Introduction
Neurogenic bladder (NGB) is a disorder of the lower
urinary tract that can lead to problematic symptoms and
complications including urinary incontinence, increased urinary
frequency and urgency, risk for infection and upper urinary tract
deterioration (1). Globally, the
reported prevalence of spinal cord injury (SCI) ranges from 236 to
1,009 per 1,000,000 individuals in 2011(2), while in Thailand, the incidence ranges
from 5.8 to 23.0 per 1,000,000(3).
The incidence of neurogenic bladder dysfunction varies depending on
the primary cause. Although, to the best of our knowledge, no
epidemiological studies on neurogenic bladder with SCI have been
conducted in Thailand, it is estimated that 70 to 84% of patients
with SCI will develop bladder dysfunction (4,5). The
symptoms of NGB vary and can range from an underactive bladder with
urinary retention or difficulties emptying the bladder to an
overactive bladder with urgency and urge incontinence, depending on
the level of SCI. These symptoms, especially those caused by
neurogenic detrusor overactivity (NDO) and detrusor external
sphincter dyssynergia, can lead to severe complications such as
upper urinary tract dilatation, urinary tract infection or renal
failure (6).
One of the goals of NGB management is to prevent
high detrusor pressure (Pdet), which can lead to upper
urinary tract deterioration (4).
Antimuscarinic drugs, a subtype of anticholinergic drugs, are the
main pharmacological treatment for NDO (7,8).
Multiple choices of antimuscarinic drugs are available, such as
oxybutynin, trospium, tolterodine, darifenacin and solifenacin,
each with its own advantages and disadvantages (7,8). The
present study focused on oxybutynin, which is widely used in
Thailand. Oxybutynin is primarily indicated for the treatment of
NDO and is the most widely prescribed compound for NDO worldwide
(9,10). Oxybutynin is a tertiary amine with
both antimuscarinic and direct muscle relaxant effects. The typical
starting dose for adults is one 5 mg tablet two to three times per
day for immediate release, and the maximum daily dose should not
exceed four 5 mg tablets (up to 45 mg daily for immediate release
as tolerated) (7,11).
Several studies have shown that oral oxybutynin is
effective in controlling symptoms of overactive bladder (12-14).
However, dry mouth is a common side effect in the oxybutynin group
compared with other treatments (13-15).
In Thailand, oxybutynin is the most commonly used first-line drug
in clinical practice due to its cost-effectiveness, (16) but published clinical evidence on
effectiveness is scarce. With guidance for optimal dosage,
cystometric parameters can be better controlled and the incidence
of adverse effects may be reduced.
While there exists a substantial body of evidence
regarding the efficacy of oxybutynin in treating NGB among
individuals with SCI (12-14),
no study to date has established the dose-response relationship of
oxybutynin. The present study aimed to determine the dose response
relationship of oxybutynin for reducing Pdet in patients
with NGB and SCI under real-world clinical care conditions. The
results of this study reflect ‘real-life’ clinical practice and may
provide guidance for the clinical use of antimuscarinic
medications.
Materials and methods
Study design and participants
The study was conducted as a retrospective cohort
study using hospital-based data at Srinagarind Hospital, Khon Kaen
University (Khon Kaen, Thailand), where the data was collected
between January 1999 and December 2016. This study was approved by
the Center for Ethics in Human Research, Khon Kaen University,
Thailand (approval no. HE651472).
