1. Introduction
In the modern world and the Fourth Industrial
Revolution (4IR), depression is one of the leading health problems
among young individuals and ~280 million individuals worldwide live
with depression (1,2). Research that was conducted in 2021 and
which examined the effect of the SARS CoV2 pandemic on mental
health, covering 204 countries, indicated that individuals in areas
that were more severely affected by the pandemic, women and younger
age groups were at greater risk of depressive disorder (3).
The COVID-19 pandemic and its consequences have
indicated the need to strengthen the mental health system in most
countries, which is in line with the shortage of psychiatrists
worldwide. It is estimated that in rich countries with high
incomes, there are ~12 psychiatrists per 100,000 individuals and in
low-income countries, ~0,1 psychiatrists per 100,000 individuals
(4). With all the facts and data in
mind, researchers and developers are developing artificial
intelligence (AI) treatments that evoke feelings of emotional
security precisely because of their accessibility and widespread
sense of emotional safety due to their accessibility and widespread
engagement.
The recent decades have witnessed great progress in
AI technology (AI is increasingly a part of digital medicine),
which opens up the possibility of applying AI both, in the
diagnosis and therapy of mental disorders. The term ‘artificial
intelligence’, which is defined as ‘the science and engineering of
making an intelligent machine’ was first used by scientists John
McCarthy, Marvin Minsky, Nathaniel Rochester and Claude Shannon in
1955(5). The use of AI in mental
health care is still limited, especially compared with the
application of AI in radiology, oncology and ophthalmology
(6,7). AI could play a key role in choosing
the best antidepressant therapy for a patient, in the sense that
based on scans of brain activity, it can accurately predict whether
an antidepressant will work. AI has enormous potential to define
diagnosis in psychiatry and to help improve understanding of mental
illness (8).
In the 4RI, there are a few treatments in
psychiatry, even in medicine, for which one can state longevity and
effectiveness in application as is the case with electroconvulsive
therapy (ECT) (9,10). In the world since 1984, electric
current has been delivered through a digital device (Somatics, LLC
or MECTA LLC) and advances in technology have brought changes in
ECT devices. ECT is still the most effective treatment for severe
mental disorders, especially depressive disorders (11). Despite evidence of safety and
effectiveness of ECT, applicability in practice is insufficient.
The explanations for the possible discrepancy between real needs
and application can be a misunderstanding on the part of the
non-medical public as well insufficient number of ECT centers and
psychiatrists who apply ECT. According to the latest research, the
percentage of application of ECT therapy (rate of use) was 5.56 per
100,000 patients (12). In Serbia,
~54 patients receive ECT annually, that is, the rate of use is
0.05/100,000(13).
2. A brief overview of the historical
development and improvement of ECT
Until the 18th century, the field of psychiatry was
limited to the classification of types of ‘madness’, and only in
the mid-18th century did they begin to consider the classification
of psychiatric disorders (14). It
was in the 18th century, that Pinel removed the shackles and chains
from the mentally ill at the Bicetre Psychiatric Hospital in Paris
(15,16). Among the drugs used were bromides,
chloral hydrate (19th century), hypnotic barbiturates (in 1903)
along with somatic therapies treatments for severe mental illness,
such as hydrotherapy, lobotomy and insulin coma until the
appearance of antipsychotics and antidepressants (in 1950)
(17).
Convulsive therapy was begun because of the belief
that convulsions helped patients with schizophrenia and this too
has its beginnings in the 16th century, when the Swiss physician
Paracelsus was the first to induce seizures by giving oral doses of
camphor. However, the first scientific account was given in the
18th century by Vienna's University physician Leopold von
Auenbrugger, who reported the beneficial effects on ‘mania vivorum’
of seizures induced by oral camphor (18). However, it was only at the beginning
of the 20th century that the Hungarian neuropathologist Ladislas
Jozef Meduna (1934) started the era of convulsive therapy
(believing that schizophrenia and epilepsy are antagonistic
disorders), by means of an intramuscular injection of camphor for
the treatment of catatonic schizophrenia. The procedure was
performed on conscious patients (without the use of anesthesia and
muscle relaxants), whereby the patient would lose consciousness
during the attack and a frequent adverse effects (AE) of this
procedure was the fracture of long bones (femur, spine), due to
strong, uncontrolled muscle contractions. Due to this, there was a
negative attitude of the public, as well as fear among patients and
employees of institutions often used it to punish and soothe
seriously ill patients (19,20).
The Italian neuropsychiatry professor Ugo Cerletti
used metrazol (cardiazol) to induce convulsions (shock) in animals
and his colleague Lucio Bini used electricity (electroshock; ECS)
as a substitute for metrazol-induced convulsive therapy. Cerletti
had the idea to use electric current to induce convulsions in
individuals. In 1938, Cerletti and Bini began using ECT on patients
and the results were significant after only 10 to 20 treatments. A
year later, in 1939, ECT was introduced to treat mental disorders
in the United States of America (20).
The trend of improving ECT started with the
application of succinyl-choline (1951), the so-called modified ECT,
which eliminated the most frequent AE, related fractures of long
bones. Blatchley (1979) had improved the neurophysiological and
biological aspects of neural refraction, which led to the
replacement of the sinusoidal current (pulse width 8-10 msec) with
a brief electric pulse (pulse width 0.5-1.5 msec) (21,22).
Since 1990, there has been interest in the ultrabrief pulse
waveform (pulse width 0.1-0.2 msec). By applying pulse stimulation,
the current does not pass continuously and only part of the charge
is released, with a sinusoidal current of the same strength
(20,23).
The next step of improvement was the replacement of
the location of the electrodes, after the bilateral [BL; namely
bifrontal (BF) and bitemporal (BT)] placed electrodes, the
unilateral position was also examined, on the right side (RUL),
which led to a reduction of side effects in the cognitive sphere.
In 2001, the American Psychiatric Association (APA) published its
latest report, which emphasizes the importance of informed consent
and the expanded role of ECT in modern medicine (23). The latest research indicates that
RUL ECT is as effective as BT ECT, only on the condition that
higher energy doses are used (24).
