Introduction
Ischemic stroke is a neurological condition that
arises from an abrupt interruption of blood flow to the brain. It
is the second most common cause of death worldwide, resulting in
~5.5 million deaths each year, according to WHO health statistics.
In addition to its high mortality rate, stroke significantly
impacts disability, with ~50% of survivors experiencing permanent
impairments (1). Currently, the
only FDA-approved thrombolytic treatment for stroke is recombinant
tissue plasminogen activator (2).
However, this therapy is applicable to only ~10% of patients with
acute ischemic stroke due to strict eligibility criteria relating
to history of hemorrhage, recent surgeries and coagulation
disorders, as well as a narrow treatment window of 4.5 h after
symptom onset (3). There is
therefore a critical need to develop neuroprotective agents that
can restore cerebral blood flow (CBF) and mitigate brain damage in
ischemic stroke.
Under normal conditions, adult CBF averages ~50
ml/100 g/min, which is essential for maintaining brain function.
However, during focal ischemia, CBF can drop below 10 ml/100 g/min,
leading to permanent neuronal injury (4). In ischemic stroke, the production of
free radicals increases, leading to oxidative stress and brain
damage (5). Oxidative stress occurs
when there is an imbalance between the generation and removal of
reactive oxygen species (ROS) (6).
When ROS levels are too high, they can harm the mitochondrial
membrane, interfere with the respiratory chain, and damage neuronal
DNA, enzymes and cell membranes, eventually causing cell death
(7-9).
Numerous in vivo studies have shown that antioxidants can
inhibit ROS-mediated reactions and protect neurons from injury in
focal cerebral ischemic stroke (10,11).
In addition to oxidative stress, the imbalanced mitochondrial
dynamics that occur during stroke can harm the patient. Imbalanced
mitochondrial dynamics result in excessive fission, leading to
fragmented mitochondria, diminished mitochondrial function,
increased ROS levels, and further brain damage. Supporting
mitochondrial fusion, especially through the mitofusin 2 (Mfn2)
protein, may lessen the harmful effects of stroke by preserving
mitochondrial integrity, enhancing energy production, reducing
oxidative stress and facilitating recovery (12,13).
Mitogen-activated protein kinase (MAPK) pathways are a third
component of stroke pathophysiology. These pathways are activated
during stroke, influencing inflammation, apoptosis and oxidative
stress. Previous studies have found that suppression of
mitochondrial fission through inhibition of the MAPK pathway can
protect against brain injury following a stroke (12-14).
Further research is needed to understand how antioxidants and
modulation of mitochondrial dynamics affect oxidative stress in
cerebral ischemia and how this could help in treating patients with
stroke. Moreover, treating permanent ischemic stroke is difficult
because of the irreversible brain damage caused by extended oxygen
deprivation. If blood flow is not restored quickly, neurons in the
affected region experience excitotoxicity, oxidative stress and
inflammation, resulting in cell death and lasting neurological
impairments. Current research is focusing on neuroprotective
treatments that target inflammation, apoptosis and mitochondrial
dysfunction in an effort to reduce further damage and support
recovery. These strategies aim to address the ongoing complex
pathological processes that persist after ischemic injury becomes
irreversible.
Galangin is a dietary flavonoid, abundant in the
rhizome of Alpinia officinarum Hance, that has been used in
China for centuries as a spice and traditional medicine (15). This compound is noted for its wide
range of bioactivities, impacting various cellular processes. These
include antioxidant (16,17), anti-inflammatory (18,19),
antiulcer (20,21), antidiabetic (22,23),
anticoagulant (24) and
anti-apoptotic (25) activities
detected in vitro and in animal models. Despite its known
bioactivities, the impact of galangin on stroke-associated brain
damage has not been widely studied. In the present study,
therefore, the effect of galangin on brain infarct volume,
oxidative stress markers, anti-oxidant defenses, and MAPK and Mfn2
pathways was examined in an in vivo model of focal ischemic
stroke.
Materials and methods
Test substances
Galangin, with a confirmed purity of 98.7% based on
high-performance liquid chromatography analysis (PubChem ID:
5281616) was sourced from Biopurify Phytochemicals Ltd. The
chemical structure of galangin is presented in Fig. 1. Piracetam (PubChem ID: 4843), which
was used as a positive control, was sourced from GlaxoSmithKline
(Thailand) Ltd. Dimethyl sulfoxide (DMSO), the vehicle used for
both piracetam and galangin, was purchased from Thermo Fisher
Scientific, Inc. (cat. no. D/4121/PB15).
Animals
A total of 60 healthy male Wistar rats, each
weighing 250-300 g, 8 weeks-old, were sourced from the Northeastern
Laboratory Animal Center at Khon Kaen University (Khon Kaen,
Thailand). The rats were housed in groups of five in standard metal
cages, under a 12/12-h light-dark cycle. Environmental conditions
were maintained with relative humidity ranging from 30-60% and a
temperature of 23±2˚C. Water and commercial food pellets were
provided ad libitum. All procedures involving animals were
performed following guidelines approved (approval no.
IACUC-KKU-105/66) by the Institutional Animal Care and Use
Committee at Khon Kaen University (Khon Kaen, Thailand).
Animal treatment
The rats were randomly divided into six groups, with
10 rats per group. Group 1, the control group, underwent a sham
operation without further treatment. Group 2, designated as the
Rt.MCAO + vehicle group, received an intraperitoneal injection of
1% (v/v) DMSO, which served as the vehicle for the test treatments,
administered 7 days after inducing focal cerebral ischemia via
right middle cerebral artery occlusion (Rt.MCAO). Group 3, the
Rt.MCAO + piracetam group, was treated with an intraperitoneal
injection of piracetam at a dose of 250 mg/kg body weight, 7 days
following Rt.MCAO, as determined by prior research (12). Groups 4 to 6, the Rt.MCAO + galangin
groups, were administered galangin intraperitoneally at doses of
25, 50 and 100 mg/kg body weight, respectively, also 7 days
post-Rt.MCAO, with the dosages selected based on preliminary
studies and existing literature (10,26).
Piracetam was chosen as the positive control because of its
demonstrated ability to reduce infarct size, increase CBF and
improve neuronal function (27-29).
DMSO was used as the vehicle for both piracetam and galangin, as a
1% (v/v) concentration is generally considered safe and non-toxic,
making it appropriate for use in biological studies (10,12,30).
All animals in the groups received their treatments via
intraperitoneal injection once daily for 7 consecutive days
following Rt. MCAO and were perfused trans-cardially on the 8th
day.
In each group, the brain infarct volume was assessed
in 5 rats using 2,3,5-triphenyltetrazolium chloride (TTC) staining.
Another 5 rats per group were utilized to analyze malondialdehyde
(MDA) levels, catalase (CAT) and glutathione peroxidase (GSH-Px)
activities in the cortex and hippocampus, while superoxide
dismutase (SOD) activity was measured in the mitochondria of the
cortex and hippocampus. Additionally, p38 MAPK and Mfn2 expression
levels were measured in the cortex and hippocampus of rats treated
with doses of galangin that produced optimal effects on infarct
volume and oxidative stress markers. The middle cerebral artery
supplies blood to parts of the frontal, temporal and parietal
cortices of the brain; therefore, tissue from these cortical areas
were collected for molecular analysis. Each test was conducted in
duplicate.
