Introduction
Humans are surrounded by microorganisms, such as
bacteria, fungi, protozoa, parasites and viruses (1,2).
Commensal bacterial flora, such as Escherichia coli, also
reside in the human digestive system; however, the spread of this
bacteria to other body parts, including the bloodstream, induces
pathogenicity (3,4), which ultimately causes pathogenic
infections.
Pathogenic microorganisms invade living organisms
with rapid self-division and acquire adaptability in new
environments, including the human body, and cause contagious
diseases. Antibiotics have been used worldwide to cure bacterial
infections (2,5). However, bacteria can evade antibiotics
via mutations, leading to resistance (6).
Commercial antibiotics are a major risk factor for
antimicrobial resistance (AMR) due to overused and other reason was
microorganism mutations has taken place spontaneously (7). Other associated external factors
contribute to AMR, such as overcrowded living conditions and the
consumption of livestock treated with antibiotics (3,4). Most
external infections that occur due to Bacillus spp. are
known as food poisoning (8).
The development of new antibacterial agents can
address the AMR crisis (5).
However, antimicrobial consumption must first be monitored as
prolonged use may trigger the occurrence of AMR (9). The discovery of novel antibacterial
agents from natural products, particularly plants, must be
sustained. Many reports on plant-derived compounds serve as a basis
for new drug development (1,10).
Notably, the use of whole herbs induces an enhanced effect compared
with single compounds; for example the combination compounds of
Ziziphus jujuba polysaccharide and ginger 6-gingerol has
synergistic effect on antioxidant and anticancer, than their dose
alone (11,12).
Pluchea indica (L.) Less from the Asteraceae
family is a native Indonesian plant also found in India and
Thailand (13,14). P. indica exerts beneficial
effects, including antidiabetic (14), antifungal (15), anti-Mycobacterium
tuberculosis (16),
antimicrobial (17) and wound
healing (18) effects. In the
present study, a computational pharmacology network analysis was
performed with molecular docking of P. indica compounds as
potential antibacterial agents against E. coli and B.
subtilis. The present study aimed to highlight promising
compounds in the ethanolic extract of P. indica that can be
explored in drug discovery.
Materials and methods
Plant collection and
identification
Dry P. indica was obtained from the Medicinal
Plant Garden (Surabaya, Indonesia) and validated at the Plant
Systematic Laboratory (Universitas Airlangga, Surabaya, Indonesia).
A voucher specimen was deposited at the Plant Systematic Laboratory
(no. PI0126012024).
Extraction
The leaves of P. indica were air-dried,
ground into a powder (20 mesh size) and macerated in absolute
ethanol (Pro Analysis; Merck KGaA) at a ratio of 1:10. The
maceration process was performed at room temperature (28±2˚C) for
24 h. The extracted products were filtered using a filter paper,
evaporated using a rotary evaporator at 60˚C, weighed to determine
the yield and stored at 4˚C, as previously described (19).
Compound profiling via gas
chromatography-mass spectrophotometry (GC-MS)
Compound profiles of ethanolic extracts of P.
indica leaves were determined using GC-MS. GC-MS analysis was
performed using an Agilent GC-MSD (Agilent Technologies Deutschland
GmbH; cat. no. 19091S-433UI) equipped with a capillary column
(30.00 m x 250.00 µm x 0.25 µm) and a mass detector in electron
impact mode with full scan (50,550 atomic mass unit). Helium was
used as the carrier gas at a flow rate of 3 ml/min (total flow
rate, 14 ml/min). The injector temperature was 280˚C and the oven
temperature ranged from 60 to 250˚C. Peaks in the chromatograms
were identified using the mass spectra. Chemicals were identified
by comparing mass spectra to those in a Standard Reference Database
(version 02. L, National Institute of Standards and Technology).
Components with quality scores >80% were selected. The relative
proportion of each component was estimated from the overall peak
area in the chromatograph, as previously described (20).
In silico pathway analysis of
antimicrobial compounds. Sample retrieval
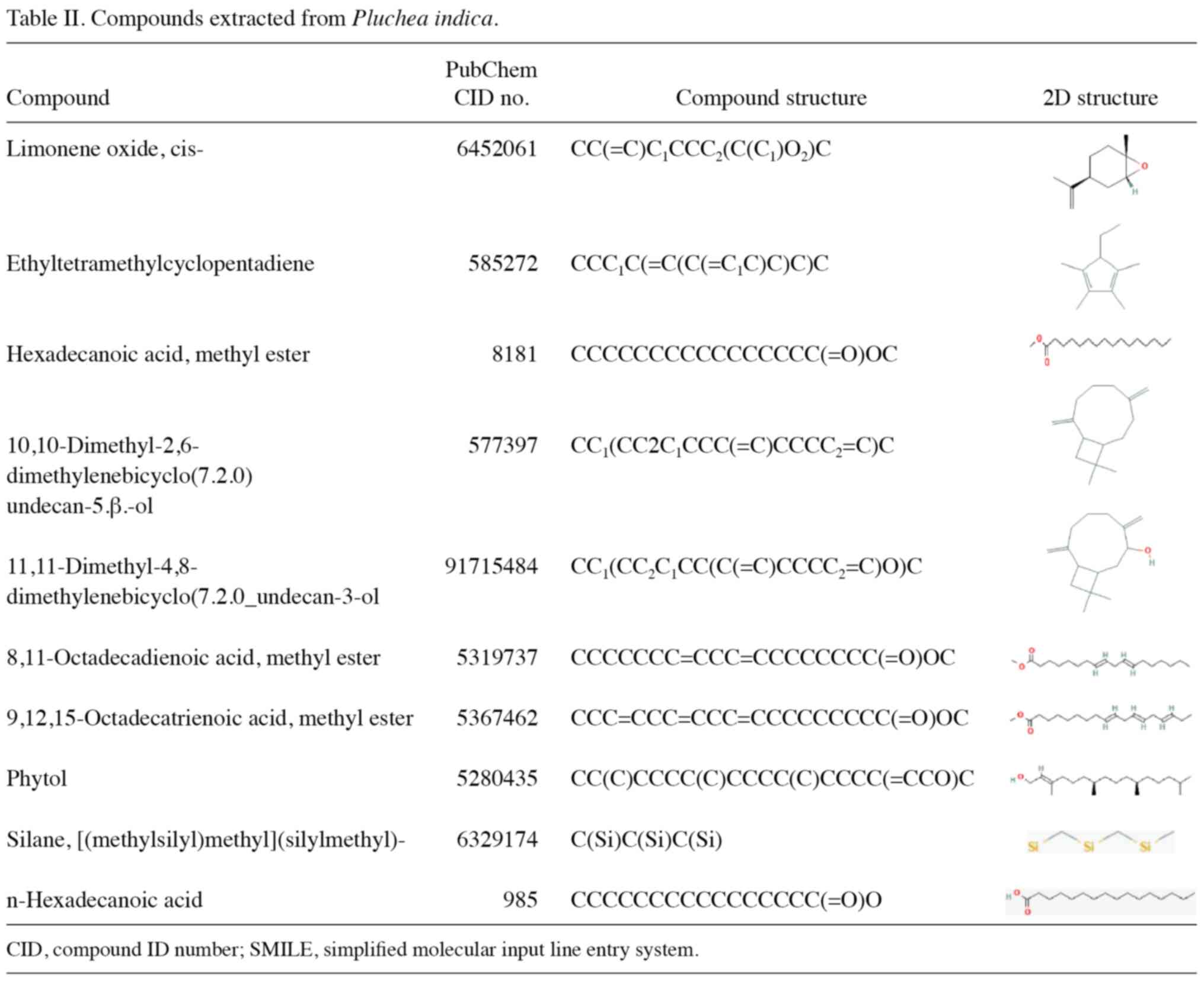
The following compounds were collected via GC-MS:
Limonene oxide, cis-, ethyltetramethylcyclopentadiene; hexadecanoic
acid, methyl ester;
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol;
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0) undecan-3-ol;
8,11-octadecadienoic acid, methyl ester; 9,12,15-octadecatrienoic
acid, methyl ester; phytol; silane,
[(methylsilyl)methyl](silylmethyl)- and n-hexadecanoic acid.
PubChem database (pubchem.ncbi.nlm.nih.gov/) was used to retrieve the
compound ID number (CID), simplified molecular input line entry
system (SMILE) Canonical and 3D files in sdf format for the ligands
(21). The Protein Data Bank (PDB)
file was input in Research Collaboratory for Structural
Bioinformatics Protein Data Bank (RCSB PDB) database (rcsb.org/) for target preparation, which involved
B. subtilis-Filamenting temperature-sensitive mutant Z
(FtsZ) (Z ring) and E. coli-Rhomboid protease
(Rpro). Water molecules and native ligands on targets
were removed using PyMOL software v.2.5.2 (Schrödinger, Inc.) under
an academic license (21,22).
