Introduction
Anaplastic thyroid cancer (ATC) is a rare and aggressive form of thyroid cancer, which commonly occurs in patients >60 years of age (1). It is characterized by the rapid growth and spread of cancerous cells in the thyroid gland (2). It is more common in older individuals and carries a poor prognosis. Although the incidence of ATC is low, accounting for 1-2% of all thyroid malignancies, it accounts for 10-30% of thyroid cancer deaths (3). As it is a rare and difficult-to-treat cancer, there is no clear evidence regarding treatment outcomes to date, and there are currently no established treatment strategies for ATC. Patients diagnosed with ATC will almost inevitably die from the disease. Despite aggressive multimodal treatment strategies, overall survival (OS) in ATC remains dismal, with median OS estimates ranging between 3 and 6 months, and less than one-fifth of patients surviving beyond one year (4,5).
Recently, a better understanding of the pathogenesis and genetic alterations of ATC has been gained. In the present study, the unusual clinical and molecular characterization of a 56-year-old male patient who died of ATC was reported. Molecular analysis of the patient's DNA revealed the presence of a variant in exon 5 of the phosphatase and tensin homolog (PTEN) gene. PTEN is a negative regulator of one of the most important cancer pathways, controlling numerous cellular processes, including proliferation, survival, energy metabolism, cell structure and motility, by inhibiting the PI3K/AKT pathway through its lipid phosphatase activity (6-9). Loss of PTEN function causes certain cells to continue to divide, leading to tumorigenesis (10,11). PTEN mutation is a rare mutation detected in thyroid cancer, with a mutation frequency of ~5% (12,13). It has been determined that PTEN mutations are associated with malignancy in 37.5% of thyroid cancer cases (14).
The present study reports the detailed clinical manifestations, pathological features and differential diagnosis of a rare case of ATC and coronary heart disease who underwent stent placement. Furthermore, a novel PTEN mutation was found, which may be associated with tumor malignancy. The present study can provide useful theoretical support for the diagnosis and treatment of ATC in the future.
Case report
The patient, a 56-year-old male, accidentally discovered a mass in the front part of his left neck 7 days before he came to the Linfen Central Hospital in August 2022. The mass was the size of a walnut and had no redness, swelling or ulceration. The patient had no fever, cough, dysphagia or chest discomfort. The patient had received a stent for coronary heart disease in 2020. Physical examination revealed that a mass of ~6x5x3 cm could be palpated in the left lobe of the thyroid gland. It was firm in texture, smooth at the surface and had unclear boundaries. It was able to move up and down with swallowing activities. There was no enlargement of the right lobe or isthmus of the thyroid gland, no tremor and no vascular murmur. There was no obvious enlargement of the bilateral cervical lymph nodes. The patient received treatment with levothyroxine sodium tablets 100 µg quaque die (Qd) since he was diagnosed.
Laboratory index examination revealed that serum thyroid-stimulating hormone (0.35, normal range: 0.56-5.91 MIU/l) and parathyroid hormone (11.94, normal range: 12-88 pg/ml) were lower than normal hormone levels. Thyroid color ultrasound showed a heterogeneous hypoechoic mass in the left lobe of the thyroid gland, thyroid imaging reporting and data system for ultrasonography (TI-RADS) (15) category 4b, solid thyroid nodules (multiple) in the right lobe, TI-RADS category 3 and enlarged lymph nodes in zone IV of the left neck (Fig. 1). Spiral CT of the neck showed diffuse enlargement of the left lobe of the thyroid gland and left lobe nodules with calcification (Fig. 2).
