Introduction
Rice is a staple food source consumed by >50% of
the global population, particularly in Asia. The demand for rice
has been predicted to continue to increase in the coming decades,
driven by global population growth. The rice production industry is
expected to maintain its sustainability efforts and continue the
production of nutraceutical by-products (1). Rice bran is a by-product of rice
milling and a valuable source of nutrients containing various
active phytochemicals, such as anthocyanins, tocopherols,
tocotrienols, oryzanols and vitamins (1). Several studies have reported that
active phytochemicals from rice bran have beneficial health
effects, including antioxidant and antibacterial properties and
cancer chemoprevention (2,3).
Anthocyanins are water-soluble flavonoids found in
certain blue- and purple-colored vegetables, fruits and grains
(4). Cyanidin-3-glycosides (C3G)
and peonidin-3-glycosides (P3G) are the most abundant anthocyanins
found in nature (5). Anthocyanins
have been reported to effectively protect and suppress chronic
diseases such as cancer, owing to their antioxidant and
anti-inflammatory properties (4,6). Our
previous study demonstrated that black rice bran-derived
anthocyanins reduced DNA damage in
H2O2-induced cell death in a cholangiocyte
cell line via the activation of the nuclear factor erythroid
2-related factor 2-NAD(P)H quinone dehydrogenase 1 axis (7). Additionally, the anticancer effects of
anthocyanins have been reported as occurring through various
mechanisms, including anti-angiogenesis (8), anti-proliferation (9) and anti-metastasis (10). C3G inhibits gastric cancer
proliferation and induces apoptosis through AKT/MAPK pathway
inhibition (11). Cohort studies
have shown that regular consumption of fruits and vegetables rich
in anthocyanins is associated with a decreased incidence of
colorectal (12), bladder (13) and gastric cancers (14).
Cholangiocarcinoma (CCA) is the second most common
form of primary liver cancer with a high incidence in Southeast
Asia, particularly in Thailand and China where liver fluke
infection is prevalent (15). CCA
originates from epithelial bile duct cells and can be caused by
chronic inflammation induced by Opisthorchis viverrini liver
fluke infection (16). CCA is an
aggressive type of cancer with high invasiveness, leading to poor
overall patient prognosis (17).
Most patients are diagnosed with advanced or metastatic CCA because
CCA has non-specific symptoms during the early stages of disease
(16). CCA predominantly
metastasizes to the liver, lungs and lymph nodes (18). Previous studies have reported that
cyanidin- and delphinidin-rich extracts from mixed plants exhibit
anti-inflammatory, anti-periductal fibrosis and anticancer effects
in Opisthorchis viverrini-infected hamsters and cell models
(19,20). However, little is known about the
inhibitory effects of black rice bran-derived anthocyanins on CCA
progression. The present study aimed to investigate the effects of
black rice bran-derived anthocyanins on CCA cell migration and
invasion, as well as their underlying mechanisms of action, in CCA
cell lines.
Materials and methods
Chemical and Reagents
Cyanidin chloride (cat. no. 80022), peonidin
chloride (cat. no. 80085), cyanidin-3-glucoside (C3G; cat. no.
89616) and peonidin-3-glucoside (P3G; cat. no. 89754) were
purchased from PhytoLab GmbH & Co. Sulforhodamine B (SRB; cat.
no. S1402) and trichloroacetic acid (TCA; cat. no. T0699) were
purchased from Sigma-Aldrich (Merck KGaA). TRIzol™ reagent (cat.
no. 15596026) and the BCA Protein Assay Kit (cat. no. 23225) were
obtained from Thermo Fisher Scientific, Inc. SensiFAST cDNA
Synthesis Kit (cat. no. BIO-65053) was purchased from Bioline.
LightCycler® 480 SYBR Green I Master Mix (cat. no.
04707516001) was purchased from Roche Diagnostics. Cell culture
reagents, including DMEM (cat. no. 12100-046), Eagle's minimum
essential medium (MEM; cat. no. 61100-061), penicillin-streptomycin
(cat. no. 15140-122) and FBS (cat. no. 10270-098), were purchased
from Gibco (Thermo Fisher Scientific, Inc.). Primary antibodies
against phosphorylated AKT (pAKT; cat. no. 4060S) and total AKT
(cat. no. 4685S) were purchased from Cell Signaling Technology Inc.
