Introduction
Myocardial infarction is a condition characterised
by inadequate blood supply due to blockage of coronary arteries,
leading to increased oxygen demand of tissues because of oxygen
deficiency caused by arterial flow restriction (1). Oxygen is an essential factor that
affects cell activity and the ultimate electron acceptor in the
electron transport chain (ETC), making it key for cell survival. A
hypoxic environment damages the ETC, limiting oxygen for
mitochondrial consumption to produce ATP via oxidative
phosphorylation (2,3). Hypoxia increases pulmonary arterial
pressure, resulting in acute pulmonary vasoconstriction (4). Hypoxia is the primary cause of
myocardial cell injury. It inhibits proliferation of myocardial
cells, induces apoptosis and decreases the viability of myocardial
cells (5-7).
Rhodiola rosea L., a perennial herb with
various biological effects, was first used in Tibetan medicine to
treat hypoxia, decrease altitude illness, lower blood pressure and
improve oxygen utilisation and tolerance to hypoxia (8-10).
R. rosea L. improves aerobic metabolic processes during
hypoxia and exerts a protective effect on cardiomyocytes damaged by
hypoxia. Salidroside (SAL), a phenolic glycoside compound isolated
from R. rosea L., exhibits various pharmacological
properties, including antioxidative, anti-apoptotic,
anti-inflammatory and cardioprotective effects (6,11). SAL
plays an important role in protecting against myocardial damage and
has significant inhibitory and protective effects on damaged
myocardial tissue via antioxidant production and inhibition of
myocardial apoptosis (12,13). In cardiovascular disease, SAL has
been reported to enhance and protect cardiac function by inhibiting
cardiomyocyte degeneration, necrosis and apoptosis (14). SAL can also stimulate the
accumulation of hypoxia inducible factor (HIF)-1α under hypoxia
conditions, decrease hypoxia-induced injury of cells and protect
cardiomyocytes from hypoxia/reoxygenation injury, indicating the
potential of SAL in combating hypoxia injury (6,15,16).
Furthermore, SAL alleviates cardiovascular emergency contraction
caused by hypoxia, induces and improves activity of key enzymes for
free radical scavenging in the body, promotes balance between free
radical generation and scavenging and inhibits lipid peroxidation
in the cell membrane (17-19).
SAL can help resist hypoxic injury, participate in signalling
pathways that antagonise hypoxic cytotoxicity and resist
hypoxia-induced apoptosis by reducing the expression of associated
proteins such as PI3K and AMPK, which effectively protects against
ischaemic anoxic cardiovascular disease (12,13,20,21).
HIF-1 is an oxygen-regulated transcription activator
that is activated because of the growth restriction of cells in
hypoxia owing to blockage of oxidative phosphorylation in normal
cells, consequently making glycolysis the primary energy supply
mode (22). HIF-1 consists of α-
and β-subunits, which are key gene regulators involved in cellular
hypoxia response, erythropoiesis regulation, angiogenesis,
anaerobic metabolism and glycolysis pathways (23,24).
HIF-1α is a key factor that influences hypoxic response in tumour
cells and is expressed cumulatively under hypoxia conditions;
however, its expression levels are low under normoxic conditions
(24). In addition, HIF-1α
regulates cell response to hypoxia and tumour biological behaviour
by influencing cell apoptosis/proliferation and energy metabolism
(25).
Egl-9 family HIF 1 (EGLN1) is the primary oxygen
receptor in the human body (26).
Under normoxic conditions, EGLN1 uses O2 as a cofactor
to hydroxylate HIF-1α. HIF-1α is recognised by the E3 ubiquitin
ligase complex formed by the von Hippel-Lindau protein, resulting
in its rapid degradation by proteasomes (27). However, under hypoxic conditions,
activity of the proline hydroxylase EGLN1 is inhibited, which
inhibits the hydroxylation and ubiquitination-based degradation of
HIF-1α. This causes HIF-1α to accumulate and enter the nucleus to
form a complex with HIF-1β (28).
This complex regulates expression of genes downstream of hypoxia
and hypoxic stress response in the body (29).
Since hypoxia-induced apoptosis of cardiomyocytes is
a notable risk factor for cardiovascular disease (30,31),
preventing cardiomyocyte apoptosis under hypoxic conditions is key.
SAL alleviates cardiomyocyte apoptosis and enhances cell viability;
however, the underlying mechanisms remain unclear. Studies on SAL
have mainly focused on its ability to resist hypoxia and the
mechanism underlying its anti-hypoxic effects (6,12,13);
however, whether SAL mitigates existing hypoxic conditions remains
to be determined. Furthermore, whether there is a regulatory
interaction between EGLN1 and HIF-1α that affects apoptosis of
cardiomyocytes is unknown. Therefore, the rescue ability of SAL in
hypoxic H9C2 cells was studied using hypoxic culture conditions
in.
Materials and methods
Materials
SAL, DMSO, trypsin, 1.5 M Tris-HCl (pH=8.8), 1.0 M
Tris-HCl (pH=6.8) and penicillin-streptomycin solution were
purchased from Beijing Solarbio Science & Technology Co., Ltd.