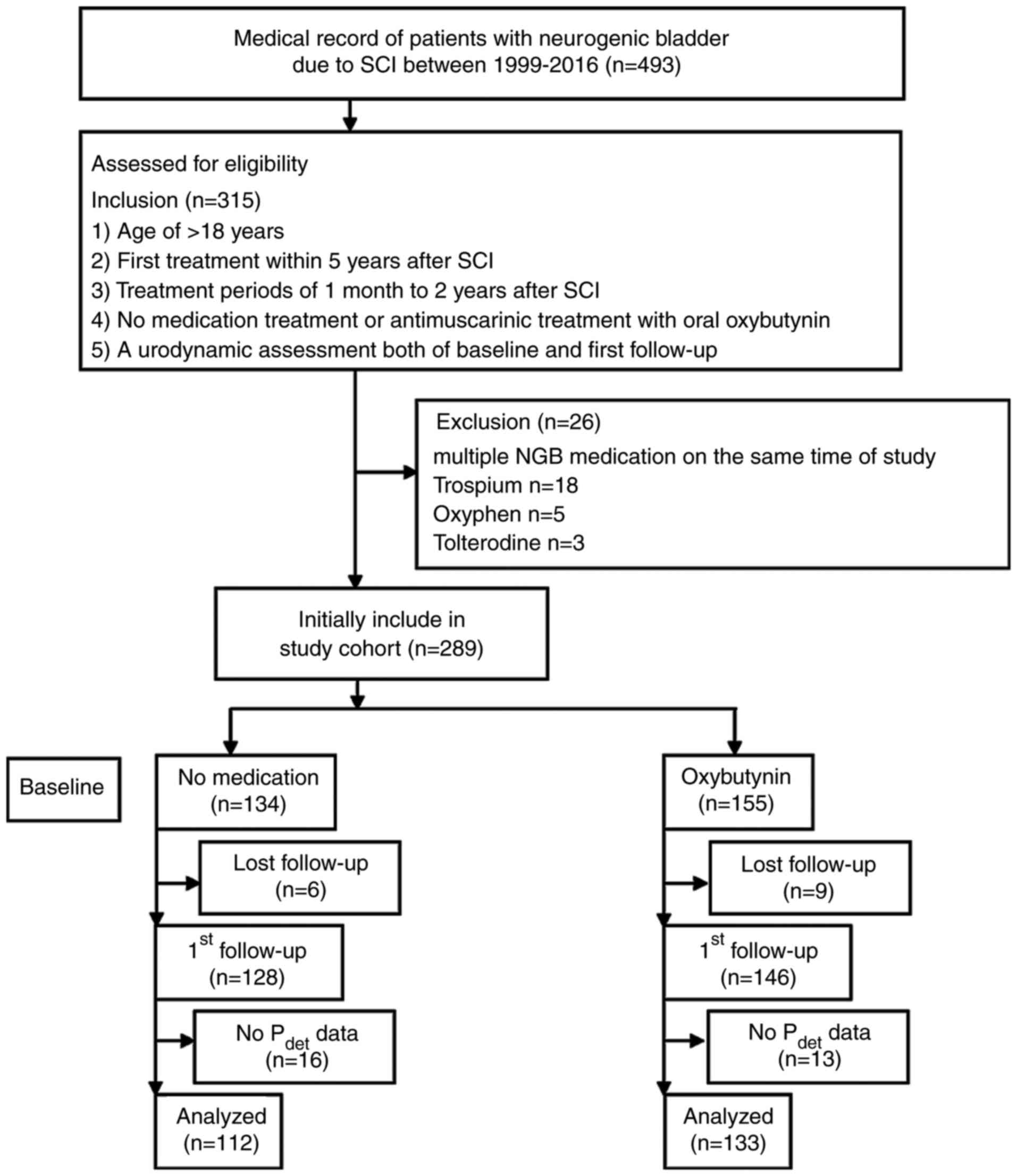
The medical records of neurogenic bladder due to
spinal cord injury were chronologically selected to meet the
following inclusion criteria: i) Patients aged >18 years; ii)
first treatment within 5 years after SCI; iii) duration time after
first treatment from ~1 month to 2 years; iv) either no medication
treatment, or antimuscarinic treatment with oral oxybutynin IR in
variable doses; and v) a urodynamic assessment both of baseline and
first follow up. Patients were excluded based on them receiving
multiple NGB medications at the same time of study. The study
flowchart is depicted in Fig.
1.
Outcomes
The primary outcome was individual Pdet
reduction after various doses of oxybutynin treatment. The
secondary outcome was the change in cystometric capacity after
oxybutynin treatment. Pdet reduction was calculated from
the difference between the highest detrusor pressure measured
during filling cystometry at baseline and follow up. Change in
cystometric capacity was calculated from the difference between
bladder volume at the end of the filling cystometrogram at baseline
and follow up. Urodynamic testing was performed using a
standardized procedure as described previously (17). The study terminology and the
urodynamic parameters followed the International Continence Society
guidelines (18).
Statistical analysis
The sample size was calculated based on the proposed
clinically meaningful Pdet reduction of 10
cmH2O. A standard deviation of 25 with a power of 80%
(two-sided test; α=0.05) was assumed. Based on the sample size
calculation using methods proposed by Borm et al (19), a sample size of 103 has a power of
80% to detect a mean difference of at least 10
cmH2O.
