Introduction
Cervical spondylotic radiculopathy (CSR) is the most
common type of degenerative disease of the cervical spine,
accounting for 60-70% of all cervical spondylosis cases. This
disease is characterized by upper extremity pain and numbness
(1). Mechanical compression of
nerve roots and release of neuroinflammatory nociceptive factors
(2) are the primary causes of upper
extremity pain. Cervical disc herniation (postero- and
anterolateral) is the primary reason for the compression of nerve
roots (3) and also stimulates
cervical sympathetic nerves, restricts cervical mobility and
induces symptoms (such as headache, vertigo and chest tightness)
(4), thereby impacting quality of
life (5). Cervicogenic headache
(CEH) may occur with CSR, however the incidence rate of CEH within
CSR is elusive (6). One of the most
common reasons is that CEH is not frequently diagnosed in the
context of CSR. Additionally, CSR is associated with lower cervical
vertebra, while CEH is associated with upper cervical vertebra
(7).
The present study aimed to investigate use of
minimally invasive surgery through a lower surgical as treatment
for both CEH associated with upper cervical vertebra and CSR
associated with lower cervical vertebra.
CEH may be anatomically associated with CSR. CEH is
defined as pain perceived in any area of the head caused by a
primary nociceptive source in musculoskeletal tissues innervated by
cervical nerves (6). CEH may occur
alone or with radicular symptoms (6) and its pathogenesis is complex, because
it is associated with the trigeminal nucleus and internal cervical
nerve, as well as intracervical pressure transmission. Studies have
shown that nerve convergence in the cervical trigeminal nucleus is
one of the mechanisms of CEH pathogenesis (7,8); other
pathogenic mechanisms includes pressure transmission within the
upper cervical joint complex (7).
Dysfunction of the cervical joint complex produces abnormal tension
on the spinal dura, leading to pain associated with CEH (9). The treatment target for CSR is
primarily C4-C7 (lower cervical spine) (10). By contrast, CEH is mainly caused by
lesions in the higher cervical spine (C1-C3) (11) and the treatment sites are
predominantly in the upper cervical spine, and traditional
treatment methods using upper cervical techniques typically yield
favorable outcomes (12,13). However, clinical trials have
demonstrated that lower cervical disc herniation may cause CEH and
may be achieved via convergence of pain transmission from lower
cervical nerves to the cervical trigeminal nucleus and nucleus
caudalis (11,14). The NDI score of CEH before and after
treatment (2.32 vs. 0.62) with upper cervical technique such as
anterior cervical fusion has been reported (15). For headache neck disability index
(NDI) scores, 66.7% of patients report a grade 0 rating at 3 months
post-surgery, but this decreases to 60% at 6 and 40% at 12 months
post-surgery (14). According to
the aforementioned studies, CEH remission rate of upper cervical
techniques is slightly higher than lower cervical techniques at 3
months after surgery; however, the sample size of lower cervical
techniques for CEH therapy study was relatively small (n=12) and
CEH remission rate assessment at 6 months after surgery was not
reported so the results need further verification in the future.
CEH and lower cervical disease, such as CSR, may be simultaneously
treated through a lower surgical approach. However, the traditional
upper surgical approach for CEH cannot achieve this combined
treatment.
It is necessary to find robust surgical approaches
for both CSR and CEH. For CSR, surgical treatments include anterior
cervical discectomy and fusion (ACDF), anterior or posterior
cervical foraminotomy, percutaneous plasma disc decompression
(PPDD) and pulsed radiofrequency (PRF) (14,15-20).
Compared with ACDF and foraminotomy, PPDD and PRF both possess
advantages of smaller incision, faster recovery and less anatomical
impact and have been utilized in the treatment of CEH and CSR
(21,22). PRF induces neuromodulation by
providing a low-energy electric field to nerve tissues and
microglia, improving excessive nociceptive signal transmission with
precise localization (23). By
contrast, PPDD ablates the herniated nucleus pulposus using plasma,
effectively preventing recurrence. Thus, PPDD can effectively
relieve compression of nerves and tissue (24). To the best of our knowledge,
however, no studies have assessed these types of surgery for
simultaneous improvement of CEH and upper extremity radicular pain
through a lower cervical approach. Therefore, the present study
aimed to investigate the efficacy and safety of PPDD and PRF
through a lower cervical approach in relieving CEH and radicular
pain of patients with CSR by analyzing clinical outcomes.
Materials and methods
Study population
The present study was approved by the Ethics
Committees of Shanghai Traditional Chinese Medicine Hospital
(Shanghai, China; approval no. 2023SHL-KY-85-01) and Jiashan County
Hospital of Traditional Chinese Medicine (Jiaxing, China; approval
no. 2023007). The study was registered in the Chinese Clinical
Trial Registry database (registration no. ChiCTR2300074113).
Informed consent was obtained by recorded verbal agreement.
Clinical data were retrospectively collected from
patients with CSR (with or without CEH) who received PPDD (n=90,
mean age, 56±12); age range (40,71), male: n=36, female: n=54) or
PRF [n=95, age:54±11; age range (39,69), male: n=38, female: n=57]
in the aforementioned hospitals between January 2022 and December
2022. The electronic medical record retrieval system was used to
collect patient data and surgical information was collected from
surgical records.
