Introduction
Li-Fraumeni syndrome (LFS) was first reported by Li
et al (1). LFS is a
hereditary cancer predisposition syndrome associated with germline
mutations in tumor suppressor gene TP53. It is most
frequently associated with soft tissue sarcoma, osteosarcoma,
breast cancer, leukemia and brain and adrenal tumors. However, they
can also cause other types of tumor (2). The prevalence of cancer is extremely
high during childhood compared with the general population
(3). Although Li-Fraumeni syndrome
has been considered a very rare genetic disorder, the frequency of
pathogenic variants of TP53 in the general population is
recently reported to be one in 5000-20,000 (4,5).
Perivascular epithelioid cell tumors (PEComas) were
first described by Bonetti et al in 1992(6). PEComas are a group of tumors defined
in the World Health Organization (WHO) Classification of bone and
soft tissue tumors in 2002(7) as
mesenchymal tumors composed of histologically and
immunohistochemically distinctive PECs. PEComas belong to a family
of tumors that include angiomyolipoma of the kidney, clear cell
sugar tumor of the lung, lymphangioleiomyomatosis of the lung,
myomelanocytic clear cell tumors of the round ligament/sickle cell
ligament, and perivascular epithelioid cell tumor not otherwise
specified (PEComa-NOS). PEComa-NOS tumors can occur at any
anatomical site, including the liver (8).
To the best of our knowledge, there are a few
reports of PEComas complicating LFS (8-11)