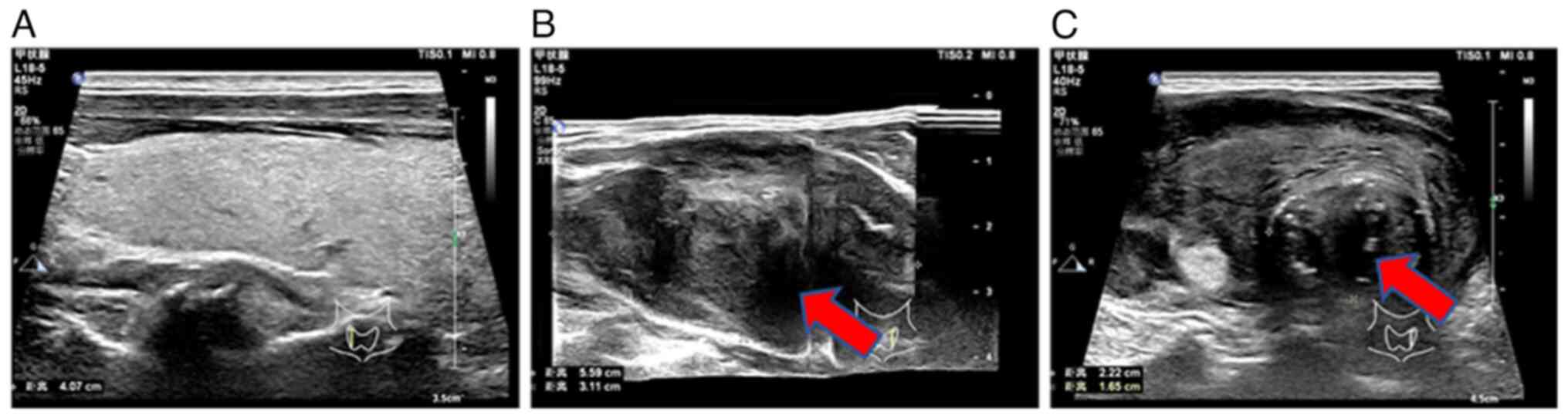 |
Figure 1
Ultrasound results of the patient's thyroid gland. (A) The normal right lobe of the thyroid gland. (B) The enlarged heterogeneous hypoechoic mass in the left lobe of the thyroid gland, as indicated by the arrow. (C) Enlarged lymph nodes in the left lobe of the thyroid gland (arrow).
|
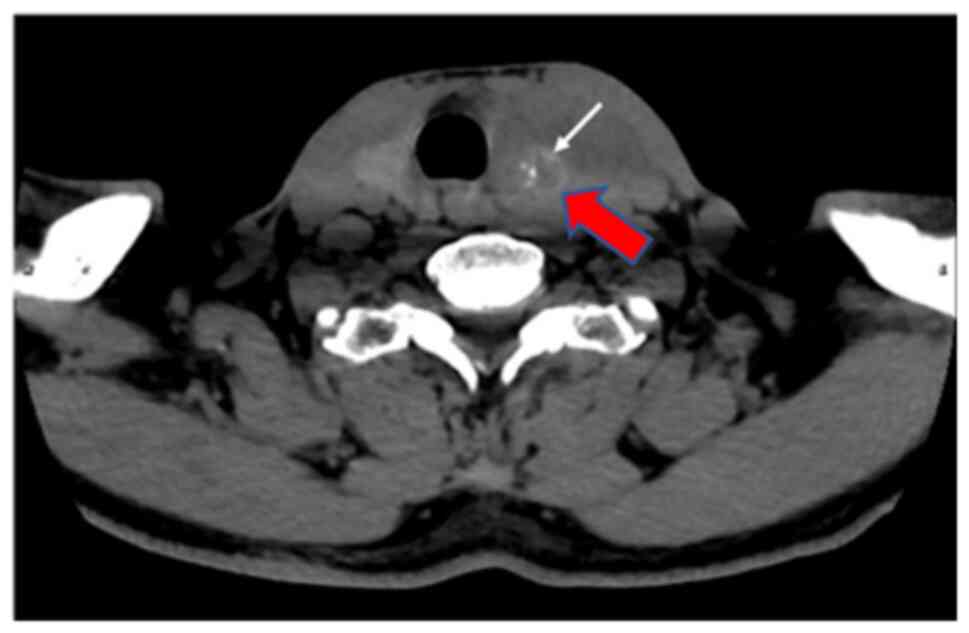 |
Figure 2
Image of a computed tomography scan of the neck performed prior to treatment. The arrows in the image indicate a diffuse enlargement of the left lobe of the thyroid gland and left lobe nodule with calcification.