Inclusion criteria were as follows: i) Diagnosis at
the time of admission of CSR with or without CEH; ii) computed
tomography and magnetic resonance imaging of the cervical spine
showed signs of nerve root compression due to cervical disc
herniation; iii) surgical therapeutic target was the C5-C8 nerve
root (PRF) or C4-C7 disc (PPDD) and iv) patient was fully compliant
with the study protocol, including attending all scheduled visits
and understanding NRS. Exclusion criteria were as follows: i)
Combination of free disc, severe spinal stenosis and calcification
of the fibrous annulus, ossification of the posterior longitudinal
ligament, carpal tunnel syndrome or frozen shoulder; ii)
combination of paraplegia or partial paralysis, cervical fracture
or dislocation, and cervical instability; iii) history of other
surgery and iv) patients with psychosocial or communication
disorders. CEH was diagnosed according to Cervicogenic Headache
International Study Group criteria as follows: i) Precipitation of
head pain by neck movement and/or sustained awkward head
positioning or by external pressure over the upper neck in the
presence or absence of ii) restriction of the range of motion in
the neck and iii) ipsilateral neck, shoulder, or arm pain of a
rather vague non-radicular nature (25,26).
The sample size was calculated using the PASS 15.0
software (ncss.com/software/pass) (two independent proportions).
The effect size (CEH remission rate at 6 months) obtained from a
pilot study (data not shown) with 20 patients was PPDD)=0.76 and
PRF)=0.51. A total of ≥144 patients (n=72/group) was required to
detect the difference between the two groups with at least P1=0.76,
P2=0.51, 80% power, 20% follow-up loss rate and type 1 error of
0.05.
Surgical techniques. PPDD
The low-temperature (40-70˚C) ablation of the
protruding part of the intervertebral disc was conducted by
percutaneous insertion of the plasma knife head under X-ray
fluoroscopy. The needle tip was accurately inserted into the
intervertebral disc, followed by plasma ablation for 30 sec,
intermission of 150 sec, shrinking for 30 sec+ intermission of 150
sec.
PRF. Under X-ray fluoroscopy, the tip of the
radiofrequency trocar needle was positioned near the diseased nerve
and pulse modulation was performed at 40 V. The needle tip was
located at the nerve root of the intervertebral foramen and the
sensory and motor nerves were tested. The radiofrequency
temperature was ~42˚C and PRF time was ~4 min.
Patient data
The age, sex, body mass index (BMI), preoperative
analgesic use and upper extremity symptoms, history of nerve block
treatment and duration of disease were collected.
Surgical data
The operation site, bleeding volume, operational
process, NRS score and NDI score of upper extremity radicular pain
and CEH events before surgery were collected.
Clinical follow-up
Primary outcomes were CEH remission rate at 6 months
after treatment; secondary outcomes were CEH remission rate at 1
and 3 months after treatment, NRS and NDI score at 1, 3 and 6
months after treatment and postoperative complication rate. NRS and
NDI score of upper extremity radicular pain and CEH remission rate
at 1, 3 and 6 months after surgery were recorded. Telephone
follow-up was performed according to questionnaire including NRS
(0-10 points), NDI (10 questions, 50 points), CEH evaluation and
evaluation of postoperative complications (postoperative hematoma
at the puncture site, infection of the puncture site and
intervertebral disc and new cervical nerve injury).
CEH remission was assessed at 1, 3 and 6 months
after surgery, including the frequency, duration, and pain degree
of CEH. Patients were asked the following: ‘How frequently do you
experience noticeable CEH daily?’, ‘what is the duration of these
episodes?’ and ‘to what extent do you feel your headache has
improved?’ Improvement was defined ≥50% enhancement in ≥2 of these
aspects.
NRS (27) was
utilized to assess upper extremity discomfort symptoms. Total NRS
score was 0-10 points as follows: 1-3, classified as mild pain
(barely interfering with sleep); 4-6, moderate pain (partially
interference with sleep) and 7-10, severe pain (inability to sleep
or waking up in pain during sleep).
NDI scoring system (28) was used to assess the effect of upper
limb disease of CSR on daily life. To ensure comprehensive
assessment across a wide range of activities, in cases where older
patients were not involved in specific activities (such as dancing,
driving, reading), similar activities (such climbing stairs,
sweeping the floor, watching television or using cell phones) were
substituted during the assessment process.
Statistical analysis
Data are presented as the mean ± SD or median and
interquartile range. Normally distributed data were analyzed by
two-way mixed ANOVA, followed by Bonferroni/Sidak's post hoc test.
Skewed data were analyzed using Mann-Whitney U test, followed by
Friedman and Nemenyi's post hoc test and Bonferroni's correction.
Rate of preoperative analgesic use nerve block and CEH remission
(1, 3 or 6 months after surgery) were compared by χ2
test. For postoperative complications analysis, Fisher's exact test
was employed. Linear regression was used to analyze the association
between surgical method (PPDD/PRF) and NRS/NDI scores. Multivariate
logistic regression analysis was used to assess association between
surgical method (PPDD/PRF) and CEH remission rate. For cases that
could not be involved in the final statistical analysis due to
missing objective data during the follow-up, deletion was performed
if the number of cases with missing data was <15. If the number
of missing data was >15 but ≤20% of the total, it was attempted
to interpolate the missing values. P<0.05 was considered to
indicate a statistically significant difference. The statistical
analysis was conducted using SPSS 26.0 software (IBM Corp.).