Serum-free DMEM and FBS were purchased from Thermo Fisher
Scientific, Inc. PBS was purchased from Lanzhou Bailing Biotech
Co., Ltd. RNApure Tissue & Cell (DNase I), UltraSYBR One Step
RT-qPCR, HiFiScript cDNA Synthesis and Cell Counting Kit-8 (CCK-8)
were purchased from Beijing ComWin Biotech Co., Ltd. SDS-PAGE
Sample Loading Buffer (5X), fluo-4/acetoxymethyl ester (Fluo-4/AM)
(2 mM) and Annexin V-FITC Apoptosis Detection kit were purchased
from Beyotime Institute of Biotechnology. BCA protein assay kit and
5% bovine serum albumin (BSA) were purchased from Beijing Labgic
Co., Ltd. L-glutamine was purchased from Suzhou Haixing Biological
Technology Co., Ltd.; 30% acrylamide (29:1) was purchased from
Sinopharm Chemical Reagent Co., Ltd. PVDF membranes was purchased
from MilliporeSigma. Tween-20 was purchased from Amresco Co., Ltd.
Anti-EGLN1 (cat. no. #4835s) was purchased from Cell Signaling
Technology, Inc. Anti-HIF-1α (cat. no. NB100-105) was purchased
from Novus Biologicals, LLC. Anti-GAPDH (cat. no. 60004-1-Ig) was
purchased from Proteintech Group, Inc. Horseradish
peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG (cat. no.
ZB2301) was purchased from OriGene Technologies, Inc. 10% SDS was
purchased from China Sinopharm International (Shanghai) Co., Ltd.
Enhanced chemiluminescence (cat. no. ECL-0011) was purchased from
Ding Guo Prosperous Co., Ltd. Primers for HIF-1α (forward,
5'-CCGCCACCACCACTGATGAATC-3' and reverse,
5'-GTGAGTACCACTGTATGCvTGATGCC-3') and GAPDH (forward,
5'-GAAGGTCGGTGTGAACGGAT-3' and reverse,
5'-CCCATTTGATGTTAGCGGGAT-3') were purchased from Beijing Tsingke
Biotech Co., Ltd. EGLN1 primers (forward,
5'-AGCTGGTCAGCCAGAAGAGT-3' and reverse, 5'-GCCCTCGATCCAGGTGATCT-3')
were purchased from Sangon Biotech Co., Ltd. All other solvents and
chemicals were of analytical grade. Pure water was produced using a
Milli-Q purification system (MilliporeSigma).
Cell culture
H9C2 cell line was purchased from Suzhou Haixing
Biological Technology Co., Ltd. The cells in the normoxia group
were cultured in DMEM containing 10 FBS and 1%
penicillin-streptomycin and incubated at 37˚C in a 5%
CO2 incubator. For the hypoxia group, cells were
cultured at 37˚C with 5 CO2 and 2% O2.
In vitro cell CCK-8 assay
The biocompatibility of SAL in H9C2 cells was
assessed using CCK-8 assay. H9C2 cells were seeded into 96-well
plates at a density of 1x105 cells/well and incubated
overnight at 37˚C, following which 200 µl SAL (10, 100, 250, 500,
750 and 1,000 nM) or DMEM (blank control) was added for an
additional 24 h at 37 ˚C. After removing the DMEM, 110 µl fresh
medium (containing 10 µl CCK-8) was added and incubated for 1.5 h.
Finally, viability was determined by measuring the absorbance of
each well at 450 nm using Spectrophotometer Multiskan (Thermo
Fisher Scientific, Inc.).
The ability of SAL to rescue hypoxia-treated H9C2
cells was also confirmed using the CCK-8 method. H9C2 cells were
cultured in 96-well plates at a density of 1x105
cells/well and incubated overnight at 37˚C. H9C2 cells were
transferred to a hypoxic incubator and cultured under hypoxic
conditions for 48 h at 37˚C. Medium was removed and 200 µl SAL (10,
100, 250, 500, 750 and 1,000 nM) or an equal volume of fresh DMEM
was added for 24 h in the hypoxic incubator at 37˚C. After removing
DMEM, the cells were incubated with CCK-8 solution for 1.5 h.
Finally, viability was calculated by measuring the absorbance of
each well at 450 nm using Spectrophotometer Multiskan (Thermo
Fisher Scientific, Inc.).
Early and late apoptosis analysis
The rescue effect of SAL on hypoxic cells was
quantitatively analysed using flow cytometry. H9C2 cells were
co-inoculated into 3-cm dishes at a density of 2.5x105
cells/ml. A total of three groups was used: Normoxia, hypoxia and
hypoxia + SAL. Cells in the normoxia group were cultured under
normoxic conditions for 48 h and incubated in fresh DMEM for an
additional 24 h at 37˚C. Cells in the hypoxia group were incubated
under hypoxic conditions for 48 h at 37˚C, followed by incubation
in DMEM for an additional 24 h. In the hypoxia + SAL group, cells
were incubated under hypoxic conditions for 48 h at 37˚C, followed
by incubation with 200 µl SAL (100 nM) for an additional 24 h at
37˚C. Cells were collected via centrifugation (500 x g for 5 min at
4˚C) and washed twice with PBS. Cells were suspended in 100 µl
binding buffer mixed with 5 µl Annexin-V/FITC and incubated for 5
min in the dark at room temperature. The cells were mixed with 10
PI stain and 400 µl PBS. The stained cells were analyzed by flow
cytometry (BD Biosciences) and analysed using WinCyte software
(CompuCyte).