Rt.MCAO model
Prior to surgery, all animals underwent an overnight
fasting period while having access to water ad libitum.
Anesthesia was induced in the rats using isoflurane, administered
at 5% for induction and maintained at 1-3% during the procedure,
delivered in 100% oxygen. The focal ischemic model was induced by
permanent occlusion of the right middle cerebral artery using a 4-0
silicone-coated monofilament, following established protocols
(31). Each monofilament was
carefully inserted into the internal carotid artery until it
reached a depth of ~17 mm or until slight resistance was
encountered. After the procedure, the incision was sutured, and a
10% povidone iodine solution was applied to the site for
postoperative antiseptic care. During subsequent brain removals
after the 7-day treatment period, images of the filaments occluding
each middle cerebral artery were captured to confirm consistent
occlusion across all animals. In the sham operation, rats underwent
the same procedure without the insertion of the monofilament.
Assessment of brain infarct
volume
Rats were anesthetized with thiopental sodium [80
mg/kg body weight (BW), administered intraperitoneally] before
undergoing cardiac perfusion with cold normal saline. The brains
were carefully removed from the skull, sectioned into 2-mm-thick
coronal slices, and stained with 2% TTC (MilliporeSigma) in normal
saline for 30 min at 37˚C. Digital images were captured using a
camera, and the infarct volume was measured using ImageJ software
(v.1.53e, National Institutes of Health). The infarct volumes were
calculated according to a previously described method by the
authors (32). The formula was as
follows: Infarct volume (%)=[(contralateral hemisphere
volume)-(non-infarct ipsilateral hemisphere volume)
x100]/(contralateral hemisphere volume).
Isolation of brain mitochondria for
biochemical assays
After perfusion, brain tissues from the cerebral
cortex and hippocampal regions were isolated and underwent
mitochondrial extraction following a protocol detailed in a
previous study by the authors (32). Briefly, the cortex and hippocampus
regions of the brain were dissected and homogenized in
mitochondrial isolation buffer, followed by centrifugation at 1,000
x g for 2 min at 4˚C. The resulting supernatant was collected into
a separate tube, while the pellet was resuspended in 0.2 ml of
isolation buffer and centrifuged again under the same conditions.
The second supernatant was combined with the first, and 0.07 ml of
an 80% Percoll solution (MilliporeSigma) was added. A 10% Percoll
solution (0.7 ml) was gently layered on top, and the mixture was
subjected to centrifugation at 18,500 x g for 10 min. The
mitochondrial pellet obtained was further purified by resuspending
it in 0.7 ml of washing buffer and centrifuging at 10,000 x g for 5
min. Afterward, the mitochondrial pellet was suspended in washing
buffer and stored at -80˚C for later use. SOD activity was
subsequently measured using a commercial SOD assay kit (cat. no.
19160; MilliporeSigma). Data are expressed as units/mg of
mitochondrial protein.
Protein quantification
After perfusion, the rat brains were quickly
extracted and dissected into the cerebral cortex and hippocampus.
The protein concentrations in these brain areas were determined
using the method outlined by Lowry et al (33), with bovine serum albumin
(MilliporeSigma) as the standard. In brief, samples were diluted
and mixed with freshly prepared Lowry reagent (containing sodium
carbonate, copper sulfate and sodium potassium tartrate). After a
10-min incubation at room temperature, Folin-Ciocalteu reagent,
diluted 1:1 with distilled water, was added, and the mixture was
incubated for 30 min to allow for color development. Absorbance was
then measured at 650 nanometers (nm) using a spectrophotometer.
Determination of the MDA level
The lipid peroxidation (LPO) product, MDA, served as
an indicator of oxidative stress. Levels of MDA were quantified in
all samples using the thiobarbituric acid (TBA; MilliporeSigma)
reaction, following the method outlined in a study by Ohkawa et
al (34). Tissue homogenates
were mixed with sodium dodecyl sulfate (SDS), acetic acid and TBA,
then heated at 95˚C for 60 min to form the MDA-TBA complex. After
cooling, the mixture was extracted with n-butanol/pyridine,
centrifuged at a speed of 4,000 x g (10 min, 4˚C), and the organic
layer was collected. The absorbance of the product was measured at
532 nm using a spectrophotometer, and MDA levels were calculated by
comparison to a standard curve prepared with
1,1,3,3-tetramethoxypropane. Results are expressed as nmol/mg
protein.
Determination of CAT activity
CAT activity was assessed following the procedure
outlined by Goldblith and Proctor (35). Initially, brain tissue underwent
homogenization in ice-cold phosphate buffer to prevent enzymatic
degradation. After centrifugation at 10,000 x g for 10 min at 4˚C,
the supernatant containing CAT was retrieved. This supernatant was
then mixed with phosphate buffer and hydrogen peroxide, and
absorbance was recorded at 240 nm using a spectrophotometer. CAT
activity is reported as units per milligram of protein (units/mg
protein).
Determination of GSH-Px activity
GSH-Px activity was assessed using a GSH-Px assay
kit obtained from MilliporeSigma (cat. no. MAK437-1KT). After the
rat brain tissue was homogenized and centrifuged at 10,000 x g for
10 min at 4˚C, the supernatant containing GSH-Px was collected.
This supernatant was then mixed with phosphate buffer, glutathione
reductase, nicotinamide adenine dinucleotide (NADPH) and hydrogen
peroxide. The reduction in NADPH absorbance at 340 nm served as an
indicator of GSH-Px activity. Enzyme activity was quantified by
monitoring the absorbance changes over time. Results are presented
as units/mg protein.
Western blot analysis
The expression levels of p38 MAPK and Mfn2 in rat
cortices and hippocampi were analyzed via western blotting as
outlined in previous studies (12,14).
Each of the cortex and hippocampus samples was homogenized in
neuronal protein extraction reagent (N-PERTM) lysis
buffer (Thermo Fisher Scientific, Inc.), and total protein
concentrations were determined using the Lowry method. Proteins (30
µg/sample) were separated on a 10% SDS-polyacrylamide gel and
transferred onto a PVDF membrane (Hybond-P; GE Healthcare; Cytiva).
To prevent non-specific binding, membranes were blocked by
incubating them for 1 h at room temperature in 5% non-fat dried
milk in TBS-T (0.1% Tween-20 in Tris-buffered saline; pH 7.4).
Subsequently, membranes were incubated overnight at 4˚C with
primary antibodies: Rabbit monoclonal anti-p38 MAPK (1:500; cat.
no. A14401; ABclonal Biotech Co., Ltd.), rabbit monoclonal
anti-mitofusin 2 (1:500; cat. no. A12771; ABclonal Biotech Co.,
Ltd.) and rabbit monoclonal anti-β-actin (1:5,000; cat. no. AC026;
ABclonal Biotech Co., Ltd.). Following washes with TBS-T, the
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit secondary antibodies (1:2,000; cat. no. AS063; ABclonal
Biotech Co., Ltd.) for 1 h at room temperature. Protein bands were
visualized using the ClarityTM Western ECL Substrate
(cat. no. 170-5060, Bio-Rad Laboratories, Inc.) and imaged with a
ChemiDoc™ MP system (Bio-Rad Laboratories, Inc.) using Image Lab
software (version 6.0.0 build 25; Bio-Rad Laboratories, Inc.). The
density of MAPK and Mfn2 bands was normalized to β-actin, with
protein expression levels quantified through ImageJ software
v.1.53e (National Institutes of Health).