ECT application through modern ECT devices has a
number of advantages compared with manual ECT application. The FDA
approved the Somatics Thimatron ECT device (Somatics, LLC) on
September 27, 1984. It is a free-standing, desktop medical
electronic device and contains transformers, electronic displays, a
printer and modular boards with microprocessors, memory chips,
optical isolators and other electronic components. Upon switching
on, it automatically performs its own integrity tests. It delivers
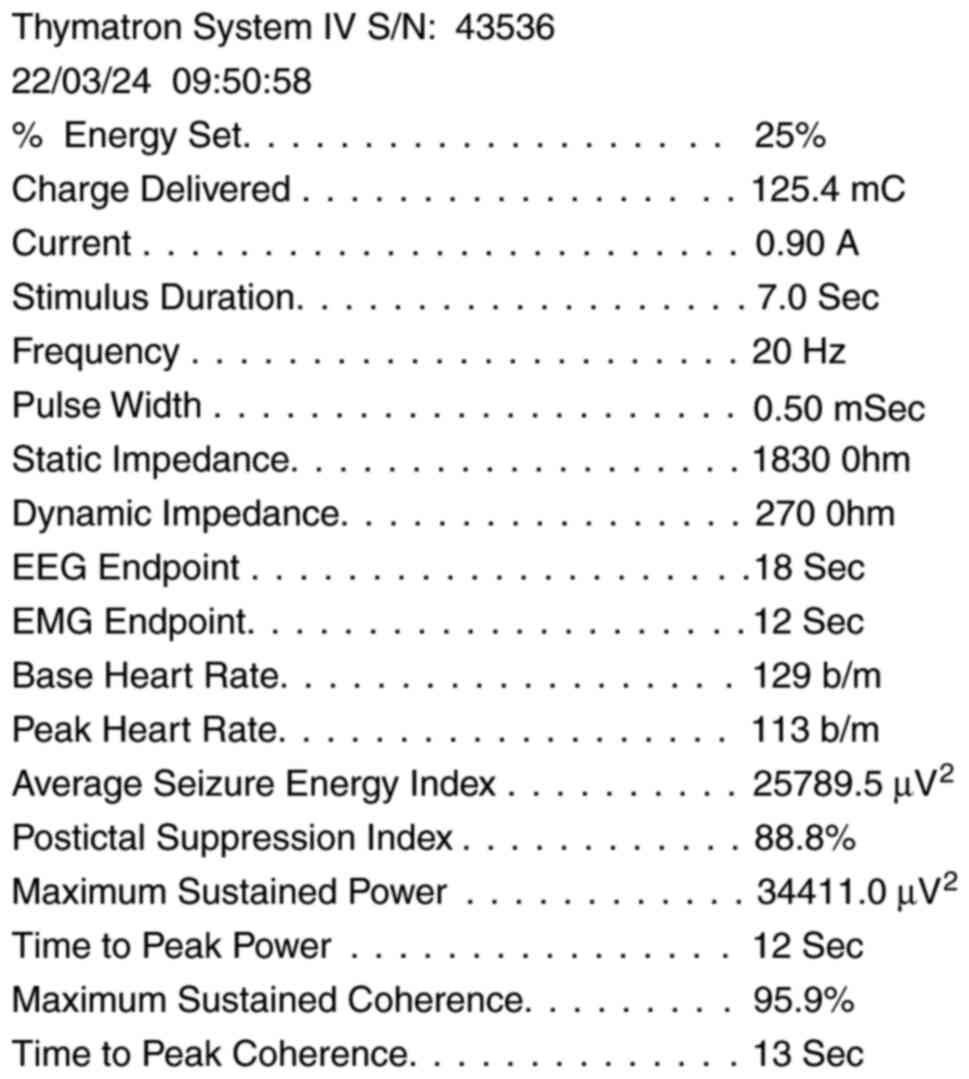
a short pulsed square wave stimulus of constant current 0.9 Amp.
Thymatron IV has all the functions of a sophisticated 4-channel
digital electroencephalogram (EEG) machine, which allows the
recording and analysis of EEG in patients, to measure slowing of
frontal EEG and other EEG manifestations that reflect the effect
and effectiveness of treatment. In addition to EEG, it is possible
to monitor electromyography (EMG) and electrocardiography (ECG).
The Thymatron IV device prints the patient's EEG, ECG, heart rate
and EMG (on the arm on which the cuff is placed; myorelaxant dose
not pass through that arm) during the treatment on a paper strip
that comes out from the front panel of the device (Fig. 1) (23,25).
When it comes to the implementation of ECT in
Serbia, it should be emphasized that in the period 1946-1975, there
were several ECT centers. Due to various organizational
difficulties, since the late 1990s, ECT has been applied
continuously to this day only in the Clinic for Psychiatry,
Military Medical Academy in Belgrade. In the 21st century, another
ECT center was established in Serbia (Clinic for psychiatry,
Clinical center Kragujevac). In Serbia, since 2001, ECT has been
applied via the Thymatron IV device (SOMATICS, LLC), BT ECT, with
the application of short-term general anesthesia (premedication;
atropine, short-acting intravenous anesthetic; propofol, muscle
relaxant; succinyl choline) and signed informed consent by the
patient (26). When the patient is
under general anesthesia and the fasciculations pass, the
psychiatrist applies electrical stimulation via stimulus electrodes
placed on both sides of the forehead in BT ECT (~2.5 cm above the
mean value of the imaginary line drawn from the tragus), in
accordance with data on efficiency BT vs. RUL (26).
