1. Introduction
Ozone (O3) is a naturally occurring
compound formed from three oxygen atoms as a result of conversion
by ultraviolet radiation (1). At
low medical concentrations, ozone acts as a powerful oxidant with
wide-spectrum antimicrobial activity and the ability to promote
healing processes and reduce inflammation through protective
antioxidant pathways, thus providing therapeutic benefits in the
treatment of a number of diseases (2-5).
The antimicrobial action is due to ozone-induced
oxidation, which causes direct and indirect damage to microbial
cell structures and metabolism (6-8).
Ozone is also involved in pharmacological immunomodulation as it
induces mild oxidative stress, which triggers antioxidant responses
through the activation of specific molecular pathways, activating
anti-inflammatory mechanisms (9,10).
Additionally, topical application of ozone has been reported to
improve the rheological properties of blood by inducing functional
and structural changes at the erythrocyte level, thereby enhancing
peripheral oxygen perfusion and general metabolism (11-13).
Due to its strong oxidizing activity, ozone has been
used as a disinfectant and germicidal agent for both industrial and
medical purposes, with a variety of applications as a sterilizing
agent for water treatment, medical equipment, dental settings and
closed environments (14-18).
Since 1930, ozone has also been studied for applications in
dentistry (1,19). However, these studies were abandoned
until the 1980s, due to the risk of inhalation toxicity and the
difficulty of achieving optimal gas concentrations without
dispersion (20). These issues have
been addressed with modern technology and appropriate delivery and
application techniques (19). A
number of parenteral and topical routes are currently used to
deliver ozone without toxic effects, with the exception of the
inhalation route, which should be avoided due to broncho-pulmonary
toxicity (11). The most widely
used formulations for the oral cavity are gaseous ozone, ozonized
water and ozonized oil (21).
The recommended concentration for a mixture of
oxygen and ozone for medical use is between 5-50 µg ozone/1 ml
oxygen (11). According to
guidelines and good clinical practice recommendations in
oxygen-ozone therapy published in the the World Federation of Ozone
Therapy's Review on Evidence Based Ozone Therapy (20), an ozone concentration of 5-10 µg/ml
is recommended for the application of ozonized products by
intralesional injection or irrigation in order to obtain a
therapeutic effect with no risk of toxicity, even in the event of
ingestion (22,23).
Along with predominantly beneficial properties, the
occurrence of some potential adverse effects must be considered,
primarily in relation to toxicity upon inhalation. Excessive or
prolonged exposure to gaseous ozone, which is more likely to occur
in operators than in patients, may cause headaches, vomiting and
irritation of the upper respiratory tract, which can manifest as a
sore throat, cough, epiphora, rhinitis and bronchoconstriction
(24,25). Additionally, certain forms of ozone
therapy, namely those that induce systemic effects, are
contraindicated in myocardial infarction, hyperthyroidism, acute
alcohol intoxication, severe anemia, thrombocytopenia, active
hemorrhage and pregnancy (21,26,27).
However, ozone therapy is non-toxic to humans and is free of side
effects when handled with care, using modern available technology
in accordance with the manufacturer's instructions and current
guidelines and recommendations (22).
At present, ozone therapy is implemented in certain
fields of dentistry, including implantology, oral surgery,
periodontology, oral medicine and management of dental caries
(28-31).
Ozone applications have yielded promising results in the treatment
of inflammatory and immune-mediated conditions of oral soft
tissues, such as oral lichen planus and aphthous stomatitis
(32,33). In addition, ozone has been applied
in the context of post-operative wounds and complications such as
alveolar osteitis, which alleviated painful symptoms and reduced
healing times (34,35). Additionally, ozone has generally
contributed to a reduction of possible side effects associated with
traditional treatments of these oral ulcerative conditions and
surgical wounds and complications (33,34,36).
Due to its antimicrobial activity, ozone has been reported to be
useful in the management of oral disease of infectious etiology,
such as herpetic stomatitis and oral candidiasis (37). In addition, a beneficial
remineralizing effect on hard dental tissues has been reported and
used to treat dental demineralization and decay (38-41).
In particular, ozone application has been suggested as a possible
alternative or complementary strategy to manage dental caries in a
less invasive and more comfortable procedure than traditional
approaches, especially in young or poorly cooperative children
(31,41,42).
Since dental caries is considered a global public health challenge,
especially in children and the socioeconomically disadvantaged
population, the implementation of effective, safe and economic
interventions to prevent and treat caries should be a priority in
oral health care policies (43-45).
Overall, ozone therapy may indirectly help restore
functional activities compromised by disease by targeting certain
biological processes as a biological response modifier (10). Although ozone therapy is now used in
a number of countries, it has mostly been adopted by private health
care practitioners (46). Over the
past four decades, practitioners in Europe have mainly used ozone
therapy empirically, potentially due to a lack of adequate
knowledge of the basic principles behind optimal use (46,47).
This approach has sometimes led to the inappropriate or dangerous
use of ozone, which has contributed to a general misconception of
its intrinsic toxicity (46,47).
These issues, combined with the difficulty and cost of conducting
extensive clinical trials, lack of interest by pharmaceutical
companies and health authorities, as well as a lack of sponsorship,
have hindered progress in this field of research, so that ozone
therapy remains a poorly known and controversial complementary
medical practice (47).
Nevertheless, a number of studies have been carried out with
promising results (2-4,29).
The general mechanisms of ozone interaction with human tissues have
been increasingly studied and progress has previously been made in
this area of research. However, the underlying mechanisms
explaining the effects of ozone on dental tissues are complex and
are not yet fully understood.
The aim of the present review was to provide an
overview of the currently available evidence on the mechanisms
involved in ozone interaction with dental tissues underlying the
beneficial effects of ozone in dentistry.
2. Literature search methods
The present review focused on the current relevant
evidence on the biological mechanisms underlying the interaction of
ozone with dental tissues, including periodontal structures, dental
cells, enamel and dentine.
A literature search was performed using the
PubMed/MEDLINE database (https://pubmed.ncbi.nlm.nih.gov) until March 22, 2024.
The online literature search was performed using combined terms
related to ozone and possible biological effects [‘ozone’ AND
‘antimicrobial’, ‘remineralization’, ‘immunomodulation’,
‘biostimulation’, ‘regeneration’, ‘dental cells’, ‘periodontal
cells’, ‘odontoblasts’, ‘cementoblasts’, ‘osteoblasts’ AND ‘(dental
OR dentistry OR oral)’] (Fig. 1).
Among all records retrieved through the literature search, reviews
or papers with an experimental design specifically addressing
ozone-related biological mechanisms in oral tissues were identified
as key references. Relevant in vitro studies, in addition to
animal and human studies, published in English were considered,
without restrictions on the publication date.
3. General mechanisms involved in the
interaction of ozone with dental tissues
A total of 487 potentially relevant records were
retrieved as a result of the literature search. The number of
studies decreased to 389 after duplicate removal, which was
performed using the Rayyan software algorithm (48). Handsearching was conducted by
manually screening the references of the considered studies to
identify possible additional relevant papers.
