Introduction
Defined as loss of skeletal muscle mass in old age,
sarcopenia is associated with a risk of physical disability,
decreased quality of life (QOL) and increased mortality (1). In Japan, because the population is
rapidly aging, sarcopenia is an important consideration when
contemplating measures to increase the healthy longevity of older
people and prevent requirements for nursing care. According to
meta-analysis (2), the sarcopenia
prevalence in individuals aged >60 years depends on the set of
classification criteria used as follows: European Working Group on
Sarcopenia in Older People 2 (EWGSOP) 2, 10; EWGSOP, 23; AWGS (the
Asian Working Group for Sarcopenia), 14; International Working
Group on Sarcopenia, 14; Foundation for the National Institutes of
Health, 12 and muscle mass definition, 27%, respectively. The Asian
Working Group for Sarcopenia (AWGS) was established in
2014(3) and renamed the AWGS 2019
Consensus. Several studies have reported that sarcopenia prevalence
ranges from 7 to 8% (4,5) in Asian countries, but varies widely
across study design, population and settings. To the best of our
knowledge, few previous reports have focused on the association
between dietary variety status and sarcopenia in older outpatients
at a university-affiliated geriatric hospital in Japan (6).
The aim of this study was to investigate, in a
multidisciplinary context, the prevalence of AWGS 2019
consensus-defined sarcopenia and its association with dietary
variety status in older outpatients at a university-affiliated
geriatric hospital.
Materials and methods
Study design
The present single-center, cross-sectional study
used the baseline data of prospective cohort study Juntendo
Sarcopenia Registration of Exploring for Predictors and Prognosis
in the elderly in Tokyo (JUSTICE-TOKYO) (7), in accordance with the guidelines
contained in the STROBE Checklist (8).
The JUSTICE-TOKYO study is a prospective,
observational cohort study of consecutive outpatients aged ≥65
years attending Juntendo Tokyo Koto Geriatric Medical Center,
Tokyo, Japan. The study enrolled 1,042 patients between November
2020 and November 2021 and will be completed in 2025. The
participants will be followed up annually for 4 years after
enrollment to determine survival, incidence of falls, number of
hospitalizations and skeletal muscle mass. At enrollment, baseline
data including patient profile [age, body mass index (BMI),
Brinkman Index (BI), drinking habits, sex, history of falls,
daycare use, social frailty, phase angle (PhA) and QOL],
comorbidities, questionnaires [Mini-Mental State Examination
(MMSE), Geriatric Depression Scale 15 (GDS-15), Abdominal
symptom-related QOL (Izumo scale) scores, constipation scoring
system (CSS), chronic obstructive pulmonary disease (COPD)
assessment test (CAT)] (9-13),
physical and skeletal muscle mass, physiological function test and
nutritional assessment data were collected and entered
prospectively into the Research Electronic Data Capture system,
which provides web-based software for the creation of secure online
forms (14).
Exclusion criteria
Patients were excluded for the following reasons: i)
Inability to walk independently because of severe osteoarthritis or
neuromuscular disease, ii) immobility, iii) delirium tremens at
presentation, iv) history of gastrointestinal, renal, acute
cerebrovascular, coronary, hepatic and respiratory events, v)
inability to be interviewed by questionnaire and vi) predicted life
expectancy <1 year because of malignant disease.
Research instruments
Inclusion was limited to patients whose information
at registration included all of the following:
i) Age, body mass index (BMI), Brinkman Index (BI)
(15), drinking habits (0, rarely
drinks alcohol; 1, drinks alcohol 1-4 days/week and 2, drinks
alcohol 5-7 days/week), sex, history of falls and daycare use,
social frailty, phase angle (PhA) and quality of life assessed by
EuroQol Visual Analogue Scale (EQ-VAS) (16-18).
Social frailty was assessed using questions regarding living alone,
going out less frequently compared with the prior year, visiting
friends sometimes, feeling helpful to friends or family and talking
with someone every day. Resistances to a multi-frequency
alternating current applied to the trunk, arms and legs were
measured to determine body composition and impedance
characteristics, including capacitive reactance and PhA, were
analyzed by an MC-780A body composition analyzer (TANITA
Corporation). PhA was calculated as follows: [- arc tangent
(reactance/resistance) x180˚/π] (19). PhA represents resistance of the cell
membrane, the volume of somatic cells and the distribution of
intra- and extracellular fluid; higher PhA generally indicates good
cell health, while lower value of PhA reflects structural damage to
the cell membrane and decreased cell density, indicating poor cell
function (20). Dual-energy X-ray
Absorptiometry) standard method for measuring an appendicular
skeletal muscle mass in sarcopenia was used (21). PhA cannot be measured by the DXA
method but can be measured by the BIA (Bioelectrical Impedance
Analysis) method to investigate whether PhA is associated with
sarcopenia in older outpatients (22). QOL was evaluated by EQ-VAS (0=worst
health, 100=perfect health), commonly used in primary care.
Anthropocentric measures, physiological performance test, walking
speed, and various questionnaires [(Izumo scale), constipation
scoring system (CSS), chronic obstructive pulmonary disease (COPD)
assessment test (CAT), Dietary Variety Score (DVS)] (11-13,23)
were conducted by nurses and nutritionists.
ii) Comorbidities [history of atrial fibrillation,
cerebral infarction/hemorrhage, diabetes mellitus (DM),
hospitalization for heart failure, hypertension, interstitial
pneumonia, malignant disease, myocardial infarction and
osteoporosis]. The age-adjusted Charlson comorbidity index was
calculated for each patient (24,25).
T-score and young adult mean (YAM) % were measured by DXA of the
total hip and lumbar spine (L2-L4). Prodigy Advance scanner (GE
Healthcare) was used to perform DXA. Osteoporosis was diagnosed in
accordance with Japanese Society for Bone and Mineral Research
criteria (26).
iii) Use of therapeutic agents (statins, acid
secretion suppressants, laxatives, steroids, analgesics,
antidementia drugs, antipsychotic drugs) and number of oral
medicines. Data on medications and number of oral medicines were
obtained by pharmacists from patient health notebooks.
iv) Neuropsychological examinations [Geriatric
Depression Scale 15 (GDS-15) and Mini-Mental State Examination
(MMSE)] (9,10) were performed by psychiatrists.
v) Abdominal symptom-related QOL (Izumo scale)
scores (constipation-, diarrhea-, fullness-, reflux- and upper
abdominal pain-related QOL) (11).