Results
Patients
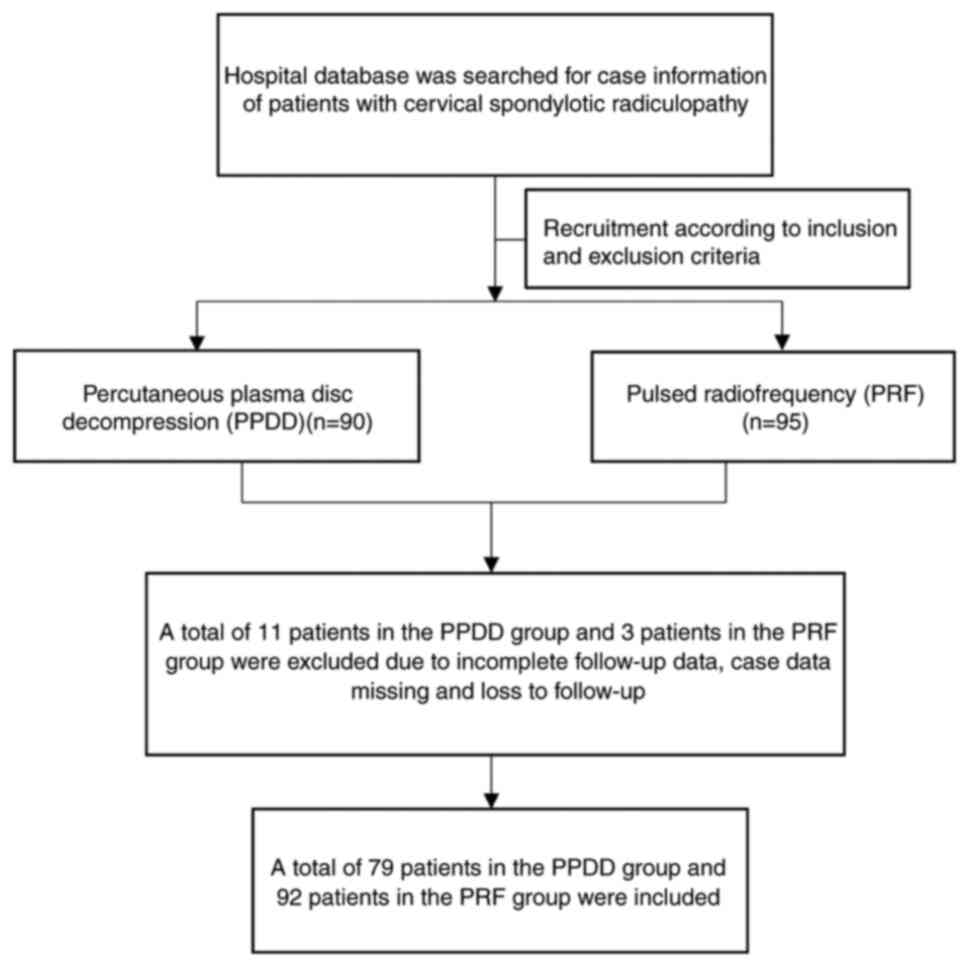
A total of 95 patients were initially recruited in
the PRF group, as well as 90 patients in the PPDD group according
to the inclusion and exclusion criteria. A total of 14 cases were
excluded due to loss of follow-up and incomplete follow-up data,
resulting in 92 patients in the PRF and 79 patients in the PPDD
group (Fig. 1).
Baseline characteristics
There were no significant differences in age, sex,
BMI, proportion of preoperative medication and numbness, treatment
rate, number of cases with upper cervical disc herniation, pain
symptoms in the upper extremity and duration of disease between PRF
and PPDD groups (P>0.05; Table
I).
 | Table IBaseline data. |
Table I
Baseline data.
| Characteristic | PRF group | PPDD group | P-value |
|---|
| Mean age,
years | 55±10 | 54±11 | 0.99 |
| Sex (%) | | | 0.75 |
|
Male | 37 (40.22) | 29 (36.71) | |
|
Female | 55 (59.78) | 50 (63.29) | |
| Mean BMI,
kg/m2 | 23.50±2.39 | 24.11±3.03 | 0.88 |
| Pre-operative
analgesic use (%) | | | 0.54 |
|
Yes | 35 (38.04) | 34 (43.04) | |
|
No | 57 (62.96) | 45 (56.96) | |
| Nerve block therapy
(%) | | | 0.75 |
|
Yes | 29 (31.52) | 27 (34.18) | |
|
No | 63 (68.48) | 52 (65.82) | |
| Upper limb symptoms
(%) | | | 0.09 |
|
Pain | 41 (44.57) | 46 (58.23) | |
|
Numbness | 51 (55.43) | 33 (41.77) | |
| Duration of disease
(%) | | | 0.10 |
|
≤1 year | 68 (73.91) | 49 (62.03) | |
|
>1
year | 24 (26.09) | 30 (37.97) | |
CEH remission rate and cervical pain
score within the two groups
PPDD group had significantly higher CEH improvement
rates than the PRF group at 1, 3 and 6 months after treatment (78.8
vs. 43.5, P=0.016; 84.8 vs. 34.8, P=0.003 and 75.8 vs. 26.1%,
P=0.005, respectively; Table II).