Drug-likeness prediction. Compounds were
assessed as drug-like molecules by referring to drug-likeness
rules, such as Lipinski (23),
Ghose (24), Veber (25), Egan (26) and Muegge (27). Bioavailability score was used to
identify the ability of candidate drug molecules to circulate in
the body. Drug-likeness analysis was performed using SwissADME
(swissadme.ch/) (20,28).
Ligand-protein docking. The binding activity
of the ligand to the target and the interaction pattern were
identified using molecular docking. Docking was performed to
determine the antibacterial potential and inhibitor mechanism
between P. indica compounds and targets. Of note, the grid
covered the entire surface of the target. In addition, the docking
grid consisted of FtsZ: Center(Å) X:28.189 Y: -7.627 Z: -4.629
dimensions (Å) X: 26.235 Y: 21.066 Z: 29.239 & Rpro:
Center (Å) X:15.407 Y: -11.974 Z: 43.963 dimensions (Å) X: 28.085
Y: 21.013 Z: 38.783. The docking simulation was performed using
PyRx 0.9.9 software (Scripps Research) under an academic license
(29,30).
Chemical interaction. The molecular
interactions between the molecular complexes from docking
simulation were identified using Discovery Studio
Visualizer™ v.16.1 (Dassault Systèmes SE). Weak bond
interactions, such as van der Waals, hydrogen, hydrophobic,
π-alkyl, and electrostatic interactions, were formed in the
ligand-protein complex. These interactions contribute to initiation
of an inhibitory response of the ligand toward specific target
domains (31).
Molecular dynamic simulation
Molecular stability analysis or docking validation
was conducted using molecular dynamics simulations in CABS-flex-2
(biocomp.chem.uw.edu.pl/CABSflex2). Molecular stability
was displayed using a root mean square fluctuation (RMSF) graph. To
achieve molecular stability, the protein-ligand complex must have
RMSF value <3 Å (32,33).
Antimicrobial activity assessment.
Media and inoculum preparation
Two bacterial strains, B. subtilis (cat. no.
1248) was purchased from Thailand Institute of Scientific and
Technology Research and E. coli (cat. no. 25922) was
purchased from The American Type Culture Collection, were tested
using antimicrobial assays. Solid and liquid media were used to
conduct antimicrobial experiments and maintain bacterial cultures.
Nutrient agar (NA; Merck, Germany) was used as the solid medium to
test antibacterial activity. NA was used in the reaction tubes to
maintain the microbial culture. Nutrient broth (NB, Merck, Germany)
was employed as liquid media for bacterial strain subcultures and
precultures. The bacterial strains were pre-cultured in culture
bottles containing sterile NB in a 37˚C incubator for 24 h, then
diluted to 10% cultures before inoculation. The absorbance of the
10% bacterial culture was adjusted with sterile water using a
spectrophotometer to meet the 0.5 McFarland standard (19).
Well diffusion assay. Well diffusion assay
was performed to determine the antimicrobial activity based on
diameter of the inhibitory zone (DIZ) on the surface of NA. A total
of 10 ml NA was placed in a sterile Petri dish and allowed to
harden. Thereafter, 30 ml NA was added. Following cooling (25±2˚C)
of the second layer, 1 ml bacterial culture was added to the NA and
the second layer was allowed to completely solidify. A total of
four wells was created on the second layer of the film for samples
and controls. Extracts diluted in 10% DMSO (250 and 500 mg/ml)
served as the samples, 10% DMSO served as the negative control and
chloramphenicol (1000 µg/ml) served as the positive control. Each
well received 10 µl diluted samples; four replicates were prepared.
The plates were incubated at 37˚C for 24 h. DIZ was measured using
Vernier calipers and the mean was calculated and compared with that
of the positive control, as previously described (20). The percentage of inhibition (PI) was
calculated as follows: PI (%)=mean DIZ of the extract/DIZ of
positive control x 100% (1). The
antimicrobial data were obtained from three independent experiments
and are expressed as the mean ± standard deviation.
Results
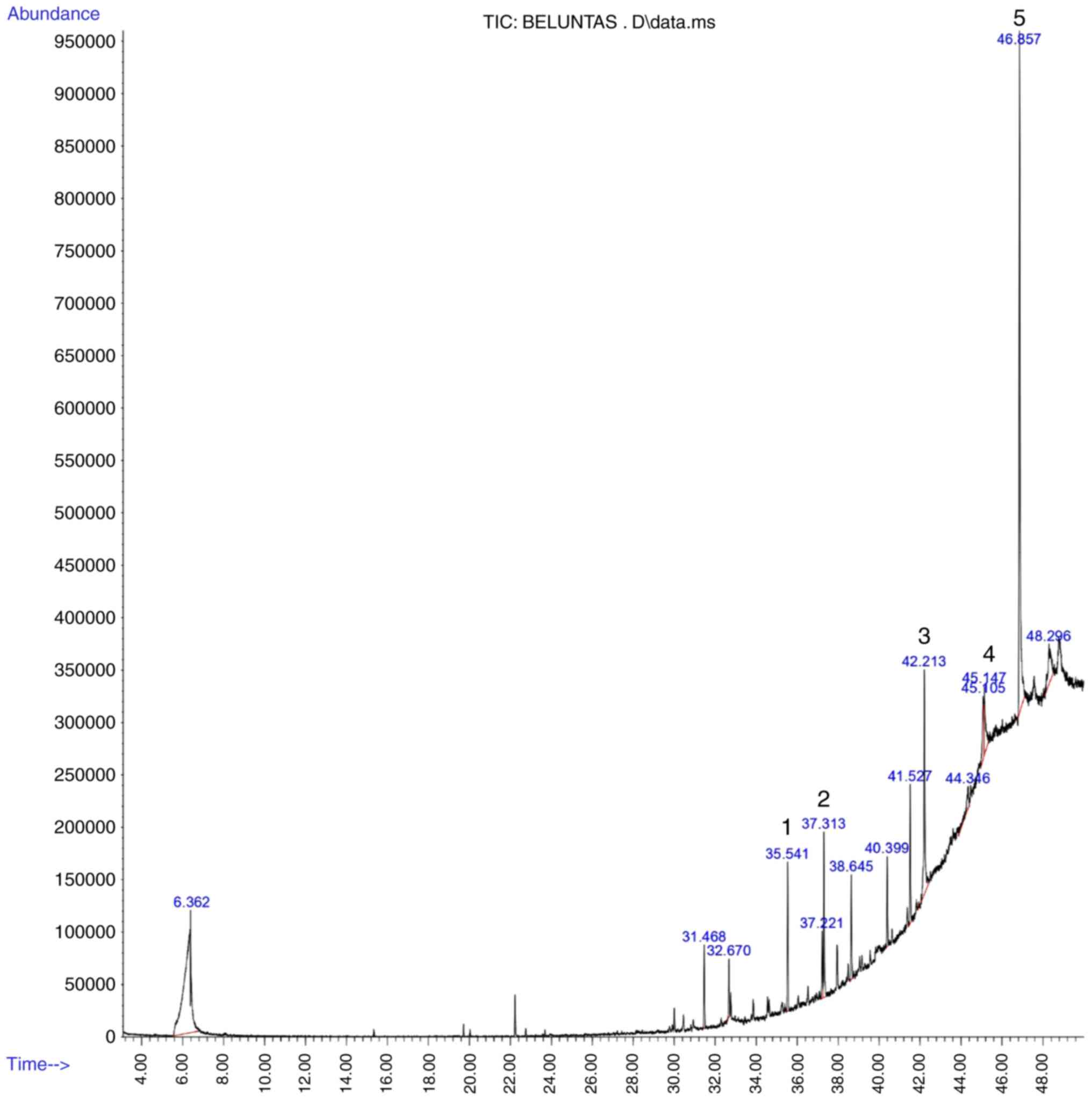
Extraction product and compound
identification
The ethanolic extraction of P. indica dried
leaves yielded ~17.79% crude extract from 88.8 g raw material
(dried leaves). A total of 12 compounds, was identified via GC-MS.
