Introduction
Metabolic dysfunction-associated steatotic liver
disease (MASLD) is the latest term used to define non-alcoholic
fatty liver disease associated with metabolic syndrome (1). MASLD is characterized by excessive
lipid accumulation associated with obesity, type 2 diabetes
mellitus, hypertension, dyslipidemia and metabolic syndrome, with
insulin resistance being the common denominator. Alternative
diagnoses such as viral hepatitis and significant alcohol intake
should also be ruled out before diagnosing MASLD (1). The initial stage of development of
this multisystem disorder shows simple hepatic steatosis that can
progress to non-alcoholic steatohepatitis associated with metabolic
dysfunction (MASH), fibrosis, cirrhosis and eventually to
hepatocellular carcinoma (HCC), depending on the clinical, genetic
and epigenetic predispositions of the patient (2-4).
MASLD is the most common cause of liver-related morbidity and
mortality, affecting >30% of individuals worldwide due to the
global prevalence of obesity and diseases associated with this
clinical condition (3,5). Additionally, cardiovascular diseases
feature among the leading causes of death in patients with MASLD,
causing ~40% of all deaths (6,7).
Overall, HCC is the sixth most common cancer and the third leading
cause of cancer-related mortality worldwide (2). However, MASLD/MASH-derived HCC shows
distinct characteristics (including lower survival rates than other
pathologies), but its underlying pathogenic mechanisms remain only
partially understood (2).
Several oncogenic mechanisms are associated with the
progression of MASLD, one of which refers to the accumulation of
lipids in hepatocytes and its associated lipotoxicity creating a
dynamic pro-inflammatory environment (3), in which multiple oncogenic pathways
are associated with HCC development, markedly changing regulatory
and signaling pathways and fostering a conducive hepatic
microenvironment for disease progression (3,8). This
process concurrently increases the risk of cardiovascular diseases
by the activation and generation of metabolic and inflammatory
components (6,9). Due to the difficulty treating and
improving HCC outcomes (10),
immune checkpoint inhibitors (ICIs) represent an effective
treatment strategy for HCC, the mechanisms of action of which are
based on activating the immune system by modulating T lymphocyte
responses and targeting immune checkpoints (11,12).
As a previous meta-analysis has shown, despite the acceptable
safety profile of ICI monotherapy and its immunological
combinations, ICIs have a specific set of treatment-related adverse
events (11,12), including a higher risk of
hypertransaminasemia, warranting liver function monitoring and the
evaluation of potential prognostic biomarkers, such as albumin,
which is related to inflammatory pressure (11,13).
Lower levels of albumin occur in cases of chronic inflammatory
disorders and cancer, acting as a negative acute-phase reactant
(13). In recent years, new
systemic therapies for advanced HCC have been developed, and it has
been suggested that the combination of new and old treatments with
locoregional approaches be implemented (14). MASLD/MASH treatment is necessary to
prevent irreversible chronic liver diseases, such as cirrhosis and
HCC. At present, regulatory agencies have not approved specific
pharmacological therapies to treat MASH, and several clinical
studies currently target its different symptoms (1,15).
Rifaximin (RIF), a non-absorbable broad-spectrum oral antibiotic,
can positively modulate the components of the intestinal
microbiota, attenuating the inflammatory process and energetic
metabolism (16-18).
These mechanisms contribute to the progression from MASLD to HCC,
making it an important target of study.
Based on the above, MASLD-related HCC configures a
global health issue as its forecast impact on HCC morbidity and
mortality is expected to rise in the future. Thus, developing
pre-clinical studies is of fundamental importance for understanding
the mechanisms linked to the development and progression of MASLD
and for evaluating prognostic markers and potential therapeutic
targets (15,19). The complex and multifaceted
pathophysiology of MASLD challenges the search for animal models
that can replicate the disease in its advanced stages, which more
urgently require treatment (15,19).
Hence, the present study aimed to evaluate the impact of RIF
treatment on MASLD-associated hepatocarcinogenesis and to assess
the hepatic and systemic inflammatory processes associated with the
risk of developing cardiovascular diseases.
Materials and methods
Animals
In total, 30 adult male Sprague Dawley rats aged 60
days and weighing 290-330 g were included in the present study. The
rats were housed in pairs in polypropylene cages with
sawdust-covered floors and allowed to acclimatize to the
maintenance room for 2 weeks prior to this experiment. The rats
were kept under a standard 12-h light/dark cycle in a
temperature-controlled environment (22±2˚C). Before starting the
study, measures for anticipating the euthanasia of animals as a
refinement procedure and to protect/preserve their well-being
whenever the animals showed altered behavior or signs of suffering
that could not be controlled with handling or analgesics were
adopted. Additionally, performing chronic gavage (16 weeks) can
result in adverse events such as irritations in the upper gastric
tract (mouth, pharynx, esophagus and stomach), physical stress,
passive reflux if the stomach is overloaded and aspiration
pneumonia. In such situations, comfort measures should also be
adopted. However, these measures were not necessary for any animal
during the present study. All experiments and procedures involving
the use of animals were approved by the Institutional Ethics
Committee of Hospital de Clinicas de Porto Alegre (Porto Alegre,
Brazil; approval no. 2019-0311). The procedures for the use of
scientific animals were conducted in accordance with the Guide for
the Care and Use of Laboratory Animals (8th edition, 2011) and Law
number 11,794 (Brazil, 2008).
Study design
Diethylnitrosamine (DEN; to stimulate the
development of HCC) doses and experimental duration for this
protocol were based on a prior study (20). Briefly, after acclimatizing to the
environment, the rats were randomly assigned to three groups based
on their weight: A control group (n=10), which received a standard
diet and water without DEN along with a daily gavage of vehicle
(Veh) solution throughout the 16-week experiment period; an HCC
group (n=10), which received a high-fat and choline-deficient diet
(HFCD), 135 mg/l DEN (MilliporeSigma) in drinking water, and a
daily gavage of Veh solution for the experimental period; and the
RIF group, which received the HFCD diet plus DEN and prophylactic
RIF (MilliporeSigma) administered daily by gavage for 16 weeks. The
experimental design is depicted in Fig. S1. The weight of the rats was logged
twice a week throughout the experiment. Additionally, naso-anal
length (cm) was measured in the initial and final week of the study
for determination of the change in Lee index. This index was
calculated as the ratio between the cube root of the body weight
and the naso-anal length of animals multiplied by 10 (g/cm)
(21). At the end of the 16-week
period, all rats were anesthetized via inhalation with isoflurane
(BioChimico) in 100% oxygen at a dose of 5% for induction and 3-4%
for maintenance, at 0.5 l/min, and then euthanized via cardiac
exsanguination. The rat livers were completely excised and weighed.
Serum samples, abdominal adipose tissue and liver fragments were
also collected under sterile conditions, flash-frozen in liquid
nitrogen and stored at -80˚C until experimental procedures were
conducted. A portion of each liver sample was fixed in 10% formalin
at room temperature for 24 h for histological analysis.
Nutritional intervention
The diet administered to the intervention groups was
selected to replicate a number of the phenotypes in humans with
MASLD, as previously shown by our research team (22). Rats in the control group were
provided with a standard rodent diet (Nuvilab CR-1; Quimtia), with
an energy content of 2.93 kcal/g (carbohydrates, 55.0%; protein,
22.0%; fat, 4.5%; other nutrients, 18.5%). Rats in the intervention
groups were fed an HFCD diet (RH19576; Rhoster) with an energy
content of 4.3 kcal/g (carbohydrates, 54.5%; protein, 14.0%; fat,
31.5, and 54.0% from trans fatty acids). The diet for all groups
was replaced every 2 days. Throughout the experimental period,
groups had ad libitum access to water and food.