The key references considered in the present review
are listed in Table I. Of these
studies, 16 were in vitro studies, seven were based on
animal models including one with an additional human clinical
phase, one was designed as a human ex vivo study and one was
a review. In the field of dentistry, the main beneficial effects of
ozone could be summarized in the following domains: Antimicrobial
activity, remineralization through direct interaction with hard
dental tissues, immunomodulation and biostimulation of dental and
periodontal cells (Fig. 2).
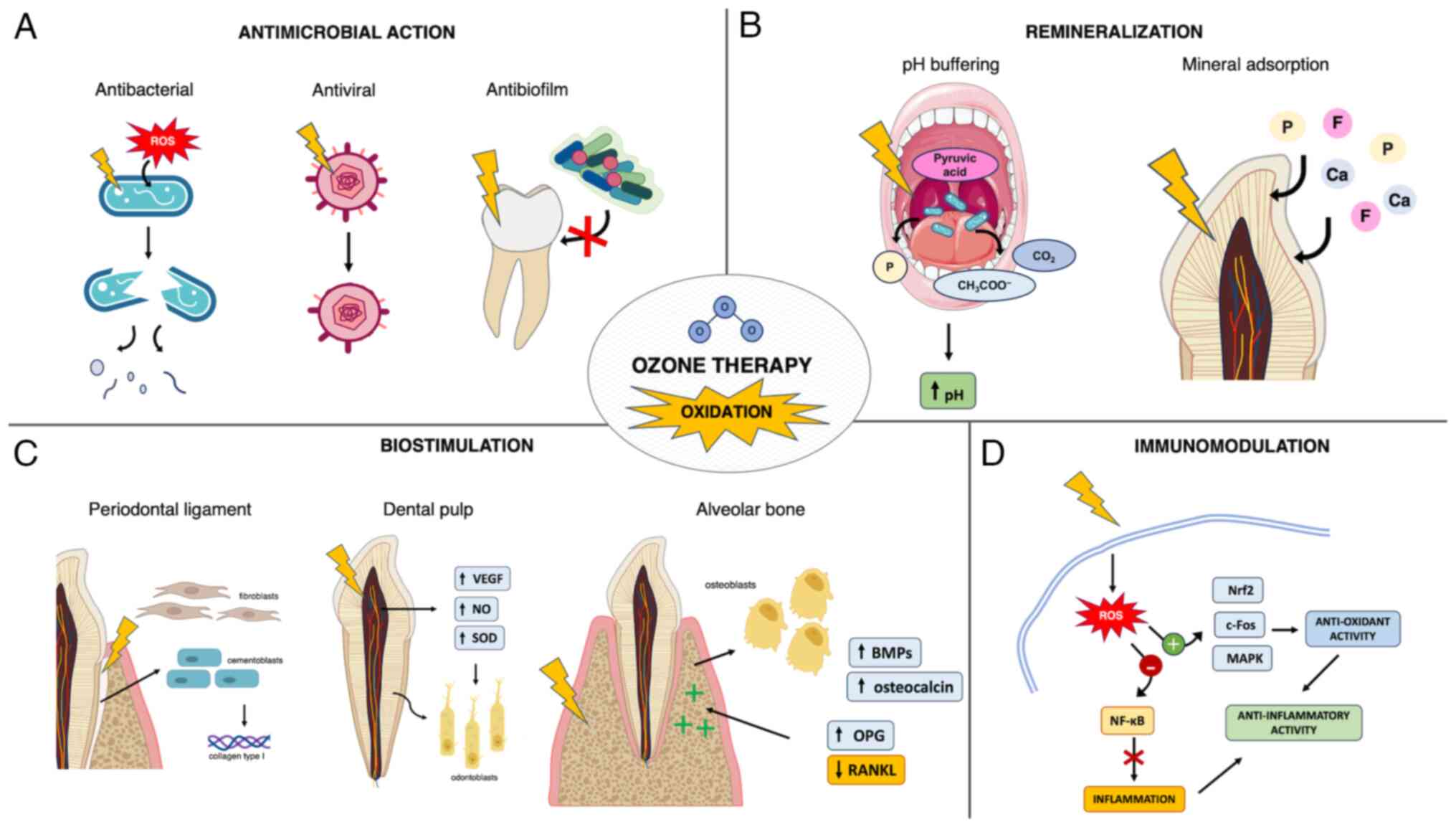 | Figure 2Mechanisms involved in ozone
interaction with dental tissues. (A) Antibacterial effect: Ozone
causes damage to cytoplasmatic membrane and cell walls of bacteria
and fungi, allowing ROS to enter cells and cause functional damage,
cell lysis and death. Antiviral effect: Ozone induces
conformational changes to viral structures such as the envelope and
spike proteins, which leads to virus inactivation. Antibiofilm
effect: Ozone induces alteration of salivary biomolecules and
related binding sites, thus hampering biofilm formation and
adhesion on dental surfaces. (B) Oxidation of the nucleic acids of
microorganisms and of pyruvic acid in the oral cavity results in a
pH buffering effect, facilitating a remineralizing environment.
Oxidation causes microstructural surface changes, removal of
surface organic components and proteins from enamel and dentine,
which leads to enhanced deposition and diffusion of minerals within
enamel and dentin, thus promoting remineralization and reduction of
dentine hypersensitivity. (C) Effects on dental pulp: Mild
oxidation promotes dental pulp homeostasis, vascularization and
regeneration associated with increased expression of VEGF, NO and
SOD, which leads to odontodifferentiation of dental pulp cells,
odontoblast proliferation and the production of mineralized matrix.
Effects on periodontal ligament: Ozone induces proliferation of
periodontal cells and enhanced collagen type I synthesis, which
results in periodontal tissue regeneration. Effects on alveolar
bone: Ozone induces increased osteoblast proliferation and
activity, increased expression of osteocalcin and BMPs, reduced
RANKL levels and increased OPG levels, which results in the
promotion of bone healing and formation. (D) Ozone byproducts and
oxidation cause mild ROS signaling and mitochondrial stress, which
lead to activation of antioxidant pathways and inhibition of
pro-inflammatory pathways, resulting in an anti-inflammatory
effects. Ozone also stimulates T-cell mediated immunity. Arrows
indicate a causal effect, lightning bolts indicate ozone-induced
oxidation and red crosses indicate prevention. ROS, reactive oxygen
species; NO, nitrous oxide; SOD, superoxide dismutase; BMP, bone
morphogenetic protein; RANKL, receptor activator of NF-κB ligand;
OPG, osteoprotegerin; Nrf2, nuclear factor erythroid 2-related
factor. |
 | Table IKey references concerning ozone
mechanisms considered in the present review ordered by publication
date. |
Table I
Key references concerning ozone
mechanisms considered in the present review ordered by publication
date.