Intercellular Ca2+
concentration analysis
The ability of SAL to maintain intracellular calcium
homeostasis was evaluated using Fluo-4/AM. H9C2 cells were cultured
in 12-well plates with cell slides at a density of
1.5x105 cells/well. Normoxia group was cultured at 37˚C
with 5% CO2 and 2% O2. Hypoxia group was
cultured at 37˚C with 5% CO2. The culture medium was
removed following incubation, the cells were washed with PBS twice
and 200 µl Fluo-4/AM (2.5 µM) was added. The cells were then
incubated at 37˚C for 15 min in the dark. The stained H9C2 cells
were washed with PBS three times, 300 µl 4% paraformaldehyde was
added to fix the cells for 30 min at 37˚C, and cells were observed
under a fluorescence microscope at 100x magnification (Leica
GmbH).
Tandem mass tag (TMT) proteomics
analysis
A total of 1x107 cells/tube (three tubes
from each group) were subjected to TMT proteomic analysis to screen
differentially expressed proteins, which were analysed at the
functional level as reported (32).
Gene Ontology (GO; david.ncifcrf.gov/tools.jsp) and Kyoto Encyclopaedia
of Genes and Genomes (KEGG) enrichment (http://www.kegg.jp) analyses were performed to
determine whether differentially expressed proteins were
significantly enriched in hypoxia-related pathways. For each
protein, the fold-change (FC) was calculated as the ratio of mean
values of all biological measurements in each two groups.
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted from cells using an RNApure Tissue
and Cell kit (DNase I) according to the manufacturer's protocol.
cDNA was synthesised using HiFiScript cDNA Synthesis kit according
to the manufacturer's protocol. UltraSYBR One Step RT-qPCR Kit was
used to detect the expression of HIF-1α and EGLN1. Thermocycling
conditions were as follows: Initial denaturation at 95˚C for 30
sec, followed by 45 cycles of 95˚C for 5 sec and 60˚C for 30 sec.
The relative expression levels of HIF-1α and EGLN1 were evaluated
by the 2-∆∆Cq method (33).
Western blotting
Cell lysate was extracted using RIPA assay buffer
and protein lysates were obtained following centrifugation (12,000
x g for 10 min at 4˚C). Proteins were quantified using a BCA assay
kit. The protein extracts were isolated and transferred onto a PVDF
membrane, which was blocked with 5% BSA at 37˚C for 2 h.
Subsequently, the membrane was incubated overnight with primary
antibody at 4˚C, followed by incubation with secondary antibody at
37˚C for 1 h. Chemiluminescent imaging system (Clinx Science) was
used to detect the signals. The bands were quantified using Image J
software 6.3 (imagej.net/software/).
Statistical analysis
All data were analysed by SPSS 16.0 software (SPSS,
Inc.). Continuous variables are expressed as the mean ± standard
deviation of three independent replicate experiments. One-way
analysis of variance followed by Tukey multiple comparisons test
were employed to determine significance between different groups.
Fisher's exact test was used to find enriched GO and KEGG terms.
The corresponding P-value was calculated as the significance index.
Benjamini-Hochberg False Discovery Rate was used to correct the
P-value. P<0.05 was considered to indicate a statistically
significant difference.
Results
Hypoxia modelling and optimal
concentration screening of SAL
Hypoxic incubator models create a hypoxic
environment and induce cell apoptosis (34,35).
Morphology of the adherent H9C2 cells was fusiform; however,
following hypoxic culture, the cell morphology became round and
some cells fell off the bottom of the culture dish (Fig. 1A). These morphological changes were
not apparent in SAL treatment group. Compared with the hypoxia
group, cells of the hypoxia + SAL group adhered to culture dishes
and exhibited spindle-shaped shape, which was notably improved.
CCK-8 assay was used to evaluate the effect of SAL
on viability of H9C2 cells under a normoxic environment (Fig. 1B). Cell viability was >90%, which
confirmed good biocompatibility of SAL. The different incubation
times had no significant effect on proliferation of H9C2 cells
under normoxic conditions; therefore, 24 h was selected as the
incubation time for SAL in subsequent experiments.
A hypoxia model was created to explore the ability
of SAL to rescue H9C2 cells following hypoxia. With higher SAL
concentrations, the survival rates of hypoxic cells were
significantly higher than those of the control group (Fig. 1C). SAL concentrations of 100, 250,
500, 750 and 1,000 nM showed good rescue ability; therefore, 100 nM
was selected for subsequent experiments.