RIF administration
The RIF dose administered followed a previous study
in the literature (8,23). The RIF group received a daily dose
of 50 mg/kg/day of RIF (Biolab Sanus Farmaceutica Ltd.) by daily
gavage until the 16th week of the experiment. The animals in the
control and HCC groups received a daily gavage with a Veh solution
(0.5 ml/kg distilled water). The therapeutic intervention by
administering gavage daily followed the same previously
standardized procedures that had been performed by our research
group (8). In summary, the
administration of RIF or the provision of Veh solution via gavage
to the respective experimental groups occurred from the first day
of the experiment until the date of euthanasia.
Biochemical analysis and atherogenic
ratios
The rats were fasted for 8 h before euthanasia via
cardiac exsanguination under isoflurane anesthesia. Aspartate
aminotransferase (AST), alanine aminotransferase (ALT), glucose,
total cholesterol (TC), low-density lipoprotein (LDL), high-density
lipoprotein (HDL) and triglyceride (TG) serum levels were
determined using Labmax 560, in the Laboratory Diagnostic Service,
Hospital de Clínicas de Porto Alegre (Porto Alegre, Brazil).
The atherogenic ratios, calculated based on the
lipid profile results, were used as a tool to predict
cardiovascular risk (CVR). The atherogenic ratios were calculated
as follows: Castelli's risk index (CRI)-I=TC/HDLc;
CRI-II=LDLc/HDLc; and atherogenic coefficient (AC)=(TC-HDLc)/HDLc
(24), where ‘c’ indicates
cholesterol.
Quantitative analysis of liver fat
deposition
Previously frozen liver tissue samples were thawed
on ice and homogenized in phosphate-buffered saline at a
concentration of 20 mg of tissue/ml, to analyze the hepatic lipid
content. From this homogenate, TG, TC and overall lipid
accumulation levels were assessed. The hepatic TG and TC levels
were enzymatically determined by colorimetric assays (Labtest
Diagnóstica S.A), at wavelengths of 505 and 500 nm, respectively.
The total lipid concentration was determined following the modified
protocol outlined by Gómez-Lechón et al (25). Briefly, the liver tissue was
homogenized in phosphate-buffered saline and incubated with 1 µl
Nile Red solution (1 mg/ml in acetone) at 37˚C for 15 min. The
fluorescence was measured at excitation and emission wavelengths of
488 and 550 nm, respectively, using a SpectraMax M3
spectrophotometer. The obtained values were normalized to the total
protein content of the homogenate (26). The results are presented as
fluorescence/µg protein. All analyses were performed in
duplicate.
Assessment of the gene expression of
hepatic inflammation
Total RNA was extracted from liver tissue fragments
using a RNeasy Mini Kit (Qiagen, Inc.). A high-capacity cDNA
reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to convert cDNA from 2 µg of RNA
according to the manufacturer's instructions. To assess the gene
expression of interleukin (IL)-1β, IL-6, IL-10, tumoral necrosis
factor-α (TNF-α), lipopolysaccharide-binding protein (LBP), myeloid
differentiation primary response 88, toll-like receptor (TLR) 4,
TLR2, transforming growth factor-β1 (TGF-β1), metalloproteinase
(MMP)2 and MMP9 in the liver, a quantitative polymerase chain
reaction with the TaqMan assay (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was performed according to the manufacturer's
instructions. The probes used are listed in Table SI. The β-actin gene was used to
normalize gene expression in the liver tissues. The changes in gene
expression levels were calculated using the formula
2-ΔΔCq (27).
Inflammatory status and endothelial
injury
By a multiplex assay using the Luminex platform
(Merck KGaA), the serum levels of inflammatory and endothelial
dysfunction markers, such as IL-1β, IL-6, TNF-α, monocyte
chemoattractant protein (MCP-1), E-selectin, intercellular adhesion
molecule (ICAM-1), plasminogen activator inhibitor (PAI-1),
insulin, leptin and adiponectin, were evaluated using the following
kits: Rat Adipokine (cat. no. RADPKMAG-80K) for the assessment of
IL-1β, IL-6, insulin, leptin, MCP-1, PAI-1 and TNFα; Rat Vascular
Injury Panel 2 (cat. no. RV2MAG-26K) for the assessment of
adiponectin, E-selectin and ICAM-1. The serum evaluation of soluble
vascular adhesion protein-1 (VAP-1) was performed by an
enzyme-linked immunosorbent assay (cat. no. MBS2515661;
MyBioSource, Inc.). Absorbance was measured in a spectrophotometer
(Zenyth 200 rt) at a wavelength of 450 nm. The results are
presented in ng/ml or pg/ml. All procedures followed the
manufacturers' instructions, and all analyses were performed in
duplicate.
Histopathological analysis
Formalin-fixed liver tissue samples (4-µm sections)
were embedded in paraffin and stained with hematoxylin and eosin
(H&E) and picrosirius red. The histopathological lesions of the
several evolutionary stages of MASLD were assessed according to the
score by Liang et al (28),
which is a highly reproducible scoring system applicable to
experimental rodent models. The degree of fibrosis was evaluated
using the slides stained with picrosirius red and cancerous lesions
were graded according to the Edmondson and Steiner classification
(29). The analysis was performed
by an experienced pathologist, who was blinded to the experimental
groups. The evaluation was conducted in the Surgical Pathology
Service at the Hospital de Clínicas de Porto Alegre (Porto Alegre,
Brazil).
Sample size calculation and
statistical analysis
The sample size was estimated using WINPEPI 11.20
software (Brixton Health) following a prior study by our group
(20,30). With a power of 80% and a
significance level of 5%, it was determined that a minimum of 10
animals per experimental group would be necessary. Data were
analyzed using SPSS version 28.0 (IBM Corp.). The normality of all
variables was assessed using the Shapiro-Wilk test and histograms.
Parametric data were analyzed using one-way analysis of variance,
followed by the Tukey's post-hoc test. Quantitative variables are
shown as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
General characteristics of the
experimental model
The animals in all experimental groups showed
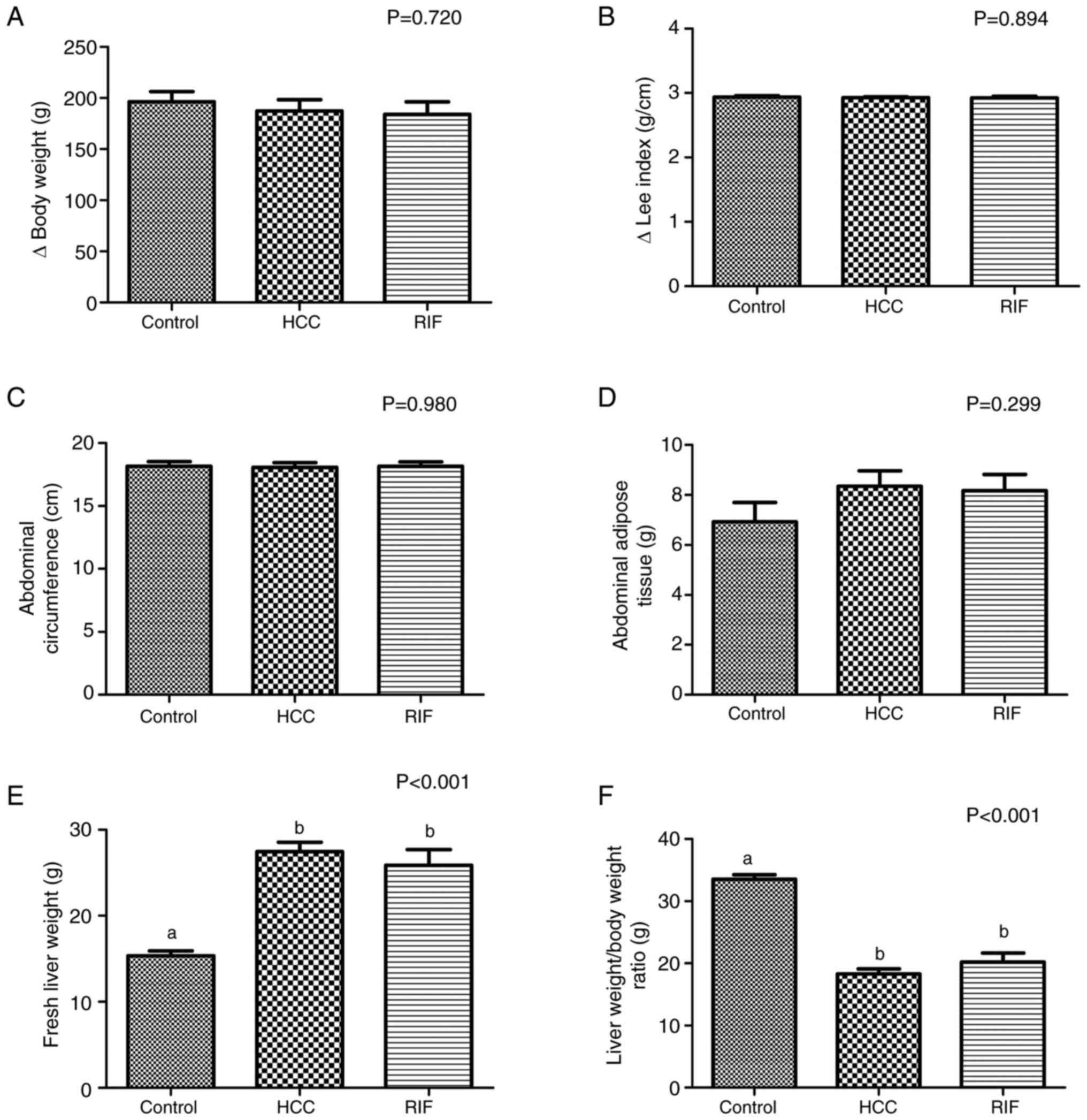
similar baseline body weights (P=0.797), evincing homogeneity.