QOL impairment was ranked from 0 (no impairment) to 15
(symptomatic).
vi) Severity of constipation was rated using the
constipation scoring system (CSS) (12), comprised of eight items: Abdominal
pain, assistance for evacuation, duration of constipation,
frequency of bowel movements, incomplete evacuation, length of
time/attempt, number of unsuccessful attempts at evacuation/24 h
and painful evacuation. The overall CSS score, which is the sum of
the item scores, ranged from 0 to 30, with a higher score
signifying constipation symptoms were worse. Gastroenterologists
performed the constipation severity assessments using CSS and Izumo
scales.
vii) Pulmonary function data [arterial oxygen
saturation (SpO2), chronic obstructive pulmonary disease
(COPD) assessment test (CAT)] results, restricted and obstructive
ventilatory impairment) (13,27).
The 8-item CAT was used to assess the impact of COPD on health
status. CAT score ≥10 indicated a high symptomatic level. Pulmonary
function tests including vital capacity (VC), forced VC (FVC) and
forced expiratory volume in 1 sec (FEV1) were performed on a Minato
System 21 (Minato Medical Science Co., Ltd.). The pulmonary
function tests, CAT and eating assessment test 10 (EAT10) (28) were evaluated by a respiratory
physician.
viii) Nutritional status [hypozincemia, controlling
nutritional status (CONUT) score, DVS)] (23,29).
Hypozincemia was defined as requiring treatment with zinc acetate
hydrate (Novelzin® Tablets, Nobelpharma K.K.) or serum
zinc levels <80 µg/dl. Zinc is a trace element essential for
life. Appetite loss (30),
depression (31), and taste
abnormality (32) are
hypozincemia-associated symptoms and risk factors for
hypoalimentation. CONUT score (0-12; calculated from serum albumin
and total cholesterol levels and total lymphocyte count) (29) was used to measure objective
nutritional status. Comprising 10 food-based components (23), DVS was calculated as follows. First,
consumption frequencies during 1 week were determined for each of
10 food items (meat, fish/shellfish, eggs, milk, soybean products,
green/yellow vegetables, potatoes, fruit, seaweed and fats/oils).
Second, scores were assigned as follows: 1, eaten almost daily and
0, not eaten almost daily. Third, the item scores were summed and
the total DVS was in the range 0 to 10, with higher scores
indicating greater dietary variety.
ix) Oral function [Oral Frailty Index (OFI)-8 and
EAT10] (28,33). Oral frailty was defined as OFI-8
score ≥4. The EAT10 tool was used to assess dysphagia severity. The
data were collected within 3 months after registration.
Definition of sarcopenia
Diagnostic algorithm recommended by AWGS 2019
Consensus was used to define (21).
The handgrip strength was measured twice each with both hands using
a handgrip dynamometer (Toei Light Co., Ltd.), and the larger value
was noted as the maximum muscle strength. Low grip strength was
defined as <28 for male and <18 kg for female patients,
according to AWGS criteria. Gait speed, manually assessed using a
stopwatch, was defined as slow when <1.0 m/sec according to AWGS
criteria. Lean mass and regional fat were assessed from whole-body
DXA scans (Prodigy Advance, GE Healthcare). Subjects were
positioned for whole-body scans in accordance with the
manufacturer's protocol. The whole-body fat mass and lean mass were
divided into arms, legs and trunk. The appendicular lean mass was
estimated as the sum of the lean mass of the upper and lower limbs.
The appendicular skeletal muscle mass index (SMI) was calculated as
the appendicular lean mass divided by the square of the height
(34). A low appendicular skeletal
muscle mass was defined as appendicular SMI <7.0 in male and
<5.4 kg/m2 in female patients.
Statistical analysis
Subjects were divided into sarcopenia and
non-sarcopenia groups and risk factors for sarcopenia were compared
by uni- and multivariate analyses. Quantitative data are expressed
as mean ± standard deviation. In univariate analyses, χ2
test was used for categorical variables and unpaired Student t
tests were used for continuous variables. Independent variables
with P<0.20 in the univariate analysis were included in
multivariate logistic regression analysis. The odds ratio (OR) and
95% confidence interval (CI) were used to assess the strength of
any associations. All statistical analyses were performed using the
SPSS version 28 software (IBM Corporation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of
patients
The clinical characteristics are summarized in
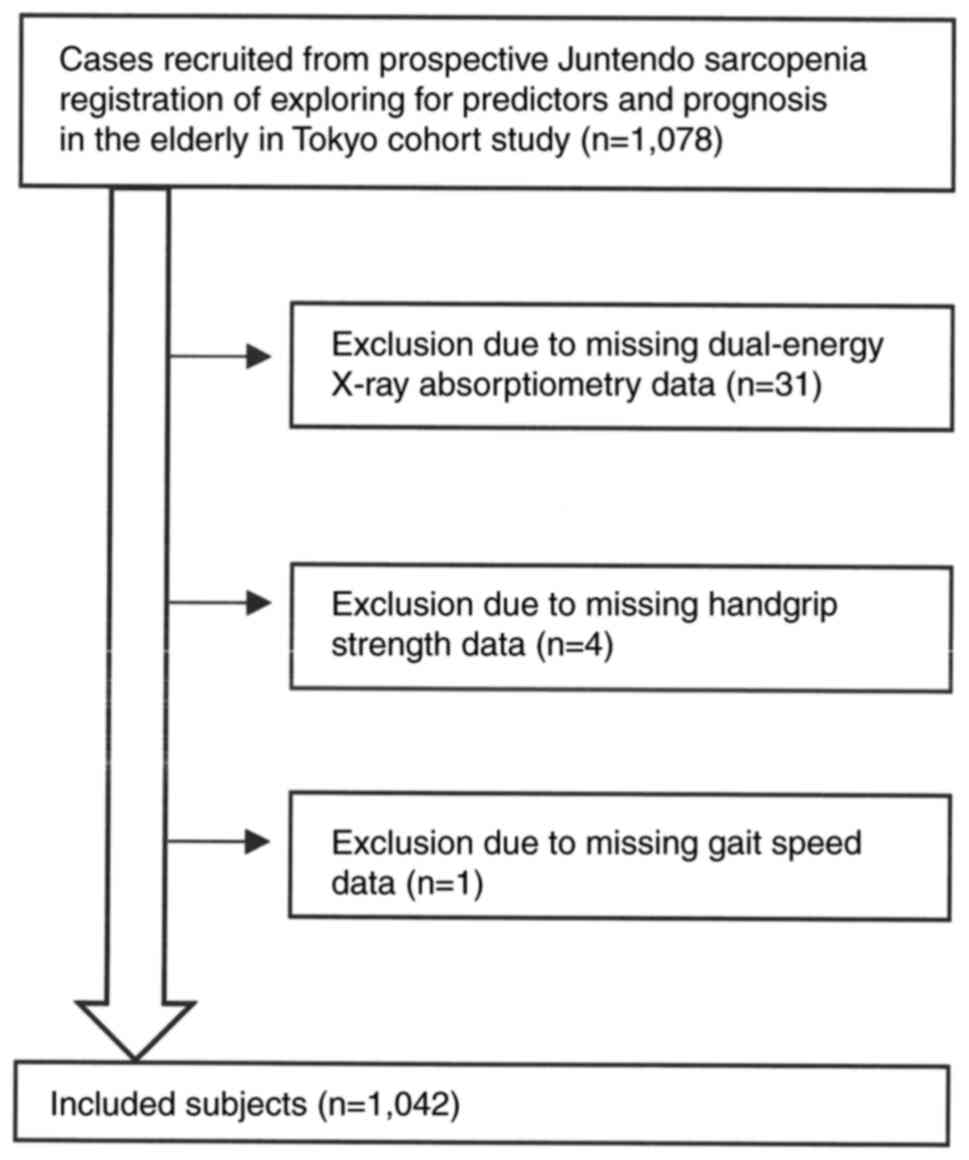
Table I. Participant recruitment
flow diagram is shown in Fig. 1.