and the association between these diseases has not been clarified.
The present study reports a rare case of liver PEComa associated
with LFS.
Case report
In March 2022, a 32-year-old female patient with a
history of LFS was referred to the Department of Gastroenterology
and Hepatology and Clinical Genomics at the Tokyo Metropolitan Tama
Medical Center (Tokyo, Japan) for a detailed examination of a liver
tumor detected by abdominal ultrasound. The patient had received a
diagnosis of LFS based on a history of sarcoma and germline
variants of TP53 7 years previously. The patient had a
history of right hamstring pleomorphic rhabdomyosarcoma at 2 years
of age, right femoral osteosarcoma at 11 years, left tibial
osteosarcoma at 22 years and sigmoid adenocarcinoma at 24 years.
Her paternal grandfather had prostate cancer, paternal grandmother
had oral and laryngeal cancer and maternal relatives had Ewing's
sarcoma. The results of blood analysis, such as complete blood
count, biochemical examination and blood coagulation test, were
within normal limits. Liver function test results and tumor marker
values were as follows: Indocyanine green retention, 3.0% (normal,
<10.0%); carcinoembryonic antigen, 1.1 ng/ml (normal, <5.0
ng/ml) and carbohydrate antigen 19-9, 9.5 U/ml (normal, <37.0
U/ml).
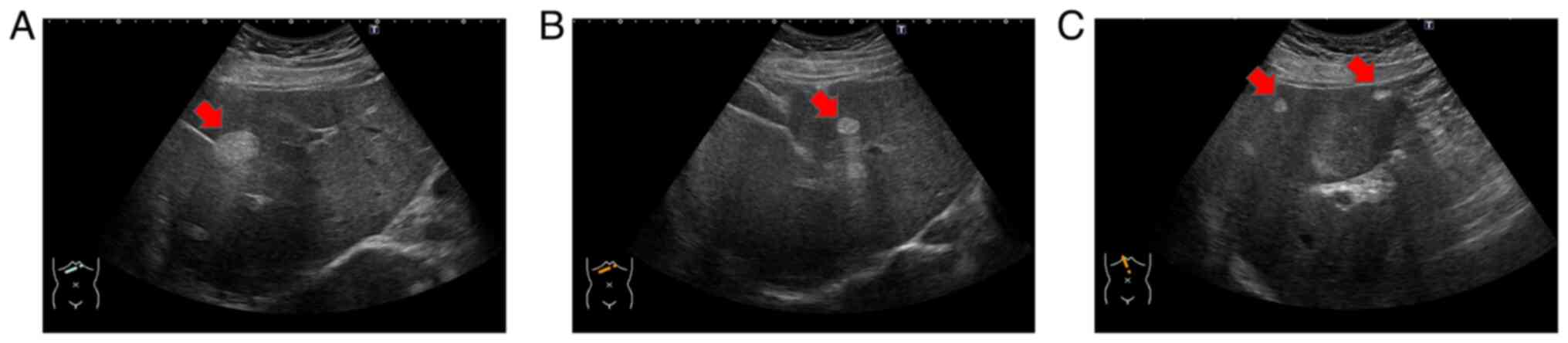
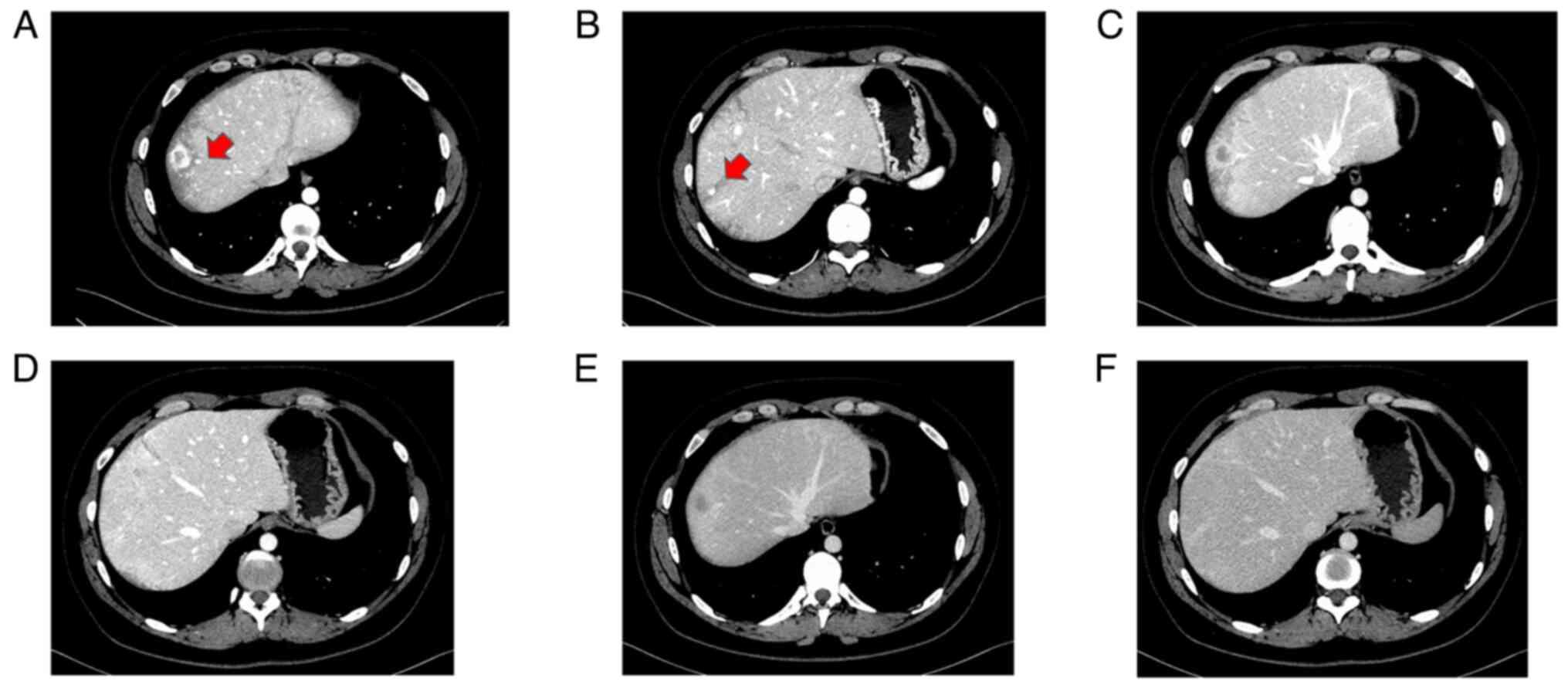
Ultrasonography revealed multiple hyperechoic tumors