Ca2+ detection and
apoptosis
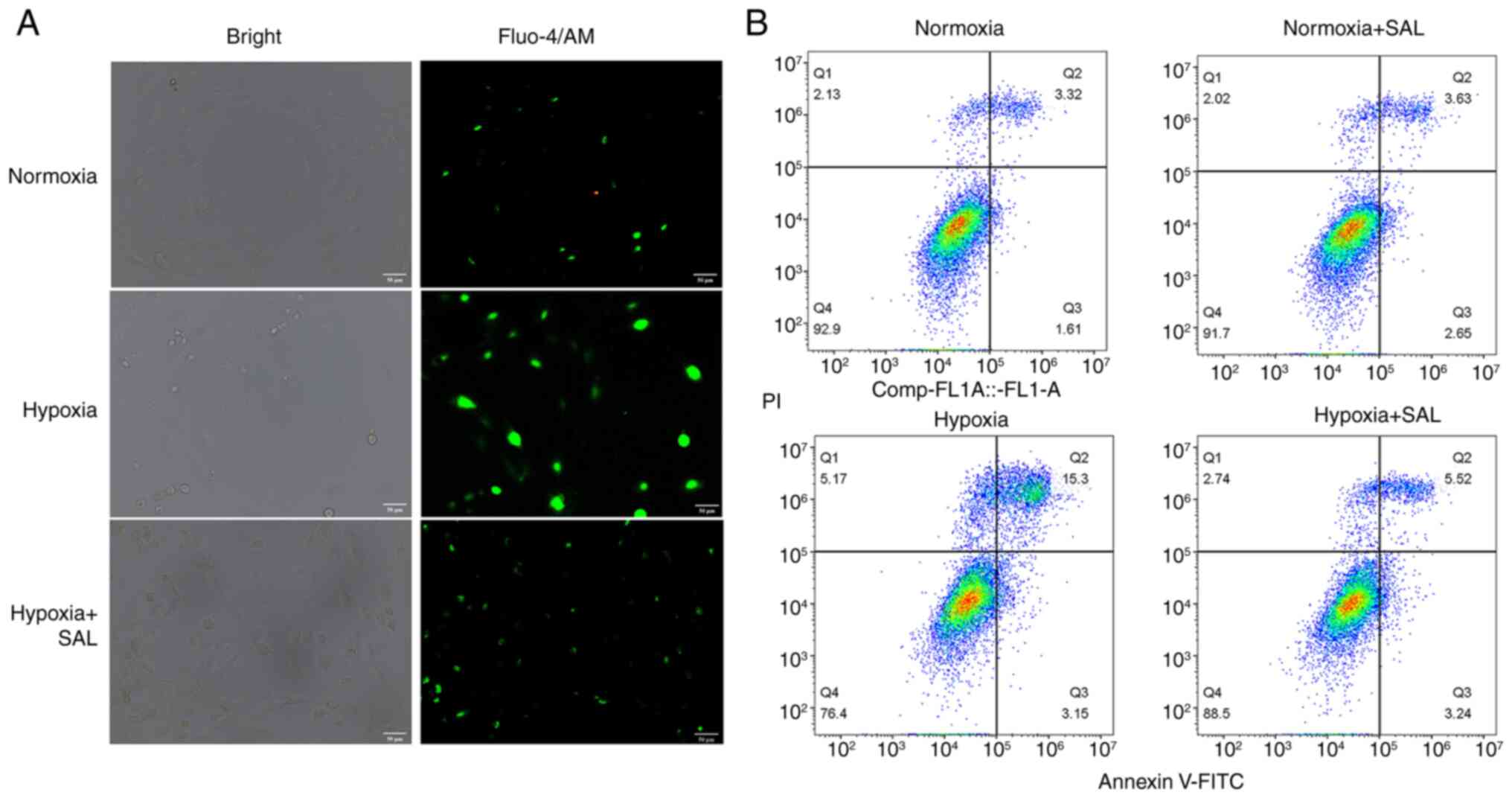
To investigate changes of intracellular
Ca2+ in the three groups, the cells were stained with
Fluo-4/AM and observed using a fluorescence microscope. Compared
with the control group, H9C2 cells in the hypoxia group showed
brighter green fluorescence, indicating that the hypoxia induced
intracellular calcium overload (Fig.
2A). Following SAL treatment in hypoxic cells, green
fluorescence intensity of Ca2+ was notably weakened
compared with the hypoxia group. These results indicated that SAL
treatment reduced intracellular calcium overload in hypoxic cells,
thereby inhibiting cell apoptosis.
Flow cytometric analysis of cells stained with
Annexin V-FITC/PI was used to assess the degree of apoptosis.
Survival rates of H9C2 cells in the normoxia and normoxia + SAL
groups were >90%, which was consistent with the cell viability
results obtained by CCK-8 assay (Fig.
2B). These results further confirmed that SAL has good
biocompatibility. After H9C2 cells were cultured under hypoxic
conditions, ~18.45% cells were apoptotic and the apoptosis rate
increased by 13.5% compared with the normoxia group, indicating
that apoptosis was induced via hypoxia-related pathways following
hypoxia. In hypoxic cells treated with SAL, the apoptosis rate was
8.76% (early and late apoptosis, 3.24 and 5.52%, respectively),
which was 9.69% lower than that in the hypoxia group. Flow
cytometry showed that SAL effectively improved survival rate of
hypoxic cells and attenuated the effects of hypoxia.
Differential protein screening
According to proteomic analysis, 63 proteins were
up- and 42 were downregulated in the normoxia group compared with
the hypoxia group. The expression of 78 proteins was up-while that
of 48 proteins was downregulated in the normoxia group compared
with the hypoxia + SAL group. The expression of four proteins was
up- and that of 12 was downregulated in the hypoxia group compared
with the hypoxia + SAL group (Fig.