|
After the operation, a morphological study of the main tumor tissue was performed. Hematoxylin and eosin (H&E) staining was performed to examine tissue morphological changes (10.1371/journal.pone.0229264), and the results showed that tumor cells were diffusely distributed and invaded the surrounding thyroid gland (Fig. 3A). The tumor cells had obvious atypia, with round and oval cells and obvious nucleoli (Fig. 3B), reflecting the typical characteristics of ATC. In addition, tumor cells in certain areas were observed to be spindle-shaped (Fig. 3C). These observations are consistent with undifferentiated carcinoma (giant cell type and spindle cell type). The size of the tumor was 6x3.5x3 cm, intravascular tumor thrombi could be seen and the cancer tissue invaded the surrounding thyroid and striated muscle. Subsequently, immunohistochemistry staining was carried out to examine the tumor marker expression levels (16). Antibodies for immunohistochemistry staining were purchased from Abcam. The results and antibody details were as follows: Cytokeratin (CK; cat. no. ab7753; 1:500 dilution) (part +; Fig. 4A), Vimentin (cat. no. ab92547; 1:250 dilution) (+; Fig. 4B), Thyroglobulin (cat. no. ab156008; 1:250 dilution) (-, data not shown), Calcitonin (cat. no. ab16697; 1:500 dilution) (-, data not shown), Synuclein (cat. no. ab138501; 1:200 dilution) (-, data not shown), CD56 (cat. no. ab119587; 1:500 dilution) (-, data not shown), thyroid transcription factor-1 (cat. no. ab76013; 1:250 dilution) (-, data not shown), P53 (wild-type; cat. no. ab32049; 1:500 dilution) (-, data not shown), CD34 (cat. no. ab81289; 1:250 dilution) (Vascular +, data not shown), actin (cat. no. ab8226; 1:500 dilution) (focus +, data not shown), myogenin (cat. no. ab124800; 1:250 dilution) (-, data not shown), CD31 (cat. no. ab28364; 1:500 dilution) (part +, data not shown), P40 (cat. no. ab76158; 1:250 dilution) (-, data not shown), Ki-67 (cat. no. ab16667; 1:500 dilution) (+, ~60%; Fig. 4C). CK is a protein expressed in epithelial cells and has been widely used for tumor diagnosis, classification and prognosis (17). Vimentin is associated