Statistical analysis
The results are presented as the mean ± standard
error of the mean (SEM). Statistical analysis was conducted using
one-way analysis of variance (ANOVA), followed by Tukey's post hoc
test, employing SPSS software v.25 (IBM Corp.). A significance
level of P<0.05 was used to determine statistical
significance.
Results
Effect of galangin on cerebral damage
in rat following Rt.MCAO
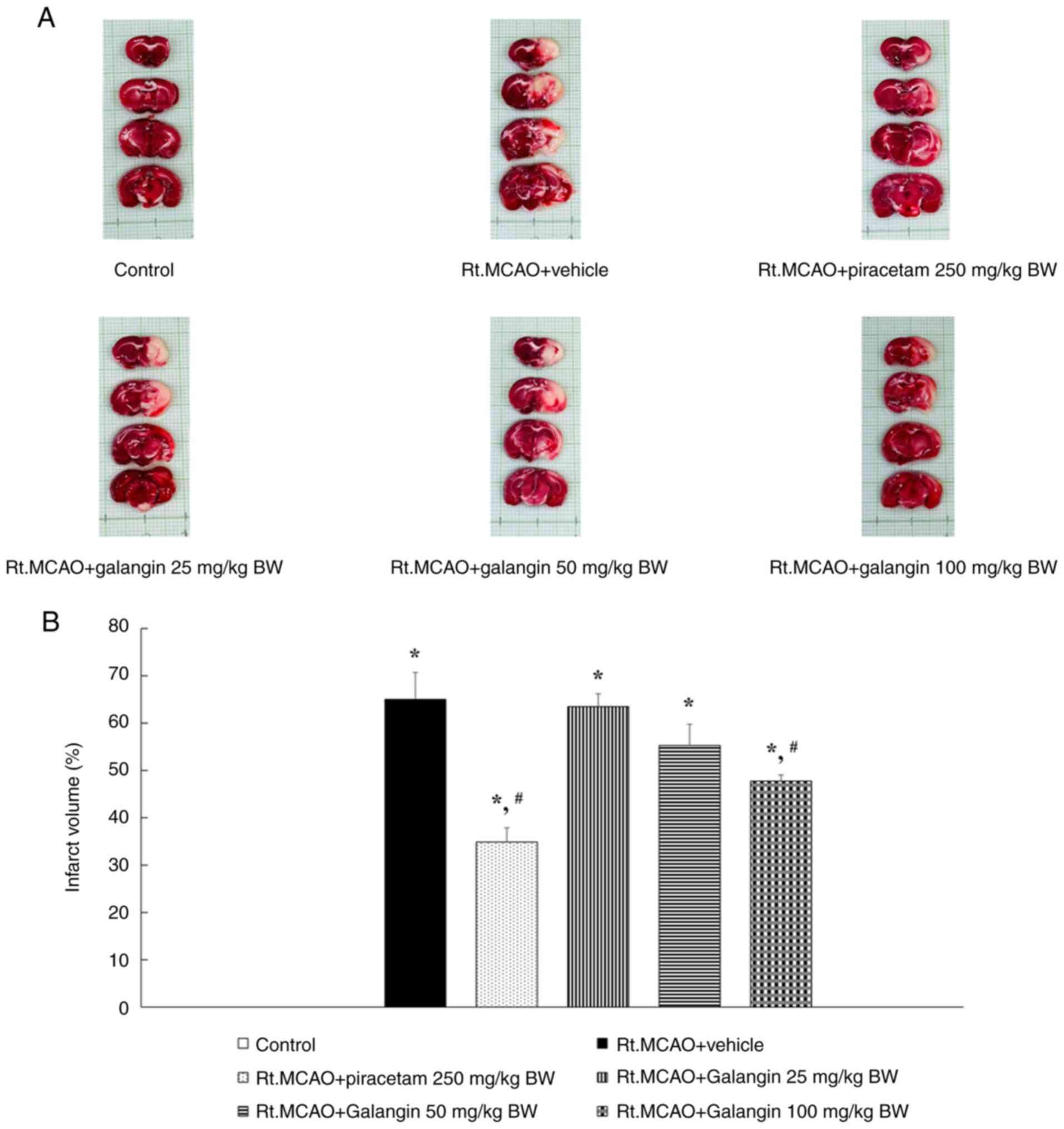
Induction of ischemic stroke by Rt.MCAO caused
substantial infarction in coronal brain sections. In the present
study, TTC staining was used to measure the extent of brain
infarction following galangin treatment in rats subjected to
permanent Rt.MCAO. Rats administered the vehicle after Rt.MCAO
exhibited a significantly larger infarct volume compared with the
control group (P<0.05) (Fig. 2).
By contrast, treatment with piracetam (250 mg/kg BW) and galangin
(100 mg/kg BW) led to a substantial decrease in infarct volumes
compared with the Rt.MCAO + vehicle group (P<0.05).
Effect of galangin on MDA levels and
endogenous antioxidant enzymes in the cortex and hippocampus
following Rt.MCAO
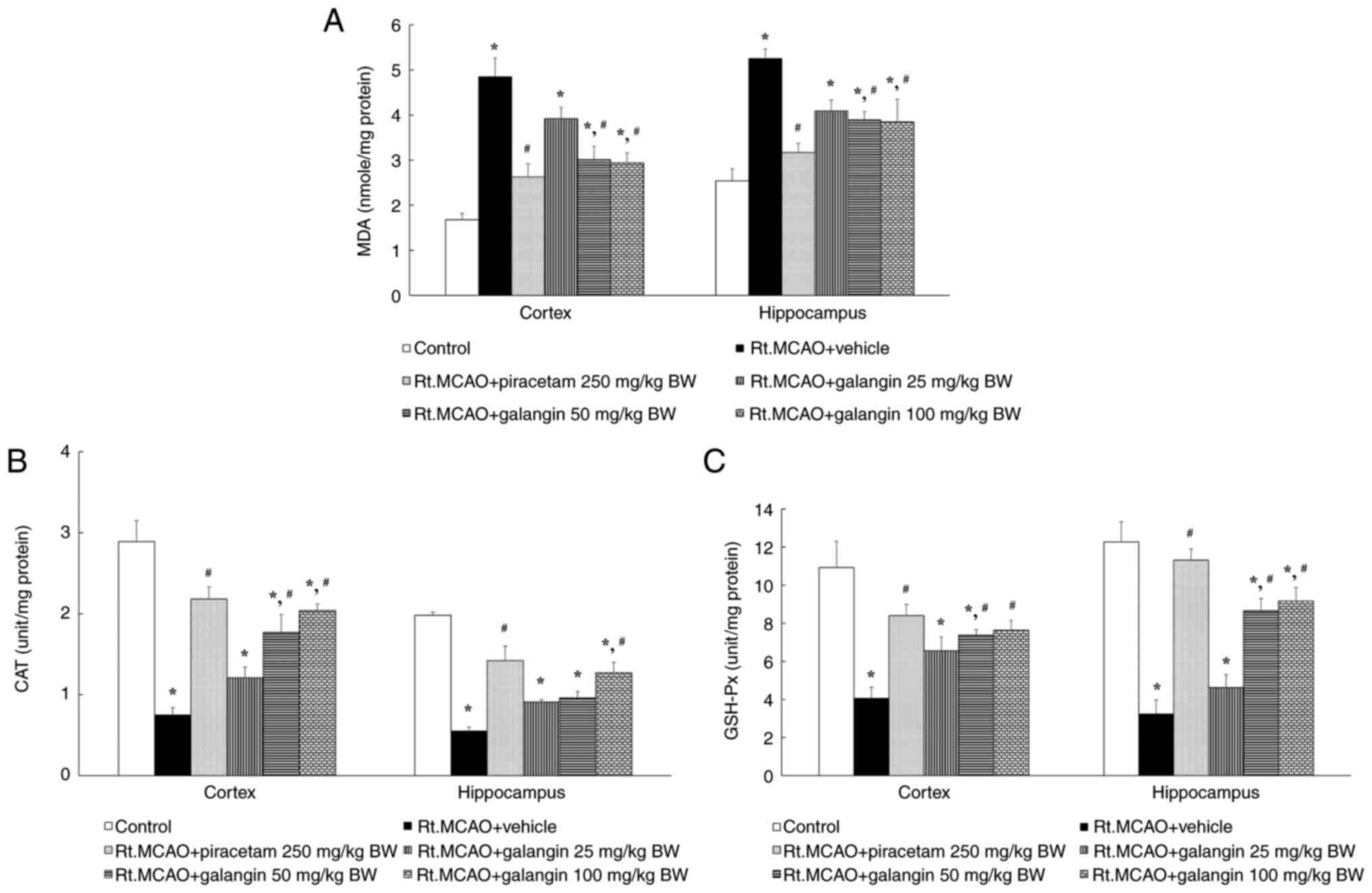
Rats with cerebral ischemia showed a marked increase
in MDA levels in both the cerebral cortex and hippocampus compared
with controls, indicating elevated lipid peroxidation in the brain.