3. The place of ECT in the treatment of
mental disorders in the 4IR
Although ECT was started because of the belief that
convulsions help patients with schizophrenia, ECT is the most
effective therapy for the treatment of an affective disorder,
namely a depressive episode in major depressive disorder (MDD) or
depressive episode in bipolar affective disorder. In all previous
studies, the effectiveness and superiority of ECT compared with
antidepressant pharmacotherapy has been proven (11). The explanation for the weaker
response to antidepressant therapy compared with ECT can perhaps be
explained by the role of the cytochrome P-450, 2C19 (CYP2C19)
enzyme, which is involved in the metabolism of most
antidepressants. In CYP2C19 slow metabolizers, the reduction in the
severity of depression was 36% less and the response rate was 75%
lower compared with normal metabolizers (27).
The superiority of ECT refers both to the reduction
of depression and to the speed of recovery; after 1 to 2 courses of
ECT, a clinical improvement is noted. The response to ECT is
between 80 and even 100%, while lower response rates of 50-60% have
been recorded in treatment resistant patients and with RUL ECT
(28).
Due to the availability and effectiveness of
lithium, affective stabilizers and antipsychotics in the treatment
of mania, primary treatment with ECT is rare today, although ECT is
an effective treatment; ~80% of patients show improvement (29).
Patients with schizophrenia have a lower remission
rate on ECT (5-10%), with a higher response if catatonia or
affective symptoms are present (40-80%) (30). Characteristics of schizophrenia,
such as positive symptoms with sudden or recent onset, catatonia or
history of good response to ECT, predict a favorable response to
ECT (31).
ECT is the therapy of choice in malignant
neuroleptic syndrome (MNS) and is a vital indication for patients
with MNS, considering that it can reduce hyperpyrexia, sweating and
delirium, probably by modulating dopamine activity in the brain.
The first effects are usually registered after the fourth course
ECT in a series (32,33).
The APA reports that ECT has no absolute
contraindications (CI), but that a risk/benefit analysis is
necessary for each individual. The latest research indicates that
pheochromocytoma and increased intracranial pressure with mass
effect can be absolute contraindications for ECT; while relative
CIs include increased intracranial pressure without mass effect,
cardiovascular and cerebral conduction disorders (23,34).
Advances in technology have contributed to the fact
that today AI is mostly used to treat depression. As
aforementioned, Thymatron IV has the function of a 4-channel
digital EEG machine, which is gaining importance considering that
EEG is widely used to study mental disorders and given that it is
non-invasive, portable, inexpensive and has a high temporal
resolution. EEG-based deep learning techniques (deep learning is a
class of machine learning algorithms that use multiple layers to
progressively extract higher-level features from raw input) help in
the early detection of patients with MDD, thus contributing to
reducing the adverse effects of MDD. Investigation of the
effectiveness of algorithms (Dimensional Convolutional Neural
Network-DCNN; Long-Short Term Memory Networks-LSTM), evaluated on
the basis of resting EEG data obtained from 30 healthy subjects and
34 patients with MDD, with 90-99.24% accuracy in distinguishing
depressed patients from healthy controls (35).
In addition to diagnostic and predictive purposes,
AI is also used for psychotherapeutic interventions, such as
cognitive-behavioral therapy (Tess application, ‘chatbots’ Sara,
Voebot and Visa). These applications work in the form of a virtual
psychiatric examination, helping patients to identify emotions and
thoughts, to learn improved functioning skills and reduce anxiety.
Research from 2017, in which the average age of the subjects was
22.2±2.33, indicates that these applications can reduce symptoms of
depression (36).
AI with an accuracy >70%, also distinguished
healthy individuals from patients with psychotic disorders; AI can
localize where incoherence occurs in speech and predict levels of
incoherence in psychotic patients compared with controls (37,38).
Research shows that AI improves adherence to therapy in patients
with schizophrenia (≤25%), quickly detects and predicts future
nonadherence to therapy (39).
Virtual reality therapy for 7 weeks in 19 patients with
schizophrenia, during a 3-month follow-up period, helped to improve
symptoms such as visual and auditory hallucinations, symptoms of
depression and improvement of quality of life, especially in
patients with treatment-resistant schizophrenia (40).
The functioning of AI is currently limited, but
further accelerated progress is expected in the near future, which
is especially important due to the recorded shortage of
psychiatrists worldwide.
4. Pharmacotherapy and ECT
