Introduction
The criteria for defining arterial aneurysms as ‘a
permanent localized dilatation of an artery having at least a 50%
increase in diameter compared with the expected normal diameter of
the artery in question’ was established by the Ad Hoc Committee on
Reporting Standards of the Society for Vascular Surgery in
1991(1). Aneurysmal dilatation of
an artery results from the structural weakening of the arterial
wall, primarily due to factors such as atherosclerosis, trauma,
infection or post-stenotic abnormalities. Peripheral arterial
aneurysms (PrAAs) exclude the aorta, iliac, cerebral and coronary
arteries (2).
True aneurysms involve all three layers of the
normal arterial wall and are the most common type. By contrast,
pseudoaneurysms, commonly referred to as false aneurysms, are
enveloped by a thin fibrous capsule in place of the typical
arterial wall layers (3-5).
Popliteal artery aneurysms (PAAs) are relatively
uncommon, with an incidence of <0.1% in the general population;
however, they account for >70% of PrAAs (6). These aneurysms predominantly affect
men and typically occur around the age of 65 years; ~50% of the
cases are bilateral and are often associated with abdominal aortic
aneurysms (AAAs). Notably, >50% of patients present with
symptoms, the most common being acute limb ischemia caused by
thromboembolic complications, which are linked to elevated rates of
morbidity and limb loss (5,6).
Within a 5-year period, ~68-74% of patients with
untreated PAAs experienced complications. These primarily included
distal embolization, thrombosis and rupture. Notably,
thromboembolic complications were associated with a marked risk of
major amputation (20-59%) and mortality (up to 11%) (7). By contrast, elective repair had a
certain risk (~10%) for long-term limb loss in patients with low
surgical risk. Thus, for PAAs reaching a diameter of 2 cm, elective
surgical intervention is generally recommended (8).
Femoral artery aneurysms (FAAs) are the second most
common type of PrAA, following PAAs. However, common FAAs (CFAAs)
are relatively uncommon, while isolated superficial FAAs (SFAAs)
and profunda femoris artery aneurysms are even rarer (9). Infra-PAAs (aneurysms of all arteries
distal to the popliteal artery, including posterior tibial,
anterior tibial and peroneal arteries) are a rare occurrence,
primarily stemming from traumatic causes, with the majority being
false aneurysms (10).
Upper limb aneurysms are an uncommon occurrence,
constituting ~1% of all cases of PrAAs. Mostly they are
subclavian-axillary artery aneurysms, which manifest at the outer
border of the first rib, and the 3rd part of the axillary artery.
These aneurysms, which are primarily post-stenotic in nature from
thoracic outlet syndrome, are induced by factors such as the
cervical rib or fibrous band (11).
Recently, endovascular treatment options have been
employed to manage PrAAs, yielding diverse outcomes. Currently, the
use of endovascular treatment for peripheral aneurysms should be
reserved for specific cases involving high-risk patients. Until the
development of devices that can counteract issues such as
thrombosis and possibility of migration, as this part of the artery
is highly mobile a tent in this position may end by extrinsic
compression due to acute knee joint flexion. Traditional open
surgery will remain the preferred treatment option (7,8,12).
Open surgical treatment options include in-situ arterial
replacement or a bypass with an autologous or prosthetic graft. To
reduce the risk of late recurrence of the aneurysm,
endoaneurysmorrhaphy is preferred compared with simple ligation of
the artery proximal and distal to the aneurysm. Thrombolysis has
also been used to improve outflow in patients with acute
presentations (6).
The primary aim of the present study was to
investigate the true PrAAs in a cohort of 30 selected patients who
underwent open surgical repair. The present study identified and
analyzed the risk factors associated with the development of PrAAs,
assessing the impact of comorbidities on the treatment outcomes of
patients with PrAAs. In addition, the study analyzed the long- and
short-term outcomes of surgical repair for true PrAAs in the
studied patient group.
Patients and methods
Study design and patients'
demographics
A retrospective analysis of prospectively collected
data was performed for 30 consecutive patients operated on for
PrAAs from different anatomical locations over 10 years. A total of
32 PrAAs were treated in the 30 patients, which comprised 22
(73.3%) men and 8 (26.7%) women. The age distribution ranged from
52 to 87 years with a mean of 61.77±6.83 years. The majority of
patients (55.3%) were in their 70s and 80s. The clinical data of
patients diagnosed with PrAAs and admitted to our institution
(Department of Cardiovascular Surgery, Shar Teaching Hospital,
Sulaymaniyah, Iraq) between January 2013 and January 2023 were
reviewed, and the outcome of open revascularization was analyzed.
The present study protocol was approved by the ethics and
scientific committees of the Kurdistan Board for Medical
Specialties (approval no. 1115) on June 6, 2023. The Rutherford
classification was used to categorize both acute and chronic limb
ischemia, and as criteria for primary and secondary patency
(13).
During the data collection, patients with true
atherosclerotic PrAAs who underwent open surgical repair and
completed follow-up for a minimum of 18 months were included in the
present study. All patients diagnosed with false PrAAs or recurrent
aneurysms, patients who had undergone a previous endovascular
repair, and patients who had not completed 18 months of follow-up
were excluded from the study. Smoking, body mass index (BMI), and
cardiac, pulmonary and renal risk factors were documented using the
criteria of the Department of Veterans Affairs National Surgical
Quality Improvement Program (14).
Finally, patients were divided into three groups:
Group 1 (asymptomatic limbs), Group 2 (symptoms of chronic limb
ischemia), and Group 3 (limbs with acute limb ischemia) (7). Indications for operation included
acute limb ischemia, PrAAs with compressive symptoms or disabling
claudication, asymptomatic PrAAs with an aneurysm 2 cm in size,
those with mural thrombi and evidence of previous
thromboembolism.
