Introduction
Type 1 diabetes (T1D) is a chronic autoimmune
condition characterized by absolute insulin deficiency and
hyperglycemia. Chronic hyperglycemia poses the risk of serious
vascular complications, including diabetic kidney disease (DKD).
DKD is characterized by a gradual decline in kidney function and an
increased risk of advancing to end-stage renal disease (ESRD)
(1).
Glycated hemoglobin (HbA1c), a traditional marker of
long-term glycemic control, has been demonstrated to be one of the
main risk factors for DKD progression (1,2).
However, with the increasing use of continuous glucose monitoring
(CGM) in T1D management, other metrics of glucose control such as
time in range (TIR), coefficient of variation (CV), time above
range (TAR), time below range (TBR), glucose management indicator
(GMI), average glucose, estimated A1C, hypoglycemia events and
duration were identified to better characterize diabetes control.
Previous studies indicate that glucose variability (GV)
characterized by CV in CGM, or fluctuations of blood glucose
levels, play a significant role in long-term T1D outcomes (3,4). The
main mechanism, which is implicated in the increased risk of
complications associated with GV, is considered to be oxidative
stress (5-8).
Some research groups have identified associations
between HbA1c and fasting GV and DKD (9). However, HbA1c variability assessment
does not provide insight into daily and hourly glucose fluctuations
(10), which can be evaluated using
a CGM approach. Regrettably, there has been limited research on the
associations between DKD progression and CGM metrics or multiple
daily glucose measurements (11)
with conflicting results. For example, there is data from the
Diabetes Control and Complications Trial (DCTT) study reporting GV
computed from 7-point daily glucose measurement profiles that were
not linked to an increased risk of diabetic nephropathy in patients
with T1D (12). Conversely, in
advanced DKD in the dialysis setting, increased GV assessed by CGM
was observed in patients with T1D and T2D (13). Notably, ESRD represents the final
stage of DKD. In addition, dialysis may be associated with
variations in glycemic control during dialysis and non-dialysis
days (14,15). Another study reported associations
between lower TIR and composite microvascular complications (MVC;
presence of neuropathy, retinopathy, or nephropathy) in 515
subjects with T1D (16). However,
studies reporting CGM parameters in patients with different DKD
stages and linking CGM parameters with DKD progression in T1D are
lacking.
There are multiple reasons for increased GV in T1D.
Postprandial glucose levels are determined by the rate of nutrient
delivery into the intestine, absorption of nutrients from the small
intestine, and the metabolism of the absorbed nutrients by the
liver. Thus, GV might be influenced by the rate of gastric
emptying, chronic gastro-intestinal diseases, autonomic neuropathy
and errors in adjustment of insulin treatment (17). In addition, impaired kidney function
is a risk factor for hypoglycemia due to a possible impairment in
kidney gluconeogenesis (18) which
might impact GV and CGM results in general.
To summarize, it is currently uncertain whether
individuals with progressive DKD, including its early stages,
exhibit distinct CGM outcomes compared with those with T1D but
without DKD. It is argued that such information is crucial for
several reasons: Firstly, to investigate the relationship between
DKD progression and real-time glucose management; secondly, to
establish that patients with advancing diabetes-related
complications should be prioritized for CGM to enhance glucose
regulation and mitigate the advancement of complications; and
thirdly, to identify CGM profile characteristics of DKD for better
patient surveillance and identification of risk factors associated
with increased GV.
Recognizing this gap in research, the present study
aimed to explore the associations between CGM parameters and DKD
progression in patients T1D, for enhanced patient care and
management strategies for the future.
Materials and methods
Patient recruitment and ethical
approval
The present study is part of the longitudinal
LatDiane study, which commenced in 2013, and is a participant in
the international InterDiane consortium. LatDiane focuses on
recruiting adult patients with T1D who were diagnosed before the
age of 40, started insulin treatment within one year of diagnosis,
and have C-peptide levels <0.3 nmol/l. Patients with chronic
kidney disease unrelated to DKD are excluded. The evaluations are
performed based on the medical records of patients (19). Follow-up visits occur every three
years. The study protocol for the overall LatDiane study and the
sub-study reported herein have received approvals from the Latvian
Central Ethics Committee (Riga, Latvia) under permission nos.
01-29.1/3 (dated, 10.07.2013), Nr.A-17/19-10-17 (dated,
17.10.2019), and Nr. 01-29.1.2/927 (dated, 09.12.2020). Biobanking,
and sample storage were conducted following the procedures of the
Genome Database of the Latvian population and are detailed in
previous studies (20,21). The present study adheres to the
principles outlined in the 1964 Declaration of Helsinki and its
subsequent amendments. Prior to inclusion in the study, written
informed consent was obtained from all participants. Recruitment
for this study took place between October 1, 2021 and July 30,
2022, at the University of Latvia (Riga, Latvia). Information
regarding the study was disseminated through the University of
Latvia's website and social media channels. The inclusion criteria
for this study required a minimum T1D duration of 8 years and
available data on the progression of DKD, including at least three
measurements of serum creatinine and three measurements of
albuminuria between the baseline visit of the LatDiane study
(2013-2021) and the present study (Fig. S1). The rationale for selecting
participants with a minimum duration of diabetes of at least 8
years is outlined as follows: In T1D, the screening (yearly
albuminuria and serum creatinine measurements) for MVC including
DKD commences 5 years after the onset of diabetes (22), and in order to calculate the eGFR
slope and assess the eGFR decline, one of the metrics to define
whether DKD is progressing, at least 3 additional years are
required, thus totaling 8 years. The definitions for progressive
DKD were used as described in a study by Colombo et al
(23).
The exclusion criteria included pregnancy, a history
of inflammatory bowel disease (such as Crohn's disease or
ulcerative colitis) and celiac disease (detected through serum
transglutaminase IgA screening), as well as a recent acute
intestinal infection within 2 months, clinical signs of acute
inflammation, and fever. On the study day, patients underwent
investigations for the collection of anthropometric measures,
completion of questionnaires, CGM sensor installation and blood
sample collection.
Clinical investigation and monitoring
of diabetic complications and co-morbidities
Blood pressure readings were obtained for all
patients. Those with a systolic blood pressure of 140 mmHg (18.7
kPa) or higher, a diastolic blood pressure of 90 mmHg (12.0 kPa) or
higher, or a history of antihypertensive drug use were classified
as having arterial hypertension. Smoking status was determined
through self-reported data via a questionnaire, with the ‘smokers’
category including patients currently smoking at least one
cigarette daily.