(Table I). Hexadecanoic acid methyl
ester, 11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol,
phytol, silane and n-hexadecanoic acid had a quality score >80%
and higher peak area than other compounds identified via GC-MS
(Fig. 1).
 | Table IGC-MS analysis of Pluchea
indica ethanolic extract. |
Table I
GC-MS analysis of Pluchea
indica ethanolic extract.
| Peak | RT | Compound | Molecular
formula | Molecular weight,
g/mol | Chromatogram peak
area, % |
|---|
| 1 | 31.468 | Limonene oxide,
cis- |
C10H16O | 152.23 | 2.34 |
| 2 | 32.670 |
Ethyltetramethylcyclopentadiene |
C11H18 | 150.26 | 1.32 |
| 3 | 35.541 | Hexadecanoic acid,
methyl ester |
C17H34O2 | 270.45 | 3.88 |
| 4 | 37.221 |
10,10-Dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol |
C15H24O | 220.35 | 1.83 |
| 5 | 37.313 |
11,11-Dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol |
C15H24O | 220.35 | 4.70 |
| 6 | 40.399 |
8,11-Octadecadienoic acid,
methylester |
C19H34O2 | 294.50 | 2.44 |
| 7 | 41.527 |
9,12,15-Octadecatrienoic acid, methyl
ester |
C19H32O2 | 292.50 | 3.82 |
| 8 | 42.213 | Phytol |
C20H40O | 296.53 | 9.33 |
| 9 | 45.147 | Silane,
[(methylsilyl)methyl](silylmethyl)- |
C3H7Si3 | 127.34 | 3.36 |
| 10 | 46.857 | n-Hexadecanoic
acid |
C16H32O2 | 256.42 | 27.36 |
Computational analysis using molecular
docking
The compounds were confirmed using CID, with
information on canonical SMILE and 2-dimentional (2D) structures
from the PubChem (Table II). Two
targets, FtsZ from B. subtilis and Rpro protein
from E. coli, were employed (Fig. 2). Before compound processing with
the target via molecular docking, all compounds were checked for
drug-likeness to assess similarity to an existing drug. Rules, such
as Lipinski's Rule of Five (Ro5), Ghose, Veber, Egan and Muegge,
were also applied. Notably, all compounds in the P. indica
extract were predicted to be drug-like molecules with a
bioavailability score of 0.55, except n-hexadecanoic acid (Table III).
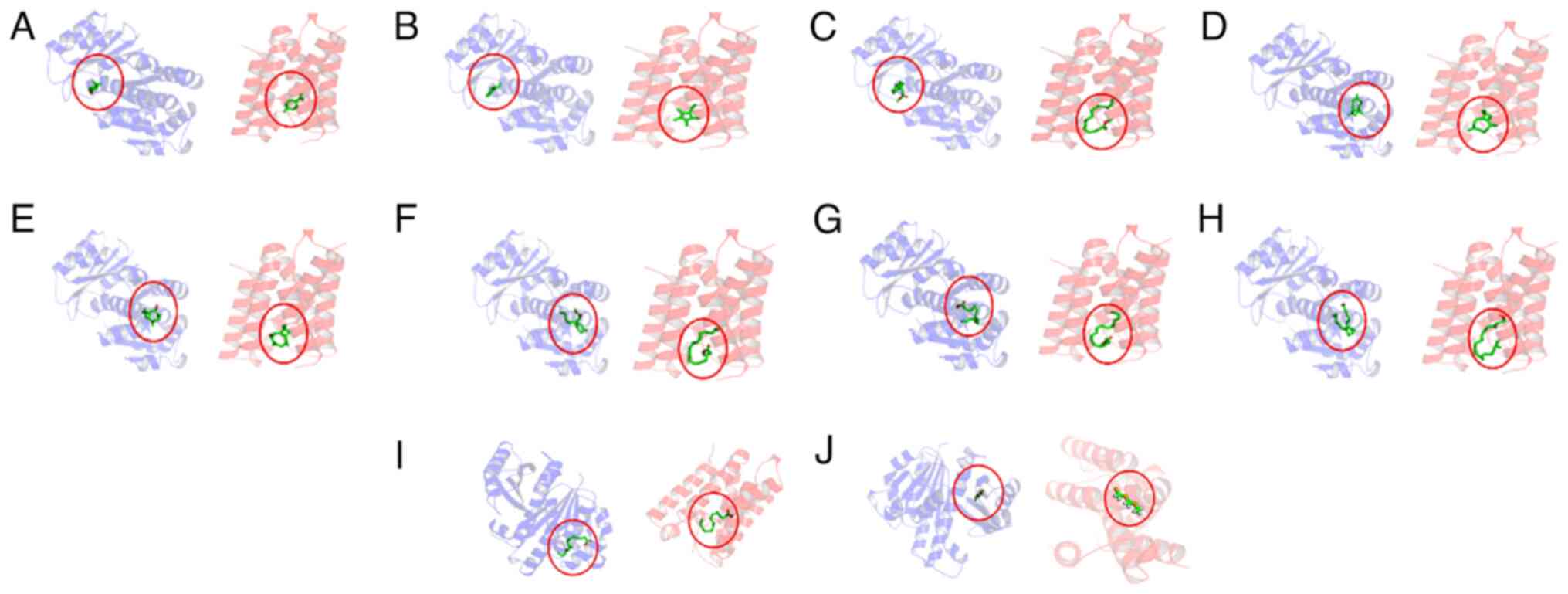 | Figure 2Molecular visualization of compounds
from Pluchea Indica and targets. (A) Limonene oxide, cis-.
(B) Ethyltetramethylcyclopentadiene. (C) Hexadecanoic acid, methyl
ester. (D)
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol. (E)
11,11-Dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol. (F)
8,11-Octadecadienoic acid, methyl ester. (G)
9,12,15-Octadecatrienoic acid, methyl ester. (H) Phytol. (I)
n-Hexadecanoic acid. (J) Silane,
[(methylsilyl)methyl](silylmethyl)-. Green, compound; circle,
Pluchea indica; blue, FtsZ-Bacillus subtilis; red,
Rpro-Escherichia coli. FtsZ, filamenting
temperature-sensitive mutant Z; Rpro, Rhomboid
protease. |
 | Table IIIPluchea indica compounds
predicted as drug-like molecules. |
Table III
Pluchea indica compounds
predicted as drug-like molecules.
| Compound | Lipinski | Ghose | Veber | Egan | Muegge | Bioavailability
score |
|---|
| Limonene oxide,
cis- | Pass | Fail | Pass | Pass | Fail | 0.55 |
|
Ethyltetramethylcyclopentadiene | Pass | Fail | Pass | Pass | Fail | 0.55 |
| Hexadecanoic acid,
methyl ester | Pass | Fail | Fail | Pass | Fail | 0.55 |
|
10,10-Dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol | Pass | Pass | Pass | Pass | Fail | 0.55 |
|
11,11-Dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol | Pass | Pass | Pass | Pass | Fail | 0.55 |
|
8,11-Octadecadienoic acid, methyl
ester | Pass | Fail | Fail | Fail | Fail | 0.55 |
|
9,12,15-Octadecatrienoic acid, methyl
ester | Pass | Fail | Fail | Pass | Fail | 0.55 |
| Phytol | Pass | Fail | Fail | Fail | Fail | 0.55 |
| Silane,
[(methylsilyl)methyl](silylmethyl)- | Pass | Fail | Pass | Pass | Fail | 0.55 |
| n-Hexadecanoic
acid | Pass | Pass | Fail | Pass | Fail | 0.85 |
Interactions of targets from bacterial proteins and
ligands from all P. indica compounds were subjected to
computational analysis using molecular docking. P. indica
compounds with the most negative binding affinities were
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol and
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol (both-6.0
kcal/mol) on FtsZ (PDB ID: 2VAM) for B. subtilis and
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.beta.-ol on
Rpro (PDB ID: 3ZMI) for E. coli (-7.8 kcal/mol;
Table IV). Molecular interaction
between targeted proteins and the pocket-binding domain of ligands
from P. indica was performed and visualized as 3D structure.
