Introduction
The most significant causes of different metabolic
diseases and malnutrition are consuming fast and contaminated food
(preservatives, pesticides, toxic metals), long-term use of drugs
and alcohol, causing problems in liver and kidney (1,2). The
liver is crucial for controlling the body's numerous physiological
and biochemical functions, including metabolism, secretion, the
delivery of nutrients and energy and vitamin storage (3,4). The
liver can detoxify endogenous or exogenous substances. Therefore,
it is very susceptible to exposure to toxic compounds from within
and outside the body, which can cause metabolic and liver diseases
(5,6).
The kidney is another vital organ with the main
function in the process of excretion. This organ has several
physiological functions, including maintaining homeostasis of body
fluids by filtering metabolites and minerals from the blood,
removing waste substances, playing a role in glucose metabolism,
erythropoiesis and regulating blood pressure, as well as producing
hormones and enzymes (7,8). The kidney filters ~180 liters of blood
per day, equivalent to four times the amount passing through other
organs. Therefore, this organ is very susceptible to exposure to
toxins in the blood that can damage the tissue and cause kidney
disease (9,10).
According to the World Health Organization, liver
disease accounts for >4% of global mortality (2 million
mortalities annually) (11) and
~10% of the world's population (850 million individuals) suffer
from kidney disease with 1.3 million mortalities each year
(12). Current pharmacological
treatment can alleviate various liver and kidney diseases according
to the main causes and delay the occurrence of end-stage liver and
renal failure. However, pharmacological treatment has not been able
to treat or restore liver and kidney function completely. Most
drugs cause liver and kidney damage to become severe and are
considered risk factors for the organs (13). In this context, alternative
treatments are needed to prevent or treat liver and kidney disease
(14). Empirically, medicinal
plants have long been used in a number of nations to cure and
prevent a wide range of illnesses (15,16).
Indonesia is the second-largest biodiversity with
28,000 plant species, comprising 2,500 medicinal plants (17,18).
In North Sumatra, traditional medicine frequently makes use of the
medicinal herb Castanopsis costata. Empirically, C.
costata leaves extract (CcE) is used to treat wounds,
inflammation, fever and to act as an analgesic (19). Previous research reported that CcE
has different pharmacological activities, such as antimalarial
(20), antidiabetic (21), antioxidant (22), antipyretic (22), antihyperlipidemic (23), antidiarrheal (24) and anti-inflammatory (25).
Medicinal plants with antioxidant, anti-inflammatory
and antihyperlipidemic activities have hepatoprotective effects,
such as in liver fibrosis and non-alcoholic fatty liver disease
(26-28).
Antioxidant, anti-inflammatory and antidiabetic activities also
have nephroprotective effects, such as in kidney fibrosis and
diabetic nephropathy (29-31).
Based on the results of previous research, CcE had antioxidant,
anti-inflammatory, antidiabetic and antihyperlipidemic activities.
This outcome validated the theory that the extract has
hepatoprotective and nephroprotective properties. Therefore, the
goal of the present study was to examine the hepatoprotective and
nephroprotective activities of CcE against paracetamol-induced
hepatotoxicity and gentamicin-induced nephrotoxicity in rat
models.
Materials and methods
Chemicals and drugs
Paracetamol, silymarin (MilliporeSigma), gentamicin
(PT. Bernofarm Pharmaceutical Company), 0.9% sodium chloride (PT.
Widatra Bhakti), diethyl ether (PT. Brataco), 10% formalin
solution, xylene, paraffin, 70% ethanol, diethyl ether,
hematoxylin-eosin stains, pulvis gummi arabicum and potassium
chloride (EMSURE®; Merck KGaA) were of analytical grade.
Kits for the estimation of total albumin (TA), aspartate
aminotransferase (AST), alanine aminotransferase (ALT), alkaline
phosphatase (ALP), total bilirubin (TB), total cholesterol (TC),
total protein (TP), serum creatinine (SCr), serum urea (SU), uric
acid (UA) were from PT. Wacana Indo Mitra, tumor necrosis factor
alpha (TNF-α), interferon gamma (IFN-γ) were from PT. Biolab
Science Universal) and superoxide dismutase (SOD), catalase (CAT),
glutathione peroxidase (GPx) and glutathione (GSH) were from
Sigma-Aldrich (Merck KGaA).
Sample collection, determination and
extraction of plants
A total of 10 kg of fresh C. costata leaves
were bought from the Pancur Batu traditional market in North
Sumatra, Indonesia in March 2022. The plant was identified at the
Herbarium Medanense, Universitas Sumatera Utara, Indonesia (voucher
number: 183/MEDA/2022). The cleaned C. costata leaves were
brought to Pharmacognosy Laboratory, Universitas Buana Perjuangan
Karawang for the extraction procedure. A total of 5 kg of C.
costata powder was macerated in 70% ethanol three times in 24
h. The liquid extract was gathered and concentrated at 50˚C using a
rotary evaporator (32).
Fourier-transform infrared
spectroscopy (FT-IR) analysis
Potassium bromide (KBr) pellets were mixed with CcE
and the results were evaluated with a Shimadzu IRPrestige-21 FT-IR
Spectrophotometer (Shimadzu Corporation). At a resolution of 4
cm-1, the spectra were collected in the 400-4,000
cm-1 range.
Randomization procedure and
blinding
For randomization, an identification number was
first assigned to each rat and then randomization was performed,
which generated random numbers and allocated rats to study groups.
Randomization was performed using online software (https://www.graphpad.com/quickcalcs/randomize1/).
Meanwhile, in blinding during the experiment, alphanumeric codes
were used to identify vials and syringes and each rat was given a
number. Then, each sample code was placed in a sealed envelope and
revealed at the end of the experiment.
Experimental animals
A total of 44 male Wistar rats in good health,
weighing 150-250 g and 8-12 weeks old, were employed in the
hepatoprotective and nephroprotective research. Rats were acquired
from CV. Mitra Putra Animal. The rats were kept at a 12-h
light/dark cycle in the Pharmacology Laboratory at Department of
Pharmacology and Clinical Pharmacy, Faculty of Pharmacy,
Universitas Buana Perjuangan Karawang with a temperature range of
20-26˚C and 30-70% humidity. In addition, the experimental animals
had unrestricted access to drinking water and normal pellets. The
human endpoints established for this study were deteriorating body
condition, weight loss, the inability to rise or ambulate and the
presence of labored respiration. No animal reached this stage.
Protocol for hepatoprotective
activity: Paracetamol-induced hepatotoxicity in rats
A hepatotoxicity model produced by paracetamol was
used to investigate hepatoprotective activity. The experimental
rats were housed in six groups of four rats each. Group I, II and
III served as normal, negative and positive control given 1% w/v
pulvis gummi arabicum (PGA) suspension, paracetamol at a dose of
1,000 mg/kg and silymarin at a dose of 50 mg/kg, respectively.
Groups IV, V and VI were each given CcE at doses of 100, 200 and
400 mg/kg orally. Determination of the CcE doses in this study
refers to previously published research (21,23).
The experimental treatments and group designs were:
Group I (normal control): For 21 days, rats were
given 1% w/v PGA suspension orally (10 ml/kg/day).
Group II (negative control): For 7 days, rats
received a dose of 1,000 mg/kg of paracetamol.
Group III (positive control): For 21 days, rats
received 50 mg/kg of silymarin.
Group IV (CcE 100): CcE was administered to rats for
21 days at a dose of 100 mg/kg.
Group V (CcE 200): CcE was administered to rats for
21 days at a dose of 200 mg/kg.
Group VI (CcE 400): CcE was administered to rats for
21 days at a dose of 400 mg/kg.
Rats in groups II-VI were given paracetamol
induction from days 15 to 21 at a dose of 1,000 mg/kg orally
(33). Meanwhile, on the 22nd day,
the treatment groups were anesthetized with diethyl ether at a dose
of 4 ml. Diethyl ether was administered to rats by simple
‘open-drop’ methods using an ether-impregnated cotton ball in a
bell jar for induction followed by inhalation via a simple face
cone. The parameters monitored to ensure the animals were
anesthetized after diethyl ether administration were ataxic,
recumbent, with a steady, slow respiratory rate, immobile and loss
of palpebral blink reflex. After a cardiac puncture, 2 ml of blood
was extracted and placed in a tube holding heparin. The rats were
euthanized by cervical dislocation. The liver was immediately
removed and washed with cold 0.9% NaCl solution to remove the blood
before weighing. For histological analysis, a portion of the
liver's median lobe was preserved in a 10% formalin solution
(fixation was carried out for 24 h at room temperature 20-22˚C)
(34). Using a motor-driven Teflon
pestle, homogenate was prepared for the liver antioxidant enzyme
level test by combining one gram of wet tissue with 9 ml of 1.25%
KCl. The homogenate was centrifuged for 10 min at 4˚C at
2,737 x g to extract the supernatant, which was then used to
measure the levels of SOD, CAT, GPx and GSH (35).
