Introduction
Toxic encephalopathy (TE) is a type of disease that
results from long-term or excessive exposure to toxic substances,
causing damage to the central nervous system. Diffuse white matter
lesions and myelin sheath destruction are the main pathological
manifestations. Acute organic solvent toxic encephalopathy (AOSTE)
usually onsets within days or weeks (1). On magnetic resonance imaging (MRI)
images, acute and subacute OSTE are mainly characterized by
bilateral symmetrical diffuse involvement of white matter areas,
basal ganglia and dentate nuclei. Some cases may involve cerebellar
white matter and the brainstem, and some severe cases can manifest
as whole brain swelling, shallow cerebral sulcus, extensive
symmetrical white matter edema and an unclear gray white matter
boundary (2,3).
Early diagnosis and treatment play a significant
role in improving the prognosis of AOSTE patients. Due to the lack
of effective treatments, the prevention of OSTE is particularly
significant. It is necessary to further improve occupational health
laws and regulations, strictly control the exposure of harmful
substances in the production environment, and improve the on-site
working environment (4). The
present study reports on the diagnosis and treatment processes of a
patient with AOSTE, which may have been caused by occasional ink
leakage (Xuzhou, Jiangsu China), and conducted a combined
literature review analysis to provide reference for the diagnosis
and treatment of this disease.
Case description
A 42-year-old male patient from Xuzhou (Jiangsu,
China), visited The Second Affiliated Hospital of Xuzhou Medical
University (Xuzhou, China) in April 2023, due to sudden dizziness
with unstable walking for 24 h, which was aggravated for 2 h. The
patient experienced dizziness 24 h ago during work with no apparent
reason, accompanied by unstable walking, leaning to one side while
walking and symptoms such as slow reaction and limb weakness. The
patient has been engaged in handling work in a printing factory for
3 years and did not come into contact with printing ink on
weekdays, so he was not classified as a toxic worker. The patient
was in good health, with no history of infectious disease, surgery,
blood transfusion or family history.
Neurological examination on admission: Clear mind,
slow reaction, decreased intelligence and lack of cooperation in
physical examination; bilateral Babinski sign was not extracted,
Romberg sign was not cooperating, finger-nose test was accurate,
neck was soft and Kernig sign was negative.
Electroencephalogram (EEG) examination: Numerous
paroxysmal medium-to-high amplitude θ waves in all leads.
Laboratory examination: Serum amyloid protein A,
>200.00 mg/l (reference range, <10.00); D-dimer, 457.00 ng/ml
(reference range, 0.00-243.00); alanine aminotransferase, 159 U/l
(reference range, ≤55.00); aspartate aminotransferase, 67 U/l
(reference range, 5.00-34.00); R-glutamyltransferase, 168 U/l
(reference range, 10.00-50.00); Urocholenogen (++); hippuric acid
(HA), 1,700 µg/ml (reference range, 40.00-70.00); blood routine
(-); tumor markers (-); serum Li, 1.60 µg/l (reference range,
0.00-72.00); Mg, 1.72 mmol/l (reference range, 1.12-2.06); Ca, 1.68
mmol/l (reference range, 1.50-2.50); Mn, 14.98 µg/l (reference
range, 3.00-36.00); Fe, 7.29 mmol/l (reference range, 4.91-9.47);
Cu, 22.11 µmol/l (reference range, 6.30-28.35); Zn, 99.20 µmol/l
(reference range, 47.33-103.00); Se, 127.81 µg/l (reference range,
69.01-147.00); Cd, 1.77 µg/l (reference range, 0.00-5.00); Hg, 1.69
µg/l (reference range, 0.00-14.90); Pb, 15.58 µg/l (reference
range, 0.00-100.00); V, 0.76 µg/l (reference range, 0.00-5.00); Cr,
1.04 µg/l (reference range, 0.00-10.00); Co, 0.19 µg/l (reference
range, 0.00-3.00); As, 0.75 µg/l (reference range, 0.00-23.00); Sr,
17.68 µg/l (reference range, 10.00-45.00); Mo, 0.89 µg/l (reference
range, 0.00-3.30).
