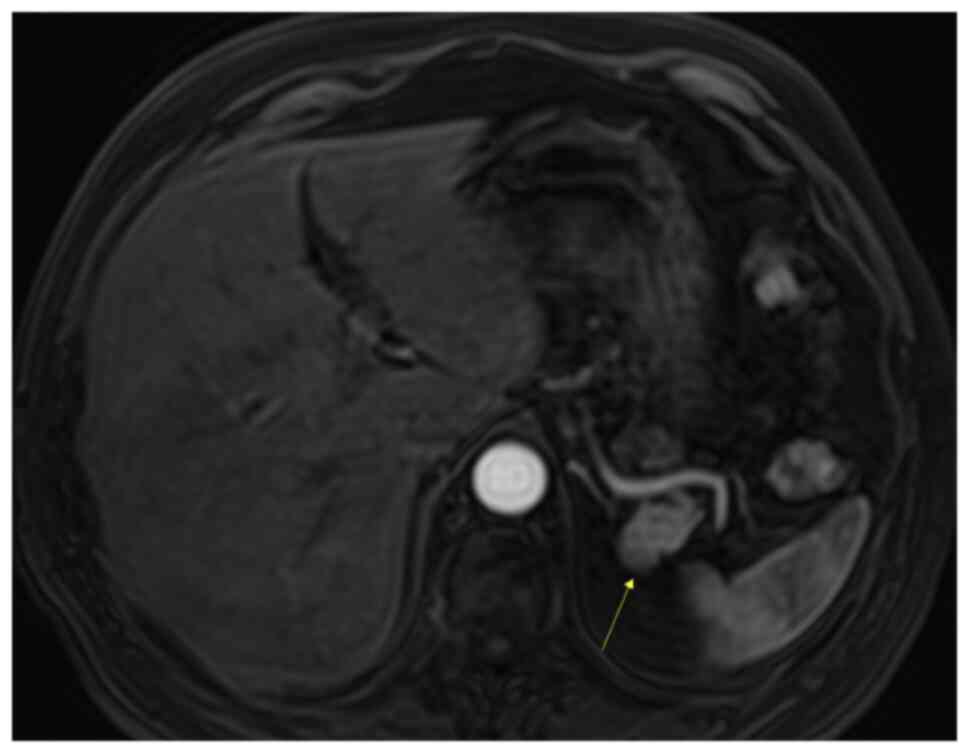
The patient was referred for an MRI. The MRI
demonstrated a mass in the pancreatic tail, which was mildly
hypointense on T1-weighted imaging (Fig. 5) and more intensive on T2-weighted
imaging (Fig. 6), when compared
with the surrounding pancreas (Fig.
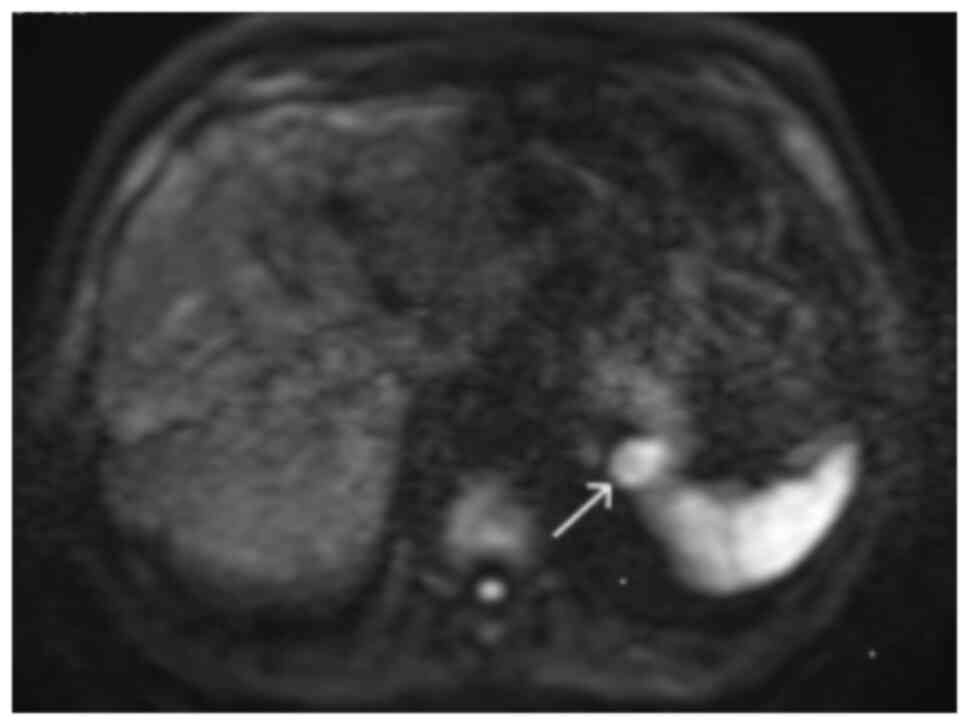
7). The mass had the same intensity as the spleen on all
unenhanced sequences, including diffusion-weighted imaging (DWI)
sequences (Fig. 8). Using dynamic
contrast-enhanced MRI, the mass showed an enhancement pattern
similar to that of the spleen (Fig.