STATA (STATA 18.0 for Windows; StataCorp LP) was
used to perform Analysis of Covariance (ANCOVA) to demonstrate
changes in Pdet or cystometric capacity between the
control group (no medication) and the experiment group (oxybutynin
in various doses). The results are reported as mean ± standard
deviation of pre- and post-treatment, mean difference, 95%
confidence interval (CI) and P-value, based on ANCOVA, adjusted for
baseline Pdet or cystometric capacity value, age
(years), sex, level of SCI, completeness of lesion, vesicoureteral
reflux (VUR) and bladder management. Additionally, analysis was
stratified by bladder management to explore whether bladder
management affects the bladder response to the reduction in
Pdet (ANCOVA). The dose-response relationship between
oxybutynin dosage and reduction in Pdet was established
by plotting a fitted line of the mean Pdet difference
using ANCOVA, with a confidence interval. For all analyses,
significance was set as α=0.05 and two-sided testing was performed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The medical records of 245 eligible patients with
NGB due to SCI were enrolled to the present study. Among the study
patients, 133 were taking oxybutynin, while 112 were not taking any
medication. The age at diagnosis in the oxybutynin group was
43.5±14.8 years vs. 48.1±15.5 years in the no-medication group. The
proportion of women in the oxybutynin group was higher compared
with that in the no medication group (34.6 vs. 25.0%). At baseline,
there was a higher prevalence of VUR in the oxybutynin group
compared with the no-medication group (18.6 vs. 5.0%). There was a
higher proportion of participants with indwelling catheter
management in the oxybutynin group (45.9 vs. 18.8%). The
distribution of level of injury, completeness of lesion, duration
after SCI, duration after first treatment and baseline values of
urodynamic variables are provided in Table I.
 | Table IDemographic characteristics of samples
between treatment groups. |
Table I
Demographic characteristics of samples
between treatment groups.
| Parameter | Oxybutynin | No medication |
|---|
| N patients | 133 | 112 |
| Age at diagnosis,
years | 43.5 (14.8) | 48.1 (15.5) |
| Sex, female/male | 34.6/65.4 | 25.0/75.0 |
| Suprasacral
injury | 88.0 | 77.7 |
| Completeness of
lesion | 35.3 | 19.6 |
| Vesicoureteral
reflux | 18.6 | 5.0 |
|
Duration
after SCI, years | 1.3 (1.3) | 0.8 (1.2) |
|
Duration
after first treatment, years | 1.0 (0.4) | 1.0 (0.3) |
| Bladder
management | | |
|
CIC and
balanced bladder | 54.1 | 81.2 |
|
Indwelling
urinary catheter | 45.9 | 18.8 |
| Baseline values | | |
|
Maximum
detrusor pressure, cmH2O | 52.3 (26.1) | 31.2 (19.0) |
|
Cystometric
capacity, ml | 264.2 (132.1) | 336.6 (156.5) |
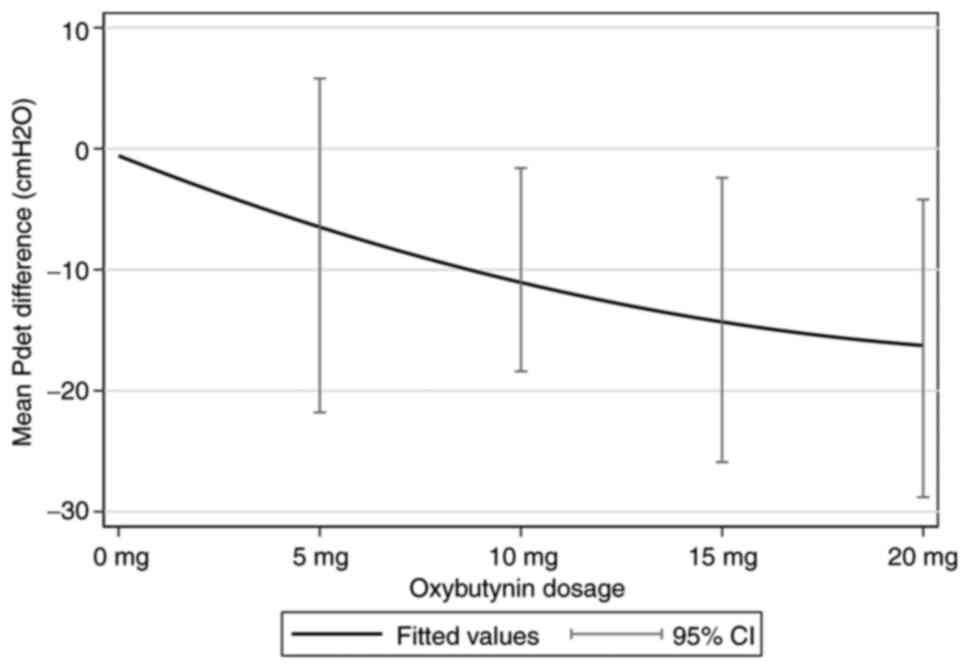
The primary outcome, the dose-response relationship
of oxybutynin for the mean of Pdet reduction, was well
described by ANCOVA, and was adjusted for baseline and age (years),
sex, level of injury, completeness of lesion, VUR and bladder
management. The decrease in Pdet by 1 mg of oxybutynin
was ~0.9 cmH2O (95% CI, -1.4 to -0.3; P=0.002). Fig. 2 shows the fit of the dose-response
relationship for the reduction in Pdet and oxybutynin
monotherapy. The secondary outcome showed that with every 1 mg
oxybutynin increase, the cystometric capacity increased by ~1.3 ml
(95% CI, -1.5 to 4.0; P=0.358). The urodynamic values at pre- and
post-treatment at various oxybutynin doses are presented in
Table II.