However, treatment with piracetam (250 mg/kg BW) and galangin (50
and 100 mg/kg BW) significantly decreased lipid peroxidation across
all examined regions affected by cerebral ischemia (Fig. 3A). Several antioxidants are known to
inhibit ROS-mediated reactions and protect neurons from focal
cerebral ischemic stroke-induced injury. In addition to measuring
MDA levels, the present study measured the endogenous antioxidant
enzymes CAT and GSH-Px. Following cerebral ischemia, the activities
of CAT and GSH-Px were significantly reduced in the Rt.MCAO +
vehicle group compared with the control group. By contrast, the
groups treated with piracetam and galangin (100 mg/kg BW) exhibited
a significant increase in CAT and GSH-Px activities across all
assessed areas compared with the Rt.MCAO + vehicle group. Galangin
at a dose of 50 mg/kg BW induced a significant increase in CAT and
GSH-Px activities only in the cortex compared with the Rt.MCAO +
vehicle group (Fig. 3B and C).
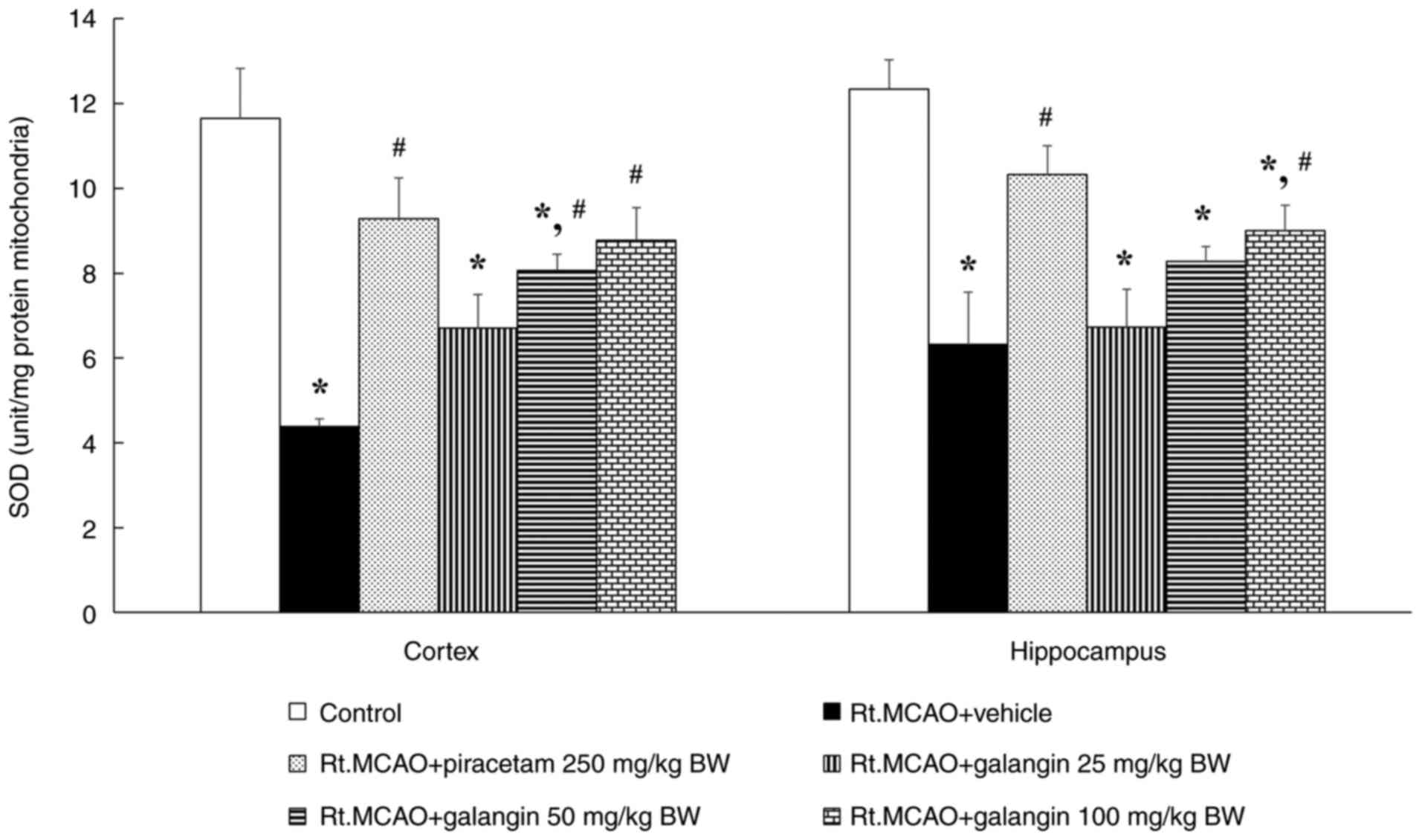
Effect of galangin on SOD activity in
mitochondria from the cortex and hippocampus of ischemic rats
Permanent occlusion of the right middle cerebral
artery caused a significant decrease in mitochondrial SOD activity
compared with the control group (P<0.05), as revealed in
Fig. 4. However, in rats treated
with piracetam (250 mg/kg BW) or galangin (100 mg/kg BW),
mitochondrial SOD activity was significantly less diminished
(P<0.05) compared with the vehicle + Rt.MCAO group in all
assessed areas. In rats treated with galangin at 50 mg/kg BW,
mitochondrial SOD activity demonstrated a significant difference
only in the cortex compared with the vehicle + Rt.MCAO group.