| First author,
year | Study type | Type of ozone and
topic of research | (Refs.) |
|---|
| Ebensberger,
2002 | In
vitro | Ozonized water;
proliferation of cementoblasts and fibroblasts | (83) |
| Celiberti,
2006 | In
vitro | Gaseous ozone;
enamel properties | (6) |
| Grootveld,
2006 | In
vitro | Gaseous ozone;
oxidation of salivary biomolecules | (56) |
| Huth, 2006 | In
vitro | Gaseous ozone,
ozonized water; cytotoxicity on oral epithelial cells and gingival
fibroblasts | (86) |
| Huth, 2007 | In
vitro | Ozonized water;
NF-κB system | (10) |
| Cardoso, 2008 | In
vitro | Ozonized water;
microbial endotoxins | (53) |
| Noguchi, 2009 | In
vitro | Ozonized water;
odontoblast-like cells | (74) |
| Raafat Abdelaziz,
2011 | In
vitro | Gaseous ozone;
dentinal tubules | (68) |
| Gürsoy, 2012 | In
vitro | Gaseous ozone;
dentinal tubules | (70) |
| Alpan, 2016 | Animal model | Gaseous ozone; bone
regeneration | (89) |
| Estrela, 2017 | In
vitro | Gaseous ozone;
dentine remineralization; biofilm | (40) |
| Amin, 2018 | Animal model | Gaseous ozone
injection; immunomodulation; candidiasis | (60) |
| Colombo, 2018 | In
vitro | Ozonized oil;
biocompatibility; gingival fibroblasts | (85) |
| Wang, 2018 | In
vitro | Ozonized oil;
gingival fibroblasts proliferation; immunomodulation | (84) |
| Krunić, 2019 | Ex vivo
human study | Gaseous ozone;
dental pulp | (81) |
| Leewananthawet,
2019 | In
vitro | Ozonized water;
oxidative stress; periodontal fibroblasts | (93) |
| Bayer Alinca,
2020 | Animal model | Gaseous ozone; bone
healing | (92) |
| Dikopova, 2021 | Animal model and
clinical human study | Gaseous ozone;
dental pulp; dentine | (72) |
| Küçük, 2021 | In
vitro | Ozonized water;
cytotoxicity; dental pulp | (73) |
| Cenci, 2022 | Review | Ozone; antiviral
immunomodulatory effects | (59) |
| Ferreira, 2022 | In
vitro | Ozonized water;
dentine metalloproteinases; dental pulp cells | (71) |
| Floare, 2023 | In
vitro | Gaseous ozone;
enamel microstructure | (24) |
| Vieira, 2023 | Animal model | Gaseous ozone; bone
inflammation and regeneration | (88) |
| De Souza, 2024 | Animal model | Gaseous ozone;
myofascial pain and inflammation related to temporomandibular
disorders | (96) |
| Lima Neto,
2024 | Animal model | Gaseous ozone; bone
metabolism following biphosphate treatment for osteoporosis | (87) |
4. Antimicrobial action
Ozone exhibits broad antimicrobial activity through
oxidation, which causes dysfunction of the bacterial cell wall and
cytoplasmic membrane, resulting in increased permeability to ozone
molecules (7). Consequently,
glycoproteins, glycolipids and amino acids are damaged and the
enzymatic system is impaired, ultimately leading to cell lysis and
death (6,8). Moreover, ozone reacts with unsaturated
fatty acids in cell membranes, producing secondary reactive species
such as aldehyde derivatives and lipid peroxides. These can reach
the nuclei of intact cells, leading to nucleic acid breakdown that
also results in the release of phosphate ions (49). The underlying mechanism by which
this occurs remains largely unknown, but may explain the ozone
buffering effect on biofilm fluids, which promotes a remineralizing
environment (40). The bactericidal
activity of ozone may be mediated by functional and structural
disruption occurring at the level of the cytoplasmic membrane
(50). Ozonized water (0.5-4.0
mg/l) has shown effective bactericidal properties against various
strains of cariogenic bacteria and oral pathogens in vitro.
Among these, however, gram-negative bacteria such as
Porphyromonas endodontalis and Porphyromonas
gingivalis were observed to be more sensitive to ozonized water
compared with gram-positive streptococci and Candida
albicans (50). Such
discrepancies have been linked to differences in the structure of
microorganism cell walls (51). The
effect of ozone was also investigated on C. albicans and
Enterococcus faecalis in infected root dentinal tubules,
alone or in association with other antimicrobial agents (52). Ozonized water significantly reduced
the number of C. albicans and E. faecalis, but showed
no residual effect and activity against lipopolysaccharide (LPS)
endotoxin (53). The authors
hypothesized that these findings were related to the poor
penetration of irrigants into the inner regions of dentinal
tubules, allowing the remaining microorganisms to multiply over the
course of the experiment (53).
The aforementioned findings were supported by a
study that investigated the effects of ozone on an oral biofilm of
Streptococcus mutans and Lactobacillus acidophilus,
which reported no evidence of bacterial colonization on
ozone-treated dentin surfaces in vitro (54). Due to the short half-life of ozone,
however, it is unlikely that ozone could have interfered with the
activity of the bacterial glycosyltransferase enzyme (54). This enzyme is responsible for the
synthesis of polysaccharides that serve an essential role in
biofilm formation and adhesion of the microorganism to tooth
surfaces (55). It is more likely
for ozone treatment to alter the dentin surface, making it more
difficult for organisms to colonize the surface (6,54).
Ozone oxidation is capable of altering organic surface components,
such as collagen, and inducing the release of specific low
molecular weight salivary biomolecules from macromolecular binding
sites (56). This may result in
‘smoothing’ of the protein molecules, thereby affecting the
wettability of the dentin surface.
Lastly, ozone has also exhibited promising virucidal
effects. A recent study on severe acute respiratory syndrome
coronavirus 2 reported that ozonized water rapidly caused
conformational changes in the S1 subunit of the S protein (57). Based on previous studies, the
authors also hypothesized that ozone could oxidize the glycoprotein
on viral particles such as the viral envelope, which may contribute
to virus inactivation (57,58). These mechanisms may be similarly
involved in viruses commonly affecting the oral cavity, such as
herpes simplex virus type 1(59).
Additionally, ozone activated the immune system in immunosuppressed
rats by stimulating T-cell mediated immunity and enhancing the
immune response against pathogens (59,60).
5. Remineralization and interaction with
dental hard tissues
With regard to the direct interaction of ozone with
hard dental tissues, studies were retrieved that investigated the
effects of ozone applications on physical and microstructural
properties of enamel and dentin.
Celiberti et al (6) reported that gaseous ozone applications
on sound enamel caused no significant changes to enamel physical
properties, such as microhardness and acid resistance, without
compromising etching and adhesive procedures for the placement of
resinous restorative materials. The authors also reported that
after initial reversible slight dehydration, ozone did not
significantly change the free surface energy of the enamel.
Consequently, the enamel-acid contact angle and interfacial area
remained unaltered, which could explain the unchanged acid
resistance. Similarly, Floare et al (24) measured an increase in enamel
microhardness by dehydration, which was based on an early
assessment after gaseous ozone application on demineralized enamel.
At a microstructural level, the aforementioned study observed a
progressive leveling of the enamel surface, opening of
interprismatic canals and enamel prism pattern homogenization
resulting from oxidation-induced disruption of the proteins
embedded in the enamel matrix. Ozone oxidative proteolytic action
can break down the organic contents of demineralized enamel and
enhance diffusion of remineralizing agents. Additionally,
ozone-induced oxidation of microbial pyruvic acid into acetate and
CO2 has a buffering effect, which counteracts the acid
environment sustaining the carious process, thus promoting surface
remineralization (24,38). Furthermore, a randomized controlled
clinical trial reported that gaseous ozone treatment of
non-cavitated carious lesions in primary teeth produced results
comparable with fluoride varnish application in terms of
fluorescence values and visual inspection index, with little or no
caries progression reported in both treatment groups (61). These findings suggested that in
young or uncooperative patients where accidental ingestion of
topical products may occur, ozone may be a valid alternative to
professionally applied topical fluorides to achieve
remineralization (62). Parents or
caregivers may also object to the use of fluorides, as early
excessive exposure to fluoride has been implicated, albeit
controversially, in a number of potential adverse health effects
(63-66).
