Introduction
Morphine, a typical opioid, is used in clinical
settings to relieve cancer-related pain, especially in patients
with bone cancer (1-4).
However, addiction and dependence remain significant issues that
affect its clinical use (5-8).
In the context of social and public health, drug abuse and
addiction are major public health concerns (9-12).
Addiction is currently viewed as a chronic and relapsing disorder
(13-15).
The process of drug addiction engages reward-related learning and
memory systems, showing synaptic plasticity within these systems
(16-28).
The mechanisms behind opioid addiction are closely related to the
central dopamine reward neural pathway, mainly involving the
prefrontal cortex (29), the
nucleus accumbens (30-32),
and the ventral tegmental area (33-38).
Exploring the neurobiological mechanisms of drug addiction is
crucial for clinical treatment.
Research on opioid addiction mechanisms often
focuses on the morphine dependence model in rodents, usually rats
or mice. The success of this model is assessed through conditioned
place preference (CPP) or self-administration (SA) experiments
(39-43).
However, these methods are time-consuming, labor-intensive, require
long-term behavioral training of animals and are subject to
experimenter bias. CPP is an experimental tool for evaluating
drug-seeking behavior or psychological craving in animals,
requiring the association of a rewarding stimulus with a
non-rewarding conditioned stimulus to confirm the presence of a
rewarding stimulus. This process is tedious, time-consuming and
requires a large sample size to avoid errors. SA experiments are
the standard used for verifying drug addiction but need specific
environments and equipment, and trained personnel. Additionally,
issues such as hemorrhage, trauma, infection and death due to
intravenous intubation in animals can affect the experimental
results and process (44).
To the best of our knowledge, there have been no
previous reports on the use of behavioral video coding analysis for
morphine addiction. Behavioral video analysis technology may
provide a convenient and efficient method for studying addictive
behaviors. Recording videos of animals during the addiction
modeling process and then coding these behaviors (45) allows for the identification of
characteristic behavioral changes following morphine addiction in
rats. Therefore, the present study assessed the behavior of
morphine-addicted rats through behavioral video analysis and to
evaluate this potentially efficient method to verify morphine
addiction model in rats, offering a new approach for future studies
on drug addiction behavior.
Materials and methods
Animals
Sprague-Dawley (SD) rats were purchased from SPF
Biotechnology Co., Ltd. Rats were housed in a
temperature-controlled environment (21-22˚C) within an animal
holding room, following a 12 h light/dark cycle. Food and tap water
were available ad libitum. The morphology, behavior, diet
and water intake of the rats were observed at 8:00 a.m. and 8:00
p.m. each day. The body weight of the rats was measured every day
before morphine injection. The experiment lasted a total of 22
days. Rats were acclimated to the laboratory for 1 week (day -6 to
day 0) before starting administration. Chronic morphine
administration and CPP assessment were performed in rats from day 1
to day 14, with video recording of rat behavior was carried out on
day 15. The ethics committee of Beijing Institute of Basic Medical
Sciences (Beijing, China; approval no. IACUC-DWZX-2022-715)
approved the experimental protocol.
The experiment would be terminated in the event of
any serious harm to animal welfare (including but not limited to):
i) Loss of 15-20% of body weight; ii) infection in brain area; iii)
other indicators such as pain, respiration (severe respiratory
tract infection, dyspnea, cyanosis and other phenomena) and
appearance (severe muscle atrophy and non-healing wounds). No rats
met the criteria for early euthanasia during the course of the
present study. After completing the experiment, the rats were
placed into a non-precharged chamber and euthanized by
CO2 inhalation (30% vol/min). Death was confirmed by
cardiac and respiratory arrest and dilated pupils.
Drugs: Morphine hydrochloride injection (10
mg/ml, Shenyang First Pharmaceutical Factory, Shenyang) was diluted
with sterile saline to achieve a 2.5 mg/ml working solution.
Apparatus
A Hot and Cold Plate Plantar Analgesia Instrument
(cat. no. 28-0010; Shenzhen Huayang Biotech Co., Ltd.) was used to
assess the thermal pain response of rats. The device included a
transparent plastic chamber (25 cm diameter, 60 cm height) and a
heating plate. Rats were placed in the plastic chamber, and the
heating plate temperature was kept at 40±1˚C using a thermal probe
and electronic feedback circuit. The timer started when the rats
were placed in the chamber and was stopped manually when the rats
showed behaviors such as lifting their hind limbs or licking their
forelimbs. If no thermal response was observed within 3 min, the
timer was stopped and the rats were promptly removed.
A Xiaomi Smart Video Camera PTZ Version 2K (cat. no.
MJSXJ09CM; Xiaomi Inc.) was placed in the rat cage for video
recording. A wooden board with a 5 cm diameter circular hole was
positioned between the camera and the rat.
For the CPP experiment, a CPP apparatus was used,
consisting of two compartments (30x60x30 cm; Zhongshi Technology).
The different compartments had distinct floors and walls, separated
by a removable plastic board. One compartment had black and white
striped walls and a white floor, while the other had black and
white checkered walls and a black floor. The test chamber was set
up under 40 lux dim lighting and shielded from white noise
(46).
The Observer XT (Noldus Information Technology BV)
software was used for coding and analyzing recorded rat videos,
focusing on typical rat behaviors (45).
Morphine administration
The morphine dependence model was established in
6-week-old (weight, 180-200 g) SD rats through a 14-day continuous
dose escalation protocol. Male (n=15) and female (n=15) rats were
randomly divided into two groups (47-52).
The morphine group received intraperitoneal morphine at a dose of 5
mg/kg on day 1, while the control group received an equivalent
volume of normal saline. The morphine dose increased gradually,
reaching 100 mg/kg on day 14 for the morphine-addicted rats, while
the control rats continued to receive an equivalent volume of
normal saline (53-57).
The initial dose of morphine group was 5 mg/kg on the first day,
and the dose gradient was 5 mg/kg per day from day 2 to day 7, 10
mg/kg per day from day 8 to day 14, and reached 100 mg/kg on day 14
(53-65).
The half-life of morphine in rats is generally 3-4 h, the drug
effect of morphine lasts for 4-6 h and morphine is largely
metabolized in 8-10 h (66-69).
Morphine was administered daily at 8 am and 8 pm, ensuring an
interval of about 12 h between doses (70).
Plantar heat tolerance test
Prior to each intraperitoneal injection of morphine,
the time from the beginning of exposure to the hot plate at 40˚C to
the lifting or licking of the hind foot was measured. The maximum
measurement time was limited to 3 min. The same measurement was
taken 1.5 h after the intraperitoneal injection of morphine under
the same conditions, recording the time to foot lifting or
licking.