Protocol for nephroprotective
activity: Gentamicin-induced nephrotoxicity in rats
A nephrotoxicity model produced by gentamicin was
used to perform the nephroprotective activity test. Random
selection was used to choose five groups of four rats each from the
experimental animals. Groups I and II as normal and negative
controls were given a 1% w/v PGA suspension and gentamicin at a
dose of 80 mg/kg, respectively. Meanwhile, groups III, IV and V
were given treatment using CcE at 100, 200 and 400 mg/kg,
respectively. The experimental treatments and group designs were as
follows:
Group I (normal control): Rats were given 1% w/v PGA
suspension orally (10 ml/kg/day) for 8 days.
Group II (negative control): Gentamicin was
administered to rats for 5 days at a dose of 80 mg/kg.
Group III (CcE 100): For 8 days, rats received a 100
mg/kg dosage of CcE.
Group IV (CcE 200): For 8 days, rats received a 200
mg/kg dosage of CcE.
Group V (CcE 400): For 8 days, rats received a 400
mg/kg dosage of CcE.
Gentamicin induction was administered
intraperitoneally to rats in groups II-V at a dose of 80 mg/kg from
days 4-8(36). On the ninth day,
the rats in each treatment group were anesthetized using diethyl
ether at a dose of 4 ml. Then 2 ml of blood was quickly collected
into a heparin tube through a cardiac puncture and rats were
euthanized by cervical dislocation. The kidney was cleaned with a
cold 0.9% NaCl solution to remove blood and foreign tissue. This
was followed by weighing and preserving the organs in 10% formalin
solution for histopathological examination (fixation was carried
out for 24 h at 20-22˚C) (37).
Determination of liver and kidney
serum biochemical parameters
Fresh rat blood samples were centrifuged for 20 min
at 503 x g and at 22˚C to produce blood serum. The serum was put in
an Eppendorf tube and its levels of ALT, AST, TB, ALP, TC, TA, TP,
SCr, SU and UA were promptly measured. In this procedure,
commercial kits were used in accordance with the manufacturer's
instructions [cat. nos. : ALT (32941-05121), AST (31335-05121), TB
(3417012999-AL2-175423984), ALP (32918-05121), TC
(3417012020-LAH-176618380), TA (3417012020-LAH-176587091), TP
(3417012020-LAH-176626657), SCr (3417012020-LAH-176623909), SU
(3417012020-BSS-211916981), UA (3417012999-LAB-205299812)] and a
HumaLyzer 2000 photometer was used for measurement (PT. Sali Polapa
Bersama).
Determination of TNF-α and IFN-γ serum
levels
TNF-α and IFN-γ levels were measured in the present
study using the ELISA technique. The collected serum was
immediately analyzed using a commercially available ELISA kit [cat.
nos. : TNF-α (MBS2707992) and IFN-γ (MBS2708210, PT. Biolab Science
Universal] containing a microtiter plate coated with specific
antibodies against TNF-α and IFN-γ standards as well as a washing
buffer and horseradish peroxidase (HRP) conjugate. Meanwhile, an
automatic microplate reader recorded optical density at 450 nm
(ELx50; BioTek; Agilent Technologies, Inc.).
Histopathological examination
After being cleaned during the autopsy, the water
content in liver and kidney tissue samples is removed using an
alcohol dehydration process. Next, clearing was performed using
xylene to remove alcohol and make the tissue transparent. Then,
paraffin penetration was performed to make the tissue harden at
room temperature and make it easier to cut using a microtome.
Paraffin blocks were sectioned at 3.4-4.6 µm and the slides were
deparaffinized in xylene, followed by H&E staining (at 30˚C:
hematoxylin ~10 min, eosin 2 min). A 100x objective lens on a light
microscope (BX-51; Olympus Corporation) with a connected camera
(Olympus Q Color-5; Olympus Corporation) and computer connection
was used to view the slides at a total magnification of x1,000. A
pathologist assessed and rated the liver and kidney sections based
on the degree of damage, somewhat modified from Zakaria et
al (34).
Statistical analysis
The experimental results were shown using the mean ±
standard error of the mean. One-way analysis of variance was used
to examine the variations in the means of the variables that were
measured. This was followed by Tukey's post hoc test using GraphPad
Prism version 8 (Dotmatics). P<0.05 was considered to indicate a
statistically significant difference. Sample size determination was
based on Federer calculation formula, which is (t-1) (n-1) ≥15;
where t is the number of the groups and n is the experimental
animal per group. (6-1) (n-1) ≥15 -> n≥4, for the testing of
hepatoprotective and (5-1) (n-1) ≥15 -> n≥4.75 for the testing
nephroprotective properties. According to this calculation, the
minimum sample size was four experimental animals in each treatment
and control group.
Results
FT-IR analysis
FT-IR revealed that there were several distinct
functional groups by identifying 27 peaks for CcE. There were
obvious peaks at 1,201.51, 1,444.98 and 1,515.13 cm-1,
showing C-O bending mode. This finding demonstrated the presence of
a number of chemicals, including ethers, alcohols, esters and
carboxylic acids. Furthermore, amines (N-H stretching), alcohol
(O-H stretching), alkanes (C-H stretching), alkynes (C≡C
stretching), carboxylic acid (C=O stretching), alkenes (C=C
stretching) and imines (C=N) were among the functional groups found
in a range of peaks that extended from 3,333.13 to 1,606.32
cm-1. Fig. 1 shows the
results of FT-IR analysis of CcE.
Hepatoprotective activity of CcE
against paracetamol-induced hepatotoxicity in rats: Effect of CcE
on liver function parameters (AST, ALT, ALP, TB, TC, TA and TP) and
liver weight
Based on the present results, administration of
paracetamol (1,000 mg/kg) to rats increased AST, ALT, ALP, TB and
TC levels and also decreased TA and TP (P<0.001-<0.0001) when
compared with normal controls. Pretreatment with CcE at all doses
caused a significant decrease (P<0.05-<0.0001) in increasing
AST, ALT, ALP, TB and TC, levels induced by paracetamol.
Furthermore, the administration of CcE at all doses also caused a
significant increase (P<0.01-<0.0001) in decreasing TA and TP
levels induced by paracetamol. The pretreatment with silymarin (50
mg/kg) had an improved effect on changes in liver biochemical serum
parameters compared with CcE. Additionally, compared with a normal
control group, there was a statistically significant increase in
liver weight (P<0.01) after the administration of paracetamol
(1,000 mg/kg). Rats' liver weight significantly decreased
(P<0.01) after receiving pretreatment with silymarin (50 mg/kg)
or CcE at all dosages when compared with the paracetamol group.
Table I shows the effects of
pretreatment with CcE on liver function parameters and liver weight
of rats.
 | Table IEffect of CcE on paracetamol-induced
liver injury in rats. For each group, the data are shown as the
mean ± standard error of the mean of four replicates. |
Table I
Effect of CcE on paracetamol-induced
liver injury in rats. For each group, the data are shown as the
mean ± standard error of the mean of four replicates.
| Treatment | Dose (mg/kg) | AST (IU/l) | ALT (IU/l) | ALP (IU/l) | TB (mg/dl) | TC (mg/dl) | TA (g/dl) | TP (g/dl) | Liver weight
(g) |
|---|
| NC | 1% PGA | 112.74±2.66 | 38.30±3.12 | 84.25±4.25 | 0.13±0.02 | 67.66±3.57 | 4.81±0.11 | 6.84±0.15 | 6.54±0.22 |
| PCT | 1,000 |
220.13±4.71h |
108.66±4.42g |
203.42±3.71h |
2.31±0.40h |
117.37±3.53g |
1.71±0.16g |
3.79±0.14h |
8.85±0.28f |
| SM | 50 |
117.74±3.58d |
45.82±3.27c |
105.83±3.74d |
0.27±0.04d |
75.29±4.15c |
4.70±0.46c |
6.31±0.19d |
6.66±0.17b |
| CcE | 100 |
191.96±3.73a |
85.62±4.03a |
186.44±3.92a |
1.51±0.06a |
98.54±4.16a |
4.37±0.19c |
5.36±0.15b |
6.83±0.16b |
| | 200 |
161.76±4.07c |
70.45±3.98b |
175.27±4.11b |
1.18±0.03c |
86.78±3.60b |
4.54±0.14c |
6.02±0.14c |
6.73±0.18b |
| | 400 |
135.69±3.57d |
54.81±4.20c |
112.26±3.67d |
0.68±0.03d |
79.27±4.84c |
4.61±0.18c |
6.21±0.12d |
6.65±0.22b |
Effect of CcE on the levels of liver
antioxidant enzymes
The findings demonstrated that, in comparison with
normal controls, the administration of paracetamol (1,000 mg/kg)
significantly reduced the activities of SOD, CAT, GPx and GSH in
liver tissue (P<0.001-<0.0001). The pretreatment with
silymarin (50 mg/kg) and CcE at all doses showed a significant
increase (P<0.05-<0.0001) in SOD, CAT, GPx and GSH activities
when compared with paracetamol group. Therefore, CcE triggered
hepatoprotective activity through the activation of endogenous
enzymatic antioxidant systems. Fig.