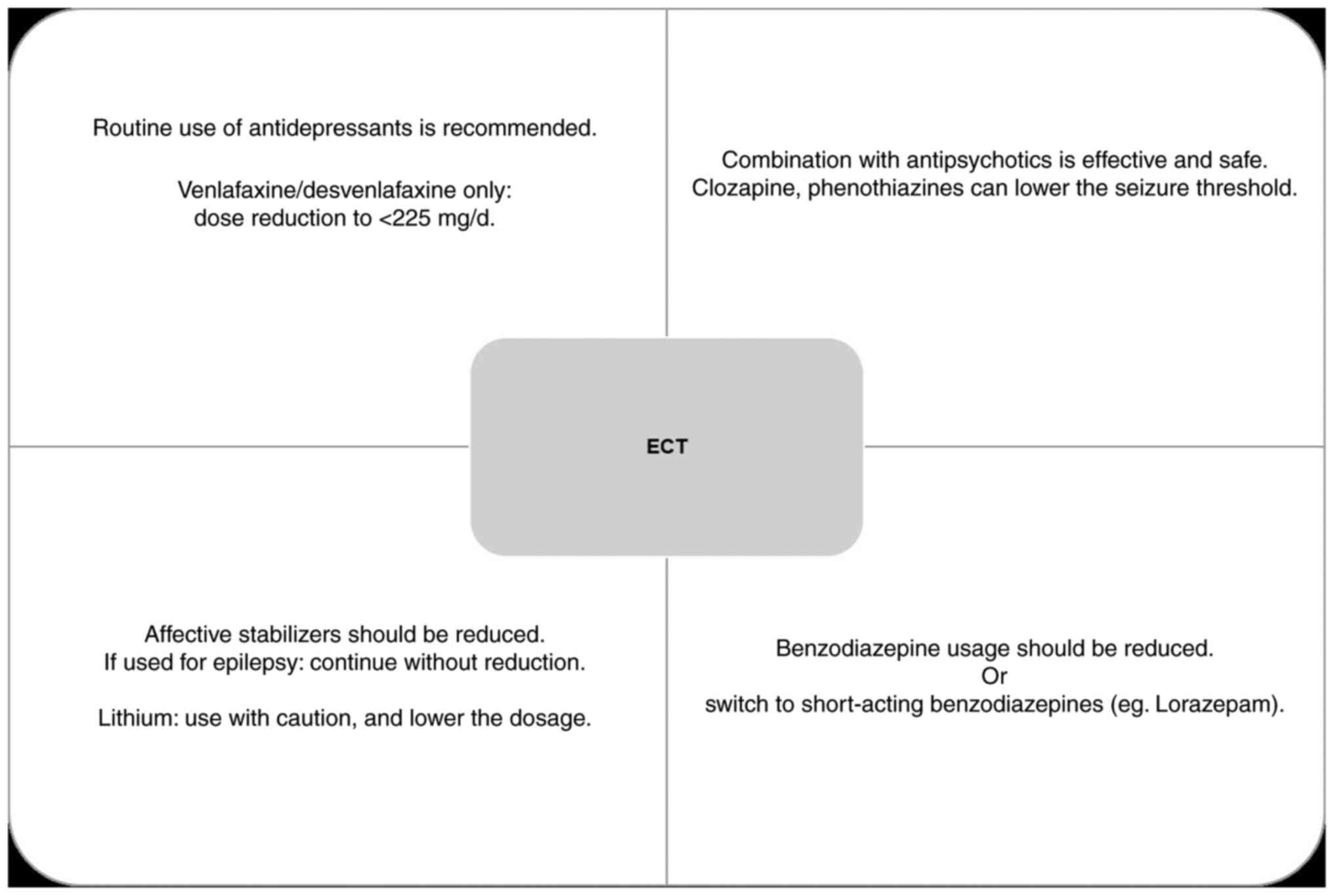
All medications taken by the patient should be
recorded, due to their possible role in increasing morbidity or
reducing the effectiveness of ECT. Although some clinicians
discontinue psychopharmacotherapy before ECT, this is not always
feasible due to the severity of mental disorder (Fig. 2).
Recent meta-analyses indicate a positive effect of
ECT in combination with antidepressants in the treatment of
depressive disorders. Tricyclic antidepressants, selective
serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline
reuptake inhibitors and monoamine oxidase inhibitors have shown
efficacy in combination with ECT. In clinical practice, the routine
use of antidepressants during ECT is recommended (41).
The combination of ECT and antipsychotics is
effective in the treatment of schizophrenia and leads to a
reduction of both positive and negative symptoms. The fastest
response was recorded in the reduction of hallucinations, delusions
and affective flattening. The combined therapy of ECT and
antipsychotics leads to a significant decrease in the severity of
symptoms in ≤60% of patients and also shortens the duration of the
current episode (4:8 months) (42,43).
Concomitant use of lithium and ECT may increase the
risk of cognitive deficits, delirium and spontaneous seizures. That
is why greater caution and the use of lower doses of lithium (if
the combination cannot be avoided) is required. In a randomized
study, a combination of lithium and high-dose, ultrabrief pulse RUL
ECT was shown to be superior to pharmacotherapy, with a relapse
rate of <15% at a 6-month follow-up period (44,45).
Affective stabilizers and benzodiazepines should be
reduced as much as possible, given that they increase the seizure
threshold and can negatively affect the effectiveness of ECT. Not
all affective stabilizers have equal effects in increase the
seizure threshold, e.g. lamotrigine increases the seizure threshold
the least (46,47).
5. Side effects of ECT
General somatic complaints (headache, nausea, muscle
pain) are frequent AE of ECT. However, a high level of animosity
when it comes to ECT, especially among the medical public, is the
prejudice that ECT leads to cognitive impairment. Memory is
special, even sacred for individuals, because it defines one's
sense of self and identity. Therefore if ECT can interfere with
one's personal memories, even just temporarily or to a lesser
extent, it puts it in a special class among medical procedures.
What happens to cognition in
depression and psychotic disorders?
Cognitive functions are impaired in severe mental
disorders. One of the diagnostic criteria for diagnosing depression
is impairment of cognitive functions and when it comes to psychotic
disorders, this deficit is found in negative symptoms. Magnetic
Resonance Imaging (MRI) studies have found that ECT leads to
neuroplasticity and reconnections of neural circuits, rather than
brain damage (48-50).
Only in recent years in 4IR, have methodologically
improved studies appeared, which use computerized
neuropsychological tests to monitor cognition during ECT, the
results of which are of great importance for both researchers and
clinicians (51-55).
The advantages of Cambridge Neuropsychological Test Automated
Battery (CANTAB), compared with other pencil-and-paper
neuropsychological tests, are that it detects and differentiates
frontal from temporal and amygdalo-hippocampal dysfunction, is
sensitive to deficits related to depression, parallel forms of
tests (thus the effect is avoided of learning during repeated
testing in test-retest situations). It is suitable for testing both
younger and older subjects, it is simple, quick to perform (the
subjects have a positive attitude towards it) and the testing
methods are culturally and linguistically independent (51).
The results of research conducted in recent years in
the world, but also in Serbia, using reliable methods of testing
cognition (e.g., CANTAB), indicate that the deficit of cognitive
functions, which is associated with the acute phase of depression,
improves during ECT treatment of depression and reduction of the
severity of depression. Improvements were recorded in the domain of
visual memory and learning [Paired Associates Learning (PAL)] and
initial thinking time for problem solving (one of the executive
functions). In other subdomains of memory no deteriorations are
observed in connection with ECT (51-55).
The improvement of PAL (sensitive to the function of
the medial temporal lobe) and executive functions (sensitive to the
function of the prefrontal cortex) can be interpreted as part of
the overall mood improvement, but this is not the only possible
explanation. It should be borne in mind that the dorsal hippocampus
contains mechanisms that support visual memory and learning and
that this is precisely the area of the brain where neurogenesis
occurs, thus supporting the theory of neurotrophic effects of ECT,
especially in the medial temporal lobe (56).