CPP Test
The CPP experiment was divided into two phases, the
pre-experimental phase and the test phase (46). The pre-experimental phase lasted
four consecutive days, with two sessions conducted daily. Rats
(n=10) were randomly selected from each of the morphine and control
groups, resulting in a total of 20 rats. Each session lasted 15
min. Conditioned reflexes were established by confining the rats to
a white drug delivery box (referred to as the white box) for 15
min, during which they received injections of either morphine (with
dosage according to the aforementioned protocol) for the morphine
group or an equivalent volume of saline for the control group,
administered intraperitoneally. On day 4, during the retention
experiment, the rats were released from the central section of the
CPP apparatus and allowed to freely explore the two chambers for 15
min.
In the final testing phase, the same procedure was
repeated for four consecutive days. CPP training was performed
twice a day for 15 min each for 3 days and the CPP test was
performed on the fourth day. The rats were again confined to the
white box for 15 min, receiving the corresponding injections
(morphine for the morphine group, with the final dose reaching 100
mg/kg, and an equivalent volume of saline for the control group).
On day 14, during the test experiment, the rats were released from
the central part of the CPP apparatus and allowed to explore the
two chambers for 15 min.
Video behavior coding
During the 14-day morphine addiction model, rats
were randomly selected from each of the two experiment groups (n=8
per group), and placed in a cage with one rat per cage. On day 15,
video recordings were made and subsequently coded and analyzed
using Observer XT software to quantify behavioral differences
between the two groups.
The observed rats were divided into two batches of 8
(each with 4 addicted rats and 4 control rats), with one batch
observed per day. Video recordings were made from 8:00 a.m. to 8:00
p.m. each day, with one rat per cage. A Xiaomi cloud platform video
camera was temporarily placed in each rat cage, isolated from the
rats by a wooden board, this setup allowed continuous recording of
the rats' activity and behavior for 12 h, with the recorded video
retained for subsequent analysis. Video coding and analysis were
conducted using Observer XT software to examine differences in
classical behaviors (such as eating, drinking, licking forelimbs,
licking hind limbs, sleeping, walking and scratching) between the
morphine group rats post-morphine addiction and the control group
rats (45).
Statistical analysis
Data analysis was performed using GraphPad Prism
(version 9; Dotmatics). The Wilcoxon rank-sum test and two-way
ANOVA with Bonferroni correction were used to assess both
experimental data and behavioral video coding recordings. P<0.05
was considered to indicate a statistically significant difference.
Data are presented as mean ± SEM.
Results
Effect of continuous morphine
administration on rat body weight
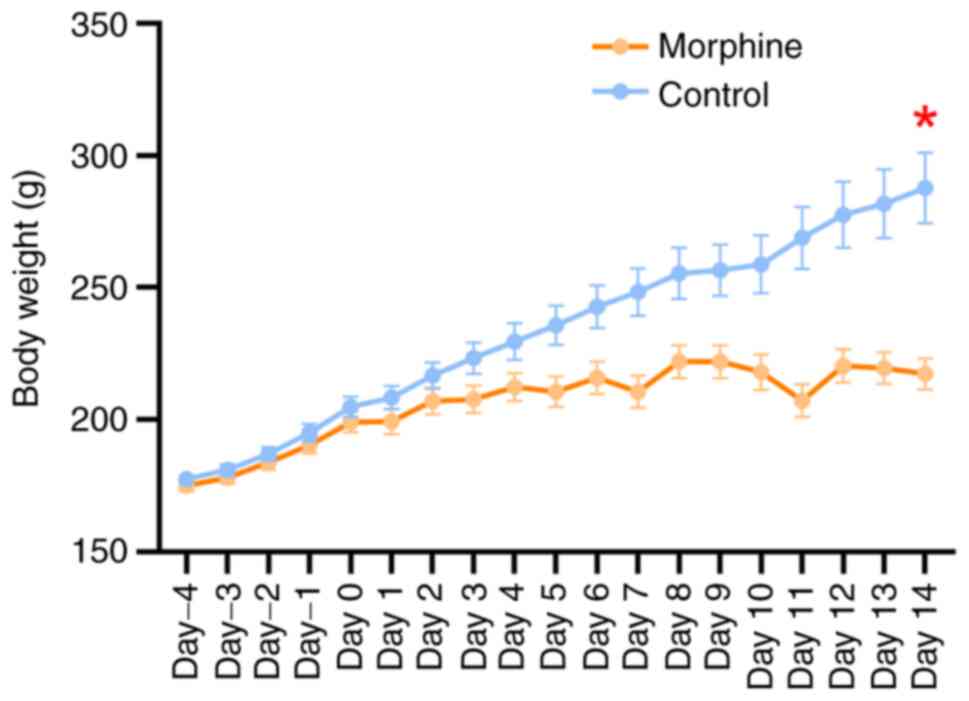
Following 14 days of escalating morphine doses, the
weight difference between the morphine group and the control group
showed a gradual and significant increase (day 14, z=2.717,
P=0.0057, Fig. 1). After 14 days,
compared with the control group, the weight growth rate of morphine
group rats was lower. The final average body weight on day 14 was
206.2±3.4 g (maximum individual body weight, 222.0 g) for the
morphine group. In contrast, the body weight of rats in the control
group showed a steady increase, with a stable growth rate. The
final average body weight on day 14 for the control group was
233.6±8.0 g (maximum individual body weight, 287.7 g). A difference
between body weight in the two groups was evident from the first
day, which increased over time. On day 14, the average body weight
of control rats exceeded that of morphine-rats by 27.4 g with the
greatest body weight difference between the two groups being 70.3
g.
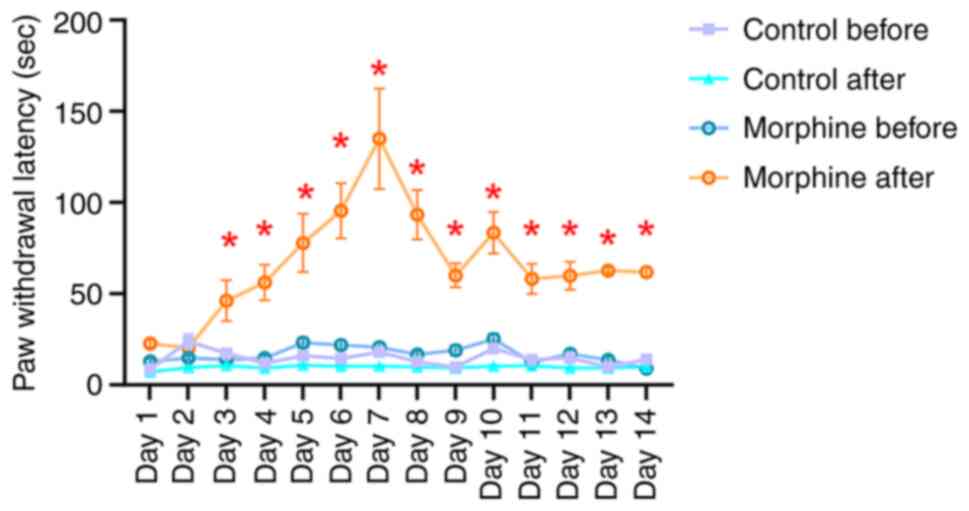
Effect of morphine injection on
thermal pain tolerance in rats
At 1.5 h post-intraperitoneal morphine injection,
the morphine group exhibited a significantly extended duration of
thermal pain tolerance compared with the same group pre-injection.