Compounds with the most negative binding affinities were
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol and
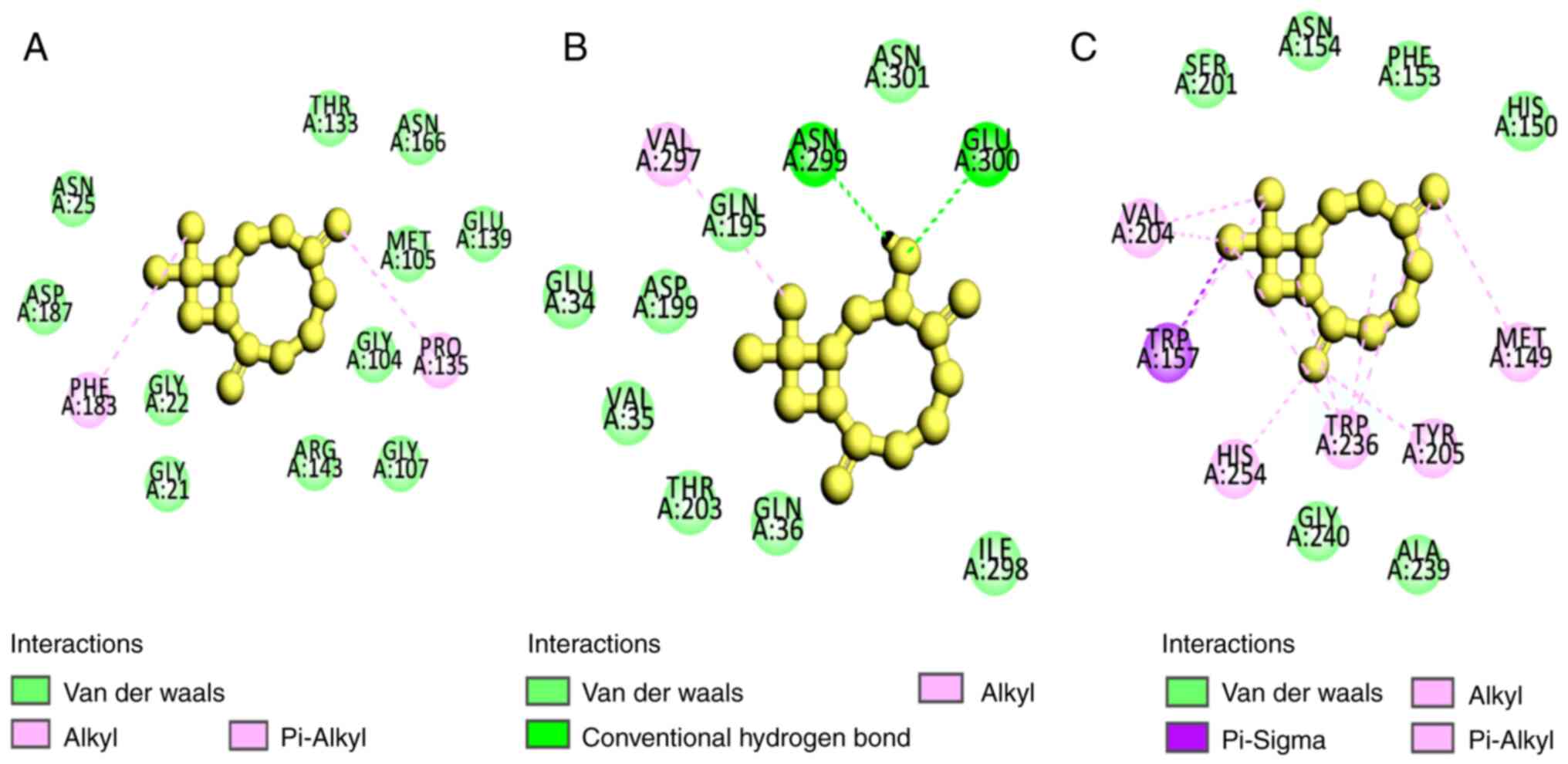
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol (Fig. 2). The analysis also revealed that
the ligands formed weak bonds, such as van der Waals and π-alkyl
bonds for
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol, on
FtsZ-B. subtilis and Rpro-E. coli. For
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol, the weak
bonds were van der Waals, hydrogen and alkyl bonds in FtsZ-B.
subtilis (Fig. 3; Table V).
 | Table IVBinding affinity from the docking
analysis. |
Table IV
Binding affinity from the docking
analysis.
| | Binding affinity,
kcal/mol |
|---|
| Compound | Bacillus
subtilis (FtsZ PDB ID: 2VAM) | Escherichia
coli (Rpro PDB ID: 3ZMI) |
|---|
| Limonene oxide,
cis- | -5.8 | -6.8 |
|
Ethyltetramethylcyclopentadiene | -5.4 | -6.3 |
| Hexadecanoic acid,
methyl ester | -4.9 | -5.8 |
|
10,10-Dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol | -6.0 | -7.8 |
|
11,11-Dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol | -6.0 | -7.2 |
|
8,11-Octadecadienoic acid, methyl
ester | -4.8 | -5.6 |
|
9,12,15-Octadecatrienoic acid, methyl
ester | -4.7 | -6.1 |
| Phytol | -4.7 | -6.1 |
| n-Hexadecanoic
acid | -5.0 | -5.5 |
| Silane,
[(methylsilyl)methyl](silylmethyl)- | -4.5 | -5.0 |
 | Table VMolecular interaction and dynamic
analysis of antimicrobial candidate compounds |
Table V
Molecular interaction and dynamic
analysis of antimicrobial candidate compounds
| | RMSF, Å |
|---|
| Compound | Target | Ligand interaction
domain | Binding site | Mean value of amino
acid residues |
|---|
|
10,10-Dimethyl-2,6-dimethylenebicyclo
(7.2.0)undecan-5.β.-ol | FtsZ PDB ID: 2VAM
(B. subtilis) | van der Waals:
Asn25, Asp187, Gly22, Gly21, Arg143, Gly104, Gly107, Met105,
Glu139, Asn166, Thr133 π-alkyl: Phe183, Pro135 | van der Waals:
0.517, 0.423, 0.390, 0.198, 0.730, 0.292, 1.251, 0.760, 1.295,
0.513, 0.281 π-alkyl: 0.688, 0.564 | 0.608 |
| | Rpro PDB
ID: 3ZMI (E. coli) | van der Waals:
Ser201, Asn154, Phe153, His150, Gly240, Ala239 π-alkyl: Val204,
Trp157, His254, Trp236, Tyr205, Met149 | van der Waals:
0.142, 0.384, 0.238, 1.014, 0.726, 0.897 π-alkyl: 0.726, 0.352,
0.343, 0.623, 0.116, 0.640 | 0.157 |
|
11,11-Dimethyl-4,8-dimethylenebicyclo
(7.2.0)undecan-3-ol | FtsZ PDB ID: 2VAM
(B. subtilis) | van der Waals:
Glu34, Asp199, Val35, Thr203, Gln36, Ile298, Asn301, Gln195
Hydrogen: Asn299, Glu300 Alkyl: Val297 | van der Waals:
1.323, 1.180, 0.912, 1.462, 1.107, 0.707, 2.226, 0.099 Hydrogen:
0.964, 1.615 Alkyl: 0.449 | 1.095 |
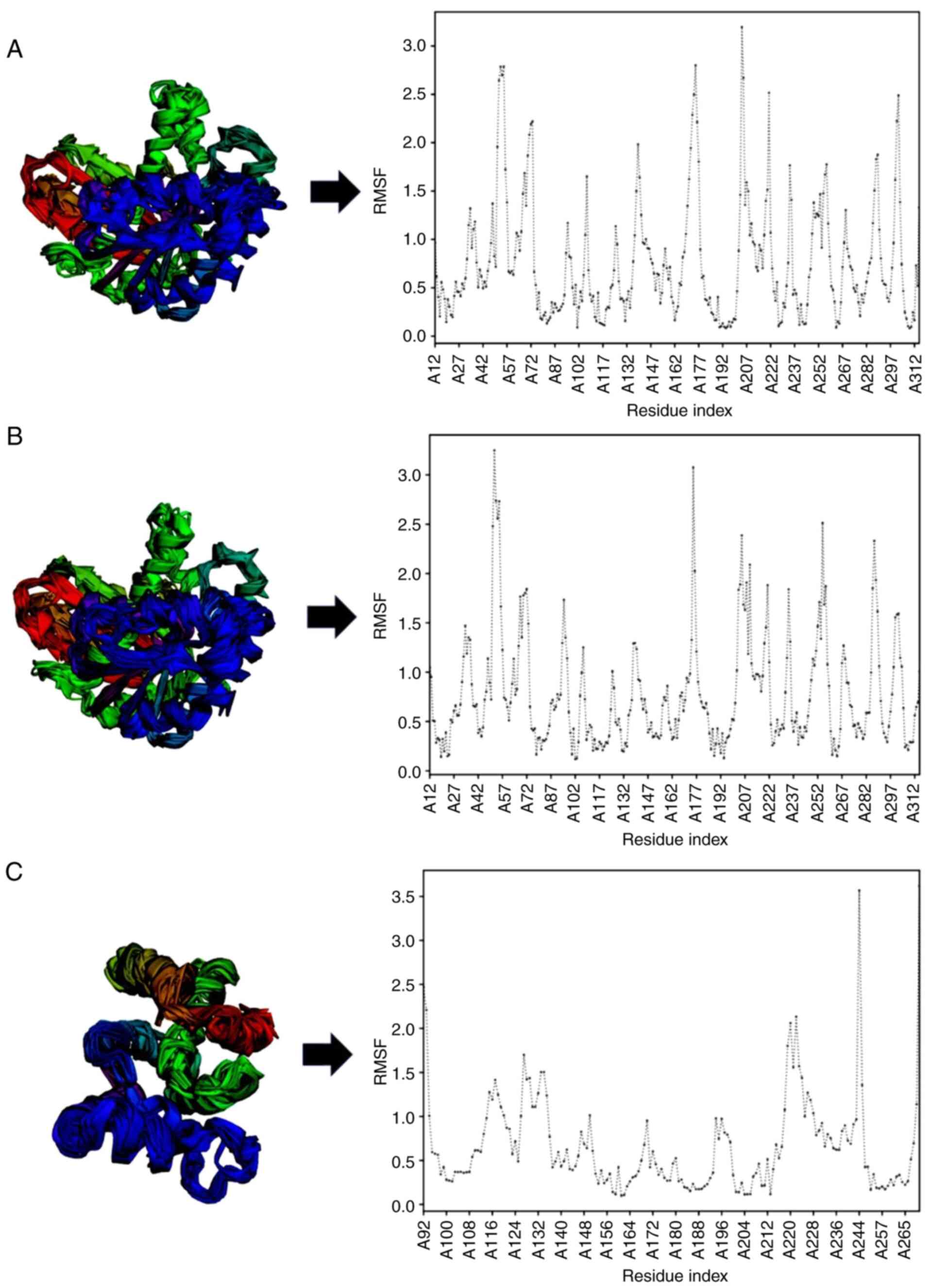
To determine the stability of protein-ligand
binding, docking validation was performed via molecular dynamics
simulation by referring to the RMSF value. The RMSF values from
binding site on ligand interaction domains were <2 Å, indicating
stability. The RMSF value was 0.608 Å for the
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol_FtsZ
complex, 0.157 Å for the
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol_FtsZ
complex, and 1.095 Å for the
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol_Rpro
complex (Table V). The molecular
interactions were due to van der Waals (Asn25, Asp187, Gly22,
Gly21, Arg143, Gly104, Gly107, Met105, Glu139, Asn166, Thr133) and
π-alkyl bonds (Phe183, Pro135) in the
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β-ol_FtsZ
complex; van der Waals bonds (Ser201, Asn154, Phe153, His150,
Gly240, Ala239) and π-alkyl bonds (Val204, Trp157, His254, Trp236,
Tyr205, Met149) in the
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol_FtsZ
complex and van der Waals (Glu34, Asp199, Val35, Thr203, Gln36,
Ile298, Asn301, and Gln195), hydrogen (Asn299 and Glu300) and alkyl
bonds (Val297) in the
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β-ol_Rpro
complex (Fig. 3). Fig. 4 shows the structural fluctuations
and RMSF graph of the target protein.