6. Mechanism of action of ECT
Decades of research have clarified the molecular
basis of ECT action. The research also included a preclinical
(animal) model of experimental ECT, the so-called electroconvulsive
shock and clinical studies. Also reviewed was research conducted
using EEG, Positron Emission Tomography, Single-Photon Emission
Computed Tomography and functional MRI.
During the last decade, much has been learned about
the molecular basis of ECT action. Several hypotheses have been put
forward about the mechanism of the antidepressant effect of ECT:
Stimulation of neurogenesis, restoration of hippocampus volume,
modulation of neurotransmitter and hormone levels, changes in
angiogenesis and cerebral circulation, changes in gene expression,
immune system and functional connectivity (57,58).
When it comes to ECT and neurogenesis, it should be
emphasized that in the hippocampus, plasticity is not limited to
synaptic plasticity, but also includes cellular plasticity, that
is, neurogenesis increases the number of immature neurons and can
increase the excitability of the hippocampus and its connection
with the limbic system. Antidepressants, e.g. SSRIs, work mainly to
increase active neuronal precursors. It is assumed that ECT
activates the ‘silenced’ neural precursors from their ‘resting
phase’ into a proliferative state, thereby increasing the amount of
active stem cells that generate new precursor cells. New neurons
generated following ECT do not differ in fate or phenotype compared
with neurons obtained under physiological conditions and they
integrate into existing networks of neurons and form appropriate
synapses. ECT induces neuroplastic processes in the hippocampus and
amygdala, i.e., it increases the volume of the hippocampus and
amygdala, which are associated with the decrease of depression
symptoms (57,58).
Research on animal models (ECS) has markedly
contributed to increasing the knowledge of ECT influence on
neurotrophic factors (59). ECS
increases the concentration of brain derived neurotrophic factor
(BDNF) protein and the expression of vascular endothelial growth
factor (VEGF) in hippocampus in rats, as well as the concentration
of nerve growth factor (NGF) in several limbic areas (60-62).
Additionally, a significant increase in hippocampal BDNF has been
noticed after a repeat ECS in the second and third week of rat age,
as well as an increase in fibroblast growth factor 2 (FGF-2) after
the third week of rat age in the rhinal cortex, which suggests a
possible maturation of neurotrophic factors on repeated ECS
(63). On the other hand, clinical
research has shown that ECT increases BDNF and VEGF serum levels,
as well as baseline NGF levels (60,64-68).
The lack of statistically significant difference between NGF levels
following the first and the last ECT may be explained by the sample
of therapy resistant schizophrenia patients, given the previous
findings of increased NGF in patients with depression and
schizophrenia (66,67). Namely, baseline levels of NGF
increased consistently following ECT in the group of patients
suffering from depression, while in patients with schizophrenia it
increased only following the first four ECTs (67). ECT also increases FGF2, but
definitive evidence explaining its role in mediating the effects of
ECT is still lacking (58,68). BDNF plays an important role in the
maintenance and survival of neural functions and in
neuroplasticity. Depressed patients have lower levels of BDNF
compared with controls and BDNF levels increased markedly only in
patients who responded to ECT and this increase was maintained even
one month after ECT (64).
ECT reduces the global functional connectivity of
neurons, especially in the dorsolateral prefrontal cortex, as well
as the regional connectivity of neurons in the dorsomedial
prefrontal cortex. Global functional connectivity is a quantitative
parameter that reveals the extent to which a certain part of the
brain is connected to other parts of the brain. Pathological
‘hyperconnectivity’ between intracortical and corticolimbic
circuits is an important aspect of depressive disorders (69).
There are an increasing number of research results
linking inflammation with depression in somatically healthy
subjects (70-72).
In patients with depression, elevated serum levels were measured:
proteins of the acute phase of inflammation (C-reactive protein),
cytokines of inflammation (IL-6 and TNF-α), as well as type 1
immune response cytokines (INF-γ); while anti-inflammatory
cytokines (type 2 immune response), IL-4 and IL-10, are markedly
lower in patients with depression. ECT reduces these differences,
leads to changes in the activity of the immune system and increases
the level of anti-inflammatory cytokines, especially IL-10(73).
7. Conclusions and perspectives
Modern, digitized ECT is still the most
controversial therapy in psychiatry. Almost all the controversy
surrounding ECT originates from sources of information about this
therapy, which are actually anecdotal representations of ECT in
movies and books and are not supported by evidence. Scientific
research related to ECT generally begins with the fact that it is
the most effective therapy for the treatment of severe mental
disorders, especially severe depression.
The reason for emphasizing the effectiveness of ECT
may lie in the need to remind the medical and psychiatric public
that ECT is part of modern psychiatry. The antidepressant
effectiveness of ECT is very high and antidepressant drugs or other
depression treatments (e.g. transcranial magnetic stimulation)
cannot yet be compared with ECT. ECT can save the life of patients
who are severely suicidal, malnourished from depression, or have
catatonic mutism. Therefore the important question is not whether
ECT is good or bad, but whether it can help individuals who truly
need it; ECT is not only an effective treatment, but in some cases
the best we currently have.
On the other hand, it is not an exaggeration to
suggest that the 4RI provided the most powerful tool so far for the
advancement of brain science at the level of human systems and
especially psychiatry. Structural and functional correlates of
numerous mental disorders have been identified, providing a
significant basis for a major step forward towards daily use for
diagnosis, prediction of response to treatment and monitoring of
therapeutic interventions. Tools such as AI, neuroimaging and blood
tests will bring significant change to psychiatry in the coming
years, but that treating the most severe mental problems will
continue to be a challenge.
Acknowledgements
The authors thank Ana Simic, student of Facoltà di
Psicologia Università Cattolica del Sacro Cuore, Milan, Italy and
Professor Vladimir Petkovic, Griffith University, Australia for
language editing.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contribution
ZS conceived the study and revised the manuscript.