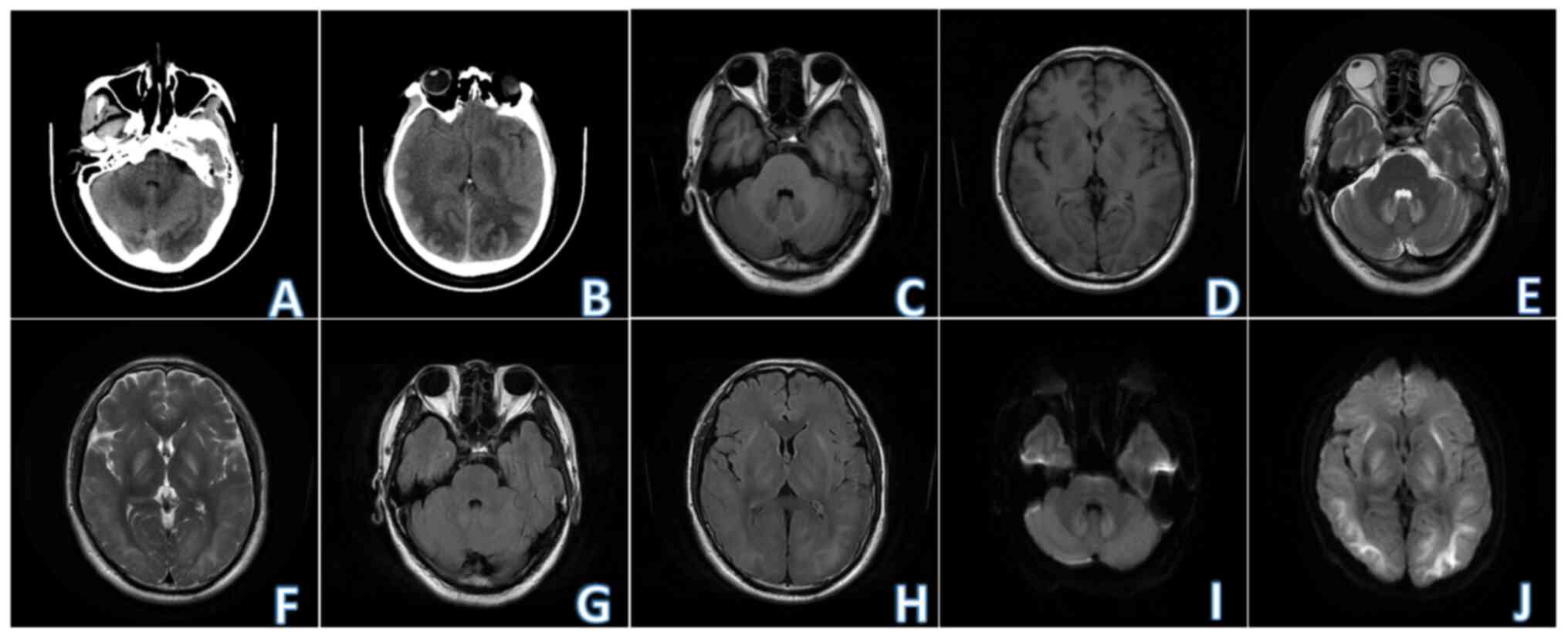
Radiological examination: Non-contrast brain
computed tomography (2023-04) revealed multiple ill-defined
symmetrical patchy low-density shadows in bilateral basal ganglia,
bilateral frontal parietal temporal occipital white matter areas
and bilateral cerebellar dentate nuclei. Non-contrast brain MRI
(2023-04) revealed the presence of multiple symmetrical patchy
T1-weighted imaging (T1WI) hypo-intensity, T2-weighted imaging
(T2WI) hyper-intensity, T2-fluid attenuated inversion recovery
hyper-intensity, diffusion-weighted imaging (DWI) hyper-intensity,
with blurred edges, in bilateral basal ganglia (outer capsule and
globus pallidus), thalamus, frontal parietal temporal occipital
white matter area and bilateral cerebellar dentate nuclei (Fig. 1).
Based on the symptoms and signs, laboratory
examinations and radiological examinations of the patient, a
preliminary diagnosis of TE was made. As disclosed by the
supervisor of the patient's printing factory, ink leakage may
occurred recently in the printing house due to reasons like machine
aging. The ink composition was as follows: 30% polyurethane resin