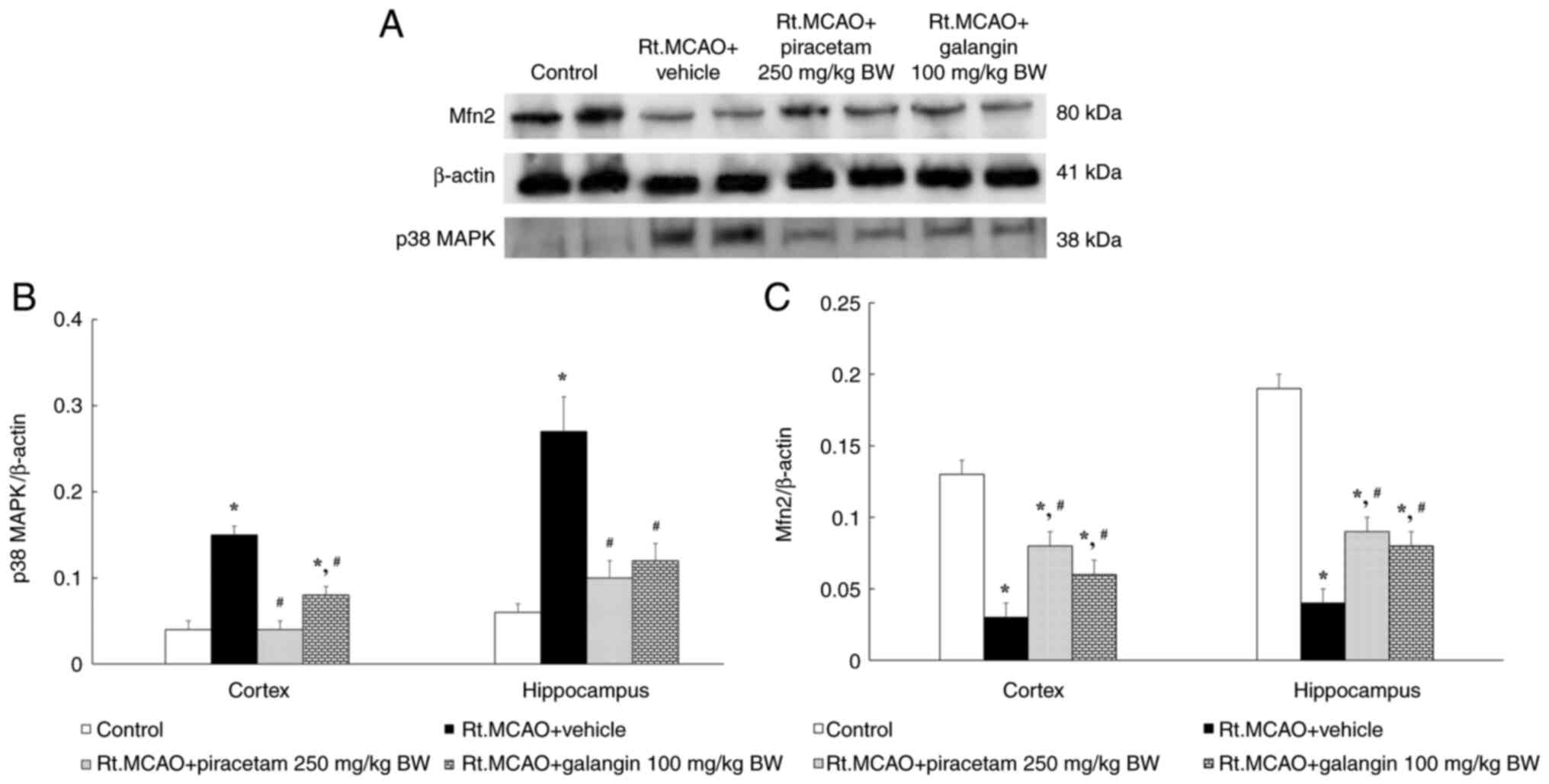
Effect of galangin on western blot
analysis of p38 MAPK and Mfn2 expression
Mitochondrial dynamics can mitigate the harmful
effects of oxidative stress in cerebral ischemia. Therefore, this
assessment examined the effect of galangin on p38 MAPK and Mfn2
protein expression using western blot analysis. Rats in the Rt.MCAO
+ vehicle group showed a marked increase in the p38 MAPK to β-actin
band density ratio and a decrease in the Mfn2 to β-actin band
density ratio compared with the control group (Fig. 5A). Treatment with piracetam at a
dose of 250 mg/kg BW or galangin at a dose of 100 mg/kg BW
significantly reduced the p38 MAPK to β-actin band density ratio
compared with the vehicle group (P<0.05; Fig. 5B). Furthermore, the Mfn2 to β-actin
band density ratio revealed a significantly smaller reduction in
these treated groups compared with the Rt.MCAO + vehicle group
(P<0.05; Fig. 5C).
Discussion
Cerebral ischemia involves a significant reduction
or interruption in blood flow to the brain, leading to a decrease
in the delivery of oxygen and nutrients to brain cells. This
condition triggers a cascade of events that can cause neuronal
damage, including excitotoxicity, oxidative stress, inflammation
and apoptosis (36). In cases of
permanent occlusion, the infarct becomes detectable within 3 to 12
h (37,38). Initially forming in the core region,
the infarct approaches its maximum size, encompassing both the core
and penumbra, and continues to expand over the following day. By 7
days post-ischemic stroke, there is a near complete depletion of
cellular elements in the affected area (39). This highlights the urgent need for
neuroprotective treatments to reduce brain injury. The present
study therefore explored the effects of galangin on brain infarct
volume in an in vivo focal ischemic stroke model. It was
demonstrated that permanent Rt.MCAO significantly increased the
brain infarct volume. However, administering piracetam at a dose of
250 mg/kg BW or galangin at a dose of 100 mg/kg BW led to a
substantial decrease in infarct volumes compared with the Rt.MCAO +
vehicle group (P<0.05). Previous studies suggested that galangin
may exert a protective effect by enhancing CBF, which could help
protect against cerebral damage from ischemic stroke (26,40).
In stroke, reduced oxygen and nutrient availability
disrupts the equilibrium between ROS production and the brain's
antioxidant defenses. ROS, such as superoxide radicals and hydrogen
peroxide, can damage lipids, proteins, DNA and other cellular
components (41). The production of
ROS, in stroke, is no longer adequately countered by endogenous
enzymatic antioxidants such as CAT, GSH-Px and SOD (42). Lipids can become oxidized,
initiating a self-sustaining chain reaction called the lipid
peroxidation cascade that generates free radicals (43). Lipid hydroperoxides and aldehydes
such as MDA are generated as products of this process. MDA has the
capacity to alter the biophysical characteristics of membranes,
affecting their fluidity and permeability, ultimately leading to
membrane disruption and lysis (44). Given the pivotal role of oxidative
stress in ischemic stroke pathology, the effect of galangin on
oxidative stress markers, particularly MDA levels, in the cortex
and hippocampus of rats subjected to Rt.MCAO was investigated. The
results revealed that both the positive control, piracetam, and the
experimental treatment, galangin (50 and 100 mg/kg BW), decreased
LPO products in the cortex and hippocampus compared with the
Rt.MCAO + vehicle group. Previous studies have shown that galangin
nanoparticles notably reduce MDA levels in models of
acetaminophen-induced liver injury (45) and cardiometabolic disorders
(46). In addition, augmenting the
activity of antioxidant enzymes has been linked to neuroprotection
and enhanced functional recovery post-stroke. Therefore, CAT and
GSH-Px activities were evaluated via biochemical assays conducted
on the cortex and hippocampus. The results of the present study
showed that piracetam and galangin effectively mitigated the
Rt.MCAO-induced decline in CAT and GSH-Px activities in both the
cortex and hippocampus compared with the Rt.MCAO + vehicle group.