in the liver (Fig. 1A-C). Magnetic
resonance imaging (MRI) was performed before a dynamic computed
tomography (CT) scan to limit radiation exposure (8,12). MRI
examinations were performed using 1.5-T MR systems (MAGNETOM Sola,
Siemens Medical Solutions, Erlangen, Germany). The gradient
strengths were 45mT/m with a slew rate of 200T/m/second.
Eighteen-element phased array matrix coil was used for signal
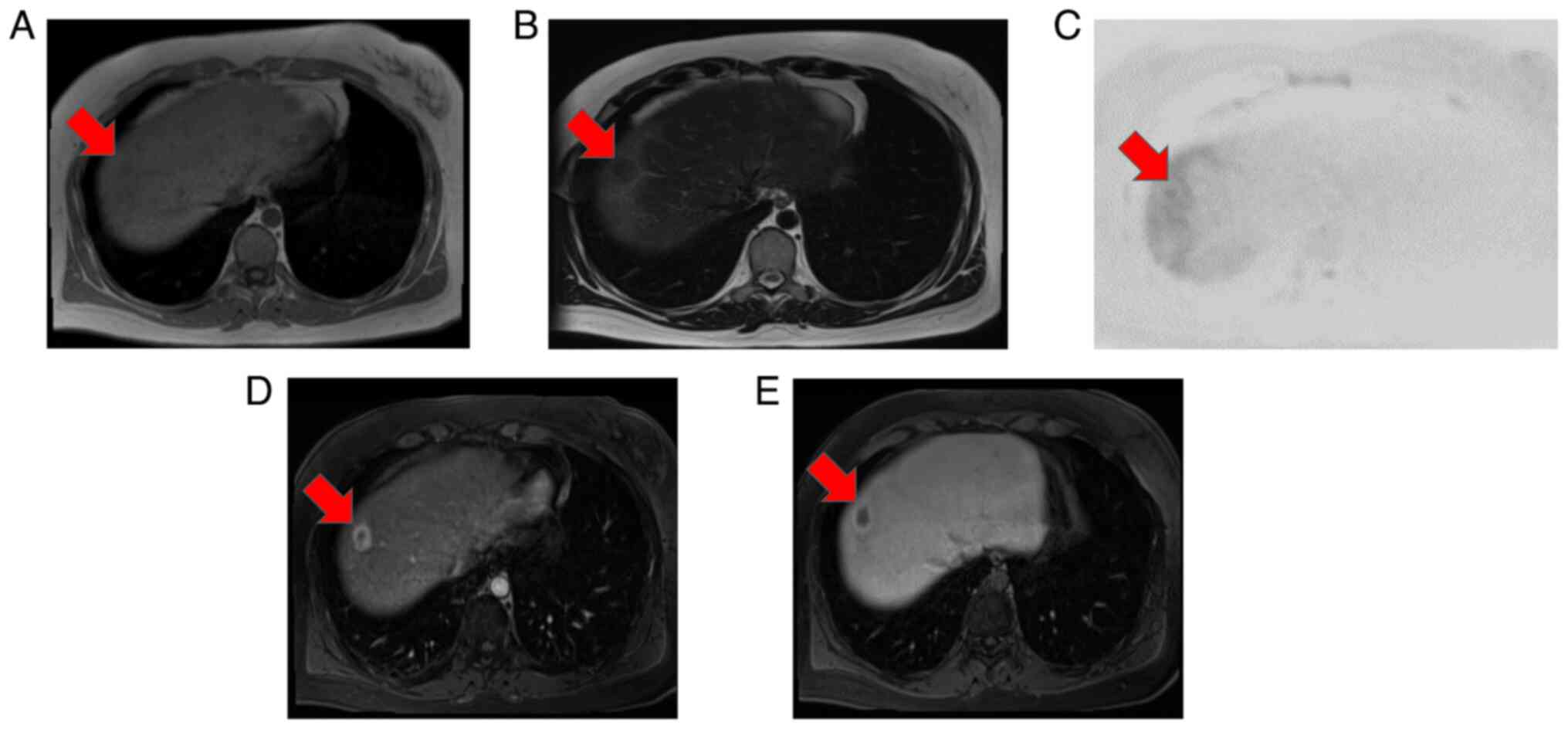
reception. MRI showed that the main tumor in segment (S)8 had low
signal intensity on both T1- and T2-weighted images and slightly
high signal intensity on diffusion-weighted imaging (Fig. 2A-C). Contrast-enhanced MRI revealed
that the S8 tumor was enhanced in a ring shape during the early
contrast phase (Fig. 2D). In the
hepatobiliary phase, the periphery had a high signal intensity and
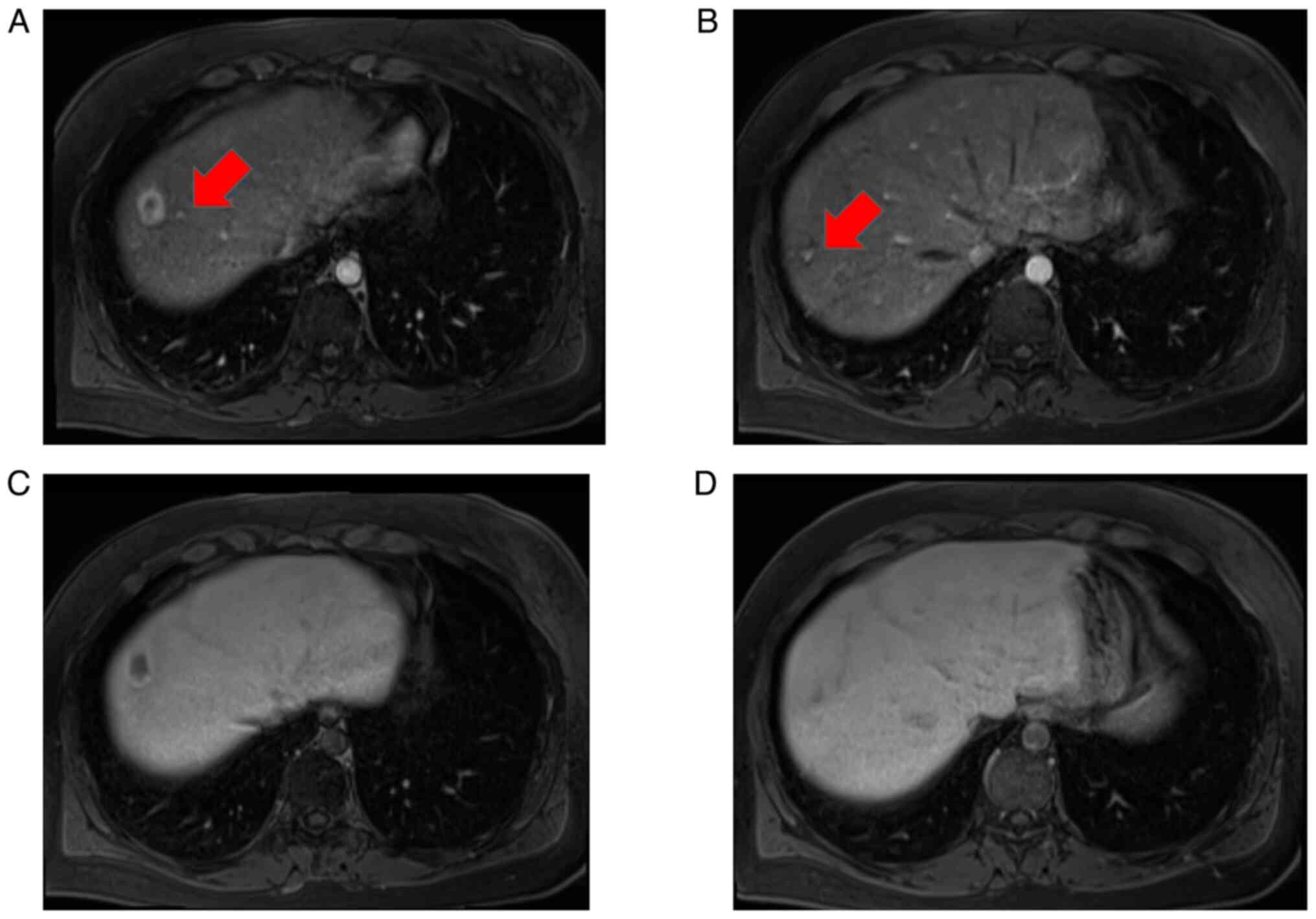
the interior had a low signal intensity (Fig. 2E). Near the main tumor in S8, two
small tumors were heavily contrasted in the early phase but were
not identified in the hepatobiliary phase (Fig. 3). Numerous tumors suspected to be
lipomas, focal nodular hyperplasia or arterio-portal shunts were
also observed in the liver.
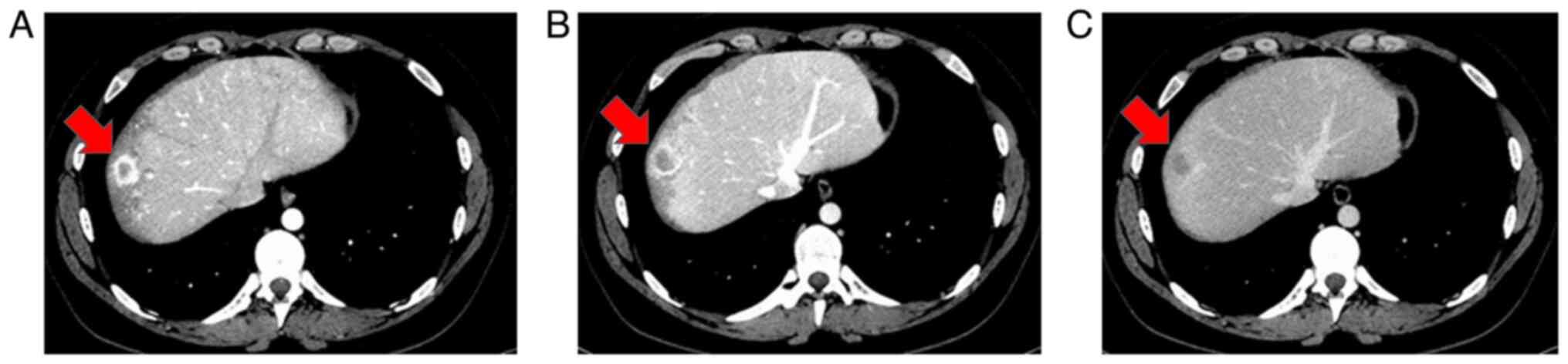
Because of the difficulty in confirming diagnosis
using MRI alone, dynamic CT was performed with informed consent,
which revealed that the main S8 tumor was contrast-enhanced in a
ring shape in the early phase and washed out in the late phase