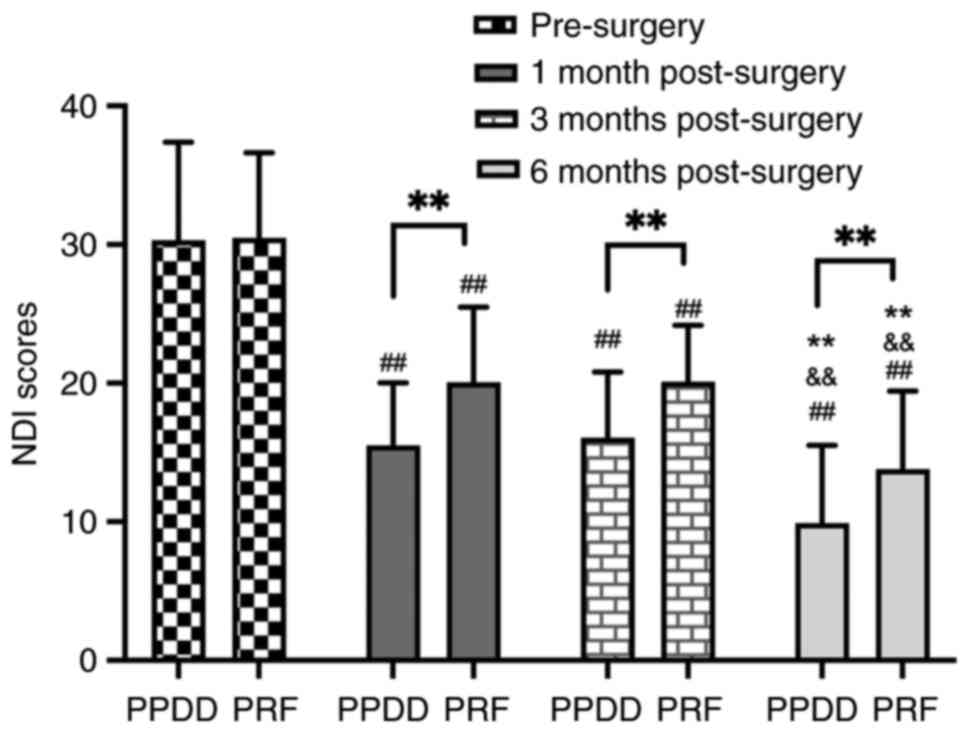
NDI scores in the PPDD group were significantly lower than those in
the PRF group at 1, 3, and 6 months post-surgery (15.49 vs. 20.05,
P=0.002; 16.06 vs. 20.10, P=0.003 and 9.90 vs. 13.80, P=0.001;
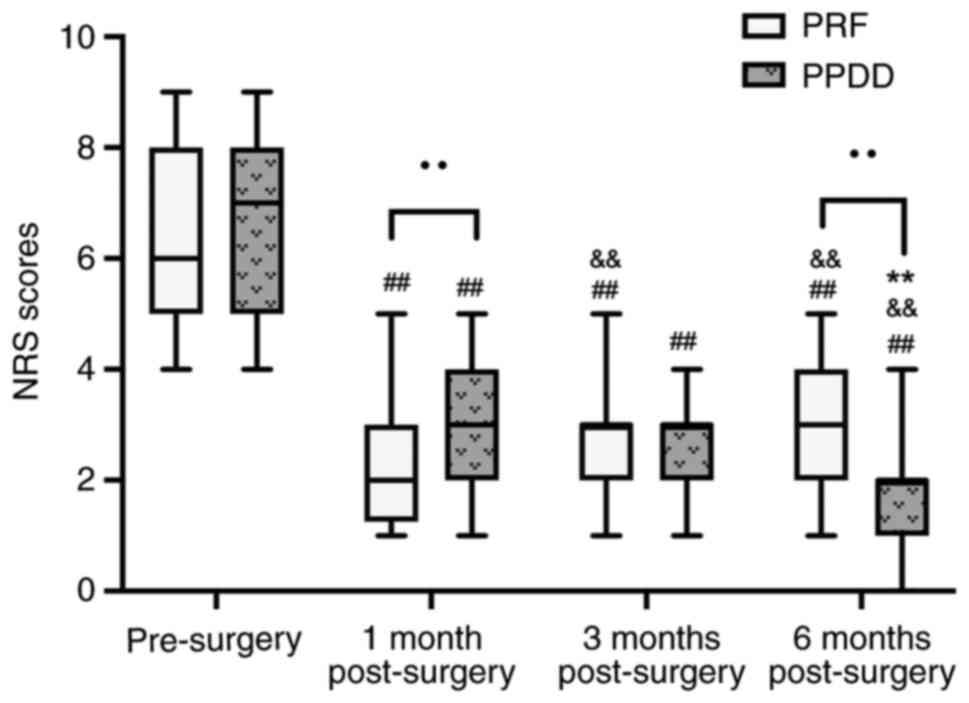
Fig. 2). PRF group showed higher
NRS scores than the PRF group at 1 month (3 vs. 2, P<0.0001). At
3 months after treatment, there was no significant difference (3
vs. 3, P=0.57) and at 6 months after treatment, the PPDD group
showed significantly lower NRS scores than the PRF group (2 vs. 3,
P<0.0001; Fig. 3). In the PPDD
group, CEH remission rate peaked at 3 months, then decreased; CEH
remission rate in the PRF group exhibited a continuous downward
trend from 1 to 6 months postoperative (Table II). In the PRF group, NDI score at
1 month after surgery showed no significant difference compared
with 3 months after surgery (20.05 vs. 20.10, P=0.99); NDI scores
between other time points [pre-surgery vs. 1 month (30.46 vs.
20.05, P<0.0001), 3 months vs. 6 months (20.10 vs. 13.80,
P<0.0001), pre-surgery vs. 6 months (30.46 vs. 13.80,
P<0.0001), pre-surgery vs. 3 months (30.46 vs. 20.10,
P<0.0001), 1 month vs. 6 months (20.05 vs. 13.80, P<0.0001)]
indicated significant differences In PPDD group, NDI score at 1
month after surgery showed no significant difference compared with
3 months after surgery (15.49 vs. 16.06, P=0.89), the comparison of
NDI between other time points [pre-surgery vs. 1 month (30.29 vs.