 | Table IIMean differences of detrusor pressure
and cystometric capacity, adjusted for their baselines at various
doses of oxybutynin after first time treatment. |
Table II
Mean differences of detrusor pressure
and cystometric capacity, adjusted for their baselines at various
doses of oxybutynin after first time treatment.
| Variable | n | Pre-treatment mean
(SD) | Post-treatment mean
(SD) | Mean difference | 95% CI | P-value |
|---|
| Detrusor
pressure | | | | | | |
|
0 mg
Oxybutynin | 112 | 31.2 (19.0) | 41.4 (27.4) | Reference | | |
|
5 mg
Oxybutynin | 15 | 36.7 (17.3) | 36.2 (22.2) | -8.0 | -21.8 to 5.8 | 0.255 |
|
10 mg
Oxybutynin | 63 | 46.3 (21.8) | 38.1 (20.2) | -10.0 | -18.4 to -1.6 | 0.019 |
|
15 mg
Oxybutynin | 29 | 63.8 (25.7) | 42.3 (28.5) | -14.2 | -25.9 to -2.4 | 0.018 |
|
20 mg
Oxybutynin | 26 | 63.0 (31.8) | 43.4 (27.6) | -16.5 | -28.8 to -4.2 | 0.009 |
|
Every 1 mg
oxybutynin increase | | - | - | -0.9 | -1.4 to -0.3 | 0.002 |
|
Every 5 mg
oxybutynin increase | | - | - | -4.4 | -7.1 to -1.6 | 0.002 |
| Cystometric
capacity | | | | | | |
|
0 mg
Oxybutynin | 112 | 336.6 (156.5) | 305.5 (148.9) | Reference | | |
|
5 mg
Oxybutynin | 15 | 242.4 (158.1) | 229.1 (132.8) | -36.6 | -115.1 to 41.8 | 0.358 |
|
10 mg
Oxybutynin | 63 | 261.2 (129.8) | 282.3 (143.4) | 8.6 | -37.0 to 54.1 | 0.711 |
|
15 mg
Oxybutynin | 29 | 274.4 (134.9) | 319.5 (139.1) | 51.1 | -10.1 to 112.3 | 0.101 |
|
20 mg
Oxybutynin | 26 | 272.8 (124.7) | 236.8 (134.5) | 1.4 | -62.3 to 65.0 | 0.966 |
|
Every 1 mg
oxybutynin increase | | | | 1.3 | -1.5 to 4.0 | 0.358 |
|
Every 5 mg
oxybutynin increase | | | | 6.4 | -7.3 to 20.1 | 0.358 |
The stratified analysis showed the effects of
oxybutynin in patients with either indwelling catheter or clean
intermittent catheterization (CIC) and balanced bladder were
different (Table III). The group
of indwelling catheter management showed that mean Pdet
was reduced during treatment of oxybutynin by 0.5 cmH2O
(95% CI, -1.4 to 0.4; P=0.270), and CIC and balanced bladder by 1.0
cmH2O (95% CI, -1.7 to -0.3; P=0.006). The mean
cystometric capacity increased by 2.9 ml (95% CI, -2.0 to 7.7;
P=0.243) in indwelling catheter with oxybutynin treatment, while it
increased by 0.03 ml (95% CI, -3.4 to 3.5; P=0.985) in patients
without indwelling catheter management.