Operative technique
A medial approach was used for revascularization of
all PAAs. If the aneurysm involved the proximal popliteal artery,
the superficial femoral or common femoral artery was used as the
inflow for the bypass. In the case of a distal PAA, one of the
tibial arteries or the peroneal artery was the site of the distal
anastomosis. Proximal and distal ligation were usually used for
small aneurysms. Endoaneurysmorrhaphy was performed most commonly
in patients with an aneurysm >2 cm or if large genicular
arteries were noted on preoperative imaging. The aneurysms were
opened, the thrombi evacuated and the lumens of backflow small
arteries are oversewn without frequently needing to transect the
medial head of the gastrocnemius muscle. Reconstruction was
performed with an autologous or prosthetic conduit or interposition
graft. A prosthetic graft was used only if a suitable autologous
vein was not available.
CFAAs were revascularized using a longitudinal
approach within the femoral triangle. Iliofemoral bypass was
conducted using polytetrafluoroethylene (PTFE) or Dacron grafts. In
the case of a SFAA, revascularization was performed by open repair
through a longitudinal mid-thigh approach and using a PTFE graft.
For both posterior tibial and PAAs, a medial approach was used,
with excision and repair performed using a great saphenous vein
(GSV) graft. Brachial artery aneurysm repairs were performed using
a right medial longitudinal approach, which involved a GSV
graft.
The case series of the present study included one
left subclavian-axillary artery aneurysm. The cervical X-ray
revealed a complete cervical rib; the approach used was
supraclavicular with extension to the infraclavicular fossa, and
the repair was performed by GSV with cervical and 1st rib
resection.
Information on concomitant aneurysms was obtained
from reports of imaging studies and from the history of previous
aneurysm repairs. Out of all the 30 patients in the present study,
25 underwent ultrasound imaging of the contralateral popliteal
fossa, and 22 underwent computed tomography angiography (CTA)
imaging of the aortoiliac vessels.
Follow-up information was obtained from the medical
records, and outpatient questionnaires were used to establish
survival and limb loss. Patients were seen 1 month after the
operation. A second follow-up was observed at 3 months. Patients
were then followed up for 6-24 months with intervals of 3 months.
Outpatient visit data were collected, and the patency rates were
calculated based on the findings of the last imaging study
performed.
Statistical analysis
The data were collected and entered into a
spreadsheet; after coding, they were transferred to SPSS (version
22; IBM Corp.) Descriptive and quantitative analyses were then
performed. The relationship between initial findings and subsequent
morbidity and mortality were determined using Fisher's exact test
for nominal variables and the continuous variables were associated
using the t-test One-way analysis of variance was used to determine
statistically significant differences between the means of three
groups. A two-tailed P<0.05 was considered to indicate
statistical significance.
Results
Patient demographics
Between 2013 and 2023, 30 patients were diagnosed
with 32 PrAAs. Open surgical revascularizations were performed in
all patients for 32 PrAAs and two associated AAAs. All patient
demographics and their comorbidities are described in Table I. Patients were classified according
to Rutherford's classification of chronic and acute limb ischemia.
Out of all the 30 patients, 22 (73.3%) were men and 8 (26.7%) were
women. The age ranged from 45 to 80 years with a mean age of 67.33
years. According to the type of clinical presentation, both men and
women were subdivided into three groups: Group 1 comprised 6
patients with asymptomatic aneurysms; Group 2 included 20 patients
with chronic symptoms; and Group 3 included 6 patients with acute
limb ischemia (Table I). Out of all
the 30 patients, 28 patients (93.3%) had unilateral PrAAs and 2
(6.7%) had bilateral PrAAs. A total of 12 patients (40.0%) had a
left-side presentation and 16 (53.3%) had a right-side presentation
(P=0.383) (Table I). Regarding risk
factors, 30.0% of the patients were non-smokers and among the
smokers, the mean pack years were 19.0±26.1 for Group 1, 35.5±24.4
for Group 2 and 47.8±23.8 for Group 3. Which means the majority
were smokers. Again, there was a significant difference among the
groups with the majority belonging to group 3.
 | Table IDemographic data and risk factors of
30 patients with 32 peripheral arterial aneurysms. |
Table I
Demographic data and risk factors of
30 patients with 32 peripheral arterial aneurysms.
| Socio-demographic
characteristic | Group 1 (n=6) | Group 2 (n=20) | Group 3 (n=6) | Total (n=32) | ANOVA test
result | P-value |
|---|
| Age, years | 60.8±16.3 | 69.0±9.03 | 73.8±4.9 | - | 2.408 | 0.109 |
|
<50 | 2 (40.0) | 2 (10.5) | 0 (0) | 4 (13.3) | 4.525 | 0.304 |
|
50-75 | 3 (60.0) | 11 (57.9) | 5 (83.3) | 21 (63.4) | | |
|
>75 | 0 (0.0) | 6 (31.6) | 1 (16.7) | 7 (23.3) | | |
| Sex | | | | | 2.876 | 0.306 |
|
Male | 3 (60.0) | 13 (68.4) | 6 (100.0) | 22 (73.3) | | |
|
Female | 2 (40.0) | 6 (31.6) | 0 (0.0) | 8 (26.7) | | |
| Presentation
side | | | | | 4.348 | 0.332 |
|
Bilateral | 1 (20.0) | 1 (5.3) | 0 (0.0) | 2 (6.7) | | |
|
Left | 3 (60.0) | 6 (31.6) | 3 (50.0) | 12 (40.0) | | |
|
Right | 1 (20.0) | 12 (63.2) | 3 (50.0) | 16 (53.3) | | |
| Smoking PY | 19.0±26.1 | 35.5±24.4 | 47.8±23.8 | - | 0.889 | 0.171 |
|
None | 3 (60.0) | 5 (26.3) | 1 (16.7) | 9 (30.0) | 5.720 | 0.209 |
|
≤50 | 2 (40.0) | 8 (42.1) | 1 (16.7) | 11 (36.7) | | |
|
>50 | 0 (0.0) | 6 (31.6) | 4 (66.6) | 10 (33.3) | | |
| BMI,
kg/m2 | 24.0±3.0 | 23.1±2.5 | 23.7±1.5 | - | 0.320 | 0.725 |
|
Underweight | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.521 | 0.516 |
|
Normal | 4 (80.0) | 14 (73.7) | 3 (50.0) | 21 (70.0) | | |
|
Overweight | 1 (20.0) | 5 (26.3) | 3 (50.0) | 9 (30.0) | | |
|
Obese | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | | |
| Comorbidities | | | | | 6.762 | 0.739 |
|
Ischemic
heart disease | 3 (50.0) | 15 (34.1) | 5 (35.7) | 23 (35.9) | | |
|
Arrhythmia | 0 (0.0) | 1 (2.3) | 2 (14.3) | 3 (4.7) | | |
|
Diabetes | 1 (16.7) | 14 (31.8) | 4 (28.6) | 19 (29.7) | | |
|
Chronic
kidney disease | 0 (0.0) | 4 (9.1) | 0 (0.0) | 4 (6.3) | | |
|
Chronic
obstructive pulmonary disease | 0 (0.0) | 3 (6.8) | 0 (0.0) | 3 (4.7) | | |
|
Hypertension | 2 (33.3) | 7 (15.9) | 3 (21.4) | 12 (18.8) | | |
| Total number of
comorbidities | 6 | 44 | 14 | 64 | | |
There was no significant difference in the BMI among
Groups 1, 2 and 3. Regarding comorbidities, 23 (35.9%) patients had
ischemic heart disease, 19 patients (29.7%) had diabetes, 12
patients (18.8%) were hypertensive, 4 patients (6.3%) had chronic
kidney disease, 3 patients (4.7%) had chronic obstructive pulmonary
disease and 3 (4.7%) patients had arrhythmia.