(Fig. 4); it also showed low
density on plain CT. A total of two small tumors near the primary
tumor were also enhanced in the early phase of dynamic CT but were
not identified in the late phase (Fig.
5). Upper and lower gastrointestinal endoscopy showed no
malignant findings.
The primary tumor in S8 could be benign, such as a
hepatic hemangioma; however, a malignant tumor, such as a
metastatic liver tumor or hepatocellular carcinoma (HCC), could not
be ruled out and it was difficult to perform a biopsy because the
tumor was localized just below the diaphragm. The lesions
surrounding the primary tumor in S8 were suspected to be
arterio-portal shunts or focal nodular hyperplasia. A number of
other liver tumors were considered lipomas. Therefore, the patient
was referred for diagnostic surgery. In August 2022, laparoscopic
partial hepatectomy of the S8 was performed without complications.
A camera port in the umbilicus, 5-mm port in the pericardial fossa
and right lateral abdomen and two 12-mm ports in the right
subxiphoid region were placed. The operative time was 5 h and 8 min
and the blood loss was 10 ml. The patient was discharged on
postoperative day 7.
Tissue samples were fixed in 10% neutral buffered
formalin solution at room temperature for 24 to 48 h, paraffin
embedded, cut to 3 µm thick, and dewaxed as per standard procedures
(13). Staining was performed at
room temperature for 10 min for Hematoxylin and 4 min for Eosin.