After the first week of the experiment, HFCD was introduced to the
HCC and RIF groups, and there was no significant difference
(P=0.720) between these experimental groups and the control group
in terms of the change in body weight (Fig. 1A). Additionally, no significant
differences in the change in Lee index (P=0.894; Fig. 1B), abdominal circumference (P=0.980;
Fig. 1C) and abdominal adipose
tissue accumulation (P=0.299; Fig.
1D) was found between the groups. However, the fresh liver
weight significantly increased and the liver weight/body weight
ratio significantly decreased in the HCC and RIF groups relative to
the control group (both P<0.001; Fig. 1E and F).
Biochemical parameters and atherogenic
ratios to assess the CVR
Table I shows the
biochemical parameter and atherogenic ratio data. The serum AST
levels in the RIF group significantly increased compared with the
control group (P=0.045). The glucose levels significantly increased
in the HCC group compared with the control group (P=0.018). There
were no significant differences in serum ALT levels between the
experimental groups (P=0.757). Regarding the lipid profiles, the
HCC and RIF groups showed significantly increases in TC, HDLc and
triglyceride serum levels relative to the control (P<0.001, for
all). However, the inverse occurred for serum LDLc levels
(P<0.001). Regarding the atherogenic ratios, rats in the HCC and
RIF groups showed a significant increase in AC, CRI-I and CR-II
(P<0.001, for all) compared with the control group.
 | Table IBiochemical parameters and
atherogenic ratios. |
Table I
Biochemical parameters and
atherogenic ratios.
| Variable | Control, n=10 | HCC, n=9 | RIF, n=10 | P-value
(ANOVA) |
|---|
| ALT, U/l | 69.2±32.9 | 61.3±19.0 | 61.5±22.6 | 0.757 |
| AST, U/l |
100.9±26.7a |
124.9±25.0a,b |
132.0±28.7b | 0.045 |
| Glucose, mg/dl |
148.0±26.4a |
114.3±9.5b |
128.2±28.6a,b | 0.018 |
| Total cholesterol,
mg/dl |
60.7±10.8a |
130.2±29.7b |
127.9±22.8b | <0.001 |
| HDL cholesterol,
mg/dl |
24.9±3.11a |
40.7±8.6b |
40.6±9.8b | <0.001 |
| LDL cholesterol,
mg/dl |
20.4±6.9a |
78.4±27.2b |
78.2±14.3b | <0.001 |
| Triglycerides,
mg/dl |
76.9±18.0a |
56.0±13.7b |
45.7±10.5b | <0.001 |
| AC |
1.43±0.3a |
2.28±0.7b |
2.22±0.4b | <0.001 |
| CRI-I |
0.77±0.3a |
1.77±0.7b |
2.49±1.1b | <0.001 |
| CRI-II |
0.8±0.3a |
2.0±0.7b |
2.0±0.4b | <0.001 |
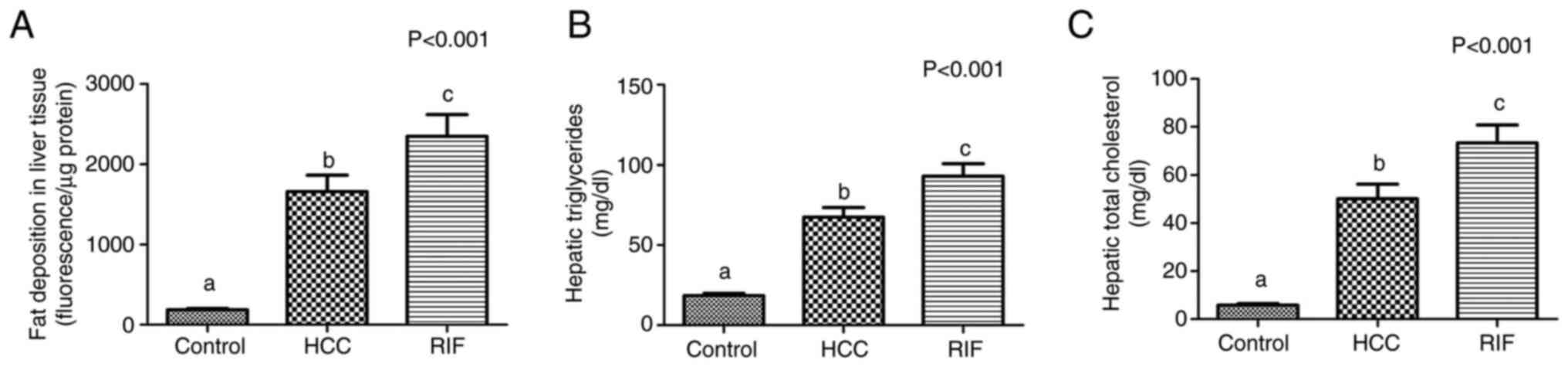
Analysis of fat deposition in liver
tissue
In the quantitative analysis of lipid deposits in
liver tissue, the rats in the HCC and RIF groups showed a
significant increase in the accumulation of lipids, TGs and TC
concentration relative to the control group (P<0.001, for all;
Fig. 2A-C).