with cancer cell epithelial-mesenchymal transition, tumor progression and metastasis (18). Ki-67 is a cell proliferation marker that is widely used to assess the proliferative activity of cancer cells (19). Taken together, the positive results of CK, vimentin and Ki-67 indicated the malignancy of tumor cells. Special staining results were as follows: Reticular fiber staining (20) showed that tumor cells are arranged in nests. Pathological mitotic figures of tumor cells (Fig. 3D) and metastasis in the lymph nodes (Fig. 3E) were also observed. In addition, tumor necrosis was present in the red framed area (Fig. 3F). The patient was diagnosed with ATC based on combined H&E and immunohistochemistry results. For whole exon sequencing (ES), total DNA was extracted from patient tumor tissue samples. ES was performed using the GenCap™ Human Whole Exon Probe V4.0 (Mygenostics), and exome libraries were sequenced on the GenCap™ Platform (Mygenostics). Published algorithms were used to identify somatic variants (21). In addition, an in-house developed method was employed to detect CNVs in normal and tumor samples. Furthermore, structural variants and genomic fusions were assessed using Manta (doi:10.1093/bioinformatics/btv710). The results indicated there is a somatic splicing variant in exon 5 of the PTEN gene (c.388C>T, p.R130X). No other notable mutations were identified in these genes based on whole-exome sequencing.
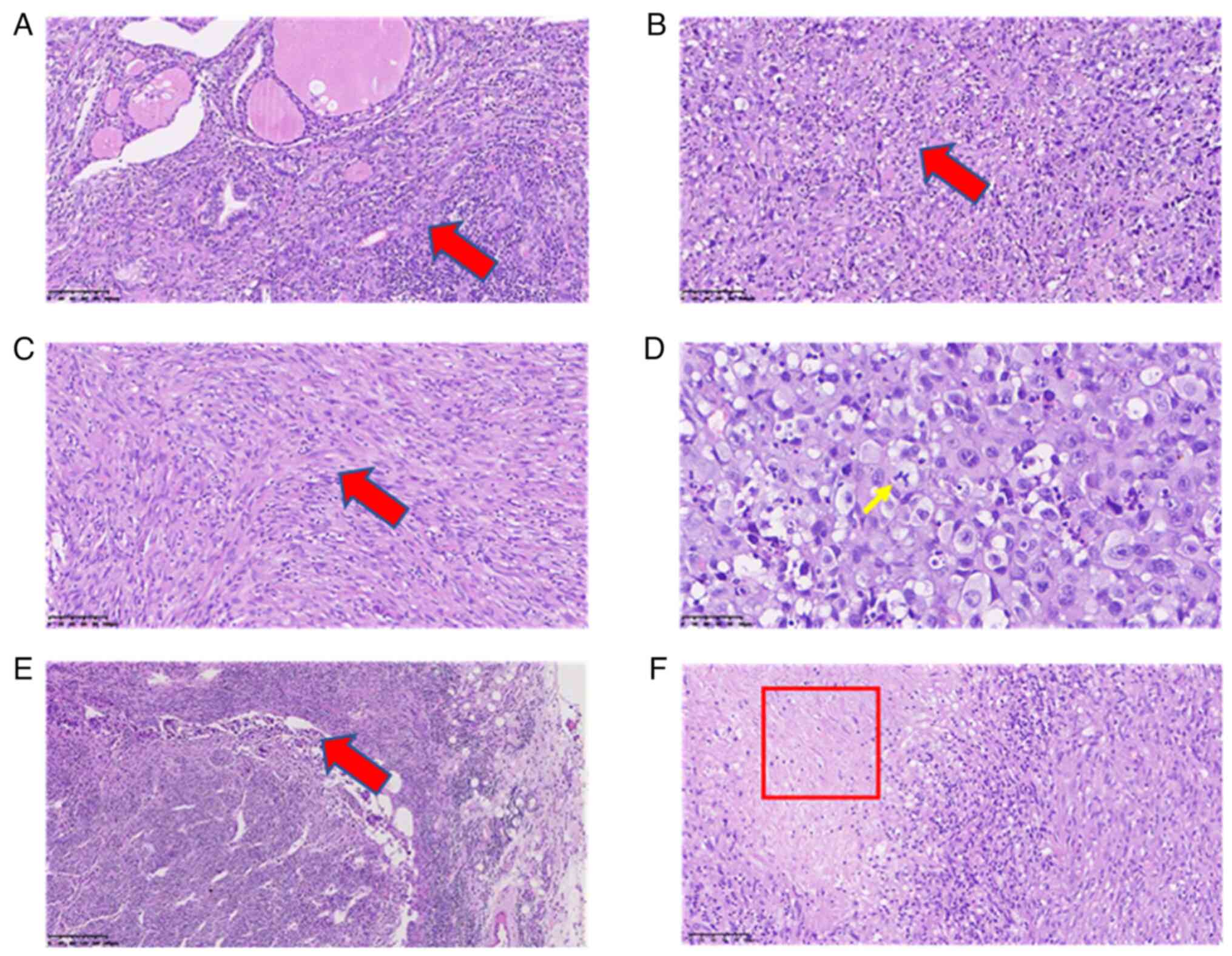 |
Figure 3
Hematoxylin and eosin staining of ATC. (A) Representative images of main tumor tissues; tumor cells are diffusely distributed and invade the surrounding thyroid gland (arrow). (B) Representative images of main tumor tissues; the tumor cells have obvious atypia, with round and oval cells and obvious nucleoli, representing the typical characteristics of ATC (arrow). (C) Representative images of main tumor tissues; tumor cells in certain areas are spindle-shaped (scale bars, 100 µm; arrow). (D) Reticular fiber staining of the main tumor tissues; the yellow arrow indicates mitotic cell (scale bar, 50 µm; arrow). (E) Representative images of lymph node tissue showing metastasis of cancer cells in the lymph node (scale bar, 200 µm; arrow). (F) Tumor necrosis is shown in the area in the boxed area (scale bar, 100 µm). ATC, anaplastic thyroid cancer.