There was no significant difference in thermal pain tolerance
following saline injection in the control group. Ultimately,
morphine-addicted rats demonstrated longer thermal tolerance than
control rats (U=74.4, P=0.039, Fig.
2).
CPP experiments in morphine-addicted
rats
CPP experiments were conducted on days 1-4 and days
11-14 for both the morphine and control groups. The analysis of CPP
results involved recording the time spent by rats in the white box.
The results before morphine addiction (day 4), demonstrated no
statistically significant difference between the morphine and
control groups (F=6.1., P=0.64, Fig.
3), indicating equivalent white box time for both groups.
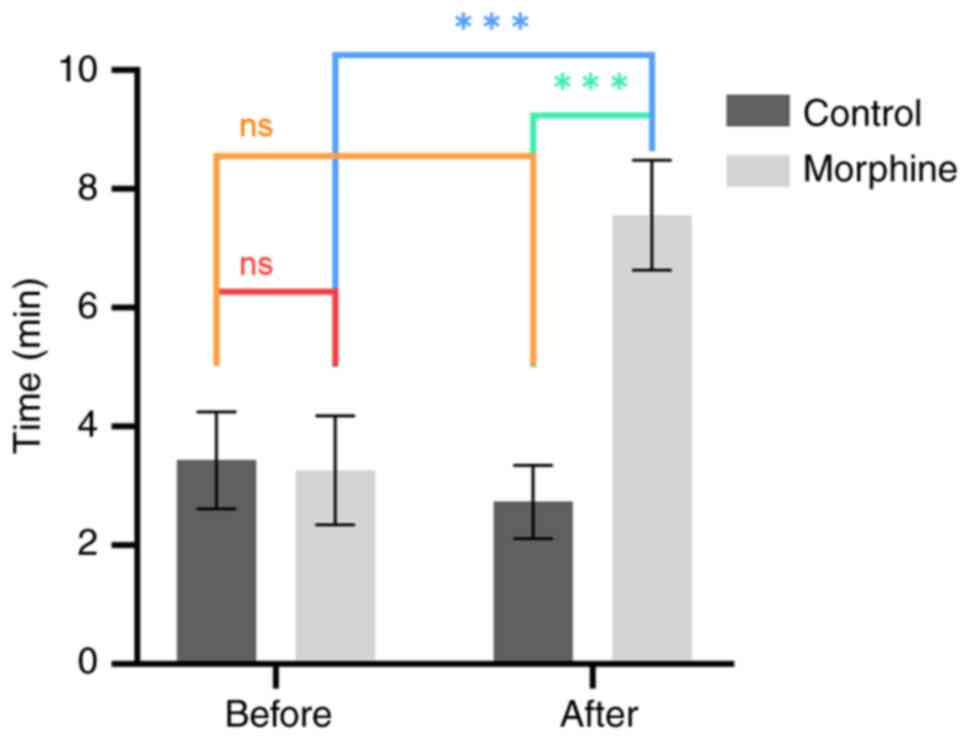 | Figure 3Comparison of white box time in CPP
experiment before and after morphine addiction modeling.
Comparative analysis of the time spent in the white box during the
CPP experiment between rats in the morphine group and the control
group. The comparisons include differences in white box time before
morphine addiction (day 4) and after morphine addiction (day 14),
and the variation in white box time for rats in the morphine group
before and after addiction. Prior to morphine addiction (day 4), no
significant difference was observed in the white box time between
rats in the morphine group and the control group (n=10, χ²=0.2,
P=0.64). However, after morphine addiction (day 14), a significant
difference in white box time was evident between the two groups
(n=10, χ²=134.8, P<0.001). Additionally, a significant
difference in white box time was identified in the morphine group
between day 4 and day 14 (n=10, χ²=170.8, P<0.001).
***P<0.001. CPP, conditioned place preference; ns,
not significant. |
In the experiment after morphine addiction (day 14),
a significant difference was demonstrated between the morphine and
control groups regarding time spent in the white box (F=5.7,
P<0.001, Fig. 3), which
demonstrated that morphine administration increased the time spent
in the white box compared to saline administration, which validated
the rat addiction model. Additionally, there was a significant
difference in the white box time of morphine group rats before and
after addiction (F=11.5, P<0.001, Fig. 3), further verifying the success of
the morphine addiction model in rats.
Video coding analysis of the effects
of morphine dependence on rat behavior
To record and statistically analyze the
characteristic behaviors of rats after addiction, a 12-h video of
rats in the morphine group and rats in the control group was
recorded. The video was then coded and analyzed using Observer XT
software, and the data was exported for analysis using the
Mann-Whitney test to compare the statistical differences in the
behaviors of the two groups. Compared with the control rats, the
morphine group rats showed significant differences in drinking and
licking the fore and hind limbs. They also exhibited characteristic
behaviors such as an increased number ball sleeping, increased
scratching, increased fur licking and changes in walking time at
specific times of the day.
Regarding feeding, rats in the morphine group did
not show significant differences compared with control rats
(U=65.5, P=0.98, Fig. 4A). However,
in terms of drinking, the morphine group drank significantly less
water than the control group (U=17, P=0.0014, Fig. 4B). For the licking of the forelimbs,
the morphine group licked significantly less compared with the
control group (U=27, P=0.014, Fig.
4C). In contrast, for the licking of the hindlimbs, the
morphine group licked significantly less than the control group
(U=32, P=0.035, Fig. 4D). There was
no significant difference between the two groups in terms of
lifting the hindlimbs (U=62, P=0.082>0.05, Fig. 4E).