Expression of genes involved in
steatohepatitis pathogenesis
Table II shows the
data obtained of the hepatic gene expression of inflammatory
parameters related to steatohepatitis pathogenesis. The HCC group
showed a significant increase in IL-1β (P=0.011) and IL-10
(P<0.001) gene expression relative to the RIF group. The RIF
group showed significantly lower expression levels of TLR2
(P=0.012), LPB (P=0.018) and MMP2 (P=0.003) than the HCC group,
obtaining values that resembled the control group. No significant
differences between the experimental groups in the gene expression
of TNF-α (P=0.174), IL-6 (P=0.187), TLR4 (P=0.140), TGF-β1
(P=0.687) and MMP9 (P=0.479) were found.
 | Table IIGene expression of liver inflammation
markers involved in steatohepatitis pathogenesis. |
Table II
Gene expression of liver inflammation
markers involved in steatohepatitis pathogenesis.
| Variables | Control, n=10 | HCC, n=9 | RIF, n=10 | P-value
(ANOVA) |
|---|
| TNF-α | 13.9±23.8 | 7.6±9.7 | 0.1±0.01 | 0.174 |
| IL-1β |
1.3±0.7a,b |
2.1±0.8b |
0.7±0.9a | 0.011 |
| IL-6 | 1.2±0.9 | 3.1±4.4 | 0.8±1.1 | 0.187 |
| IL-10 |
1.3±0.7a |
1.6±0.5a |
0.4±0.5b | <0.001 |
| TLR4 | 1.6±1.1 | 1.2±0.9 | 0.7±0.9 | 0.140 |
| TLR2 |
1.2±0.7a |
4.4±4.2b |
0.8±1.3a | 0.012 |
| LPB |
1.3±0.7a |
2.4±1.2b |
1.2±0.8a | 0.018 |
| Myd88 |
1.3±0.8a |
1.0±0.9a,b |
0.3±0.4b | 0.009 |
| TGF-β1 | 1.2±0.8 | 1.6±1.9 | 1.1±0.1 | 0.687 |
| MMP2 |
1.3±0.8a |
3.9±2.3b |
1.0±1.5a | 0.003 |
| MMP9 | 1.5±1.4 | 2.7±1.5 | 3.2±4.6 | 0.479 |
Systemic inflammation and endothelial
dysfunction
Table III shows
the data obtained on the protein concentrations of inflammatory and
endothelial dysfunction parameters. The HCC and RIF groups showed a
significant increase in the serum concentrations of PAI-1 (P=0.013
and P<0.001, respectively), ICAM-1 (P<0.001, for both) and
E-selectin (P<0.001, for both) relative to the control group.
The RIF group showed a significant increase in MCP-1 protein
concentration compared with the HCC and control groups (P<0.001,
for both). The RIF group had a significantly higher concentration
of VAP-1 than the control group (P=0.041). No significant
differences in the protein concentration of IL-1β (P=0.194), IL-6
(P=0.393) and TNF-α (P=0.918) between the groups were found.
 | Table IIIInflammation and endothelial
dysfunction. |
Table III
Inflammation and endothelial
dysfunction.
| Variables | Control, n=10 | HCC, n=9 | RIF, n=10 | P-value
(ANOVA) |
|---|
| IL-1β, pg/ml | 1.5±2.1 | 6.4±6.6 | 7.5±11.1 | 0.194 |
| IL-6, pg/ml | 39.0±31.6 | 17.5±18.7 | 29.7±44.3 | 0.393 |
| TNF-α, pg/ml | 1.6±0.9 | 1.7±0.4 | 1.7±0.5 | 0.918 |
| PAI-1, pg/ml |
25.2±14.5a |
91.3±60.2b |
135.6±82.8b | 0.001 |
| MCP-1, pg/ml |
304.3±109.3a |
336.6±119.9a |
534.6±84.4b | <0.001 |
| ICAM-1, ng/ml |
0.1±0.001a |
1.6±0.8b |
1.8±0.7b | 0.001 |
| E-selectin,
ng/ml |
1.2±0.4a |
2.6±0.2b |
2.6±0.4b | 0.001 |
| VAP-1, ng/ml |
5.7±1.5a |
7.1±1.4a,b |
7.8±1.6b | 0.008 |
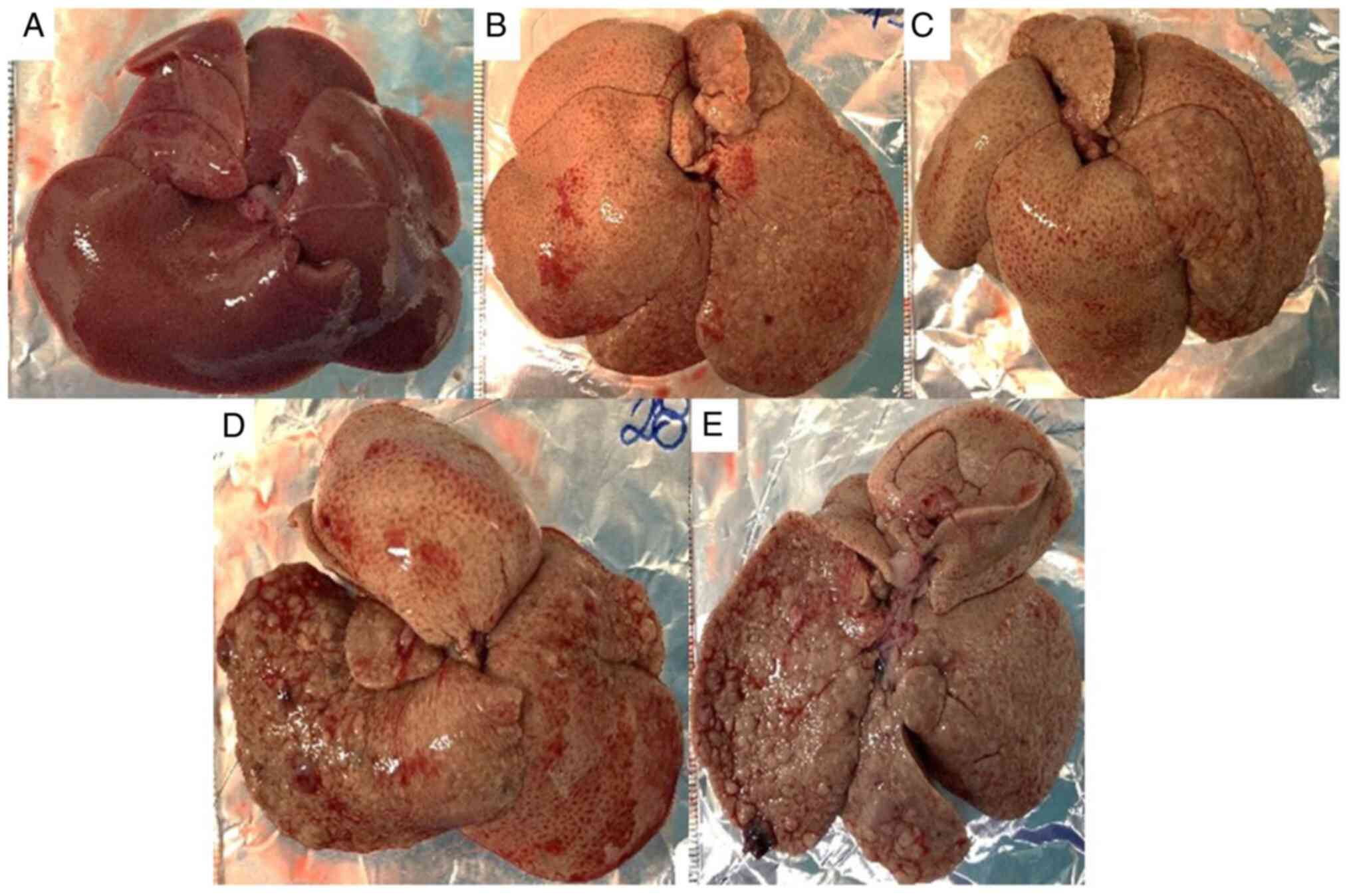
Liver histopathological analysis
No abnormalities in the macroscopic appearance of
the liver of the control rats (Fig.