liquid, 30% titanium dioxide, 10% ternary chlorine resin liquid, 7%
isopropyl alcohol, 7% n-propyl acetate and 16% ethyl acetate.
Combined with an abnormally elevated HA on urinalysis, the patient
was ultimately diagnosed with AOSTE.
The patient received the following treatment
measures, including: i) Hyperbaric oxygen therapy to increase blood
oxygen levels; ii) dehydration of mannitol and glycerol fructose to
reduce intracranial pressure; iii) antioxidants to protect the
brain and nerve cells and promote brain function recovery; iv) high
dose B vitamins to nourish nerves; v) Butylphthalide to improve
mitochondrial metabolism, and Edaravone to eliminate free radicals;
vi) antibiotics to prevent secondary infections; and vii) potassium
chloride and sodium chloride to maintain water electrolyte and
acid-base balance.
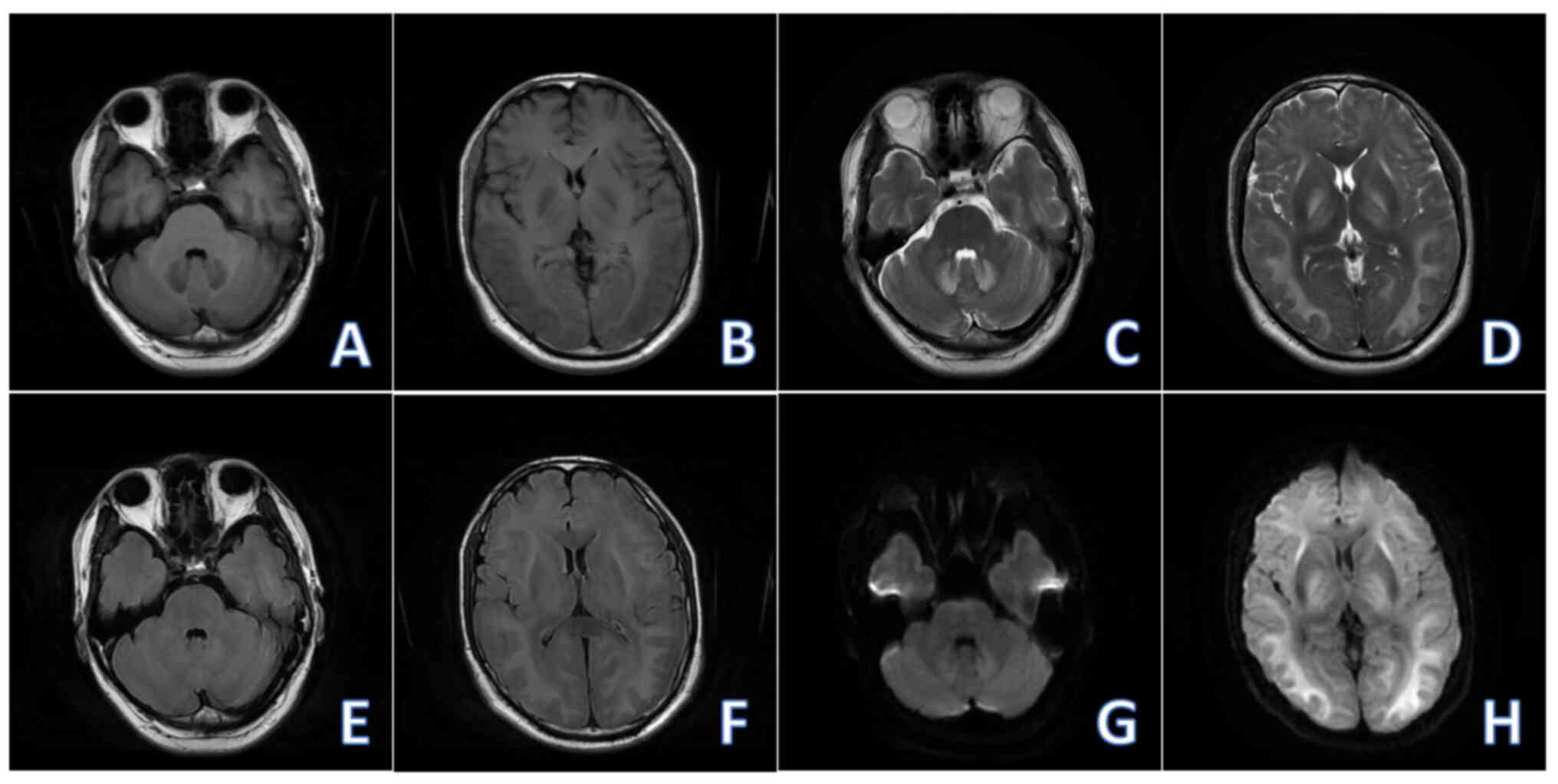
Non-contrast brain MRI (2023-04) revealed reductions
in the extent of the abnormal signal in the brain and in the DWI
intensity of the lesion compared with the MRI performed on 2023-04
(Fig. 2). After 14 days of
treatment, the symptoms of dizziness and unstable walking in the