Anti-claudin-1 (cat. no. sc-81796), vimentin (cat. no. sc-6260),
slugs (cat. no. sc-166476) and β-actin (cat. no. sc-47778)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
HRP-conjugated anti-mouse (cat. no. NXA931V) and anti-rabbit (cat.
no. NA934V) secondary antibodies, ECL prime blocking reagent (cat.
no. RPN415V) and ECL prime western blot detection (cat. no.
RPN2236) were obtained from Cytiva. HRP-conjugated anti-goat
secondary antibodies (cat. no. A15999) were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). A total of 16
biotinylated lectins and ABC-peroxidase solution (cat. no. PK-4000)
were purchased from Vector Laboratories, Inc. (Maravai
LifeSciences). The SignalStain® DAB substrate kit (cat.
no. 8059) was obtained from Cell Signaling Technology, Inc. Alexa
fluor 488 conjugated streptavidin (cat. no. S11223; 1:400) was
purchased from Thermo Fisher Scientific, Inc.
BBR-M-10 preparation
Black rice bran from black-pigmented rice (Oryza
sativa L.) was collected from a rice mill in Krabueang Yai
(Nakhon Ratchasima, Thailand). The black rice bran extract enriched
in anthocyanins was prepared as previously described (7). Briefly, black rice bran was soaked in
n-hexane followed by 0.1% HCl in MeOH at room temperature for 24 h.
The MeOH extracts were filtered and evaporated to obtain powdered
extracts. The powder extracts were subjected to silica column
chromatography (CC) using a gradient solvent system of hexane
[hexane-ethylacetate (EtOAc), EtOAc, EtOAc-MeOH and MeOH]. A total
of eight fractions were obtained, and the anthocyanin contents were
investigated via thin-layer chromatography (TLC) and compared with
the cyanidin, peonidin, C3G and P3G standards. Fractions with high
contents of C3G and P3G via TLC were further purified using
Sephadex LH20 CC in MeOH:H2O (80:20) to produce
fractions. A total of four fractions, including BBR-M-10 from
Sephadex LH20 CC were analyzed via TLC. TLC analysis demonstrated
that the main anthocyanin components in BBR-M-10 were C3G and P3G
when compared with the standard compounds. Anthocyanin content was
further analyzed using high-performance liquid chromatography and
compared with the cyanidin, peonidin, C3G and P3G standards. HPLC
analyses were performed using the 1260 Infinity instrument (Agilent
Technologies, Inc.) The separation of anthocyanins was analyzed
using a ZORBAX SB-C18 StableBond Analytical 4.6x250 mm 3.5 µM
column. The mobile phases used were: A, 0.1% TFA in deionized water
and B, acetonitrile. The gradient conditions were as follows: B at
10% for 5 min, followed by a linear increase to 15% B over the
following 15 min, a hold at 15% B for 5 min, then an increase in B
from 15-18% over 5 min followed, by 18-35% B over 20 min, then B at
35-90% for 10 min. The detection wavelength was 520 nm. The
chromatographic conditions were as follows: Flow rate, 1 ml/min;
column temperature, 35˚C; 20 µl injection; stop time, 60 min; and
post time, 10 min. The total anthocyanin content of BBR-M-10 was
108 mg CGE/100 g DW (100 mg/l). BBR-M-10 comprises C3G (94.5 mg/l)
and P3G (2.47 mg/l).
Cell culture
Human CCA cell lines (KKU-055 and KKU-213A), a
normal human lung fibroblast line (IMR-90) and an immortalized
human cholangiocyte (MMNK-1) cell line were used. KKU-055 (cat. no.
JCRB1551) and KKU-213A (cat. no. JCRB1557) were previously
established and authenticated (21). Certificates of analysis were
obtained from the Japanese Collection of Research Bioresources Cell
Bank. The immortalized human cholangiocyte MMNK-1 cell line was
provided by Dr. Sopit Wongkham (Khon Kaen University, Thailand).
CCA cell lines and MMNK-1 were cultured in DMEM supplemented with
inactivated 10% FBS and 100 U/ml penicillin-streptomycin. IMR-90
cells (cat. no. CCL-186) were obtained from the American Type
Culture Collection and cultured in EMEM supplemented with
inactivated 10% FBS and 100 U/ml penicillin-streptomycin. All the
cell lines were incubated at 37˚C in a humidified incubator
containing 5% CO2. PCR assays were used to verify the
lack of mycoplasma contamination in all cell lines.