Although the effect of ozone on dentin is less well
studied, it has been reported that, in addition to improving
diffusion of salivary ions to the surface of degraded dentin, ozone
can neutralize acidic proteins produced by cariogenic bacteria.
These constitute the osmotic stimuli responsible for the movement
of fluids in dentin tubules that causes hypersensitivity (67,68).
Moreover, promising results have been reported in preliminary
studies investigating ozone management of dentine hypersensitivity
(67,68). Ozone oxidation of organic matter
occurs through a selective reaction with molecules containing
double bonds, amines or activated organic groups (68). Additionally, the resulting ozone
byproducts, such as free hydroxyl radicals, undergo a stronger
oxidizing action that selectively involves the organic components
of dentin (69). These direct and
indirect reactions may be responsible for the preferential
degradation of demineralized peritubular dentin, which results in
an increase in dentinal tubule diameter (68). This, associated with the local
presence of remineralizing agents, enhances their deposition in
open dentinal tubules, which can lead to increased tubular
occlusion rates (68). Furthermore,
in vitro evidence suggested that gaseous ozone autonomously
induces occlusion of dentinal tubules, similarly to erbium-doped
yttrium-aluminum-garnet laser treatment. This resulted in a
significant reduction in the diameter and number of open dentinal
tubules, which may be due to the immediate precipitation of
minerals from oxidation induced-disruption of the surface matrix
(70). Such ozone-induced effects
could be affected by specific experimental setup and protocols,
which have not been compared in the currently available
literature.
Lastly, it has been reported that ozone interferes,
albeit partially, with the activation of embedded dentin
metalloproteases (MMPs) more than other common antiseptic agents
(71). Dentin MMPs serve an
important role in the progression of dental caries, as they can
degrade the collagen matrix and negatively affect the adhesive
interface of restorations (71).
6. Biostimulation of dental and periodontal
structures
With regard to the effect on dental cells, ozone was
reported to stimulate the odontodifferentiation potential of dental
pulp cells and the production of mineral nodules in vitro,
possibly acting as an indirect biostimulator of tertiary dentin
formation in vivo (71).
Evidence of ozone enhancing dental pulp regenerative potential has
been reported by additional previous studies (72-74).
An in vitro study by Noguchi et al (74) reported that, while bacterial LPS
toxin inhibits the formation of mineralized nodules by odontoblast
cells, ozonized water exposure can restore this property by
reducing inflammatory response in odontoblast cells, exhibiting a
direct toxic effect against bacterial LPS in vitro.
Similarly, gaseous ozone application followed by the placement of
mineral trioxide aggregate (MTA) was reported to improve pulp
regenerative potential by increasing odontoblast proliferation and
dentin bridge formation in an in vivo animal study, when
compared with the placement of MTA alone (72). The increased ability to normalize
pulp microcirculation and reduce inflammation was also observed in
a concurrent human clinical study in cases of accidental pulpal
exposure (72). The interaction of
ozone with protein-lipid complexes of cell membranes and blood
plasma promotes the synthesis of biologically active compounds,
enhances the activity of immunocompetent cells and improves
rheology and oxygen-carrying capacity of the blood (75). Similar findings were reported by
Küçük et al (73) who
observed that ozonized water at different concentrations
demonstrated a good biocompatibility according to cell viability
and enhanced the proliferation of primary dental pulp cells in
vitro. This finding may also support the potential application
of ozone as an adjuvant irrigant in the field of regenerative
endodontics.
Ozone has also been reported to stimulate tissue
regeneration of dental pulp. Similar to healing and regeneration
processes occurring in other types of tissues, the mechanisms
involved in the aforementioned process include increased expression
of VEGF, increased levels of nitric oxide (NO) and reduced
oxidative stress due to increased activity of antioxidant enzymes
such as superoxide dismutase (SOD) (76-79).
NO is involved in pulp healing as a regulator of vascular
homeostasis and pulp afferent sensitivity, as an indicator of cell
differentiation and as a mediator of inflammatory activity
(80). In particular, one of the
three isoenzymes of NO synthase (NOS), neuronal NOS (nNOS), is
typically present in healthy human pulp tissue, which demonstrates
its involvement in pulp homeostasis (81). VEGF is responsible for the
angiogenesis, activity and differentiation of odontoblasts and a
slight increase in VEGF levels may be related to an increase in
pulp vascularization inducing a beneficial response (81,82). A
previous study reported increased VEGF and nNOS levels and
decreased SOD levels in pulp tissue following a single application
of gaseous ozone in the deep cavities of healthy teeth, where
increased SOD activity and subsequent depletion may have occurred
in response to mild oxidative stress induced by ozone (81). These findings also indicated that
ozone could effectively diffuse through dentin to pulp tissue after
a single application (81).
Additionally, promising effects of ozone on
periodontal cells and structures have been previously reported,
which may support the notion that ozone has biostimulating effects.
Cementoblasts and fibroblasts in the residual periodontal ligament
(PDL) adhering to the root surface of healthy avulsed teeth showed
a higher proliferation rate when irrigated with ozonized water
compared with saline (83). This
suggested that ozone, in addition to effectively decontaminating
the root surface, may also promote periodontal regeneration after
the reimplantation of avulsed teeth. These findings have been
supported by several studies investigating the biostimulatory
potential of ozone on the periodontal structures and bone
remodeling. Ozonized oil has been reported to significantly promote
the production of type I collagen by human gingival fibroblasts,
which was associated with the regenerative capacity of periodontal
tissues (84). Furthermore, ozone,
particularly ozonized water, has shown optimal biocompatibility
with gingival fibroblasts, outperforming other antiseptics commonly
used in oral care such as chlorhexidine digluconate (85,86).
Beneficial effects of ozone on bone metabolism have
also been reported. The application of gaseous ozone was reported
to improve bone metabolism and homeostasis in a zoledronate-treated
rat model of osteoporosis, which showed a synergistic effect
between zoledronate and ozone associated with increased bone
regeneration (87). This
ozone-induced biostimulatory effect was further confirmed by other
previous studies that reported increased bone regeneration of
calvarial defects of both healthy rats and rats with diabetes
mellitus (88,89). Diabetes mellitus and hyperglycemia
are associated with impaired healing processes due to activation of
the NF-κB pathway, as well as increased alkaline phosphatase
expression and suppressed osteocalcin, MMP-13, VEGF and glycolytic
enzyme levels in osteoblasts, resulting in osteoporosis (90,91).
Conversely, an increased number of osteoblastic cells and increased
expression levels of osteocalcin and bone morphogenetic protein-2
(BMP-2) were observed in diabetic rat calvarial defects treated
with xenografts and ozone compared with a xenograft-only group,
which suggested ozone-induced improved bone formation and
remodeling (89).
Similar results have been reported for periodontal
disease-induced bone destruction (92,93).