9). The apparent diffusion coefficient (ADC) value of the mass
was notably decreased compared with that of a normal pancreas, but
similar to that of the spleen.
No enlarged lymph nodes or other pathological
processes were observed in the abdomen or the rest of the pancreas
on either CT or MRI scans.
Due to comorbidities, the patient refused further
evaluation or surgery. The lesion was periodically monitored using
CT scans every 1-2 years. At the last medical check-up in November
2023, the patient was doing well with no new symptoms and the CT
scan revealed that the mass had remained static (Fig. 10). Since the tumour remained static
during the 7-year follow-up, it was concluded that it was an
IPAS.
Discussion
Congenital foci of splenic tissue, which are common
(with a prevalence of 10-30% found in an autopsy series of 3,000
people) and in most cases present without symptoms, are termed ASs
(8,9). They are usually located near the
splenic hilum, with ~20% located in or near the pancreatic tail
(8). In order to avoid unnecessary
surgery and reduce possible patient morbidity and mortality, it is
necessary to diagnose IPAS non-invasively (9). For an accurate non-invasive diagnosis
of IPAS, multimodal imaging is important.
Using sonography, IPASs are well-defined structures
that can be round, ovoid or lobulated in shape (8). Pancreatic parenchyma has an increased
echogenicity compared with that of IPASs (10). All IPASs exhibit echogenicity, which
is homogenous and identical to that of the main spleen (10). Sonography has advantages and
disadvantages for its use in diagnosis; while it is cost-effective,
it is operator-dependent and lacks sensitivity, which makes it a
less useful method in several circumstances. For example, in
patients that are obese, the pancreatic tail is hard to visualise
(11). However, contrast-enhanced
sonography may improve the diagnostic power of US. In the arterial
phase, there is a typical inhomogeneous enhancement due to the
different flow rates through the splenic cords (5). In the venous phase, IPASs show dense
end persistent enhancement lasting 3-5 min (5,8).
Endoscopic US (EUS), particularly when used with elastography, has
an increased resolution and sensitivity compared with abdominal US
(1). According to the study by Li
et al (12), EUS-fine-needle
aspiration (FNA) biopsy has a low diagnostic value, and is
associated with a risk of intra-abdominal bleeding and a
misdiagnosis for PNET. However, the study by Marques et al
(1) considered that EUS-FNA is an
effective tool to obtain a definitive IPAS diagnosis.
Scintigraphy and single-photon emission CT both have
a reduced spatial resolution compared with other cross-sectional
imaging techniques, such as the plain and the contrast-enhanced CT
and MRI, which can result in false-negative findings for small
IPASs. Therefore, these methods are used in combination with other
cross-sectional imaging techniques (12).
IPAS may show heterogeneous enhancement on the early
CT phase (usually within 70 sec after contrast administration),
which is explained by the different flow rates through the cords of
the red and white pulp (2,5,8,10,11).
The same pattern of enhancement is seen in the spleen (2,5,8,10,11).