Mean age-adjusted Charlson Comorbidity Index score was 5.5±1.4.
Sex- and age-specific prevalence of sarcopenia is shown in Table II. Sarcopenia and non-sarcopenia
were diagnosed in 223 and 819 patients, respectively. The total
prevalence of sarcopenia was 31.0 in male and 13.9% in female
patients.
 | Table IClinical characteristics of
participants (n=1,042). |
Table I
Clinical characteristics of
participants (n=1,042).
| Characteristic | Value |
|---|
| Mean age,
years | 78.2±6.1 |
| Sex (%) | |
|
Male | 458 (44.0) |
|
Female | 584 (56.0) |
| Mean BMI,
kg/m2 | 22.9±3.9 |
| Mean Brinkman
Index | 359.1±615.5 |
| Mean alcohol | 0.5±0.8 |
| Mean phase angle,
˚ | -4.7±0.8 |
| Mean EQ-5D
score | 75.0±17.0 |
| History of falls
(%) | |
|
Yes | 203 (19.5) |
|
No | 839 (80.5) |
| History of daycare
use (%) | |
|
Yes | 91 (8.7) |
|
No | 951 (91.3) |
| Social frailty
(%) | |
|
Yes | 679 (65.2) |
|
No | 363 (34.8) |
| Cerebral
infarction/hemorrhage (%) | |
|
Yes | 79 (7.6) |
|
No | 963 (92.4) |
| Myocardial
infarction (%) | |
|
Yes | 44 (4.2) |
|
No | 998 (95.8) |
| Hospitalization for
heart failure (%) | |
|
Yes | 41 (3.9) |
|
No | 1,001 (96.1) |
| Interstitial
pneumonia (%) | |
|
Yes | 54 (5.2) |
|
No | 988 (94.8) |
| Malignant disease
(%) | |
|
Yes | 228 (21.9) |
|
No | 814 (78.1) |
| Hypertension
(%) | |
|
Yes | 606 (58.2) |
|
No | 436 (41.8) |
| Diabetes mellitus
(%) | |
|
Yes | 180 (17.3) |
|
No | 862 (82.7) |
| Atrial fibrillation
(%) | |
|
Yes | 87 (8.3) |
|
No | 955 (91.7) |
| Osteoporosis
(%) | |
|
Yes | 343 (32.9) |
|
No | 699 (67.1) |
| Mean age-adjusted
Charlson comorbidity index | 5.5±1.4 |
| Statin use (%) | |
|
Yes | 434 (41.7) |
|
No | 608 (58.3) |
| Acid secretion
suppressant use (%) | |
|
Yes | 573 (55.0) |
|
No | 469 (45.0) |
| Laxative use
(%) | |
|
Yes | 228 (21.9) |
|
No | 814 (78.1) |
| Steroid use
(%) | |
|
Yes | 50 (4.8) |
|
No | 992 (95.2) |
| Analgesic drug use
(%) | |
|
Yes | 114 (10.9) |
|
No | 928 (89.1) |
| Antidementia drug
use (%) | |
|
Yes | 29 (2.8) |
|
No | 1013 (97.2) |
| Antipsychotic drug
use (%) | |
|
Yes | 261 (25.0) |
|
No | 781 (75.0) |
| Mean number of oral
medicines | 6.1±3.5 |
| Mean MMSE
score | 26.5±3.1 |
| Mean GDS-15
score | 4.2±3.0 |
| Mean reflux-related
QOL score | 1.8±2.4 |
| Mean upper
abdominal pain-related QOL score | 1.1±2.0 |
| Mean
fullness-related QOL score | 1.6±2.4 |
| Mean
constipation-related QOL score | 2.2±2.6 |
| Mean
diarrhea-related QOL score | 2.0±2.6 |
| Mean CSS score | 3.5±3.7 |
| Mean
SpO2,% | 97.2±2.1 |
| Mean CAT score | 8.5±6.6 |
| Restricted
ventilatory impairment (%) | |
|
Yes | 172 (16.5) |
|
No | 870 (83.5) |
| Obstructive
ventilatory impairment (%) | |
|
Yes | 260 (25.0) |
|
No | 782 (75.0) |
| Hypozincemia
(%) | |
|
Yes | 820 (78.7) |
|
No | 222 (21.3) |
| Mean CONUT
score | 1.0±1.1 |
| Mean DVS | 3.7±2.2 |
| Oral frailty
(%) | |
|
Yes | 522 (50.1) |
|
No | 520 (49.9) |
| Mean EAT10
score | 1.6±3.7 |
 | Table IIPrevalence of sarcopenia. |
Table II
Prevalence of sarcopenia.