The microscope was an Olympus BX53 (light microscope) and the
magnification was 200x. The following primary antibodies were used:
smooth muscle actin (SMA) (1:5; clone1A4; Cat. No.: IR61161-2;
Dako), and human melanocyte black-45 (HMB45) (1:50, clone HMB45;
Cat. No.: M063401-2; Dako). CC1 buffer (Cat. No.: 950-124; Roche)
was used for antigen retrieval. The antigen retrieval step was
performed at 95˚C for 64 min. Primary antibody incubation was
performed at 36˚C for 32 min. Secondary antibody (ultraView
Universal DAB Detection Kit; cat. No.; 05269806001; Roche)
incubation was performed at 36˚C for 32 min.
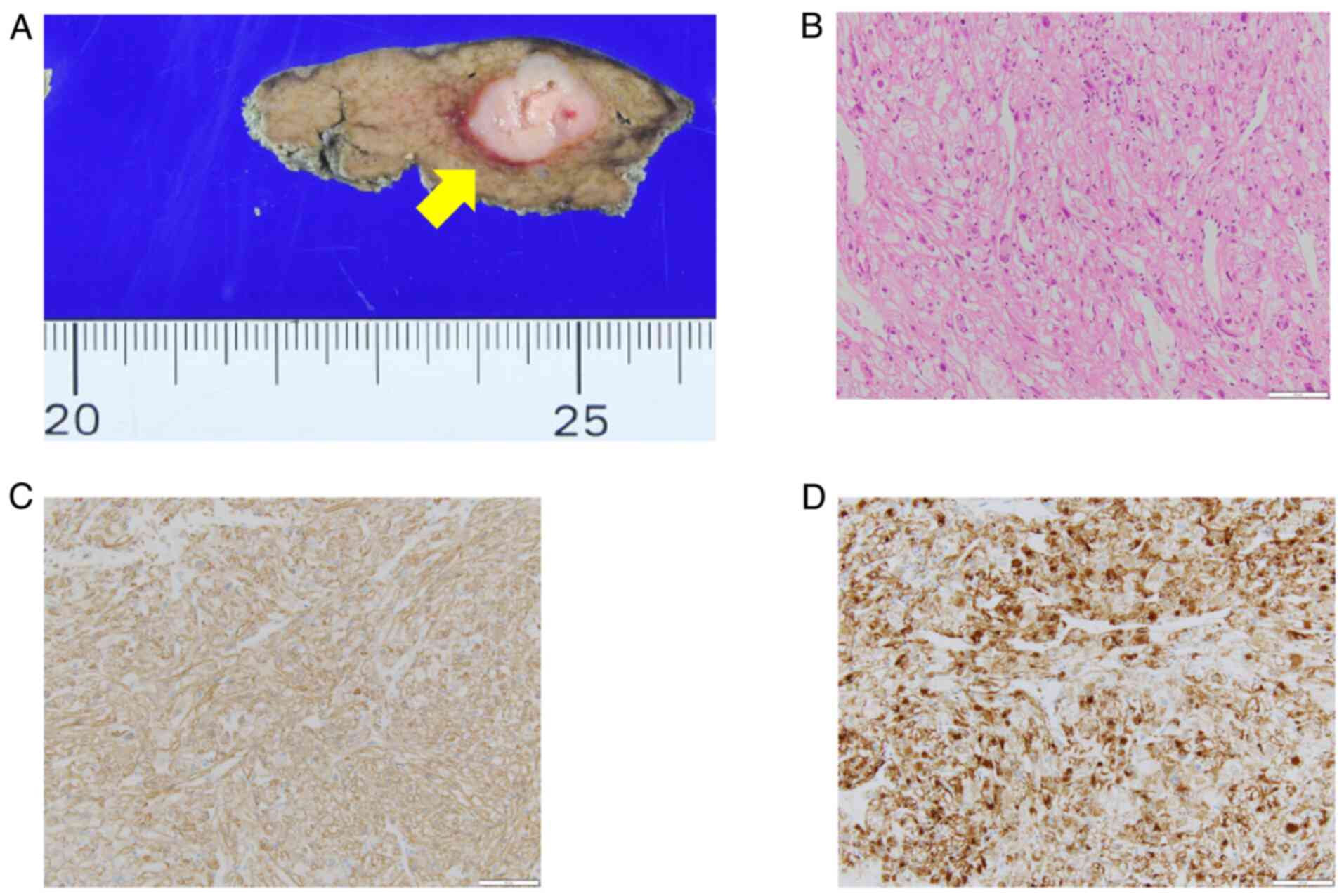
Histopathological findings showed a well-demarcated
white mass with a necrotic center (Fig.
6A). Hematoxylin-Eosin staining showed round, spindle-shaped
cells with pale sporangia against a background of tumor vascular
growth. This indicated nuclear polymorphism and a lack of nuclear
fission (Fig. 6B).
Immunohistochemical staining was positive for SMA and HMB-45
(Fig. 6C and D), leading to diagnosis of PEComa.