BBR-M-10 treatment and cell
viability
KKU-055, KKU-213A, IMR-90 and MMNK-1 cells were
seeded at 7x103 cells/well into 96-well plates and
incubated overnight. The cells were then treated with various
concentrations of BBR-M-10 from 0-5,000 µg/ml and incubated for 24
h. Cell viability was assessed using the SRB assay. Briefly,
treated cells were fixed with 10% TCA at 4˚C for overnight and
stained with 0.4% (w/v) SRB in 1% (v/v) acetic acid at room
temperature for 30 min. The unbound SRB was removed, washed three
times with 1% (v/v) acetic acid and dried. Next, the stained cells
were solubilized in 10 mM Tris-base (pH 10) and mixed in a shaker
at room temperature for 15 min. Absorbance at 564 nm was measured
using a TECAN Infinite 200 Pro microplate reader (Tecan Group,
Ltd.). The IC50 was calculated using GraphPad Prism
(version 8; Dotmatics).
RNA extraction and quantitative
PCR
KKU-055 and KKU-213A cells were seeded at
3.5x105 cells/well into 6-well plates. After 24 h, cells
were treated with BBR-M-10 at 0 and 200 µg/ml for 24 h. Total RNA
was extracted using TRIzol™ reagent according to the manufacturer's
instructions. RNA quantity and quality were measured using a
NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.) and agarose gel electrophoresis,
respectively. A SensiFAST cDNA Synthesis Kit was used to synthesize
cDNA. Quantitative PCR was performed using the
LightCycler® 480 SYBR Green I Master Mix to investigate
gene expression. The primer sequences for all genes were obtained
from previous studies (Table I)
(22,23). Gene amplification was performed by
initial denaturation at 95˚C for 5 min, followed by 40 cycles of
denaturation at 95˚C for 10 sec, annealing at 50˚C for ST6GAL1,
annealing at 60˚C for ST3GAL1, 3, 4, and 6 for 10 sec and a final
extension at 72˚C for 10 sec. b-actin was used as the endogenous
control. The relative mRNA levels of each gene were normalized to
b-actin and calculated using the 2-∆∆Cq method (24).
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| ST3GAL1 | F:
GGACCCTGAAAGTGCTCA |
| | R:
TCTCCAGCATAGGGTCCA |
| ST3GAL3 | F:
GTATGATCGGTTGGGCTTC |
| | R:
CGCTCGTACTGCTCAGG |
| ST3GAL4 | F:
GTCAGCAAGTCCCGCT |
| | R:
CTTGTTGATGGCATCTCCC |
| ST3GAL6 | F:
GGTATCTTGTGGCCATATTCC |
| | R:
CTCCATTACCAACCACCAC |
| ST6 β-galactoside
α-2,6-sialyltransferase 1 | F:
CTTGTTTTCCTGCTCAGA |
| | R:
GCAAACAGAAGAAAGACCA |
| β-actin | F:
GATCAGCAAGCAGGAGTATGACG |
| | R:
AAGGGTGTAACGCAACTAAGTCATAG |
Wound-healing assay
KKU-055 and KKU-213A cells were seeded at a density
of 3x105 cells/well in 24-well plates. After 24 h of
growth to 90% cell confluence, a vertical wound was scratched
through the cell monolayer using a sterile 200 µl plastic
micropipette tip. Cell debris was removed and replaced with
BBR-M-10 at 50, 100 and 200 µg/ml in serum-free DMEM. Cell
migration during the wound healing process was observed and
digitally photographed using a light microscope at 0, 12, 18 and 24
h (magnification, x100). The wound area was evaluated using Image J
software (version 1.53a; National Institutes of Health) and
calculated as follows: (Area of original wound-area of wound during
healing)/area of the original wound.
Transwell migration and invasion
assay
Transwell inserts were coated with 100 µl of
Matrigel 200 µg/ml and incubated at 37˚C for 2 h. KKU-055 and
KKU-213A cells at a density of 1x105 cells in 200 µl of
FBS-free medium with or without BBR-M-10 at 50, 100 and 200 µg/ml
were seeded into Transwell inserts for the migration assay
Matrigel-coated Transwell inserts for the invasion assay. Next, 600
µl of 10% FBS-containing medium was loaded into the lower chamber
to create a chemotactic gradient and incubated at 37˚C. After 12 h,
the Transwell inserts were removed from the plate and a
cotton-tipped applicator was used to remove the cells on the upper
side of the membranes. The migrated cells on the bottom side of the
membranes were fixed with 10% TCA at 4˚C overnight, stained with
SRB at room temperature for 30 min, washed with 10% acetic acid and
dried. Images of migrated cells were captured using a light
microscope and counted. Migrated cells were counted in five fields
of view. The presented values were the number of total migrated
cells per fields of view at x100 magnification.