Untreated periodontal pockets caused by the presence of anaerobic
pathogens, vascular endothelial damage, edema and increased
inflammatory cell infiltration, exhibit low oxygen levels (94). HIF-1α (hypoxia-inducible factor 1α)
is activated by pro-inflammatory signals in periodontal cells and
gingival tissue and is the major regulatory protein that responds
to hypoxia (94). Hypoxia also
causes an increase in the release of receptor activator of NF-κB
ligand (RANKL), which causes bone resorption and a decrease in the
level of osteoprotegerin (OPG), which is responsible for bone
formation (95). Furthermore, a
study investigating the effects of ozone therapy on periodontal
disease-induced bone destruction in rats reported a lower number of
HIF-1-α positive cells in the ozone-treated group compared with
both positive and negative controls, as well as significantly fewer
RANKL-stained cells. Conversely, OPG levels were higher in the
ozone-treated group, which suggests that ozone therapy may
potentially be effective in promoting bone healing (92).
7. Immunomodulation
Knowledge of the underlying immunomodulatory
mechanisms of ozone is developing and is important in a number of
medical fields, including oral medicine. The pharmacological
activity of medical ozone mainly depends on the ability of ozone
byproducts to induce mild reactive oxygen species (ROS) signaling
or mitochondrial stress that triggers an antioxidant response
(9). This occurs through the
activation of the Nrf2 (nuclear factor erythroid 2-related
factor)-mediated system and inhibition of NF-κB pathway, which
modulates immunity toward anti-inflammatory mechanisms (9). Ozone has also been used to treat
myofascial pain and inflammation associated with temporomandibular
disorders in rats (96). Despite a
lack of improvement in nociception and inflammation that could have
been due to the local infiltrative route of ozone administration
and mechanically damaged muscle tissue, there was significant
evidence of ozone stimulating collagen deposition and tissue repair
(96). This finding was attributed
to ozone-induced stimulation of fibroblast migration and increased
immunohistochemical expression of platelet-derived growth factor,
TGF-β and VEGF, which serve essential roles in tissue repair
(96).
With specific reference to the dental field, Huth
et al (10) investigated the
effect of aqueous ozone on NF-κB-associated signaling both in
periodontal ligament tissue from root surfaces of periodontally
damaged teeth and in oral cells stimulated with TNF. It was
reported that the NF-κB pro-inflammatory pathway was inhibited
following incubation with ozonized medium, through ozone-mediated
prevention of NF-κB inhibitor IκBα proteolysis, cytokine expression
and κB-dependent transcription (10,59).
Furthermore, the inhibitory effect was not directly caused by
ozone, but was mediated, to varying degrees, by the formation of
specific ozonized amino acids (10). Similarly, Leewananthawet et
al (93) reported that ozone
ultrafine bubble water induced oxidative stress through ROS
production in human primary periodontal ligament fibroblasts.
Consequently, certain genes and pathways involved in oxidative
stress responses, such as c-Fos, Nrf2 and
p38-MAPK signaling were upregulated after ozone treatment.
Furthermore, the MAPK pathways have been reported to be associated
with BMP-9-mediated differentiation of PDL fibroblasts to
osteoblastic-like cells, possibly serving a pivotal role in
regenerating mineralized dental tissues (93,97).
8. Limitations
There are certain limitations to the present review,
mainly related to the available evidence. A number of the studies
considered were published >10 years ago. However, a number of
these studies were informative and were important sources for the
purposes of the present review. Furthermore, most of the evidence
reviewed was based on in vitro studies which, unlike animal
models, may not be able to replicate real clinical conditions and
may therefore only provide partial insights into the in vivo
mechanisms of action of ozone. Finally, the limited availability
and heterogeneity of the relevant literature prevented the
assessment of the specific effects of different ozone formulations
and protocols of application.
9. Clinical relevance and future
perspectives
Future studies should focus on investigating
different formulations, concentrations and application protocols of
ozone in order to induce the specific desired effects. In addition,
further research is warranted in the less-studied fields, such as
dental cell biostimulation and pulp regeneration. These are topics
of increasing interest to clinicians, therefore, in-depth research
could expand clinical applications of ozone in a number of areas of
dentistry, including cariology and regenerative endodontics. The
development of in vitro experimental models through modern
technologies could help overcome the limitations of animal studies,
such as cost and ethical issues and traditional in vitro
settings, which fail to replicate the biological environment.
Selected optimal protocols should then be tested in well-designed
randomized clinical trials. This would allow comparisons between
established traditional treatments for specific oral health
conditions, both in terms of clinical efficacy and patient
acceptance. For example, the use of ozone for oral ulcerative
lesions of various etiologies, as well as for the treatment of
dental caries in young children, has shown promising results
(31,32). This has provided an alternative
treatment option that is less invasive compared with traditional
approaches, free of side effects and with good patient
compliance.
10. Conclusions
A variety of biological mechanisms acting through
multiple biochemical target pathways are responsible for the
therapeutic effects of ozone. These mechanisms are complex and at
present not fully understood, especially with respect to specific
effects on the oral and dental environment. Overall, they include
antimicrobial action, immunomodulatory and biostimulatory effects
and the direct modifications of hard dental tissues. Additional
research in this area could provide further insights, enhance the
use of ozone for broader medical purposes and dentistry
applications and assist in the selection of targeted and more
effective ozone treatment protocols.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grant 'FAR UNIMORE
2023' from the University of Modena and Reggio Emilia.
Availability of data and materials
Not applicable.
Authors' contributions
FV, TF and LG conceptualized the topic of the review
and methodology. FV retrieved the relevant literature. FV and TF
prepared the manuscript draft. UC, MV and LG reviewed and edited
the manuscript. All authors read and approved of the final version
of the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El Meligy OA, Elemam NM and Talaat IM:
Ozone therapy in medicine and dentistry: A review of the
literature. Dent J (Basel). 11(187)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Costa T, Linhares D, Ribeiro da Silva M
and Neves N: Ozone therapy for low back pain. A systematic review.
Acta Reumatol Port. 43:172–181. 2018.PubMed/NCBI
|
|
3
|
Oliveira Modena DA, de Castro Ferreira R,
Froes PM and Rocha KC: Ozone therapy for dermatological conditions:
A systematic review. J Clin Aesthet Dermatol. 15:65–73.
2022.PubMed/NCBI
|
|
4
|
Shang W, Wang Y, Wang G and Han D:
Benefits of ozone on mortality in patients with COVID-19: A
systematic review and meta-analysis. Complement Ther Med.
72(102907)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Song M, Zeng Q, Xiang Y, Gao L, Huang J,
Huang J, Wu K and Lu J: The antibacterial effect of topical ozone
on the treatment of MRSA skin infection. Mol Med Rep. 17:2449–2455.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Celiberti P, Pazera P and Lussi A: The
impact of ozone treatment on enamel physical properties. Am J Dent.