In the specimen, there were two nodules in addition
to the primary lesion, which were also PEComas. All lesions lacked
nuclear fission. The three PEComas in the specimen suggest the
possibility of multiple PEComas remaining in the liver. MRI
performed 7 months after surgery showed no significant changes. The
patient is currently under follow-up, with no recurrence at 16
months post-surgery. Regular follow-ups, breast MRI, mammography
and abdominal ultrasound and MRI annually and upper- and
lower-gastrointestinal endoscopy every 2-5 years are planned.
Discussion
LFS is an autosomal dominant syndrome that results
in multiple types of cancer. To the best of our knowledge, <400
families have been reported with this syndrome. Mutations in the
TP53 gene are present in 50-80% of affected families. This
syndrome has a high incidence of malignancy at a young age and a
high frequency of breast cancer, osteosarcoma, soft tissue sarcoma
[penetrance: 14.3-26.7%, SIR: 302.8 (130.4-596.8), RR: 61
(33-102)], brain tumor [penetrance: 5.4-13.0%, SIR: 45.0
(9.0-131.5), RR: 35 (19-60)] and adrenocortical cancer [penetrance:
1.7-13.0%, SIR: unknown, RR: 2047 (455-9212)]. These tumors are
defined as ‘core tumors’ in LFS. Other types of cancer, such as
hematological malignancy, epithelial cancer and pediatric cancers
such as neuroblastoma, can also develop (14). The lifetime probability of
developing cancer of a TP53 pathological variant carrier is
~75% in males and almost 100% in females (15).
PEComas were first described by Bonetti et al
(6) in 1992 and are defined as
tumors resulting from PEC differentiation. PEComa is a mesenchymal
tumor composed of cells associated with blood vessel walls that
express melanocytes and smooth muscle markers and is more common in
female patients. PEComa can occur in any organ of the body, but the
kidneys, genitourinary organs and uterus are the most common sites,
whereas tumors of a hepatic origin are relatively rare (16-19).
These include angiomyolipoma, clear-cell tumor and
lymphangioleiomyomatosis. They are mostly benign, but they could
also be malignant (20-22).
Folpe et al (23) reported
PEComa grading based on tumor diameter and pathological findings;
high-risk factors include: i) Tumor diameter >5 cm; ii) invasive
growth pattern; iii) severe nuclear atypia and increased cell
density; iv) fission pattern (>1/50 high-power fields); v)
necrosis and vi) vascular invasion. Cases with ≥2 high-risk factors
are classified as malignant disease, one risk factor (a nuclear
polymorphism or multinucleated giant cells or a tumor diameter
>5 cm) is classified as disease of uncertain malignant potential
and no risk factors are considered benign, as in the present
case.
Hepatic PEComas typically show low signal intensity
on T1-weighted MRI and high signal intensity on T2-weighted MRI
(24); however, the present mass
showed low signal intensity on both T1- and T2-weighted images,
which was atypical, making preoperative diagnosis difficult. This
also suggested a benign tumor, such as a hepatic hemangioma;
nonetheless, the possibility of malignancy (such as metastatic
liver tumor or HCC) could not be ruled out. Some studies have
reported that outflow vessels of hepatic PEComas consist of the
hepatic venous system, distinguishing them from HCC (25,26).
In the present case, the primary tumor was contrast-enhanced in a
ring shape in the early phase and washed out in the late phase;
therefore, the possibility of malignancy could not be ruled out. As
PEComa is associated with upregulation of the mTOR pathway, which
regulates glucose transporter-1 function,
fluorodeoxyglucose-positron emission tomography/CT findings would
likely have been positive (27,28).
The primary lesion was located at the S8 of the
liver, just below the diaphragm; therefore, liver biopsy was
difficult. Laparoscopic excisional biopsy is less invasive and
useful for lesions for which malignancy cannot be ruled out.
Alterations in three main pathways have been
described in PEComa pathogenesis. LOH (loss of function) in
tuberous sclerosis complex subunits 1 (~27%) and 2 (~73%) is the
most common (29,30). Rearrangement affecting transcription
factor binding to immunoglobulin heavy contrast mu enhancer 3,
which is implicated in cell differentiation, is another key
molecular feature of PEComa pathogenesis (23%) (29,30).
Rearrangements of RAD51 binding protein B have been identified in
uterine PEComas (29).
To the best of our knowledge, there have only been
six reported cases of LFS complicated by PEComa, including the
present case, with two other cases being siblings of the present
patient, both diagnosed with LFS (Table
I) (8-11).
All six patients (four female and two male) were aged <50 years.