CAT breaks down hydrogen peroxide, preventing the formation of
highly reactive hydroxyl radicals that could harm cells. Reduced
glutathione aids in converting GSH-Px and glutathione-S-transferase
into non-toxic products (17).
Previous studies have shown that reduced activity of these enzymes
may result from free radical damage or enzymatic glycation
(47,48). Flavonoids help mitigate oxidative
damage by scavenging free radicals, offering protection against
various diseases (49). Moreover,
galangin can neutralize free radicals by releasing hydrogen atoms
from its hydroxyl group.
Because mitochondrial dysfunction and low SOD levels
contribute to neuronal death and larger cerebral infarctions, the
present study also examined the effect of galangin on mitochondrial
SOD activity in the cortex and hippocampus. After Rt.MCAO, rats
administered with the vehicle exhibited a significant reduction in
SOD activity compared with the control group. Treatment with
piracetam or galangin effectively prevented this decline in SOD
activity in both the cortex and hippocampus mitochondria compared
with the Rt.MCAO + vehicle group. This effect may be attributable
to the antioxidant properties of galangin (16,17).
Several antioxidants have been reported to inhibit ROS-mediated
reactions and protect neurons from injury caused by focal cerebral
ischemic stroke (10,11).
During a stroke, mitochondrial dynamics can mitigate
the harmful effects of oxidative stress in cerebral ischemia.
Therefore, the present study examined the effect of galangin on p38
MAPK and Mfn2 protein expression using western blot analysis. In
accordance with expectations, a notable increase was detected in
the p38 MAPK to β-actin band density ratio and a decrease was
detected in the Mfn2 to β-actin band density ratio in rats with
permanent Rt.MCAO compared with the control group. However,
administration of piracetam at a dose 250 mg/kg BW or galangin at a
dose of 100 mg/kg BW led to a significant decrease in the p38 MAPK
to β-actin band density ratio compared with the vehicle-treated
group. Furthermore, the reduction in the Mfn2 to β-actin band
density ratio was less pronounced in these treatment groups than
the Rt.MCAO + vehicle group. After a stroke, disruptions in
mitochondrial dynamics skew the balance between mitochondrial
fission and fusion, favoring increased fission, which results in
neuronal damage. Signaling pathways involving p38 MAPK and
dynamin-related protein 1 (DRP1) are associated with various
biological mechanisms during cerebral hypoxia/ischemia, such as
programmed cell death, oxidative stress, mitochondrial function,
calcium signaling and synaptic activity (13,50,51).
In rat models of MCAO, inhibiting DRP1 was shown to reduce both
cytochrome c release and apoptosis (52). Suppressing DRP1 may help alleviate
mitochondrial dysfunction and prevent cell death by reducing
mitochondrial fission (51).
Additionally, previous studies suggested that inhibition of
mitochondrial fission, including the MAPK pathways, could help
mitigate brain damage following a stroke (12-14).
Therefore, blocking p38 MAPK or DRP1 directly halts neuronal
degeneration by decreasing mitochondrial fission, which in turn
helps recover mitochondrial membrane potential, restore
mitochondrial function, and minimize cell death. Moreover, a 7-day
treatment period for galangin based on the pathophysiological
changes during the subacute phase of ischemic stroke was selected.
This phase involves ATP depletion, ionic imbalance and the
generation of reactive species, leading to tissue damage. Both
defense mechanisms and inflammatory responses are activated,
worsening neuronal injury. Thus, this timeframe was chosen to
evaluate the potential antioxidant and mitochondrial effects of
galangin in reducing neuronal damage in a focal ischemic stroke
model. Recent research indicated that flavonoids play a role in
preserving mitochondrial function and protecting against
disruptions in mitochondrial dynamics caused by oxidative stress
(53). Flavonoids, categorized as
secondary metabolites, primarily comprise a benzopyrone ring with
phenolic or polyphenolic groups positioned variably (49). These compounds are present in a
range of sources including fruits, herbs, stems, cereals, nuts,
vegetables, flowers and seeds (54). Flavonoids offer a variety of
potential health benefits, such as anticancer, antioxidant,
anti-inflammatory and antiviral properties, along with
neuroprotective and cardio-protective effects (55-58).
Previous research has shown that Alpinia officinarum
flavonoids can modulate transient receptor potential vanilloid
subtype 1 (TRPV1) expression to safeguard the gastric lining
(59). Galangin may similarly
interact with TRPV1 to inhibit the release of neurotransmitters and
inflammatory agents, thereby easing pain and inflammation (59). Galangin also appears to reduce
neurogenic pain, particularly that caused by capsaicin, by
influencing TRPV1-associated pathways, including those involving
NF-κB/TNF-α and COX-2(60).
The present study has several limitations. First,
post-stroke behavioral impairments were not investigated in the
current study and there are no data available on the decline in
neurological function over time. It would have been useful to
assess functional outcomes such as motor, sensory and cognitive
abilities in the rat model after the stroke. Second, although DRP1
and phosphorylated MAPK are key regulators of mitochondrial fission
and play crucial roles in stroke-induced damage, the authors were
unable to measure DRP1 and phosphorylated MAPK due to funding
constraints. This will be addressed in future research. Lastly, the
small number of replicates used in the biochemical assays is a
different limitation. While three or more replicates would have
been preferable, only two were used. Nonetheless, the assays were
performed by a skilled researcher, and the standard curves
displayed high R² values.
Future studies should explore the neuroprotective
mechanisms of galangin beyond its antioxidant properties and impact
on mitochondrial dynamics, specifically examining its role in
inflammation and apoptosis. Research is needed to evaluate its
long-term effectiveness and determine the optimal dosing.
Investigating the potential of galangin in combination with other
therapeutic agents could further enhance its efficacy.
Additionally, it would be beneficial to examine how different
mitochondrial subtypes respond to treatment. Ultimately, clinical
trials are necessary to validate its safety and effectiveness in
human populations.
In summary, galangin exhibits notable in vivo
efficacy in reducing the brain damage induced by cerebral ischemia.
This favorable outcome is associated, at least in part, with its
antioxidant activity and its effect on mitochondrial dynamics.
Acknowledgements
The authors would like to thank Dr Tim Cushnie
(Faculty of Medicine, Mahasarakham University, Mahasarakham 44000,
Thailand) for language-editing assistance. The authors would like
to extend their sincere gratitude to the Northeastern Laboratory
Animal Center at Khon Kaen University, Khon Kaen, Thailand, for
providing the animals used in the present study.