A total of 6 patients (21.8%) with PrAAs were
asymptomatic (Group 1), 20 patients (59.37%) had chronic
limb-threatening ischemia (Group 2) and 6 patients (18.75%) had
acute limb ischemia (Group 3) (Table
II). Group 2, composed of 19 (59.37%) participants, presented
with chronic limb-threatening ischemia and was further subdivided
according to the Rutherford classification as follows: Class II
moderate claudication, comprising 3 (10%) patients; class III
severe claudication, also comprising 4 patients (10%); class IV
ischemic rest pain, comprising 2 (6.7%) patients; class V minor
tissue loss, comprising 5 (16.7%) patients; and class VI major
tissue loss, comprising 6 (20%) patients. Group 3 (acute limb
ischemia) was subdivided into classes IIa, ‘marginally threatened’,
comprising 2 (6.7%) patients, and IIb, ‘immediately threatened’,
comprising 4 (13.3%) patients.
 | Table IIClinical data of 30 limbs with 32
PrAAs. |
Table II
Clinical data of 30 limbs with 32
PrAAs.
| Clinical data | Limbs with
PrAA |
|---|
| Group 1 | 6 (18.8) |
|
Asymptomatic | 6 |
| Group
2a | 20 (62.5) |
|
I Mild
claudication | 0 |
|
II Moderate
claudication | 3 |
|
III Severe
claudication | 4 |
|
IV Ischemic
rest pain | 2 |
|
V Minor
tissue loss | 5 |
|
VI Major
tissue loss | 6 |
| Group
3a | 6 (18.8) |
|
I
Viable | 0 |
|
IIa
Marginally threatened | 2 |
|
IIb
Immediately threatened | 4 |
|
III
Irreversible | 0 |
Intraoperative findings
Intraoperative measurements were used when recorded;
12 PrAAs (12/32, 37.5%) had a thrombus. After the initial round of
imaging and investigations, a runoff was found in 12/32 (37.5%) of
the cases.
PAAs comprised 23 (71.8%) patients; PAA was the most
prevalent PrAA. After PAA, the most common aneurysms were CFAAs,
comprising 4 (12.5%) patients; aneurysms of the brachial artery,
comprising 2 (6.25%) patients; SFAAs, affecting 1 (3.1%) patient;
PTAAs, affecting 1 (3.1%) patient; and arteries of the subclavian
and axillary, affecting 1 (3.1%) patient. CTA was performed in 22
cases (73.3%). There was at least one remote aneurysm in 4 (13.2%)
patients, and 2 (6.7%) of these were afflicted bilaterally.
The diameter of the aneurysm was measured with
ultrasound in 15 affected patient limbs and a CTA scan in 16 limbs.
The mean aneurysmal diameter on Doppler ultrasound was
3.4250±1.06355 cm (range: 2.20-6 cm), and on CTA it was
3.4167±1.00438 cm (range: 2.00-5.9 cm).
Current or previous deep vein thrombosis (DVT) was
present in two limbs (6.6%) (P=0.262). In both limbs, the aneurysm
was in the popliteal artery. Furthermore, both affected patients
were in Group 2. Neither patient had an aneurysm rupture. An
association was observed between AAAs and PrAAs, with two patients
observed to have concomitant AAAs and PAAs (Table III).
 | Table IIIPeripheral arterial aneurysms with
AAA association and deep vein thrombosis. |
Table III
Peripheral arterial aneurysms with
AAA association and deep vein thrombosis.
| | AAAa | |
|---|
| Variable | Not present | Present | ANOVA test | P-value |
|---|
| Deep vein
thrombosis | | | 0.217 | 0.262 |
|
No | 30 (93.8) | 29 (90.6) | | |
|
Yes | 2 (6.6) | 3 (9.37) | | |
| Diseased
artery | 2 (6.3) | 0 (0.0) | 4.523 | 0.649 |
|
Common
femoral artery | 4 (12.5) | 1 (3.1) | | |
|
Popliteal
artery | 23 (71.8) | 2 (6.3) | | |
|
Posterior
tibial artery | 1 (3.1) | 0 (0.0) | | |
|
Superficial
femoral artery | 1 (3.1) | 0 (0.0) | | |
|
Subclavian
and axillary arteries | 1 (3.1) | 0 (0.0) | | |
All 32 PrAAs were repaired by interposition graft
(endoaneurysm repair). A 1st rib resection was required in one
case. The medial popliteal approach was used for all isolated PAAs
(n=20, 66.7%). Laparotomy and medial approaches were used for PAAs
associated with AAA (n=2, 6.7%). The longitudinal approach in the
femoral triangle or cubital fossa was used for 7 (23.3%) cases,
including CFAAs (n=4, 13.3%), SFAAs (n=1, 3.3%) and brachial artery
aneurysms (n=2, 6.7%). The supraclavicular longitudinal approach
was used for the only subclavian artery aneurysm (n=1, 3.1%). No
significant association was present among the different approaches,
P=0.215 (Table IV). The conduits
used were autogenous GSV (n=21, 70.0%), or prosthetic grafts,
including Dacron (n=3, 10.0%) or expanded PTFE (ePTFE) (n=6, 20.0%)
(Table IV).