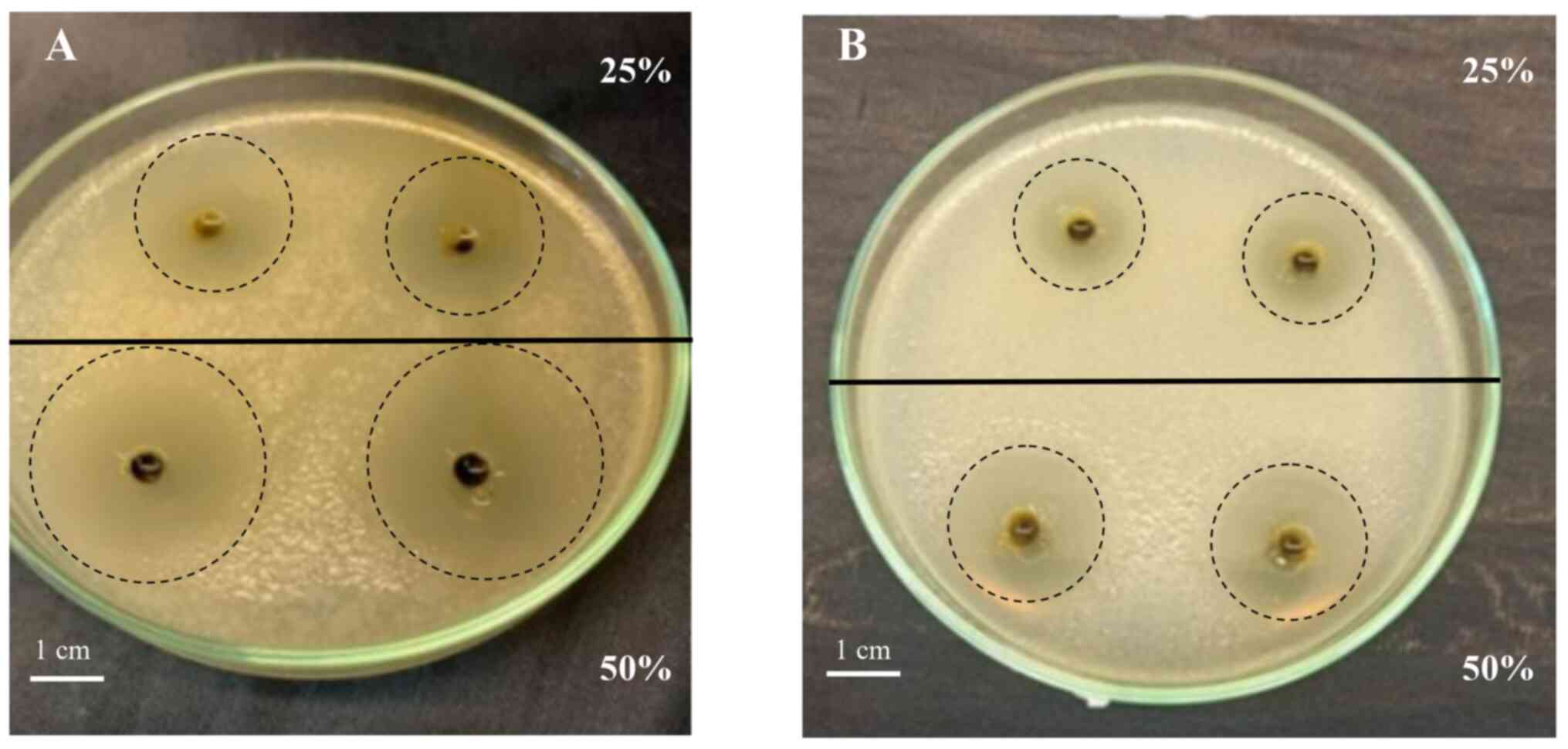
In vitro antibacterial activity based
on well diffusion assay
The antibacterial activity of P. indica
extract was determined using the well diffusion method. Notably,
50% P. indica extract exhibited the strongest inhibitory
activity against E. coli growth (DIZ, 31.86±1.63 mm), with
PI >80% that of the positive control (chloramphenicol).
Moreover, 25% extract exhibited strong inhibitory activity (DIZ,
21.29±1.02 mm), with PI >~50% (Table VI). The 50% extract caused a larger
DIZ than the 25% extract against B. subtilis, with PI
>40% at both concentrations compared with that of the positive
control (Fig. 5). Therefore, the
higher the concentration of the extract, the greater the
antibacterial activity.
 | Table VIWell diffusion of Pluchea
indica ethanolic extract. |
Table VI
Well diffusion of Pluchea
indica ethanolic extract.
| | DIZ, mm | PI, % |
|---|
| Group | E. coli | B.
subtilis | E. coli | B.
subtilis |
|---|
| 25% extract | 21.29±1.02 | 17.76±1.23 | 55.60 | 44.53 |
| 50% extract | 31.86±1.63 | 21.09±0.09 | 82.13 | 50.74 |
|
Chloramphenicol | 38.79±0.43 | 39.88±0.32 | 100.00 | 100.00 |
Discussion
To the best of our knowledge, novel drugs from
plant-derived compounds have not been developed recently. The field
of ethnopharmacology, which involves use traditional medicinal
plants, can be applied in modern medical practice as therapeutic
agent (34,35). P. indica was used as an
antibacterial agent in the present study. Various factors,
including solvents, can affect the proportion of bioactive
compounds in an extract (36,37).
In the present study, P. indica was extracted with ethanol,
a universal solvent, to obtain bioactive compounds with
antibacterial properties. Ethanol extract of P. indica is an
antibacterial agent against B. cereus, E. coli,
Pseudomonas fluorescens, Staphylococcus aureus and
Salmonella typhimurium (38).
In the present study, E. coli was employed as
a representative gram-negative bacterium that commonly causes
infection when it occupies the gastrointestinal and urinary
systems, leading to individuals becoming immunocompromised
(1,39). Gram-positive bacteria, including
Bacillus spp., are commonly detected in the blood, stool and
respiratory systems of infected patients (8). One of the 29 strains of B.
subtilis was previously identified as MDR, with high resistance
to norfloxacin (40).
Plant-derived compounds have gained popularity in
drug development owing to minimal side effects on human health
(41). Phytochemicals in P.
indica extract have been demonstrated to exhibit notable
wound-healing activity (18) and
anti-venom potential (42).
Recently, to accelerate drug discovery, conventional methods, such
as high-throughput screening (HTS) and virtual HTS, have been
developed (16). HTS frequently
produces bulky hydrophobic metabolites that are poorly suited to
chemical alterations (16). Thus,
in silico docking studies are a bioinformatics tool to
define the binding form and binding affinity score (43).