Lectin cytochemistry
KKU-055 and KKU-213A cells were seeded at a density
of 3x104 cells/well in 24-well plates and incubated
overnight. The cells were treated with 0 and 200 µg/ml BBR-M-10 for
24 h. The cells were fixed with 4% paraformaldehyde in PBS for 15
min and permeabilized with 0.1% Triton X-100 in PBST for 10 min at
room temperature. The endogenous hydrogen peroxide-generating
activity was blocked with 0.3% hydrogen peroxide for 30 min at room
temperature. Nonspecific binding was blocked with 3% BSA (HIMedia
Laboratories, LLC) for 30 min at room temperature. A 1:500 dilution
of biotinylated lectins (Table II)
was added and incubated at 4˚C overnight on a shaker. The
ABC-Peroxidase Solution was then used as the secondary antibodies
for 1 h at room temperature to determine the lectin signal. A
SignalStain® DAB substrate kit was used to visualize the
signal under a light microscope at x100 magnification.
 | Table IIGlycan expression in CCA cell lines
evaluated by staining with a panel of 16 lectins. |
Table II
Glycan expression in CCA cell lines
evaluated by staining with a panel of 16 lectins.
| | KKU-055 | KKU-213A |
|---|
| Lectin | Major sugar | 0 µg/ml | 200 µg/ml | 0 µg/ml | 200 µg/ml |
|---|
| Arachis
hypogaea (peanut) agglutinin | Galactose | ++ | ++ | + | + |
| Glycine max
(soybean) |
N-acetylgalactosamine | + | + | + | + |
| Ulex europaeus
agglutinin I; | Fucose | ++ | ++ | +++ | ++ |
| Triticum
vulgaris (wheat germ) |
N-acetylglucosamine | +++ | +++ | +++ | +++ |
| Dolichos
biflorus agglutinin |
N-acetylgalactosamine | +++ | +++ | +++ | +++ |
| Ricinus
communis agglutinin |
N-acetylgalactosamine | +++ | +++ | +++ | ++ |
| Concanavalin A | Mannose | +++ | +++ | +++ | +++ |
| Lycopersicon
esculentum (tomato) lectin |
N-acetylglucosamine | +++ | +++ | ++ | ++ |
| Erythrina
cristagalli lectin | Galactose | +++ | +++ | +++ | +++ |
| Solanum
tuberosum (potato) lectin |
N-acetylglucosamine | + | + | + | + |
| Jacalin | Galactose | + | + | + | + |
| Datura
Stramonium lectin |
N-acetylglucosamine | ++ | ++ | ++ | ++ |
| Vicia
villosa agglutinin |
N-acetylgalactosamine | + | + | ++ | ++ |
| Griffonia
(Bandeiraea) simplicifolia lectin II |
N-acetylglucosamine | ++ | ++ | ++ | ++ |
| Sambucus
nigra lectin | (α2,6) linked
sialic acid | +++ | +++ | +++ | ++ |
| Maackia
Amurensis lectin II | (α2,3) linked
sialic acid | - | - | + | + |
Lectin staining by flow cytometry
KKU-055 and KKU-213A cells were seeded at a density
of 3x105 cells/well in 6-well plates and incubated at
37˚C. After 24 h, the cells were treated with 0 and 200 µg/ml
BBR-M-10 and incubated at 37˚C for 24 h. The cell pellet was
harvested and washed with 2% FBS in PBS. The cell pellet was fixed
with 4% paraformaldehyde in PBS at room temperature for 15 min and
nonspecific binding was blocked with 3% BSA for 30 min on ice. The
cell pellet was then incubated for 1 h on ice with biotinylated SNA
lectins (cat. no. PK-4000; 1:500). Alexa fluor 488 conjugated
streptavidin (cat. no. S11223; 1:400) was then added for 1 h on ice
to determine the lectin signal. Cell pellets were resuspended in 2%
FBS in PBS and analyzed by flow cytometry using Attune NxT
fluorescent detector and Attune Cytometric Software (version
5.3.2415.0; Thermo Fisher Scientific, Inc.). Then mean fluorescence
intensity was analyzed using the Flowing software (version 2; Turku
Bioscience).