| A, Total
(n=1,042) |
|---|
| Age, years | Number of
patients | Number of
cases | Prevalence, % |
|---|
| 65-69 | 85 | 15 | 17.6 |
| 70-74 | 231 | 33 | 14.3 |
| 75-79 | 278 | 38 | 13.7 |
| 80-84 | 274 | 70 | 25.5 |
| ≥85 | 174 | 67 | 38.5 |
| B, Male
(n=458) |
| Age, years | Number of
patients | Number of
cases | Prevalence, % |
| 65-69 | 42 | 11 | 26.2 |
| 70-74 | 94 | 14 | 14.9 |
| 75-79 | 123 | 23 | 18.7 |
| 80-84 | 113 | 47 | 41.6 |
| ≥85 | 86 | 47 | 54.7 |
| C, Female
(n=584) |
| Age, years | Number of
patients | Number of
cases | Prevalence, % |
| 65-69 | 43 | 4 | 9.3 |
| 70-74 | 137 | 19 | 13.9 |
| 75-79 | 155 | 15 | 9.7 |
| 80-84 | 161 | 23 | 14.3 |
| ≥85 | 88 | 20 | 22.7 |
Association between sarcopenia and
covariates in univariate analysis
Table III shows
the association between sarcopenia and covariates in univariate
analysis and the prevalence of sarcopenia. Between the sarcopenia
and non-sarcopenia groups, there were significant differences in
age, BMI, Brinkman Index, cerebral infarction/hemorrhage, history
of falls and daycare use, myocardial infarction, number of oral
medicines, PhA, proportion of males, QOL, history of
hospitalization for heart failure, malignant disease and diabetes
mellitus, age-adjusted Charlson comorbidity index, use of
antidementia drugs, number of oral medicines, MMSE, GDS-15,
reflux-related QOL and CSS score, restricted ventilatory
impairment, CONUT score, DVS, oral frailty and EAT10 score.
 | Table IIIAssociation between sarcopenia and
covariates in univariate analysis. |
Table III
Association between sarcopenia and
covariates in univariate analysis.
| Covariate | Sarcopenia
(n=223) | Non-sarcopenia
(n=819) | P-value |
|---|
| Mean age,
years | 80.6±6.3 | 77.6±5.8 | <0.001 |
| Sex (%) | | | |
|
Male | 142 (63.7) | 316 (38.6) | |
|
Female | 81 (36.3) | 503 (61.4) | <0.001 |
| Mean BMI,
kg/m2 | 20.8±3.1 | 23.4±3.9 | <0.001 |
| Mean Brinkman
Index | 543.0±309.0 | 309.0±568.9 | <0.001 |
| Mean alcohol | 0.5±0.5 | 0.5±0.8 | 0.366 |
| Mean phase angle,
˚ | -4.4±0.9 | -4.8±0.8 | <0.001 |
| Mean EQ-5D
score | 71.6±17.5 | 76.0±16.7 | <0.001 |
| History of falls
(%) | | | |
|
Yes | 54 (24.2) | 149 (18.2) | |
|
No | 169 (75.8) | 670 (81.8) | 0.044 |
| History of daycare
use (%) | | | |
|
Yes | 36 (16.1) | 55 (6.7) | |
|
No | 187 (83.9) | 764 (93.3) | <0.001 |
| Social frailty
(%) | | | |
|
Yes | 155 (69.5) | 524 (64.0) | |
|
No | 68 (30.5) | 295 (36.0) | 0.125 |
| Cerebral
infarction/hemorrhage (%) | | | |
|
Yes | 26 (11.7) | 53 (6.5) | |
|
No | 197 (88.3) | 766 (93.5) | 0.009 |
| Myocardial
infarction (%) | | | |
|
Yes | 16 (7.2) | 28 (3.4) | |
|
No | 207 (92.8) | 791 (96.6) | 0.013 |
| Hospitalization for
heart failure (%) | | | |
|
Yes | 14 (6.3) | 27 (3.3) | |
|
No | 209 (93.7) | 792 (96.7) | 0.042 |
| Interstitial
pneumonia (%) | | | |
|
Yes | 17 (7.6) | 37 (4.5) | |
|
No | 206 (92.4) | 782 (95.5) | 0.064 |
| Malignant disease
(%) | | | |
|
Yes | 63 (28.3) | 165 (20.1) | |
|
No | 160 (71.7) | 654 (79.9) | 0.009 |
| Hypertension
(%) | | | |
|
Yes | 140 (62.8) | 466 (56.9) | |
|
No | 83 (37.2) | 353 (43.1) | 0.114 |
| Diabetes mellitus
(%) | | | |
|
Yes | 58 (26.0) | 122 (14.9) | |
|
No | 165 (74.0) | 697 (85.1) | <0.001 |
| Atrial fibrillation
(%) | | | |
|
Yes | 21 (9.4) | 66 (8.1) | |
|
No | 202 (90.6) | 753 (91.9) | 0.516 |
| Osteoporosis
(%) | | | |
|
Yes | 83 (37.2) | 260 (31.7) | |
|
No | 140 (62.8) | 559 (68.3) | 0.125 |
| Mean age-adjusted
Charlson comorbidity index | 6.1±1.5 | 5.4±1.4 | <0.001 |
| Statin use (%) | | | |
|
Yes | 86 (38.6) | 348 (42.5) | |
|
No | 137 (61.4) | 471 (57.5) | 0.292 |
| Acid secretion
suppressant use (%) | | | |
|
Yes | 127 (57.0) | 446 (54.5) | |
|
No | 96 (43.0) | 373 (45.5) | 0.507 |
| Laxative use
(%) | | | |
|
Yes | 55 (24.7) | 173 (21.1) | |
|
No | 168 (75.3) | 646 (78.9) | 0.257 |
| Steroid use
(%) | | | |
|
Yes | 12 (5.4) | 38 (4.6) | |
|
No | 211 (94.6) | 781 (95.4) | 0.646 |
| Analgesic drug use
(%) | | | |
|
Yes | 22 (9.9) | 92 (11.2) | |
|
No | 201 (90.1) | 727 (88.8) | 0.562 |
| Antidementia drug
use (%) | | | |
|
Yes | 11 (4.9) | 18 (2.2) | |
|
No | 212 (95.1) | 801 (97.8) | 0.0278 |
| Antipsychotic drug
use (%) | | | |
|
Yes | 57 (25.6) | 204 (24.9) | |
|
No | 166 (74.4) | 615 (75.1) | 0.842 |
| Mean number of oral
medicines | 6.8±3.6 | 6.0±3.5 | 0.002 |
| Mean MMSE
score | 25.3±3.5 | 26.8±2.9 | <0.001 |
| Mean GDS-15
score | 5.0±3.2 | 4.0±3.0 | <0.001 |
| Mean reflux-related
QOL score | 1.5±2.1 | 1.8±2.4 | 0.030 |
| Mean upper
abdominal pain-related QOL score | 1.0±2.1 | 1.1±2.0 | 0.652 |
| Mean
fullness-related QOL score | 1.6±2.5 | 1.7±2.4 | 0.804 |
| Mean
constipation-related QOL score | 2.3±2.8 | 2.2±2.5 | 0.616 |
| Mean
diarrhea-related QOL score | 2.2±2.9 | 2.0±2.5 | 0.375 |
| Mean CSS score | 4.0±4.1 | 3.3±3.6 | 0.013 |
| Mean
SpO2 (%) | 97.0±4.0 | 97.3±1.1 | 0.156 |
| Mean CAT score | 9.1±6.9 | 8.4±6.5 | 0.118 |
| Restricted
ventilatory impairment (%) | | | |
|
Yes | 60 (26.9) | 112 (13.7) | |
|
No | 163 (73.1) | 707 (86.3) | <0.001 |
| Obstructive
ventilatory impairment (%) | | | |
|
Yes | 66 (29.6) | 194 (23.7) | |
|
No | 157 (70.4) | 625 (76.3) | 0.071 |
| Hypozincemia
(%) | | | |
|
Yes | 186 (83.4) | 634 (77.4) | |
|
No | 37 (16.6) | 185 (22.6) | 0.053 |
| Mean CONUT
score | 1.4±1.3 | 0.9±1.1 | <0.001 |
| Mean DVS | 3.3±2.3 | 3.8±2.2 | <0.001 |
| Oral frailty
(%) | | | |
|
Yes | 127 (57.0) | 395 (48.2) | |
|
No | 96 (43.0) | 424 (51.8) | 0.021 |
| Mean EAT10
score | 2.2±4.2 | 1.4±3.5 | 0.004 |
Association between sarcopenia and
covariates in multivariate analysis
In multivariate analysis, age, male sex, BMI,
Brinkman Index, PhA, QOL, history of daycare use, diabetes
mellitus, osteoporosis, MMSE and DVS were related to sarcopenia
(Table IV).