The principal mechanism of docking involves
identifying plant metabolites as candidates for drug improvement
via binding to the protein target of a cell (16,43,44).
In the present study, computational analysis using docking
simulations required bacterial protein targets. Several subcellular
protein targets are present in B. subtilis and E.
coli, including Murein cluster e-B protein (MreB), MreC, The
partitioning motor protein (ParM), Z-associated protein D (ZapD)
and FtsZ (45). MreB and MreC
localize in a helical pattern along the longitudinal axis of the
entire cell (46,47), ParM is present in intercellular
filaments along the cell length (48) and ZapD is focally localized to the
mid-cell of the septum in an FtsZ-dependent manner (49).
FtsZ protein plays a crucial role in bacterial
division and is arranged in a large protein complex in the middle
of dividing cells called divisomes (50). As the regulation and function of the
divisome are dependent on FtsZ, this protein is the first to be
recruited for division (51).
Inhibition of FtsZ may be a promising approach to combat antibiotic
resistance. FtsZ is conserved in most bacteria but absent in
eukaryote cells. The structures of FtsZ protein are available in
the PDB (ID numbers, such as PDB ID: 2VXY, 3VPA, 4DXD, 5H5G) and ID
2VAM, which identified the FtsZ protein from B. subtilis as
the protein target in this present study (51). Rpro is an intramembrane
protease implicated in critical regulation of different cellular
signaling processes (52,53). Thus, the inhibition of these
bacterial proteins inhibits growth (52).
Computational approaches to drug discovery have led
to the development of alternative tools to decrease costs and
determine the effectiveness of potential drug candidates.
Computational screening from ligand-based sources has been
performed to identify potential compound inhibitors for drug
repurposing (16,43). However, information such as CID,
(SMILE) Canonical and 3D files about the candidate ligands must
first be obtained. The present study revealed 10 compounds in the
P. indica leaf extract using GC-MS screening.
Drug-likeness analysis can assist in increasing
probability of a natural chemical progressing through clinical
trials (54). The present study
used Lipinski's Ro5, Ghose, Veber and Muegge. Ro5 assesses
lipophilicity (LogP), molecular mass, hydrogen bonding and molar
refractivity (55). Veber measures
oral bioavailability based on molecular weight, topological surface
area and hydrogen and rotatable bonds. Ghose evaluates drug
likeness using LogP, refractivity and the number of atoms. Muegge
evaluation predicts drug-likeness using drug databases and
pharmacophore calculations (56,57).
Two compounds,
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.beta.-ol and
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol, passed
four (Lipinski, Ghose, Veber, and Egan) of these rules. In this
analysis, a compound must pass at least one rule to proceed to the
next step in the molecular docking and dynamic simulation.
Implementation of drug-likeness rules improves physicochemical and
pharmacokinetic profiles of active substances (58,59).
The Veber's rule-based bioavailability score, which
indicates the capacity for absorption and circulation, determines
the pharmacokinetic profile of drug-like molecules. Here, the best
performance indicator of antibacterial drug effectiveness against
target microorganisms, such as E. coli and B.
subtilis, had a bioavailability score of 0.55 (60,61).
Ligands form interaction patterns or pocket-binding
regions on weakly bonded targets. The binding affinity is the
binding energy of the ligand during its interaction with the
target. According to Gibbs' rule, a lower binding affinity value
indicates increased ligand activity, which means the compound with
the most negative value is the predicted ligand (62,63).
Candidate antimicrobial agents with inhibitory
activity should have a lower (the most negative) binding affinity
(64-66).
Based on docking simulation,
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol and
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol from P.
indica may be antimicrobial drugs with inhibitory activities
against FtsZ and Rpro proteins.
10,10-Dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol is a
terpenoid derivative found in the extracts of Mammea
siamensis flower and young leaves (67); this compound is also one of the
volatile compounds found in the Artemisia argyi fruit
extract (68).
11,11-Dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol (bicyclo
(7.2.0) undecan-3-ol) and 11,11-dimethyl-4,8-bis(methylene)-are
volatile compounds in Achillea millefolium essential oil
(69), propolis (70), and this compound in Syzygium
aromaticum extract has potential as an antifungal and
nematicidal (71). Notably,
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol is
positively associated with antibacterial activity (72).
Further analysis revealed that these two ligands
could form van der Waals, hydrogen, and π-alkyl bonds. Weak-bond
interactions in protein inhibitors, serve a role in promoting
biological responses such as disrupt the regulate enzyme,
interfering the metabolic role and blocking or slowing enzymatic
function. The existence of van der Waals, hydrogen, hydrophobic,
π-alkyl, and electrostatic interactions can increase stability of
ligand-protein interactions (73).
In the present study, docking validation was
performed through molecular dynamics simulation of a ligand-protein
complex. The molecular complexes,
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.-ol_FtsZ,
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol_FtsZ, and
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β-ol_Rpro,
were identified based on their molecular stability. RMSF of
molecular complexes with stable fluctuations is <3 Å (74,75).
In vitro, higher concentrations of the
extract induced stronger antibacterial activity. Based on a
previous study, this activity could be classified as very strong as
DIZ was >15 mm (76). The
compounds in the P. indica leaf extract exhibited strong
antibacterial activity against gram-negative E. coli. The
compounds predicted as potential antibacterial agents were
10,10-dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol and
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol. However,
the present study had some limitations. The in vitro
experiments must be supplemented by additional analysis to confirm
that the compounds have effective activity against the protein
target. The in silico approach requires more extensive
protein screening to ensure compounds affect bacterial cells. The
present study excluded the non-proteins analysis and only focus on
essential bacterial proteins, such as bacterial proteins that serve
key role in division and growth (Rpro and FtsZ). The present study
only compared the compounds in the extract and excluded the
positive control in molecular docking analysis to reduce false
positive and to improve reliability and efficiency as previously
described (77,78).
Overall, 10 compounds were identified in the P.
indica leaf extract.
10,10-Dimethyl-2,6-dimethylenebicyclo(7.2.0)undecan-5.β.-ol and
11,11-dimethyl-4,8-dimethylenebicyclo(7.2.0)undecan-3-ol formed a
ligand-protein complex with FtsZ from B. subtilis and
Rpro from E. coli. Based on in vitro
experiments, the 50% P. indica extract had the strongest
inhibitory effect on the growth of E. coli and B.
subtilis. Therefore, these ligands from P. indica leaf
extract may serve as candidate inhibitors of targeted proteins that
contribute to pathogenicity in the bacterial life cycle.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Universitas
Airlangga (Contract no. 215/UN3.15/PT/2022).
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contributions
DKW, JJ, CR, SS, PP, SP and HP designed the study.
CR, VDK, AJS and CTR performed experiments and analyzed data. DKW,
VDK and AJS wrote and edited the manuscript. DKW, JJ, SS, PP, SP
and HP confirm the authenticity of all the raw data and reviewed
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Periferakis A, Periferakis K, Badarau IA,
Petran EM, Popa DC, Caruntu A, Costache RS, Scheau C, Caruntu C and
Costache DO: Kaempferol: Antimicrobial properties, sources,
clinical, and traditional applications. Int J Mol Sci.
23(15054)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hikmawanti NPE, Saputri FC, Yanuar A,
Jantan I, Ningrum RA and Mun'im A: Insights into the anti-infective
effects of Pluchea indica (L.) Less and its bioactive
metabolites against various bacteria, fungi, viruses, and
parasites. J Ethnopharmacol. 320(117387)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Erb A, Stürmer T, Marre R and Brenner H:
Prevalence of antibiotic resistance in Escherichia coli:
Overview of geographical, temporal, and methodological variations.
Eur J Clin Microbiol Infect Dis. 26:83–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mageiros L, Méric G, Bayliss SC, Pensar J,
Pascoe B, Mourkas E, Calland JK, Yahara K, Murray S, Wilkinson TS,
et al: Genome evolution and the emergence of pathogenicity in avian
Escherichia coli. Nat Commun. 12(765)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
World Health Organization (WHO):
Antimicrobial resistance. WHO, Geneva, 2023.
|
|
6
|
Martinez JL: General principles of
antibiotic resistance in bacteria. Drug Discov Today Technol.
11:33–39. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tang KWK, Millar BC and Moore JE:
Antimicrobial resistance (AMR). Br J Biomed Sci.
80(11387)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kobayashi A, Higashi H, Shimada T and
Suzuki S: Baseline and seasonal trends of Bacillus cereus
and Bacillus subtilis from clinical samples in Japan. Infect
Prev Pract. 5(100272)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
World Health Organization (WHO):
Optimizing surveillance of antimicrobial consumption (AMC). WHO,
Geneva, 2022.
|
|
10
|
Seidel V: Plant-derived chemicals: A
source of inspiration for new drugs. Plants (Basel).