 | Table IIIMean differences of detrusor pressure
and cystometric capacity, adjusted for their baselines at various
doses of oxybutynin after first time treatment stratified by
catheter management method. |
Table III
Mean differences of detrusor pressure
and cystometric capacity, adjusted for their baselines at various
doses of oxybutynin after first time treatment stratified by
catheter management method.
| Variable | Mean
difference | 95% CI | P-value |
|---|
| Indwelling catheter
(n=75) | | | |
|
Detrusor
pressure | | | |
|
Every
1 mg oxybutynin increase | -0.5 | -1.4 to 0.4 | 0.270 |
|
Every
5 mg oxybutynin increase | -2.5 | -7.1 to 2.0 | 0.270 |
|
Cystometric
capacity | | | |
|
Every
1 mg oxybutynin increase | 2.9 | -2.0 to 7.7 | 0.243 |
|
Every
5 mg oxybutynin increase | 14.3 | -10.0 to 38.7 | 0.243 |
| CIC & balanced
bladder (n=143) | | | |
|
Detrusor
pressure | | | |
|
Every
1 mg oxybutynin increase | -1.0 | -1.7 to -0.3 | 0.006 |
|
Every
5 mg oxybutynin increase | -5.0 | -8.5 to -1.5 | 0.006 |
|
Cystometric
capacity | | | |
|
Every
1 mg oxybutynin increase | 0.03 | -3.4 to 3.5 | 0.985 |
|
Every
5 mg oxybutynin increase | 0.2 | -17.1 to 17.4 | 0.985 |
Discussion
This retrospective cohort study aimed to investigate
the dose-response relationship of oxybutynin for reducing detrusor
pressure in individuals with NGB following SCI under real-world
clinical care conditions. The present study effectively
demonstrated a dose response for oxybutynin: With every 1 mg
increase in dose, Pdet was decreased by 0.9
cmH2O.
Although an indwelling urethral catheter increases
the risk of UTI, renal impairment, bladder stone formation,
urethral stricture, urethral erosion and bladder cancer, some
patients require this bladder management method due to personal
restrictions such as impaired hand function for self-intermittent
cathetherization or limited assistance from a caregiver (20-22).
The present study further stratified patients based on bladder
management methods to explore whether bladder management method
affected the response in Pdet and bladder capacity. The
study found that Pdet in patients with indwelling
catheters responded less to oxybutynin compared with patients
without indwelling catheters (-0.5 cmH2O vs. -1.0
cmH2O for every 1 mg increase in oxybutynin), although
they regained more bladder capacity (2.9 ml). The current study
showed the same trend as the previous study of Kim et al
(23), where patients who require
chronic indwelling catheters for bladder management who take
oxybutynin regularly have improved bladder compliance and lower
bladder leak point pressures.
The usual starting dose of oxybutynin for adults
with NGB is 5 mg (11). The present
study categorized oxybutynin into four groups based on the
available doses in the market. During this study, patients received
doses ranging from 5 to 20 mg/day. Common adverse effects of
oxybutynin are dry mouth, constipation, headache, dyspepsia and dry
eyes (24). In particular,
dose-dependent dry mouth is most commonly reported (25). Several trials and meta-analyses have
shown that oxybutynin has a clinically superior efficacy profile
(26-28),
but it is limited by low tolerance due to side effects, which leads
to higher withdrawals rates compared with other antimuscarinic
medications, such as tolteridine (27). In fact, patients receiving low-dose
oxybutynin should have fewer adverse effects leading to improved
compliance with an antimuscarinic treatment than those receiving
the high dose. Based on our findings, physicians could adjust the
optimal dosage of oxybutynin for individual patients based on their
detrusor pressure and bladder management methods. This will result
in a more precise dosage of anticholinergic treatment for each
patient. For example, if a clinically meaningful reduction of
Pdet of 10 cmH2O is desired in patients with
CIC and balanced bladder, a recommended oxybutynin dosage would be
10 mg. However, if a larger decrease in Pdet is desired,
then increasing the dosage, combining with other anticholinergic
drugs or changing the method of administration, should be
implemented (29,30).