2 shows the effects of the extracts on liver antioxidant
enzymes.
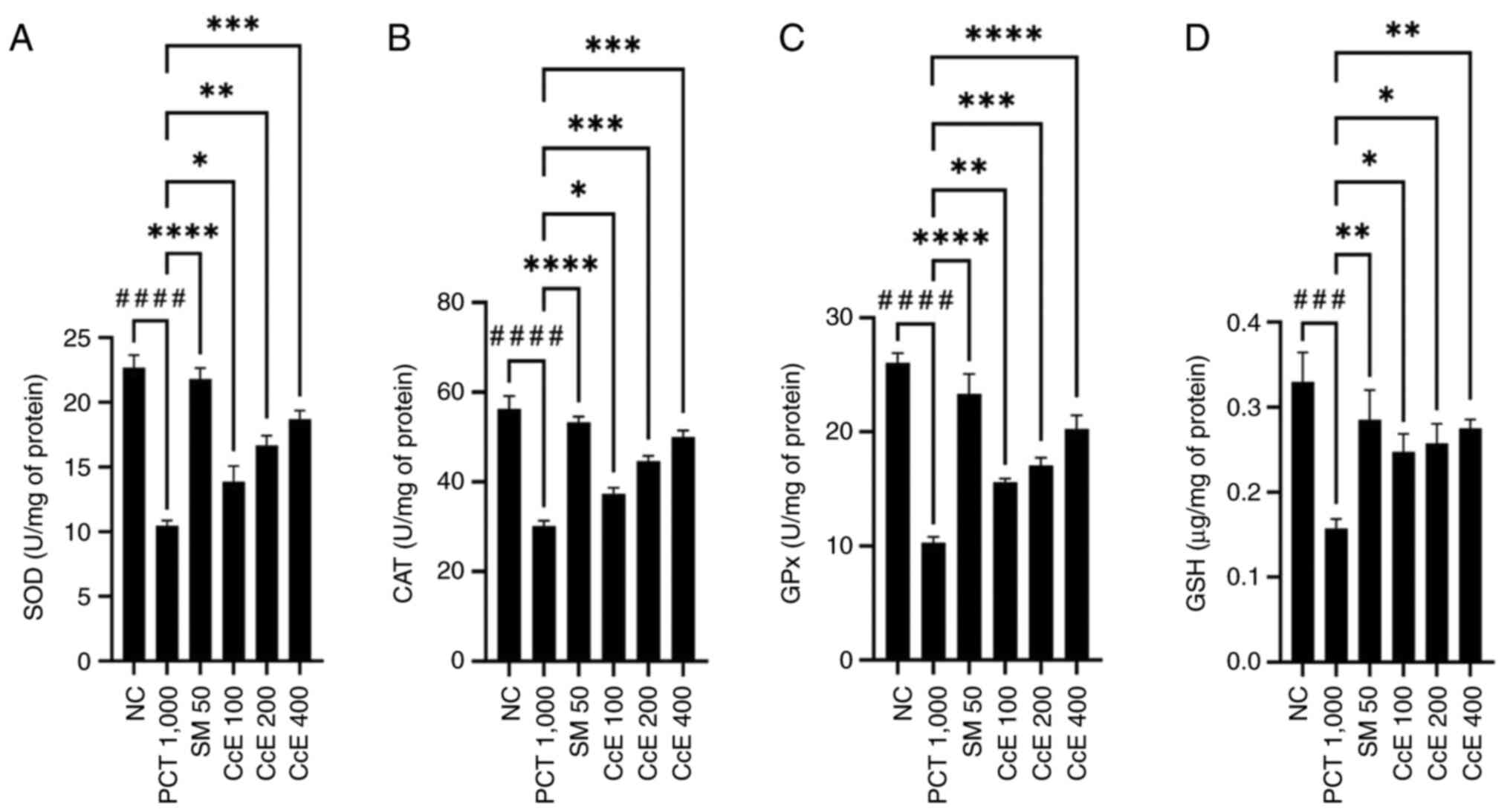 | Figure 2Effect of CcE on liver antioxidant
enzymes in rats with hepatotoxicity induced by paracetamol. The
data in each group is displayed as the mean ± standard error of the
mean of four replicates showed significant differences
*P<0.05, **P<0.01,
***P<0.001; ****P<0.0001 vs.
paracetamol group. ###P<0.001 and
####P<0.0001 vs. normal group. (A) SOD levels. (B)
CAT levels. (C) GPx levels. (D) GSH levels. CcE, Castanopsis
costata extract; SOD, superoxide dismutase; CAT, catalase; GPx,
glutathione peroxidase; GSH, glutathione; NC, normal control; PCT,
paracetamol; SM, silymarin. |
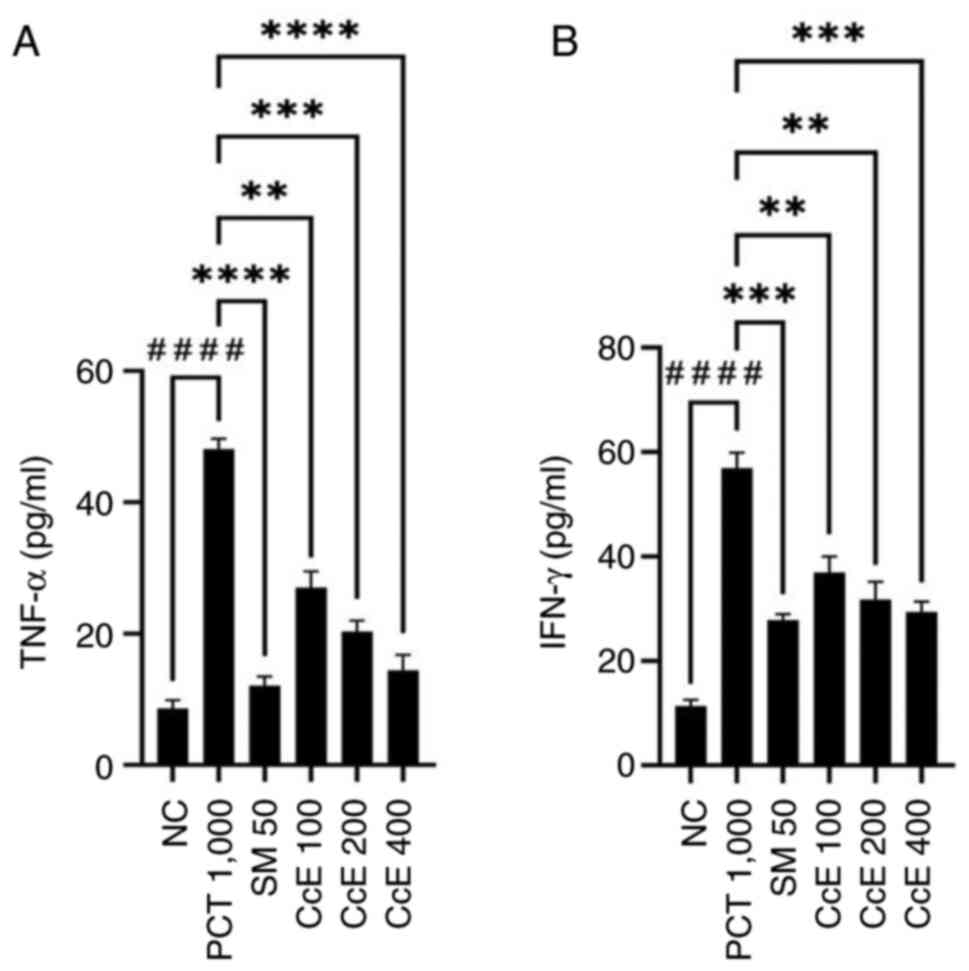
Effect of CcE on TNF-α and IFN-γ serum
levels in paracetamol-induced hepatotoxicity rats
Based on the present results, administration of
paracetamol (1,000 mg/kg) induced a substantial increase in TNF-α
and IFN-γ levels (P<0.0001) when compared with normal control.