Immunofluorescent analysis
KKU-055 and KKU-213A cells were seeded at a density
of 2.5x104 cells/well in 8-well cell culture slides and
incubated at 37˚C and 5% CO2 overnight. After 24 h of
incubation, cells were treated with 0 and 200 µg/ml BBR-M-10 for 24
h. The cells were washed with PBS and fixed with 4%
paraformaldehyde at room temperature for 15 min. The fixed cells
were permeabilized with 0.1% Triton-X100 in PBS for 10 min on ice.
A blocking solution (2% BSA in 0.1% PBST) was added to the cells
for 30 min on ice, and then the cells were incubated at 4˚C
overnight with phalloidin Alexa Fluor 647 antibodies (cat. no.
#8940S; 1:50; Cell Signaling Technology, Inc.). After washing with
PBS, nuclear counterstaining with Hoechst 33258 solution (1:1,000)
was performed on ice for 10 min. Stained cells were visualized
using a confocal microscope (Nikon Corporation).
SDS-PAGE and western blotting
analysis
KKU-055 and KKU-213A cell lysates were extracted
using protein lysis buffer containing 10% TritonX, protease
inhibitor cocktail (Roche diagnostics GmbH) and Tris-lysis buffer
pH 7.5. The protein determination method was a BCA protein assay
kit (Thermo Fisher Scientific Inc.). A total of 40 µg of protein
extracts were separated using 10-15% SDS-PAGE and transferred to a
nitrocellulose membrane (Cytiva). Non-specific binding was blocked
with 5% ECL prime blocking reagent (Cytiva) containing 0.05% Tween
20 at room temperature for 1 h. Then, the membranes were probed
with primary antibodies at dilutions of 1:1,000 for vimentin,
claudin-1, pAKT and AKT and 1:500 for ST6GAL1 in 0.05% PBST at 4˚C
overnight. The membranes were then washed three times with 0.1%
PBST three times for 10 min each. Membranes were incubated with
secondary antibodies at room temperature for 1 h. ECL™ Prime
Western blotting detection reagents (Cytiva) were used to determine
the target protein signals and visualized using the ImageQuant™ LAS
500 (Cytiva). The density of each target protein was determined
using ImageJ software and normalized to β-actin.
Statistical analysis
The results were expressed as the mean ± SD (n=3).
The one-way ANOWA followed by Bonferroni's multiple comparisons
test was used to analyze data. GraphPad Prism was used for data
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of BBR-M-10 on cell
viability
To evaluate the effect of BBR-M-10 on CCA cell
viability, KKU-055 and KKU-213A cells were treated with BBR-M-10 at
various concentrations. A normal fibroblast cell line (IMR-90) was
used as the normal control cell line because IMR-90 cells were
derived from a human embryo with a normal karyotype and have a
finite lifespan (25). The cell
viability test demonstrated that BBR-M-10 was not toxic to KKU-055,
KKU213A and IMR-90 cells. The respective IC50 value for
BBR-M-10 was 2.94 and 3.47 mg/ml, for the CCA cell lines KKU-055
and KKU-213A, respectively, and for IMR-90 cells it was 4.30 mg/ml
(Fig. 1A). Additionally, the effect
of BBR-M-10 in MMNK-1 cells was measured. The IC50 value
for BBR-M-10 was 0.75 mg/ml for MMNK-1 (Fig. S1). Taken together, the decreases in
cell viability of MMNK-1 and CCA cell lines were observed at high
doses of BBR-M-10 (1,250-5,000 µg/ml), whereas high doses of
BBR-M-10 showed less toxicity to the IMR-90 cell line (Fig. S2). Our previous study demonstrated
that the main anthocyanin components in BBR-M-10 are C3G and P3G
(7). Thus, the effects of C3G and
P3G on CCA cell viability were investigated in both CCA cell lines.
These results showed that CCA cell viability was not significantly
impacted by C3G or P3G treatment at 0-500 µM (Fig. 1B and C). Thus, low doses of BBR-M-10 (50, 100
and 200 µg/ml) were selected to assess the effect of BBR-M-10 on
CCA cell migration and invasion.