If the enhancement of the focal lesion tracks that of the spleen in
all CT phases, particularly if the typical ‘zebra’ pattern of the
spleen is observed in the arterial phase, IPAS can be diagnosed
with near certainty using CT (2).
This was the case in the present study. Typically, the attenuation
of IPAS is increased compared with that of the pancreas in the
arterial, pancreatic and portal venous phases (2). However, this typical pattern is not
always visible in the IPAS. This may be due to the small size of
the IPAS or due to a different mixture of red and white pulp, as
compared with the main spleen (12). In contrast to IPAS, other
hypervascular pancreatic tumours show signs of hyperattenuation in
the arterial phase and isoattenuation or hypoattenuation to the
adjacent pancreas in the venous phase (2).
When visualized using MRI, IPASs are hypointense on
T1-weighted images and hyperintense [or more intense (as in the
present case)] on T2-weighted images compared with the pancreas
that is surrounding it (2). In
addition, IPASs exhibit a heterogeneous enhancement on
arterial-phase gadolinium-based-enhanced MRI, which is comparative
to that of the normal spleen (2,9,12). In
the portal venous phase, the IPAS is homogeneously hyperintense,
and in the delayed phase it is isointense compared with the
adjacent pancreas (2).
DWI has been used to successfully diagnose IPAS, and
it has the ability to differentiate it from a solid pancreatic
tumour. It also shows notable differences between pancreatic and
splenic tissue (13). The signal
intensity from DWI with high B values and the ADC are useful in
differentiating IPAS from other solid pancreatic tumours, since the
spleen shows the lowest ADC among the organs of the upper abdomen
(13,14). IPASs have a reduced ADC value and an
increased high signal intensity on DWI compared with other
pancreatic tumours (13,14). The signal intensities of IPASs are
identical to those of the spleen on multiple MRI sequences, which
is key in diagnosis (15).
On CT and MRI, an IPAS is often described as a small
(1-3-cm), well-defined lesion that has a density and intensity
comparable to that of the spleen in all plain and contrast-enhanced
phases. Its feature is that it remains stable over consecutive
imaging (6). Although biopsies are
useful for diagnosing primary and secondary tumours throughout the
body and can improve the outcomes of patients by enabling a prompt
diagnosis and the appropriate treatments, in the case of IPASs it
is important to avoid biopsy (16).
An IPAS is a benign entity, which does not require
therapy, except when it is combined with idiopathic
thrombocytopenic purpura (ITP) (12,17).
Accessory splenectomy should be considered in any patient with a
recurrence of ITP if clinical examinations are suggestive of
residual functional splenic tissue (17). Only in rare cases do IPASs cause
symptoms, and these are due to the following events: Compression,
torsion, spontaneous rupture and haemorrhage (12). To the best of our knowledge, only 2
cases of symptomatic ASs have been reported in the English
literature (18,19).
Unnecessary surgeries performed due to IPASs being
falsely diagnosed as primary pancreatic tumours, or even
hypervascular metastases, have revealed most of the previously
reported IPASs (2). Despite the
high diagnostic accuracy of imaging studies, IPAS is often
unidentified and unnecessary surgery is performed in up to 66.6% of
cases (20).