The current retrospective study at a supertertiary
hospital may inherently introduce selection bias. The included
population likely represents a more complex patient profile
compared with a general hospital setting. Additionally, inherent
limitations in retrospective data, including potentially incomplete
records and patients lost to follow-up, may further compromise
generalizability. There were no data regarding the compliance for
medications and medical adverse effects of each participant. Apart
from an improvement in urodynamic data, which may imply reduced
risk of upper urinary tract deterioration in the future, one of the
aims of anticholinergic treatment is to achieve continence to allow
better quality of life in patients without indwelling
catheterization (29,31). However, the present study could not
consider on this aspect due to incomplete medical records.
Hospital-based data from single institutions may not be
generalizable to other settings with different patient
demographics, practices, healthcare infrastructure and resources.
This study's setting in a supertertiary center, which handles more
complex cases, may skew results if the patient population is not
representative of the broader community. The change of cystometric
capacity in relation to oxybutynin dosage should be interpreted
with caution due to the non-linear nature of the relationship. The
duration after SCI may affect the response to antimuscarinic
medication: Since patients began their first antimuscarinic
medication at ~1.3 years after their SCI, they may have a lower
response to medication compared to groups with earlier treatment
(31).
In conclusion, the findings of the present study
offer insights for determining the initial dosage of medications
with variable responses in individuals with neurogenic bladder due
to SCI. We found that increasing the dose by 5 mg resulted in a
decrease of Pdet by 4.4 cmH2O.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors were responsible for the research
conceptualization, study design, and manuscript drafting. SB, JS,
PS and BT were involved in data collection, analysis, and
interpretation. SB and JS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics of approval statement and patient
consent statement
Ethical approval for this study was obtained from
the Khon Kaen University Ethics Committee in Human Research
(approval no. HE651472). The studies were conducted in accordance
with the local legislation and institutional requirements. The
Ethics Committee/Institutional Review Board waived the requirement
of written informed consent for participation from the participants
or the participants' legal guardians/next of kin because due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
References
|
1
|
Ginsberg D: The epidemiology and
pathophysiology of neurogenic bladder. Am J Manag Care.
19:S191–S196. 2013.PubMed/NCBI
|
|
2
|
Cripps RA, Lee BB, Wing P, Weerts E,
Mackay J and Brown D: A global map for traumatic spinal cord injury
epidemiology: Towards a living data repository for injury
prevention. Spinal Cord. 49:493–501. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Przydacz M, Denys P and Corcos J: What do
we know about neurogenic bladder prevalence and management in
developing countries and emerging regions of the world? Ann Phys
Rehabil Med. 60:341–346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dorsher PT and McIntosh PM: Neurogenic
bladder. Adv Urol. 2012(816274)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Manack A, Motsko SP, Haag-Molkenteller C,
Dmochowski RR, Goehring EL Jr, Nguyen-Khoa BA and Jones JK:
Epidemiology and healthcare utilization of neurogenic bladder
patients in a US claims database. Neurourol Urodyn. 30:395–401.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Amarenco G, Ismaël SS, Chesnel C,
Charlanes A and Breton F: Diagnosis and clinical evaluation of
neurogenic bladder. Eur J Phys Rehabil Med. 53:975–980.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cameron AP: Medical management of
neurogenic bladder with oral therapy. Transl Androl Urol. 5:51–62.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sartori AM, Kessler TM, Castro-Díaz DM, de
Keijzer P, Del Popolo G, Ecclestone H, Frings D, Groen J, Hamid R,
Karsenty G, et al: Summary of the 2024 update of the European
Association of Urology guidelines on neurourology. Eur Urol.
85:543–555. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Neurogenic Bladder Turkish Research Group.
Yildiz N, Akkoc Y, Erhan B, Gündüz B, Yılmaz B, Alaca R, Gök H,
Köklü K, Ersöz M, et al: Neurogenic bladder in patients with
traumatic spinal cord injury: Treatment and follow-up. Spinal Cord.
52:462–467. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Szollar SM and Lee SM: Intravesical
oxybutynin for spinal cord injury patients. Spinal Cord.
34:284–287. 1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dwyer J, Tafuri SM and LaGrange CA:
Oxybutynin. In: StatPearls [Internet] Treasure Island (FL),
StatPearls Publishing, 2024.
|
|
12
|
Andersson KE and Chapple CR: Oxybutynin
and the overactive bladder. World J Urol. 19:319–323.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Madersbacher H, Stöhrer M, Richter R,
Burgdörfer H, Hachen HJ and Murtz G: Trospium chloride versus
oxybutynin: A randomized, double-blind, multicentre trial in the
treatment of detrusor hyper-reflexia. Br J Urol. 75:452–456.