 | Table IVAssociation between sarcopenia and
covariates in multivariate analysis. |
Table IV
Association between sarcopenia and
covariates in multivariate analysis.
| Covariate | Standardized
coefficient | OR | 95% CI | P-value |
|---|
| Age | 0.061 | 1.0632 | 1.028-1.100 | <0.001 |
| Male | 1.903 | 6.7028 | 3.818-11.767 | <0.001 |
| BMI | -0.272 | 0.7619 | 0.715-0.812 | <0.001 |
| Brinkman Index | 0.000 | 1.0004 | 1.000-1.001 | 0.021 |
| Phase angle | 0.619 | 1.8576 | 1.385-2.491 | <0.001 |
| EQ-5D | -0.015 | 0.9853 | 0.975-0.996 | 0.007 |
| History of
falls | 0.242 | 1.2735 | 0.789-2.057 | 0.323 |
| History of day care
use | 0.646 | 1.9071 | 1.038-3.504 | 0.038 |
| Social frailty | 0.230 | 1.2583 | 0.822-1.927 | 0.291 |
| Cerebral
infarction/hemorrhage | 0.209 | 1.2322 | 0.600-2.530 | 0.569 |
| Myocardial
infarction | 0.515 | 1.6733 | 0.725-3.860 | 0.227 |
| History of
hospitalization for heart failure | 0.382 | 1.4649 | 0.523-4.102 | 0.467 |
| Interstitial
pneumonia | 0.504 | 1.6555 | 0.764-3.587 | 0.201 |
| Malignant
disease | 0.188 | 1.2067 | 0.773-1.884 | 0.409 |
| Hypertension | 0.174 | 1.1902 | 0.786-1.802 | 0.411 |
| Diabetes
mellitus | 0.780 | 2.1823 | 1.381-3.449 | 0.001 |
| Osteoporosis | 0.595 | 1.8130 | 1.078-3.048 | 0.025 |
| Age-adjusted
Charlson comorbidity index | -0.019 | 0.9814 | 0.707-1.362 | 0.910 |
| Antidementia drug
use | -0.573 | 0.5638 | 0.141-2.256 | 0.418 |
| Number of oral
medicines | 0.013 | 1.0130 | 0.953-1.077 | 0.678 |
| MMSE score | -0.085 | 0.9184 | 0.862-0.978 | 0.008 |
| GDS-15 score | 0.045 | 1.0459 | 0.981-1.115 | 0.171 |
| Reflux-related QOL
score | -0.053 | 0.9480 | 0.867-1.036 | 0.240 |
| CSS score | -0.003 | 0.9974 | 0.946-1.051 | 0.922 |
|
SpO2 | 0.021 | 1.0214 | 0.955-1.093 | 0.538 |
| CAT score | 0.004 | 1.0043 | 0.968-1.042 | 0.816 |
| Restricted
ventilatory impairment | 0.238 | 1.2692 | 0.787-2.048 | 0.329 |
| Obstructive
ventilatory impairment | 0.143 | 1.1540 | 0.747-1.783 | 0.519 |
| Hypozincemia | -0.364 | 0.6950 | 0.432-1.119 | 0.134 |
| CONUT score | -0.015 | 0.9851 | 0.835-1.162 | 0.859 |
| DVS | -0.092 | 0.9120 | 0.834-0.997 | 0.043 |
| Oral frailty | 0.043 | 1.0436 | 0.690-1.578 | 0.840 |
| EAT10 score | -0.005 | 0.9949 | 0.943-1.049 | 0.850 |
Discussion
To the best of our knowledge, the present study is
the first large cross-sectional, multidisciplinary study examining
the prevalence of sarcopenia according to AWGS 2019 consensus
criteria and the association between dietary variety status and
sarcopenia in older outpatients of a university-affiliated
geriatric hospital in Japan. Sarcopenia prevalence was higher than
previously reported in community-dwelling individuals (2) and dietary variety status was
associated with sarcopenia.
According to a systematic review and meta-analysis,
muscle mass is most commonly assessed by BIA, followed by DXA and
computed tomography scan (35). The
most frequently employed sarcopenia classification criteria
established by the European Working Group on Sarcopenia in Older
People (EWGSOP) (36) and AWGS,
were used in 95 and 55 studies, respectively (31). According to the aforementioned
meta-analysis, the sarcopenia prevalence in individuals aged >60
years depended on the set of classification criteria used as
follows: EWGSOP2, 10; EWGSOP, 23; AWGS, 14; International Working
Group on Sarcopenia, 14; Foundation for the National Institutes of
Health, 12 and muscle mass definition, 27%, respectively. Here,
sarcopenia prevalence was higher (21.4%) than the mean of the
reported prevalence of AWGS-defined sarcopenia (2). This discrepancy may be attributed to
the higher number of patients with multiple severe disease at a
university-affiliated geriatric hospital. Additionally, the mean
age of participants was higher in here (78.2±6.1 years) than in
other studies (71-74 years) (37-40).