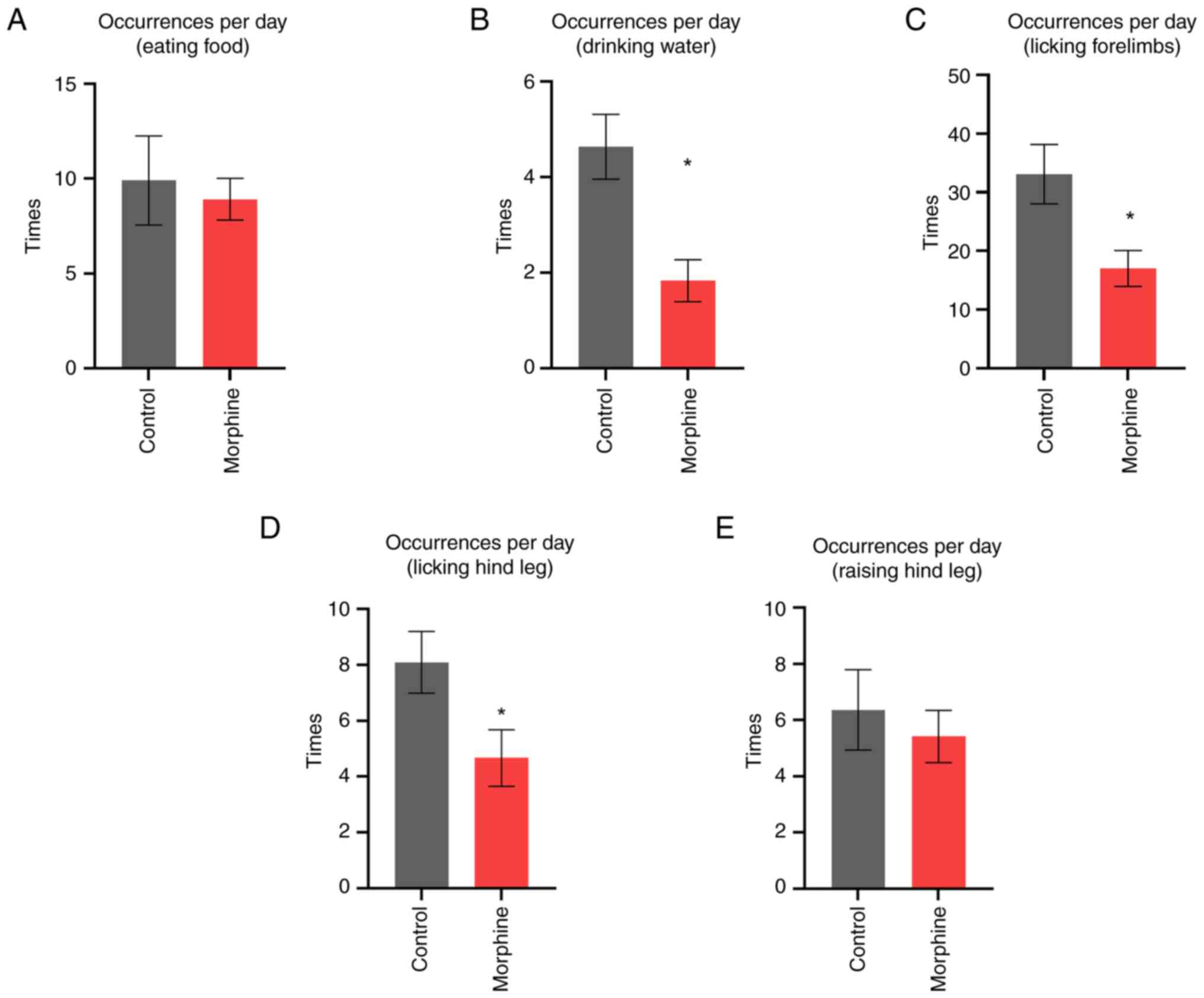 | Figure 4Behavioral analysis by video coding
comparing rats in the morphine group and control group. (A) No
significant difference was shown in feed intake between the
morphine and control groups (n=8, U=65.5, P=0.98). (B) A
significant difference was shown in water intake, with the morphine
group drinking less than the control group (n=8, U=17, P=0.0014).
(C) A significant difference was shown in foreleg licking, with the
morphine group licking less than the control group (n=8, U=27,
P=0.014). (D) A significant difference was shown in hind limb
licking, with the morphine group licking more than the control
group (n=8, U=32, P=0.035). (E) No significant difference was shown
in hind leg lifting between the morphine and control groups (n=8,
U=62, P=0.082>0.05). *P<0.05. |
In the present study, ‘marten sleeping’ means
sleeping curled up in the lateral arch of the rat with a clear view
of the entire head. ‘Ball sleeping’ is when the rat is resting with
its head curled up on its abdomen and its eyes closed. In the 12-h
observation period, morphine-addicted rats displayed significantly
more ‘ball sleeping’ behavior compared with control rats during the
2:30-3:00 p.m. period (z=-2.412, P=0.0159, Fig. 5A). There was no significant
difference between the groups for ‘marten sleeping’ at any recorded
time period. The frequency of scratching showed a statistically
significant difference only during the 1:00-1:30 p.m. period
(z=-1.964, P=0.0495; Fig. 5C). The
frequency of licking showed a significant difference between the
groups during the 3:30-4:00 p.m. period (z=-2.29, P=0.022, Fig. 5D). Additionally, the morphine group
walked significantly less than the control group during the 12:00
a.m.-12:30 p.m. and 6:00-6:30 p.m. periods (z=-2.093, P=0.036;
z=-2.039, P=0.0415; Fig. 5E). These
results indicate that rats chronically injected with morphine
exhibit characteristic behaviors such as increased ball sleeping,
increased scratching, increased hair licking, and changes in
walking behaviorat specific times of the day.
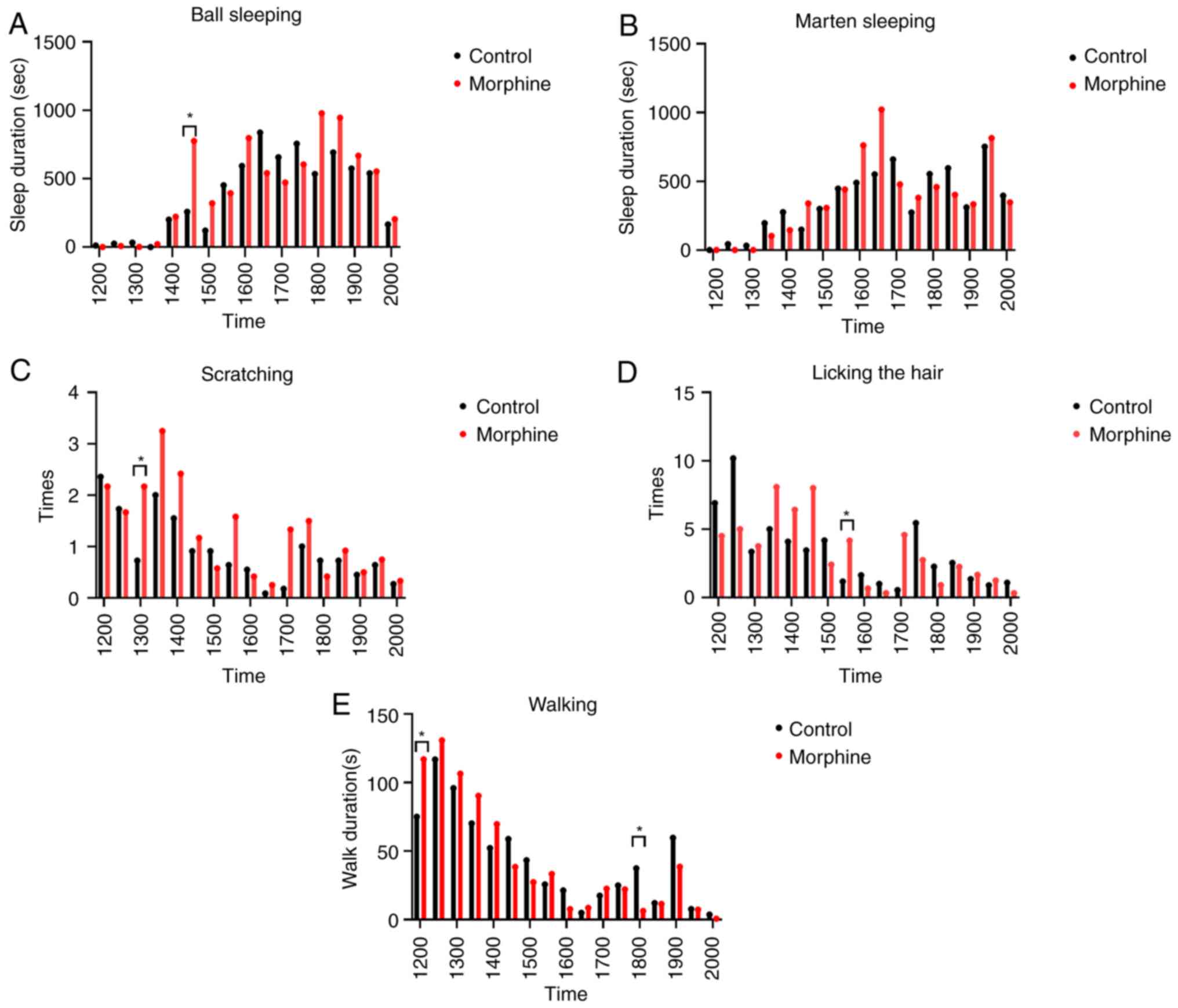 | Figure 5Behavioral comparison between
morphine and control group rats over a 9-h period using video
coding. (A) Comparison of ‘ball sleeping’ behavior, a significant
difference was observed only during the 2:30-3:00 p.m. period (n=8,
z=-2.412, P=0.0159). (B) Analysis of ‘marten sleeping behavior
(n=8, P=0.8>0.05). (C) Frequency of scratching actions, a
significant difference was found only during the 1:00-1:30 p.m.