 | Table IVSurgical techniques and early
postoperative outcomes (30 days post-operation). |
Table IV
Surgical techniques and early
postoperative outcomes (30 days post-operation).
| Item | Group 1 (n=7) | Group 2 (n=19) | Group 3 (n=6) | Total | ANOVA test | P-value |
|---|
| Surgical technical
approach | | | | | 6.740 | 0.298 |
|
Laparotomy
and medial approach | 1 (20.0) | 1 (5.3) | 0 (0.0) | 2 (6.7) | | |
|
Longitudinal
approach in the femoral triangle or cubital fossa | 0 (0.0) | 4 (26.3) | 2 (33.3) | 6 (23.3) | | |
|
Medial
approach to popliteal artery | 4 (60.0) | 15 (68.4) | 4 (66.7) | 23 (66.7) | | |
|
Supraclavicular
and longitudinal approach | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | | |
| Procedure | | | | | 3.834 | 0.167 |
|
Repair | 4 (80.0) | 19 (100.0) | 6 (100.0) | 29 (96.7) | | |
|
Repair and
bypass | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | | |
| Graft type | | | | | 6.148 | 0.112 |
|
Great
saphenous vein | 5 (100.0) | 12 (63.2) | 4 (66.7) | 21 (70.0) | | |
|
Dacron | 0 (0.0) | 1 (5.3) | 2 (33.3) | 3 (10.0) | | |
|
Expanded
polytetrafluoroethylene | 0 (0.0) | 6 (31.6) | 0 (0.0) | 6 (20.0) | | |
| Duration,
minutes | 152.0±23.9 | 148.9±28.1 | 160.0±16.7 | - | 0.422 | 0.662 |
| Amount of blood
lost, ml | 740.0±181.7 | 194.5±44.6 | 245.8±100.3 | - | 0.346 | 0.710 |
| Hospitalization
time, days | 1.8±0.81 | 3.98±0.94 | 1.83±0.74 | - | 0.672 | 0.521 |
| Surgical site
infection | | | | | 4.282 | 0.126 |
|
No | 4 (80.0) | 19 (100.0) | 5 (83.3) | 28 (93.3) | | |
|
Yes | 1 (20.0) | 0 (0.0) | 1 (16.7) | 2 (6.7) | | |
| Bleeding | | | | | NS | |
|
No | 5 (100.0) | 18 (100.0) | 6 (100.0) | 29 (100.0) | | |
|
Yes | 0 (0.0) | 1 (3.3) | 0 (0.0) | 1 (3.3) | | |
| Early
post-operative ischemia | | | | | 7.237 | 0.126 |
|
48 h
post-operative graft occlusion | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (3.3) | | |
|
ALI on 3rd
day post-operation | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | | |
|
No signs of
distal ischemia were noticed | 4 (80.0) | 19 (100.0) | 5 (83.3) | 28 (93.3) | | |
|
post-operatively,
no more rest pain | | | | | | |
| Systemic
complication, n (%) | | | | | 1.311 | 0.509 |
|
No | 4 (80.0) | 17 (89.5) | 6 (100.0) | 27 (90.0) | | |
|
Yes | 1 (20.0) | 2 (10.5) | 0 (0.0) | 3 (10.0) | | |
| Total number of
treated aneurysms | 6 | 20 | 6 | 32 | | |
Post-operative outcome
In the first 30 days, systemic complications
occurred in 3 patients. Group 2 suffered the most complications
(9.37%, 3/32). Within Group 2, 2 patients suffered from anterior
acute coronary syndrome. The first patient suffered an acute
anterior myocardial infarction (MI), which was treated by coronary
catheterization and stenting at 1 week postoperatively. The second
patient suffered an anterior MI, which was treated conservatively.
Within Group 1, patient 3 suffered a chest infection following the
second operation. On post-operative day 3, the patient also
developed acute limb ischemia (ALI), which was treated by revision
of the anastomosis and distal embolectomy (Table IV). In addition, in the first 30
days, two patients developed ALI post-operatively (2/32, 6.25%,
P=0.055). Patient 1 was from Group 3, whose first presentation was
acute limb ischemia (immediately threatened), and developed
thrombosis of the graft (Dacron graft was used). Revision and
embolectomy were performed 48 h post-operatively. The second
patient from Group 1 developed ALI, which was treated by revision
of the anastomosis and distal embolectomy (Table IV).
Mean ICU and hospital stays were 3-8 days in Group
1, 3-15 days in Group 2 and 5-0 days in Group 3 (P=0.521) (Table IV). In the first 30 days
post-operation, 2 patients developed surgical site infections
(SSIs). Specifically, a patient from Group 1 developed superficial
SSI treated by antibiotics, and the other patient from Group 3
developed deep SSI treated by repeated debridement (Table IV).
The 2-year primary patency rate was 26/32 (81.25%),
and the overall secondary patency rate was 28/32 (87.5%). For GSV
graft, the primary patency was 19/21 (90.47%) and the secondary
patency was 20/21 (95.23%). Primary and secondary patency of the
synthetic conduit (Dacron) graft were 1/3 (33.33%) and 2/3
(66.66%), respectively, and for ePTFE grafts, primary and secondary
patency were 6/6 (100%) (Fig.
1).
No patients underwent amputation in the first 30
days. There were no deaths up to the 30-day post-operative period.
To salvage a failing operation (n=2), two reinterventions were
performed in the first 30 days. During follow-up (first 30 days
post-operation), 2 patients underwent two salvage operations. The
first had acute ischemia, and the second had chronic ischemia with
rest pain. At long-term follow-up, two grafts became occluded.