Although the observation period was ≤3 years in each case, none of
the patients experienced a recurrence of PEComa. In all six cases,
the tumors did not appear to be highly malignant.
 | Table IReported cases of Li-Fraumeni syndrome
complicated by perivascular epithelioid cell tumor. |
Table I
Reported cases of Li-Fraumeni syndrome
complicated by perivascular epithelioid cell tumor.
| Case no. | Age, years | Sex | Organ | Treatment | Follow-up | First author,
year | (Refs.) |
|---|
| 1 | 24 | F | Kidney, liver | Surgery | No recurrence at 1
year | Neofytou et
al, 2015 | (9) |
| 2 | 29 | F | Liver | Surgery | No recurrence at 1
year | Galera López et
al, 2020 | (8) |
| 3 | 27 | M | Liver | Surgery | No recurrence at 9
months | Galera López et
al, 2020 | (8) |
| 4 | 38 | M | Femoral muscle, lung
metastasis | Surgery;
chemoradiation | No recurrence at 7
months | Butz et al,
2022 | (10) |
| 5 | 49 | F | Liver | Surgery | No recurrence at 3
years | Yang et al,
2024 | (11) |
| 6 | 32 | F | Liver | Surgery | No recurrence at 16
months | Present case,
2024 | - |
The etiology of PEComa and its association with LFS
are unknown; however, Butz et al (10) identified site-specific LOH in PEComa
tissue with respect to a novel TP53 pathogenic variant. This
suggests a role of the defective TP53 pathway in the
pathogenesis of PEComa, which is also associated with the malignant
and metastatic form of this tumor type.
Cancer surveillance and treatment for the present
patient will be continued in accordance with the medical guidelines
for LFS 2019, version 1.1(14). The
most common cause of PEComa recurrence is residual liver; however,
pulmonary metastases have also been reported (31). When PEComa recurs, re-resection
should be considered if possible. Nab-sirolimus, an mTOR inhibitor,
was recently demonstrated as effective against PEComa (32). The patient will undergo breast MRI,
mammography and abdominal ultrasound and MRI annually and upper-
and lower-gastrointestinal endoscopy every 2-5 years due to the
presence of LFS. An orthopedic surgeon and dermatologist will also
have annual consultations.
Further case series are required to determine the
association between LFS and PEComa.
Although rare, the possibility of a PEComa should be
considered when liver tumors are found in patients with LFS.
Laparoscopic liver resection is beneficial as a diagnostic
treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RT and MT conceived and designed the study. MT
revised the manuscript. MT, THa and RT performed surgery. DI
performed preoperative examination and postoperative follow-up. JA
made the diagnosis based on the imaging findings. HO performed the
pathological diagnosis. THi and YM decided on a treatment plan for
the patient. RT and MT confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li FP, Fraumeni JF Jr, Mulvihill JJ,
Blattner WA, Dreyfus MG, Tucker MA and Miller RW: A cancer family
syndrome in twenty-four kindreds. Cancer Res. 48:5358–5362.
1988.PubMed/NCBI
|
|
2
|
Yoshida GJ, Fuchimoto Y, Osumi T, Shimada
H, Hosaka S, Morioka H, Mukai M, Masugi Y, Sakamoto M and Kuroda T:
Li-Fraumeni syndrome with simultaneous osteosarcoma and liver
cancer: Increased expression of a CD44 variant isoform after
chemotherapy. BMC Cancer. 12(444)2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Amadou A, Achatz MIW and Hainaut P:
Revisiting tumor patterns and penetrance in germline TP53 mutation
carriers: Temporal phases of Li-Fraumeni syndrome. Curr Opin Oncol.
30:23–29. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peng G, Bojadzieva J, Ballinger ML, Li J,
Blackford AL, Mai PL, Savage SA, Thomas DM, Strong LC and Wang W:
Estimating TP53 mutation carrier probability in families with
Li-Fraumeni syndrome using LFSPRO. Cancer Epidemiol Biomarkers
Prev. 26:837–844. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gonzalez KD, Noltner KA, Buzin CH, Gu D,
Wen-Fong CY, Nguyen VQ, Han JH, Lowstuter K, Longmate J, Sommer SS
and Weitzel JN: Beyond Li Fraumeni syndrome: Clinical
characteristics of families with p53 germline mutations. J Clin
Oncol. 27:1250–1256. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bonetti F, Pea M, Martignoni G and Zamboni
G: PEC and sugar. Am J Surg Pathol. 16:307–308. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Folpe AL and Kwiatkowski DJ: Perivascular
epithelioid cell neoplasms: Pathology and pathogenesis. Hum Pathol.
41:1–15. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Galera López MDM, Márquez Rodas I, Agra
Pujol C, García Pérez Á, Velasco Sánchez E and Álvarez Álvarez R:
Simultaneous diagnosis of liver PEComa in a family with known
Li-Fraumeni syndrome: A case report. Clin Sarcoma Res.
10(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Neofytou K, Famularo S and Khan AZ: PEComa
in a young patient with known Li-Fraumeni syndrome. Case Rep Med.
2015(906981)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Butz H, Lövey J, Szentkereszty M, Bozsik
A, Tóth E and Patócs A: Case report: A novel pathomechanism in
PEComa by the loss of heterozygosity of TP53. Front Oncol.
12(849004)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Y, Lee J, Woo CG, Lee OJ and Son SM:
Epithelioid angiomyolipoma of the liver in a patient with
Li-Fraumeni syndrome: A case report. Diagn Pathol.
19(16)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Funato M, Tsunematsu Y, Yamazaki F, Tamura
C, Kumamoto T, Takagi M, Kato S, Sugimura H and Tamura K:
Characteristics of Li-Fraumeni syndrome in Japan; a review study by
the special committee of JSHT. Cancer Sci. 112:2821–2834.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sato M, Kojima M, Nagatsuma AK, Nakamura
Y, Saito N and Ochiai A: Optimal fixation for total preanalytic
phase evaluation in pathology laboratories: A comprehensive study
including immunohistochemistry, DNA, and mRNA assays. Pathol Int.