9(1562)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu Z, Gao R, Li H, Wang Y, Luo Y, Zou J,
Zhao B and Chen S: New insight into the joint significance of
dietary jujube polysaccharides and 6-gingerol in antioxidant and
antitumor activities. RSC Adv. 11:33219–33234. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Habtemariam S: Going back to the good old
days: The merit of crude plant drug mixtures in the 21st century.
Int J Complement Alt Med. 6(00182)2017.
|
|
13
|
Jena GSJP, Mishra R, Nayak S and Satapathy
KB: Addition of five new generic asteraceous members to the flora
of Odisha, India. J Indian Bot Soc. 100:53–61. 2020.
|
|
14
|
Syabana MA, Yuliana ND, Batubara I and
Fardiaz D: Antidiabetic activity screening and nmr profile of
vegetable and spices commonly consumed in Indonesia. Food Sci
Technol. 41:254–264. 2021.
|
|
15
|
Demolsky WL, Sugiaman VK and Pranata N:
Antifungal activity of Beluntas ‘Indian Camphorweed’(Pluchea
indica) ethanol extract on Candida albicans in vitro using
different solvent concentrations. Eur J Dent. 16:637–642.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kumar M, Singh SK, Singh PP, Singh VK, Rai
AC, Srivastava AK, Shukla L, Kesawat MS, Kumar Jaiswal A, Chung SM
and Kumar A: Potential anti-Mycobacterium tuberculosis
activity of plant secondary metabolites: Insight with molecular
docking interactions. Antioxidants (Basel). 10(1990)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cadamuro RD, da Silveira Bastos IMA, Silva
IT, da Cruz ACC, Robl D, Sandjo LP, Alves S Jr, Lorenzo JM,
Rodríguez-Lázaro D, Treichel H, et al: Bioactive compounds from
mangrove endophytic fungus and their uses for microorganism
control. J Fungi (Basel). 7(455)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiangnoon R, Samee W, Uttayarat P,
Jittachai W, Ruksiriwanich W, Sommano SR, Athikomkulchai S and
Chittasupho C: Phytochemical analysis, antioxidant, and wound
healing activity of Pluchea indica L.(Less) branch extract
nanoparticles. Molecules. 27(635)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wahyuni DK, Nariswari A, Supriyanto A,
Purnobasuki A, Punnapayak H, Bankeeree W, Prasongsuk S and Ekasari
W: Antioxidant, antimicrobial, and antiplasmodial activities of
Sonchus arvensis L. leaf ethyl acetate fractions. Pharmacogn J. 14
(Suppl):S993–S998. 2022.
|
|
20
|
Wahyuni DK, Wacharasindhu S, Bankeeree W,
Punnapayak H and Prasongsuk S: In silico anti-SARS-CoV-2,
antiplasmodial, antioxidant, and antimicrobial activities of crude
extracts and homopterocarpin from heartwood of Pterocarpus
macrocarpus Kurz. Heliyon. 9(e13644)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Listiyani P, Kharisma VD, Ansori ANM,
Widyananda MH, Probojati RT, Murtadlo AAA, Turista DDR, Ullah ME,
Jakhmola V and Zainul R: In silico phytochemical compounds
screening of Allium sativum targeting the Mpro of SARS-CoV-2.
Pharmacogn J. 14:604–609. 2022.
|
|
22
|
El Mchichi L, El Aissouq A, Kasmi R,
Belhassan A, El-Mernissi R, Ouammou A, Lakhlifi T and Bouachrine M:
In silico design of novel Pyrazole derivatives containing thiourea
skeleton as anti-cancer agents using: 3D QSAR, drug-likeness
studies, ADMET prediction and molecular docking. Mater Today Proc.
45:7661–7674. 2021.
|
|
23
|
Lipinski CA, Lombardo F, Dominy BW and
Feeney PJ: Experimental and computational approaches to estimate
solubility and permeability in drug discovery and development
settings. Adv Drug Deliv Rev. 23:3–25. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ghose AK, Viswanadhan VN and Wendoloski
JJ: A knowledge-based approach in designing combinatorial or
medicinal chemistry libraries for drug discovery. 1. A qualitative
and quantitative characterization of known drug databases. J Comb
Chem. 1:55–68. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Veber DF, Johnson SR, Cheng HY, Smith BR,
Ward KW and Kopple KD: Molecular properties that influence the oral
bioavailability of drug candidates. J Med Chem. 45:2615–2623.
2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Egan WJ, Merz KM Jr and Baldwin JJ:
Prediction of drug absorption using multivariate statistics. J Med
Chem. 43:3867–3877. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Muegge I, Heald SL and Brittelli D: Simple
selection criteria for drug-like chemical matter. J Med Chem.
44:1841–1846. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Johnson TO, Adegboyega AE, Iwaloye O,
Eseola OA, Plass W, Afolabi B, Rotimi D, Ahmed EI, Albrakati A,
Batiha GE and Adeyemi OS: Computational study of the therapeutic
potentials of a new series of imidazole derivatives against
SARS-CoV-2. J Pharmacol Sci. 147:62–71. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dibha AF, Wahyuningsih S, Ansori ANM,
Kharisma VD, Widyananda MH, Parikesit AA, Sibero MT, Probojati RT,
Murtadlo AAA, Trinugroho JP, et al: Utilization of secondary
metabolites in algae Kappaphycus alvarezii as a breast cancer drug
with a computational method. Pharmacogn J. 14:536–543. 2022.
|
|
30
|
Amer HH, Eldrehmy EH, Abdel-Hafez SM,
Alghamdi YS, Hassan MY and Alotaibi SH: Antibacterial and molecular
docking studies of newly synthesized nucleosides and Schiff bases
derived from sulfadimidines. Sci Rep. 11(17953)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jaszczyszyn I, Bielska W, Gawlowski T,
Dudzic P, Satława T, Kończak J, Wilman W, Janusz B, Wróbel S,
Chomicz D, et al: Structural modeling of antibody variable regions
using deep learning-progress and perspectives on drug discovery.
Front Mol Biosci. 10(1214424)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wijaya RM, Hafidzhah MA, Kharisma VD,
Ansori ANM and Parikesit AA: Makara journal of scienc e. Makara J
Sci. 162(171)2021.
|
|
33
|
Peach R, Arnaudon A and Barahona M:
Relative, local and global dimension in complex networks. Nat
Commun. 13(3088)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pirintsos S, Panagiotopoulos A, Bariotakis
M, Daskalakis V, Lionis C, Sourvinos G, Karakasiliotis I, Kampa M
and Castanas E: From traditional ethnopharmacology to modern
natural drug discovery: A methodology discussion and specific
examples. Molecules. 27(4060)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bhagawan WS, Ekasari W and Agil M:
Ethnopharmacology of medicinal plants used by the Tenggerese
community in Bromo Tengger Semeru National Park, Indonesia.
Biodiversitas J Biol Divers. 24:5464–5477. 2023.
|
|
36
|
Moldovan ML, Iurian S, Puscas C,
Silaghi-Dumitrescu R, Hanganu D, Bogdan C, Vlase L, Oniga I and
Benedec D: A design of experiments strategy to enhance the recovery
of polyphenolic compounds from Vitis vinifera by-products through
heat reflux extraction. Biomolecules. 9(529)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang QW, Lin LG and Ye WC: Techniques for
extraction and isolation of natural products: A comprehensive
review. Chin Med. 13(20)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Srimoon R and Ngiewthaisong S: Antioxidant
and antibacterial activities of Indian marsh fleabane (Pluchea
indica (L.) Less). KKU Res J. 20:144–154. 2015.
|
|
39
|
Kaper JB, Nataro JP and Mobley HL:
Pathogenic Escherichia coli. Nat Rev Microbiol. 2:123–140.
2004.
|
|
40
|
Adamski P, Byczkowska-Rostkowska Z,
Gajewska J, Zakrzewski AJ and Kłębukowska L: Prevalence and
antibiotic resistance of Bacillus sp. isolated from raw
milk. Microorganisms. 11(1065)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dehelean CA, Marcovici I, Soica C, Mioc M,
Coricovac D, Iurciuc S, Cretu OM and Pinzaru I: Plant-derived
anticancer compounds as new perspectives in drug discovery and
alternative therapy. Molecules. 26(1109)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ibrahim SRM, Bagalagel AA, Diri RM, Noor
AO, Bakhsh HT and Mohamed GA: Phytoconstituents and pharmacological
activities of Indian Camphorweed (Pluchea Indica): A
multi-potential medicinal plant of nutritional and ethnomedicinal
importance. Molecules. 27(2383)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kitchen DB, Decornez H, Furr JR and
Bajorath J: Docking and scoring in virtual screening for drug
discovery: Methods and applications. Nat Rev Drug Discov.