Pretreatment with CcE at all doses caused a significant decrease in
increasing the levels of TNF-α (P<0.01-<0.0001) and IFN-γ
(P<0.01-<0.001) induced by paracetamol. However, pretreatment
with silymarin (50 mg/kg) had an improved effect on decreasing
TNF-α levels (P<0.0001) and IFN-γ (P<0.001) than CcE. The
effect of CcE on TNF-α and IFN-γ levels in rats with
paracetamol-induced hepatotoxicity is depicted in Fig. 3.
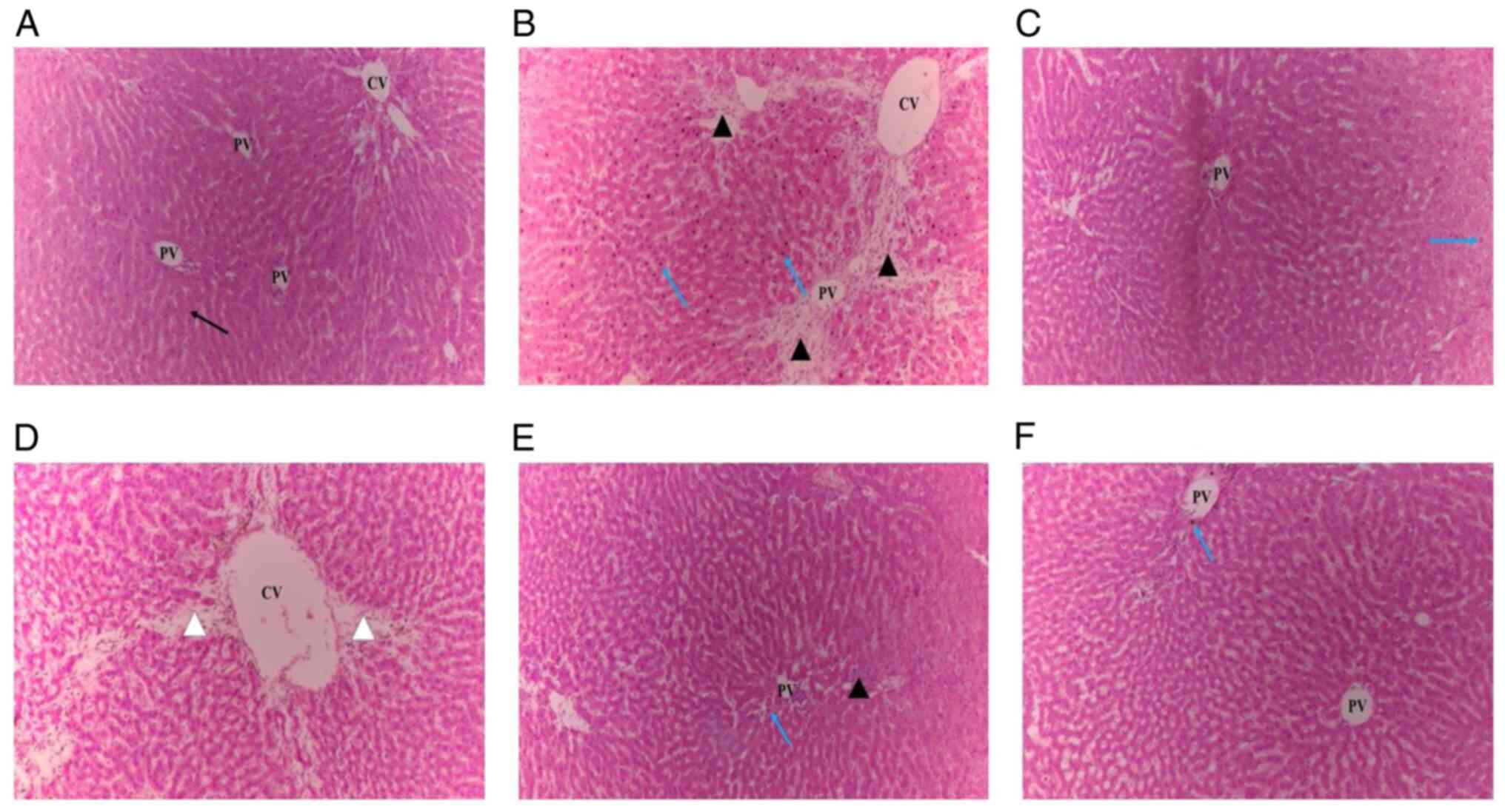
Effect of CcE on histopathological
analyses of liver of rats in paracetamol-induced
hepatotoxicity
Fig. 4A illustrated
the cellular architecture with clear cells, sinusoidal gaps and
central veins observed in the histopathological analysis of liver
slices in the normal control group. However, the paracetamol group
showed the most severe damage to cellular architecture, with
centrilobular necrosis, hyperplasia, vascular and cellular
degeneration, inflammation, polymorphonuclear aggregation,
extensive lymphocyte infiltration and loss of cellular boundaries
(Fig. 4B). Pretreatment with
silymarin (50 mg/kg) showed complete improvement in cellular
architecture, such as necrotic hepatocyte patches (Fig. 4C). In comparison with the
paracetamol group, the pretreatment with CcE at all doses resulted
in a lobular pattern that was nearly normal with mild degrees of
necrosis and lymphocyte infiltration (Fig. 4D-F). Table II shows the histopathological
scores of the changes.
 | Table IIEffect of CcE on liver section
histological score in rats with hepatotoxicity induced by
paracetamol. |
Table II
Effect of CcE on liver section
histological score in rats with hepatotoxicity induced by
paracetamol.
| Treatment | Dose (mg/kg) | Steatosis | Necrosis | Inflammation | Hemorrhage |
|---|
| NC | 1% PGA | - | - | - | - |
| PCT | 1,000 | + | +++ | ++ | ++ |
| SM | 50 | - | + | - | - |
| CcE | 100 | + | ++ | + | - |
| | 200 | - | + | - | - |
| | 400 | - | + | - | - |
Nephroprotective activity of CcE
against gentamicin-induced nephrotoxicity in rats: Effect of CcE on
kidney function parameters (SCr, SU and UA) and kidney weight
In comparison with normal controls, the
administration of gentamicin (80 mg/kg) to rats resulted in a
significant rise (P<0.001) in the levels of SCr, SU and UA.
Gentamicin-induced levels of SCr, SU and UA were significantly
(P<0.05-<0.01) reduced after pretreatment with CcE at all
dosages. Rat kidney weight increased significantly (P<0.01)
after receiving gentamicin (80 mg/kg) in comparison with the normal
control group. Furthermore, in comparison with the gentamicin
group, the pretreatment with CcE at all dosages resulted in a
significant (P<0.01) drop in the kidney weight of the rats.
Table III shows the effects of
CcE pretreatment on kidney function parameters and kidney weight of
rats.
 | Table IIIEffect of CcE on renal damage caused
by gentamicin in rats. For each group, the data are shown as the
mean ± standard error of the mean of four replicates. |
Table III
Effect of CcE on renal damage caused
by gentamicin in rats. For each group, the data are shown as the
mean ± standard error of the mean of four replicates.