patient improved, but there were still symptoms such as decreased
memory and delayed reactions, and the patient requested
discharge.
After discharge, the patient took medicine
regularly: Butylphthalein soft capsules for 2 capsules/Tid;
Coenzyme Q10 for 1 capsule/Tid; and Mecobalamin capsules for 1
capsule/Tid. After 1 month of discharge, the patient was followed
up at the outpatient department, and his symptoms such as
dizziness, unstable walking and decreased memory improved.
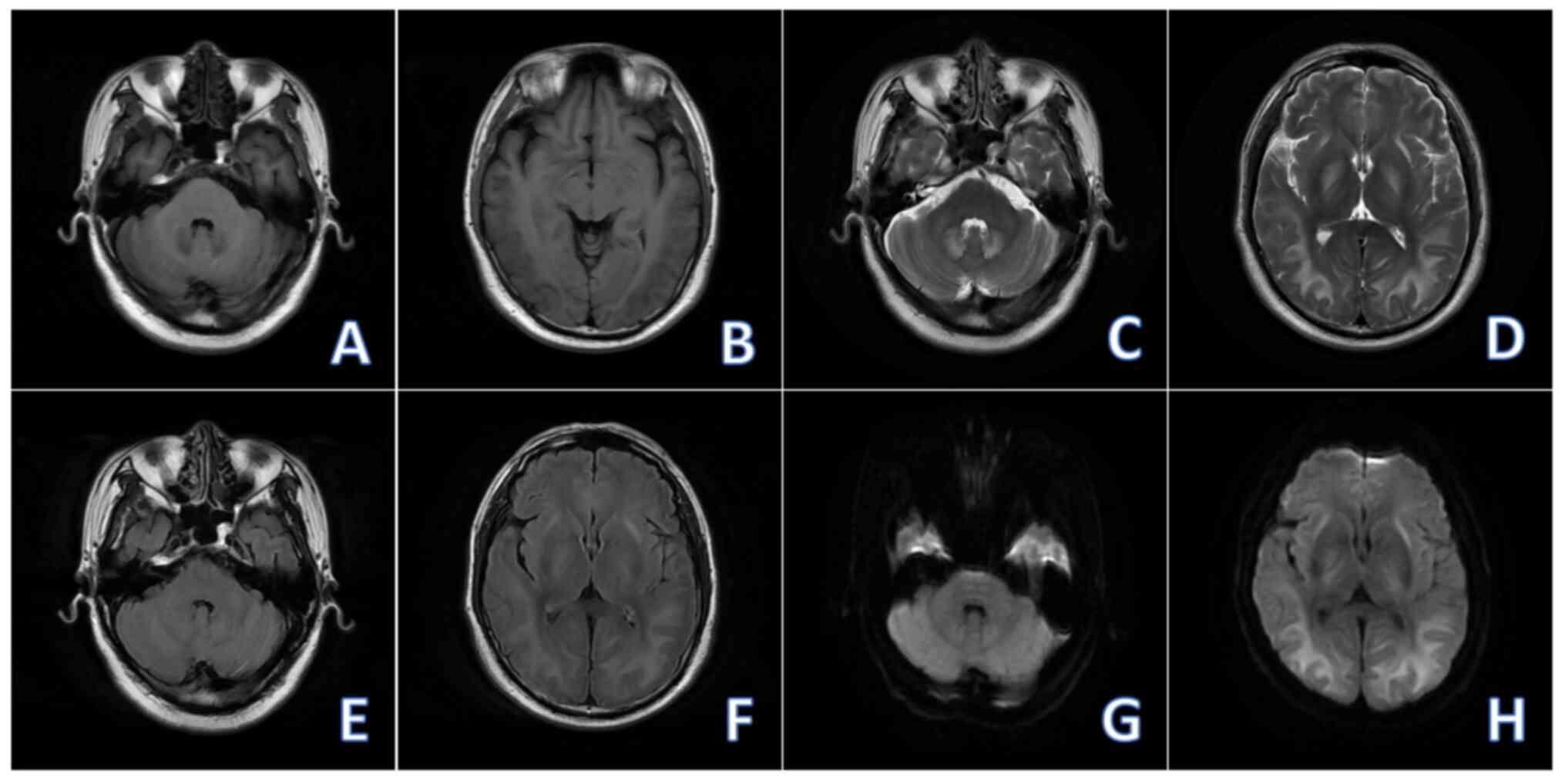
Non-contrast brain MRI (2023-05) revealed reductions in the extent
of the abnormal signal in the brain and in the DWI intensity of the
lesion compared with the MRI performed on 2023-04 (Fig. 3). At 6 months of discharge, the
patient was followed up at the outpatient department, and his
symptoms such as dizziness, unstable walking and decreased memory
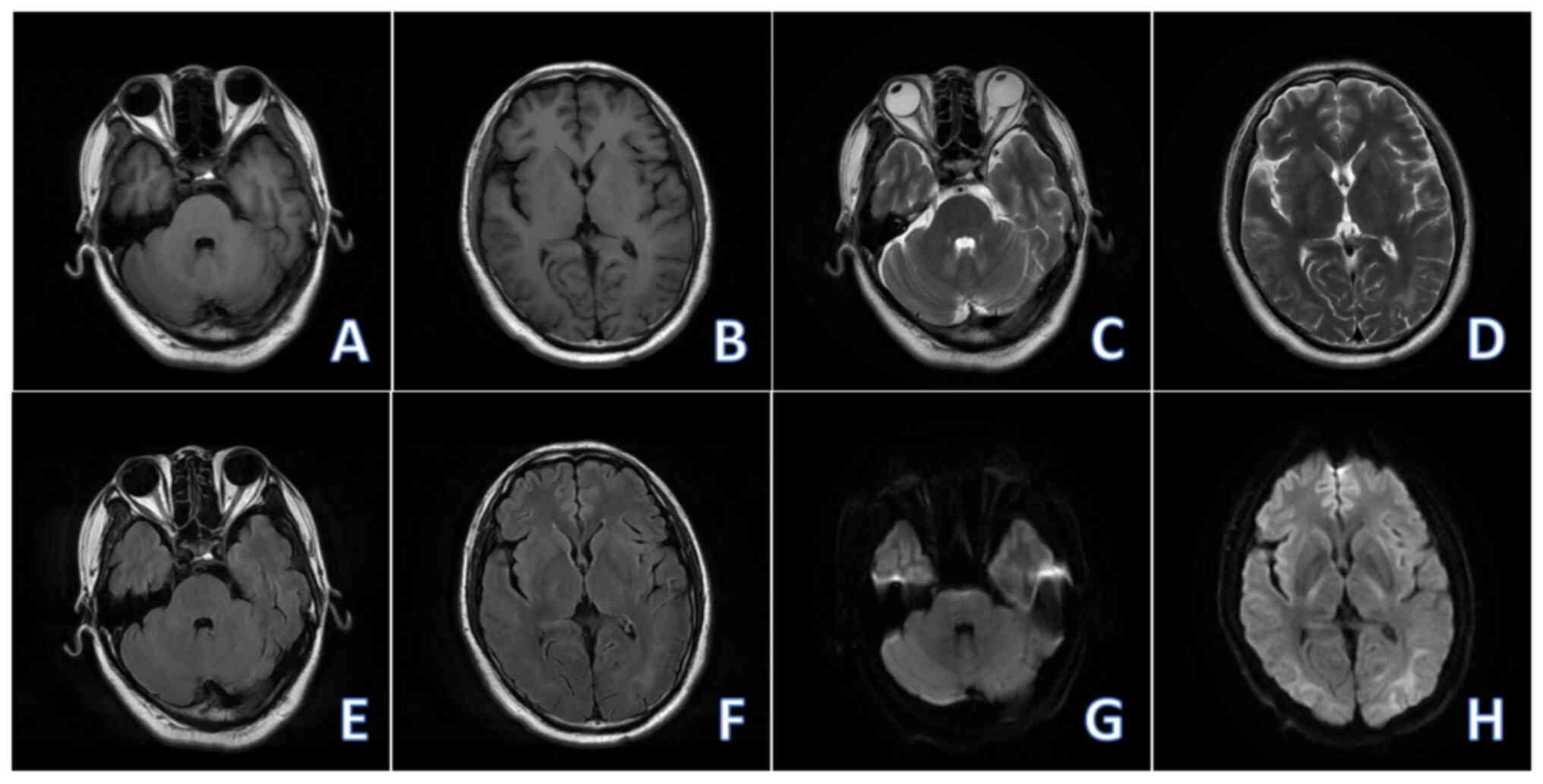
significantly improved. Non-contrast brain MRI (2023-10) revealed
the decreased range of abnormal signals in the brain compared with
the MRI performed on (2023-05), and the DWI signal of the lesion
tended to be normal, with no new lesions being observed (Fig. 4).