The assessment of cardiovascular disease (CVD) and
diabetes-related complications, such as retinopathy, neuropathy,
and DKD, was conducted through a review of medical records. CVD was
defined based on a history of acute myocardial infarction, coronary
bypass or percutaneous transluminal coronary angioplasty, stroke,
amputation, or peripheral vascular disease. The determination of
albuminuria involved calculating the albumin-to-creatinine ratio
from two out of three collected morning spot urine samples. The
estimated glomerular filtration rate (eGFR) was calculated
according to the Chronic Kidney Disease Epidemiology Collaboration
(CKD-EPI) equation (24)
In this equation, the creatinine value is measured
in milligrams per deciliter (mg/dl), and the age is expressed in
years.
ESRD was defined as an eGFR <15 ml/min/1.73 m²,
requiring dialysis or kidney transplantation. Progressive DKD was
defined as an eGFR decline exceeding 3 ml/min/1.73 m² per year
utilizing at least three serum creatinine measurements, or an
increase in the stage of albuminuria during the follow-up period
(23).
Insulin resistance was estimated via the estimated
glucose disposal rate (eGDR) formula. The lower the eGDR, the
higher the insulin resistance.
eGDR=24.4-12.97 x Waist/Hips-3.39 x Hypertension-0.6
x HbA1c%
If the blood pressure was ≥140/90 mmHg and/or the
patient regularly took antihypertensive medication, hypertension
was indicated as 1; otherwise, it was indicated as 0(25).
Blood samples
Blood samples were collected through venipuncture
and, along with morning spot urine samples, were promptly sent to a
certified clinical laboratory for measurement of clinical
analytes.
Monitoring of compliance level to
diabetes self-management
Diabetes diaries were used to monitor compliance
levels. Patients were asked to document at least four capillary
blood glucose levels each day, carbohydrates consumed, insulin
doses administered, and other glucose-influencing factors for 14
days. Patients measured glucose levels at waking, before meals, 2 h
post-meal, and before bed, with an additional 2:00 a.m. check every
third day. The compliance level was assessed based on the number of
entries made for the necessary parameters over a 14-day period. The
compliance levels were categorized as follows:
• Low: <6 days with necessary entries
• Moderate: 6-10 days with necessary entries
• High: ≥10 days with necessary entries
CGM
FreeStyle Libre ProiQ Sensors (diagnostic or
‘blinded’ glucose sensors; Abbott GmbH) were used for CGM.
Participants had to wear the sensor for 14 days. Average glucose,
CV, estimated HbA1c, glucose management indicator (GMI), percentage
of TAR (%TAR), percentage of TIR (%TIR), percentage of TBR (%TBR),
and low glucose events were analyzed. TIR was defined as the
percentage (%) of time when glucose levels were between 3.9 and 10
mmol/l, according to the recommendations applicable to most
subjects with T1D (10).
Out of the 78 subjects initially recruited, 75 had
glucose sensor data after 14 days of CGM. Therefore, data on 75
subjects were analyzed.
Statistical analysis
Statistical analyses were performed using SPSS
version 27 (IBM Corp.), and statistical visualizations were created
using the open-source software R version 4.3.1(26), along with the ggplot2 package
version 3.5.1(27).
The one-sample Kolmogorov-Smirnov test was employed
along with histogram analysis to assess the normality of the
variables of the study. All continuous variables were found to
deviate from a normal distribution. Descriptive statistical
analyses were performed on all variables in the study. Categorical
variables were presented as counts and percentages of the total
samples of the study. Continuous variables were summarized using
median values and interquartile ranges (IQR). Univariate analyses
were conducted to compare socio-demographic variables between the
study groups. Categorical variables were analyzed using the
Chi-square test, and continuous variables were evaluated using the
Mann-Whitney U test.
Spearman's correlation analysis was conducted to
examine the relationships between CGM parameters, kidney and liver
function markers, body mass index (BMI), T1D disease duration, and
eGDR within each study group, with results presented as Spearman's
correlation coefficients (95% CI).
Multiple binary logistic regression models were
constructed to assess the association between DKD progression and
CGM parameters. Models were adjusted for clinically relevant
variables including sex, BMI, age, and diabetes duration and tested
for fit by performing the Hosmer-Lemeshow test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Description of the whole study
cohort
The present study included a sample of 75 patients
with T1D from the longitudinal LatDiane cohort. The cohort was
followed for a median duration of 7.46 years (6.50-8.16 years). The
majority of included subjects were women (n=44, 58.67%). The median
age of the participants was 44.00 years (34.00-53.00 years), and
their BMI was 24.50 kg/m2 (22.95-28.30
kg/m2). Most of the participants were non-smokers 41
(54.67 %), and 44 (58.67%) had hypertension. The median duration of
diabetes in the group was 25.00 years (16.50-33.00 years), and the
HbA1c level was 8.20% (7.30-9.55%). Among the subjects, 39 (52.00%)
had retinopathy, 22 (29.33%) had autoimmune thyroid disease, 14
(18.67%) had other autoimmune diseases, and 14 (18.67%) had CVD.
Out of the total subjects included in the study, 10 individuals
(13.33%) were found to have macroalbuminuria. Data are summarized
in Table SI. At the time of the
study, 28 subjects had progressive DKD.
Description of the study groups
The study groups, categorized based on the presence
of stable DKD or progressive DKD, did not differ in median age, sex
distribution, median BMI and median HbA1c. Subjects with
progressive DKD had a higher prevalence of hypertension (P=0.02)
and a longer history of diabetes (P=0.01) compared with patients
with stable DKD. Additionally, significant differences were
observed in the presence of retinopathy, CVD, and albuminuria
between the two groups (all P<0.01). Triglyceride levels were
higher in the progressive DKD group compared with the stable DKD
group (P=0.04). As anticipated, eGFR was lower in the progressive
DKD group compared with the stable DKD group (P=0.02). The dialysis
occurrence 5 (17.9%) and kidney transplantation occurrence 7
(25.0%), both markers of ESRD, were more frequent in the
progressive DKD group. Characteristics of the groups are summarized
in Table I.
 | Table ISocio-demographic and disease-related
data of the two study groups. |
Table I
Socio-demographic and disease-related
data of the two study groups.