| Treatment | Dose (mg/kg) | SCr (mg/dl) | SU (mg/dl) | UA (mg/dl) | Kidney weight
(g) |
|---|
| NC | 1% PGA | 0.51±0.11 | 19.66±0.99 | 3.83±0.23 | 1.02±0.04 |
| GM | 80 |
1.55±0.19b |
57.28±3.78b |
7.07±0.59b |
2.85±0.21a |
| CcE | 100 |
0.98±0.23c |
39.08±2.14c |
5.95±0.23c |
1.84±0.06d |
| | 200 |
0.83±0.14c |
33.11±2.09c |
5.42±0.55c |
1.76±0.03d |
| | 400 |
0.71±0.16d |
28.88±1.43d |
4.35±0.48d |
1.47±0.12d |
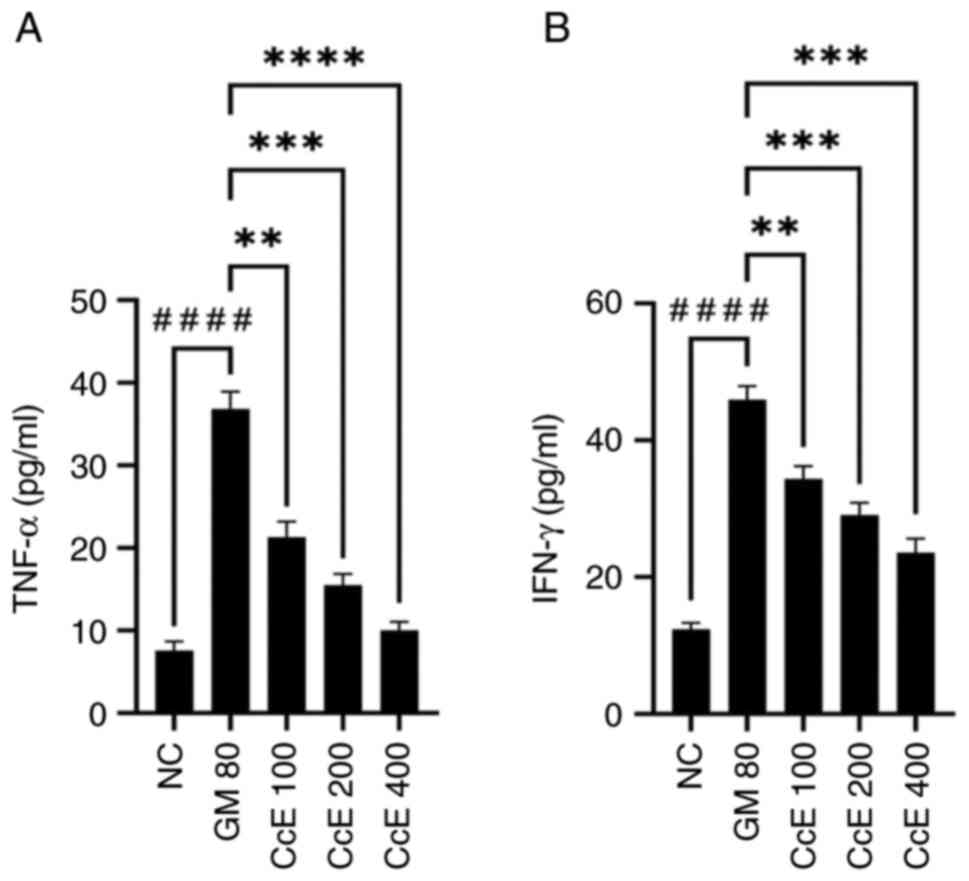
Effect of CcE on TNF-α and IFN-γ serum
levels in gentamicin-induced nephrotoxicity in rats
Compared with normal controls, the administration of
80 mg/kg of gentamicin resulted in a statistically significant rise
(P<0.0001) in the levels of TNF-α and IFN-γ. The levels of TNF-α
(P<0.01-<0.0001) and IFN-γ (P<0.01-<0.001) generated by
gentamicin were significantly reduced following pretreatment with
CcE at all dosages. Fig. 5
illustrates how CcE affects TNF-α and IFN-γ levels in rats that
have gentamicin-induced nephrotoxicity.
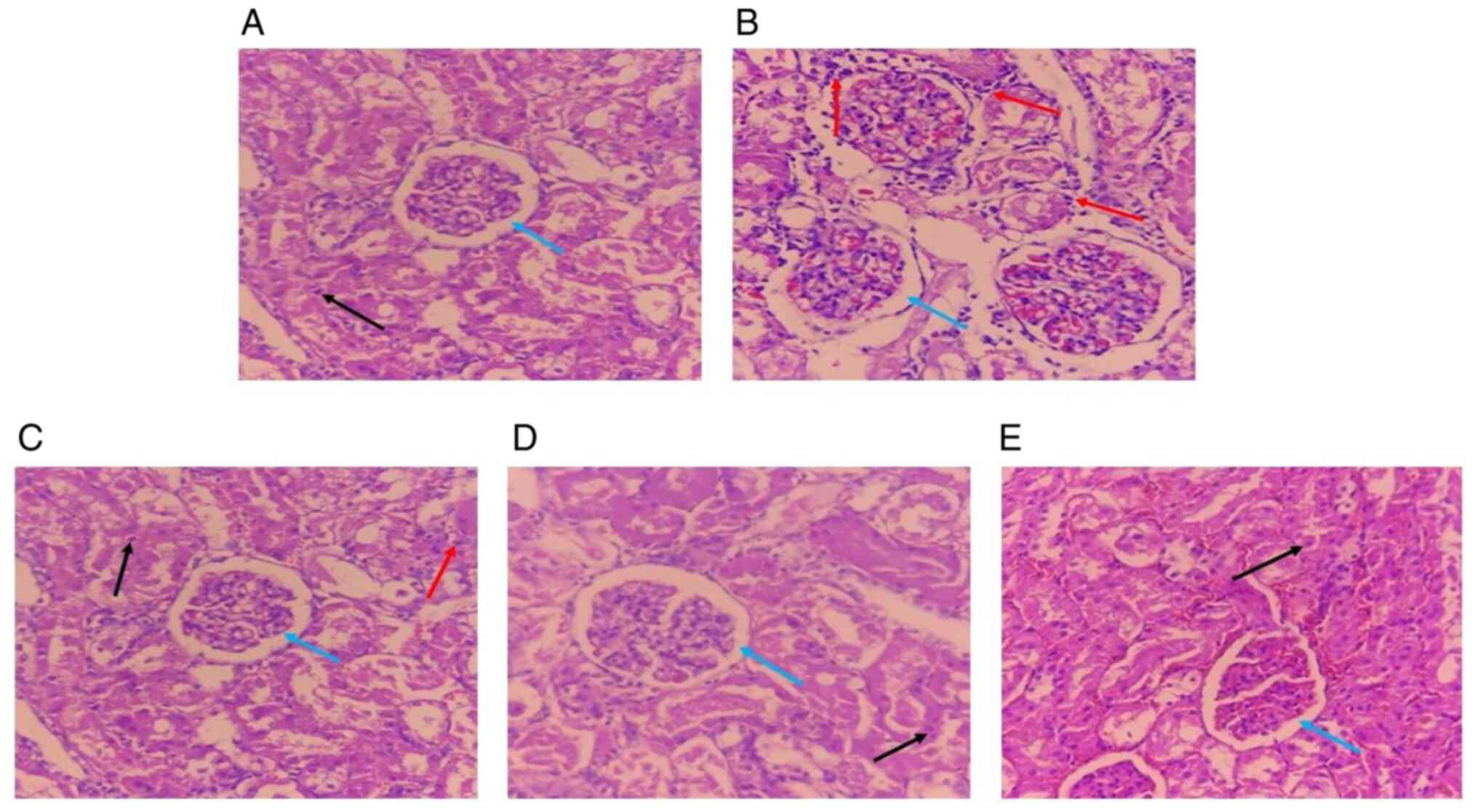
Effect of CcE on histopathological
analyses of kidney of rats in gentamicin-induced
nephrotoxicity
When evaluated histopathologically, the kidney
sections from the normal control group revealed normal tubules and
glomeruli without any evident abnormalities (Fig. 6A). The gentamicin group showed
severe acute glomerular and tubular necrosis, characterized by
total obliteration of the tubular lumen, as well as intertubular
hemorrhage and acute leukocyte infiltration (Fig. 6B). Meanwhile, pretreatment with CcE
at all doses showed normal glomeruli, relatively normal tubular
dilation, no interstitial edema and capillary congestion when
compared with the gentamicin group (Fig. 6C-E). Table IV shows the histopathological
scores of the changes.
 | Table IVEffect of CcE on histopathological
scoring of kidney section in gentamicin-induced nephrotoxicity
rats. |
Table IV
Effect of CcE on histopathological
scoring of kidney section in gentamicin-induced nephrotoxicity
rats.
| Treatment | Dose (mg/kg) | Necrosis | Inflammation | Hemorrhage |
|---|
| NC | 1% PGA | - | - | - |
| GM | 80 | +++ | ++ | ++ |
| CcE | 100 | + | + | - |
| | 200 | - | - | - |
| | 400 | - | - | - |
Discussion
Despite recent therapeutic advances and significant
developments in modern medicine, liver and kidney diseases remain a
global health problem (38,39). The most common cause of liver and
kidney damage is long-term use of drugs [especially nonsteroidal
anti-inflammatory drugs (NSAIDs), antibiotics and chemotherapy
drugs]. This can cause the ability of these organs to regenerate to
eventually become dysfunctional, leading to scarring and fibrosis
(40,41). Currently, the conventional treatment
focuses on symptom management and transplantation in severe cases
of liver and kidney disease (42,43).
However, there are no drugs used to increase the detoxification
power of these organs (44). The
quest to discover hepatoprotective and nephroprotective agents has
become a significant challenge over the past decades (45,46).
CcE has emerged as a promising alternative hepatoprotective and
nephroprotective agent.