period (n=8, z=-1.964, P=0.0495). (D) Frequency of fur licking, a
significant difference was observed only during the 3:30-4:00 p.m.
period (n=8, z=-2.29, P=0.022). (E) Comparison of walking
frequency, significant differences were noted during the
12:00-12:30 p.m. (n=8, z=-2.093, P=0.036) and 6:00-6:30 p.m.
periods (n=8, z=2.039, P=0.0415). *P<0.05. |
Discussion
Morphine addiction and dependence are recognized as
neuropsychiatric diseases, involving various behavioral,
neurobiological and molecular changes (55,71-75).
In animal models, especially in rats, studying behavioral
manifestations after morphine addiction is crucial for
understanding the neural mechanisms of addiction and finding
treatments. Video coding and analysis technology record animal
behavior under specific conditions, using computer software to
encode, identify and analyze the behavioral data. This technology
enables comprehensive recording and accurate analysis of animal
behavior, which is safe and non-invasive, and does not interfere
with the animals' activities. Thus, it reveals the behavioral
characteristics of rats after morphine addiction more accurately
than traditional behavioral observation.
At present, the mechanism of morphine addiction has
been studied and evaluated using various animal models, among which
the rat model of chronic morphine administration is commonly used
(48,49,51,76).
In studies of rat or mouse behavior, most analyses use experiments
to verify behavior, such as the rotarod, open field test, elevated
plus maze test, cliff hanging, passive avoidance test, Morris water
maze, light/dark box or light spot tests. The study of daily
behaviors in rats mainly focuses on neuropathic pain and other
diseases, using video coding analysis for assessment of daily
walking gait (77-81).
Research on addictive behavior primarily involves
self-administration and CPP tests, with little use of daily
behavior video coding analysis (82,83).
However, after morphine administration, whether rats are addicted
or not is usually judged by CPP test and self-administration
experiment, and these methods have certain limitations and
shortcomings, such as time-consuming, labor-intensive, requiring
long-term behavioral training of animals and risk of infection and
death due to invasive procedures. Therefore, in the present study,
video coding was used to analyze the characteristic behavior of
chronic morphine administration in rats, which was expected to
provide a simple and quick method to analyze and evaluate morphine
addiction through the behavioral changes before and after morphine
addiction. Video behavior observation and analysis has many
applications in studying animal behaviors such as pain, depression
and anxiety. For example, Braw et al (84) demonstrated the depression and
anxiety behavior of rats with different genetic models, finding
differential expression of anxiety in pre-pubertal rats belonging
to the ‘depressed’ strains, suggesting that these strains may be
suitable for modelling different sub-groups of depression at young
ages. Medvedev et al (85)
demonstrated MK-801 and memantine acted against tactile allodynia
induced by sciatic nerve ligatio. Yuan and Devine (86) demonstrated the self-injury behavior
of rats induced by anxiety drugs, and finding the rats given
anxiety-inducing drugs showed stronger self-injurious behavior. To
the best of our knowledge, no previous studies have performed video
behavioral coding analysis of morphine addiction behavior in rats.
Therefore, using video coding analysis to study addiction behavior
characteristics introduces innovative changes and has potential
value for the researching of addiction mechanisms and
treatments.
In the present study, weight observation, heat
tolerance test, CPP test and behavioral video coding analysis were
performed using a morphine addiction model in rats. The results
indicated that morphine-addicted rats had increased heat pain
tolerance and reduced weight gain. The present study used the
method of video behavior coding analysis for the first time to find
that rats with chronic morphine administration exhibited slower
weight gain, smaller body size, and fragile fur (87). Behavioral changes were mainly
characterized by decreased water intake, decreased toe licking and
increased daytime sleep in a spherical posture. The present study
analyzed various behavioral changes in rats before and after
morphine addiction and provided a new method for verifying the
morphine addiction rat model through behavioral video analysis
(46-49,51,52).
Behavioral video analysis of morphine-addicted rats
revealed significant differences in drinking and licking of the
fore and hind limbs, as well as in sleeping postures and scratching
during the middle of the day. According to Kon et al
(88), morphine increases the
expression of aquaporin-3 water channels in the colon by increasing
the secretion of serotonin, which enhances water absorption from
the lumen to the vasculature of the colon. Deroche et al
(89) reported that opioids bind to
MOP receptors in enteric neurons, delaying gastrointestinal transit
time, and stimulating non-propulsive GI motility and the pylorus
and ileocecal sphincters. Consequently, chronic morphine
administration results in diminished thirst and reduced water
excretion, leading to decreased water intake. Morphine group rats
exhibited more frequent ball sleeping, especially during the
2:00-2:30 p.m. timeframe. The altered sleep posture suggests that
long-term morphine exposure may lead to functional or structural
changes in the limbic system and motor cortex, which are involved
in mediating instinctive and emotional behaviors through Papez
circuits (90). It is speculated
that morphine affects the neurons and neural circuits in the limbic
system or cortical nuclei, resulting in changes in sleep
posture.
The present study analyzed the behavioral
characteristics of morphine-addicted rats using the minimum sample
size necessary to achieve statistical significance. Increasing the
sample size could provide more precise behavior analysis and more
convincing experimental conclusions. Additionally, the 14-day
morphine addiction model is currently complex; future optimizations
could make the model more efficient and reduce animal
suffering.