|
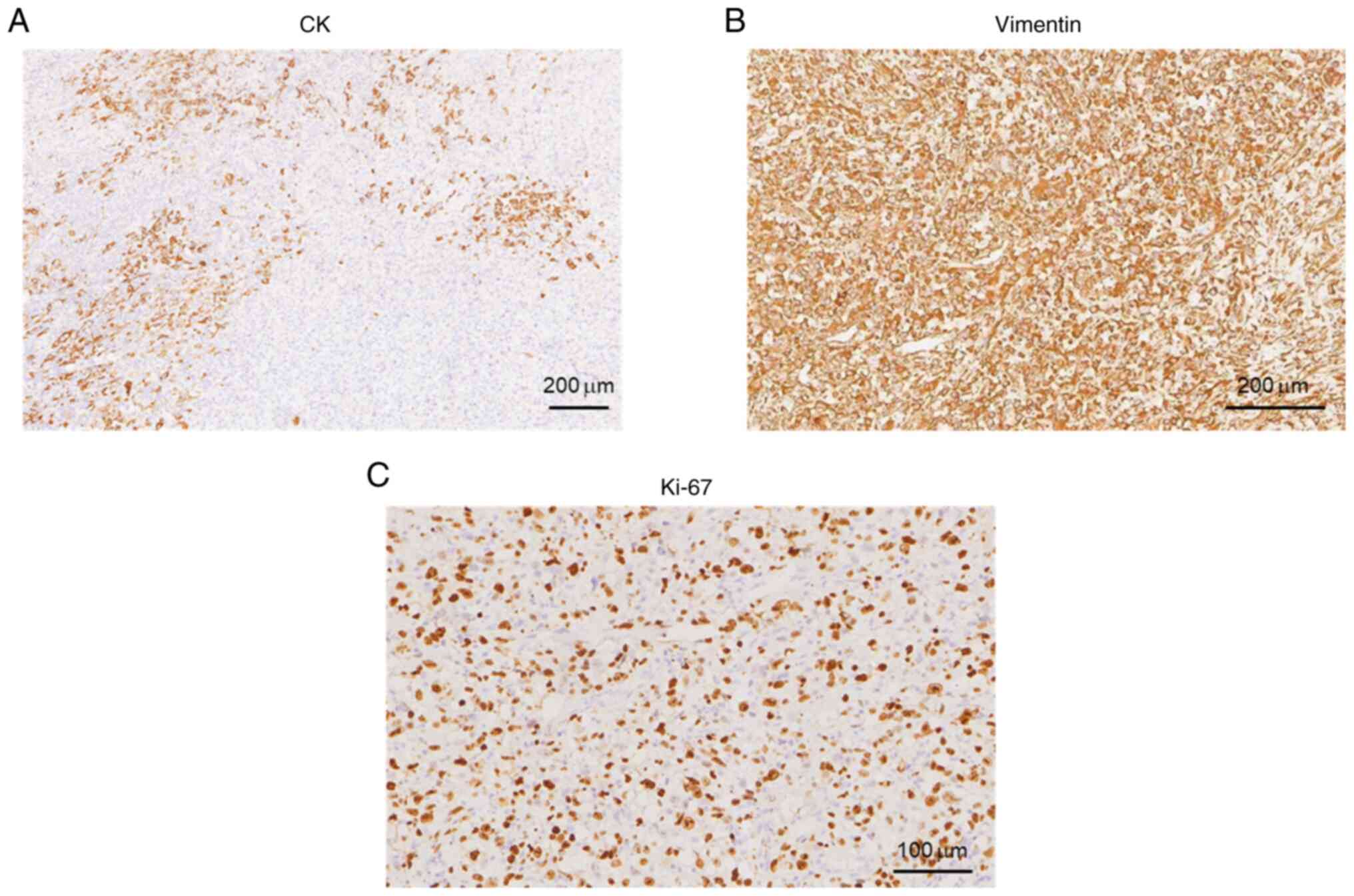 |
Figure 4
Immunohistochemical images indicated that the tumor was positive (yellow or brown areas) for (A) CK, (B) vimentin (scale bars, 200 µm) and (C) Ki-67 (scale bar, 100 µm). CK, cytokeratin.
|
The patient had surgery in August 2022, was hospitalized again 1 month later and received radiotherapy in December 2022. The total dose was 95% planning target volume (PTV)1 66 Gy/33 fractions (f), 95% PTV2 59.4 Gy/33 f. During radiotherapy, oxygen inhalation, asthma relief, phlegm reduction, dehydration, nutritional support and electrolyte imbalance correction were supplemented. At discharge, the doctor's instructions were as follows: Make sure to get sufficient rest and nutrition; continue oral treatment with levothyroxine sodium tablets 100 µg Qd and review five thyroid function items after one month; further systemic treatment was recommended to control the condition and follow-up for discomfort. The patient visited the hospital for follow-ups in the first and third months after surgery. Subsequently, the hospital staff conducted a follow-up call with the patient every month. In December 2023, the patient passed away.
Discussion
ATC is an undifferentiated tumor derived from the follicular epithelium of the thyroid gland. The disease progresses rapidly, treatment is ineffective and patient survival is usually 3-5 months (22). The incidence rate of ATC is 1-2 cases per 1,000,000 individuals, accounting for 0.9-9.8% of the total number of thyroid cancer cases worldwide (22). It is more aggressive than differentiated thyroid cancer, with disease-specific mortality approaching 100% (2). The present study reported the case of a patient with ATC who survived for >1 year, providing useful clues for understanding this rare disease. In particular, a novel nonsense mutation (c.388C>T, p.R130X) of the PTEN gene in exon 5 was found, which may be associated with tumor malignancy.