Patients who underwent endoaneurysmorrhaphy during the first
operation did not receive re-intervention in 100% of cases.
Overall limb loss was 2/32 (6.25%), and in Group 2,
it was 10.5% (2/19) (Table VI and
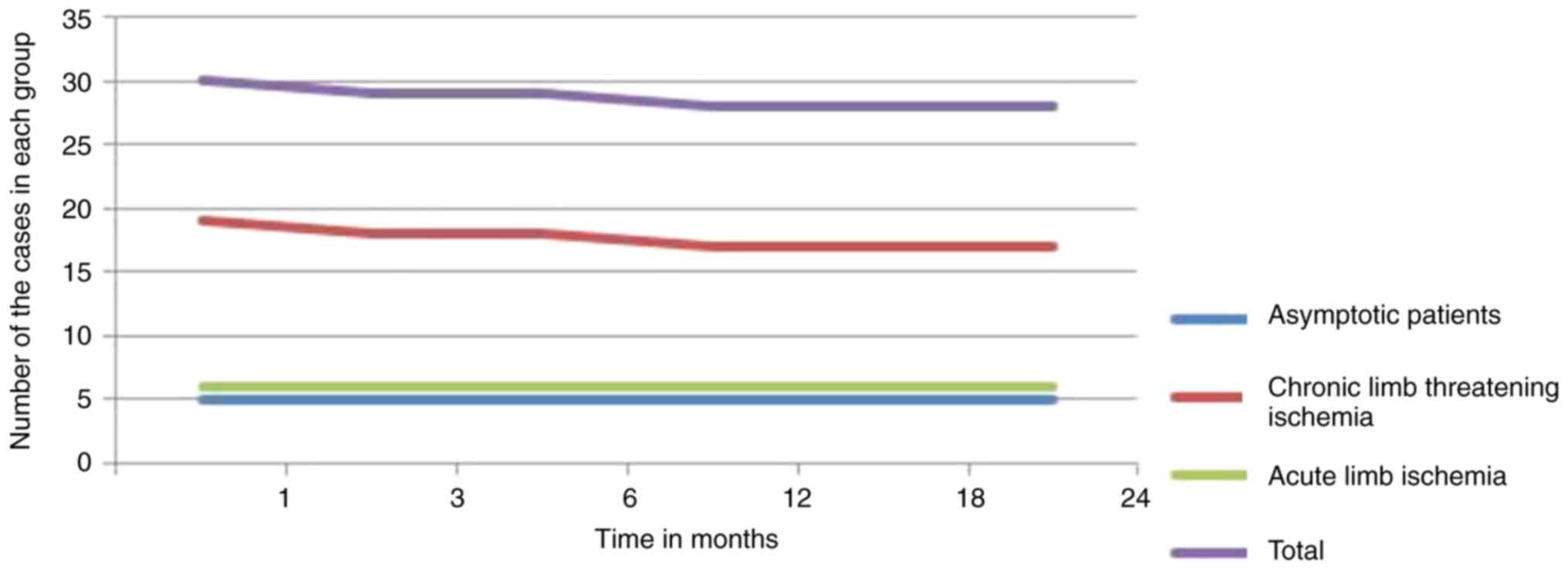
Fig. 1). The graph shows the graft
patency over time in the 3 groups. The rate of patency was
comparable over time. The 2-year cumulative limb salvage rate was
100% for Group 1, 89.5% for Group 2 and 100% for Group 3 (Table VI). Patients with GSV grafts had
less limb salvage at 2 years than those with PTFE grafts (90.5% vs.
100%, P=0.632). In Group 3, the 2-year limb salvage with GSV and
Dacron grafts was 100%. There was no limb loss in Group 1 and none
in Group 3 (GSV, Dacron, ePTFE grafts used for the bypass).
 | Table VIAssociation between graft type and
amputation, and graft patency. |
Table VI
Association between graft type and
amputation, and graft patency.
| | Graft type | |
|---|
| Item | Dacron (n=3) | Expanded
polytetrafluoroethylene (n=6) | Great saphenous
vein (n=21) | Total (n=32) | P-value |
|---|
| Amputation | | | | | 0.999 |
|
No | 3 (100.0) | 6 (100.0) | 19 (90.5) | 28 (93.3) | |
|
Yes | 0 (0.0) | 0 (0.0) | 2 (9.5) | 2 (6.7) | |
| Graft patency | | | | | 0.517 |
|
Early graft
failurea | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (3.3) | |
|
No
patency | 0 (0.0) | 0 (0.0) | 1 (4.8) | 1 (3.3) | |
|
Patency | 3 (100.0) | 5 (83.3) | 20 (95.2) | 28 (93.3) | |
In Group 2, two amputations, consisting of an
above-knee amputation and one distal phalanx amputation, were
performed. The patient who required an above-knee amputation had
comorbid hypertension and diabetes mellitus with moderate
intermittent claudication; they were treated with GSV
endoaneurysmorrhaphy. At 12 months post-operation, the patient
presented with acute irreversible ischemia. A total of 1 patient
succumbed at 3 months post-operation from acute coronary syndrome;
they were from Group 2 and had been implanted with an ePTFE graft
(Table V).
 | Table VEarly and late post-operative
outcomes. |
Table V
Early and late post-operative
outcomes.