|
Characteristics | Stable DKD
(N=47) | Progressive DKD
(N=28) | P-value |
|---|
| Male, n (%) | 22 (46.81%) | 9 (32.14%) | 0.21 |
| Age, years | 39.00
(31.50-51.00) | 48.00
(36.75-54.50) | 0.05 |
| Smoking, n (%) | 12 (25.53%) | 8 (28.57%) | 0.39 |
| Body mass index,
kg/m2 | 24.40
(22.95-27.90) | 25.40
(22.42-28.62) | 0.84 |
| Duration of
diabetes, years, | 23.00
(13.00-27.00) | 31.00
(19.25-37.00) | 0.01 |
| Hypertension, n
(%) | 23 (48.93%) | 21 (75.00%) | 0.02 |
| Retinopathy, n
(%) | 19 (40.43%) | 20 (71.43%) | <0.01 |
| Severe retinopathy,
n (%) | 16 (34.04%) | 18 (64.3%) | 0.01 |
| Autoimmune thyroid
disease, n (%) | 12 (25.4%) | 10 (35.7%) | 0.16 |
| Other autoimmune
diseases, n (%) | 6 (12.76%) | 8 (28.6%) | 0.10 |
| CVD, n (%) | 3 (6.38%) | 11 (39.3%) | <0.01 |
| Kidney
transplantation, n (%) | 0 (0.0%) | 7 (25.0%) | <0.01 |
| Dialysis, n
(%) | 0 (0.0%) | 5 (17.9%) | <0.01 |
| HbA1c | | | |
|
% | 8.00
(7.20-9.45) | 8.65
(7.62-9.75) | 0.14 |
|
mmol/mol | 64.00
(55.00-79.50) | 71.0
(60.75-81.78) | |
| eGFR ml/min/1.73
m2 | 101.54
(85.56-114.05) | 80.43
(40.78-100.58) | 0.02 |
| Albuminuria,
mg/mmol | 0.90
(0.45-1.85) | 6.48
(2.90-70.35) | <0.01 |
| High-density
lipoprotein cholesterol, mmol/l | 1.81
(1.49-2.14) | 1.74
(1.42-2.17) | 0.65 |
| Low-density
lipoprotein cholesterol, mmol/l | 2.70
(2.17-3.40) | 2.46
(1.79-3.39) | 0.40 |
| Total cholesterol,
mmol/l | 4.90
(4.17-5.58) | 4.98
(3.98-5.98) | 0.70 |
| Triglycerides,
mmol/l | 0.93
(0.65-1.37) | 1.32
(0.79-2.25) | 0.04 |
| Gamma-glutamyl
transferase U/l | 15.00
(12.00-21.50) | 20.00
(14.00-30.50) | 0.09 |
| Alanine
transaminase U/I | 23.00
(18.00-32.50) | 23.00
(16.50-30.00) | 0.68 |
| Aspartate
aminotransferase, U/I | 25.00
(19.00-31.00) |
26.50(19.25-35.50) | 0.36 |
| C-reactive protein,
mg/l | 0.89
(0.49-2.66) |
1.43(0.57-3.38) | 0.25 |
| Estimated glucose
disposal rate, mg/kg/min | 7.20
(4.50-9.25) |
4.90(3.75-8.33) | 0.08 |
CGM metrics and DKD
In total, 75 individuals had available CGM data
after sensor wear for 14 days. The median duration of CGM sensor
wear among T1D patients was 15 days (15.00-15.00 days). The cohort
exhibited a median average glucose level of 9.50 mmol/l (8.10-11.40
mmol/l), with a CV of 39.59% (35.67-43.67%). The GMI was 7.40%
(6.80-8.20%). The distribution of glucose levels revealed that the
median TAR was 43.00% (27.00-59.00%), TIR was 51.00%
(37.00-66.00%), and the TBR was 4.00% (2.00-10.00%). Low glucose
events were quantified at 9.00 (4.00-15.00), with the median
duration of 110 min (76.00-143.50 min). The estimated A1C for the
cohort was 7.60% (6.65-8.75%). Overnight hypoglycemia was prevalent
in 55 participants during the monitoring period, constituting
74.32% of the cohort. Data are summarized in Table SII.
The analysis of CGM data between the study groups
revealed that individuals with progressive DKD had higher average
glucose levels, while those with stable DKD exhibited lower results
(P=0.03). TAR was increased in individuals with progressive DKD
compared with stable DKD (P=0.05). Conversely, the stable DKD group
had a higher percentage of TIR compared with individuals with
progressive DKD (P=0.03). Individuals with progressive DKD had
longer median duration of low glucose events compared with those
with stable DKD (P=0.03). Furthermore, estimated A1C levels were
higher in the progressive DKD group compared with the stable DKD
group (P=0.02). Similarly, GMI was higher in the progressive DKD
group compared with the stable DKD group (P=0.03). The observed
differences across a range of factors are summarized in Table II.
 | Table IICGM results of the two study
groups. |
Table II
CGM results of the two study
groups.
| CGM | Stable DKD
(N=47) | Progressive DKD
(N=28) | P-value |
|---|
| Sensor days | 15.00
(15.00-15.00) | 15.00
(15.00-15.00) | 0.98 |
| Average glucose,
mmol/l | 8.90
(7.60-10.70) | 10.50
(8.82-13.10) | 0.03 |
| Coefficient of
variance, % | 39.30
(35.60-42.70) | 39.70
(35.70-45.80) | 0.52 |
| Glucose management
indicator, % | 7.10
(6.60-7.90) | 7.85
(7.10-8.90) | 0.03 |
| Time above range,
% | 37.00
(25.00-53.00) | 53.00
(35.50-68.75) | 0.05 |
| Time in range,
% | 56.00
(37.00-70.00) | 43.00
(29.75-55.50) | 0.03 |
| Time below range,
% | 5.00
(6.00-11.00) | 3.50
(1.00-7.75) | 0.38 |
| Low glucose events,
n | 11.00
(6.00-17.00) | 6.00
(4.00-14.00) | 0.09 |
| Average duration of
low glucose events, min | 95.00
(74.00-125.00) | 117.50
(87.25-171.75) | 0.03 |
| Estimated A1C,
% | 7.20
(6.30-8.40) | 8.20
(7.20-9.90) | 0.02 |
| Overnight
hypoglycemia, n (%) | 37 (78.7%) | 18 (66.7%) | 0.25 |
Compliance to diabetes self-management
and DKD
In total, 74 participants provided diabetes diary
data. To elucidate the distribution of compliance levels (low,
moderate, high) among the cohort a comprehensive analysis was
conducted. The findings revealed that 15 individuals (20.27%)
exhibited low levels of compliance, 14 individuals (18.92%)
demonstrated moderate compliance, while the majority, comprising 45
individuals (60.81%), exhibited high compliance. Furthermore, 32
patients (43.24%) identified hypoglycemia in <50% of instances,
22 patients (29.73%) recognized hypoglycemia in 50 to 70% of
occurrences, and 20 patients (27.03%) acknowledged hypoglycemia in
70 to 100% of instances. Recognized hypoglycemia in the cohort was
50.00% (31.25-73.75%) of all hypoglycemia cases registered in the
sensor. Data are summarized in Table
SIII. CV was significantly associated with hypoglycemia events
registered in the sensor (P<0.01) (Table SIV). Patients with overnight
hypoglycemia had a higher CV level (P<0.01) (Table SIV).