19:67–72. 2006.PubMed/NCBI
|
|
7
|
Foroughi M, Khiadani M, Kakhki S, Kholghi
V, Naderi K and Yektay S: Effect of ozonation-based disinfection
methods on the removal of antibiotic resistant bacteria and
resistance genes (ARB/ARGs) in water and wastewater treatment: A
systematic review. Sci Total Environ. 811(151404)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Santos GM, Pacheco RL, Bussadori SK,
Santos E, Riera R, De Oliveira Cruz Latorraca C, Mota P, Benavent
Caldas Bellotto EF and Martmbianco ALC: Effectiveness and safety of
ozone therapy in dental caries treatment: Systematic review and
meta-analysis. J Evid Based Dent Pract. 20(101472)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chirumbolo S, Valdenassi L, Simonetti V,
Bertossi D, Ricevuti G, Franzini M and Pandolfi S: Insights on the
mechanisms of action of ozone in the medical therapy against
COVID-19. Int Immunopharmacol. 96(107777)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huth KC, Saugel B, Jakob FM, Cappello C,
Quirling M, Paschos E, Ern L, Hickel R and Brand K: Effect of
aqueous ozone on the NF-kappaB system. J Dent Res. 86:451–456.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bocci V: Ozone: A new medical drug.
Springer, Dordrecht, The Netherlands ; Norwell, MA, 2005.
|
|
12
|
Górnicki A and Gutsze A: In vitro effects
of ozone on human erythrocyte membranes: An EPR study. Acta Biochim
Pol. 47:963–971. 2000.PubMed/NCBI
|
|
13
|
Tükel SS, Bilgin R and Gül S: Effects of
ozone on the activity of erythrocyte membrane Na(+)-K+ ATPase.
Biochem Mol Biol Int. 33:1033–1040. 1994.PubMed/NCBI
|
|
14
|
Azuma T, Usui M and Hayashi T:
Inactivation of antibiotic-resistant bacteria in hospital
wastewater by ozone-based advanced water treatment processes. Sci
Total Environ. 906(167432)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bardellini E, Amadori F, Veneri F, Conti G
and Majorana A: Coronavirus disease-2019 and dental practice: A
project on the use of ozonized water in the water circuit of the
dental armchair. Stomatologija. 22:35–38. 2020.PubMed/NCBI
|
|
16
|
Irie MS, Dietrich L, Souza GLD, Soares
PBF, Moura CCG, Silva GRD and Paranhos LR: Ozone disinfection for
viruses with applications in healthcare environments: A scoping
review. Braz Oral Res. 36(e006)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lim S, Shi JL, Von Gunten U and McCurry
DL: Ozonation of organic compounds in water and wastewater: A
critical review. Water Res. 213(118053)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Uppal T, Khazaieli A, Snijders AM and
Verma SC: Inactivation of human coronavirus by FATHHOME's dry
sanitizer device: Rapid and eco-friendly ozone-based disinfection
of SARS-CoV-2. Pathogens. 10(339)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Beretta M and Federici Canova F: A new
method for deep caries treatment in primary teeth using ozone: A
retrospective study. Eur J Paediatr Dent. 18:111–115.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lynch E: Ozone: The revolution in
dentistry. Quintessence, Copenhagen, 2004.
|
|
21
|
Gupta S and Deepa D: Applications of ozone
therapy in dentistry. J Oral Res Rev. 8:86–91. 2016.
|
|
22
|
International Scientific Committee of
Ozone Therapy-ISCO3: Learning methodology instructions and
perfection in ozone therapy for medical doctors, 2015. Available
online at: https://isco3.org/wp-content/uploads/2015/09/ISCO3-HUM-00-01.pdf.
|
|
23
|
World Federation of Ozone Therapy: WFOT'S
review on evidence based ozone therapy, 2015. Available online at:
https://spozonoterapia.pt/wp-content/uploads/2016/02/WFOT-OZONE-2015-ENG.compressed.pdf.
|
|
24
|
Floare AD, Focht D, Hajdu AI, Niculescu
Talpoş IC, Bălean OJ, Muntean CV, Sebeşan D, Jumanca DE and
Găluşcan A: Ozone and microstructural morphological changes of
tooth enamel. Rom J Morphol Embryol. 63:539–544. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
D'Amario M, Di Carlo M, Natale SM, Memè L,
Marzo G, Matarazzo G and Capogreco M: Application of ozone therapy
in paediatric dentistry. Appl Sci. 12(11100)2022.
|
|
26
|
Nogales CG, Ferrari PH, Kantorovich EO and
Lage-Marques JL: Ozone therapy in medicine and dentistry. J Contemp
Dent Pract. 9:75–84. 2008.PubMed/NCBI
|
|
27
|
Re K, Gandhi J, Liang R, Patel S, Joshi G,
Smith NL, Reid I and Khan SA: Clinical utility of ozone therapy and
hyperbaric oxygen therapy in degenerative disc disease. Med Gas
Res. 13:1–6. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Randi CJ, Heiderich CMC, Serrano RV,
Morimoto S, de Moraes LOC, Campos L and Palma LF: Use of ozone
therapy in Implant Dentistry: A systematic review. Oral Maxillofac
Surg. 28:39–49. 2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rapone B, Ferrara E, Santacroce L, Topi S,
Gnoni A, Dipalma G, Mancini A, Di Domenico M, Tartaglia GM, Scarano
A and Inchingolo F: The gaseous ozone therapy as a promising
antiseptic adjuvant of periodontal treatment: A randomized
controlled clinical trial. Int J Environ Res Public Health.
19(985)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tricarico G, Rodrigues Orlandin J,
Rocchetti V, Ambrosio CE and Travagli V: A critical evaluation of
the use of ozone and its derivatives in dentistry. Eur Rev Med
Pharmacol Sci. 24:9071–9093. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Veneri F, Filippini T, Consolo U, Vinceti
M and Generali L: Ozone treatment for the management of caries in
primary dentition: A systematic review of clinical studies. Dent J
(Basel). 12(69)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kumar T, Arora N, Puri G, Aravinda K,
Dixit A and Jatti D: Efficacy of ozonized olive oil in the
management of oral lesions and conditions: A clinical trial.
Contemp Clin Dent. 7:51–54. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Al-Omiri MK, Alhijawi M, AlZarea BK, Abul
Hassan RS and Lynch E: Ozone treatment of recurrent aphthous
stomatitis: A double blinded study. Sci Rep.
6(27772)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Torul D, Omezli MM and Avci T:
Investigation of the clinical efficacy of CGF and ozone in the
management of alveolar osteitis: A randomized controlled trial.
Clin Oral Investig. 27:4521–4529. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kogila AV, Kishore M, Padma Rayulu K, Raju
BHRK, Tyro D and Bhupathi A: A comparative study of pain and
healing in post-dental extraction sockets treated with ozonated
water/oil and normal saline. J Maxillofac Oral Surg. 21:1119–1125.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Veneri F, Bardellini E, Amadori F, Conti G
and Majorana A: Efficacy of ozonized water for the treatment of
erosive oral lichen planus: A randomized controlled study. Med Oral
Patol Oral Cir Bucal. 25:e675–e682. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khatri I, Moger G and Kumar NA: Evaluation
of effect of topical ozone therapy on salivary Candidal carriage in
oral candidiasis. Indian J Dent Res. 26:158–162. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Samuel SR, Dorai S, Khatri SG and Patil
ST: Effect of ozone to remineralize initial enamel caries: In situ
study. Clin Oral Investig. 20:1109–1113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jena D, Manas A, Venkateswararao CH,
Salama MT, Ismail PMS and Basha SR: Comparative evaluation of
efficacy of bioactive glass, tricalcium phosphate, and ozone
remineralizing agents on artificial carious lesion. J Pharm Bioall
Sci. 14 (Suppl 1):S959–S961. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Estrela C, Chaves RM, Cardoso PC, De Je
Barata T, De Souza JB, De Torres ÉM, Estrela CR, Magalhães AP and
Lopes LG: Ozone gas effect on mineral content of dentin exposed to
Streptococcus mutans Biofilm: An energy-dispersive x-ray
evaluation. J Contemp Dent Pract. 18:265–269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dähnhardt JE, Jaeggi T and Lussi A:
Treating open carious lesions in anxious children with ozone. A
prospective controlled clinical study. Am J Dent. 19:267–270.