3A) were found, whereas those in the HCC (Fig. 3B) and RIF (Fig. 3C-E) groups had the yellowish and
greasy livers that characterize steatosis. Additionally, no
abnormalities in the liver histopathological evaluation of the
control group (Fig. 4A and B) were observed, whereas the rats in the
HCC group had predominantly microvesicular steatosis, mild or
moderate macrovesicular steatosis, inflammatory activity, and local
fibrosis (Fig. 4C and D) and rats in the RIF group had
predominantly microvesicular steatosis, moderate or severe
macrovesicular steatosis, inflammatory activity, and local fibrosis
(Fig. 4E and F).
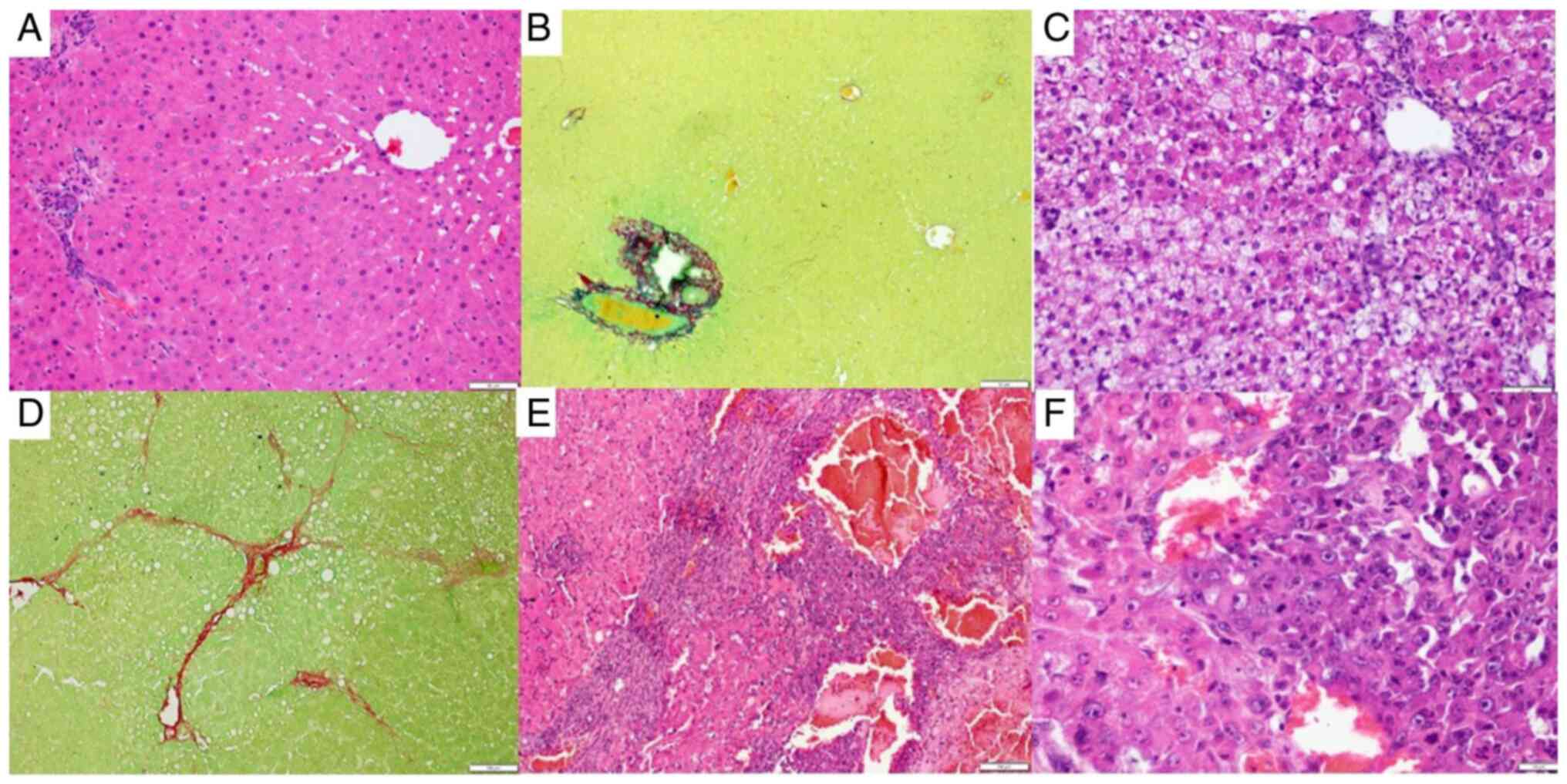 | Figure 4Hepatic histological evaluation. (A)
H&E stain in the control group; magnification, x10. (B)
Picrosirius stain in the control group; magnification, x10. (C)
H&E stain in the HCC group, magnification, x20; (D) Picrosirius
stain in the HCC group, magnification, x10. (E) H&E stain in
the RIF group, magnification, x10. (F) H&E stain in the RIF
group (magnification, x40) demonstrating metabolic
dysfunction-associated liver disease-related liver injury secondary
to the development of HCC. Scale bar, 10 µm. HCC, hepatocellular
carcinoma; RIF, rifaximin. |
Rodent-standardized MASLD activity scores showed
that 7 (77.8%) rats in the HCC group developed steatosis and 2
(22.2%) steatohepatitis; 1 animal from this experimental group
died, the biological samples of which were ignored in the proposed
analyses. In the RIF group, 7 (70.0%) rats developed steatosis and
3 (30.0%) steatohepatitis. No animals in the HCC group developed
liver cancer and only 2 (20.0%) in the RIF group developed grade IV
(Fig. 3D) and grade II (Fig. 3E) HCC. The control group showed no
hepatic histopathological changes. Table IV summarizes the data obtained in
the evaluation of the hepatic histopathological scores.
 | Table IVDistribution of liver
histopathological findings. |
Table IV
Distribution of liver
histopathological findings.
| | General NAFLD
scoring system for the rat models | |
|---|
| Variables | No NAFLD, n
(%) | Steatosis, n
(%) | Steatohepatitis, n
(%) | HCC development, n
(%) |
|---|
| Control, n=10 | 10 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| HCC, n=9 | 0 (0.0) | 7 (77.8) | 2 (22.2) | 0 (0.0) |
| RIF, n=10 | 0 (0.0) | 7 (70.0) | 3 (30.0) | 2 (20.0) |
Discussion
Recent decades have seen a significant increase in
the prevalence of MASLD, which is associated with cardiometabolic
risk factors. The progression of the disease is not only linked to
the development of cardiovascular diseases, the main cause of
mortality in this clinical condition, but also to the development
of HCC (6,31). This context evinces the utmost
importance of experimental studies to evaluate the
pathophysiological mechanisms and the potential therapeutic targets
of HCC. Indeed, there is a growing body of evidence that shows that
the use of RIF can contribute to reducing the complications of
cirrhosis by relieving portal pressure (8,32-34).
However, this is not the same as protection against HCC, whose
pathogenesis does not involve portal hypertension. A search on
PubMed (https://pubmed.ncbi.nlm.nih.gov/) using the keywords
‘RIF’ and ‘HCC’ yields 20 articles. Careful reading shows that only
one article, published by our group, investigated this issue
(8). On the contrary, a study has
shown that the use of antibiotics, including RIF, can worsen the
outcome of patients with HCC treated with sorafenib (34). Based on this evidence, the present
study introduces a brand-new aspect to the discussion. In the
present study, an experimental model of HCC secondary to MASLD was
developed with the overall objective of evaluating the effect of
prophylactic RIF treatment on the inflammatory markers and CVR of
the disease. It was shown that the HCC and RIF experimental groups
generally evinced alterations in the serum lipid profile, increased
lipid and cholesterol deposition in hepatic tissue and significant
changes in the atherogenic indices and the concentration of
systemic inflammatory markers and endothelial adhesion molecules
when compared with healthy rats. This indicated the triggering of
metabolic and CVR-associated changes in the development of MASLD.