In summary, high-resolution video equipment was used
to record the behavior of morphine-addicted rats, and behavioral
video coding and identification analysis were performed to
systematically study the characteristics of morphine addiction
behavior. By analyzing the behavioral characteristics of
morphine-addicted rats, we can better understand the occurrence and
development of addiction, reveal the impact of addiction on rat
behavior and its potential neural mechanisms, and provide important
theoretical and methodological support for further research on the
neural circuit mechanisms of addiction and the development of
related treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant no. 82073833) and the National
Key R&D Plan (grant nos. 2022YFC3600500 and
2022YFC3600502).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
JY performed multiple experiments, acquired data and
wrote the first draft of the manuscript. JY, TZ, DL, HL, XP, and FL
performed data analysis. YZ, FX, and XW contributed to the study
design and participated in revising the manuscript. YZ, FX, and XW
confirmed the authenticity of all raw data and all authors agreed
to be accountable for all aspects of the study. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Experiments were conducted in compliance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. The experimental procedures were approved by
the ethics committee of Beijing Institute of Basic Medical Sciences
(approval no. IACUC-DWZX-2022-715; Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Häuser W, Welsch P, Radbruch L, Fisher E,
Bell RF and Moore RA: Cannabis-based medicines and medical cannabis
for adults with cancer pain. Cochrane Database Syst Rev.
6(Cd014915)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Luger NM, Mach DB, Sevcik MA and Mantyh
PW: Bone cancer pain: From model to mechanism to therapy. J Pain
Symptom Manage. 29 (5 Suppl):S32–S46. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mercadante S: Intravenous morphine for
management of cancer pain. Lancet Oncol. 11:484–489.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nguyen QN, Chun SG, Chow E, Komaki R, Liao
Z, Zacharia R, Szeto BK, Welsh JW, Hahn SM, Fuller CD, et al:
Single-Fraction stereotactic vs conventional multifraction
radiotherapy for pain relief in patients with predominantly
nonspine bone metastases: A Randomized phase 2 trial. JAMA Oncol.
5:872–878. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mantsch JR, Baker DA, Funk D, Lê AD and
Shaham Y: Stress-Induced reinstatement of drug seeking: 20 years of
progress. Neuropsychopharmacology. 41:335–356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reiner DJ, Fredriksson I, Lofaro OM,
Bossert JM and Shaham Y: Relapse to opioid seeking in rat models:
Behavior, pharmacology and circuits. Neuropsychopharmacology.
44:465–477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shalev U, Grimm JW and Shaham Y:
Neurobiology of relapse to heroin and cocaine seeking: A review.
Pharmacol Rev. 54:1–42. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trujillo KA and Akil H: Inhibition of
morphine tolerance and dependence by the NMDA receptor antagonist
MK-801. Science. 251:85–87. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garofoli M: Adolescent Substance Abuse.
Prim Care. 47:383–394. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lewis DC: Drug overdose, addiction and
binge drinking: Medical problems with public health consequences. R
I Med J (2013). 97:18–19. 2014.PubMed/NCBI
|
|
11
|
McCarty D, Argeriou M, Huebner RB and
Lubran B: Alcoholism, drug abuse, and the homeless. Am Psychol.
46:1139–1148. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lu L and Wang X: Drug addiction in China.
Ann N Y Acad Sci. 1141:304–317. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Leshner AI: Addiction is a brain disease,
and it matters. Science. 278:45–47. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu JF and Li JX: Drug addiction: A
curable mental disorder? Acta Pharmacol Sin. 39:1823–1829.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nestler EJ: Molecular basis of long-term
plasticity underlying addiction. Nat Rev Neurosci. 2:119–128.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Chiamulera C, Piva A and Abraham WC:
Glutamate receptors and metaplasticity in addiction. Curr Opin
Pharmacol. 56:39–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fourgeaud L, Mato S, Bouchet D, Hémar A,
Worley PF and Manzoni OJ: A single in vivo exposure to cocaine
abolishes endocannabinoid-mediated long-term depression in the
nucleus accumbens. J Neurosci. 24:6939–6945. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gipson CD, Kupchik YM and Kalivas PW:
Rapid, transient synaptic plasticity in addiction.
Neuropharmacology. 76 Pt B:276–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hafenbreidel M, Rafa Todd C and Mueller D:
Infralimbic GluN2A-Containing NMDA receptors modulate
reconsolidation of cocaine self-administration memory.
Neuropsychopharmacology. 42:1113–1125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hyman SE: Addiction: A disease of learning
and memory. Am J Psychiatry. 162:1414–1422. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kauer JA and Malenka RC: Synaptic
plasticity and addiction. Nat Rev Neurosci. 8:844–858.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Keralapurath MM, Briggs SB and Wagner JJ:
Cocaine self-administration induces changes in synaptic
transmission and plasticity in ventral hippocampus. Addict Biol.
22:446–456. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lüscher C and Malenka RC: Drug-evoked
synaptic plasticity in addiction: From molecular changes to circuit
remodeling. Neuron. 69:650–663. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mameli M, Bellone C, Brown MT and Lüscher
C: Cocaine inverts rules for synaptic plasticity of glutamate
transmission in the ventral tegmental area. Nat Neurosci.
14:414–416. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Tronson NC and Taylor JR: Molecular
mechanisms of memory reconsolidation. Nat Rev Neurosci. 8:262–275.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Tzschentke TM and Schmidt WJ:
Glutamatergic mechanisms in addiction. Mol Psychiatry. 8:373–382.
2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van Huijstee AN and Mansvelder HD:
Glutamatergic synaptic plasticity in the mesocorticolimbic system
in addiction. Front Cell Neurosci. 8(466)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ossipov MH, Lai J, King T, Vanderah TW,
Malan TP Jr, Hruby VJ and Porreca F: Antinociceptive and
nociceptive actions of opioids. J Neurobiol. 61:126–148.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mickiewicz AL and Napier TC: Repeated
exposure to morphine alters surface expression of AMPA receptors in
the rat medial prefrontal cortex. Eur J Neurosci. 33:259–265.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boudreau AC and Wolf ME: Behavioral
sensitization to cocaine is associated with increased AMPA receptor
surface expression in the nucleus accumbens. J Neurosci.