There were no differences in compliance levels and
recognition of hypoglycemia between the DKD study groups. Patients
with progressive DKD marked fewer hypoglycemia events in their
diary compared with those with stable DKD, but this difference was
not statistically significant (P=0.08). A comprehensive summary of
these findings is presented in Table
III, which provides a detailed breakdown of the results.
 | Table IIICompliance level of the two study
groups. |
Table III
Compliance level of the two study
groups.
| Parameters of
compliance | Stable DKD
(N=47) | Progressive DKD
(N=27) | P-value |
|---|
| Low compliance
level | 8 (17.0%) | 7 (25.9%) | 0.66 |
| Moderate compliance
level | 10 (21.3%) | 4 (14.8%) | |
| High compliance
level | 29 (61.7%) | 16 (59.3%) | |
| Recognition of
hypoglycemia in <50% of occurrences | 22 (46.8%) | 10 (37.0%) | 0.83 |
| Recognition of
hypoglycemia in 50-70% of occurrences | 11 (23.4%) | 11 (40.7%) | |
| Recognition of
hypoglycemia in 70-100% of occurrences | 14 (29.8%) | 6 (22.2%) | |
| Marked hypoglycemia
in diary | 6.00
(2.00-9.00) | 3.00
(1.00-6.00) | 0.08 |
Correlations and regression
analysis
CGM data, clinical markers and DKD
progression. Patterns of correlations between CGM metrics and
clinical variables were similar for several parameters and differed
in other cases between the groups.
Correlations in the whole study group are presented
in Table SIV. With regard to the
kidney markers, eGFR was weakly negatively correlated with the
median duration of low glucose events (ρ=-0.253; P<0.03) in the
whole study group (Table SIV).
However, eGFR slope and albuminuria were not statistically
significantly correlated with CGM metrics in any of the study
groups and neither in the whole cohort (Table SIV, Table SV and Table SVI).
The differences in the correlation patterns between
CGM metrics and clinical factors between both study groups is
highlighted in Fig. 1.
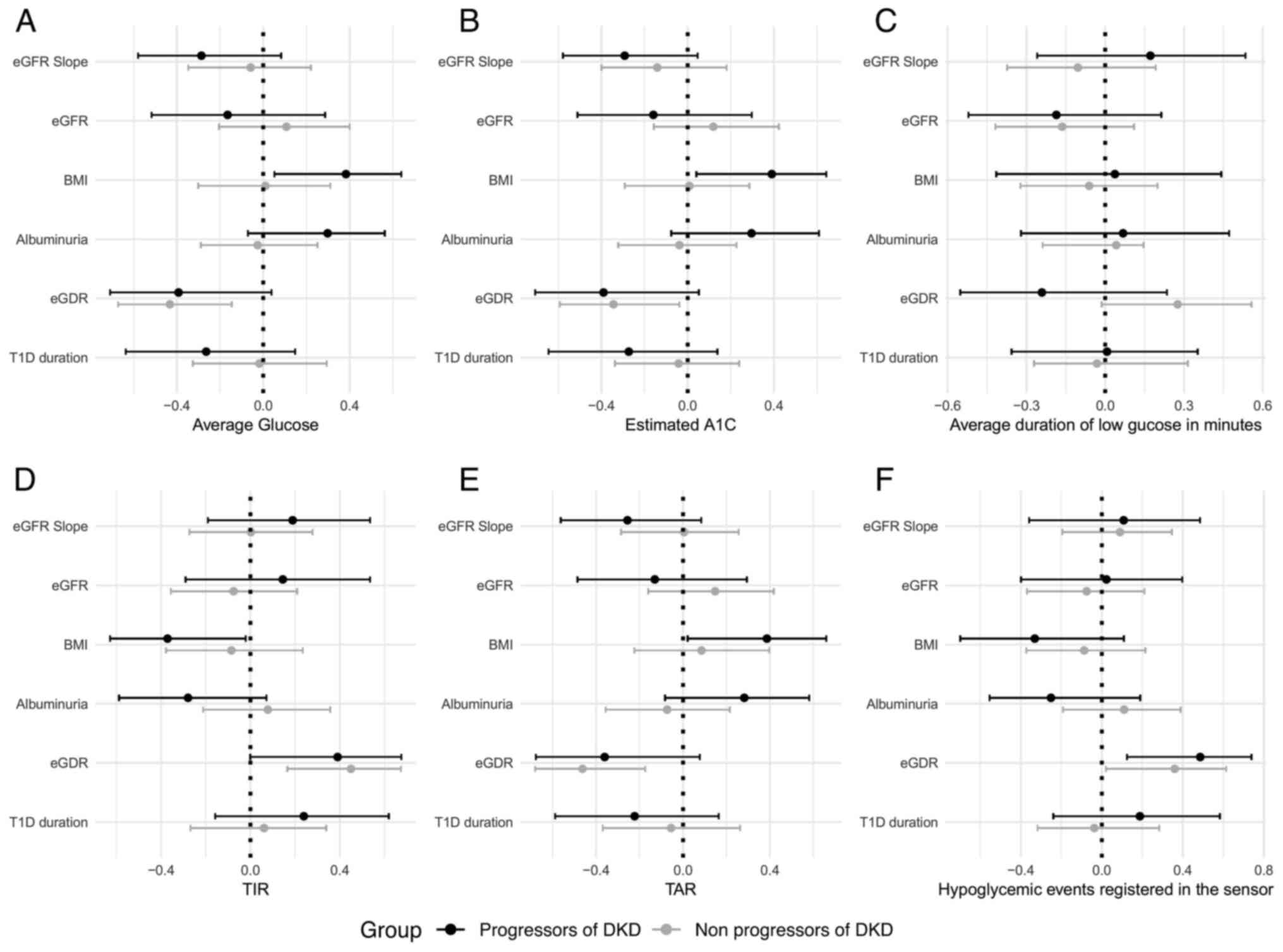 | Figure 1Forest plots demonstrating the
correlations between continuous glucose monitoring parameters,
kidney function markers, BMI, T1D duration, eGDR in both study
groups. Spearman's correlation coefficients (95% confidence level)
are illustrated by forest plots for (A) average glucose, (B)
estimated A1C, (C) average duration of low glucose in minutes, (D)
TIR, (E) TAR, and (F) hypoglycemic events registered in the sensor.
The eGFR slope was calculated as a regression line using at least
three eGFR calculations over the follow-up period (at least three
years). BMI, body mass index; T1D, type 1 diabetes; eGDR, estimated
glucose disposal rate; TIR, time in range; TAR, time above range;
eGFR, estimated glomerular filtration rate. |
Furthermore, in the group with progressive DKD, the
insulin resistance marker eGDR showed a moderate positive
correlation with the number of low glucose events (ρ=0.422; P=0.03)
and TIR (ρ=0.390; P=0.04), and statistically significant negative
correlations with average glucose (ρ=-0.392; P=0.04), GMI
(ρ=-0.406; P=0.03), and estimated A1c (ρ=-0.390; P=0.04) (Table SVI). Similar correlations were
observed in the group with stable DKD (Table SV). As regards the BMI, it was
negatively correlated with the number of low glucose events
(ρ=-0.507; P<0.01), positively correlated with TAR (ρ=0.386;
P=0.04), average glucose (ρ=0.383; P=0.04), GMI (ρ=0.406; P=0.03)
and estimated A1c (ρ=0.390; P=0.04) in subjects with progressive
DKD (Table SVI), but in the group
with stable DKD, statistically significant correlations between
these markers were not observed (Table
SV). This might indicate that being overweight impacts glucose
control at a greater degree in the case of DKD.