S1). Subsequently, differentially expressed proteins were
screened for those associated with the hypoxia pathway.
Relative protein abundance of EGLN1(974) in the
hypoxia group was significantly higher than that in the control
group (461) following hypoxia treatment; however, the protein
abundance decreased to 737 following treatment with SAL (P<0.05;
Fig. 3A). EGLN1 expression was
negatively associated with oxygen concentration under hypoxic
conditions.
GO and KEGG enrichment analysis
GO enriched terms were ‘response to glucose’,
‘response to hypoxia’ ‘glycolysis/gluconeogenesis’ (Fig. 3B). Therefore, cells responded to
hypoxia by expressing hypoxia-related proteins; hypoxia treatment
affected cellular glucose metabolism pathways. KEGG enrichment
analysis showed that HIF-1 signalling pathway was enriched in the
hypoxia vs. hypoxia + SAL group; seven proteins were down- and one
was upregulated (Fig. 4A and
B). Hypoxia affected the
glycolysis/gluconeogenesis pathway, a central pathway in energy
metabolism (Fig. 3C and D).
Expression of HIF-1α and EGLN1
Expression of HIF-1α and EGLN1 in the hypoxia group
was significantly higher than those in the normoxia group (Fig. 5A). For HIF-1α, mRNA expression under
hypoxia was 4.98-fold that of the control group, whereas treatment
with SAL decreased its expression. The mRNA expression levels of
EGLN1 showed a similar trend. mRNA expression of EGLN1 under
hypoxia was 2.40-fold higher than that in the normoxia group and
the expression of EGLN1 was reduced following co-incubation with
SAL. Non-hydroxylated HIF-1α is more stable than hydroxylated
HIF-1α, resulting in increased mRNA expression. EGLN1 mRNA
expression increased under hypoxia.
Compared to the normoxia group, expression of HIF-1α
protein significantly increased following hypoxia treatment, which
was consistent with mRNA levels (Fig.
5B and C). EGLN1 protein
expression increased following hypoxia treatment. However,
following treatment with SAL for 24 h, expression of HIF-1α and
EGLN1 protein decreased.
Discussion
Under hypoxic conditions, the ETC in the
mitochondria of cardiomyocytes is blocked due to insufficient
oxygen supply (36). Electrons leak
out of the respiratory chain and combine with O2 to
produce reactive oxygen species (ROS), causing lipid peroxidation
of mitochondrial and cell membranes and ultimately apoptosis
(36,37). Therefore, it is key to prevent
hypoxia-induced loss of cardiomyocytes. Studies have shown that SAL
exerts anti-hypoxic and protective effects on cardiac function
(12,13,38).
Following hypoxic stimulation in vitro, SAL treatment
improves cardiomyocyte activity, protects cardiomyocytes from
hypoxia-induced injury and decreases apoptosis (39). The present study further
demonstrated that SAL could rescue cardiomyocytes under hypoxia,
decrease apoptosis and regulate the hypoxia-associated EGLN1/HIF-1α
pathway.
Cobalt chloride (CoCl2) is used to mimic
hypoxic conditions because it promotes apoptosis and increases ROS
production, leading to mitochondrial abnormality (40). However, CoCl2 mimics
hypoxia by stabilising HIF-1α expression (41). Therefore, the present study used
true hypoxia (2% O2). The present study showed that SAL
could effectively relieve Ca2+ overload in hypoxic
cells. Hypoxia changes cell membrane potential, leading
Ca2+ to enter the cells, thereby causing calcium
overload. Calcium ions play an important role in several
physiological processes, such as energy production and apoptosis
(42,43). Mitochondrial calcium overload
induces mitochondrial swelling, mitochondrial membrane potential
disorder and the release of apoptotic factors into cytoplasm, which
in turn leads to apoptosis (44,45).
In cardiomyocytes, mitochondria contain large calcium pools; when
mitochondrial Ca2+ levels exceed their capacity,
mitochondrial permeability transition pores are irreversibly
opened, lowering mitochondrial membrane potential and ultimately
causing apoptosis. Decreasing mitochondrial Ca2+
overload can therefore prevent cardiac injury (46). Here, SAL decreased Ca2+
concentration in cardio myocytes under hypoxic condition, which may
also play a protective role.
TMT proteomics identified differentially expressed
proteins associated with glucose metabolism and the hypoxia
response pathway, among which EGLN1 plays an important role in
hypoxia (26,27). Hypoxia inhibits activation of EGLN1,
which in turn inhibits hydroxylation of the two proline residues of
HIF-1α, causing HIF-1α to accumulate in the cell and promoting
apoptosis. The addition of SAL alleviates effects of hypoxia
environment, EGLN1 protein is activated and hydroxylates the
oxygen-dependent degradation domain of HIF-1α, causing its
degradation (27). SAL treatment
downregulated HIF-1α and EGLN1 expression, which was increased
under hypoxia. GO and KEGG analysis showed that EGLN1 protein
expression was related to HIF-1α hypoxia pathway. As the upstream
gene of HIF-1α, EGLN1 expression and activity directly affect the
expression of HIF-1α, thus affecting apoptosis. However, SAL
decreased expression of HIF-1α and EGLN1 and increased the vitality
of cells.