| Item | Group 1 (n=7) | Group 2 (n=19) | Group 3 (n=6) | Total | ANOVA test | P-value |
|---|
| AAA | | | | | 1.311 | 0.509 |
|
Not
present | 4 (80.0) | 17 (89.6) | 6 (100.0) | 27 (90.0) | | |
|
Present | 1 (20.0) | 2 (10.5) | 0 (0.0) | 3 (10.0) | | |
| Vein
thrombosis | | | | | 0.801 | >0.999 |
|
No | 5 (100.0) | 17 (89.5) | 6 (100.0) | 28 (93.3) | | |
|
Yes | 0 (0.0) | 2 (10.5) | 0 (0.0) | 2 (6.7) | | |
| Amputation | | | | | 0.801 | >0.999 |
|
No | 5 (100.0) | 17 (89.5) | 6 (100.0) | 28 (93.3) | | |
|
Yes | 0 (0.0) | 2 (10.5) | 0 (0.0) | 2 (6.7) | | |
| Death | | | | | 0.164 | >0.999 |
|
No | 5 (100.0) | 18 (94.7) | 6 (100.0) | 29 (96.7) | | |
|
Yes | 0 (0.0) | 1 (5.3) | 0 (0.0) | 1 (3.3) | | |
| Graft type | | | | | 6.148 | 0.112 |
|
Dacron | 0 (0.0) | 1 (5.3) | 2 (33.3) | 3 (10.0) | | |
|
ePTFE | 0 (0.0) | 6 (31.6) | 0 (0.0) | 6 (20.0) | | |
|
Great
saphenous vein | 5 (100.0) | 12 (63.2) | 4 (66.7) | 21 (70.0) | | |
| Graft
patencya | | | | | 2.370 | >0.999 |
|
Early
failure of the graft | 0 (0.0) | 1 (5.3) | 0 (0.0) | 1 (3.3) | | |
|
No patency
(late) | 0 (0.0) | 1 (5.3) | 0 (0.0) | 1 (3.3) | | |
|
Patency | 5 (100.0) | 17 (89.5) | 6 (100.0) | 28 (93.3) | | |
Discussion
The present study aimed to examine the existing
evidence concerning the natural progression and treatment of PrAAs
and, more precisely, PAAs. It also aimed to apply decision theory
techniques to establish the circumstances in which conservative
management would be considered suitable. The present study
confirmed the effectiveness of open surgical repair, which aligns
with findings from previous studies that reported similar patency
rates and minimal complications (12,14-16).
In the present study, PrAA rupture was not observed,
and the most frequent primary complication observed was
thromboembolism leading to acute limb ischemia. Notably, patients
with PAAs exhibited an increase in local pressure on the popliteal
vein, thus increasing the risk of popliteal vein thrombosis. This
risk factor was associated with aneurysm size, with both PAA cases
in this study measuring 3.6x3.2 cm. In addition, both patients in
Group 2 presented with chronic limb ischemia, including major
tissue loss. Furthermore, ~6.7% of the patients had experienced
DVT, either during the study or preceding it. These findings are
partially comparable with a number of other studies (12-14).
For asymptomatic patients and patients with
mild-to-moderate claudication, the indication for intervention is
less clear. Other factors that influence surgical decision-making
are the development of symptoms, aneurysm size, mural thrombi,
evidence of previous thromboembolism and anatomical risks of
surgical repair (15,17).
The findings of Huang et al (17) suggested that thromboembolism may be
the predominant complication in cases of PAA, while rupture remains
rare. This previous study emphasized that the heightened risk of
popliteal vein thrombosis was due to local compression, with 6% of
the participants in the study having a history of current or
previous DVT. Michaels and Galland (18) used Markov decision analysis to
determine the ideal management of PAAs. This previous study
indicated that conversative management could be used for
asymptomatic patients, as the rate of aneurysmal enlargement was
<10% per year and they were less likely to suffer adverse
events.
A retrospective analysis (19,20) of
popliteal aneurysms suggested that small aneurysms (≤2 cm) may not
be as benign as previously considered. Despite their small size,
these aneurysms may be lined with thrombi that can embolize the
infra-popliteal arteries. Notably, 64% of the small aneurysms in
these two series were partially thrombosed, which was not
significantly different from the incidence of thrombosis in larger
aneurysms (70%). In addition, complete thrombosis of the popliteal
aneurysm was not associated with aneurysm size in the present
series. Similar findings were obtained in the case series of the
present study.
A review of 12 publications revealed that the
development of symptoms was at an average of 14% per year (range,
5-24%) (18). In a study conducted
by Lowell et al (20),
asymptomatic patients with PAAs were monitored without surgical
intervention, with an average follow-up duration of 17 months.
During this timeframe, 18% of the patients exhibited symptoms. Risk
factors associated with the development of symptoms included
aneurysm size exceeding 2 cm, poor runoff and the presence of a
mural thrombus (P<0.05).
Treatment for PAAs has become more aggressive to
prevent limb loss. The current literature on PAAs has been limited
primarily to surgical series, with a minor description of patients
treated conservatively (21,22).
Following PAAs, FAAs represent the most prevalent PrAAs. These
aneurysms can occur bilaterally and are commonly associated with
additional arterial aneurysms, particularly those in the aortic
region. It is recommended that symptomatic FAAs larger than 3 cm
undergo treatment (23). In the
upper extremities, aneurysmal development at the level of the
brachial and radial arteries is most commonly secondary to trauma,
invasive interventions and atrioventricular fistulae for
hemodialysis. True aneurysms of the subclavian artery are uncommon,
accounting for only 0.1% of all PrAAs. In addition, post-stenotic
dilatation or atherosclerotic degeneration serve a role in their
etiology (24-26).
In the present study, the size of PrAAs measured by
duplex scan (3.4±1.06 cm) emerged as a critical risk factor for
thrombus formation. The significance of thrombus formation size
indicated that even small aneurysms pose a heightened risk of
thrombus development, leading to limb ischemia and the potential
for amputation. Consequently, in the present study, even
asymptomatic patients underwent surgical repair as a preventive
measure to avert limb loss. Notably, no notable difference was
detected in the diameter of PrAAs when assessed using duplex
ultrasound and CTA, both yielding a mean measurement of 3.4±1.06355
cm. Consequently, neither method demonstrated superiority in size
measurement.
Others have also reported a heightened incidence of
complications in this particular group (patients with acute limb
ischemia) (27,28). However, it is worth noting that one
publication covering 51 patients, including 14 who underwent
emergency repair, indicated no instances of early mortality or
cardiac complications (28,29). In the series of the present study,
acute ischemia was associated with an 8% risk of early limb loss
and a 15% risk of late limb loss.
In another study, the preferred graft was a GSV,
which was implanted through a medial approach. The GSV is
advantageous due to the ease of vein harvesting and convenient
access to the anastomotic sites to ensure optimal inflow and
outflow. In two thirds of the cases, the femoral artery served as
the location for the proximal anastomosis, while the
infra-popliteal artery was utilized distally in a quarter of the
operations (30-32).