The most recent follow-up of the patient was in
April 2024. The patient has resigned from the printing factory and
become a mall cleaner. The patient's symptoms such as dizziness,
unstable walking and decreased memory have basically disappeared.
The patient only underwent EEG examination in April 2024, and the
result was negative. We will conduct long-term follow-up on this
patient and strengthen the management of similar patients.
Discussion
TE is a disease caused by long-term or excessive
exposure to toxic substances, which can lead to damage to the
central nervous system (CNS). It mainly displays the pathological
manifestations of diffuse white matter lesions and myelin sheath
destruction (1,2). OSTE is a subtype of TE. Organic
solvents are volatile at room temperature and show strong
lipophilicity (4). They can enter
human body through respiratory inhalation and direct contact with
skin and mucous membranes, and are easily deposited in the
lipid-rich brain tissue through the blood-brain barrier, thereby
causing damage to the myelin sheath (3,4).
The common organic solvent components with CNS
toxicity include alkanes, alcohols, esters, ethers, ketones,
nitrosamines and aldehydes. In most cases, organic solvents exist
in the form of composite components. Organic solvents can cause
lipid peroxidation and excessive generation of free radicals in
metabolism, thereby disrupting the stability of cell membranes and
leading to myelin degeneration (5,6).
Organic solvents can also directly interfere with the function of
central neurons and induce acute CNS damage (7,8). OSTE
lacks specific clinical symptoms, and is determined by the specific
damaged brain area, while the severity of symptoms mainly depends
on the concentration and duration of exposure to toxins (9).
Referring to the brain biopsy results of some cases
of OSTE (10), some patients have
white matter atrophy, patchy myelin sheath loss and granular
inclusions with birefringence in macrophages under periodic
acid-Schiff staining (7). No
significant axonal loss was observed using the modified
Bielschewsky method and immunofluorescence neurofilament staining
(11). Glial fibrillary acidic
protein immunofluorescence and modified phosphotungstic acid
hematoxylin staining (12,13) showed small astrocyte microfilaments,
indicating mild chronic reactive astrocyte proliferation. Partial
pathological results still show lymphocyte infiltration in the
white matter of the brain and slight Purkinje cell loss in the
vermis of the cerebellum.
The patient in the present case was a porter from a
printing factory and does not belong to the category of toxic
workers. It is hypothesized that the illness in this patient is
that the printing factory may have recently experienced ink
leakage, and the organic solvent component toluene in the ink can
be directly absorbed into the bloodstream through the respiratory
tract, finally causing damage to the CNS. The absorbed toluene can
be metabolized into benzoic acid via a mixed oxidase system, and it
then combines with glycine to produce the product HA, which is
excreted into urine (8). Therefore,
the concentration of HA in urine can indirectly reflect the level
of toluene exposure (14,15). EEG can display the electrical
activity of the brain, and patients with OSTE may experience whole
brain or focal slow waves, which may serve as an auxiliary means
for early diagnosis (16). MRI is
an important tool for the diagnosis of OSTE, especially in the
acute and subacute stages. MRI can display bilateral symmetrical or
diffuse white matter damage and involvement of gray matter nuclei.
In addition, imaging changes may partially or completely recover
after the improvement of the patient's clinical symptoms (17). In the present case, the non-contrast
brain MRI of the patient revealed symmetrical abnormal signals in
the bilateral basal ganglia, thalamus, frontal parietal temporal
occipital white matter area, and bilateral cerebellar dentate
nuclei. As the symptoms improved after treatment, the abnormal
signal range and signal intensity on the MRI images gradually
decreased.
The symptoms of AOSTE usually develop a few days or
weeks after contracting the disease. Mild cases may present with
symptoms including headache, dizziness, nausea and vomiting,
fatigue, delayed response, and ataxia, while severe cases may
experience symptoms such as blurred consciousness, epileptic
seizures, coma and even death (18-20).
Mahavar et al (21) reported
a case of bilateral basal ganglia cerebral hemorrhage caused by
accidental ingestion of toluene, and the patient ultimately died of
cardiac arrest. Although AOSTE can cause severe damage to the CNS
in the short term, most patients have a good prognosis so long as
the exposure to toxins is removed early and appropriate treatment
is received. Therefore, timely detection and identification of
AOSTE and targeted treatment for patients are of particular
significance (22). The patients
reported in the present article had an improved prognosis thanks to
timely diagnosis and treatment.
Acute and subacute OSTE are mainly characterized by
bilateral symmetrical, diffuse involvement of white matter areas,
basal ganglia and dentate nuclei on MRI images. Some cases may
involve cerebellar white matter and the brainstem, and some severe
cases can manifest as whole brain swelling, shallow cerebral
sulcus, extensive symmetrical white matter edema and unclear gray
white matter boundary. As the clinical symptoms in the patient
improve, the aforementioned radiological manifestations can
partially or completely disappear (23). Chronic OSTE is mainly featured by
mild to moderate cortical and cerebellar atrophy on MRI images,
multifocal white matter damage mainly involving the paraventricular
and semioval center or diffuse symmetrical supratentorial white
matter lesions (24,25). The degree of imaging damage is
positively correlated with the duration of exposure to toxins
(17,26).
Based on the analysis of clinical data from five
patients with subacute OSTE and neurological injury, He et
al (27) assumed that the
latent period of subacute neurological injury caused by OSTE is
mostly 3 months, with a short exposure time and hidden onset.