The hepatoprotective and nephroprotective properties
of CcE were assessed in the present study on hepatotoxicity and
nephrotoxicity caused by paracetamol and gentamicin, respectively.
Several tests were performed to examine hepatoprotective effect of
CcE, namely liver function tests, liver antioxidant enzyme levels,
inflammatory cytokine levels and histopathology research in
paracetamol-induced rats. Administration of high doses of
paracetamol (1,000 mg/kg) is known to cause liver damage in rats
(6). This happens as a result of
the bioactivation of paracetamol, which creates the potentially
dangerous reactive metabolite N-acetyl-p-benzoquinone imine.
Specifically, CYP2E1 and CYP3A4 enzymes of the cytochrome P-450
(CYP) system produces these metabolite chemicals (47), which oxidize lipids or other
significant sulfhydryl groups and bond covalently to tissue
macromolecules (6). All treatment
groups had their serum levels of AST, ALT and ALP tested in order
to evaluate any changes in liver function parameters. Aspartic or
alanine amino groups are transferred to ketoglutaric acid through
the action of AST and ALT during the gluconeogenesis process,
resulting in the production of pyruvic and oxaloacetate,
respectively (48). Meanwhile, ALP
functions to transport metabolites across cell membranes (49). AST, ALT and ALP were found in high
concentrations when hepatopathy occurs. These enzymes leak into the
bloodstream and are used as markers of hepatocyte damage (50). In the present study, the rats
administered paracetamol had significantly higher serum levels of
AST, ALT and ALP compared with the normal control group. It has
been observed that pretreatment with CcE at different doses
considerably lessens the rise in blood levels of AST, ALT and ALP
in rats given paracetamol. This is due to the ability of CcE to
prevent intracellular leakage of the enzymes by stabilizing the
activity of hepatocyte membranes (35).
An additional method of evaluating liver function is
to estimate serum levels of TB, TC, TA and TP (34,35). A
class of enzymes known as uridine-diphosphoglucuronic
glucuronosyltransferase transforms bilirubin, a byproduct of
hemoglobin metabolism, into glucuronic acid in hepatocyte cells to
increase the solubility in water. However, the bilirubin
conjugation process is disrupted in the case of liver damage,
resulting in hyperbilirubinemia (51). Previous research reports that liver
damage affects the structure and function of membranes, as well as
lipid metabolism, disrupting fluidity, permeability and the
transport system (52,53). This condition decreases the number
of hepatocytes and the capacity to synthesize protein and albumin
(35).
The results of the present study showed that the
rats given paracetamol had lower levels of TA and TP and greater
levels of TB and TC compared with the normal control group.
Pretreatment with CcE at all doses was reported to restore TB, TC,
TA and TP levels, as shown in Table
I. This was due to the antioxidant (22), antihyperlipidemic (23) and anti-inflammatory (25) effects of CcE. Consequently, there
was an increase in hepatocyte count and liver function, as shown by
the rise in TA and TP and fall in TB and TC levels.
The administration of high-dose paracetamol
exacerbates oxidative stress, reactive oxygen species (ROS) and
reactive nitrogen species, leading to alterations in the
antioxidant enzyme system, a notable reduction in hepatic GSH and
an upsurge in inflammatory cytokines (TNF-α and INF-γ) (54). Figs.
2 and 3 illustrate how
pretreatment with CcE at all doses improved the activity of liver
antioxidant enzymes and was found to reduce TNF-α and INF-γ levels.
This was due to the strong antioxidant effect of CcE (22) which stimulated an increase in liver
antioxidant enzyme levels. Therefore, pretreatment with CcE can
metabolized ROS and neutralized free radicals, as well as
non-radical oxidants to prevent or reduce oxidative damage
(55). The reduction in TNF-α and
INF-γ levels resulting from CcE was due to its anti-inflammatory
properties (25). The present study
found that CcE showed hepatoprotective action based on the data,
which were corroborated by histological investigations. Thus, CcE
was shown to enhance the architecture of liver tissue by lowering
the degree of necrosis, enlarging cell borders and guarding against
a significant infiltration of lymphocytes, as illustrated in
Fig. 4.
However, based on the present results, pretreatment
with silymarin (50 mg/kg) had an improved effect on changes in
liver biochemical serum parameters, liver enzyme levels and
cytokine levels compared with CcE. Silymarin is an active component
of Silybum marianum L. which is a medicinal plant that has
been used for centuries to treat various liver diseases (56). Silymarin administration prevents
hepatic dysfunction and restored normal liver functionality in
studies on hepatotoxicity in rats. Silymarin also functions as an
antioxidant by reducing oxidative stress and preventing the loss of
glutathione, increasing the regenerative ability of the liver cells
by enhancing the synthesis of deoxyribonucleic acid (DNA) and
ribonucleic acid (RNA) and inhibits elevated intrahepatic messenger
RNA (mRNA) levels of interleukins (IL-2, IL-4), IFN-γ and TNF-α
significantly (57,58).
Several tests were conducted to assess the
nephroprotective effect of CcE including renal function,
inflammatory cytokine level and renal histopathology analyses in
gentamicin-induced rats. Administration of gentamicin (80 mg/kg) is
reported to cause kidney organ damage in rats (37). It causes acute tubular necrosis,
which is followed by renal failure, by inhibiting protein synthesis
in the proximal tubules (59).
Serum SCr, SU and UA levels were measured in all treatment groups
to assess changes in renal functions due to gentamicin induction.
SCr is a waste product derived from muscle breakdown and protein
digestion. SU and UA are waste products produced by the liver when
breaking down proteins and purines, respectively. These substances
are filtered by the kidney and excreted in the urine (60). In a healthy kidney, SCr, SU and UA
are found in urine, but filtering the substances is difficult
during nephropathy (61). The rats
exposed to gentamicin showed greater serum levels of SCr, SU and UA
compared with the normal control group. Pretreatment with CcE at
all doses significantly reduced serum levels of SCr, SU and UA in
rats induced by gentamicin, as shown in Table III. This was due to the strong
antioxidant effect of CcE (22),
which prevented kidney damage caused by free radical exposure and
quenched ROS formed due to gentamicin induction (62).
Gentamicin is reported to cause tubular injury,
triggering infiltrated renal epithelial cells to express
proinflammatory cytokines in renal tissue (63). In the present study, pretreatment
with CcE significantly reduced TNF-α and INF-γ, leading to an
increase due to gentamicin induction (Fig. 5). The effect of reducing TNF-α and
INF-γ levels by CcE was due to anti-inflammatory activity (25). According to histological analysis,
CcE improved renal tissue architecture by averting capillary
congestion, tubular necrosis, interstitial edema, glomerular
congestion and interstitial with inflammatory cells (Fig. 6).
A limitation in the present study was that the
sample size was too small, which had the potential to cause the
loss of significant differences even if they exist in the
population and may not be applicable to studies with larger
populations. The authors suggest the use of freely downloadable
software G Power for sample size calculation (64).
In conclusion, CcE had hepatoprotective activity in
paracetamol-induced rats by improving liver function, increasing
antioxidant enzyme levels, decreasing pro-inflammatory cytokine
levels (TNF-α and IFN-γ) and enhancing liver tissue architecture.
CcE also possessed nephroprotective activity in gentamicin-induced
rats through improving kidney function, decreasing pro-inflammatory
cytokine levels (TNF-α and IFN-γ) and enhancing renal tissue
architecture. Thus, CcE served as a significant natural chemical
source for the creation of novel hepatoprotective and
nephroprotective medications. Further research in vitro was
recommended to determine the exact mechanisms of hepatoprotective
and nephroprotective effects of C. costata leaves.
Acknowledgements
Not applicable.
Funding
Funding: The Regular Fundamental Research of the Ministry of
Education, Culture, Research and Technology of Indonesia (grant no.
0459/E5/PG.02.00/2024) provided funding for the present study.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Author's contributions
MYA designed the present study. AS, EA and FH
contributed to the methodology. ZO, MFWS and PRAS wrote the
manuscript and participated in the literature collection and
evaluation. DW, NH and SWS confirm the authenticity of all the raw
data. NAS, WI and SS contributed to the data collection and
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of Universitas
Padjadjaran in Bandung, Indonesia has accepted the present study
protocol under the numbers 568/UN6.KEP/EC/2022 and
109/UN6.KEP/EC/2023 for the testing of hepatoprotective and
nephroprotective properties, respectively, in compliance with
ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Narayanan M, Gopi A, Natarajan D,
Kandasamy S, Saravanan M, El Askary A, Elfasakhany A and
Pugazhendhi A: Hepato and nephroprotective activity of methanol
extract of Hygrophila spinosa and its antibacterial
potential against multidrug resistant Pandoraea sputorum.