This HIF-1α pathway is key for cellular adaptation
and survival in hypoxic environments. When cells are exposed to
hypoxia, HIF-1α is stabilised and dimerises with HIF-1β, which
regulates the expression of genes involved in metabolism, cell
proliferation and apoptosis. Several other differentially expressed
proteins involved in the HIF-1 signalling pathway have been
identified by TMT proteomics, including downstream metabolic
regulators of HIF-1α, such as pyruvate dehydrogenase kinase (PDK1)
and lactate dehydrogenase (LDHA) (47). Under hypoxic conditions,
HIF-1-mediated PDK1 expression shunts glucose away from
mitochondria, attenuating mitochondrial respiration and preventing
toxic ROS production (48). LDHA is
a key enzyme that converts pyruvate into lactic acid during
glycolysis. Hypoxia-induced LDHA is reported to promote
inflammation (47,49). SAL alleviates hypoxia-induced
upregulation of PDK1 and LDHA, suggesting that SAL may alleviate
physiological stress caused by hypoxia (47,49).
The expression of HIF-1α and EGLN1 increased under hypoxia
treatment; the expression of HIF-1α decreased following treatment
with SAL, demonstrating that SAL decreased expression of hypoxia
factors. Similarly, EGLN1 showed a consistent trend with HIF-1α.
These results showed that SAL inhibited expression of
hypoxia-related factors, inhibiting their effects in the signalling
pathway and achieving cell rescue. SAL could effectively regulate
the expression of HIF-1α and EGLN1 by regulating the EGLN1/HIF-1α
hypoxia signalling pathway and inhibit apoptosis.
The GO and KEGG enrichment analysis also showed
hypoxia affected the glycolysis/gluconeogenesis pathway, a central
pathway in energy metabolism Gluconeogenesis refers to the process
by which non-sugar substances are converted into glycogen or
glucose by enzymes in organs such as the liver and kidney (50,51).
HIF-1 activation by hypoxia induces glycolysis, the anaerobic
oxidation of glucose, resulting in conversion of normoxic aerobic
respiratory metabolic pathway to a different energy production
pathway with lower oxygen consumption (50).
The rescue effect and underlying mechanism of action
of SAL on cardiomyocytes remain to be confirmed in cell lines other
than H9C2 cells. The concentrations required to achieve potential
effects of SAL may vary in different cells; therefore, further
investigation is warranted to optimize the concentration of SAL for
hypoxia treatment.
In conclusion, SAL enhanced the viability of
cardiomyocytes, inhibited intracellular mitochondrial calcium
overload, decreased the expression of hypoxia-associated factors
HIF-1α and EGLN1 and inhibited cell apoptosis through the
EGLN1/HIF-1α pathway, suggesting that SAL effectively rescued the
damage of cardiomyocytes caused by hypoxia (Fig. 6).
Supplementary Material
Proteomic analysis result. (A) 63
proteins were up- and 42 were downregulated in the normoxia group
compared with the hypoxia group. (B) The expression of 78 proteins
was up-while that of 48 proteins was downregulated in the normoxia
group compared with the hypoxia + SAL group. (C) The expression of
four proteins was up- and that of 12 was downregulated in the
hypoxia group compared with the hypoxia + SAL group.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Hunan Province (grant no. 2023JJ50289) and Scientific
Research Project of Hunan Provincial Health Commission (grant no.
202103011065).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WZ wrote and reviewed the manuscript and conceived
and designed the study. ZL performed experiments and analyzed data.
CX performed experiments. XL conceived and designed the study and
edited the manuscript. WZ and XL confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veldhuizen J, Chavan R, Moghadas B, Park
JG, Kodibagkar VD, Migrino RQ and Nikkhah M: Cardiac ischemia
on-a-chip to investigate cellular and molecular response of
myocardial tissue under hypoxia. Biomaterials.
281(121336)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ichiki T and Sunagawa K: Novel roles of
hypoxia response system in glucose metabolism and obesity. Trends
Cardiovasc Med. 24:197–201. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kishimoto I, Tokudome T, Hosoda H,
Miyazato M and Kangawa K: Ghrelin and cardiovascular diseases. J
Cardiol. 59:8–13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wilkins MR, Ghofrani HA, Weissmann N,
Aldashev A and Zhao L: Pathophysiology and treatment of
high-altitude pulmonary vascular disease. Circulation. 131:582–590.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guo J, Zhu K, Li Z and Xiao C: Adiponectin
protects hypoxia/reoxygenation-induced cardiomyocyte injury by
suppressing autophagy. J Immunol Res. 2022(8433464)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu B, Wei H, Lan M, Jia N, Liu J and
Zhang M: MicroRNA-21 mediates the protective effects of salidroside
against hypoxia/reoxygenation-induced myocardial oxidative stress
and inflammatory response. Exp Ther Med. 19:1655–1664.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rabinovich-Nikitin I, Blant A, Dhingra R,
Kirshenbaum LA and Czubryt MP: NF-κB p65 attenuates cardiomyocyte
PGC-1α expression in hypoxia. Cells. 11(2193)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen Y, Tang M, Yuan S, Fu S, Li Y, Li Y,
Wang Q, Cao Y, Liu L and Zhang Q: Rhodiola rosea: A
therapeutic candidate on cardiovascular diseases. Oxid Med Cell
Longev. 2022(1348795)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Y, Zhao Y, Li X, Liu T, Jiang X and Han
F: Characterization of global metabolic profile of Rhodiola
crenulata after oral administration in rat plasma, urine, bile and
feces based on UHPLC-FT-ICR MS. J Pharm Biomed Anal. 149:318–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xie N, Fan F, Jiang S, Hou Y, Zhang Y,
Cairang N, Wang X and Meng X: Rhodiola crenulate alleviates
hypobaric hypoxia-induced brain injury via adjusting
NF-κB/NLRP3-mediated inflammation. Phytomedicine.