ZS, KS, VTO, ZG and APĆ participated in the writing of the
manuscript. ZS and KS reviewed the manuscript. Data authentication
is not applicable. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Zvezdana Stojanović: https://orcid.org/0000-0003-0695-1563.
References
|
1
|
Kontopoulou L, Vasara E, Paraskevadaki E,
Karpetas G, Papathanasiou IV and Gourgoulianis KI: Dietary
supplementation practices among undergraduate students in greece
during the COVID-19 pandemic and their association with
COVID-19-related Anxiety. Mater Sociomed. 35:140–147.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Silva C, Fonseca C, Ferreira R, Pinho L,
Schneider BC, Weidner A, Morgado B and Lopes MJ: Depression in
older adults during the COVID-19 pandemic: A systematic review
protocol. BMJ Open. 12(e065610)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
COVID-19 Mental Disorders Collaborators.
Global prevalence and burden of depressive and anxiety disorders in
204 countries and territories in 2020 due to the COVID-19 pandemic.
Lancet. 398:1700–1712. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kokota D, Lund C, Ahrens J, Breuer E and
Gilfillan S: Evaluation of mhGAP training for primary healthcare
workers in Mulanje, Malawi: A quasi-experimental and time series
study. Int J Ment Health Syst. 14(3)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McCarthy J, Minsky ML, Rochester N and
Shannon CE: A Proposal for the Dartmouth Summer Research Project on
Artificial Intelligence, August 31, 1955. AI Magazine. 27:12–15.
2006.
|
|
6
|
Bi WL, Hosny A, Schabath MB, Giger ML,
Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, et
al: Artificial intelligence in cancer imaging: Clinical challenges
and applications. CA Cancer J Clin. 69:127–157. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zakhem GA, Fakhoury JW, Motosko CC and Ho
RS: Characterizing the role of dermatologists in developing
artificial intelligence for assessment of skin cancer. J Am Acad
Dermatol. 85:1544–1556. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu W, Zhang Y, Jiang J, Lucas MV, Fonzo
GA, Rolle CE, Cooper C, Chin-Fatt C, Krepel N, Cornelssen CA, et
al: An electroencephalographic signature predicts antidepressant
response in major depression. Nat Biotechnol. 38:439–447.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nothdurfter C, Eser D, Schüle C, Zwanzger
P, Marcuse A, Noack I, Möller HJ, Rupprecht R and Baghai TC: The
influence of concomitant neuroleptic medication on safety,
tolerability and clinical effectiveness of electroconvulsive
therapy. World J Biol Psychiatry. 7:162–170. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hays R: The relevance of medical history
to current practice. Aust J Gen Pract. 53:157–160. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Trifu S, Sevcenco A, Stănescu M, Drăgoi AM
and Cristea MB: Efficacy of electroconvulsive therapy as a
potential first-choice treatment in treatment-resistant depression
(Review). Exp Ther Med. 22(1281)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wilkinson ST, Agbese E, Leslie DL and
Rosenheck RA: Identifying recipients of electroconvulsive therapy:
Data from privately insured americans. Psychiatr Serv. 69:542–548.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Spiric Z, Stojanovic Z, Samardzic R,
Milovanović S, Gazdag G and Marić NP: Electroconvulsive therapy
practice in Serbia today. Psychiatr Danub. 26:66–69.
2014.PubMed/NCBI
|
|
14
|
Surís A, Holliday R and North CS: The
evolution of the classification of psychiatric disorders. Behav Sci
(Basel). 6(5)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Berlin I: The Salpetriere Hospital: from
confining the poor to freeing the insane. Am J Psychiatry.
160(1579)2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ruiz-Gomez N and Rosenstock ML: The ties
that bind past and present: Tony robert-fleury, philippe pinel and
the salpêtrière. Foren Sci Int Mind Law. 2(100049)2021.
|
|
17
|
Lehmann HE: Before they called it
psychopharmacology. Neuropsychopharmacology. 8:291–303.
1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Faedda GL, Becker I, Baroni A, Tondo L,
Aspland E and Koukopoulos A: The origins of electroconvulsive
therapy: Prof. Bini's first report on ECT. J Affect Disord.
120:12–15. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fink M: The origins of convulsive therapy.
Am J Psychiatry. 141:1034–1041. 1984.
|
|
20
|
Kaliora SC, Zervas IM and Papadimitriou
GN: Electroconvulsive therapy: 80 years of use in psychiatry.
Psychiatriki. 29:291–302. 2018.PubMed/NCBI View Article : Google Scholar : (In Greek,
Modern).
|
|
21
|
Fink M: Induced Seizures as Therapy in
Man. In Larry R. Squire editor. Encyclopedia of Neuroscience.
Academic Press 117-123, 2009.
|
|
22
|
Leiknes KA, Jarosh-von Schweder L and Høie
B: Contemporary use and practice of electroconvulsive therapy
worldwide. Brain Behav. 2:283–344. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
American Psychiatric Association Task
Force on Electroconvulsive Therapy: The Practice of
Electroconvulsive Therapy: Recommendations for Treatment, Training,
and Privileging. 2nd edition. American Psychiatric Association,
Washington, DC, 2001.
|
|
24
|
Dominiak M, Antosik-Wójcińska AZ, Goetz Z,
Sikorska O, Stefanowski B, Gorostiza D and Święcicki Ł: Efficacy,
safety and tolerability of formula-based unilateral vs bilateral
electroconvulsive therapy in the treatment of major depression: A
randomized open label controlled trial. J Psychiatr Res. 133:52–59.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Waite J and Easton A (eds): The ECT
Handbook. 3rd edition. Royal College of Psychiatrists, London,
2013.
|
|
26
|
Stojanovic Z: Evaluation
ofelectroconvulsive therapyeffects on the
cognitiveandmnesticfunctionsin patients with depression.