PTEN, a tumor suppressor gene, is frequently mutated in human cancers and PTEN is also present in aggressive thyroid cancers (23). The human PTEN gene encodes a protein composed of 403 amino acids with a molecular weight of ~54 kDa. The majority of PTEN consists of the N-terminal phosphatase domain (7-185 amino acids) and the closely related C2 domain (186-351 amino acids), which are required for protein stability and catalysis (Fig. 5D) (24,25). To date, 110 germline PTEN mutations have been reported in patients with two tumor predisposition syndromes, each with overlapping clinical features: Cowden disease and Bannayan-Riley-Ruvalcaba syndrome (26). Somatic PTEN mutations are primarily found in endometrial cancer and glioblastoma, but PTEN mutations have also been detected to a lesser extent in other types of malignancies, such as breast and thyroid cancer (26). The majority (70%) of somatic PTEN mutations are nonsense, frameshift or splicing mutations that result in protein truncation (26,27). The ONCOKB database (www.oncokb.org) was used to summarize the mutations of the PTEN gene in thyroid cancer cases, as shown in Fig. 5D. In this case report, a novel mutation was found in PTEN, a nonsense mutation in exon 5 of the PTEN gene (c.388C>T, p.R130X). HomoloGene in the National Center for Biotechnology Information (NCBI) database (www.ncbi.nlm.nih.gov) was used to query PTEN species homology sequences and the UGENE software (version 50.0; https://ugene.net/) was used for sequence visualization and it was found that R130 is a highly conserved amino acid (Fig. 5A). Therefore, once this amino acid is mutated, it is likely to affect the structure and function of the protein. Next, Swiss-Model (https://swissmodel.expasy.org/) was used with P60484-F1 as a template to conduct homology modeling of PTEN protein wild-type and p.R130X mutant proteins (28,29), and PyMOL (https://pymol.org/2/) software was used for sequence visualization. Fig. 5B shows the structure of wild-type PTEN. As shown in Fig. 5C, the nonsense mutation (c.388C>T, p.R130X) in exon 5 of the PTEN gene prevents the efficient expression of the protein, resulting in protein truncation. The PTEN (c.388C>T, p.R130X) mutation has been reported in colon carcinoma (30), renal cell carcinoma (31), Cowden syndrome (32), endometrial cancer (33) and glioblastoma (34). This mutation has an impact on the tumor suppressive function of PTEN, leads to the activation of oncogenic pathways and is related to patient prognosis (26). Despite previous reports on PTEN mutations in thyroid cancer, effective clinical treatments remain lacking (14,35). Further research is needed on whether this mutation is related to tumor occurrence and malignancy.
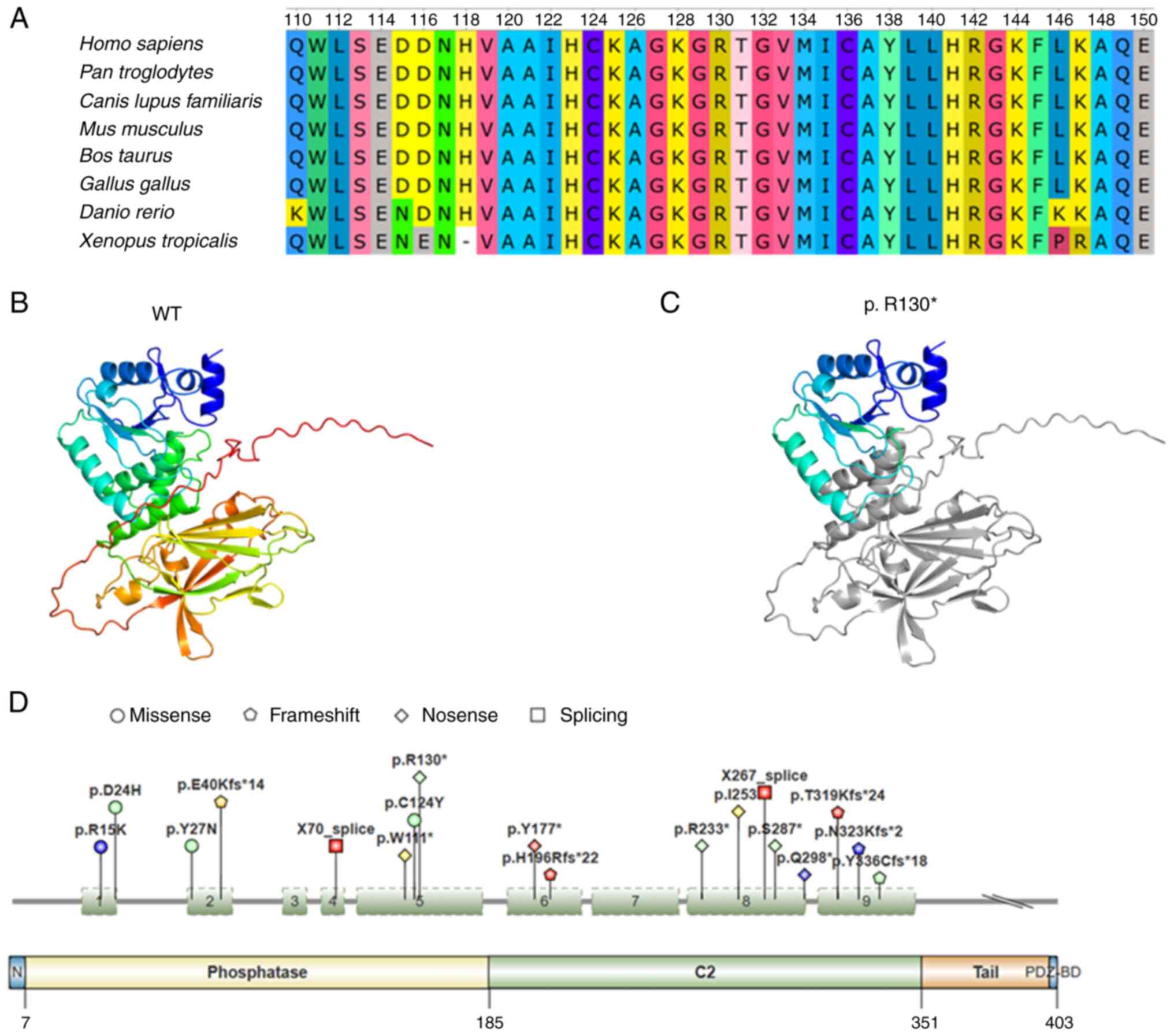 |
Figure 5
(A) Conservation analysis of PTEN gene R130. (B and C) Swiss-Model (https://swissmodel.expasy.org/) was used to conduct homology modeling of PTEN. Structure of (B) WT and (C) p.R130X mutant protein using P60484-F1 as a template. The image shows the Cartoon structure in rainbow colors and the truncated sequence is represented in gray. (D) PTEN gene variation in thyroid cancer cases. Red indicates anaplastic thyroid cancer, blue is papillary thyroid cancer, green is poorly differentiated thyroid cancer and yellow is Hurthle cell thyroid cancer. The data were obtained from the ONCOKB database (www.oncokb.org) and the figure was produced by IBS 2.0. PTEN, phosphatase and tensin homolog; WT, wild-type.
|
In conclusion, the present study reported a rare case of a patient exhibiting distinct pathological features of ATC. Notably, genetic analysis revealed a PTEN mutation not previously reported in thyroid cancer. Protein structure prediction suggested that this mutation may result in truncation of the PTEN protein, potentially contributing to the malignancy of ATC. Further study is needed in the future to verify the role of this mutation in tumor development and progression, as well as to elucidate its underlying mechanism.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be found in the NCBI database under accession no. SRR29333118 (https://ncbi.nlm.nih.gov/sra/?term=SRR29333118).