Funding
Funding: The present study was supported by a grant from Faculty
of Medicine, Mahasarakham University (grant no. MED MSU
01/010/2567).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AS handled the funding acquisition, investigation,
methodology, writing and editing of the manuscript. NP contributed
to the investigation and methodology, reviewed and edited the
manuscript. JJ was responsible for conceptualization, data
curation, formal analysis, funding acquisition, investigation,
methodology, project administration, supervision, validation and
both writing the original draft and reviewing and editing the
manuscript. JJ and AS confirm the authenticity of all raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experimentation protocols were carefully
designed to minimize potential suffering. These protocols were
carried out in strict compliance with the approval (approval no.
IACUC-KKU-105/66) from the Institutional Animal Care and Use
Committee at Khon Kaen University (Khon Kaen, Thailand).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Donkor ES: Stroke in the 21st Century: A
Snapshot of the Burden, epidemiology, and quality of life. Stroke
Res Treat. 2018(3238165)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shirley R, Ord EN and Work LM: Oxidative
Stress and the Use of Antioxidants in Stroke. Antioxidants (Basel).
3:472–501. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rabinstein AA: Treatment of acute ischemic
stroke. Continuum (Minneap Minn). 23:62–81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jaffer H, Morris VB, Stewart D and
Labhasetwar V: Advances in stroke therapy. Drug Deliv Transl Res.
1:409–419. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Garcia-Sanchez A, Miranda-Diaz AG and
Cardona-Munoz EG: The Role of oxidative stress in physiopathology
and pharmacological treatment with pro- and antioxidant properties
in chronic diseases. Oxid Med Cell Longev.
2020(2082145)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kiechl S and Willeit J: Alteplase in acute
ischaemic stroke: no time to slow down. Lancet Neurol. 15:893–895.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Feng S, Yang M, Liu S, He Y, Deng S and
Gong Y: Oxidative stress as a bridge between age and stroke: A
narrative review. J Intensive Med. 3:313–319. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Olufunmilayo EO, Gerke-Duncan MB and
Holsinger RMD: Oxidative stress and antioxidants in
neurodegenerative disorders. Antioxidants (Basel).
12(517)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu
W, Bennett MVL and Chen J: Oxidative stress and DNA damage after
cerebral ischemia: Potential therapeutic targets to repair the
genome and improve stroke recovery. Neuropharmacology. 134:208–217.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kongsui R and Jittiwat J: Ameliorative
effects of 6-gingerol in cerebral ischemia are mediated via the
activation of antioxidant and anti-inflammatory pathways. Biomed
Rep. 18(26)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jittiwat J, Suksamrarn A, Tocharus C and
Tocharus J: Dihydrocapsaicin effectively mitigates cerebral
ischemia-induced pathological changes in vivo, partly via
antioxidant and anti-apoptotic pathways. Life Sci.
283(119842)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kongsui R and Jittiwat J: In vivo
protective effects of 6-gingerol in cerebral ischemia involve
preservation of antioxidant defenses and activation of
anti-apoptotic pathways. Biomed Rep. 20(85)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li C, Chen C, Qin H, Ao C, Chen J, Tan J
and Zeng L: The role of mitochondrial dynamin in stroke. Oxid Med
Cell Longev. 2022(2504798)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang XM, Zhang L, Wang G, Niu W, He Z,
Ding L and Jia J: Suppression of mitochondrial fission in
experimental cerebral ischemia: The potential neuroprotective
target of p38 MAPK inhibition. Neurochem Int. 90:1–8.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Heo MY, Sohn SJ and Au WW:
Anti-genotoxicity of galangin as a cancer chemopreventive agent
candidate. Mutat Res. 488:135–150. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aloud AA, Veeramani C, Govindasamy C,
Alsaif MA and Al-Numair KS: Galangin, a natural flavonoid reduces
mitochondrial oxidative damage in streptozotocin-induced diabetic
rats. Redox Rep. 23:29–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aloud AA, Veeramani C, Govindasamy C,
Alsaif MA, El Newehy AS and Al-Numair KS: Galangin, a dietary
flavonoid, improves antioxidant status and reduces
hyperglycemia-mediated oxidative stress in streptozotocin-induced
diabetic rats. Redox Rep. 22:290–300. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thangaiyan R, Arjunan S, Govindasamy K,
Khan HA, Alhomida AS and Prasad NR: Galangin attenuates
isoproterenol-induced inflammation and fibrosis in the cardiac
tissue of albino Wistar rats. Front Pharmacol.
11(585163)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lee HN, Shin SA, Choo GS, Kim HJ, Park YS,
Kim BS, Kim SK, Cho SD, Nam JS, Choi CS, et al: Anti-inflammatory
effect of quercetin and galangin in LPS-stimulated RAW264.7
macrophages and DNCB-induced atopic dermatitis animal models. Int J
Mol Med. 41:888–898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin K, Wang Y, Gong J, Tan Y, Deng T and
Wei N: Protective effects of total flavonoids from Alpinia
officinarum rhizoma against ethanol-induced gastric ulcer in vivo
and in vitro. Pharm Biol. 58:854–862. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gong J, Zhang Z, Zhang X, Chen F, Tan Y,
Li H, Jiang J and Zhang J: Effects and possible mechanisms of
Alpinia officinarum ethanol extract on indomethacin-induced gastric
injury in rats. Pharm Biol. 56:294–301. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aloud AA, Chinnadurai V, Govindasamy C,
Alsaif MA and Al-Numair KS: Galangin, a dietary flavonoid,
ameliorates hyperglycaemia and lipid abnormalities in rats with
streptozotocin-induced hyperglycaemia. Pharm Biol. 56:302–308.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abukhalil MH, Althunibat OY, Aladaileh SH,
Al-Amarat W, Obeidat HM, Al-Khawalde AAA, Hussein OE, Alfwuaires
MA, Algefare AI, Alanazi KM, et al: Galangin attenuates diabetic
cardiomyopathy through modulating oxidative stress, inflammation
and apoptosis in rats. Biomed Pharmacother.
138(111410)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jin Y, Yang P and Wang L, Gao Z, Lv J, Cui
Z, Wang T, Wang D and Wang L: Galangin as a direct inhibitor of
vWbp protects mice from Staphylococcus aureus-induced pneumonia. J
Cell Mol Med. 26:828–839. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang YC, Tsai MS, Hsieh PC, Shih JH, Wang
TS, Wang YC, Lin TH and Wang SH: Galangin ameliorates
cisplatin-induced nephrotoxicity by attenuating oxidative stress,
inflammation and cell death in mice through inhibition of ERK and
NF-kappaB signaling. Toxicol Appl Pharmacol. 329:128–139.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li S, Wu C, Zhu L, Gao J, Fang J, Li D, Fu
M, Liang R, Wang L, Cheng M and Yang H: By improving regional
cortical blood flow, attenuating mitochondrial dysfunction and
sequential apoptosis galangin acts as a potential neuroprotective
agent after acute ischemic stroke. Molecules. 17:13403–13423.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tortiglione A, Minale M, Pignataro G,
Amoroso S, DiRenzo G and Annunziato L: The 2-oxopyrrolidinacetamide
piracetam reduces infarct brain volume induced by permanent middle
cerebral artery occlusion in male rats. Neuropharmacology.