In a prospective study of the relationship between sarcopenia and
falls in persons aged ≥80 years (mean age, 86.7 years) in Italy,
sarcopenia was identified in 25.4% of participants (41). Hence, interpretation of sarcopenia
prevalence should consider diagnostic criteria used and the
characteristics of the population under study (37-41).
The present study showed a higher prevalence of
sarcopenia among male compared with female patients. However, a
meta-analysis of studies using the AWGS definition did not find a
difference in the sarcopenia prevalence based on sex (2). Participants in the present study were
all outpatients at a university-affiliated geriatric hospital. This
resulted in a higher mean age compared with previously reported
community-dwelling older populations (2). Since a previous Japanese cohort study
showed that sarcopenia prevalence increases with age in males
(37), the higher mean age may
underlie the present high sarcopenia prevalence among males.
In the present study, sarcopenia was significantly
associated with Brinkman Index, history of daycare use, PhA, QOL
and DM. Previous reports indicate a higher prevalence of smokers in
male patients with sarcopenia (38)
although the association is not confirmed (40). Because PhA reflects cell membrane
fragility, muscle mass and strength and nutritional status, its use
as a proxy for predicting falls and identifying individuals at risk
of disability has been suggested (42). Matsumoto et al (43) demonstrated that a history of falls
may serve as a simple screening tool to help prevent osteoporosis
and sarcopenia. A 2-year prospective observational study indicated
that sarcopenia prevalence is a significant predictor of falling
(44). In older adults, injuries
and fractures from falls lead to reduced physical activity and
strength and confinement to bed (38,39).
PhA from BIA is a valuable and simple prognostic tool for
identifying older individuals at risk of disability who may benefit
from preventive treatment (45).
The present study also evaluated quality of life using the EQ-VAS
questionnaire, which is commonly used in primary care to assess
health. Previous studies have demonstrated the value of EQ-VAS in
assessing frailty and decline in EQ-VAS in predicting frailty
(46,47). DM and heart failure are risk factors
for skeletal muscle mass decrease (48,49).
Studying sarcopenia and its association with DM and heart failure
may provide insight into sarcopenia prevention, diagnosis and
management.
The present study also showed that osteoporosis and
sarcopenia are significantly associated. Hida et al
(50) suggested that sarcopenia
increases the risk for osteoporotic vertebral fracture.
Simultaneous muscle loss and abnormal bone metabolism caused by
sarcopenia-associated systemic disorder, including malnutrition and
diabetes, could contribute to this association (50). Neuropsychological examination in the
present study revealed that sarcopenia was associated with low MMSE
and high GDS-15 score. Several studies have suggested that
cognitive impairment is associated with sarcopenia (38,51,52).
Nishikawa et al (53)
reported an independent association between decreased grip strength
and increased risk for depression progression in patients with
chronic liver disease.
A systematic review conducted by Jang et al
(54) concluded that dietary
variety can decrease risk of sarcopenia. The present study found a
significant association CSS and sarcopenia in univariate analysis.
A previous study in Juntendo Tokyo Koto Geriatric Medical Center,
Tokyo, Japan) demonstrated that sarcopenia is an independent
predictor for CSS score (55).
Decreased abdominal pressure due to low muscle mass for sarcopenia
may contribute to functional defecation disorder (49). DVS was associated with sarcopenia.
Momoki et al reported that DVS is associated with sarcopenia
in elderly female community residents in Japan (56).
The present study had limitations. First,
participants were outpatients aged ≥65 years at a single university
hospital. Background variables such as exercise routines, dietary
pattern, occupation, education level and marital status was not
investigated. Therefore, it is possible that the present findings
cannot be generalized. Due to the higher number of patients with
high degrees of multimorbidity, sarcopenia prevalence may be
overestimated because of unhealthy subject bias. Furthermore, as
the present study was a cross-sectional study, it is not possible
to infer a causal relationship with sarcopenia. A longitudinal
study should be conducted to investigate the effects of sarcopenia
prevention.
In conclusion, the present large cross-sectional
study demonstrated that sarcopenia was more prevalent in
outpatients than previously reported in community-dwelling older
individuals and dietary variety status was associated with
sarcopenia.
Acknowledgements
The authors would like to thank Dr Hisafumi Yamagata
and Dr. Maiko Suzuki (Department of Gastroenterology, Juntendo
Tokyo Koto Geriatric Medical Center, Tokyo, Japan), Dr. Ryota
Kanemaru and Dr. Tomoko Yamada (Department of Respiratory Medicine,
Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan), Dr.
Yurina Sugita (Department of Cardiology, Juntendo Tokyo Koto
Geriatric Medical Center, Shinsuna, Koto, Tokyo, Japan) and Dr. Ran
Matsudaira (Department of Rheumatology and Internal Medicine,
Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan) for
patient referral. The authors would also like to thank Ms. Mutsuko
Hatanaka and Ms. Ryoko Yamaguchi (Department of Gastroenterology,
Juntendo Tokyo Koto Geriatric Medical Center, Tokyo, Japan) for
data input.
Funding
Funding: The present study was supported by Japan Society for
the Promotion of Science KAKENHI (Grants-in-Aid for Scientific
Research; grant nos. 21K11633 and 16K09042).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DA and KMi confirm the authenticity of all the raw
data. DA, KS, AN and KMi designed the study. DA and KMa interpreted
data. DA wrote the manuscript. NY and YN performed statistical
analysis. NSi, HS, NE, YI, MT, NSa, MI, MN, TM, SI and YM
administered questionnaires. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki. The Juntendo Tokyo Koto Geriatric
Medical Center Ethics Committee approved the study (approval no.
G20-0011). Informed consent was obtained from each subject.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delmonico MJ, Harris TB, Lee JS, Visser M,
Nevitt M, Kritchevsky SB, Tylavsky FA and Newman AB: Health, Aging
and Body Composition Study. Alternative definitions of sarcopenia,
lower extremity performance, and functional impairment with aging
in older men and women. J Am Geriatr Soc. 55:769–774.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Petermann-Rocha F, Balntzi V, Gray SR,
Lara J, Ho FK, Pell JP and Celis-Morales C: Global prevalence of
sarcopenia and severe sarcopenia: A systematic review and
meta-analysis. J Cachexia Sarcopenia Muscle. 13:86–99.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen LK, Liu LK, Woo J, Assantachai P,
Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al:
Sarcopenia in Asia: Consensus report of the Asian Working Group for
Sarcopenia. J Am Med Dir Assoc. 15:95–101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoshimura N, Muraki S, Oka H, Iidaka T,
Kodama R, Kawaguchi H, Nakamura K, Tanaka S and Akune T: Is
osteoporosis a predictor for future sarcopenia or vice versa?