25:9144–9151. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hemby SE, Tang W, Muly EC, Kuhar MJ,
Howell L and Mash DC: Cocaine-induced alterations in nucleus
accumbens ionotropic glutamate receptor subunits in human and
non-human primates. J Neurochem. 95:1785–1793. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sutton MA, Schmidt EF, Choi KH, Schad CA,
Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL and Self
DW: Extinction-induced upregulation in AMPA receptors reduces
cocaine-seeking behaviour. Nature. 421:70–75. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bachtell RK, Choi KH, Simmons DL, Falcon
E, Monteggia LM, Neve RL and Self DW: Role of GluR1 expression in
nucleus accumbens neurons in cocaine sensitization and
cocaine-seeking behavior. Eur J Neurosci. 27:2229–2240.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Conrad KL, Tseng KY, Uejima JL, Reimers
JM, Heng LJ, Shaham Y, Marinelli M and Wolf ME: Formation of
accumbens GluR2-lacking AMPA receptors mediates incubation of
cocaine craving. Nature. 454:118–121. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kasanetz F, Deroche-Gamonet V, Berson N,
Balado E, Lafourcade M, Manzoni O and Piazza PV: Transition to
addiction is associated with a persistent impairment in synaptic
plasticity. Science. 328:1709–1712. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
LaLumiere RT and Kalivas PW: Glutamate
release in the nucleus accumbens core is necessary for heroin
seeking. J Neurosci. 28:3170–3177. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Degoulet M, Stelly CE, Ahn KC and Morikawa
H: L-type Ca²+ channel blockade with antihypertensive
medication disrupts VTA synaptic plasticity and drug-associated
contextual memory. Mol Psychiatry. 21:394–402. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lane DA, Lessard AA, Chan J, Colago EE,
Zhou Y, Schlussman SD, Kreek MJ and Pickel VM: Region-specific
changes in the subcellular distribution of AMPA receptor GluR1
subunit in the rat ventral tegmental area after acute or chronic
morphine administration. J Neurosci. 28:9670–9681. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Billa SK, Sinha N, Rudrabhatla SR and
Morón JA: Extinction of morphine-dependent conditioned behavior is
associated with increased phosphorylation of the GluR1 subunit of
AMPA receptors at hippocampal synapses. Eur J Neurosci. 29:55–64.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cai YQ, Wang W, Hou YY, Zhang Z, Xie J and
Pan ZZ: Central amygdala GluA1 facilitates associative learning of
opioid reward. J Neurosci. 33:1577–1588. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun Y, Chen G, Zhou K and Zhu Y: A
conditioned place preference protocol for measuring incubation of
craving in rats. J Vis Exp. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Tzschentke TM: Measuring reward with the
conditioned place preference (CPP) paradigm: Update of the last
decade. Addict Biol. 12:227–462. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lin XJ, Zhang JJ and Yu LC: GluR2-3Y
inhibits the acquisition and reinstatement of morphine-induced
conditioned place preference in rats. Neurosci Bull. 32:177–182.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tzschentke TM: Measuring reward with the
conditioned place preference paradigm: A comprehensive review of
drug effects, recent progress and new issues. Prog Neurobiol.
56:613–672. 1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang X, Bey AL, Katz BM, Badea A, Kim N,
David LK, Duffney LJ, Kumar S, Mague SD, Hulbert SW, et al: Altered
mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3
complete knockout model of autism. Nat Commun.
7(11459)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Domínguez-Salazar E, Naser HF and
Velázquez-Moctezuma J: D1-like antagonist blocks conditioned place
preference induced by ejaculation in male rats. Behav Brain Res.
269:15–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chang SL, Moldow RL, House SD and Zadina
JE: Morphine affects the brain-immune axis by modulating an
interleukin-1 beta dependent pathway. Adv Exp Med Biol. 402:35–42.
1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Graf JA, Patel JA and Chang SL: Chronic
exposure to morphine, but not ethanol, attenuates the expression of
interleukin-1 beta converting enzyme in rat spleen. Immunol Lett.
58:153–157. 1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
House SD, Mao X, Wu G, Espinelli D, Li WX
and Chang SL: Chronic morphine potentiates the inflammatory
response by disrupting interleukin-1beta modulation of the
hypothalamic-pituitary-adrenal axis. J Neuroimmunol. 118:277–285.
2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lobo MK, Covington HE III, Chaudhury D,
Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW,
Kennedy PJ, et al: Cell type-specific loss of BDNF signaling mimics
optogenetic control of cocaine reward. Science. 330:385–390.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ocasio FM, Jiang Y, House SD and Chang SL:
Chronic morphine accelerates the progression of
lipopolysaccharide-induced sepsis to septic shock. J Neuroimmunol.
149:90–100. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zadina JE, Kastin AJ, Harrison LM, Ge LJ
and Chang SL: Opiate receptor changes after chronic exposure to
agonists and antagonists. Ann N Y Acad Sci. 757:353–361.
1995.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Askari N, Mousavi A and Vaez-Mahdavi MR:
Maternal deprivation effect on morphine-induced CPP is related to
changes in opioid receptors in selected rat brain regions
(hippocampus, prefrontal cortex, and nucleus accumbens). Behav
Processes. 197(104607)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rodgers HM, Lim SA, Yow J, Dinkins ML,
Patton R, Clemens S and Brewer KL: Dopamine D1 or
D3 receptor modulators prevent morphine tolerance and
reduce opioid withdrawal symptoms. Pharmacol Biochem Behav.
194(172935)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu LW, Lu J, Wang XH, Fu SK, Li Q and Lin
FQ: Neuronal apoptosis in morphine addiction and its molecular
mechanism. Int J Clin Exp Med. 6:540–545. 2013.PubMed/NCBI
|
|
56
|
Papaleo F and Contarino A: Gender- and
morphine dose-linked expression of spontaneous somatic opiate
withdrawal in mice. Behav Brain Res. 170:110–118. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rahmati B and Beik A: Prevention of
morphine dependence and tolerance by Nepeta menthoides was
accompanied by attenuation of Nitric oxide overproduction in male
mice. J Ethnopharmacol. 199:39–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Koek W: Morphine-induced conditioned place
preference and effects of morphine pre-exposure in adolescent and
adult male C57BL/6J mice. Psychopharmacology (Berl). 233:2015–2024.
2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Li X, Kshatriya D and Bello NT:
Weight-gain propensity and morphine withdrawal alters locomotor
behavior and regional norepinephrine-related gene expression in
male and female mice. Pharmacol Biochem Behav.
213(173329)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Madayag AC, Gomez D, Anderson EM,
Ingebretson AE, Thomas MJ and Hearing MC: Cell-type and
region-specific nucleus accumbens AMPAR plasticity associated with
morphine reward, reinstatement, and spontaneous withdrawal. Brain
Struct Funct. 224:2311–2324. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
McDevitt DS, McKendrick G and Graziane NM:
Anterior cingulate cortex is necessary for spontaneous opioid
withdrawal and withdrawal-induced hyperalgesia in male mice.
Neuropsychopharmacology. 46:1990–1999. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Nakamura A, Ono H, Ando A, Hinata M,
Niidome K, Omachi S, Sakaguchi G and Shinohara S: Suppression of
the acute upregulation of phosphorylated-extracellular regulated
kinase in ventral tegmental area by a µ-opioid receptor agonist is
related to resistance to rewarding effects in a mouse model of bone
cancer. J Pharmacol Sci. 133:9–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Piccin A and Contarino A: Long-lasting
pseudo-social aggressive behavior in opiate-withdrawn mice. Prog
Neuropsychopharmacol Biol Psychiatry. 97(109780)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Piccin A and Contarino A: The CRF(1)
receptor mediates social behavior deficits induced by opiate
withdrawal. J Neurosci Res. 100:309–321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Varshneya NB, Walentiny DM, Stevens DL,
Walker TD, Akinfiresoye LR and Beardsley PM: Structurally diverse
fentanyl analogs yield differential locomotor activities in mice.