Therefore, for organic solvent workers with central and peripheral
nervous system injuries, the first physician should closely monitor
the patient's occupational exposure history to avoid misdiagnosis
and missed diagnosis.
The diagnosis of AOSTE is mainly based on a clear
history of organic solvent exposure, acute onset, clinical symptoms
such as headache, dizziness, drowsiness, blurred consciousness,
coma and epileptic seizures, combined with radiological features
such as bilateral symmetrical white matter damage, involvement of
gray matter nuclei or diffuse involvement of the whole brain, while
excluding other triggers including infection, medication, and
cerebrovascular accidents (28).
Additionally, the diagnosis of chronic OSTE mainly
relies on a clear long-term history of exposure to organic
solvents, symptoms and signs of CNS damage, corresponding
radiological manifestations and the exclusion of other diseases
that may cause similar clinical or radiological manifestations
(29). In line with the diagnostic
criteria established by the World Health Organization in 1985,
chronic OSTE is diagnosed based on the following four criteria
(30): i) A clear history of
exposure to neurotoxic organic solvents, with a certain incubation
period before the onset of neurological symptoms; ii) typical
subjective symptoms of CNS damage, such as decreased memory and
attention, depression, irritability, sleep disorders and fatigue;
iii) objective evidence of decreased memory and attention found
from standard neuropsychological tests; and iv) exclusion of
primary organic or primary psychiatric symptoms. In 2012, a
European conference consensus proposed that the diagnosis of
chronic OSTE should encompass at least 5-10 years of exposure to
organic solvents, and objective examination of cognitive function
and quantitative evaluation of clinical symptoms should be
emphasized during diagnosis (31).
Meanwhile, joint evaluation by neurologists, occupational disease
specialists and neuropsychologists is warranted before the
diagnosis is made. If necessary, the participation of psychiatrists
and toxicologists is also required (31).
Zheng (32)
retrospectively analyzed the radiology characteristics of eight
patients with acute/subacute OSTE and concluded that acute/subacute
OSTE extensively affects white matter, but the lateral ventricles,
corpus callosum, and temporal polar white matter are not easily
affected. This may be a key point in distinguishing it from other
TE on radiology examinations.
The MRI manifestations of AOSTE are similar to those
caused by various diseases, therefore, differential diagnosis
should be conducted as follows (33): i) Wernicke's encephalopathy: A
metabolic disorder of the CNS caused by vitamin B1 deficiency due
to chronic alcoholism, which mainly affects the white matter around
the third ventricle and midbrain aqueduct. ii) Carbon monoxide
toxic encephalopathy: Can be divided into three types, including
mainly involving white matter, mainly involving neural nuclei and
mainly involving the cortex. MRI findings are characterized by
limited diffusion of bilateral pallidum, and the disease can be
distinguished based on a history of toxic contact (34). iii) Neuronal intranuclear inclusion
body disease (NIBD): MRI manifestations of adult NIBD show a high
DWI signal at the corticomedullary junction, which appears as
‘serrated’ or ‘flame-like’, but does not involve the deep white
matter (35). iv) Hepatolenticular
degeneration (HLD): Clinical manifestations include progressive
liver damage, neuropsychiatric disorders and corneal pigment ring
(36). Brain MRI findings reveal a
low intensity on T1WI image and a high intensity on T2WI image of
the lenticular nucleus, age-unmatched brain atrophy and brainstem
atrophy (mainly medullary atrophy), while white matter lesions of
OSTE are mainly characterized by atrophy (37). In addition, the ‘panda face sign’ in
the midbrain is a specific radiological manifestation of HLD
(38).
At present, due to the lack of corresponding
antidotes, there is no specific treatment for OSTE. Typically,
early diagnosis and treatment play a significant role in improving
the prognosis of patients with AOSTE. In this regard, it is
necessary to strengthen the understanding of AOSTE in medical
personnel, especially emergency medical personnel. When receiving
patients with suspected OSTE, it is necessary to inquire if they
have a history of exposure to organic solvents, such as those
working in the chemical or printing industry, understand the types
of organic solvents the patients have been exposed to, the exposure
time, environmental ventilation and personal protective measures,
and ask if the patients have symptoms such as dizziness, headache,
nausea, fatigue and lack of concentration (39,40).
Besides, it is important to detect the metabolites of organic
solvents, such as HA (an indicator of toluene exposure),
methylhippuric acid (an indicator of xylene exposure) and
2-thiothiazolidine-4-carboxylic acid (an indicator of carbon
disulfide exposure), by using biological samples including blood
and urine, so as to assess the patients' recent exposure to organic
solvents (41,42).
The treatment principles for TE mainly include
removing the cause, reducing cerebral edema, improving cerebral
circulation and protecting nerve cells. Notably, comprehensive
treatment measures such as hyperbaric oxygen therapy, hormone
anti-inflammatory therapy, dehydration and intracranial pressure
reduction, have important application value in the treatment of TE.
For patients with acute onset, symptomatic supportive treatment
remains the main approach. For those with severe conditions, the
early application of high-dose glucocorticoids and sufficient
treatment duration of mannitol can timely control symptoms and
prevent disease deterioration. For patients with combined emotional
disorders and sleep disorders, symptomatic treatment should be
given (43).