15.49, P<0.0001), 3 months vs. 6 months (16.06 vs. 9.90,
P<0.0001), pre-surgery vs. 3 months (30.29 vs. 16.06,
P<0.0001), pre-surgery vs. 6 months (30.29 vs. 9.90,
P<0.0001), 1 month vs. 6 months (15.49 vs. 9.90, P<0.0001)]
both indicated significant differences (Fig. 2); In the PRF group, NRS score at 3
months after surgery showed no significant difference compared with
6 months after surgery (3 vs. 3, P=0.22); NRS scores between other
time points [pre-surgery vs. 1 month (6 vs. 2, P<0.0001), 1
month vs. 3 months (2 vs. 3, P=0.003), pre-surgery vs. 3 months (6
vs. 3, P<0.0001), pre-surgery vs. 6 months (6 vs. 3,
P<0.0001), 1 month vs. 6 months (2 vs. 3, P=0.004)] indicated
significant differences. In PPDD group, NRS score at 1 month after
surgery showed no significant difference compared with 3 months
after surgery (3 vs. 3, P=0.42); NRS scores between other time
points [pre-surgery vs. 1 month (7 vs. 3, P<0.0001), 3 months
vs. 6 months (3 vs. 2, P=0.002), pre-surgery vs. 6 months (7 vs. 2,
P<0.0001), pre-surgery vs. 3 months (7 vs. 3, P<0.0001), 1
month vs. 6 months (3 vs. 2, P=0.004)] indicated significant
differences (Fig. 3). There was no
significant difference in the incidence of postoperative
complications between the two groups (P>0.999) (Table II).
 | Table IICEH remission rate and postoperative
complications. |
Table II
CEH remission rate and postoperative
complications.
| Characteristic | PRF group
(n=92) | PPDD group
(n=79) | OR (95% CI) | P-value |
|---|
| CEH remission at 1
month post-surgery (%) | | | | |
|
Yes | 40 (43.48) | 62 (78.48) | 4.83 (1.49,
15.61) | 0.016a |
|
No | 52 (56.52) | 17 (21.52) | | |
| CEH remission at 3
months post-surgery (%) | | | | |
|
Yes | 32 (34.78) | 67 (84.81) | 10.51(2.92,
37.81) | 0.003b |
|
No | 60 (65.22) | 12 (15.19) | | |
| CEH remission at 6
months post-surgery (%) | | | | |
|
Yes | 24 (26.09) | 60 (75.95) | 8.85 (2.60,
30.13) | 0.005b |
|
No | 68 (73.91) | 19 (24.05) | | |
| Postoperative
complications (%) | | | | 0.999 |
|
None | 86 (94.48) | 75 (94.94) | | |
|
Infection | 1 (1.08) | 1 (1.27) | | |
|
Hematoma | 2 (2.17) | 2 (2.53) | | |
|
Nerve
injury | 2 (2.17) | 1 (1.27) | | |
Linear regression of NRS and NDI
Surgical methods (PPDD/PRF) and confounders were
imported into linear regression to analyze the independent impact
of surgical methods on NRS and NDI score and CEH remission.
Confounders included age, sex, BMI, preoperative analgesic use and
upper extremity symptoms, history of nerve block treatment and
duration of disease. Compared with the PRF group, NRS score
[β=-1.14, 95% confidence interval (CI; -1.66, -0.62), P<0.0001]
and NDI score [β=-3.98, 95%CI (-6.03, -1.92), P=0.011] at 6 months
after treatment were significantly decreased in the PPDD group.
Compared with the PRF group, the CEH improvement at 6 months after
treatment was significantly elevated in the PPDD group [odds ratio,
9.87, 95%CI (2.73,13.30), P=0.003; Table III]. Therefore, surgical method
was an independent factor for NRS and NDI score, and CEH remission
rate.
 | Table IIIAssociation between surgical method
and primary outcomes. |
Table III
Association between surgical method
and primary outcomes.
| | CEH improvement
rate at 6 months after treatment | NDI score at 6
months after treatment |
|---|
| Variable | OR (95% CI) | P-value | β (95% CI) | P-value |
|---|
| Age | 1.01
(0.95,1.07) | 0.70 | 0.00
(-0.09,0.09) | 0.99 |
| Sex | 0.21
(0.04,1.06) | 0.26 | 1.65
(-0.41,3.71) | 0.12 |
| BMI | 0.90
(0.70,1.17) | 0.41 | -0.14
(-0.49,0.22) | 0.44 |
| Upper limb
symptoms | 1.02
(0.20,5.16) | 0.98 | 0.42
(-1.61,2.45) | 0.68 |
| Duration of
disease | 0.74
(0.18,3.00) | 0.67 | 2.52
(0.36,4.67) | 0.02a |
| Pre-operative
analgesic use | 0.30
(0.07,1.32) | 0.21 | 1.37
(-0.66,3.39) | 0.19 |
| Nerve block
therapy | 0.21
(0.04,1.14) | 0.23 | 1.23
(-0.87,3.34) | 0.25 |
| Surgical
method | 9.87
(2.73,13.30) | 0.002b | -3.98
(-6.03,-1.92) | 0.01a |
Discussion
The present study indicated that both PPDD and PRF
improved CEH and upper limb pain of patients with CSR, however the
efficacy and stability of PRF were inferior to PPDD. Compared with
1 month after surgery, the NDI score in the PRF group at 3 months
and 6 months after surgery was significantly increased, indicating
that patients in the PRF group may require repeated treatment,
whereas the NRS and NDI scores in the PPDD group decreased. The CEH
remission rate in the PPDD group was higher than that in the PRF
group. This indicated that within 6 months of treatment, patients
who received PPDD exhibited significantly greater relief from
CEH.