103(154240)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bai XL, Deng XL, Wu GJ, Li WJ and Jin S:
Rhodiola and salidroside in the treatment of metabolic disorders.
Mini Rev Med Chem. 19:1611–1626. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tian X, Huang Y, Zhang X, Fang R, Feng Y,
Zhang W, Li L and Li T: Salidroside attenuates myocardial
ischemia/reperfusion injury via AMPK-induced suppression of
endoplasmic reticulum stress and mitochondrial fission. Toxicol
Appl Pharmacol. 448(116093)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan W, Li K, Buhe A, Li T, Tian P and Hong
J: Salidroside inhibits the proliferation and migration of gastric
carcinoma cells and tumor growth via the activation of
ERS-dependent autophagy and apoptosis. RSC Adv. 9:25655–25666.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X, Kou Y, Lu Y and Pu Y: Salidroside
ameliorated hypoxia-induced tumorigenesis of BxPC-3 cells via
downregulating hypoxia-inducible factor (HIF)-1α and LOXL2. J Cell
Biochem. 121:165–173. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang Y, Hou Y, Zeng Y, Hu Y, Zhang Y, Wang
X and Meng X: Salidroside attenuates CoCl2-simulated
hypoxia injury in PC12 cells partly by mitochondrial protection.
Eur J Pharmacol. 912(174617)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hou Y, Zhang Y, Jiang S, Xie N, Zhang Y,
Meng X and Wang X: Salidroside intensifies mitochondrial function
of CoCl2-damaged HT22 cells by stimulating PI3K-AKT-MAPK
signaling pathway. Phytomedicine. 109(154568)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang YF, Chang YY, Zhang XM, Gao MT, Zhang
QL, Li X, Zhang L and Yao WF: Salidroside protects against
osteoporosis in ovariectomized rats by inhibiting oxidative stress
and promoting osteogenesis via Nrf2 activation. Phytomedicine.
99(154020)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xing Y, Peng HY, Li X, Zhang MX, Gao LL
and Yang XE: Extraction and isolation of the salidroside-type
metabolite from zinc (Zn) and cadmium (Cd) hyperaccumulator Sedum
alfredii Hance. J Zhejiang Univ Sci B. 13:839–845. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu MC, Shi HM, Gao XF and Wang H:
Salidroside attenuates myocardial ischemia-reperfusion injury via
PI3K/Akt signaling pathway. J Asian Nat Prod Res. 15:244–252.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhu Y, Shi YP, Wu D, Ji YJ, Wang X, Chen
HL, Wu SS, Huang DJ and Jiang W: Salidroside protects against
hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt
dependent pathway. DNA Cell Biol. 30:809–819. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Godet I, Shin YJ, Ju JA, Ye IC, Wang G and
Gilkes DM: Fate-mapping post-hypoxic tumor cells reveals a
ROS-resistant phenotype that promotes metastasis. Nat Commun.
10(4862)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Infantino V, Santarsiero A, Convertini P,
Todisco S and Iacobazzi V: Cancer cell metabolism in hypoxia: Role
of HIF-1 as key regulator and therapeutic target. Int J Mol Sci.
22(5703)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janbandhu V, Tallapragada V, Patrick R, Li
Y, Abeygunawardena D, Humphreys DT, Martin EMMA, Ward AO, Contreras
O, Farbehi N, et al: Hif-1a suppresses ROS-induced proliferation of
cardiac fibroblasts following myocardial infarction. Cell Stem
Cell. 29:281–297.e12. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qin Y, Liu HJ, Li M, Zhai DH, Tang YH,
Yang L, Qiao KL, Yang JH, Zhong WL, Zhang Q, et al: Salidroside
improves the hypoxic tumor microenvironment and reverses the drug
resistance of platinum drugs via HIF-1α signaling pathway.
EBioMedicine. 38:25–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun L, Wu C, Ming J, Guo E, Zhang W, Li L
and Hu G: EGLN1 induces tumorigenesis and radioresistance in
nasopharyngeal carcinoma by promoting ubiquitination of p53 in a
hydroxylase-dependent manner. J Cancer. 13:2061–2073.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang J, Deng H, Wang Z, Zha H, Liao Q, Zhu
C, Chen X, Sun X, Jia S, Ouyang G, et al: EGLN1 prolyl
hydroxylation of hypoxia-induced transcription factor HIF1α is
repressed by SET7-catalyzed lysine methylation. J Biol Chem.
298(101961)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou Y, Ouyang N, Liu L, Tian J, Huang X
and Lu T: An EGLN1 mutation may regulate hypoxic response in
cyanotic congenital heart disease through the PHD2/HIF-1A pathway.