Due to the lack of effective treatments, the
prevention of OSTE is particularly significant. It is necessary to
further improve occupational health laws and regulations, strictly
control the exposure of harmful substances in the production
environment and improve the on-site working environment. Meanwhile,
it is necessary to strengthen safety protection and regular health
checks for toxic workers. In addition, it is also greatly needed to
enhance occupational health promotion, raise workers' awareness of
protection and reduce exposure to toxic substances. Once suspicious
symptoms appear, one should immediately leave the relevant work
environment and receive early diagnosis and treatment (44).
The main clinical manifestations of the present
patient were dizziness with unstable walking, and non-contrast
brain MRI showed symmetrical abnormal signals in the bilateral
basal ganglia, thalamus, frontal parietal temporal occipital white
matter area and bilateral cerebellar dentate nuclei. In laboratory
tests, the level of HA significantly increased, indirectly
reflecting the exposure level of toluene (an organic solvent
component in ink). Therefore, in this case, a possible history of
ink exposure, brain MRI findings and HA testing were indispensable
factors in the diagnosis.
In summary, AOSTE should be a part of the
differential diagnosis in any patient with acute neurobehavioral
and neurological deficits. Brain MRI and urine testing are
important for diagnosis. In addition, early diagnosis and treatment
play a significant role in improving the prognosis of patients with
AOSTE. Therefore, when AOSTE is detected and recognized in a timely
manner, patients should be treated with strategies such as
hyperbaric oxygen, anti-inflammatory, cranial pressure lowering and
neurotrophic therapy.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Key R&D Project of
Xuzhou Science and Technology Bureau (grant no. KC23208).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW and PD conceived and designed the study,
collected and assembled the data and wrote and revised the
manuscript. All authors contributed to the article and read and
approved the final manuscript. YJW and PD confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The study involving human participant was reviewed
and approved by Ethics Committee of The Second Affiliated Hospital
of Xuzhou Medical University (grant no. KY-20241201). The patient
provided his written informed consent to participate in this
study.
Patient consent for publication
The patient provided consent for his/her information
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dobbs MR: Toxic encephalopathy. Semin
Neurol. 31:184–193. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Agarwal A and Vancil T: Toxic
encephalopathy. J Gen Intern Med. 27:876–877. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li XL, Zhao HT, Han J, Yan ZR and Wang HY:
Toxic encephalopathy induced by radix Sophorae tonkinensis. Acta
Neurol Belg. 122:855–858. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sainio MA Sr: Neurotoxicity of solvents.
Handb Clin Neurol. 131:93–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ikeda M: Public health problems of organic
solvents. Toxicol Lett. 64-65:191–201. 1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hedström AK, Hössjer O, Katsoulis M,
Kockum I, Olsson T and Alfredsson L: Organic solvents and MS
susceptibility: Interaction with MS risk HLA genes. Neurology.
91:e455–e462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Filley CM, Halliday W and
Kleinschmidt-DeMasters BK: The effects of toluene on the central
nervous system. J Neuropathol Exp Neurol. 63:1–12. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Win-Shwe TT and Fujimaki H: Neurotoxicity
of toluene. Toxicol Lett. 198:93–99. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Greenberg MM: The central nervous system
and exposure to toluene: A risk characterization. Environ Res.
72:1–7. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ridgway P, Nixon TE and Leach JP:
Occupational exposure to organic solvents and long-term nervous
system damage detectable by brain imaging, neurophysiology or
histopathology. Food Chem Toxicol. 41:153–187. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eaker EY, Shaw G and Sninsky CA:
Neurofilament immunoreactivity in myenteric neurons differs from
that found in the central nervous system. Gastroenterology.
99:1364–1371. 1990.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heimfarth L, Passos FRS, Monteiro BS,
Araújo AAS, Quintans Júnior LJ and Quintans JSS: Serum glial
fibrillary acidic protein is a body fluid biomarker: A valuable
prognostic for neurological disease-A systematic review. Int
Immunopharmacol. 107(108624)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shum MW and Hon JK: A modified
phosphotungstic acid haematoxylin stain for formalin-fixed tissues.
J Med Lab Technol. 26:38–42. 1969.PubMed/NCBI
|
|
14
|
Oginawati K, Anka AAH, Susetyo SH,
Febriana SA, Tanziha I and Prakoeswa CRS: Urinary hippuric acid
level as a biological indicator of toluene exposure on batik
workers. Heliyon. 7(e07775)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shan X, Zhang L, Ye H, Shao J, Shi Y, Tan
S, Zhang L and Su K: Analytical techniques for monitoring of
toluene and xylene exposure biomarkers hippuric acid and
methylhippuric acid in human urine samples. Bioanalysis.
13:1569–1584. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Keski-Säntti P, Kovala T, Holm A,
Hyvärinen HK and Sainio M: Quantitative EEG in occupational chronic
solvent encephalopathy. Hum Exp Toxicol. 27:315–320.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aydin K, Sencer S, Demir T, Ogel K, Tunaci
A and Minareci O: Cranial MR findings in chronic toluene abuse by
inhalation. AJNR Am J Neuroradiol. 23:1173–1179. 2002.PubMed/NCBI
|
|
18
|
Mi T, Han C, Wang Y, Ma H, Jia J, Ding Y,
Esmail F, Chen J, Peng L, Xu J and Sun YX: Acute toxic
leukoencephalopathy in migrant workers exposed to organic solvents
in construction materials. Occup Environ Med. 70:435–436.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu JR, Fang S, Ding MP, Chen ZC, Zhou JJ,
Sun F, Jiang B and Huang J: Toxic encephalopathy caused by
occupational exposure to 1, 2-Dichloroethane. J Neurol Sci.