Environ Res. 201(111594)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parasuraman S, Qi YZ and Jie LW: Hepato-
and nephroprotective effects of ethanolic extract of seeds of
Macrotyloma uniflorum on paracetamol-induced hepato- and
nephrotoxicity in rats. Indian J Pharm Educ Res. 56:772–779.
2022.
|
|
3
|
Li M, Xie F, Wang L, Zhu G, Qi LW and
Jiang S: Celastrol: An update on its hepatoprotective properties
and the linked molecular mechanisms. Front Pharmacol.
13(857956)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alkandahri MY, Pamungkas BT, Oktoba Z,
Shafirany MZ, Sulastri L, Arfania M, Anggraeny EN, Pratiwi A,
Astuti FD, Indriyani , et al: Hepatoprotective effect of
kaempferol: A review of the dietary sources, bioavailability,
mechanisms of action, and safety. Adv Pharmacol Pharm Sci.
2023(1387665)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Iranshahy M, Iranshahi M, Abtahi SR and
Karimi G: The role of nuclear factor erythroid 2-related factor 2
in hepatoprotective activity of natural products: A review. Food
Chem Toxicol. 120:261–276. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Okokon JE, Udobang JA, Bassey AI, Edem UA
and Agu EC: Hepatoprotective and nephroprotective activities of
husk extract of Zea mays against paracetamol-induced liver
and kidney injuries in rats. Trop J Nat Prod Res. 4:67–76.
2020.
|
|
7
|
Abdel-Hady H, El-Sayed M, Abdel-hady AA,
Hashash MM, Abdel-Hady AM, Aboushousha T, Abdel-Hameed ES,
Abdel-Lateef EE and Morsi EA: Nephroprotective activity of
methanolic extract of Lantana camara and squash
(Cucurbita pepo) on cisplatin-induced nephrotoxicity in rats
and identification of certain chemical constituents of Lantana
camara by HPLC-ESI-MS. Pharmacogn J. 10:136–147. 2018.
|
|
8
|
Al-Snafi AE and Talab TA: A review of
medicinal plants with nephroprotective effects. GSC Biol Pharm Sci.
8:114–122. 2019.
|
|
9
|
Sujana D, Saptarini NM, Sumiwi SA and
Levita J: Nephroprotective activity of medicinal plants: A review
on in-silico, in-vitro, and in-vivo based studies. J Appl Pharm
Sci. 11:113–127. 2021.
|
|
10
|
Wannes WA and Tounsi MS: Tunisian
nephroprotective plants: A review. J Explor Res Pharmacol. 8:74–91.
2023.
|
|
11
|
Devarbhavi H, Asrani SK, Arab JP, Nartey
YA, Pose E and Kamath PS: Global burden of liver disease: 2023
update. J Hepatol. 79:516–537. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bello AK, Okpechi IG, Levin A, Ye F,
Damster S, Arruebo S, Donner JA, Caskey FJ, Cho Y, Davids MR, et
al: An update on the global disparities in kidney disease burden
and care across world countries and regions. Lancet Glob Health.
12:e382–e395. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
El-Rabey HA, Rezk SM, Sakran MI, Mohammed
GM, Bahattab O, Balgoon MJ, Elbakry MA and Bakry N: Green coffee
methanolic extract and silymarin protect against
CCl4-induced hepatotoxicity in albino male rats. BMC
Complement Med Ther. 21(19)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tienda-Vázquez MA, Morreeuw ZP,
Sosa-Hernández JE, Cardador-Martínez A, Sabath E, Melchor-Martínez
EM, Iqbal HMN and Parra-Saldívar R: Nephroprotective plants: A
review on the use in pre-renal and post-renal diseases. Plants
(Basel). 11(818)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lawson SK, Satyal P and Setzer WN: The
volatile phytochemistry of seven native american aromatic medicinal
plants. Plants (Basel). 10(1061)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alkandahri MY, Maulana YE, Subarnas A,
Kwarteng A and Berbudi A: Antimalarial activity of extract and
fractions of Cayratia trifolia (L.) domin. Int J Pharm Res.
12:1435–1441. 2020.
|
|
17
|
Elfahmi , Woerdenbag HJ and Kayser
O: Jamu: Indonesian traditional herbal medicine towards rational
phytopharmacological use. J Herb Med. 4:51–73. 2014.
|
|
18
|
Farhamzah Kusumawati AH, Alkandahri MY,
Hidayah H, Sujana D, Gunarti NS, Yuniarsih N, Apriana SD and
Agustina LS: Sun protection factor activity of black glutinous rice
emulgel extract (Oryza sativa var glutinosa). Indian J Pharm
Educ Res. 56:302–310. 2022.
|
|
19
|
Salim E, Fatimah C and Fanny DY: Analgetic
activity of Cep-cepan (Saurauia cauliflora Dc.) leaves
extract. J Nat. 17:31–38. 2017.
|
|
20
|
Alkandahri MY, Berbudi A, Utami NV and
Subarnas A: Antimalarial activity of extract and fractions of
Castanopsis costata (Blume) A.DC. Avicenna J Phytomed.
9:474–481. 2019.PubMed/NCBI
|
|
21
|
Alkandahri MY, Sujana D, Hasyim DM,
Shafirany MZ, Sulastri L, Arfania M, Frianto D, Farhamzah
Kusumawati AH and Yuniarsih N: Antidiabetic activity of extract and
fractions of Castanopsis costata leaves on alloxan-induced
diabetic mice. Pharmacogn J. 13:1589–1593. 2021.
|
|
22
|
Alkandahri MY, Arfania M, Abriyani E,
Ridwanuloh D, Farhamzah F, Fikayuniar L, Hasyim DM and Nurul and
Wardani D: Evaluation of antioxidant and antipyretic effects of
ethanolic extract of cep-cepan leaves (Castanopsis costata
(Blume) A.DC). J Adv Pharm Educ Res. 12:107–112. 2022.
|
|
23
|
Alkandahri MY, Kusumiyati K, Renggana H,
Arfania M, Frianto D, Wahyuningsih ES and Maulana YE:
Antihyperlipidemic activity of extract and fractions of
Castanopsis costata leaves on rats fed with high cholesterol
diet. RASĀYAN J Chem. 15:2350–2358. 2022.
|
|
24
|
Alkandahri MY, Sholih MG, Fadilah NN,
Arfania M, Amal S, Frianto D, Mardiana LA, Astuti D and Hasyim DM:
Evaluation of antidiarrheal, antispasmodic, and antisecretory
activities of extract and fractions of Castanopsis costata
leaves in animal models. Pharmacogn J. 15:31–37. 2023.
|
|
25
|
Alkandahri MY, Sadino A, Pamungkas BT,
Oktoba Z, Arfania M, Yuniarsih N, Wahyuningsih ES and Putri DE:
Pharmacological evaluation of anti-inflammatory, antipyretic,
analgesic, and antioxidant activities of Castanopsis costata
leaf fractions (water, ethyl acetate, and n-hexane fractions): The
potential medicinal plants from North Sumatra, Indonesia. Res Pharm
Sci. 19:251–266. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ielciu I, Sevastre B, Olah NK, Turdean A,
Chișe E, Marica R, Oniga I, Uifălean A, Sevastre-Berghian AC,
Niculae M, et al: Evaluation of hepatoprotective activity and
oxidative stress reduction of Rosmarinus officinalis L.
shoots tincture in rats with experimentally induced hepatotoxicity.
Molecules. 26(1737)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Song X, Liu Z, Zhang J, Yang Q, Ren Z,
Zhang C, Liu M, Gao Z, Zhao H and Jia L: Anti-inflammatory and
hepatoprotective effects of exopolysaccharides isolated from
Pleurotus geesteranus on alcohol-induced liver injury. Sci
Rep. 8(10493)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang W, Liu H, Zhang Y, Feng Y, Yuan F,
Song X, Gao Z, Zhang J, Song Z and Jia L: Antihyperlipidemic and
hepatoprotective properties of alkali- and enzyme-extractable
polysaccharides by Dictyophora indusiata. Sci Rep.
9(14266)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wardani G, Nugraha J, Mustafa MR and
Sudjarwo SA: Antioxidative stress and anti-inflammatory activity of
fucoidan nanoparticles against nephropathy of
streptozotocin-induced diabetes in rats. Evid Based Complement
Alternat Med. 2022(3405871)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Perez-Meseguer J, Torres-González L,
Gutiérrez-González JA, Alarcón-Galván G, Zapata-Chavira H, Torres
NW, Moreno-Peña DP, Muñoz-Espinosa LE and Cordero-Pérez P:
Anti-inflammatory and nephroprotective activity of Juglans
mollis against renal ischemia-reperfusion damage in a wistar
rat model. BMC Complement Altern Med. 19(186)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Al-Harbi LN, Alshammari GM, Shamlan G,
Binobead MA, AlSedairy SA, Al-Nouri DM, Arzoo S and Yahya MA:
Nephroprotective and anti-diabetic potential of Beta
vulgaris L. Root (Beetroot) methanolic extract in a rat model
of type 2 diabetes mellitus. Medicina (Kaunas).