1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stöhrer M, Mürtz G, Kramer G, Schnabel F,
Arnold EP and Wyndaele JJ: Propiverine Investigator Group.
Propiverine compared to oxybutynin in neurogenic detrusor
overactivity-results of a randomized, double-blind, multicenter
clinical study. Eur Urol. 51:235–242. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Diokno A, Sand P, Labasky R, Sieber P,
Antoci J, Leach G, Atkinson L and Albrecht D: Long-term safety of
extended-release oxybutynin chloride in a community-dwelling
population of participants with overactive bladder: A one-year
study. Int Urol Nephrol. 34:43–49. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Armstrong EP, Malone DC and Bui CN:
Cost-effectiveness analysis of anti-muscarinic agents for the
treatment of overactive bladder. J Med Econ. 15 (Suppl 1):S35–S44.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sirasaporn P and Saengsuwan J: Incidence
and predictive factors for developing vesicoureteric reflux in
individuals with suprasarcral spinal cord injury: A historical
cohort study. Spinal Cord. 59:753–760. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abrams P, Cardozo L, Fall M, Griffiths D,
Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A and Wein A:
Standardisation Sub-Committee of the International Continence
Society. The standardisation of terminology in lower urinary tract
function: Report from the standardisation sub-committee of the
international continence society. Urology. 61:37–49.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Borm GF, Fransen J and Lemmens WA: A
simple sample size formula for analysis of covariance in randomized
clinical trials. J Clin Epidemiol. 60:1234–1238. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Consortium for Spinal Cord Medicine.
Bladder management for adults with spinal cord injury: A clinical
practice guideline for health-care providers. J Spinal Cord Med.
29:527–573. 2006.PubMed/NCBI
|
|
21
|
De Ruz AE, Leoni EG and Cabrera RH:
Epidemiology and risk factors for urinary tract infection in
patients with spinal cord injury. J Urol. 164:1285–1289.
2000.PubMed/NCBI
|
|
22
|
West DA, Cummings JM, Longo WE, Virgo KS,
Johnson FE and Parra RO: Role of chronic catheterization in the
development of bladder cancer in patients with spinal cord injury.
Urology. 53:292–297. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim YH, Bird ET, Priebe M and Boone TB:
The role of oxybutynin in spinal cord injured patients with
indwelling catheters. J Urol. 158:2083–2086. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lam S and Hilas O: Pharmacologic
management of overactive bladder. Clin Interv Aging. 2:337–345.
2007.PubMed/NCBI
|
|
25
|
Sussman D and Garely A: Treatment of
overactive bladder with once-daily extended-release tolterodine or
oxybutynin: The antimuscarinic clinical effectiveness trial (ACET).
Curr Med Res Opin. 18:177–184. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chapple C, Khullar V, Gabriel Z and Dooley
JA: The effects of antimuscarinic treatments in overactive bladder:
A systematic review and meta-analysis. Eur Urol. 48:5–26.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Harvey MA, Baker K and Wells GA:
Tolterodine versus oxybutynin in the treatment of urge urinary
incontinence: A meta-analysis. Am J Obstet Gynecol. 185:56–61.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shen SH, Jia X, Peng L, Zeng X, Shen H and
Luo DY: Intravesical oxybutynin therapy for patients with
neurogenic detrusor overactivity: A systematic review and
meta-analysis. Int Urol Nephrol. 54:737–747. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Amend B, Hennenlotter J, Schafer T,
Horstmann M, Stenzl A and Sievert KD: Effective treatment of
neurogenic detrusor dysfunction by combined high-dosed
antimuscarinics without increased side-effects. Eur Urol.
53:1021–1028. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pannek J, Sommerfeld HJ, Botel U and Senge
T: Combined intravesical and oral oxybutynin chloride in adult
patients with spinal cord injury. Urology. 55:358–362.
2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hadiji N, Previnaire JG, Benbouzid R,
Robain G, Leblond C, Mieusset R, Enjalbert M and Soler JM: Are
oxybutynin and trospium efficacious in the treatment of detrusor
overactivity in spinal cord injury patients? Spinal Cord.
52:701–705. 2014.PubMed/NCBI View Article : Google Scholar
|