PPDD and PRF are both viable options for treating
CEH and exhibit varying efficacy in relieving CEH and alleviating
upper limb pain. The surgical approach should prioritize PPDD,
which directly targets the intervertebral disc by relieving disc
pressure. By contrast, PRF primarily targets nerves and surrounding
tissues. The occurrence of CEH is associated with the
intervertebral disc and intracervical pressure. Prior research has
proposed two potential mechanisms for CEH (13): Pressure transmission within the
upper cervical joint complex and dural connectivity between the
lower and the upper cervical spine. Furthermore, related
experiments (13,29) have demonstrated alleviation of
spinal cord pressure via cervical laminoplasty, highlighting the
role of intracervical pressure in CEH. Therefore, PPDD could
significantly alleviate CEH. PRF treatment for CEH may be achieved
by regulation of the posterior branch nerve and decreased pressure
on the cervical fascia tissue. The radiofrequency scalpel is placed
around the posterior nerve, to suppress the transmission of
nociceptive electrical signals and decrease pro-inflammatory factor
(such as IL-6 and TNF-α) release through pulse current regulation
(30) to block pain signal
crosstalk between the internal cervical nerve and trigeminal
nucleus. PRF can decrease the tension of the cervical fascia by
acting on surrounding nerve tissue, thereby decreasing cervical
pressure and alleviating CEH. This may be achieved by reducing the
levels of Iba1 around the fascia, thereby inhibiting inflammatory
cell adhesion and tissue remodeling (31). PRF is commonly used to treat CEH due
to its ability to electrically modulate nerves but it does not
address intervertebral disc issues. As disc herniation is the
primary cause of nerve compression and increased cervical pressure,
PRF may be less beneficial for treating CEH associated with
cervical disc herniation compared with PPDD (31). This may also explain why the
remission rate of CEH in the PRF group was not as high as that in
the PPDD group.
As previous studies (11,29)
have indicated, CEH is primarily associated with C1-C3 nerves and
discs. How PPDD effectively improves both CEH symptoms and upper
limb pain via a low cervical spine approach was not clear yet.
Another mechanism of PPDD involves the association between anterior
vertebral fascia and internal neck pressure. The convergence of
C1-C3 cervical nerves in the trigeminocervical nucleus forms the
neurophysiological foundation for CEH (11,29).
In addition to the anatomical basis of the upper cervical nerves,
previous studies highlighted the role of the dura mater in CEH
(13,29,32).
Hack et al (32)
demonstrated that the myodural bridge, comprised of the rectus
capitis posterior minor muscle, dura mater, occipital bone,
atlantoaxial joint and the connecting tissues, transmits forces
from the upper cervical joint complex, which includes connective
tissue, dura mater, C1 cervical nerve root and rectus capitis
posterior minor muscle, to the pain-sensitive dura mater. Lower
cervical pressure is transmitted to the upper cervical joint via
anterior vertebral fascia, and cervical pressure can act on upper
cervical joint complex. Reducing cervical pressure in these areas
may be the mechanism by which PPDD via a lower surgical approach
improves CEH.
NRS and NDI scores in the PPDD group were
significantly decreased compared with the PRF group after
treatment. PPDD yielded greater and longer lasting effects than
PRF, which may be due to the more stable chemical effects of PPDD
compared with the electrical effects of PRF. PPDD exhibits an
anti-inflammatory and stress-reducing double effect, including
dorsal root ganglion decompression and inactivation of inflammatory
factors around the sinuvertebral nerve (33,35). A
herniated cervical disc within or outside the intervertebral
foramen frequently induces radicular pain in the upper extremity by
stimulating or compressing the dorsal root ganglion (33). Compression of the dorsal root
ganglion results in abnormal discharge of A and C fibers,
potentially underlying development of cervical radicular pain
(34). PPDD contributes to
mitigation of radicular pain by decreasing disc volume (35) and alleviating nerve root compression
via vaporization and retraction of the herniated disc. Furthermore,
the sinuvertebral nerve is activated by inflammatory factors,
leading to pain. It has been demonstrated that phospholipase A2
(PLA2) exhibits elevated activity in herniated intervertebral disc
tissue (36,37). PLA2 has the capacity of exciting
nociceptors, resulting in pain in the innervated region, and can
also directly stimulate the nerve root, causing chemical
radiculitis (37,38). PPDD generates plasma with sufficient
energy to disrupt the molecular bonds of PLA2(38). Consequently, PPDD may alleviate pain
by decreasing inflammatory factor release in the intervertebral
disc and deactivating pain-inducing factors surrounding the
sinuvertebral nerve, including PLA2. By contrast, PRF blocks the
transmission of nociceptive stimulation in the nerve. PRF is more
rapid and can achieve the inactivation of pain-inducing factors by
acting on targets such as neurotransmitters and ion channels
(39,40). When the radio frequency electrode is
in operation, the blade directly acts on surrounding tissue of the
nerve root, enhancing release of GABA neurotransmitters without
damaging the nerve (39), and
inhibiting the transmission of harmful signals by enhancing the
activity of Na+/K+ ATP plasma channels, thus
quickly exerting analgesic effects (40). While it primarily acts by electrical
pulse control rather than directly decreasing pressure on
protrusions that cause nerve root compression, the therapeutic
effect is relatively limited and symptoms may return.
In summary, PPDD had a dual effect of physical
decompression of intervertebral discs and inactivation of chemical
inflammation around the nerves, which makes it more thorough in
relieving cervical pressure and peripheral nerve compression,
weakening pain nerve conduction of CEH and reducing the generation
of pain signal impulses in the upper limb nerve roots. In contrast,
PRF mainly acts on local tissue surrounding nerves, exerting its
effects via neurotransmitter inactivation and ion channel
inhibition. The site of action is limited and there is no deep
pressure release effect, which cannot alleviate nerve root
compression caused by intervertebral disc herniation. Its analgesic
and anti-inflammatory effects are limited. CEH and upper limb pain
are associated with nerve compression and release of inflammatory
cytokines around nerves.