Additionally, when comparing the two intervention groups, a
significant increase in the gene expression of inflammatory
mediators and hepatic fibrogenesis in the HCC group were observed
when compared with the RIF-treated rats. These differences suggest
a certain attenuation of the inflammatory and metabolic stimulus
due to RIF. The hepatic histological evaluation showed that all
animals in the HCC and RIF groups developed the local steatosis,
inflammation and fibrosis that characterize MASLD. However, the
development of HCC, the main objective of the present study,
occurred in only 2 animals in the RIF group, showing the difficulty
in reproducing experimental models previously described in the
literature.
The need to fully understand the pathogenesis and
progression of MASLD and to conduct preclinical tests for potential
therapeutic agents has led to the development of experimental
models that can reproduce hepatic phenotypes that resemble that in
humans with MASLD and that can progress to inflammation, MASH
cirrhosis, and HCC (35,36). The results of the present study
showed that the use of HFCD for 16 weeks was able to induce changes
in the serum and hepatic lipid profile, serum concentration of
systemic inflammatory markers and adhesion molecules and in the
atherogenic indices. These results corroborate other studies from
our research group, which have reported a significant increase in
the risk of cardiovascular disease associated with MASLD, which
configures a pathophysiological mechanism that needs to be better
evaluated with possible therapeutic targets (22,37).
Regarding the serum levels of hepatic transaminases in the present
study, significant differences in ALT levels between the
experimental groups were not observed; however, rats in the RIF
group showed a significant increase in AST levels compared with the
control group. In the context of MASLD, the evaluation of both
markers, but especially ALT due to its localization in the
hepatocellular cytosol, serves as an indicator of liver damage.
However, these transaminase levels fluctuate, and imaging or
histological studies are necessary for diagnosis. Additionally,
normal levels of liver enzymes are observed in individuals across
the spectrum of MASLD, which may underestimate the presence of the
disease (38,39). The present study was primarily
developed to evaluate these hepatic inflammatory and CVR parameters
in an experimental model of HCC secondary to MASLD. Analysis was
hindered by the absence of HCC in most studied animals. The
literature includes several experimental models that promote the
development of HCC by diet, chemicals, xenografts and genetic
induction (19,40). The previously described and
standardized mixed experimental model of HCC secondary to MASLD
induced by DEN and HFCD mimics the disease phenotype in humans,
including excessive caloric intake, the development of obesity and
dyslipidemia and a similar evolutionary profile to that in humans
living with the several evolutionary stages of disease progression
up to HCC (20). Recently, our
research group published studies that used this same experimental
model to evaluate the therapeutic effect of RIF on modulating the
composition of the intestinal microbiota, epigenetic markers and
autophagy. These studies observed the development of HCC secondary
to MASLD, and treatment with RIF showed a beneficial effect on
modulating the intestinal microbiota and epigenetic markers,
preventing/retarding hepatic carcinogenesis (8,30).
Considerable efforts have been made to generate experimental models
that share numerous physiological, anatomical and metabolic
characteristics with humans (35,41).
However, in addition to these factors, the limitation in
reproducing standardized experimental models in the literature
configures a significant factor to be considered.
RIF is an oral, safe and poorly absorbed antibiotic
that is widely used in clinical practice, especially to treat
irritable bowel syndrome, traveler's diarrhea and hepatic
encephalopathy (17,42,43).
RIF plays a notable role in modulating the intestinal microbiota
due to its selective antimicrobial activity in the intestine,
affecting both gram-positive and gram-negative bacteria (17,42).
Due to these characteristics, the use of RIF has been the subject
of preclinical and clinical studies to treat MASLD at its different
evolutionary stages (8,43,44).
However, the effect of RIF on MASLD/MASH must be better understood
due to controversial results in the literature. Cheng et al
(45) showed an adverse effect of
prolonged (6-month long) administration of RIF in mice, resulting
in the activation of genes involved in lipid uptake, leading to
hepatic steatosis. Fujinaga et al (44) reported that the use of RIF combined
with an angiotensin II receptor blocker was able to reduce
intestinal permeability, portal endotoxemia and hepatic
fibrogenesis by suppressing the TLR4/NF-κB signaling pathway in an
experimental model of non-alcoholic steatohepatitis. Clinically,
studies have reported that short-term treatment with RIF is
beneficial in reducing endotoxemia, inflammatory cytokine levels
and insulin resistance (18,46).
In the present study, although MASLD failed to progress to HCC,
both intervention groups showed mild to moderate steatosis,
inflammation and local fibrosis. In this context, an interesting
result in the present study refers to the significant reduction in
the gene expression of inflammatory mediators and markers of
hepatic fibrogenesis in rats treated with RIF compared with the HCC
group (which received no treatment). A critical factor for the
development and progression of MASLD refers to intestinal dysbiosis
and, as shown in a previous study by our research group, treatment
with RIF managed to promote modulation of the intestinal microbiota
(8). This previous study found a
significant decrease in the gene expression of LBP and TLR2, and
consequently a reduction in the expression of IL-1β and MMP2 in
animals treated with RIF compared with animals with HCC. LBP is a
soluble acute phase protein that binds to bacterial
lipopolysaccharide, which in turn can activate TLRs (including
TLR-2) thereby triggering an inflammatory and hepatic fibrogenesis
response (44,47,48).
In this scenario, the differences in the present study resulting
from RIF treatment suggest the partial attenuation in inflammatory
and metabolic stimulation. In this context, a prior experimental
model study recently published shows the potential beneficial
effect of RIF in preventing/delaying the development of
carcinogenesis (8). Additionally,
our research group is developing in vitro studies with
hexachlorobenzene, which can stimulate hepatic proliferation.
Unpublished results show that RIF reduces cell proliferation in
Huh-7 cells through antiproliferative, antimigratory and
pro-apoptotic effects. The lack of in vitro experiments is a
limitation of the present study. However, as aforementioned, the
in vitro data should be published soon.
Although the potential beneficial effect of RIF
administration was shown, the main limitation of the present study
refers to the absence of HCC development. A previously described
mixed HCC experimental model that has been reproduced by our
research group in the past was utilized in the present study.
However, the same success was not obtained. Animal models are
essential for studying the initiation and progression of MASLD. In
MASLD, an ideal preclinical model is triggered by the same causes
of the disease in humans (such as caloric excess) and is associated
with the same risk factors (49,50).
In this context, the ideal assessment of HCC secondary to MASLD
would trigger the lesion by the progression of the disease, rather
than administering a chemical carcinogen (49). However, the use of chemical
additives is very common, as spontaneous development of HCC only
via diet occurs from 50 weeks of experimentation, increasing the
costs of studies (49,50). The present study likely showed no
development of HCC due to an issue regarding the DEN used, dosage
and/or administration since its histopathological evaluation showed
that the studied animals had steatosis and inflammation, probably
due to the use of HFCD. In the present study, the histopathological
evaluation of liver tissue was conducted only through staining with
H&E and picrosirius red. The lack of evaluation by α-smooth
muscle actin, fibronectin and Masson staining is therefore a
limitation. The issue widely stems from the notion that
experimental models must be reproducible, reliable, simple, easy
and accessible for the development and preclinical validation of
new therapeutic targets (49-51).
However, the literature shows few reports on experimental models
that have failed to reproduce the expected phenotype in a disease
(as occurred in the present study), complicating the discussion of
the topic.