Author's contributions
YZ advised on patient treatment, analyzed patient results, checked and confirmed authenticity of the raw data and drafted the manuscript. YZ read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Linfen Central Hospital of Shanxi (Linfen, China; approval no. 2023-38-1).
Patient consent for publication
Written informed consent was obtained from the patient for the publication of anonymized data and any accompanying images.
Competing interests
The author declares that there are no competing interests.
References
|
1
|
Graceffa G, Salamone G, Contino S, Saputo F, Corigliano A, Melfa G, Proclamà MP, Richiusa P, Mazzola S, Tutino R, et al: Risk factors for anaplastic thyroid carcinoma: A case series from a tertiary referral center for thyroid surgery and literature analysis. Front Oncol. 12(948033)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rao SN and Smallridge RC: Anaplastic thyroid cancer: An update. Best Pract Res Clin Endocrinol Metab. 37(101678)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Talbott I and Wakely PE Jr: Undifferentiated (anaplastic) thyroid carcinoma: Practical immunohistochemistry and cytologic look-alikes. Semin Diagn Pathol. 32:305–310. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, Deschamps F, Lamartina L, Lacroix L, Dupuy C, et al: Anaplastic thyroid carcinoma: An Update. Cancers (Basel). 14(1061)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Ridder M, Nieveen van Dijkum E, Engelsman A, Kapiteijn E, Klumpen HJ and Rasch CRN: Anaplastic thyroid carcinoma: A nationwide cohort study on incidence, treatment and survival in the Netherlands over 3 decades. Eur J Endocrinol. 183:203–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S, Cognetti F and Ciuffreda L: PTEN: Multiple functions in human malignant tumors. Front Oncol. 5(24)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Haddadi N, Lin Y, Travis G, Simpson AM, Nassif NT and McGowan EM: PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol Cancer. 17(37)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cain RJ and Ridley AJ: Phosphoinositide 3-kinases in cell migration. Biol Cell. 101:13–29. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ward PS and Thompson CB: Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol. 4(a006783)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yehia L, Keel E and Eng C: The clinical spectrum of PTEN Mutations. Annu Rev Med. 71:103–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH and Baker SJ: Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 64:7773–7779. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagy R, Ganapathi S, Comeras I, Peterson C, Orloff M, Porter K, Eng C, Ringel MD and Kloos RT: Frequency of germline PTEN mutations in differentiated thyroid cancer. Thyroid. 21:505–510. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rustad CF, Bjornslett M, Heimdal KR, Maehle L, Apold J and Moller P: Germline PTEN mutations are rare and highly penetrant. Hered Cancer Clin Pract. 4:177–185. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bandargal S, Rajab M, Forest VI, Pusztaszeri MP, Hier MP, da Silva SD and Payne RJ: Characteristics of PTEN mutation in thyroid tumours: A retrospective chart review. Cancers (Basel). 15(1575)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou J, Song Y, Zhan W, Wei X, Zhang S, Zhang R, Gu Y, Chen X, Shi L, Luo X, et al: Thyroid imaging reporting and data system (TIRADS) for ultrasound features of nodules: Multicentric retrospective study in China. Endocrine. 72:157–170. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Zheng H, Xu M, Maeda N, Tsunedomi R, Kishi H, Nagano H and Kobayashi S: Fyn-Mediated paxillin tyrosine 31 phosphorylation regulates migration and invasion of breast cancer cells. Int J Mol Sci. 24(15980)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dum D, Menz A, Volkel C, De Wispelaere N, Hinsch A, Gorbokon N, Lennartz M, Luebke AM, Hube-Magg C, Kluth M, et al: Cytokeratin 7 and cytokeratin 20 expression in cancer: A tissue microarray study on 15,424 cancers. Exp Mol Pathol. 