2006.PubMed/NCBI
|
|
42
|
Luppieri V, Manfra A, Ronfani L, Chermetz
M and Cadenaro M: Ozone therapy for early childhood caries (ECC)
treatment: An in vivo prospective study. Appl Sci.
12(1964)2022.
|
|
43
|
Petersen PE and Kwan S: Equity, social
determinants and public health programmes-the case of oral health.
Community Dent Oral Epidemiol. 39:481–487. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
The Lancet: Big sugar and neglect by
global health community fuel oral health crisis, 2019. Available
online at: https://www.eurekalert.org/pub_releases/2019-07/tl-tlb071619.php.
|
|
45
|
Veneri F, Vinceti SR and Filippini T:
Fluoride and caries prevention: A scoping review of public health
policies. Ann Ig: Jan 17, 2024 (Epub ahead of print).
|
|
46
|
Bocci V, Borrelli E, Travagli V and
Zanardi I: The ozone paradox: Ozone is a strong oxidant as well as
a medical drug. Med Res Rev. 29:646–682. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bocci VA: Scientific and medical aspects
of ozone therapy. State of the art. Arch Med Res. 37:425–435.
2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ouzzani M, Hammady H, Fedorowicz Z and
Elmagarmid A: Rayyan-a web and mobile app for systematic reviews.
Syst Rev. 5(210)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cataldo F: DNA degradation with ozone. Int
J Biol Macromol. 38:248–254. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nagayoshi M, Fukuizumi T, Kitamura C, Yano
J, Terashita M and Nishihara T: Efficacy of ozone on survival and
permeability of oral microorganisms. Oral Microbiol Immunol.
19:240–246. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yamayoshi T and Tatsumi N: Microbicidal
effects of ozone solution on methicillin-resistant Staphylococcus
aureus. Drugs Exp Clin Res. 19:59–64. 1993.PubMed/NCBI
|
|
52
|
Camacho-Alonso F, Salmerón-Lozano P and
Martínez-Beneyto Y: Effects of photodynamic therapy, 2%
chlorhexidine, triantibiotic mixture, propolis and ozone on root
canals experimentally infected with Enterococcus faecalis:
An in vitro study. Odontology. 105:338–346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cardoso MG, De Oliveira LD, Koga-Ito CY
and Jorge AOC: Effectiveness of ozonated water on Candida
albicans, Enterococcus faecalis, and endotoxins in root
canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
105:e85–e91. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Knight GM, McIntyre JM, Craig GG, Mulyani
and Zilm PS: The inability of Streptococcus mutans
and Lactobacillus acidophilus to form a biofilm in vitro on
dentine pretreated with ozone. Aust Dent J. 53:349–353.
2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tamesada M, Kawabata S, Fujiwara T and
Hamada S: Synergistic effects of streptococcal glucosyltransferases
on adhesive biofilm formation. J Dent Res. 83:874–879.
2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Grootveld M, Silwood CJL and Lynch E: High
resolution 1H NMR investigations of the oxidative consumption of
salivary biomolecules by ozone: Relevance to the therapeutic
applications of this agent in clinical dentistry. Biofactors.
27:5–18. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Takeda Y, Jamsransuren D, Makita Y, Kaneko
A, Matsuda S, Ogawa H and Oh H: Inactivation activities of ozonated
water, slightly acidic electrolyzed water and ethanol against
SARS-CoV-2. Molecules. 26(5465)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rowen RJ and Robins H: A plausible ‘penny’
costing effective treatment for corona virus-ozone therapy. J
Infect Dis Epidemiol. 6(113)2020.
|
|
59
|
Cenci A, Macchia I, La Sorsa V, Sbarigia
C, Di Donna V and Pietraforte D: Mechanisms of action of ozone
therapy in emerging viral diseases: Immunomodulatory effects and
therapeutic advantages with reference to SARS-CoV-2. Front
Microbiol. 13(871645)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Amin LE: Biological assessment of ozone
therapy on experimental oral candidiasis in immunosuppressed rats.
Biochem Biophys Rep. 15:57–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Johansson E, Van Dijken JWV, Karlsson L
and Andersson-Wenckert I: Treatment effect of ozone and fluoride
varnish application on occlusal caries in primary molars: A
12-month study. Clin Oral Invest. 18:1785–1792. 2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
American Academy of Pediatric Dentistry:
Fluoride therapy. In: The Reference Manual of Pediatric Dentistry.
American Academy of Pediatric Dentistry, Chicago, Ill., pp352-358,
2023.
|
|
63
|
Veneri F, Vinceti M, Generali L, Giannone
ME, Mazzoleni E, Birnbaum LS, Consolo U and Filippini T: Fluoride
exposure and cognitive neurodevelopment: Systematic review and
dose-response meta-analysis. Environ Res.
221(115239)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Iamandii I, De Pasquale L, Giannone ME,
Veneri F, Generali L, Consolo U, Birnbaum LS, Castenmiller J,
Halldorsson TI, Filippini T and Vinceti M: Does fluoride exposure
affect thyroid function? A systematic review and dose-response
meta-analysis. Environ Res. 242(117759)2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fiore G, Veneri F, Di Lorenzo RD, Generali
L, Vinceti M and Filippini T: Fluoride exposure and ADHD: A
systematic review of epidemiological studies. Medicina (Kaunas).
59(797)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Veneri F, Filippini T, Cecchini M, Vinceti
M, Consolo U and Generali L: Early fluoride intake and molar
incisor hypomineralisation (MIH) defects: A systematic review and
dose-response meta-analysis. Acta Biomed. 95(e2024079)2024.
|
|
67
|
Lena K and Marianne K: Ozone treatment on
dentin hypersensitivity surfaces-a pilot study. Open Dent J.
11:65–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Raafat Abdelaziz R, Mosallam RS and Yousry
MM: Tubular occlusion of simulated hypersensitive dentin by the
combined use of ozone and desensitizing agents. Acta Odontol Scand.
69:395–400. 2011.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Summerfelt ST and Hochheimer JN: Review of
ozone processes and applications as an oxidizing agent in
aquaculture. Prog Fish Cult. 59:94–105. 1997.
|
|
70
|
Gürsoy H, Çakar G, İpçi ŞD, Kuru B and
Yilmaz S: In vitro evaluation of the effects of different treatment
procedures on dentine tubules. Photomed Laser Surg. 30:695–698.