The present study presented a novel case of IPAS
that was reliably recognized using non-invasive imaging techniques,
so that unnecessary surgery was avoided. In conclusion, the
characteristic location and features of an IPAS on non-invasive
diagnostic imaging techniques, such as CT and MRI, may be specific
enough to allow for a close follow-up of patients that present with
small well-circumscribed lesions in the pancreatic tail without
proven malignant disease and are either poor candidates for surgery
or refuse surgery. If the lesion remains static during follow-up
for 5-6 years, an IPAS could be diagnosed with a high degree of
certainty.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KK collected the data, assisted in drafting the case
report section, critically revised the manuscript and wrote the
manuscript. DOK and BJP collected the data, assisted in drafting
the case report section and critically revised the manuscript. All
authors confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of the
patient's clinical information and images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marques S, Bispo M and Noia L:
Intrapancreatic accessory spleen: A diagnosis not to forget! Case
Rep. Gastroenterol. 10:749–754. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim SH, Lee JM, Han JK, Lee JY, Kim KW,
Cho KC, Cho KC and Choi BI: Intrapancreatic accessory spleen:
Findings on MR imaging, CT, US and scintigraphy, and the pathologic
analysis. Korean J Radiol. 9:162–174. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hwang HS, Lee SS, Kim SC, Seo DW and Kim
J: Intrapancreatic accessory spleen: Clinicopathologic analysis of
12 cases. Pancreas. 40:956–965. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Osher E, Scapa E, Klausner J, Greenman Y,
Tordjman K, Melhem A, Nachmany I, Sofer Y, Geva R, Blachar A, et
al: Pancreatic incidentaloma: Differentiating nonfunctioning
pancreatic neuroendocrine tumours from intrapancreatic accessory
spleen. Endocr Pract. 22:773–779. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Massani M, Maccatrozzo P, Morana G, Fabris
L, Ruffolo C, Bonariol L, Pauletti B and Bassi N: Diagnostic
difficulties and therapeutic choices in intrapancreatic accessory
spleen: Case reports. Open Access Surg. 9:15–20. 2016.
|
|
6
|
Spencer LA, Spizarny DL and Williams TR:
Imaging features of intrapancreatic accessory spleen. Br J Radiol.
83:668–673. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Santo E and Bar-Yishay I: Pancreatic solid
incidentalomas. Endosc Ultrasound. 6 (Suppl 3):S99–S103.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rahbar H, Bhargava P, Vaidya S and Medverd
JR: Intrapancreatic accessory spleen. Radiol Case Rep.
5(386)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Loureiro AL, Ferreira AO, Palmeiro M and
Penedo JP: Intrapancreatic accessory spleen: A misleading
diagnosis. BMJ Case Rep. 2013(bcr2012008471)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo W, Han W, Liu J, Jin L, Li JS, Zhang
ZT and Wang Y: Intrapancreatic accessory spleen: A case report and
review of the literature. World J Gastroenterol. 15:1141–1143.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kykalos S, Machairas N, Molmenti EP and
Sotiropoulos G: Intrapancreatic accessory spleen: Two case reports
of a rare entity. Cureus. 12(e8797)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li BQ, Xu XQ and Guo JC: Intrapancreatic
accessory spleen: A diagnostic dilemma. HPB (Oxford). 20:1004–1011.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Munk-Madsen MZ, Zakarian K, Sandor Oturai
P, Hansen CP, Federspiel B, Fallentin E and Linno Willemoe G:
Intrapancreatic accessory spleen mimicking malignant tumour: Three
case reports. Acta Radiol Open. 8(2058460119859347)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Contractor U, Henderson I and Zaitoun MD:
Intrapancreatic accessory spleen: An important differential to
consider before surgery. Hum Pathol Case Rep. 1:49–51. 2014.
|
|
15
|
Chavan N, Desai GS, Tampi C and Wagle P:
Intrapancreatic accessory spleen: An enigmatic entity. BMJ Case
Rep. 12(e228510)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hashimoto K, Nishimura S, Ito T, Oka N and
Akagi M: Limitations and usefulness of biopsy techniques for the
diagnosis of metastatic bone and soft tissue tumours. Ann Med Surg
(Lond). 68(102581)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
García AF and Sanjuanbenito Dehesa A:
Intrapancreatic accessory spleen: A rare cause of recurrence of
immune thrombocytopenic purpura. Clin Case Rep. 4:979–981.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Obuchi T, Takagane A, Sato K, Yonezawa H,
Funato O and Kobayashi M: Single-incision laparoscopic excision of
symptomatic accessory spleen in the pelvis: An initial report. J
Minim Access Surg. 13:321–322. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hems TE and Bellringer JF: Torsion of an
accessory spleen in an elderly patient. Postgrad Med J. 66:838–839.
1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Riordan B, Rodendo O and Stafford A:
Laparoscopic distal pancreatectomy and splenectomy for an
intrapancreatic accessory spleen-a case report and review of the
literature. Mesentery Peritoneum. 6(AB154)2022.
|