Several differences were identified in the
correlation patterns of CGM metrics in the study groups (Table SIV, Table SV and Table SVI). For example, CV was
significantly negatively correlated with average glucose (ρ=-0.433;
P=0.02) in the progressive DKD group. In addition, TIR was
significantly positively correlated with CV in the progressive DKD
group (ρ=0.403; P=0.03), but not in the stable DKD group (ρ=-0.138;
P=0.36) (Table SV and Table VI). Conversely, TAR and CV
exhibited a negative statistically significant correlation in the
progressive DKD group (ρ=-0.548; ρ<0.01; Table SVI), but not in the stable DKD
group (ρ=-0.002; P=0.99; Table
SV). These data indicated that the achievement of improved
diabetes control is linked to an increase in GV in subjects with
progressive DKD (Table SIV,
Table SV, Table SVI and Table SVII).
The present study explored cross-sectional
associations between glycemic indicators and the progression of DKD
(Table IV). Notably, a longer TIR
was associated with a significant 3% decrease in the odds of DKD
progression (P=0.04). Conversely, a higher TAR had a significant
2.5% increase in the odds of DKD progression (P=0.04). The average
duration of low glucose events were associated with a 0.8% increase
in the odds of progression for each additional minute (P=0.05),
indicating a statistically significant but borderline effect.
Elevated estimated A1C levels were significantly associated with a
39.5% increase in the odds of DKD progression (P=0.03). An increase
in the frequency of low glucose events was not significantly
associated with DKD progression (P=0.18). These findings highlight
the nuanced relationships between glycemic indicators and the
likelihood of DKD progression.
 | Table IVAssociation between the progression
of DKD and CGM parameters. |
Table IV
Association between the progression
of DKD and CGM parameters.
| Variable | Model | Hosmer-Lemeshow
test | OR | 95% CI | P-value |
|---|
| Time in range
(%) | 1 | 0.64 | 0.970 | 0.944; 0.998 | 0.04 |
| Time above range
(%) | 2 | 0.72 | 1.025 | 1.001; 1.050 | 0.04 |
| Average duration of
low glucose events (min) | 3 | 0.59 | 1.008 | 1.000; 1.016 | 0.05 |
| Estimated A1C | 4 | 0.99 | 1.395 | 1.040; 1.872 | 0.03 |
| GMI | 5 | 0.60 | 1.582 | 0.999; 2.505 | 0.05 |
| Low glucose
events | 6 | 0.72 | 0.954 | 0.890; 1.023 | 0.18 |
| Average
glucose | 7 | 0.53 | 1.217 | 0.998; 1.489 | 0.05 |
Discussion
In the present study, it was demonstrated that
subjects with T1D and progressive DKD have less controlled glycemia
as assessed by CGM in comparison to subjects with stable DKD.
Moreover, varying correlation patterns were identified between CGM
metrics and clinical markers in the study groups. Ultimately, it
was determined that CGM metrics were statistically significant
predictors of progressive DKD in the present study.
CGM metrics in the whole study cohort were quite
poor as compared with what the guidelines recommend (10), including a median CV of 39%, a TAR
of 43%, a median TIR of 51%, and overnight hypoglycemia prevalent
in 74,32% of the cohort. Notable differences in glucose management
were observed between patients with stable DKD and those with
progressive DKD. Particularly, patients with progressive DKD
demonstrated higher average glucose levels, and spent less TIR and
more TAR, as compared with subjects with stable DKD. An extended
duration of low glucose events were noted in patients with
progressive DKD, indicating an increased risk of
hypoglycemia-related complications. This finding underscores the
challenges of glycemic control as DKD progresses. Several factors
may be involved in this process. The patients in the progressive
DKD group had longer diabetes duration, a higher prevalence of CVD
and retinopathy, indicating more severe disease burden (28). As previously demonstrated by the
authors of the present study, the prevalence of diabetic
gastroenteropathy was higher in patients with progressive DKD
(29). This complication may lead
to changes in food passage through the gastrointestinal tract and
abnormal absorption of nutrients. Although not assessed in this
study, autonomic nervous system dysfunction which develops in
numerous patients with long-standing diabetes, may cause decreased
hypoglycemia awareness (30).
Altogether, the aforementioned factors may complicate insulin
dosing and lead to increased GV (17). On the other hand, patients with DKD
are more prone to hypoglycemia due to decreased gluconeogenesis in
the kidney, but intermittent hypoglycemia induces a hormonal surge,
leading to renal stress and exacerbating DKD (31-33).
Additionally, GV can contribute to endothelial dysfunction and
increased oxidative stress, key factors in DKD pathogenesis
(34,35). However, the present findings
indicate that achieving adequate glucose control is challenging in
subjects with progressive DKD. Indeed, it was shown that TIR was
significantly positively correlated with CV, while average glucose,
estimated A1C and GMI were negatively correlated with CV in
patients with DKD. At the same time, such a situation is not
observed in participants with stable DKD. That poses a therapeutic
challenge for glucose management in patients with DKD and
underlines the necessity for setting personalized glycemic targets
for subjects with DKD (10).
Due to the recent introduction of CGM into diabetes
care, only a few studies report data on correlations between CGM
metrics and diabetes complications, which underlines the novelty of
the present study. For example, associations were reported between
lower TIR and higher prevalence of composite MVC (presence of
neuropathy, retinopathy, or nephropathy) in 515 subjects with T1D
(16). In the aforementioned study
and in contrast to the findings of the present study, patients with
and without diabetic nephropathy did not significantly differ in
TIR. It is plausible that this finding resulted from the small
number of patients with nephropathy in the study (37 out of 550).
In a meta-analysis which reviewed studies devoted to an association
between CGM and diabetes complications (36), six studies addressed nephropathy and
only one study (37) included data
on MVC in T1D related to DKD. In this study (37) microalbuminuria was assessed in
conjunction with fundoscopy. Notably, only 16 out of 32 patients
were classified as having MVC, and these patients had significantly
higher GV calculated from CGM data compared with patients without
MVC. In contrast to the findings reviewed in previous studies
(36,37), the analysis in the present study
focused on CGM metric comparison between DKD groups (and not MVC as
a composite endpoint). The present study defined DKD progression as
a combination of decreased eGFR and increased albuminuria, and it
revealed the association between CGM metrics and progressing DKD,
using data from the longitudinal LatDiane study. It is stressed
that comparison of CGM results with regard to DKD, between studies
with patients with DKD in type 2 diabetes and T1D is not possible.