There were certain limitations in the present study.
Firstly, there may be risk of selection bias due to the
retrospective study design and lack of randomization. Data was not
acquired in real-time, which may decrease accuracy. In future
research, the inner mechanism of internal cervical pressure
conduction should be further investigated, including the
association between increased pressure on the cervical fascia and
abnormal discharge of pain fibers in the trigeminal nucleus, as
well as the impact of lower cervical disc decompression on upper
cervical disc pressure, which may facilitate better treatment of
CEH and associated types of cervical disease. Further prospective
studies with larger patient cohorts remain to be conducted.
In conclusion, PPDD via a lower surgical approach
significantly relieved CEH symptoms and upper extremity radicular
pain in patients with CSR. In addition, PPDD was more effective
than PRF for long-term CEH remission and upper limb pain
alleviation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shanghai
University of Traditional Chinese Medicine (TCM) Xinglin Young
Talent Training System-Xinglin Scholars Project [grant no. TCM
(2020)23], Shanghai University of TCM Excellent Talents Training
Program [grant no. TCM (2020)10)] and Future Plan for TCM
Development of Science and Technology of Shanghai Municipal
Hospital of TCM (grant no. WL-HBMS-2022002K).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SK conceived the study, analyzed data and wrote the
manuscript. XQ and JC analyzed data. JW contributed to analysis and
interpretation of data. KW conceived and supervised the study. All
the authors have read and approved the final manuscript. SK and XQ
and JC and JW and KW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Shanghai Traditional Chinese Medicine Hospital (No.
2023SHL-KY-85-01) (Shanghai, China) and Jiashan County Hospital of
Traditional Chinese Medicine (No. 2023007) (Jiaxing, China). The
study was registered in the Chinese Clinical Trial Registry
database (registration no. ChiCTR2300074113). Participants provided
verbal consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang C, Gu Z, Yu J, Zhang P and Yang F:
Clinical observation of long chiropractic treatment on patients
with neurogenic cervical spondylosis: Study protocol for a
randomized controlled trial. Medicine (Baltimore).
101(e28861)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang P, Zuo G, Du SQ, Gao TC, Liu RJ, Hou
XZ, Ji X, Yin J, Li KM and Zhang Q: Meta-analysis of the
therapeutic effect of acupuncture and chiropractic on cervical
spondylosis radiculopathy: A systematic review and meta-analysis
protocol. Medicine (Baltimore). 99(e18851)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ye LQ, Chen C, Liu YH, Li Z and Lu GL:
Effect of cervical spine motion on displacement of posterolateral
annulus fibrosus in cervical spondylotic radiculopathy with
contained posterolateral disc herniation: A three-dimensional
finite element analysis. J Orthop Surg Res. 17(548)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thind H, Ramanathan D, Ebinu J, Copenhaver
D and Kim KD: Headache relief is maintained 7 years after anterior
cervical spine surgery: Post hoc analysis from a multicenter
randomized clinical trial and cervicogenic headache hypothesis.
Neurospine. 17:365–373. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sun Y, Muheremu A, Yan K, Yu J, Zheng S
and Tian W: Effect of different surgical methods on headache
associated with cervical spondylotic myelopathy and/or
radiculopathy. BMC Surg. 15(105)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pang X, Liu C and Peng B: Anterior
cervical surgery for the treatment of cervicogenic headache caused
by cervical spondylosis. J Pain Res. 13:2783–2789. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Antonaci F, Bono G and Chimento P:
Diagnosing cervicogenic headache. J Headache Pain. 7:145–148.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chua NHL, Suijlekom HV, Wilder-Smith OH
and Vissers KC: Understanding cervicogenic headache. Anesth Pain
Med. 2:3–4. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Goyal S, Kumar A, Mishra P and Goyal D:
Efficacy of interventional treatment strategies for managing
patients with cervicogenic headache: A systematic review. Korean J
Anesthesiol. 75:12–24. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen C, Yuchi CX, Gao Z, Ma X, Zhao D, Li
JW, Xu B, Zhang CQ, Wang Z, Du CF and Yang Q: Comparative analysis
of the biomechanics of the adjacent segments after minimally
invasive cervical surgeries versus anterior cervical discectomy and
fusion: A finite element study. J Orthop Translat. 23:107–112.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alix ME and Bates DK: A proposed etiology
of cervicogenic headache: The neurophysiologic basis and anatomic
relationship between the dura mater and the rectus posterior
capitis minor muscle. J Manipulative Physiol Ther. 22:534–539.
1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Suijlekom H, Van Zundert J, Narouze S,
van Kleef M and Mekhail N: 6. Cervicogenic headache. Pain Pract.
10:124–130. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shimohata K, Hasegawa K, Onodera O,
Nishizawa M and Shimohata T: The clinical features, risk factors,
and surgical treatment of cervicogenic headache in patients with
cervical spine disorders requiring surgery. Headache. 57:1109–1117.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Diener HC, Kaminski M, Stappert G, Stolke
D and Schoch B: Lower cervical disc prolapse may cause cervicogenic
headache: Prospective study in patients undergoing surgery.