2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ferreira LDAQ, Anestino TA, Branco NTT,
Diniz LA, Diniz MG, de Magalhães CS, Peixoto RTRDC, Moreira AN,
Dias DR, Madeira MFM and Diniz IMA: Adjunctive therapies for in
vitro carious lesions: Antimicrobial activity, activation of dentin
metalloproteinases and effects on dental pulp cells. Photodiagnosis
Photodyn Ther. 40(103168)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Dikopova NZH, Volkov AG, Kopecki IS,
Nikolskaya IA, Margaryan EG, Budina TV, Samokhlib YA, Kondratiev
SA, Paramonov YOU and Arakelyan MG: Clinical and experimental
validation of the ozone therapy effectiveness in case of accidental
exposure of the dental pulp. New Armen Med J. 15(77)2021.
|
|
73
|
Küçük F, Yıldırım S and Çetiner S:
Cytotoxicity assessment of different doses of ozonated water on
dental pulp cells. BMC Oral Health. 21(32)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Noguchi F, Kitamura C, Nagayoshi M, Chen
KK, Terashita M and Nishihara T: Ozonated water improves
lipopolysaccharide-induced responses of an odontoblast-like cell
line. J Endod. 35:668–672. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Seidler V, Linetskiy I, Hubálková H,
Stanková H, Smucler R and Mazánek J: Ozone and its usage in general
medicine and dentistry. A review article. Prague Med Rep. 109:5–13.
2008.PubMed/NCBI
|
|
76
|
Lu W, Xu W, Li J, Chen Y, Pan Y and Wu B:
Effects of vascular endothelial growth factor and insulin growth
factor-1 on proliferation, migration, osteogenesis and
vascularization of human carious dental pulp stem cells. Mol Med
Rep. 20:3924–3932. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yildirim AO, Eryilmaz M, Kaldirim U, Eyi
YE, Tuncer SK, Eroğlu M, Durusu M, Topal T, Kurt B, Dilmen S, et
al: Effectiveness of hyperbaric oxygen and ozone applications in
tissue healing in generated soft tissue trauma model in rats: An
experimental study. Ulus Travma Acil Cerrahi Derg. 20:167–175.
2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zhang J, Guan M, Xie C, Luo X, Zhang Q and
Xue Y: Increased growth factors play a role in wound healing
promoted by noninvasive oxygen-ozone therapy in diabetic patients
with foot ulcers. Oxid Med Cell Longev. 2014(273475)2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zhang W, Zhang X, Ling J, Wei X and Jian
Y: Osteo-/odontogenic differentiation of BMP2 and VEGF
gene-co-transfected human stem cells from apical papilla. Mol Med
Rep. 13:3747–3754. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Korkmaz Y, Baumann MA, Steinritz D,
Schröder H, Behrends S, Addicks K, Schneider K, Raab WHM and Bloch
W: NO-cGMP signaling molecules in cells of the rat molar
dentin-pulp complex. J Dent Res. 84:618–623. 2005.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Krunić J, Stojanović N, Đukić L, Roganović
J, Popović B, Simić I and Stojić D: Clinical antibacterial
effectiveness and biocompatibility of gaseous ozone after
incomplete caries removal. Clin Oral Invest. 23:785–792.
2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ebensberger U, Pohl Y and Filippi A:
PCNA-expression of cementoblasts and fibroblasts on the root
surface after extraoral rinsing for decontamination. Dent
Traumatol. 18:262–266. 2002.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wang PL, Tachi Y, Masuno K, Okusa N and
Imamura Y: The study of ozone ointment on human gingival
fibroblasts cell proliferation ability and anti-inflammatory. J
Hard Tissue Biol. 27:209–212. 2018.
|
|
85
|
Colombo M, Ceci M, Felisa E, Poggio C and
Pietrocola G: Cytotoxicity evaluation of a new ozonized olive oil.
Eur J Dent. 12:585–589. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Huth KC, Jakob FM, Saugel B, Cappello C,
Paschos E, Hollweck R, Hickel R and Brand K: Effect of ozone on
oral cells compared with established antimicrobials. Eur J Oral
Sci. 114:435–440. 2006.PubMed/NCBI View Article : Google Scholar
|
|
87
|
de Lima Neto TJ, Delanora LA, Sá Simon ME,
Carmo Ribeiro KH, Matsumoto MA, Quírino Louzada MJ, Shibli JA,
Ervolino E and Faverani LP: Ozone improved bone dynamic of female
rats using zoledronate. Tissue Eng Part C Methods. 30:1–14.
2024.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Vieira VSJG, da Rosa ÂR, Montagner PG, de
Campos FUF, Teixeira LN, Aura JM, Joly JC, Passador-Santos F and
Martinez EF: Effect of ozone therapy on the modulation of
inflammation and on new bone formation in critical defects of rat
calvaria filled with autogenous graft. J Stomatol Oral Maxillofac
Surg. 124(101292)2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Alpan AL, Toker H and Ozer H: Ozone
therapy enhances osseous healing in rats with diabetes with
calvarial defects: A morphometric and immunohistochemical study. J
Periodontol. 87:982–989. 2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Botolin S and McCabe LR: Chronic
hyperglycemia modulates osteoblast gene expression through osmotic
and non-osmotic pathways. J Cell Biochem. 99:411–424.
2006.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Sarkar PD and Choudhury AB: Relationships
between serum osteocalcin levels versus blood glucose, insulin
resistance and markers of systemic inflammation in central Indian
type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 17:1631–1635.
2013.PubMed/NCBI
|
|
92
|
Bayer Alinca S, Sağlam E, Zengin Celik T,
Hacisalihoglu P and Doğan MA: Is low level laser therapy or ozone
therapy more effective for bone healing? Understanding the
mechanisms of HIF-1α, RANKL and OPG. Biotech Histochem. 95:597–604.
2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Leewananthawet A, Arakawa S, Okano T,
Daitoku Kinoshita R, Ashida H, Izumi Y and Suzuki T: Ozone
ultrafine bubble water induces the cellular signaling involved in
oxidative stress responses in human periodontal ligament
fibroblasts. Sci Technol Adv Mat. 20:589–598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Afacan B, Öztürk VÖ, Paşalı Ç, Bozkurt E,
Köse T and Emingil G: Gingival crevicular fluid and salivary
HIF-1α, VEGF, and TNF-α levels in periodontal health and disease. J
Periodontol. 90:788–797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Yu XJ, Xiao CJ, Du YM, Liu S, Du Y and Li
S: Effect of hypoxia on the expression of RANKL/OPG in human
periodontal ligament cells in vitro. Int J Clin Exp Pathol.
8:12929–12935. 2015.PubMed/NCBI
|
|
96
|
de Souza KBR, Almeida Guerra LR, da Silva
Guerreiro ML, Casais-E-Silva LL and Aguiar MC: Nociceptive and
histomorphometric evaluation of the effects of ozone therapy on the
rat masseter muscle in a carrageenan model of myofascial pain. Arch
Oral Biol. 160(105893)2024.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Fuchigami S, Nakamura T, Furue K, Sena K,
Shinohara Y and Noguchi K: Recombinant human bone morphogenetic
protein-9 potently induces osteogenic differentiation of human
periodontal ligament fibroblasts. Eur J Oral Sci. 124:151–157.
2016.PubMed/NCBI View Article : Google Scholar
|