In addition to being two different diseases with varying
treatments, courses of disease and rates of complications, the
histological appearance and pathogenesis of DKD in these two
diabetes types differ (38-40).
In addition, there are numerous studies demonstrating associations
between HbA1c variability and DKD progression, but these data
cannot be compared with CGM results. A systematic review
consolidating data from 14 studies with over 62,000 participants,
demonstrated the link between high GV (assessed by HbA1c
variability) and increased incidence and progression of chronic
kidney disease, including a heightened risk of advancing to ESRD
(41,42).
Considering clinical factors that were identified as
being involved in glucose control in progressive DKD, higher BMI
was positively correlated with longer TAR, higher average glucose,
GMI and estimated A1C in subjects with progressive DKD in contrast
to participants with stable kidney markers. This may indicate that
being overweight impacts glucose control to a greater extent in the
case of DKD. Previous studies have reported associations between
metabolic syndrome, metabolic-associated fatty liver disease and
visceral obesity with DKD (43,44).
The present study also demonstrated a direct correlation between
insulin resistance parameter eGDR and TIR in both study groups,
indicating that high insulin sensitivity is linked to improved
glycemic control, in agreement with previous findings (45).
In addition, longer duration of hypoglycemic events
in DKD, identified in the present study as well as in other studues
(18,46), might contribute to weight gain, fear
of hypoglycemia and deterioration of glycemic control. On the other
hand, longer hypoglycemia poses additional stress on kidneys
through a hormonal surge, forming a vicious cycle between DKD
progression and deterioration of glycemic control (47,48).
Dialysis represents an additional risk factor for GV
in DKD due to abrupt changes in glucose and insulin concentrations
during and after the dialysis session (13). These factors may have contributed to
the poorer CGM metrics observed in the progressive DKD group. Among
the 28 participants, 12 had ESRD, with 7 having undergone kidney
transplantation and 5 receiving dialysis. Immunosuppressive
treatment may cause an increase in insulin resistance resulting in
poor glycemic control and CGM metrics (49).
Compliance to diabetes self-management can also
impact CGM results. Compliance might be compromised with advancing
diabetes due to fatigue caused by disease burden (28), impact of depression, anxiety, mild
cognitive impairment, dementia (50). However, differences in compliance to
diabetes self-management were not observed in the study groups
during the CGM period, except for a trend to fewer marked
hypoglycemia events in the progressive DKD group. Therefore, even
sufficient diabetes self-management is not enough to achieve
optimal glycemic control in patients with advancing DKD, and these
patients have reduced hypoglycemia awareness. These patients may
need more frequent structured diabetes education and sessions with
a diabetes nurse and nutritionist (51), and are definite candidates for CGM
(10,52). Unfortunately, numerous countries,
including Latvia, still do not provide an opportunity to use CGM
for all patients with T1D. The present study confirmed that
patients with progressive DKD should be prioritized for this
diabetes management method, along with children, pregnant
individuals, and subjects with frequent hypoglycemia (53). Indeed, it was demonstrated that
patients with ESRD resulting from DKD may improve glycemic control
due to CGM usage (49). Although
certain studies (54,55) indicate that the effectiveness of CGM
to restore hypoglycemia awareness in T1D varies due to individual
patient factors, CGM remains one of the most effective methods in
reducing hypoglycemia-related complications in T1D.
The cross-sectional nature of the present study
limits the ability to draw definitive causal conclusions as the
temporal sequence of events (for example, what came first-poor
daily glucose profiles or rapid progression of DKD), cannot be
established. The relatively small sample size, especially in the
progressive DKD group, might have influenced the results. Future
research with a larger and more diverse sample would further
strengthen the external validity of the present findings. In
addition, CGM may have accuracy limitations, such as the delay in
registering blood glucose changes in dynamic situations, however,
this aspect is not that crucial in the case of diagnostic CGM which
was used. By contrast, a rather short period of CGM (14 days) in
the present study might not be sufficient for a thorough assessment
of a daily glucose profile of a subject, constituting another
limitation. The strengths of the present study include examination
of CGM metrics in patients with different DKD progression statuses
(adding to markedly limited studies in the field), considering
diabetes self-management in the analysis of results, and
identification of differences in correlation patterns between CGM
metrics in subjects with stable and progressive DKD, which warrant
a personalized approach to CGM interpretation and establishment of
therapeutic targets in subjects with DKD.
To conclude, in the present study, the associations
between CGM metrics and DKD status in T1D were revealed. The
findings indicate the necessity for regular CGM in patients with
progressive DKD for improvement of their glycemic control and DKD
outcomes, but also call for the development of a personalized
approach to CGM data interpretation and establishing therapeutic
targets in these subjects.
Supplementary Material
Study recruitment flow diagram. T1D,
type 1 diabetes; eGFR, estimated glomerular filtration rate; CGM,
continuous glucose monitoring; DKD, diabetic kidney disease.
Socio-demographic and disease-related
data of the whole study cohort.
CGM results of the whole study
cohort.
Compliance level of the whole study
cohort.
Correlations in the whole study
group.
Correlations in the group with stable
DKD.
Correlations in the group with
progressive DKD.
Acknowledgements
The authors express their gratitude to Mrs Irena
Puzirevska from the Faculty of Medicine and Life Sciences,
University of Latvia (Riga, Latvia) for her invaluable contribution
to the coordination of recruitment.
Funding
Funding: Project Lzp-2020/1-0138 ‘Association between glucose
variability, intestinal disorders and progression of diabetic
nephropathy in type 1 diabetes patients’ funded the present
research conducted. This project provided the essential financial
support for data collection, analysis, and the overall study
framework. In addition, MikroTik supported the present research
through a scholarship administered by the University of Latvia
Foundation.
Availability of data and materials
The data generated in the present study are not
publicly available due to privacy and ethical restrictions.
However, access to the data may be requested from the corresponding
author, subject to appropriate ethical approvals.
Authors' contributions
AF and JS confirm the authenticity of all raw data.
AF, JJ, LK and JS contributed to the study design and methodology.
AF, LK and JJ performed the experiments. AF led the formal analysis
and data curation. JJ performed part of the statistical analysis.
JS provided conceptual guidance, supervision and obtained funding.
AF and JS interpreted the data and wrote the manuscript, with JJ
and LK contributing to the review and editing. AF performed the
statistical analysis. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study protocol of the general LatDiane and
sub-study devoted to continuous glucose monitoring described herein
were approved by the Latvian Central Ethics Committee and received
permission nos. 01-29.1/3 (dated 10.07.2013), Nr.A-17/19-10-17
(dated 17.10.2019), and Nr. 01-29.1.2/927 (dated 09.12.2020). In
compliance with ethical standards, written informed consent was
obtained from all individual participants involved in the
study.