(unpublished PhDThesis). Faculty of Medicine, University of
Kragujevac, 2016.(In Serbian).
|
|
27
|
Joković D, Milosavljević F, Stojanović Z,
Šupić G, Vojvodić D, Uzelac B, Jukić MM and Petković Ćurčin A:
CYP2C19 slow metabolizer phenotype is associated with lower
antidepressant efficacy and tolerability. Psychiatry Res.
312(114535)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gurel SC, Mutlu E, Başar K and Yazıcı MK:
Bi-temporal electroconvulsive therapy efficacy in bipolar and
unipolar depression: A retrospective comparison. Asian J Psychiatr.
55(102503)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Elias A, Thomas N and Sackeim HA:
Electroconvulsive Therapy in Mania: A review of 80 years of
clinical experience. Am J Psychiatry. 178:229–239. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sinclair DJ, Zhao S, Qi F, Nyakyoma K,
Kwong JS and Adams CE: Electroconvulsive therapy for
treatment-resistant schizophrenia. Cochrane Database Syst Rev.
3(CD011847)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Grover S, Sahoo S, Rabha A and Koirala R:
ECT in schizophrenia: A review of the evidence. Acta
Neuropsychiatr. 31:115–127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stojanovic Z and Spiric Z: Acute psychosis
followed by fever-Malignant Neuroleptic Syndrome or viral
encephalitis? Vojnosanit Pregl. 71:603–607. 2014.PubMed/NCBI
|
|
33
|
Morcos N, Rosinski A and Maixner DF:
Electroconvulsive therapy for neuroleptic malignant Syndrome: A
case series. J ECT. 35:225–230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Buday J, Albrecht J, Mareš T, Podgorná G,
Horáčková K, Kališová L, Raboch J and Anders M: Brain Tumors and
electroconvulsive therapy: A literature overview of the last 80
years. Front Neurol. 11(723)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Saeedi A, Saeedi M, Maghsoudi A and
Shalbaf A: Major depressive disorder diagnosis based on effective
connectivity in EEG signals: A convolutional neural network and
long short-term memory approach. Cogn Neurodyn. 15:239–252.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fitzpatrick KK, Darcy A and Vierhile M:
Delivering cognitive behavior therapy to young adults with symptoms
of depression and anxiety using a fully automated conversational
agent (Woebot): A Randomized controlled trial. JMIR Ment Health.
4(e19)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Elvevåg B, Foltz PW, Rosenstein M and
Delisi LE: An automated method to analyze language use in patients
with schizophrenia and their first-degree relatives. J
Neurolinguistics. 23:270–284. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Antonucci LA, Raio A, Pergola G, Gelao B,
Papalino M, Rampino A, Andriola I, Blasi G and Bertolino A: Machine
learning-based ability to classify psychosis and early stages of
disease through parenting and attachment-related variables is
associated with social cognition. BMC Psychol. 9(47)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bain EE, Shafner L, Walling DP, Othman AA,
Chuang-Stein C, Hinkle J and Hanina A: Use of a novel artificial
intelligence platform on mobile devices to assess dosing compliance
in a phase 2 clinical trial in subjects with schizophrenia. JMIR
Mhealth Uhealth. 5(e18)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
du Sert OP, Potvin S, Lipp O, Dellazizzo
L, Laurelli M, Breton R, Lalonde P, Phraxayavong K, O'Connor K,
Pelletier JF, et al: Virtual reality therapy for refractory
auditory verbal hallucinations in schizophrenia: A pilot clinical
trial. Schizophr Res. 197:176–181. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pluijms EM, Kamperman AM, Hoogendijk WJ,
Birkenhäger TK and van den Broek WW: Influence of an adjuvant
antidepressant on the efficacy of electroconvulsive therapy: A
systematic review and meta-analysis. Aust N Z J Psychiatry.
55:366–380. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ipekcioglu D, Yazar MS, Canbek O, Yuksel
O, Meterelliyoz KS and Ilnem MC: Electroconvulsive therapy combined
with antipsychotic therapy in the treatment of acute schizophrenia
inpatients: Symptom profile of the clinical response. Psychiatry
and Clinical Psychopharmacol. 28:363–370. 2018.
|
|
43
|
Pawełczyk T, Kołodziej-Kowalska E,
Pawełczyk A and Rabe-Jabłońska J: Effectiveness and clinical
predictors of response to combined ECT and antipsychotic therapy in
patients with treatment-resistant schizophrenia and dominant
negative symptoms. Psychiatry Res. 220:175–180. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Patel RS, Bachu A and Youssef NA:
Combination of lithium and electroconvulsive therapy (ECT) is
associated with higher odds of delirium and cognitive problems in a
large national sample across the United States. Brain Stimul.
13:15–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kellner CH, Husain MM, Knapp RG, McCall
WV, Petrides G, Rudorfer MV, Young RC, Sampson S, McClintock SM,
Mueller M, et al: A novel strategy for continuation ECT in
geriatric depression: Phase 2 of the PRIDE Study. Am J Psychiatry.
173:1110–1118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Delamarre L, Galvao F, Gohier B, Poulet E
and Brunelin J: How much do benzodiazepines matter for
electroconvulsive therapy in patients with major depression? J ECT.
35:184–188. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zolezzi M: Medication management during
electroconvulsant therapy. Neuropsychiatr Dis Treat. 12:931–939.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kalisova L, Kubinova M, Michalec J,
Albrecht J, Madlova K and Raboch J: Cognitive functioning in
patients treated with electroconvulsive therapy. Neuropsychiatr Dis
Treat. 14:3025–3031. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ousdal OT, Brancati GЕ, Kessler U,
Erchinger V, Dale AM, Abbott C and Oltedal L: The neurobiological
effects of electroconvulsive therapy studied through magnetic
resonance: What Have we learned, and where do we go? Biol
Psychiatry. 91:540–549. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jolly AJ and Singh SM: Does
electroconvulsive therapy cause brain damage: An update. Indian J
Psychiatry. 62:339–353. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Falconer DW, Cleland J, Fielding S and
Reid IC: Using the CambridgeNeuropsychological Test Automated
Battery (CANTAB) to assess the cognitive impact ofelectroconvulsive
therapy on visual and visuospatial memory. Psychol Med.