3:935–949. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sandeep G, Nagasree KP, Hanisha M and
Kumar MMK: AUDocker LE: A GUI for virtual screening with AUTODOCK
Vina. BMC Res Notes. 4(445)2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sharavanan VJ, Sivaramakrishnan M,
Kothandan R, Muthusamy S and Kandaswamy K: Molecular docking
studies of phytochemicals from Leucas aspera targeting
Escherichia coli and Bacillus subtilis subcellular
proteins. Pharmacogn J. 11:278–285. 2019.
|
|
46
|
Domínguez-Cuevas P, Porcelli I, Daniel RA
and Errington J: Differentiated roles for MreB-actin isologues and
autolytic enzymes in Bacillus subtilis morphogenesis. Mol
Microbiol. 89:1084–1098. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Leaver M and Errington J: Roles for MreC
and MreD proteins in helical growth of the cylindrical cell wall in
Bacillus subtilis. Mol Microbiol. 57:1196–1209.
2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Møller-Jensen J, Jensen RB, Löwe J and
Gerdes K: Prokaryotic DNA segregation by an actin-like filament.
EMBO J. 21:3119–3127. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Durand-Heredia J, Rivkin E, Fan G, Morales
J and Janakiraman A: Identification of ZapD as a cell division
factor that promotes the assembly of FtsZ in Escherichia
coli. J Bacteriol. 194:3189–3198. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Du RL, Sun N, Fung YH, Zheng YY, Chen YW,
Chan PH, Wong WL and Wong KY: Discovery of FtsZ inhibitors by
virtual screening as antibacterial agents and study of the
inhibition mechanism. RSC Med Chem. 13:79–89. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ortiz C, Natale P, Cueto L and Vicente M:
The keepers of the ring: Regulators of FtsZ assembly. FEMS
Microbiol Rev. 40:57–67. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tichá A, Stanchev S, Vinothkumar KR,
Mikles DC, Pachl P, Began J, Škerle J, Švehlová K, Nguyen MTN,
Verhelst SHL, et al: General and modular strategy for designing
potent, selective, and pharmacologically compliant inhibitors of
rhomboid proteases. Cell Chem Biol. 24:1523–1536.e4.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Aljohny BO, Rauf A, Anwar Y, Naz S and
Wadood A: Antibacterial, antifungal, antioxidant, and docking
studies of potential dinaphthodiospyrols from Diospyros lotus Linn
roots. ACS Omega. 6:5878–5885. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jia CY, Li JY, Hao GF and Yang GF: A
drug-likeness toolbox facilitates ADMET study in drug discovery.
Drug Discov Today. 25:248–258. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ononamadu C and Ibrahim A: Molecular
docking and prediction of ADME/drug-likeness properties of
potentially active antidiabetic compounds isolated from
aqueous-methanol extracts of Gymnema sylvestre and Combretum
micranthum. B BioTechnologia (Pozn). 102:85–99. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bickerton GR, Paolini GV, Besnard J,
Muresan S and Hopkins AL: Quantifying the chemical beauty of drugs.
Nat Chem. 4:90–98. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sympli HD: Estimation of drug-likeness
properties of GC-MS separated bioactive compounds in rare medicinal
Pleione maculata using molecular docking technique and SwissADME in
silico tools. Netw Model Anal Health Inform Bioinform.
10(14)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Protti ÍF, Rodrigues DR, Fonseca SK, Alves
RJ, de Oliveira RB and Maltarollo VG: Do drug-likeness rules apply
to oral prodrugs? ChemMedChem. 16:1446–1456. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ursu O, Rayan A, Goldblum A and Oprea TI:
Understanding drug-likeness. WIREs Comput Mol Sci. 1:760–781.
2011.
|
|
60
|
Alghamdi AA, ALAM M and Nazreen S: In
silico ADME predictions and in vitro antibacterial evaluation of
2-hydroxy benzothiazole-based 1,3,4-oxadiazole derivatives. Turk J
Chem. 44:1068–1084. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Okolo EN, Ugwu DI, Ezema BE, Ndefo JC, Eze
FU, Ezema CG, Ezugwu JA and Ujam OT: New chalcone derivatives as
potential antimicrobial and antioxidant agent. Sci Rep.
11(21781)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Aliye M, Dekebo A, Tesso H, Abdo T,
Eswaramoorthy R and Melaku Y: Molecular docking analysis and
evaluation of the antibacterial and antioxidant activities of the
constituents of Ocimum cufodontii. Sci Rep.
11(10101)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ballante F: Protein-ligand docking in drug
design: Performance assessment and binding-pose selection. Methods
Mol Biol. 1824:67–88. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Abishad P, Niveditha P, Unni V, Vergis J,
Kurkure NV, Chaudhari S, Rawool DB and Barbuddhe SB: In silico
molecular docking and in vitro antimicrobial efficacy of
phytochemicals against multi-drug-resistant enteroaggregative
Escherichia coli and non-typhoidal Salmonella spp.
Gut Pathog. 13(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yao X, Hu H, Wang S, Zhao W, Song M and
Zhou Q: Synthesis, antimicrobial activity, and molecular docking
studies of aminoguanidine derivatives containing an acylhydrazone
moiety. Iran J Pharm Res. 20(536)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Saqallah FG, Hamed WM, Talib WH, Dianita R
and Wahab HA: Antimicrobial activity and molecular docking
screening of bioactive components of Antirrhinum majus (snapdragon)
aerial parts. Heliyon. 8(e10391)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kumutanat W, Hongthong S, Thanasansurapong
S, Kongkum N and Chumnanvej N: GC-MS and bioassay-guided isolation
of xanthones from Mammea siamensis. Indones J Chem.
23(716)2023.
|
|
68
|
Ye X, Lu J and Cao L: GC-MS analysis of
volatile components extracted from fruit of Artemisia argyi.
Med Plant. 8:10–13. 2017.
|
|
69
|
Radzhabov GK, Aliev AM, Musaev AM and
Islamova FI: Variability of the constituent composition of
Achillea millefolium essential oils in the wild flora of
Dagestan. Pharm Chem J. 56:661–666. 2022.
|
|
70
|
Jasaitytė J, Kubilienė L, Stankevičius M
and Maruška AS: Determination of propolis volatile compounds by
GC-MS method. The vital nature sign [elektroninis išteklius]: 8th
International scientific conference: Abstract book. Kaunas:
Vytautas Magnus University, 8, 2014. https://www.vdu.lt/cris/entities/publication/797d1f5f-212f-4d6c-8f01-3c6b474c13d6.
|
|
71
|
Carrillo-Morales M, Wong-Villarreal A,
Aguilar-Marcelino L, Castañeda-Ramírez GS, Antonio Pineda-Alegría J
and Hernández-Núñez E: Chemical composition and antifungal and
nematicidal activities of the hexanic and methanolic extracts of
Syzygium aromaticum. ScienceAsia. 49:124–130. 2023.
|
|
72
|
Lin F and Long C: GC-TOF-MS-based
metabolomics correlated with bioactivity assays unveiled seasonal
variations in leaf essential oils of two species in Garcinia L. Ind
Crops Prod. 194(116356)2023.
|
|
73
|
Šudomová M, Hassan STS, Khan H, Rasekhian
M and Nabavi SM: A multi-biochemical and in silico study on
anti-enzymatic actions of pyroglutamic acid against PDE-5, ACE, and
urease using various analytical techniques: Unexplored
pharmacological properties and cytotoxicity evaluation.
Biomolecules. 9(392)2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Samarakoon S: Phosphodiesterase-5 (PDE5)
inhibitory potential of major phytochemicals of withania somnifera
and cardiospermum halicacabum: An in silico comparison with
approved PDE5 inhibitors. Authorea Preprints, 2023.
|
|
75
|
Anyubaga SB, Shallangwa GA, Uzairu A and
Abechi SE: Chemo-informatics applications in the design of novel
7-keto-sempervirol derivatives as SmCB1 inhibitors with potential
for treatment of Schistosomiasis. Heliyon.
10(e23115)2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kačániová M, Galovičová L, Borotová P,
Valková V, Ďúranová H, Kowalczewski PŁ, Said-Al Ahl HAH, Hikal WM,
Vukic M, Savitskaya T, et al: Chemical composition, in vitro and in
situ antimicrobial and antibiofilm activities of Syzygium
aromaticum (Clove) essential oil. Plants (Basel).
10(2185)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Coleman RG, Carchia M, Sterling T, Irwin
JJ and Shoichet BK: Ligand pose and orientational sampling in
molecular docking. PLoS One. 8(e75992)2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Peach ML and Nicklaus MC: Combining
docking with pharmacophore filtering for improved virtual
screening. J Cheminform. 1(6)2009.PubMed/NCBI View Article : Google Scholar
|