Four-year observations between the second and third ROAD study
surveys. Osteoporos Int. 28:189–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoshida D, Suzuki T, Shimada H, Park H,
Makizako H, Doi T, Anan Y, Tsutsumimoto K, Uemura K, Ito T and Lee
S: Using two different algorithms to determine the prevalence of
sarcopenia. Geriatr Gerontol Int. 14 (Suppl 1):S46–S51.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kiuchi Y, Tsutsumimoto K, Doi T, Kurita S,
Nishimoto K, Makizako H and Shimada H: Effect of dietary diversity
on incident of disability in community-dwelling older adults with
sarcopenia: A 40-month follow-up longitudinal study. Maturitas.
179(107887)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsuno K, Asaoka D, Sugano K, Takahashi K
and Miyauchi K: Rationale and design of Juntendo Sarcopenia
Registration to explore the predictors and prognosis of sarcopenia
and frailty in the elderly in TOKYO (JUSTICE-TOKYO). Geriatr
Gerontol Int. 24:168–172. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative. The
strengthening the reporting of observational studies in
epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Int J Surg. 12:1495–1499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Folstein MF, Folstein SE and McHugh PR:
‘Mini-mental state’. A practical method for grading the cognitive
state of patients for the clinician. J Psychiatr Res. 12:189–198.
1975.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sugishita K, Sugishita M, Hemmi I, Asada T
and Tanigawa T: A validity and reliability study of the Japanese
version of the geriatric depression scale 15 (GDS-15-J). Clin
Gerontol. 40:233–240. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Furuta K, Ishihara S, Sato S, Miyake T,
Ishimura N, Koshino K, Tobita H, Moriyama I, Amano Y, Adachi K, et
al: Development and verification of the Izumo Scale, new
questionnaire for quality of life assessment of patients with
gastrointestinal symptoms. Nihon Shokakibyo Gakkai Zasshi.
106:1478–1487. 2009.PubMed/NCBI(In Japanese).
|
|
12
|
Agachan F, Chen T, Pfeifer J, Reissman P
and Wexner SD: A constipation scoring system to simplify evaluation
and management of constipated patients. Dis Colon Rectum.
39:681–685. 1996.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jones PW, Harding G, Berry P, Wiklund I,
Chen WH and Kline Leidy N: Development and first validation of the
COPD assessment test. Eur Respir J. 34:648–654. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harris PA, Taylor R, Thielke R, Payne J,
Gonzalez N and Conde JG: Research electronic data capture
(REDCap)-a metadata-driven methodology and workflow process for
providing translational research informatics support. J Biomed
Inform. 42:377–381. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brinkman GL and Coates EO Jr: The effect
of bronchitis, smoking, and occupation on ventilation. Am Rev
Respir Dis. 87:684–693. 1963.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Makizako H, Shimada H, Tsutsumimoto K, Lee
S, Doi T, Nakakubo S, Hotta R and Suzuki T: Social frailty in
community-dwelling older adults as a risk factor for disability. J
Am Med Dir Assoc. 16:1003.e7–e11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baumgartner RN, Chumlea WC and Roche AF:
Bioelectric impedance phase angle and body composition. Am J Clin
Nutr. 48:16–23. 1988.PubMed/NCBI View Article : Google Scholar
|
|
18
|
EuroQol Group: EuroQol-a new facility for
the measurement of health-related quality of life. Health Policy.
16:199–208. 1990.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bellido D, García-García C, Talluri A,
Lukaski HC and García-Almeida JM: Future lines of research on phase
angle: Strengths and limitations. Rev Endocr Metab Disord.
24:563–583. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Norman K, Stobäus N, Pirlich M and
Bosy-Westphal A: Bioelectrical phase angle and impedance vector
analysis-clinical relevance and applicability of impedance
parameters. Clin Nutr. 31:854–861. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
working group for sarcopenia: 2019 Consensus update on sarcopenia
diagnosis and treatment. J Am Med Dir Assoc. 21:300–307.e2.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mushnick R, Fein PA, Mittman N, Goel N,
Chattopadhyay J and Avram MM: Relationship of bioelectrical
impedance parameters to nutrition and survival in peritoneal
dialysis patients. Kidney Int. Suppl:S53–S56. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kumagai S, Watanabe S, Shibata H, Amano H,
Fujiwara Y, Shinkai S, Yoshida H, Suzuki T, Yukawa H, Yasumura S
and Haga H: Effects of dietary variety on declines in high-level
functional capacity in elderly people living in a community. Nihon
Koshu Eisei Zasshi. 50:1117–1124. 2003.PubMed/NCBI(In Japanese).
|
|
24
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Charlson M, Szatrowski TP, Peterson J and
Gold J: Validation of a combined comorbidity index. J Clin
Epidemiol. 47:1245–1251. 1994.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Soen S, Fukunaga M, Sugimoto T, Sone T,
Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, et al:
Diagnostic criteria for primary osteoporosis: Year 2012 revision. J
Bone Miner Metab. 31:247–257. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
No authors listed. Guideline of
respiratory function tests-spirometry, flow-volume curve, diffusion
capacity of the lung. Nihon Kokyuki Gakkai Zasshi. Suppl:S1–S56.
2004.PubMed/NCBI(In Japanese).
|
|
28
|
Belafsky PC, Mouadeb DA, Rees CJ, Pryor
JC, Postma GN, Allen J and Leonard RJ: Validity and reliability of
the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol.
117:919–924. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ignacio de Ulíbarri J, González-Madroño A,
de Villar NG, González P, González B, Mancha A, Rodríguez F and
Fernández G: CONUT: A tool for controlling nutritional status.
First validation in a hospital population. Nutr Hosp. 20:38–45.
2005.PubMed/NCBI
|
|
30
|
Levenson CW: Zinc regulation of food
intake: New insights on the role of neuropeptide Y. Nutr Rev.
61:247–249. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nowak G, Szewczyk B and Pilc A: Zinc and
depression. An update. Pharmacol Rep. 57:713–718. 2005.PubMed/NCBI
|
|
32
|
Kinomoto T, Sawada M, Ohnishi Y, Yamaguchi
T, Tsuge S, Ogawa S, Washizuka M, Minaguchi J, Mera Y and Takehana
K: Effects of polaprezinc on morphological change of the tongue in
zinc-deficient rats. J Oral Pathol Med. 39:617–623. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tanaka T, Hirano H, Ohara Y, Nishimoto M
and Iijima K: Oral Frailty Index-8 in the risk assessment of
new-onset oral frailty and functional disability among
community-dwelling older adults. Arch Gerontol Geriatr.