This line of research of our group has developed
unpublished in vitro, experimental and clinical studies and
shows significant potential in the field of hepatology and
metabolic disorders. Overall, it has been observed that RIF can
reduce the expression of inflammatory mediators and modulate the
expression of epigenetic markers, autophagy and the composition of
the gut microbiota (8,30). This suggests the beneficial effect
of RIF beyond its current clinical uses, particularly in the
modulation of inflammatory and metabolic pathways, including the
cardiometabolic pathways involved in MASLD. However, some knowledge
gaps still require further exploration in future studies, including
the reproducibility of experimental models. The development and
reproduction of reliable and consistent experimental models of HCC
secondary to MASLD are of utmost importance to evaluate the
pathophysiological mechanisms associated with disease progression
and to identify new biomarkers and therapeutic targets. In this
context, the primary objective in developing the present study was
to evaluate markers of autophagy and epigenetics and to assess
their relationship with the microbiota composition in these
different study groups. However, due to the non-development of HCC,
the objectives had to be modified. It is difficult to explain the
reason for this limitation as the experimental parameters in our
previous study (the species of rats, the diet and the medication
doses) in which the tumor developed were repeated exactly in the
present study (8). Considering all
the variables, perhaps DEN itself could be responsible for the
negative results. Thus, the long-term effects of RIF on MASLD/MASH
and its progression to HCC remain unclear. Therefore, further
studies should evaluate inflammatory and metabolic pathways to
assess the potential beneficial effects associated with this
process and ensure their clinical applicability.
Despite the aforementioned gaps, the present study
showed that, although most rats studied did not develop HCC, RIF
treatment reduced metabolic stimulus and inflammatory markers
compared with rats that received no MASLD treatment. As the
reproducibility of experimental models is key to allowing the
evaluation of pathophysiological mechanisms associated with disease
progression and to identify new biomarkers and therapeutic targets,
it is important to show negative results to the academic
community.
Supplementary Material
Experimental design. The control group
(n=10) received a standard diet, water without DEN and gavage with
the Veh solution; the HCC group (n=10) received a HFCD diet, water
with DEN and gavage with the Veh solution; the RIF group (n=10)
received a HFCD diet, water with DEN and gavage with RIF during the
16-week experiment, after which all animals were euthanized (†).
DEN, diethylnitrosamine; HCC, hepatocellular carcinoma; HFCD,
high-fat and choline-deficient; RIF, rifaximin; Veh, vehicle.
TaqMan probes used to evaluate the
gene expression of liver inflammatory markers.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the following Brazilian
funding agencies: Financiamento e Incentivo à Pesquisa from
Hospital de Clínicas de Porto Alegre (grant no. 2019-0311), Biolab
Sanus Farmacêutica, the National Council for Scientific and
Technological Development (CNPq) and the Coordination for the
Improvement of Higher Education Personnel.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LL and MRA performed the conceptualization,
methodology, formal analysis, investigation, data curation, writing
of the original draft and the review and editing of the manuscript;
GTSG, LB, MHMP, CEP, CTSC and CUC performed the methodology, formal
analysis and the review and editing of the manuscript. LL, CUC and
MRA confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments and procedures involving the use of
animals were approved by the Institutional Ethics Committee of
Hospital de Clinicas de Porto Alegre (Porto Alegre, Brazil;
approval no. 2019-0311). The procedures for the use of scientific
animals were conducted in accordance with the Guide for the Care
and Use of Laboratory Animals (8th edition, 2011) and Law number
11,794 (Brazil, 2008).
Patient consent for publication
Not applicable.
Competing interests
Biolab Sanus Farmaceutica Ltd. donated the rifaximin
used in our study, although this company had no influence on either
the design or conduct of the study, the analysis or interpretation
of the data or the writing of the manuscript. Therefore, we do not
believe this constitutes a competing interest.
References
|
1
|
Rinella ME, Lazarus JV, Ratziu V, Francque
SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab
JP, et al: A multisociety Delphi consensus statement on new fatty
liver disease nomenclature. J Hepatol. 79:1542–1556.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Apostolo D, Ferreira LL, Vincenzi F,
Vercellino N, Minisini R, Latini F, Ferrari B, Burlone M, Pirisi M
and Bellan M: From MASH to HCC: the role of Gas6/TAM receptors.
Front Immunol. 15(1332818)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Phoolchund AGS and Khakoo SI: MASLD and
the Development of HCC: Pathogenesis and Therapeutic Challenges.
Cancers (Basel). 16(259)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meroni M, Chiappori F, Paolini E, Longo M,
De Caro E, Mosca E, Chiodi A, Merelli I, Badiali S, Maggioni M, et
al: A novel gene signature to diagnose MASLD in metabolically
unhealthy obese individuals. Biochem Pharmacol.
218(115925)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Younossi ZM, Golabi P, Paik JM, Henry A,
Van Dongen C and Henry L: The global epidemiology of nonalcoholic
fatty liver disease (NAFLD) and nonalcoholic steatohepatitis
(NASH): A systematic review. Hepatology. 77:1335–1347.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mellemkjær A, Kjær MB, Haldrup D, Grønbæk
H and Thomsen KL: Management of cardiovascular risk in patients
with metabolic dysfunction-associated steatotic liver disease. Eur
J Intern Med. 122:28–34. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Younossi ZM: Non-alcoholic fatty liver
disease-A global public health perspective. J Hepatol. 70:531–544.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Michalczuk MT, Longo L, Keingeski MB,
Basso BS, Guerreiro GTS, Ferrari JT, Vargas JE, Oliveira CP,
Uribe-Cruz C, Cerski CTS, et al: Rifaximin on epigenetics and
autophagy in animal model of hepatocellular carcinoma secondary to
metabolic-dysfunction associated steatotic liver disease. World J
Hepatol. 16:75–90. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Maliakkal BJ: Pathogenesis of
non-alcoholic fatty liver disease and implications on
cardiovascular outcomes in liver transplantation. Transl
Gastroenterol Hepatol. 5(36)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim H, Lee DS, An TH, Park HJ, Kim WK, Bae
KH and Oh KJ: Metabolic spectrum of liver failure in type 2
diabetes and obesity: From NAFLD to NASH to HCC. Int J Mol Sci.
22(4495)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rizzo A, Mollica V, Tateo V, Tassinari E,
Marchetti A, Rosellini M, De Luca R, Santoni M and Massari F:
Hypertransaminasemia in cancer patients receiving immunotherapy and
immune-based combinations: The MOUSEION-05 study. Cancer Immunol
Immunother. 72:1381–194. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rizzo A, Ricci AD and Brandi G: Systemic
adjuvant treatment in hepatocellular carcinoma: Tempted to do
something rather than nothing. Future Oncol. 16:2587–2589.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guven DC, Sahin TK, Erul E, Rizzo A, Ricci
AD, Aksoy S and Yalcin S: The association between albumin levels
and survival in patients treated with immune checkpoint inhibitors:
A systematic review and meta-analysis. Front Mol Biosci.
9(1039121)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rizzo A, Ricci AD and Brandi G:
Trans-Arterial chemoembolization plus systemic treatments for
hepatocellular carcinoma: An Update. J Pers Med.
12(1788)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang S and Friedman SL: Found in
translation-Fibrosis in metabolic dysfunction-associated
steatohepatitis (MASH). Sci Transl Med. 15(eadi0759)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cobbold JFL, Atkinson S, Marchesi JR,
Smith A, Wai SN, Stove J, Shojaee-Moradie F, Jackson N, Umpleby AM,
Fitzpatrick J, et al: Rifaximin in non-alcoholic steatohepatitis:
An open-label pilot study. Hepatol Res. 48:69–77. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leone P, Mincheva G, Balzano T,
Malaguarnera M, Felipo V and Llansola M: Rifaximin Improves spatial
learning and memory impairment in rats with liver Damage-Associated
neuroinflammation. Biomedicines. 10(1263)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gangarapu V, Ince AT, Baysal B, Kayar Y,
Kılıç U, Gök Ö, Uysal Ö and Şenturk H: Efficacy of rifaximin on
circulating endotoxins and cytokines in patients with nonalcoholic
fatty liver disease. Eur J Gastroenterol Hepatol. 27:840–845.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fang J, Celton-Morizur S and Desdouets C:
NAFLD-Related HCC: Focus on the latest relevant preclinical models.