40:1017–1025. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fernie G, Bennett DM, Currie J, Perrin JS
and Reid IC: Detecting objective and subjective cognitive effects
of electroconvulsive therapy: Intensity, duration and test utility
in a large clinical sample. Psychol Med. 44:2985–2994.
2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kalogerakou S, Oulis P, Anyfandi E,
Konstantakopoulos G, Papakosta VM, Kontis D, Theochari E,
Angelopoulos E, Zervas IM, Mellon RC, et al: Episodic Visual
learning/memory and attentional flexibility in patients with major
depressive disorder after clinically effective electroconvulsive
therapy. J ECT. 31:246–252. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Maric NP, Stojanovic Z, Andric S,
Soldatovic I, Dolic M and Spiric Z: The acute and medium-term
effects of treatment with electroconvulsive therapy on memory in
patients with major depressive disorder. Psychol Med. 46:797–806.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Stojanovic Z, Andric S, Soldatovic I,
Dolic M, Spiric Z and Maric NP: Executive function in
treatment-resistant depression before and after electroconvulsive
therapy. World J Biol Psychiatry. 18:624–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
van Buel EM, Sigrist H, Seifritz E, Fikse
L, Bosker FJ, Schoevers RA, Klein HC, Pryce CR and Eisel UL: Mouse
repeated electroconvulsive seizure (ECS) does not reverse social
stress effects but does induce behavioral and hippocampal changes
relevant to electroconvulsive therapy (ECT) side-effects in the
treatment of depression. PLoS One. 12(e0184603)2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Maffioletti E, Carvalho Silva R,
Bortolomasi M, Baune BT, Gennarelli M and Minelli A: Molecular
biomarkers of electroconvulsive therapy effects and clinical
response: Understanding the present to shape the future. Brain Sci.
11(1120)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Singh A and Kar SK: How Electroconvulsive
Therapy Works?: Understanding the neurobiological mechanisms. Clin
Psychopharmacol Neurosci. 15:210–221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
An X and Wang Y: Electroconvulsive shock
increases neurotrophy and neurogenesis: Time course and treatment
session effects. Psychiatry Res. 309(114390)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Polyakova M, Schroeter ML, Elzinga BM,
Holiga S, Schoenknecht P, de Kloet ER and Molendijk ML:
Brain-Derived neurotrophic factor and antidepressive effect of
electroconvulsive therapy: Systematic review and meta-analyses of
the preclinical and clinical literature. PLoS One.
10(e0141564)2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen F, Danladi J, Wegener G, Madsen TM
and Nyengaard JR: sustained ultrastructural changes in rat
hippocampal formation after repeated electroconvulsive seizures.
Int J Neuropsychopharmacol. 23:446–458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Conti G, Gale K and Kondratiev A:
Immunohistochemical evaluation of the protein ekpression of nerve
grovth factor and its TrkA receptor in rat limbic regions after
electroshock seizures. Neurosci Res. 65:201–209. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kim J, Gale K and Kondratyev A: Effects of
repeated minimal electroshock seizures on NGF, BDNF and FGF-2
protein in the rat brain during postnatal development. Int J Dev
Neurosci. 28:227–232. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
van Zutphen EM, Rhebergen D, van Exel E,
Oudega ML, Bouckaert F, Sienaert P, Vandenbulcke M, Stek M and Dols
A: Brain-derived neurotrophic factor as a possible predictor of
electroconvulsive therapy outcome. Transl Psychiatry.
9(155)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang T, Tang X, Wei Y, Xu L, Hu Y, Cui H,
Zeng J, Ye J, Xie Y, Tang Y, et al: Serum angioneurin levels
following electroconvulsive therapy for mood disorders. Bipolar
Disord. 25:671–682. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pacitti F, Bersani G, Aloe L, Caredda M,
Orsi P, Quartini A, Vitali M, Ceccanti M, Tirassa P, Fiore M and
Iannitelli A: Nerve growth factor serum levels in
treatment-resistant schizophrenic patients following
electroconvulsive therapy. Clin Ter. 171:e67–e74. 2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Iannitelli A, Aloe L, Fiore M and Bersani
G: Changes in Nerve Growth Factor plasma levels during
Electroconvulsive Therapy-Variazioni nei livelli plasmatici del
nerve growth factor in risposta alla terapia elettroconvulsivante.
Giornale Italiano di Psicopatologia. 7:275–282. 2001.(In
Italian).
|
|
68
|
Kato M, Okugawa G, Wakeno M, Takekita Y,
Nonen S, Tetsuo S, Nishida K, Azuma J, Kinoshita T and Serretti A:
Effect of basic fibroblast growth factor (FGF2) gene polymorphisms
on SSRIs treatment response and side effects. Eur
Neuropsychopharmacol. 19:718–725. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Deng Y, Li W and Zhang B: Functional
Activity in the Effect of Transcranial Magnetic Stimulation Therapy
for Patients with Depression: A Meta-Analysis. J Pers Med.
13(405)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Křenek P, Hořínková J and Bartečků E:
Peripheral inflammatory markers in subtypes and core features of
depression: A systematized review. Psychopathology. 56:403–416.
2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Foley ÉM, Parkinson JT, Kappelmann N and
Khandaker GM: Clinical phenotypes of depressed patients with
evidence of inflammation and somatic symptoms. Compr
Psychoneuroendocrinol. 8(100079)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lee CH and Giuliani F: The role of
inflammation in depression and fatigue. Front Immunol.
10(1696)2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Yrondi A, Sporer M, Péran P, Schmitt L,
Arbus C and Sauvaget A: Electroconvulsive therapy, depression, the
immune system and inflammation: A systematic review. Brain Stimul.
11:29–51. 2018.PubMed/NCBI View Article : Google Scholar
|