126(104762)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Berr AL, Wiese K, Dos Santos G, Koch CM, Anekalla KR, Kidd M, Davis JM, Cheng Y, Hu YS and Ridge KM: Vimentin is required for tumor progression and metastasis in a mouse model of non-small cell lung cancer. Oncogene. 42:2074–2087. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Davey MG, Hynes SO, Kerin MJ, Miller N and Lowery AJ: Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers (Basel). 13(4455)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu X, He S, Jiang X, Wei P, Zhou X, Shi Z, Li X, Lu J, Zhao H, Wei B and Jin M: Reticular fibre structure in the differential diagnosis of parathyroid neoplasms. Diagn Pathol. 18(79)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen X, Schulz-Trieglaff O, Shaw R, Barnes B, Schlesinger F, Källberg M, Cox AJ, Kruglyak S and Saunders CT: Manta: Rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 32:1220–1222. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nagaiah G, Hossain A, Mooney CJ, Parmentier J and Remick SC: Anaplastic thyroid cancer: A review of epidemiology, pathogenesis, and treatment. J Oncol. 2011(542358)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luongo F, Colonna F, Calapa F, Vitale S, Fiori ME and De Maria R: PTEN Tumor-Suppressor: The dam of stemness in cancer. Cancers (Basel). 11(1076)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Leslie NR and Longy M: Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 52:30–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song MS, Salmena L and Pandolfi PP: The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 13:283–296. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bonneau D and Longy M: Mutations of the human PTEN gene. Hum Mutat. 16:109–122. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dillon LM and Miller TW: Therapeutic targeting of cancers with loss of PTEN function. Curr Drug Targets. 15:65–79. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al: Highly accurate protein structure prediction with AlphaFold. Nature. 596:583–589. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, et al: AlphaFold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50(D1):D439–D444. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Danielsen SA, Lind GE, Bjornslett M, Meling GI, Rognum TO, Heim S and Lothe RA: Novel mutations of the suppressor gene PTEN in colorectal carcinomas stratified by microsatellite instability- and TP53 mutation-status. Hum Mutat. 29:E252–E262. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim RH, Wang X, Evans AJ, Campbell SC, Nguyen JK, Farncombe KM and Eng C: Early-onset renal cell carcinoma in PTEN harmatoma tumour syndrome. NPJ Genom Med. 5(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pistorius S, Klink B, Pablik J, Rump A, Aust D, Garzarolli M, Schröck E and Schackert HK: An unusual case of Cowden syndrome associated with ganglioneuromatous polyposis. Hered Cancer Clin Pract. 14(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fusco N, Sajjadi E, Venetis K, Gaudioso G, Lopez G, Corti C, Rocco EG, Criscitiello C, Malapelle U and Invernizzi M: PTEN alterations and their role in cancer management: Are we making headway on precision medicine? Genes (Basel). 11(719)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Owen S, Alken S, Alshami J, Guiot MC, Kavan P, Reardon DA, Muanza T, Gibson N, Pemberton K, Solca F, et al: Genomic analysis of tumors from patients with glioblastoma with long-term response to afatinib. Onco Targets Ther. 15:367–380. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee Y and Oh YL: Thyroid pathology, a clue to PTEN hamartoma tumor syndrome. J Pathol Transl Med. 57:178–183. 2023.PubMed/NCBI View Article : Google Scholar
|



