43:427–433. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kessler J, Thiel A, Karbe H and Heiss WD:
Piracetam improves activated blood flow and facilitates
rehabilitation of poststroke aphasic patients. Stroke.
31:2112–2116. 2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Y, Feng J, Xu F and Wang J: Piracetam
inhibits ethanol (EtOH)-induced memory deficit by mediating
multiple pathways. Brain Res. 1676:83–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Janyou A, Wicha P, Jittiwat J, Suksamrarn
A, Tocharus C and Tocharus J: Dihydrocapsaicin attenuates blood
brain barrier and cerebral damage in focal cerebral
ischemia/reperfusion via oxidative stress and inflammatory. Sci
Rep. 7(10556)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jittiwat J: Baihui point laser acupuncture
ameliorates cognitive impairment, motor deficit, and neuronal loss
partly via antioxidant and anti-inflammatory effects in an animal
model of focal ischemic stroke. Evid Based Complement Alternat Med.
2019(1204709)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jittiwat J, Chonpathompikunlert P and
Sukketsiri W: Neuroprotective effects of Apium graveolens against
focal cerebral ischemia occur partly via antioxidant,
anti-inflammatory, and anti-apoptotic pathways. J Sci Food Agric.
101:2256–2263. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
34
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Goldblith SA and Proctor BE: Photometric
determination of catalase activity. J Biol Chem. 187:705–709.
1950.PubMed/NCBI
|
|
36
|
Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z
and Gu L: Targeting oxidative stress and inflammation to prevent
ischemia-reperfusion injury. Front Mol Neurosci.
13(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakano S, Iseda T, Kawano H, Yoneyama T,
Ikeda T and Wakisaka S: Correlation of early CT signs in the deep
middle cerebral artery territories with angiographically confirmed
site of arterial occlusion. AJNR Am J Neuroradiol. 22:654–659.
2001.PubMed/NCBI
|
|
38
|
Allen LM, Hasso AN, Handwerker J and Farid
H: Sequence-specific MR imaging findings that are useful in dating
ischemic stroke. Radiographics. 32:1285–1289. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang D, Ren J, Luo Y, He Q, Zhao R, Chang
J, Yang Y and Guo ZN: T cell response in ischemic stroke: From
mechanisms to translational insights. Front Immunol.
12(707972)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guan X, Li Z, Zhu S, Cheng M, Ju Y, Ren L,
Yang G and Min D: Galangin attenuated cerebral ischemia-reperfusion
injury by inhibition of ferroptosis through activating the
SLC7A11/GPX4 axis in gerbils. Life Sci. 264(118660)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: Chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khanum R and Thevanayagam H: Lipid
peroxidation: Its effects on the formulation and use of
pharmaceutical emulsions. Asian J Pharm Sci. 12:401–411.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Montine KS, Quinn JF, Zhang J, Fessel JP,
Roberts LJ II, Morrow JD and Montine TJ: Isoprostanes and related
products of lipid peroxidation in neurodegenerative diseases. Chem
Phys Lipids. 128:117–124. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mohammadi A, Kazemi S, Molayousefian I,
Pirzadeh M and Moghadamnia AA: Galangin nanoparticles protect
acetaminophen-induced liver injury: A biochemical and
histopathological approach. Evid Based Complement Alternat Med.
2022(4619064)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Prasatthong P, Meephat S, Rattanakanokchai
S, Khamseekaew J, Bunbupha S, Prachaney P, Maneesai P and
Pakdeechote P: Galangin resolves cardiometabolic disorders through
modulation of AdipoR1, COX-2, and NF-ĸB expression in rats fed a
high-fat diet. Antioxidants (Basel). 10(769)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gusti AMT, Qusti SY, Alshammari EM, Toraih
EA and Fawzy MS: Antioxidants-related superoxide dismutase (SOD),
Catalase (CAT), glutathione peroxidase (GPX),
Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS)
gene variants analysis in an obese population: A preliminary
case-control study. Antioxidants (Basel). 10(595)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lubrano V and Balzan S: Enzymatic
antioxidant system in vascular inflammation and coronary artery
disease. World J Exp Med. 5:218–224. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ullah A, Munir S, Badshah SL, Khan N,
Ghani L, Poulson BG, Emwas AH and Jaremko M: Important flavonoids
and their role as a therapeutic agent. Molecules.
25(5243)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gui C, Ren Y, Chen J, Wu X, Mao K, Li H,
Yu H, Zou F and Li W: p38 MAPK-DRP1 signaling is involved in
mitochondrial dysfunction and cell death in mutant A53T α-synuclein
model of Parkinson's disease. Toxicol Appl Pharmacol.
388(114874)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Huan Y, Hao G, Shi Z, Liang Y, Dong Y and
Quan H: The role of dynamin-related protein 1 in cerebral
ischemia/hypoxia injury. Biomed Pharmacother.
165(115247)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhao YX, Cui M, Chen SF, Dong Q and Liu
XY: Amelioration of ischemic mitochondrial injury and Bax-dependent
outer membrane permeabilization by Mdivi-1. CNS Neurosci Ther.
20:528–538. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kumar S, Chhabra V, Shenoy S, Daksh R,
Ravichandiran V, Swamy RS and Kumar N: Role of flavonoids in
modulation of mitochondria dynamics during oxidative stress. Mini
Rev Med Chem. 24:908–919. 2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Faleye OS, Lee JH and Lee J: Selected
flavonoids exhibit antibiofilm and antibacterial effects against
Vibrio by disrupting membrane integrity, virulence and metabolic
activities. Biofilm. 6(100165)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Parmenter BH, Croft KD, Hodgson JM,
Dalgaard F, Bondonno CP, Lewis JR, Cassidy A, Scalbert A and
Bondonno NP: An overview and update on the epidemiology of
flavonoid intake and cardiovascular disease risk. Food Funct.
11:6777–6806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhao L, Yuan X, Wang J, Feng Y, Ji F, Li Z
and Bian J: A review on flavones targeting serine/threonine protein
kinases for potential anticancer drugs. Bioorg Med Chem.
27:677–685. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Patel DK: Medicinal importance,
pharmacological activity and analytical aspects of flavonoid
‘Irisflorentin’ from Belamcanda chinensis (L.) DC. Curr Drug Res
Rev. 15:222–227. 2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang D, Chen J, Pu L, Yu L, Xiong F, Sun
L, Yu Q, Cao X, Chen Y, Peng F and Peng C: Galangin: A food-derived
flavonoid with therapeutic potential against a wide spectrum of
diseases. Phytother Res. 37:5700–5723. 2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lin K, Deng T, Qu H, Ou H, Huang Q, Gao B,
Li X and Wei N: Gastric protective effect of Alpinia officinarum
flavonoids: Mediating TLR4/NF-ĸB and TRPV1 signalling pathways and
gastric mucosal healing. Pharm Biol. 61:50–60. 2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lin K, Fu D, Wang Z, Zhang X and Zhu C:
Analgesic and anti-inflammatory effects of galangin: A potential
pathway to inhibit transient receptor potential vanilloid 1
receptor activation. Korean J Pain. 37:151–163. 2024.PubMed/NCBI View Article : Google Scholar
|