Pharmacol Biochem Behav. 222(173496)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kalvass JC, Olson ER, Cassidy MP, Selley
DE and Pollack GM: Pharmacokinetics and pharmacodynamics of seven
opioids in P-glycoprotein-competent mice: Assessment of unbound
brain EC50,u and correlation of in vitro, preclinical, and clinical
data. J Pharmacol Exp Ther. 323:346–355. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Melzacka M: Pharmacokinetic aspects of
some behavioral effects of psychotropic drugs. Pol J Pharmacol
Pharm. 36:117–136. 1984.PubMed/NCBI
|
|
68
|
Regenthal R, Krueger M, Koeppel C and
Preiss R: Drug levels: Therapeutic and toxic serum/plasma
concentrations of common drugs. J Clin Monit Comput. 15:529–544.
1999.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sulimai NH, Ko JC, Jones-Hall YL, Weng HY,
Deng M, Breur GJ and Knipp GT: Evaluation of 25% poloxamer as a
slow release carrier for morphine in a rat model. Front Vet Sci.
5(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Santos-Vera B, Vaquer-Alicea ADC,
Maria-Rios CE, Montiel-Ramos A, Ramos-Cardona A, Vázquez-Torres R,
Sanabria P and Jiménez-Rivera CA: Protein and surface expression of
HCN2 and HCN4 subunits in mesocorticolimbic areas after cocaine
sensitization. Neurochem Int. 125:91–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cao DN, Song R, Zhang SZ, Wu N and Li J:
Nucleus accumbens hyperpolarization-activated cyclic
nucleotide-gated channels modulate methamphetamine
self-administration in rats. Psychopharmacology (Berl).
233:3017–3029. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Caruso Brown AE: Treating addiction as a
terminal disease. N Engl J Med. 382:207–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Mu L, Liu X, Yu H, Vickstrom CR, Friedman
V, Kelly TJ, Hu Y, Su W, Liu S, Mantsch JR and Liu QS:
cAMP-mediated upregulation of HCN channels in VTA dopamine neurons
promotes cocaine reinforcement. Mol Psychiatry. 28:3930–3942.
2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Settley C: The physical and psychological
wellbeing of caregivers of individuals suffering from substance
addiction. Arch Psychiatr Nurs. 34:107–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Xiao ZW, Cao CY, Wang ZX, Li JX, Liao HY
and Zhang XX: Changes of dopamine transporter function in striatum
during acute morphine addiction and its abstinence in rhesus
monkey. Chin Med J (Engl). 119:1802–1807. 2006.PubMed/NCBI
|
|
76
|
Aramjoo H, Riahi-Zanjani B, Farkhondeh T,
Forouzanfar F and Sadeghi M: Modulatory effect of opioid
administration on the activity of cholinesterase enzyme: A
systematic review of mice/rat models. Environ Sci Pollut Res Int.
28:52675–52688. 2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Djabirska I, Delaval L, Tromme A, Blomet
J, Desmecht D and Van Laere AS: Longitudinal quantitative
assessment of TMEV-IDD-induced MS phenotypes in two inbred mouse
strains using automated video tracking technology. Exp Neurol.
379(114851)2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kulbeth HJ, Fukuda S and Brents LK:
Automated quantification of opioid withdrawal in neonatal rat pups
using Ethovision® XT software. Neurotoxicol Teratol.
84(106959)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Richmond-Hacham B, Tseitlin L, Bikovski L
and Pick CG: Investigation of mild traumatic brain injury home cage
behavior: The home cage assay advantages. J Neurotrauma.
41:e1780–e1792. 2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Timotius IK, Roelofs RF, Richmond-Hacham
B, Noldus LPJJ, von Hörsten S and Bikovski L: CatWalk XT gait
parameters: A review of reported parameters in pre-clinical studies
of multiple central nervous system and peripheral nervous system
disease models. Front Behav Neurosci. 17(1147784)2023.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Watkins J, Ghosh A, Keerie AFA, Alix JJP,
Mead RJ and Sreedharan J: Female sex mitigates motor and
behavioural phenotypes in TDP-43Q331K knock-in mice. Sci
Rep. 10(19220)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lebedev IV, Pleskacheva MG and Anokhin KV:
C57BL/6 mice open field behaviour qualitatively depends on arena
size. Zh Vyssh Nerv Deiat Im I P Pavlova. 62:485–496.
2012.PubMed/NCBI(In Russian).
|
|
83
|
Novati A, Manfré G, Flunkert S, Van der
Harst JE, Homberg JR, Wronski R and Nguyen HP: Validation of
behavioral phenotypes in the BACHD rat model. Behav Brain Res.
393(112783)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Braw Y, Malkesman O, Dagan M, Bercovich A,
Lavi-Avnon Y, Schroeder M, Overstreet DH and Weller A: Anxiety-like
behaviors in pre-pubertal rats of the Flinders Sensitive Line (FSL)
and Wistar-Kyoto (WKY) animal models of depression. Behav Brain
Res. 167:261–269. 2006.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Medvedev IO, Malyshkin AA, Belozertseva
IV, Sukhotina IA, Sevostianova NY, Aliev K, Zvartau EE, Parsons CG,
Danysz W and Bespalov AY: Effects of low-affinity NMDA receptor
channel blockers in two rat models of chronic pain.
Neuropharmacology. 47:175–183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Yuan X and Devine DP: The role of anxiety
in vulnerability for self-injurious behaviour: studies in a rodent
model. Behav Brain Res. 311:201–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Le T, Xia M, Jia M, Sarkar N, Chen J, Li
H, Wynn GH, Ursano RJ and Choi KH: Association between initial
morphine intake and body weight change, acoustic startle reflex and
drug seeking in rats. Psychopharmacology (Berl). 231:4569–4577.
2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kon R, Ikarashi N, Hayakawa A, Haga Y,
Fueki A, Kusunoki Y, Tajima M, Ochiai W, Machida Y and Sugiyama K:
Morphine-induced constipation develops with increased aquaporin-3
expression in the colon via increased serotonin secretion. Toxicol
Sci. 145:337–347. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Deroche V, Piazza PV, Casolini P, Maccari
S, Le Moal M and Simon H: Stress-induced sensitization to
amphetamine and morphine psychomotor effects depend on
stress-induced corticosterone secretion. Brain Res. 598:343–348.
1992.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Yunusa S, Müller CP and Hassan Z:
Mitragynine (Kratom)-Withdrawal behaviour and cognitive impairments
can be ameliorated by an epigenetic mechanism. Br J Pharmacol.
181:2070–2084. 2024.PubMed/NCBI View Article : Google Scholar
|