Cancers (Basel). 15(3723)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de Lima VM, Oliveira CP, Alves VA, Chammas
MC, Oliveira EP, Stefano JT, de Mello ES, Cerri GG, Carrilho FJ and
Caldwell SH: A rodent model of NASH with cirrhosis, oval cell
proliferation and hepatocellular carcinoma. J Hepatol.
49:1055–1061. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Moura RF, Ribeiro C, de Oliveira JA,
Stevanato E and de Mello MA: Metabolic syndrome signs in Wistar
rats submitted to different high-fructose ingestion protocols. Br J
Nutr. 101:1178–1184. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Longo L, Tonin Ferrari J, Rampelotto PH,
Hirata Dellavia G, Pasqualotto A, P Oliveira C, Thadeu Schmidt
Cerski C, Reverbel da Silveira T, Uribe-Cruz C and Álvares-da-Silva
MR: Gut dysbiosis and increased intestinal permeability Drive
microRNAs, NLRP-3 inflammasome and liver fibrosis in a nutritional
model of Non-Alcoholic steatohepatitis in adult male sprague dawley
rats. Clin Exp Gastroenterol. 13:351–368. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sujatha R and Kavitha S: Atherogenic
indices in stroke patients: A retrospective study. Iran J Neurol.
16:78–82. 2017.PubMed/NCBI
|
|
25
|
Gómez-Lechón MJ, Donato MT,
Martínez-Romero A, Jiménez N, Castell JV and O'Connor JE: A human
hepatocellular in vitro model to investigate steatosis. Chem Biol
Interact. 165:106–116. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liang W, Menke AL, Driessen A, Koek GH,
Lindeman JH, Stoop R, Havekes LM, Kleemann R and van den Hoek AM:
Establishment of a general NAFLD scoring system for rodent models
and comparison to human liver pathology. PLoS One.
9(e115922)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ferrari JT, Guerreiro GTS, Longo L,
Silveira TR, Cerski CTS, Tozawa E, Oliveira CP, Álvares-da-Silva MR
and Uribe-Cruz C: Potential beneficial effect of rifaximin in the
prevention of hepatocellular carcinoma through the modulation of
the microbiota in an experimental model of non-alcoholic fatty
liver disease. Acta Gastroenterol Latinoam. 53:265–282. 2023.
|
|
31
|
Guerreiro GTS, Longo L, Fonseca MA, de
Souza VEG and Álvares-da-Silva MR: Does the risk of cardiovascular
events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int,
2021.
|
|
32
|
Zacharias HD, Kamel F, Tan J, Kimer N,
Gluud LL and Morgan MY: Rifaximin for prevention and treatment of
hepatic encephalopathy in people with cirrhosis. Cochrane Database
Syst Rev. 7(CD011585)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Patel VC, Lee S, McPhail MJW, Da Silva K,
Guilly S, Zamalloa A, Witherden E, Støy S, Manakkat Vijay GK, Pons
N, et al: Rifaximin-α reduces gut-derived inflammation and mucin
degradation in cirrhosis and encephalopathy: RIFSYS randomised
controlled trial. J Hepatol. 76:332–342. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pomej K, Balcar L, Scheiner B, Semmler G,
Meischl T, Mandorfer M, Reiberger T, Müller C, Trauner M, Pinter M,
et al: Antibiotic therapy is associated with worse outcome in
patients with hepatocellular carcinoma treated with sorafenib. J
Hepatocell Carcinoma. 8:1485–1493. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Flessa CM, Nasiri-Ansari N, Kyrou I, Leca
BM, Lianou M, Chatzigeorgiou A, Kaltsas G, Kassi E and Randeva HS:
Genetic and Diet-Induced Animal Models for Non-Alcoholic fatty
liver disease (NAFLD) research. Int J Mol Sci.
23(15791)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Denk H, Abuja PM and Zatloukal K: Animal
models of NAFLD from the pathologist's point of view. Biochim
Biophys Acta Mol Basis Dis. 1865:929–942. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Longo L, Rampelotto PH, Filippi-Chiela E,
de Souza VEG, Salvati F, Cerski CT, da Silveira TR, Oliveira CP,
Uribe-Cruz C and Álvares-da-Silva MR: Gut dysbiosis and systemic
inflammation promote cardiomyocyte abnormalities in an experimental
model of steatohepatitis. World J Hepatol. 13:2052–2070.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hadizadeh F, Faghihimani E and Adibi P:
Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J
Gastrointest Pathophysiol. 8:11–26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sanyal D, Mukherjee P, Raychaudhuri M,
Ghosh S, Mukherjee S and Chowdhury S: Profile of liver enzymes in
non-alcoholic fatty liver disease in patients with impaired glucose
tolerance and newly detected untreated type 2 diabetes. Indian J
Endocrinol Metab. 19:597–601. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Uehara T, Pogribny IP and Rusyn I: The DEN
and CCl4-Induced mouse model of fibrosis and
inflammation-associated hepatocellular carcinoma. Curr Protoc
Pharmacol. 66(14.30.1-10)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Oseini AM, Cole BK, Issa D, Feaver RE and
Sanyal AJ: Translating scientific discovery: The need for
preclinical models of nonalcoholic steatohepatitis. Hepatol Int.
12:6–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lee S and Saffo S: Evolution of care in
cirrhosis: Preventing hepatic decompensation through
pharmacotherapy. World J Gastroenterol. 29:61–74. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jian J, Nie MT, Xiang B, Qian H, Yin C,
Zhang X, Zhang M, Zhu X and Xie WF: Rifaximin ameliorates
non-alcoholic steatohepatitis in mice through regulating gut
microbiome-related bile acids. Front Pharmacol.
13(841132)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fujinaga Y, Kawaratani H, Kaya D, Tsuji Y,
Ozutsumi T, Furukawa M, Kitagawa K, Sato S, Nishimura N, Sawada Y,
et al: Effective combination therapy of Angiotensin-II receptor
blocker and rifaximin for hepatic fibrosis in rat model of
nonalcoholic steatohepatitis. Int J Mol Sci.
21(5589)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cheng J, Krausz KW, Tanaka N and Gonzalez
FJ: Chronic exposure to rifaximin causes hepatic steatosis in
pregnane X receptor-humanized mice. Toxicol Sci. 129:456–468.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Abdel-Razik A, Mousa N, Shabana W, Refaey
M, Elzehery R, Elhelaly R, Zalata K, Abdelsalam M, Eldeeb AA, Awad
M, et al: Rifaximin in nonalcoholic fatty liver disease: hit
multiple targets with a single shot. Eur J Gastroenterol Hepatol.
30:1237–1246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang L, Xie Z, Yu H, Du H, Wang X, Cai J,
Qiu Y, Chen R, Jiang X, Liu Z, et al: TLR2 inhibition ameliorates
the amplification effect of LPS on lipid accumulation and
lipotoxicity in hepatic cells. Ann Transl Med.
9(1429)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ranoa DRE, Kelley SL and Tapping RI: Human
lipopolysaccharide-binding protein (LBP) and CD14 independently
deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and
TLR2 and enhance formation of the ternary signaling complex. J Biol
Chem. 288:9729–9741. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Asgharpour A, Cazanave SC, Pacana T,
Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK,
Mirshahi F, et al: A diet-induced animal model of non-alcoholic
fatty liver disease and hepatocellular cancer. J Hepatol.
65:579–588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lau JK, Zhang X and Yu J: Animal models of
non-alcoholic fatty liver disease: Current perspectives and recent
advances. J Pathol. 241:36–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Febbraio MA, Reibe S, Shalapour S, Ooi GJ,
Watt MJ and Karin M: Preclinical models for studying NASH-Driven
HCC: How useful are they? Cell Metab. 29:18–26. 2019.PubMed/NCBI View Article : Google Scholar
|