Patient consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gupta S, Dominguez M and Golestaneh L:
Diabetic kidney disease: An update. Med Clin North Am. 107:689–705.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miller RG and Orchard TJ: Understanding
metabolic memory: A tale of two studies. Diabetes. 69:291–299.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou Z, Sun B, Huang S, Zhu C and Bian M:
Glycemic variability: Adverse clinical outcomes and how to improve
it? Cardiovasc Diabetol. 19(102)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
DeVries JH: Glucose variability: Where it
is important and how to measure it. Diabetes. 62:1405–1408.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Papachristoforou E, Lambadiari V, Maratou
E and Makrilakis K: Association of glycemic indices (hyperglycemia,
glucose variability, and hypoglycemia) with oxidative stress and
diabetic complications. J Diabetes Res.
2020(7489795)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wentholt IME, Kulik W, Michels RPJ,
Hoekstra JBL and DeVries JH: Glucose fluctuations and activation of
oxidative stress in patients with type 1 diabetes. Diabetologia.
51:183–190. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Colomo N, López-Siguero JP, Leiva I,
Fuentes N, Rubio-Martín E, Omiste A, Guerrero M, Tapia MJ,
Martín-Tejedor B, Ruiz de Adana MS and Olveira G: Relationship
between glucose control, glycemic variability, and oxidative stress
in children with type 1 diabetes. Endocrinol Diabetes Nutr (Engl
Ed). 66:540–549. 2019.PubMed/NCBI View Article : Google Scholar : (English,
Spanish).
|
|
8
|
Dimova R, Chakarova N, Grozeva G, Kirilov
G and Tankova T: The relationship between glucose variability and
insulin sensitivity and oxidative stress in subjects with
prediabetes. Diabetes Res Clin Pract. 158(107911)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wadén J, Forsblom C, Thorn LM, Gordin D,
Saraheimo M and Groop PH: A1C variability predicts incident
cardiovascular events, microalbuminuria, and overt diabetic
nephropathy in patients with type 1 diabetes. Diabetes.
58:2649–2655. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Battelino T, Danne T, Bergenstal RM, Amiel
SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL,
et al: Clinical targets for continuous glucose monitoring data
interpretation: Recommendations from the international consensus on
time in range. Diabetes Care. 42:1593–1603. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Purandare VB, Bale C, Kakrani A and
Unnikrishnan AG: Understanding glycemic variability. J Diabetology.
12:275–284. 2021.
|
|
12
|
Lachin JM, Bebu I, Bergenstal RM,
Pop-Busui R, Service FJ, Zinman B and Nathan DM: Association of
glycemic variability in type 1 diabetes with progression of
microvascular outcomes in the diabetes control and complications
trial. Diabetes Care. 40:777–783. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ling J, Ng JKC, Chan JCN and Chow E: Use
of continuous glucose monitoring in the assessment and management
of patients with diabetes and chronic kidney disease. Front
Endocrinol (Lausanne). 13(869899)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bomholt T, Rix M, Almdal T, Knop FK,
Rosthøj S, Jørgensen MB, Feldt-Rasmussen B and Hornum M: Glucose
variability in maintenance hemodialysis patients with type 2
diabetes: Comparison of dialysis and nondialysis days. Hemodial
Int. 27:126–133. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bushljetik IR, Trajceska L, Pusevski V and
Spasovski G: Glucose levels during dialysis with glucose-free
versus glucose-rich dialysate fluid. Prilozi. 40:41–46.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
El Malahi A, Van Elsen M, Charleer S,
Dirinck E, Ledeganck K, Keymeulen B, Crenier L, Radermecker R, Taes
Y, Vercammen C, et al: Relationship between time in range, glycemic
variability, HbA1c, and complications in adults with type 1
diabetes mellitus. J Clin Endocrinol Metab. 107:e570–e581.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kovatchev B: Glycemic variability: Risk
factors, assessment, and control. J Diabetes Sci Technol.
13:627–635. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Galindo RJ, Beck RW, Scioscia MF,
Umpierrez GE and Tuttle KR: Glycemic monitoring and management in
advanced chronic kidney disease. Endocr Rev. 41:756–774.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahola AJ, Radzeviciene L, Zaharenko L,
Bulum T, Skrebinska S, Prakapiene E, Blaslov K, Roso V, Rovite V,
Pirags V, et al: Association between symptoms of depression,
diabetes complications and vascular risk factors in four European
cohorts of individuals with type 1 diabetes-InterDiane Consortium.
Diabetes Res Clin Pract. 170(108495)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zamora-Ros R, Biessy C, Rothwell JA, Monge
A, Lajous M, Scalbert A, López-Ridaura R and Romieu I: Dietary
polyphenol intake and their major food sources in the Mexican
Teachers' Cohort. Br J Nutr. 120:353–360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sokolovska J, Dekante A, Baumane L,
Pahirko L, Valeinis J, Dislere K, Rovite V, Pirags V and Sjakste N:
Nitric oxide metabolism is impaired by type 1 diabetes and diabetic
nephropathy. Biomed Rep. 12:251–258. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
American Diabetes Association Professional
Practice Committee. 11. Chronic Kidney Disease and Risk Management:
Standards of Care in Diabetes-2024. Diabetes Care. 47 (Suppl
1):S219–S230. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Colombo M, Valo E, McGurnaghan SJ,
Sandholm N, Blackbourn LAK, Dalton RN, Dunger D, Groop PH, McKeigue
PM, Forsblom C, et al: Biomarker panels associated with progression
of renal disease in type 1 diabetes. Diabetologia. 62:1616–1627.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150(604)2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wadén J, Tikkanen H, Forsblom C, Fagerudd
J, Pettersson-Fernholm K, Lakka T, Riska M and Groop PH: FinnDiane
Study Group. Leisure time physical activity is associated with poor
glycemic control in type 1 diabetic women. Diabetes Care.
28:777–782. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
R Core Team: A language and environment
for statistical computing, 2023.
|
|
27
|
Wickham H: ggplot2: Create Elegant Data
Visualisations Using the Grammar of Graphics, 2024.
|
|
28
|
Griggs S and Morris NS: Fatigue among
adults with type 1 diabetes mellitus and implications for
self-management: An integrative review. Diabetes Educ. 44:325–339.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fedulovs A, Tzivian L, Zalizko P, Ivanova
S, Bumane R, Janeviča J, Krūzmane L, Krustins E and Sokolovska J:
Progression of diabetic kidney disease and gastrointestinal
symptoms in patients with type I diabetes. Biomedicines.