94(104340)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baumgartner RN, Koehler KM, Gallagher D,
Romero L, Heymsfield SB, Ross RR, Garry PJ and Lindeman RD:
Epidemiology of sarcopenia among the elderly in New Mexico. Am J
Epidemiol. 147:755–763. 1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shafiee G, Keshtkar A, Soltani A, Ahadi Z,
Larijani B and Heshmat R: Prevalence of sarcopenia in the world: A
systematic review and meta-analysis of general population studies.
J Diabetes Metab Disord. 16(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cruz-Jentoft AJ, Baeyens JP, Bauer JM,
Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y,
Schneider SM, et al: Sarcopenia: European consensus on definition
and diagnosis: Report of the European working group on sarcopenia
in older people. Age Ageing. 39:412–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kurose S, Nishikawa S, Nagaoka T, Kusaka
M, Kawamura J, Nishioka Y, Sato S, Tsutsumi H and Kimura Y:
Prevalence and risk factors of sarcopenia in community-dwelling
older adults visiting regional medical institutions from the Kadoma
sarcopenia study. Sci Rep. 10(19129)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kitamura A, Seino S, Abe T, Nofuji Y,
Yokoyama Y, Amano H, Nishi M, Taniguchi Y, Narita M, Fujiwara Y and
Shinkai S: Sarcopenia: Prevalence, associated factors, and the risk
of mortality and disability in Japanese older adults. J Cachexia
Sarcopenia Muscle. 12:30–38. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Uemura K, Makizako H, Lee S, Doi T, Lee S,
Tsutsumimoto K and Shimada H: The impact of sarcopenia on incident
homebound status among community-dwelling older adults: A
prospective cohort study. Maturitas. 113:26–31. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakamura K, Yoshida D, Honda T, Hata J,
Shibata M, Hirakawa Y, Furuta Y, Kishimoto H, Ohara T, Kitazono T,
et al: Prevalence and mortality of sarcopenia in a
community-dwelling older japanese population: The Hisayama study. J
Epidemiol. 31:320–327. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Landi F, Liperoti R, Russo A, Giovannini
S, Tosato M, Capoluongo E, Bernabei R and Onder G: Sarcopenia as a
risk factor for falls in elderly individuals: results from the
ilSIRENTE study. Clin Nutr. 31:652–568. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Matsumoto Y, Tada M, Yamada Y, Mandai K,
Hidaka N and Koike T: The bioimpedance phase angle is more useful
than sarcopenia as a predictor of falls in patients with rheumatoid
arthritis: Results from a 2-y prospective cohort study. Nutrition.
102(111729)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Matsumoto H, Tanimura C, Kushida D, Osaka
H, Kawabata Y and Hagino H: FRAX score and recent fall history
predict the incidence for sarcopenia in community-dwelling older
adults: A prospective cohort study. Osteoporos Int. 31:1985–1994.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Matsumoto H, Tanimura C, Tanishima S,
Osaki M, Noma H and Hagino H: Sarcopenia is a risk factor for
falling in independently living Japanese older adults: A 2-year
prospective cohort study of the GAINA study. Geriatr Gerontol Int.
17:2124–2130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Uemura K, Doi T, Tsutsumimoto K, Nakakubo
S, Kim MJ, Kurita S, Ishi H and Shimada H: Predictivity of
bioimpedance phase angle for incident disability in older adults. J
Cachexia Sarcopenia Muscle. 11:46–45. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kim S, Won CW, Kim BS, Kim S, Yoo J, Byun
S, Jang HC, Cho BL, Son SJ, Lee JH, et al: EuroQol visual analogue
scale (EQ-VAS) as a predicting tool for frailty in Older Korean
adults: The Korean frailty an aging cohort study (KFACS). J Nutr
Health Aging. 22:1275–1280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Beaudart C, Demonceau C, Reginster JY,
Locquet M, Cesari M, Cruz Jentoft AJ and Bruyère O: Sarcopenia and
health-related quality of life: A systematic review and
meta-analysis. J Cachexia Sarcopenia Muscle. 14:1228–1243.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Park SW, Goodpaster BH, Lee JS, Kuller LH,
Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA,
Nevitt M, et al: Excessive loss of skeletal muscle mass in older
adults with type 2 diabetes. Diabetes Care. 32:1993–1997.
2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fülster S, Tacke M, Sandek A, Ebner N,
Tschöpe C, Doehner W, Anker SD and von Haehling S: Muscle wasting
in patients with chronic heart failure: Results from the studies
investigating co-morbidities aggravating heart failure (SICA-HF).
Eur Heart J. 34:512–519. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hida T, Shimokata H, Sakai Y, Ito S,
Matsui Y, Takemura M, Kasai T, Ishiguro N and Harada A: Sarcopenia
and sarcopenic leg as potential risk factors for acute osteoporotic
vertebral fracture among older women. Eur Spine J. 25:3424–3431.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maeda K and Akagi J: Cognitive impairment
is independently associated with definitive and possible sarcopenia
in hospitalized older adults: The prevalence and impact of
comorbidities. Geriatr Gerontol Int. 17:1048–1056. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nishiguchi S, Yamada M, Shirooka H, Nozaki
Y, Fukutani N, Tashiro Y, Hirata H, Yamaguchi M, Tasaka S,
Matsushita T, et al: Sarcopenia as a risk factor for cognitive
deterioration in community-dwelling older adults: A 1-year
prospective study. J Am Med Dir Assoc. 17:372.e5–e8.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Nishikawa H, Yoh K, Enomoto H, Ikeda N,
Takashima T, Aizawa N, Nishimura T, Nishiguchi S and Iijima H:
Reduced grip strength is associated with progression of depressive
status in chronic liver diseases. Ann Palliat Med. 10:3976–3987.
2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jang EH, Han YJ, Jang SE and Lee S:
Association between diet quality and sarcopenia in older adults:
Systematic review of prospective cohort studies. Life (Basel).
11(811)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Asaoka D, Takeda T, Inami Y, Abe D,
Shimada Y, Matsumoto K, Ueyama H, Matsumoto K, Komori H, Akazawa Y,
et al: Association between the severity of constipation and
sarcopenia in elderly adults: A single-center university
hospital-based, cross-sectional study. Biomed Rep.
14(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Momoki C, Habu D, Ogura J, Tada A, Hasei
A, Sakurai K and Watanabe H: Relationships between sarcopenia and
household status and locomotive syndrome in a community-dwelling
elderly women in Japan. Geriatr Gerontol Int. 17:54–60.
2017.PubMed/NCBI View Article : Google Scholar
|