292:111–113. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dick FD: Solvent neurotoxicity. Occup
Environ Med. 63:221–179. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mahavar S, Chaturvedi A, Singh A, Kumar R,
Dariya SS and Sharma R: Toluene poisoning presenting as bilateral
basal ganglia haemorrhage. J Assoc Physicians India. 66:93–94.
2018.PubMed/NCBI
|
|
22
|
Basant N, Gupta S and Singh KP: Predicting
the acute neurotoxicity of diverse organic solvents using
probabilistic neural networks based QSTR modeling approaches.
Neurotoxicology. 53:45–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gaosheng X, Tao J and Min M: The analysis
of clinical and imaging features in 15 patients with occupational
organic solvent poisoning. Stroke Nervous Dis. 28:672–675. 2021.(In
Chinese).
|
|
24
|
Liu J, Zhang L, He B, Zhuang JH, Xu J,
Huang LY and Peng H: Roles of neuroimage in toxic encephalopathy
induced by 1, 2-Dichloroethane. Clin Neurol Neurosurg.
184(105398)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dietemann JL, Botelho C, Nogueira T,
Vargas MI, Audibert C, Abu Eid M, Bogorin A, Bernardo R, Jacques C,
Kremer S and Zöllner G: Imagerie des encephalopathies toxiques
aigues Imaging in acute toxic encephalopathy. J Neuroradiol.
31:313–326. 2004.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
26
|
Keski-Säntti P, Mäntylä R, Lamminen A,
Hyvärinen HK and Sainio M: Magnetic resonance imaging in
occupational chronic solvent encephalopathy. Int Arch Occup Environ
Health. 82:595–602. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He L, Kong D and Jing H: Analysis on
clinical characteristics of five cases of sub-acute organic solvent
poisoning nervous system injuries. Chin J Industrial Med.
36:326–328. 2023.(In Chinese).
|
|
28
|
Donoghue AM, Dryson EW and Wynn-Williams
G: Contrast sensitivity in organic-solvent-induced chronic toxic
encephalopathy. J Occup Environ Med. 37:1357–1363. 1995.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seo S and Kim J: An aggravated
return-to-work case of organic solvent induced chronic toxic
encephalopathy. Ann Occup Environ Med. 30(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Baker EL: Chronic toxic encephalopathy
caused by occupational solvent exposure. Ann Neurol. 63:545–547.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Van Valen E, van Thriel C, Akila R, Nilson
LN, Bast-Pettersen R, Sainio M, van Dijk F, van der Laan G, Verberk
M and Wekking E: Chronic solvent-induced encephalopathy: European
consensus of neuropsychological characteristics, assessment, and
guidelines for diagnostics. Neurotoxicology. 33:710–726.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng X: Imaging features of acute and
subacute toxic encephalopathy induced by organic solvents. J
Practical Radiology. 40:19–21. 2024.
|
|
33
|
Biswas S, Pendharkar HS and Murumkar VS:
MRI Spectrum of toxic encephalopathy-an institutional experience.
Neurol India. 70:1525–1533. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang X, Li Z, Berglass J, He W, Zhao J,
Zhang M, Gao C, Zhang C, Zhang H and Yi X: MRI and clinical
manifestations of delayed encephalopathy after carbon monoxide
poisoning. Pak J Pharm Sci. 29 (Suppl 6):S2317–S2320.
2016.PubMed/NCBI
|
|
35
|
Sone J, Mori K, Inagaki T, Katsumata R,
Takagi S, Yokoi S, Araki K, Kato T, Nakamura T, Koike H, et al:
Clinicopathological features of adult-onset neuronal intranuclear
inclusion disease. Brain. 139:3170–3186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mulligan C and Bronstein JM: Wilson
disease: An overview and approach to management. Neurol Clin.
38:417–432. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bandmann O, Weiss KH and Kaler SG:
Wilson's disease and other neurological copper disorders. Lancet
Neurol. 14:103–113. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Panda AK, Mehta VJ, Dung AA and Kushwaha
S: Face of giant panda sign in Wilson's disease. J Assoc Physicians
India. 62:707–708. 2014.PubMed/NCBI
|
|
39
|
Haibing Z, Guilan O and Yanwei L: Clinical
characteristics of occupational acute organic solvent poisoning
encephalopathy. J Gannan Med Univ. 40:891–895. 2020.
|
|
40
|
Chuan F and Xianwen C: Research progress
on organic solvent toxic encephalopathy. Chin J Neurol. 47:55–58.
2014.
|
|
41
|
Mestdagh I, van Bergen L, Kocken C,
Heyvaert V, Cras P and Van Den Eede F: Diagnosing solvent-induced
chronic toxic encephalopathy: The effect of underperformance in
neuropsychological testing. Int J Psychiatry Clin Pract.
23:171–177. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dryson EW and Ogden JA: Organic solvent
induced chronic toxic encephalopathy: Extent of recovery, and
associated factors, following cessation of exposure.
Neurotoxicology. 21:659–665. 2000.PubMed/NCBI
|
|
43
|
Van Valen E, van Hout ES, Wekking EM,
Lenderink AF, van der Laan G and Hageman G: Brain damage caused by
exposure to organic solvents; diagnostics and disease course of
chronic solvent-induced encephalopathy. Ned Tijdschr Geneeskd.
159(A9431)2015.PubMed/NCBI
|
|
44
|
Van Valen E, Wekking E, van der Laan G,
Sprangers M and van Dijk F: The course of chronic solvent induced
encephalopathy: A systematic review. Neurotoxicology. 30:1172–1186.
2009.PubMed/NCBI View Article : Google Scholar
|