Genes Dis. 6:35–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu G, Zhao W, Zhang H, Wang T, Han Z and
Ji X: rs1769793 variant reduces EGLN1 expression in skeletal muscle
and hippocampus and contributes to high aerobic capacity in
hypoxia. Proc Natl Acad Sci USA. 117:29283–29285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang G, Zhang D, Zhang X, Yu K and Jiang
A: Saprirearine protects H9c2 cardiomyocytes against
hypoxia/reoxygenation-induced apoptosis by activating Nrf2. Acta
Biochim Pol. 69:429–436. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Su Y, Tian H, Wei L, Fu G and Sun T:
Integrin β3 inhibits hypoxia-induced apoptosis in cardiomyocytes.
Acta Biochim Biophys Sin (Shanghai). 50:658–665. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang W, Li Q, Huang G, Lin BY, Lin D, Ma
Y, Zhang Z, Chen T and Zhou J: Tandem mass tag-based proteomic
analysis of potential biomarkers for hepatocellular carcinoma
differentiation. Onco Targets Ther. 14:1007–1020. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liang RP, Jia JJ, Li JH, He N, Zhou YF,
Jiang L, Bai T, Xie HY, Zhou L and Sun YL: Mitofusin-2 mediated
mitochondrial Ca2+ uptake 1/2 induced liver injury in
rat remote ischemic perconditioning liver transplantation and alpha
mouse liver-12 hypoxia cell line models. World J Gastroenterol.
23:6995–7008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Salyha N and Oliynyk I: Hypoxia modeling
techniques: A review. Heliyon. 9(e13238)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou W, Yang W, Fan K, Hua W and Gou S: A
hypoxia-activated NO donor for the treatment of myocardial hypoxia
injury. Chem Sci. 13:3549–3555. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wen SY, Tamilselvi S, Shen CY, Day CH,
Chun LC, Cheng LY, Ou HC, Chen RJ, Viswanadha VP, Kuo WW and Huang
CY: Protective effect of HDL on NADPH oxidase-derived super oxide
anion mediates hypoxia-induced cardiomyocyte apoptosis. PLoS One.
12(e0179492)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sun S, Tuo Q, Li D, Wang X, Li X, Zhang Y,
Zhao G and Lin F: Antioxidant effects of salidroside in the
cardiovascular system. Evid Based Complement Alternat Med.
2020(9568647)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ni J, Li Y, Xu Y and Guo R: Salidroside
protects against cardiomyocyte apoptosis and ventricular remodeling
by AKT/HO-1 signaling pathways in a diabetic cardiomyopathy mouse
model. Phytomedicine. 82(153406)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li M, Li K and Ren Y: Nesfatin-1 protects
H9c2 cardiomyocytes against cobalt chloride-induced hypoxic injury
by modulating the MAPK and Notch1 signaling pathways. J Biol Res
(Thessalon). 28(21)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lee HR, Leslie F and Azarin SM: A facile
in vitro platform to study cancer cell dormancy under hypoxic
microenvironments using CoCl2. J Biol Eng.
12(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu Z, Zhang D, He X, Huang Y and Shao H:
Transport of calcium ions into mitochondria. Curr Genomics.
17:215–219. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Y, Li L, Yue J, Cao L, Liu P, Dong
WF and Liu G: Yttrium-mediated red fluorescent carbon dots for
sensitive and selective detection of calcium ions. Luminescence.
36:1969–1976. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bao W, Liu M, Meng J, Liu S, Wang S, Jia
R, Wang Y, Ma G, Wei W and Tian Z: MOFs-based nanoagent enables
dual mitochondrial damage in synergistic antitumor therapy via
oxidative stress and calcium overload. Nat Commun.
12(6399)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zheng P, Ding B, Jiang Z, Xu W, Li G, Ding
J and Chen X: Ultrasound-augmented mitochondrial calcium ion
overload by calcium nanomodulator to induce immunogenic cell death.
Nano Lett. 21:2088–2093. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhou Q, Xie M, Zhu J, Yi Q, Tan B, Li Y,
Ye L, Zhang X, Zhang Y, Tian J and Xu H: PINK1 contained in
huMSC-derived exosomes prevents cardiomyocyte mitochondrial calcium
overload in sepsis via recovery of mitochondrial Ca2+
efflux. Stem Cell Res Ther. 12(269)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen SF, Pan MX, Tang JC, Cheng J, Zhao D,
Zhang Y, Liao HB, Liu R, Zhuang Y, Zhang ZF, et al: Arginine is
neuroprotective through suppressing HIF-1α/LDHA-mediated
inflammatory response after cerebral ischemia/reperfusion injury.
Mol Brain. 13(63)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu D, Wang S, Wang F, Zhang Q, Zhang Z and
Li X: Lactate dehydrogenase A (LDHA)-mediated lactate generation
promotes pulmonary vascular remodeling in pulmonary hypertension. J
Transl Med. 22(738)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Meng F, Zhang W and Wang Y: RASAL1
inhibits HepG2 cell growth via HIF-2α mediated gluconeogenesis.
Oncol Lett. 14:7344–7352. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wu X and Chen S: Advances in natural small
molecules on pretranslational regulation of gluconeogenesis. Drug
Dev Res. 84:613–628. 2023.PubMed/NCBI View Article : Google Scholar
|