The potential benefits of employing tibial arteries for distal
anastomosis as needed have been underscored by a number of authors.
For all but small, thrombosed aneurysms, endoaneurysmorrhaphy
should be performed to prevent late recurrence, and this
recommendation extends to other PrAAs (33-35).
In our center, endoaneurysmorrhaphy was performed
for all patients. Re-intervention was observed in only two cases.
This approach demonstrated a notably high patency rate when
compared with alternative techniques observed in other studies.
Surgical intervention for upper-extremity aneurysms should be
initiated without delay. Risk factors combined with the minimal
morbidity associated with repair suggest that surgical repair
should be performed routinely for true upper extremity arterial
aneurysms (36,37).
In the present study, the follow-up for graft
patency was 2 years. All 6 patients with PTFE grafts had 100%
patency. In those with GSV grafts, primary patency was 90.47% and
secondary patency was 95.23%, which was significantly higher for
GSV compared with Dacron grafts. Dacron graft primary patency was
33.33% and secondary patency was 66.66%. The standard approach for
interposition grafting typically involves using a prosthetic graft,
with PTFE being the most commonly utilized material. The choice of
graft material has become a topic of some debate. This is because a
prosthetic graft, especially PTFE, is well suited in size to the
enlarged popliteal artery (32,36).
Additionally, a considerable number of patients with
PAAs exhibit good-to-excellent runoff, ensuring high flow and
prolonged patency of a prosthesis (17). In a study by Pulli et al
(29), no significant difference in
patency at 60 months was observed between 118 PTFE grafts (71.5%)
and 34 vein grafts (79.9%). However, it is worth noting that the
number of limbs at risk during this time interval was not
reported.
The present study is limited by its interpretations
being drawn from a single center only and further by its
retrospective analysis; however, the data were collected
prospectively. The limitations of the present study include the
single-center design and a relatively small sample size, which may
introduce bias. Additionally, the follow-up period of a minimum of
18 months might not capture long-term outcomes or complications.
Longer follow-up durations would provide more comprehensive
insights into the durability of the interventions and the
occurrence of late complications or recurrences.
Although general management guidelines as outlined
in the present study report were followed, the selection of graft
material, surgical technique, and indications for elective surgery
were at the discretion of the operating vascular surgeon. No
randomization was performed to evaluate different techniques. The
present study supports the efficacy of open surgical repair,
particularly endoaneurysmorrhaphy, in achieving high primary and
secondary patency rates, and low rates of limb loss and mortality.
These findings suggest that open repair should remain a preferred
treatment option for PrAAs. Despite the challenges posed by acute
limb ischemia and systemic complications, early intervention and
meticulous follow-up contribute to reducing adverse events.
Moving forward, future research should focus on the
risk factors and pathophysiological mechanisms underlying PrAA
development, and evaluate the comparative effectiveness of
endovascular and open surgical approaches in larger, multi-center
studies. Future research should focus on identifying specific risk
factors and pathophysiological mechanisms underlying PrAA
development, as well as comparing the long-term outcomes of
endovascular vs. open surgical approaches in a larger, multi-center
cohort.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RSS assisted in surgeries, follow-up, data
collection, study design and statistical analysis. AYI performed
manuscript revision and statistical analysis. AB was the surgeon in
charge of the present study design, performed follow-up, collected
the data, revised the manuscript and performed statistical
analysis. RSS and AB confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients to participate in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnston KW, Rutherford RB, Tilson MD,
Shah DM, Hollier L and Stanley JC: Suggested standards for
reporting on arterial aneurysms. Subcommittee on Reporting
Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting
Standards, Society for Vascular Surgery and North American Chapter,
International Society for Cardiovascular Surgery. J Vasc Surg.
13:452–458. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sokhal BS, Ma Y and Rajagopalan S: Femoral
artery aneurysms. Br J Hosp Med (Lond). 83:1–10. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Corriere MA and Guzman RJ: True and false
aneurysms of the femoral artery. Semin Vasc Surg. 18:216–223.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lai PMR, Akama-Garren E, Can A, Tirado SR,
Castro VM, Dligach D, Finan S, Gainer VS, Shadick NA, Savova G, et
al: Family history as the strongest predictor of aortic and
peripheral aneurysms in patients with intracranial aneurysms. J
Clin Neurosci. 126:128–134. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim TI and Sumpio BE: Management of
asymptomatic popliteal artery aneurysms. Int J Angiol. 28:5–10.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kropman RH, van Santvoort HC, Teijink J,
van de Pavoordt HD, Belgers HJ, Moll FL and de Vries JP: The medial
versus the posterior approach in the repair of popliteal artery
aneurysms: A multicenter case-matched study. J Vasc Surg. 46:24–30.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hanson J, Etemady-Deylamy A, Frisby J,
D'Addario J, Smeds M, Chamberland R, Guo H and Abate G: Femoral
artery aneurysm with large hematoma from Pasteurella: Case report
and literature review. BMC Infect Dis. 22(170)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sagar J and Button M: Posterior tibial
artery aneurysm: A case report with review of literature. BMC Surg.
14(37)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morrissey NJ: Endovascular treatment of
peripheral arterial aneurysms. Mt Sinai J Med. 71:1–3.
2004.PubMed/NCBI
|
|
10
|
Rutherford RB, Baker DJ, Ernst C, Johnson
WK, Porter JM, Ahn S and Jones DN: Recommended standards for
reports dealing with lower extremity ischemia: Revised version. J
Vasc Surg. 26:517–538. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van der Heijden LLM, Marang-van de Mheen
PJ, Thielman L, Stijnen P, Hamming JF and Fourneau I: Validity of
routinely reported rutherford scores reported by clinicians as part
of daily clinical practice. Int J Angiol. 33:148–155.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khuri SF, Daley J, Henderson W, Hur K,
Demakis J, Aust JB, Chong V, Fabri PJ, Gibbs JO, Grover F, et al:
The Department of Veterans Affair's NSQIP: The first national,
validated, outcome-based, risk-adjusted, and peer-controlled
program for the measurement and enhancement of the quality of
surgical care National VA Surgical Quality Improvement Program. Ann
Surg. 228:491–507. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dzieciuchowicz Ł, Łukaszuk M, Figiel J,
Klimczak K, Krasiński Z and Majewski W: Factors influencing the
clinical course of popliteal artery aneurysm. Med Sci Monit.