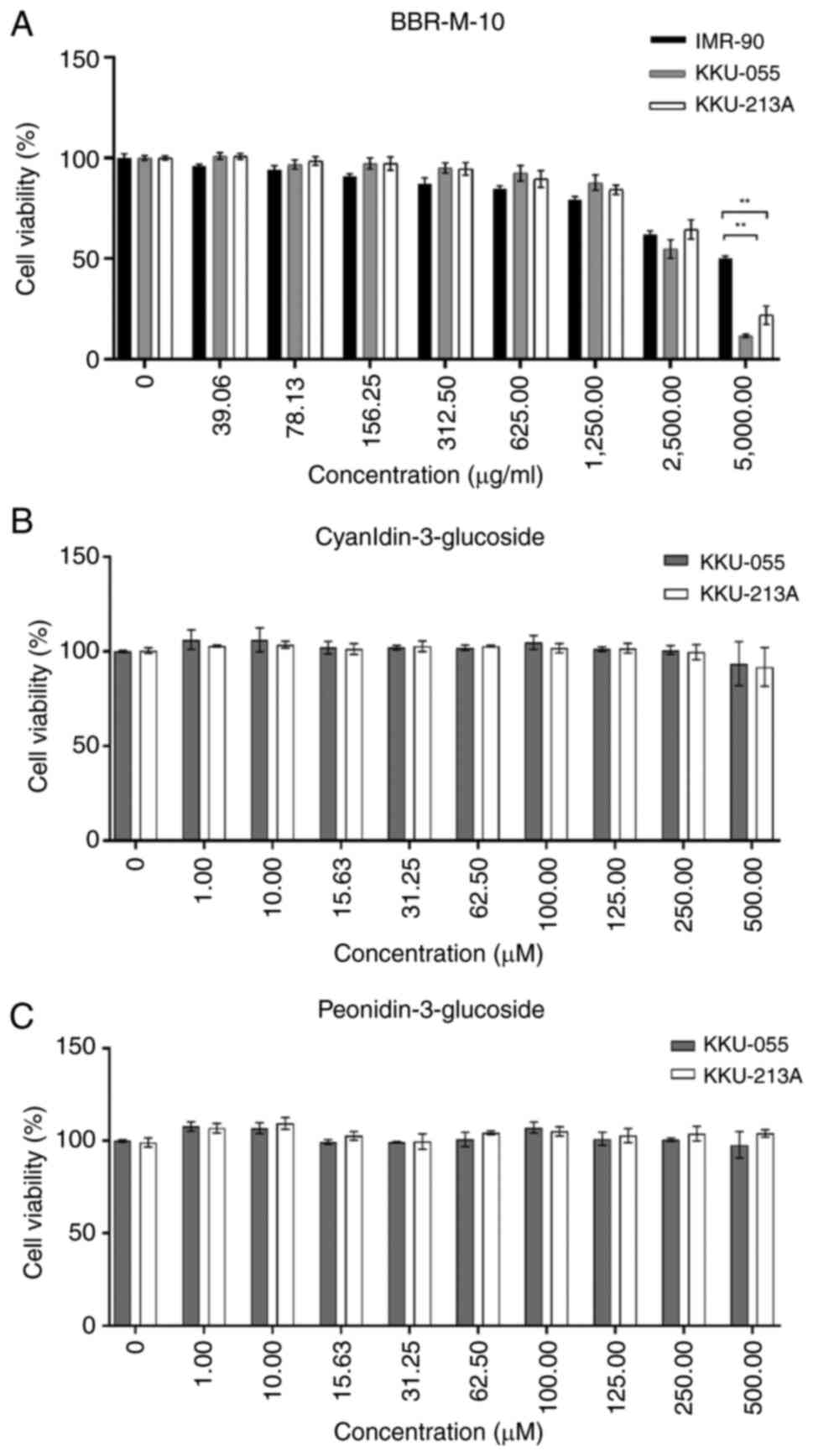 | Figure 1Anti-proliferative effect of BBR-M-10
on CCA cell lines. (A) BBR-M-10 treatment at concentration 0,
39.06, 78.13, 156.25, 312.50, 625.00, 1,250.00, 2,500.00 and
5,000.00 µg/ml in CCA cell lines and normal lung fibroblasts
(IMR-90). (B) Cyanidin-3-glucoside and (C) peonidin-3-glucoside
treatment at concentration 0, 1,10, 15.63, 31.25, 62.50, 100, 125,
250, and 500 µM in CCA cell lines. Data were presented as mean ± SD
(n=3). **P<0.01. CCA, cholangiocarcinoma. |
BBR-M-10 inhibited CCA cell migration
and invasion
The effects of anthocyanins on crucial cancer cell
properties, including cell proliferation, migration and invasion,
have been documented in various types of cancers such as squamous
cell carcinoma, liver cancer, CCA and cervical cancer (10,19).