64:209–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kumamoto T, Yamazaki F, Nakano Y, Tamura
C, Tashiro S, Hattori H, Nakagawara A and Tsunematsu Y: Medical
guidelines for Li-Fraumeni syndrome. 2019, version 1.1. Int J Clin
Oncol. 26:2161–2178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bougeard G, Renaux-Petel M, Flaman JM,
Charbonnier C, Fermey P, Belotti M, Gauthier-Villars M,
Stoppa-Lyonnet D, Consolino E, Brugières L, et al: Revisiting
Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol.
33:2345–2352. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jeon IS and Lee SM: Multimodal treatment
using surgery, radiotherapy, and chemotherapy in a patient with a
perivascular epithelioid cell tumor of the uterus. J Pediatr
Hematol Oncol. 27:681–684. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu CH, Chao WT, Lin SC, Lau HY, Wu HH and
Wang PH: Malignant perivascular epithelioid cell tumor in the
female genital tract: Preferred reporting items for systematic
reviews and meta-analyses. Medicine (Baltimore).
98(e14072)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khaja F, Carilli A, Baidas S, Sriharan A
and Norford S: PEComa: A perivascular epithelioid cell tumor in the
liver-a case report and review of the literature. Case Rep Med.
2013(904126)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fang SH, Zhou LN, Jin M and Hu JB:
Perivascular epithelioid cell tumor of the liver: A report of two
cases and review of the literature. World J Gastroenterol.
13:5537–5539. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Armah HB and Parwani AV: Perivascular
epithelioid cell tumor. Arch Pathol Lab Med. 133:648–654.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kirste S, Kayser G, Zipfel A, Grosu AL and
Brunner T: Unresectable hepatic PEComa: A rare malignancy treated
with stereotactic body radiation therapy (SBRT) followed by
complete resection. Radiat Oncol. 13(28)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin KH, Chang NJ, Liou LR, Su MS, Tsao MJ
and Huang ML: Successful management of perivascular epithelioid
cell tumor of the rectum with recurrent liver metastases: A case
report. Medicine (Baltimore). 97(e11679)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Folpe AL, Mentzel T, Lehr HA, Fisher C,
Balzer BL and Weiss SW: Perivascular epithelioid cell neoplasms of
soft tissue and gynecologic origin: A clinicopathologic study of 26
cases and review of the literature. Am J Surg Pathol. 29:1558–1575.
2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tan Y, Zhang H and Xiao EH: Perivascular
epithelioid cell tumour: Dynamic CT, MRI and clinicopathological
characteristics-analysis of 32 cases and review of the literature.
Clin Radiol. 68:555–561. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng RQ, Kudo M, Ishikawa E, Chung H,
Minami Y, Ogawa C, Sakaguchi Y, Kitano M, Kawasaki T and Maekawa K:
Hepatic angiomyolipoma: Identification of an efferent vessel as a
hepatic vein by contrast-enhanced harmonic sonography. J Med
Ultrason (2001). 32:191–196. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Akitake R, Kimura H, Sekoguchi S, Nakamura
H, Seno H, Chiba T and Fujimoto S: Perivascular epithelioid cell
tumor (PEComa) of the liver diagnosed by contrast-enhanced
ultrasonography. Intern Med. 48:2083–2086. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun L, Sun X, Li Y and Xing L: The role of
(18)F-FDG PET/CT imaging in patient with malignant PEComa treated
with mTOR inhibitor. Onco Targets Ther. 8:1967–1970.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takahashi M, Nojima H, Kuboki S, Horikoshi
T, Yokota T, Yoshitomi H, Furukawa K, Takayashiki T, Takano S and
Ohtsuka M: Comparing prognostic factors of Glut-1 expression and
maximum standardized uptake value by FDG-PET in patients with
resectable pancreatic cancer. Pancreatology. 20:1205–1212.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Agaram NP, Sung YS, Zhang L, Chen CL, Chen
HW, Singer S, Dickson MA, Berger MF and Antonescu CR: Dichotomy of
genetic abnormalities in PEComas with therapeutic implications. Am
J Surg Pathol. 39:813–825. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bing Z, Yao Y, Pasha T, Tomaszewski JE and
Zhang PJ: p53 in pure epithelioid PEComa: An immunohistochemistry
study and gene mutation analysis. Int J Surg Pathol. 20:115–122.
2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rakheja R, Abikhzer G, Alabed YZ, Nahal A
and Lisbona R: The appearance of osseous PEComa on F-18 FDG PET/CT.
Clin Nucl Med. 37:190–192. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wagner AJ, Ravi V, Riedel RF, Ganjoo K,
Van Tine BA, Chugh R, Cranmer L, Gordon EM, Hornick JL, Du H, et
al: nab-Sirolimus for patients with malignant perivascular
epithelioid cell tumors. J Clin Oncol. 39:3660–3670.
2021.PubMed/NCBI View Article : Google Scholar
|