15:CR231–CR235. 2009.PubMed/NCBI
|
|
14
|
Verikokos C, Karaolanis G, Doulaptsis M,
Kouvelos G, Kotzadimitriou A, Palla VV and Klonaris C: Giant
popliteal artery aneurysm: Case report and review of the
literature. Case Rep Vasc Med. 2014(780561)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tshomba Y, Papa M, Marone EM, Kahlberg A,
Rizzo N and Chiesa R: A true posterior tibial artery aneurysm - A
case report. Vasc Endovascular Surg. 40:243–249. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ascher E, Markevich N, Schutzer RW,
Kallakuri S, Jacob T and Hingorani AP: Small popliteal artery
aneurysms: Are they clinically significant? J Vasc Surg.
37:755–760. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang Y, Gloviczki P, Noel AA, Sullivan
TM, Kalra M, Gullerud RE, Hoskin TL and Bower TC: Early
complications and long-term outcome after open surgical treatment
of popliteal artery aneurysms: Is exclusion with saphenous vein
bypass still the gold standard? J Vasc Surg. 45:706–713; discussion
713-715. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Michaels JA and Galland RB: Management of
asymptomatic popliteal aneurysms: The use of a Markov decision tree
to determine the criteria for a conservative approach. Eur J Vasc
Surg. 7:136–143. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Douglas DA, Adelman MA, Esposito R and
Rockman C: Surgical repair of a left subclavian artery aneurysm
causing stenosis of a left internal mammary graft - A case report.
Vasc Endovascular Surg. 39:281–285. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lowell RC, Gloviczki P, Hallett JW Jr,
Naessens JM, Maus TP, Cherry KJ Jr, Bower TC and Pairolero PC:
Popliteal artery aneurysms: The risk of nonoperative management.
Ann Vasc Surg. 8:14–23. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bahcivan M, Keceligil HT, Kolbakir F and
Gol MK: Surgical treatment of peripheral artery aneurysms. Hellenic
J Cardiol. 51:37–41. 2010.PubMed/NCBI
|
|
22
|
Farber A, Angle N, Avgerinos E, Dubois L,
Eslami M, Geraghty P, Haurani M, Jim J, Ketteler E, Pulli R, et al:
The Society for Vascular Surgery clinical practice guidelines on
popliteal artery aneurysms. J Vasc Surg. 75 (1S):109S–120S.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guzzardi G, Natrella M, Del Sette B,
Petullà M, Fanelli G, Porta C, Carriero A and Laganà D:
Endovascular repair of popliteal artery aneurysms: An Italian
multicenter study. Radiol Med. 124:79–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pittathankal AA, Dattani R, Magee TR and
Galland RB: Expansion rates of asymptomatic popliteal artery
aneurysms. Eur J Vasc Endovasc Surg. 27:382–384. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Galland RB and Magee TR: Popliteal
aneurysms: Distortion and size related to symptoms. Eur J Vasc
Endovasc Surg. 30:534–538. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mahmood A, Salaman R, Sintler M, Smith SR,
Simms MH and Vohra RK: Surgery of popliteal artery aneurysms: A
12-year experience. J Vasc Surg. 37:586–593. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Antonello M, Frigatti P, Battocchio P,
Lepidi S, Cognolato D, Dall'Antonia A, Stramanà R, Deriu GP and
Grego F: Open repair versus endovascular treatment for asymptomatic
popliteal artery aneurysm: Results of a prospective randomized
study. J Vasc Surg. 42:185–193. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Stone PA, Armstrong PA, Bandyk DF, Keeling
WB, Flaherty SK, Shames ML, Johnson BL and Back MR: The value of
duplex surveillance after open and endovascular popliteal aneurysm
repair. J Vasc Surg. 41:936–941. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pulli R, Dorigo W, Troisi N, Innocenti AA,
Pratesi G, Azas L and Pratesi C: Surgical management of popliteal
artery aneurysms: Which factors affect outcomes? J Vasc Surg.
43:481–487. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rowlands C, Youssef S and Rajagopalan S:
Popliteal arterial aneurysms. Br J Hosp Med (Lond). 83:1–7.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kassem MM, Oropallo A and Gonzalez L:
Popliteal Artery Aneurysm. In: StatPearls (Internet). StatPearls
Publishing, Treasure Island, FL, 2024.
|
|
32
|
Gouny P, Bertrand P, Duedal V,
Cheynel-Hocquet C, Lancelin C, Escourolle F, Nussaumeand O and
Vayssairat M: Limb salvage and popliteal aneurysms: Advantages of
preventive surgery. Eur J Vasc Endovasc Surg. 19:496–500.
2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Henry M, Amor M, Henry I, Klonaris C,
Tzvetanov K, Buniet JM, Amicabile C and Drawin T: Percutaneous
endovascular treatment of peripheral aneurysms. J Cardiovasc Surg
(Torino). 41:871–883. 2000.PubMed/NCBI
|
|
34
|
Diwan A, Sarkar R, Stanley JC, Zelenock GB
and Wakefield TW: Incidence of femoral and popliteal artery
aneurysms in patients with abdominal aortic aneurysms. J Vasc Surg.
31:863–869. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Davidović LB, Marković DM, Pejkić SD,
Kovacević NS, Colić MM and Dorić PM: Subclavian artery aneurysms.
Asian J Surg. 26:7–11; discussion 12. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hudorović N, Lovričević I, Franjić DB,
Brkić P and Tomas D: True aneurysm of brachial artery. Wien Klin
Wochenschr. 122:588–591. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Beseth BD and Moore WS: The posterior
approach for repair of popliteal artery aneurysms. J Vasc Surg.
43:940–944; discussion 944-945. 2006.PubMed/NCBI View Article : Google Scholar
|