60(394)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hidayah H, Amal S, Yuniarsih N, Farhamzah
F, Kusumawati AH, Gunarti NS, Abriyani E, Mursal ILP, Sundara AK
and Alkandahri MY: Sun protection factor activity of Jamblang
leaves serum extract (Syzygium cumini). Pharmacogn J.
15:134–140. 2023.
|
|
33
|
Kiran PM, Raju AV and Rao BG:
Investigation of hepatoprotective activity of Cyathea
gigantea (Wall. ex. Hook.) leaves against paracetamol-induced
hepatotoxicity in rats. Asian Pac J Trop Biomed. 2:352–356.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zakaria ZA, Kamisan FH, Kek TL and Salleh
MZ: Hepatoprotective and antioxidant activities of Dicranopteris
linearis leaf extract against paracetamol-induced liver
intoxication in rats. Pharm Biol. 58:478–489. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Okokon JE, Simeon JO and Umoh EE:
Hepatoprotective activity of the extract of Homalium
letestui stem against paracetamol-induced liver injury.
Avicenna J Phytomed. 7:27–36. 2017.PubMed/NCBI
|
|
36
|
Dewi IP, Aldiana M, Viadina ZA, Fajrin FA,
Holidah D and Christianty FM: Nephroprotective effect of sugarcane
(Saccharum officinarum L.) leaves ethanol extract on
gentamicin-induced nephrotoxicity in rats. J Adv Pharm Technol Res.
15:208–213. 2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Iqbal SM, Hussain L, Hussain M, Akram H,
Asif M, Jamshed A, Saleem A and Siddique R: Nephroprotective
potential of a standardized extract of Bambusa arundinacea:
In vitro and in vivo studies. ACS Omega. 7:18159–18167.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Foghis M, Tit DM, Bungau SG, Ghitea TC,
Pallag CR, Foghis AM, Behl T, Bustea C and Pallag A: Highlighting
the use of the hepatoprotective nutritional supplements among
patients with chronic diseases. Healthcare (Basel).
11(2685)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Basist P, Parveen B, Zahiruddin S, Gautam
G, Parveen R, Khan MA, Krishnan A, Shahid M and Ahmad S: Potential
nephroprotective phytochemicals: Mechanism and future prospects. J
Ethnopharmacol. 283(114743)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hosack T, Damry D and Biswas S:
Drug-induced liver injury: A comprehensive review. Therap Adv
Gastroenterol. 16(17562848231163410)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Perazella MA and Rosner MH: Drug-induced
acute kidney injury. Clin J Am Soc Nephrol. 17:1220–1233.
2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU,
Lee TH, Jang BK, Kim SG, Ahn SB, Kim H, et al: KASL clinical
practice guidelines: Management of nonalcoholic fatty liver
disease. Clin Mol Hepatol. 27:363–401. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Japanese Society of Nephrology. Essential
points from evidence-based clinical practice guideline for chronic
kidney disease 2023. Clin Exp Nephrol. 28:473–495. 2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Meharie BG, Amare GG and Belayneh YM:
Evaluation of hepatoprotective activity of the crude extract and
solvent fractions of Clutia abyssinica (Euphorbiaceae) leaf
against CCl4-induced hepatotoxicity in mice. J Exp
Pharmacol. 12:137–150. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pandey B, Baral R, Kaundinnyayana A and
Panta S: Promising hepatoprotective agents from the natural
sources: A study of scientific evidence. Egypt Liver J.
13(14)2023.
|
|
46
|
Molaei E, Molaei A, Abedi F, Hayes AW and
Karimi G: Nephroprotective activity of natural products against
chemical toxicants: The role of Nrf2/ARE signaling pathway. Food
Sci Nutr. 9:3362–3384. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chidiac AS, Buckley NA, Noghrehchi F and
Cairns R: Paracetamol (acetaminophen) overdose and hepatotoxicity:
Mechanism, treatment, prevention measures, and estimates of burden
of disease. Expert Opin Drug Metab Toxicol. 19:297–317.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Oh RC, Hustead TR, Ali SM and Pantsari MW:
Mildly elevated liver transaminase levels: Causes and evaluation.
Am Fam Physician. 96:709–715. 2017.PubMed/NCBI
|
|
49
|
Giannini EG, Testa R and Savarino V: Liver
enzyme alteration: A guide for clinicians. CMAJ. 172:367–379.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nkosi CZ, Opoku AR and Terblanche SE:
Effect of pumpkin seed (Cucurbita pepo) protein isolate on
the activity levels of certain plasma enzymes in
CCl4-induced liver injury in low-protein fed rats.
Phytother Res. 19:341–345. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ruiz ARG, Crespo J, Martínez RML,
Iruzubieta P, Mercadal GC, Garcés ML, Lavin B and Ruiz MM:
Measurement and clinical usefulness of bilirubin in liver disease.
Adv Lab Med. 2:352–372. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Arguello G, Balboa E, Arrese M and
Zanlungo S: Recent insights on the role of cholesterol in
non-alcoholic fatty liver disease. Biochim Biophys Acta.
1852:1765–1778. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Maretti-Mira AC, Salomon MP, Hsu AM, Kanel
GC and Golden-Mason L: Hepatic damage caused by long-term high
cholesterol intake induces a dysfunctional restorative macrophage
population in experimental NASH. Front Immunol.
13(968366)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang X, Wu Q, Liu A, Anadón A, Rodríguez
JL, Martínez-Larrañaga MR, Yuan Z and Martínez MA: Paracetamol:
Overdose-induced oxidative stress toxicity, metabolism, and
protective effects of various compounds in vivo and in vitro. Drug
Metab Rev. 49:395–437. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Khademalhosseini M, Ranjbar E, Mohammadi
R, Khalili P, Mehran M, Jalali N, Rajabi Z and Jamali Z: Dietary
antioxidants and liver enzymes in Rafsanjan, a region in Southeast
Iran. Sci Rep. 13(8555)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gillessen A and Schmidt HHJ: Silymarin as
supportive treatment in liver diseases: A narrative review. Adv
Ther. 37:1279–1301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Karimi G, Vahabzadeh M, Lari P, Rashedinia
M and Moshiri M: ‘Silymarin’, a promising pharmacological agent for
treatment of diseases. Iran J Basic Med Sci. 14:308–317.
2011.PubMed/NCBI
|
|
58
|
Akhtar MN, Saeed R, Saeed F, Asghar A,
Ghani S, Ateeq H, Ahmed A, Rasheed A, Afzaal M, Waheed M, et al:
Silymarin: A review on paving the way towards promising
pharmacological agent. Int J Food Prop. 26:2256–2272. 2023.
|
|
59
|
Balakumar P, Rohilla A and
Thangathirupathi A: Gentamicin-induced nephrotoxicity: Do we have a
promising therapeutic approach to blunt it? Pharmacol Res.
62:179–186. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tesfa E, Munshea A, Nibret E, Mekonnen D,
Sinishaw MA and Gizaw ST: Maternal serum uric acid, creatinine and
blood urea levels in the prediction of pre-eclampsia among pregnant
women attending ANC and delivery services at Bahir Dar city public
hospitals, northwest Ethiopia: A case-control study. Heliyon.
8(e11098)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen L, Zhu Z, Ye S and Zheng M: The serum
uric acid to serum creatinine ratio is an independent risk factor
for diabetic kidney disease. Diabetes Metab Syndr Obes.
15:3693–3703. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bustos PS, Deza-Ponzio R, Páez PL, Cabrera
JL, Virgolini MB and Ortega MG: Flavonoids as protective agents
against oxidative stress induced by gentamicin in systemic
circulation. Potent protective activity and microbial synergism of
luteolin. Food Chem Toxicol. 118:294–302. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Karimi Z, Pakfetrat Z, Roozbeh J and
Janfeshan S: Toll-like receptor-2 mediates systemic inflammation in
gentamicin-induced rat nephrotoxicity. Clin Exp Pharmacol Physiol.
47:1584–1590. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Faul F, Erdfelder E, Lang AG and Buchner
A: G*Power 3: A flexible statistical power analysis
program for the social, behavioral, and biomedical sciences. Behav
Res Methods. 39:175–191. 2007.PubMed/NCBI View Article : Google Scholar
|