11(2679)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Williams S, Raheim SA, Khan MI, Rubab U,
Kanagala P, Zhao SS, Marshall A, Brown E and Alam U: Cardiac
autonomic neuropathy in type 1 and 2 diabetes: Epidemiology,
pathophysiology, and management. Clin Ther. 44:1394–1416.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yun JS, Park YM, Han K, Kim HW, Cha SA,
Ahn YB and Ko SH: Severe hypoglycemia and the risk of end stage
renal disease in type 2 diabetes. Sci Rep. 11(4305)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sagmeister MS, Harper L and Hardy RS:
Cortisol excess in chronic kidney disease-A review of changes and
impact on mortality. Front Endocrinol (Lausanne).
13(1075809)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hamilton A, Zhang Q, Salehi A, Willems M,
Knudsen JG, Ringgaard AK, Chapman CE, Gonzalez-Alvarez A, Surdo NC,
Zaccolo M, et al: Adrenaline stimulates glucagon secretion by
Tpc2-dependent Ca2+ mobilization from acidic stores in pancreatic
α-cells. Diabetes. 67:1128–1139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ceriello A, Esposito K, Piconi L, Ihnat
MA, Thorpe JE, Testa R, Boemi M and Giugliano D: Oscillating
glucose is more deleterious to endothelial function and oxidative
stress than mean glucose in normal and type 2 diabetic patients.
Diabetes. 57:1349–1354. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Costantino S, Paneni F, Battista R,
Castello L, Capretti G, Chiandotto S, Tanese L, Russo G, Pitocco D,
Lanza GA, et al: Impact of glycemic variability on chromatin
remodeling, oxidative stress, and endothelial dysfunction in
patients with type 2 diabetes and with target HbA1c levels.
Diabetes. 66:2472–2482. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yapanis M, James S, Craig ME, O'Neal D and
Ekinci EI: Complications of diabetes and metrics of glycemic
management derived from continuous glucose monitoring. J Clin
Endocrinol Metab. 107:e2221–e2236. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Šoupal J, Škrha J, Fajmon M, Horová E,
Mráz M, Škrha J and Prázný M: Glycemic variability is higher in
type 1 diabetes patients with microvascular complications
irrespective of glycemic control. Diabetes Technol Ther.
16:198–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gheith O, Farouk N, Nampoory N, Halim MA
and Al-Otaibi T: Diabetic kidney disease: World wide difference of
prevalence and risk factors. J Nephropharmacol. 5:49–56.
2016.PubMed/NCBI
|
|
39
|
Ohta M, Babazono T, Uchigata Y and Iwamoto
Y: Comparison of the prevalence of chronic kidney disease in
Japanese patients with Type 1 and Type 2 diabetes. Diabetic
Medicine. 27:1017–1023. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Marshall SM: Natural history and clinical
characteristics of CKD in Type 1 and Type 2 diabetes mellitus. Adv
Chronic Kidney Dis. 21:267–272. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bergenstal RM: Glycemic variability and
diabetes complications: Does it matter? Simply put, there are
better glycemic markers! Diabetes Care. 38:1615–1621.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Habte-Asres HH, Wheeler DC and Forbes A:
The association between glycaemic variability and progression of
chronic kidney disease: A Systematic Review. Compr Clin Med.
4(102)2022.
|
|
43
|
Jansson Sigfrids F, Lithovius R, Groop P
and Thorn LM: Lessons learned from the FinnDiane Study:
Epidemiology and metabolic risk factors for diabetic kidney disease
in type 1 diabetes. Diabet Med. (e15431)2024.PubMed/NCBI View Article : Google Scholar : doi:
10.1111/dme.15431 (Epub ahead of print).
|
|
44
|
Targher G, Mantovani A, Pichiri I,
Mingolla L, Cavalieri V, Mantovani W, Pancheri S, Trombetta M,
Zoppini G, Chonchol M, et al: Nonalcoholic fatty liver disease is
independently associated with an increased incidence of chronic
kidney disease in patients with type 1 diabetes. Diabetes Care.
37:1729–1736. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Clinck I, Mertens J, Wouters K, Dirinck E
and De Block C: Insulin resistance and CGM-Derived parameters in
people with type 1 diabetes: Are they associated? J Clin Endocrinol
Metab. 109:e2131–e2140. 2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Grube D, Wei G, Boucher R, Abraham N, Zhou
N, Gonce V, Carle J, Simmons DL and Beddhu S: Insulin use in
chronic kidney disease and the risk of hypoglycemic events. BMC
Nephrol. 23(73)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Amiel SA: The consequences of
hypoglycaemia. Diabetologia. 64:963–970. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bumbu A, Moutairou A, Matar O, Fumeron F,
Velho G, Riveline JP, Gautier JF, Marre M, Roussel R and Potier L:
Non-severe hypoglycaemia is associated with weight gain in patients
with type 1 diabetes: Results from the Diabetes Control and
Complication Trial. Diabetes Obes Metab. 20:1289–1292.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jakubowska Z and Malyszko J: Continuous
glucose monitoring in people with diabetes and end-stage kidney
disease-review of association studies and Evidence-Based
discussion. J Nephrol. 37:267–279. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
van Duinkerken E, Snoek FJ and de Wit M:
The cognitive and psychological effects of living with type 1
diabetes: A narrative review. Diabet Med. 37:555–563.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Szadkowska A, Czyżewska K, Pietrzak I,
Mianowska B, Jarosz-Chobot P and Myśliwiec M: Hypoglycaemia
unawareness in patients with type 1 diabetes. Pediatr Endocrinol
Diabetes Metab. 24:126–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Vloemans AF, van Beers CAJ, de Wit M,
Cleijne W, Rondags SM, Geelhoed-Duijvestijn PH, Kramer MHH, Serné
EH and Snoek FJ: Keeping safe. Continuous glucose monitoring (CGM)
in persons with Type 1 diabetes and impaired awareness of
hypoglycaemia: A qualitative study. Diabet Med. 34:1470–1476.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Friedman JG, Cardona Matos Z, Szmuilowicz
ED and Aleppo G: Use of continuous glucose monitors to manage type
1 diabetes mellitus: Progress, challenges, and recommendations.
Pharmgenomics Pers Med. 16:263–276. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rickels MR, Peleckis AJ, Dalton-Bakes C,
Naji JR, Ran NA, Nguyen HL, O'Brien S, Chen S, Lee I and Schutta
MH: Continuous glucose monitoring for hypoglycemia avoidance and
glucose counterregulation in long-standing type 1 diabetes. J Clin
Endocrinol Metab. 103:105–114. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Choudhary P, Ramasamy S, Green L, Gallen
G, Pender S, Brackenridge A, Amiel SA and Pickup JC: Real-time
continuous glucose monitoring significantly reduces severe
hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes.
Diabetes Care. 36:4160–4162. 2013.PubMed/NCBI View Article : Google Scholar
|