Cephalalgia. 27:1050–1054. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang L, Li Y, Dai C, Pang X, Li D, Wu Y,
Chen X and Peng B: Anterior cervical decompression and fusion
surgery for cervicogenic headache: A multicenter prospective cohort
study. Front Neurol. 13(1064976)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Radhakrishnan K, Kitchy WJ, O'Fallon M and
Kurland LT: Epidemiology of cervical radiculopathy. A
population-based study from Rochester, Minnesota, 1976 through
1990. Brain. 117:325–335. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Luyao H, Xiaoxiao Y, Tianxiao F, Yuandong
L and Wang P: Management of cervical spondylotic radiculopathy: A
systematic review. Global Spine J. 12:1912–1924. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jeon HC, Kim CS, Kim SC, Kim TH, Jang JW,
Choi KY, Moon BJ and Lee JK: Posterior cervical microscopic
foraminotomy and discectomy with laser for unilateral
radiculopathy. Chonnam Med J. 51:129–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Birnbaum K: Percutaneous cervical disc
decompression. Surg Radiol Anat. 31:379–387. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kwak SG, Lee DG and Chang MC:
Effectiveness of pulsed radiofrequency treatment on cervical
radicular pain: A meta-analysis. Medicine (Baltimore).
97(e11761)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bonaldi G, Baruzzi F, Facchinetti A,
Fachinetti P and Lunghi S: Plasma radio-frequency-based diskectomy
for treatment of cervical herniated nucleus pulposus: Feasibility,
safety, and preliminary clinical results. AJNR Am J Neuroradiol.
27:2104–2111. 2006.PubMed/NCBI
|
|
22
|
Snidvongs S and Mehta V: Pulsed radio
frequency: A non-neurodestructive therapy in pain management. Curr
Opin Support Palliat Care. 4:107–110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sam J, Catapano M, Sahni S, Ma F,
Abd-Elsayed A and Visnjevac O: Pulsed radiofrequency in
interventional pain management: Cellular and molecular mechanisms
of action-an update and review. Pain Physician. 24:525–532.
2021.PubMed/NCBI
|
|
24
|
Kasch R, Mensel B, Schmidt F, Ruetten S,
Barz T, Froehlich S, Seipel R, Merk HR and Kayser R: Disc volume
reduction with percutaneous nucleoplasty in an animal model. PLoS
One. 7(e50211)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sjaastad O, Fredriksen TA and Pfaffenrath
V: Cervicogenic headache: Diagnostic criteria. The cervicogenic
headache international study group. Headache. 38:442–445.
1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Blumenfeld A and Siavoshi S: The
challenges of cervicogenic headache. Curr Pain Headache Rep.
22(47)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alo KM, Yland MJ, Redko V, Feler C and
Naumann C: Lumbar and sacral nerve root stimulation (NRS) in the
treatment of chronic pain: A novel anatomic approach and neuro
stimulation technique. Neuromodulation. 2:23–31. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Reisener MJ, Okano I, Zhu J, Salzmann SN,
Miller CO, Shue J, Sama AA, Cammisa FP, Girardi FP and Hughes AP:
Workers' compensation status in association with a high NDI score
negatively impacts post-operative dysphagia and dysphonia following
anterior cervical fusion. World Neurosurg. 154:e39–e45.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bogduk N: The anatomical basis for
cervicogenic headache. J Manipulative Physiol Ther. 15:67–70.
1992.PubMed/NCBI
|
|
30
|
Das B, Conroy M, Moore D, Lysaght J and
McCrory C: Human dorsal root ganglion pulsed radiofrequency
treatment modulates cerebrospinal fluid lymphocytes and
neuroinflammatory markers in chronic radicular pain. Brain Behav
Immun. 70:157–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cho HK, Kang JH, Kim SY, Choi MJ, Hwang
SJ, Cho YW and Ahn SH: Changes in neuroglial activity in multiple
spinal segments after caudal epidural pulsed radiofrequency in a
rat model of lumbar disc herniation. Pain Physician.
19:E1197–E1209. 2016.PubMed/NCBI
|
|
32
|
Hack GD, Koritzer RT, Robinson WL,
Hallgren RC and Greenman PE: Anatomic relation between the rectus
capitis posterior minor muscle and the dura mater. Spine (Phila Pa
1976). 20:2484–2486. 1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee DG, Ahn SH and Lee J: Comparative
effectivenesses of pulsed radiofrequency and transforaminal steroid
injection for radicular pain due to disc herniation: A prospective
randomized trial. J Korean Med Sci. 31:1324–1330. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song XJ, Hu SJ, Greenquist KW, Zhang JM
and LaMotte RH: Mechanical and thermal hyperalgesia and ectopic
neuronal discharge after chronic compression of dorsal root
ganglia. J Neurophysiol. 82:3347–3358. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gerges FJ, Lipsitz SR and Nedeljkovic SS:
A systematic review on the effectiveness of the nucleoplasty
procedure for discogenic pain. Pain Physician. 13:117–132.
2010.PubMed/NCBI
|
|
36
|
Lee JH and Lee SH: Comparison of clinical
efficacy between interlaminar and transforaminal epidural injection
in patients with axial pain due to cervical disc herniation.
Medicine (Baltimore). 95(e2568)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tang ZY, Shu B, Cui XJ, Zhou CJ, Shi Q,
Holz J and Wang YJ: Changes of cervical dorsal root ganglia induced
by compression injury and decompression procedure: A novel rat
model of cervical radiculoneuropathy. J Neurotrauma. 26:289–295.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ren D, Zhang Z, Sun T and Li F: Effect of
percutaneous nucleoplasty with coblation on phospholipase A2
activity in the intervertebral disks of an animal model of
intervertebral disk degeneration: A randomized controlled trial. J
Orthop Surg Res. 10(38)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu C and Sun D: GABA receptors in brain
development, function, and injury. Metab Brain Dis. 30:367–379.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tsantoulas C and McMahon SB: Opening paths
to novel analgesics: The role of potassium channels in chronic
pain. Trends Neurosci. 37:146–158. 2014.PubMed/NCBI View Article : Google Scholar
|