To address whether BBR-M-10 affected cancer cell activity, the
migration and invasion properties of CCA cell lines upon BBR-M-10
treatment were assessed using wound healing, Transwell migration
and invasion assays. KKU-055 and KKU213A cells were treated with
BBR-M-10 at 0, 50, 100 and 200 µg/ml. The wound healing assay
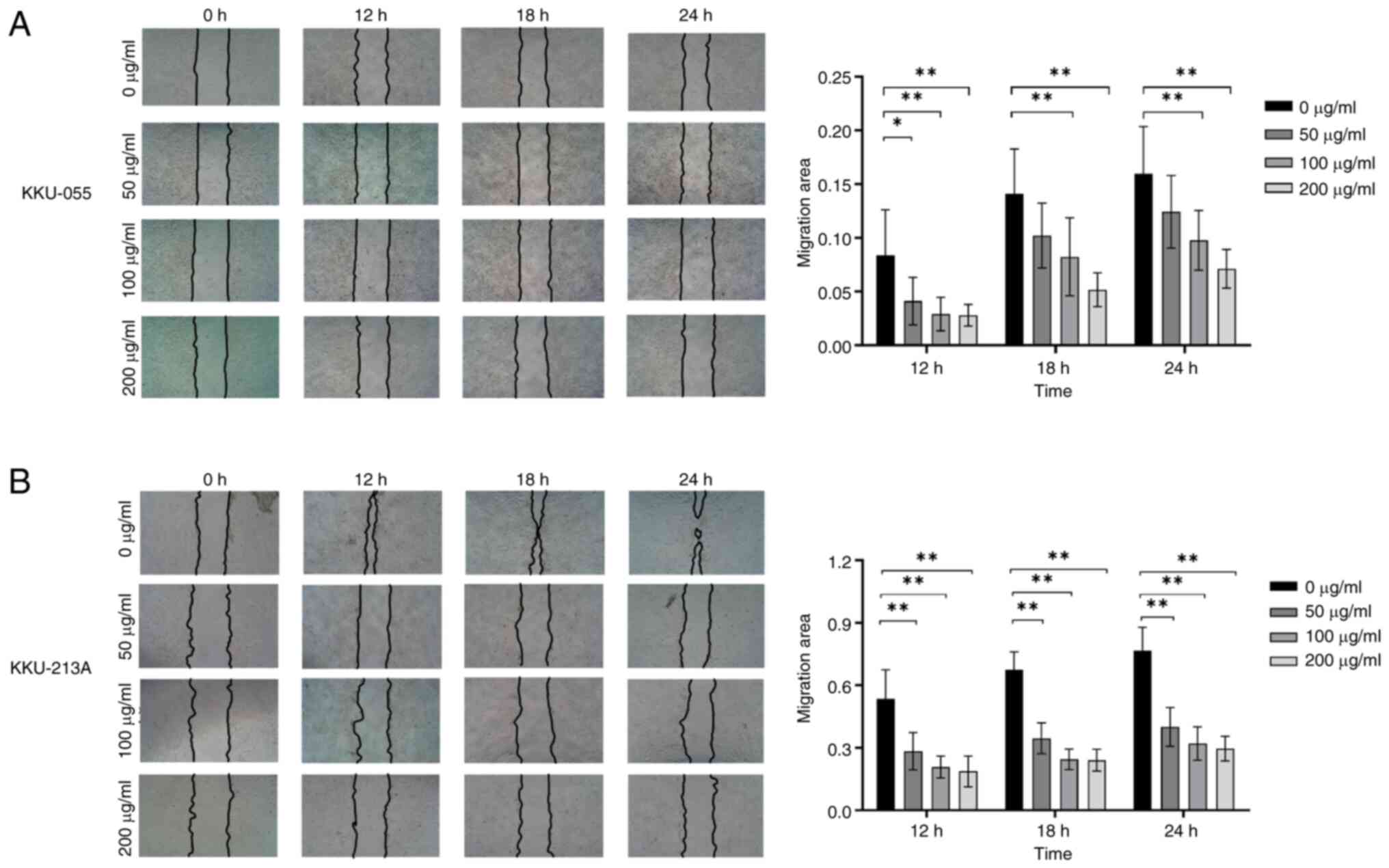
results demonstrated a statistically significant dose-dependent
decrease in the migration ability of both KKU-055 and KKU-213A
cells (Fig. 2A and B). Additionally, BBR-M-10 treatment at 100
and 200 µg/ml significantly decreased the migration area in both
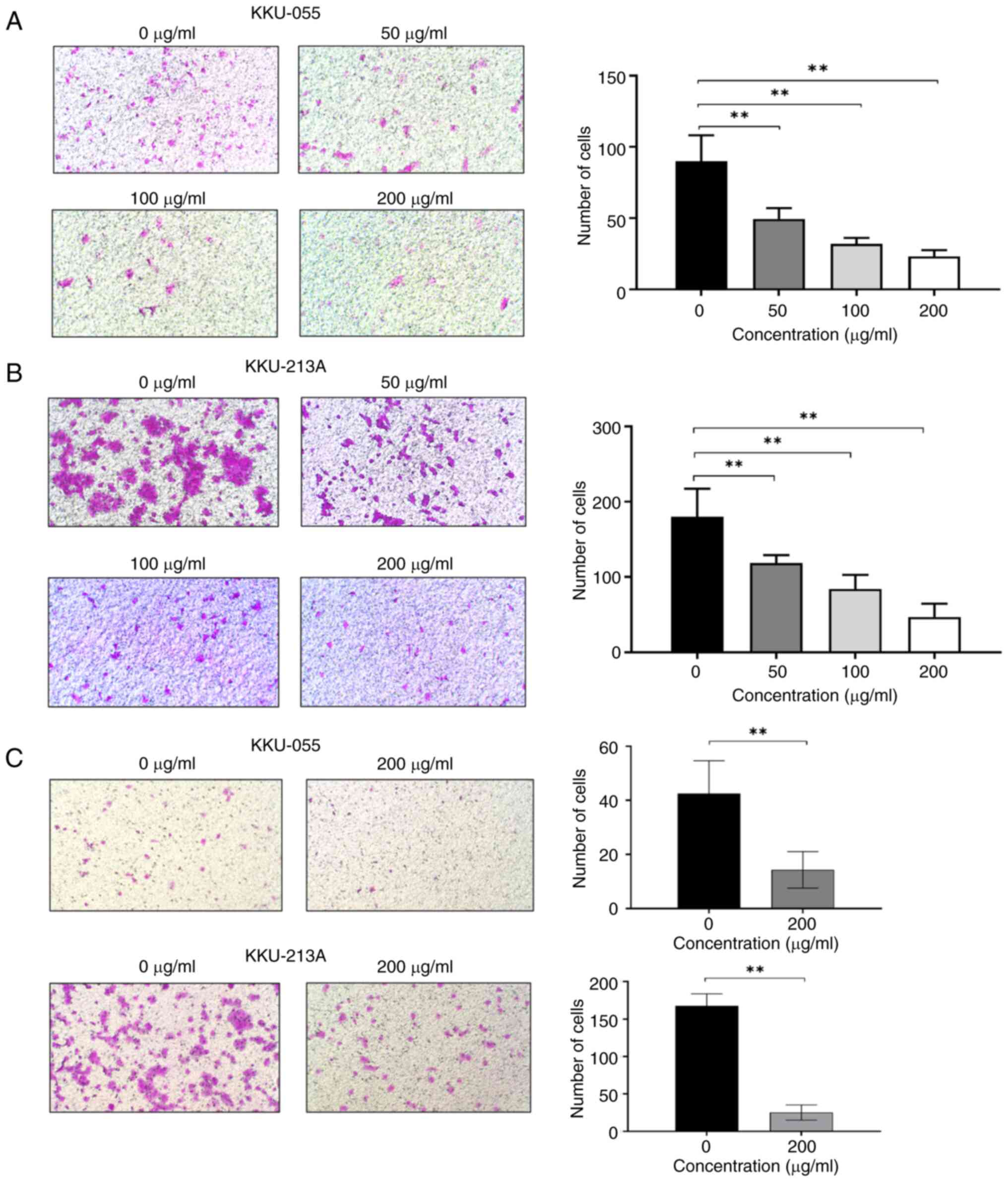
KKU-055 and KKU-213A cells. Transwell migration assay results
demonstrated that BBR-M-10 treatment at 50, 100 and 200 µg/ml
significantly reduced the migration ability of KKU-055 and KKU-213A
cells (Fig. 3A and B). Moreover, BBR-M-10 treatment at 200
µg/ml significantly decreased the cell invasion ability of KKU-055
and KKU-213A cells (Fig. 3C). These
findings suggested that BBR-M-10 may inhibit the signaling pathway
that promotes CCA cell migration and invasion.
BBR-M-10 attenuated
epithelial-mesenchymal transition in CCA via the AKT pathway
Actin polymerization serves a crucial role in
regulating cell structure during cancer cell migration and invasion
(26). To address the effect of
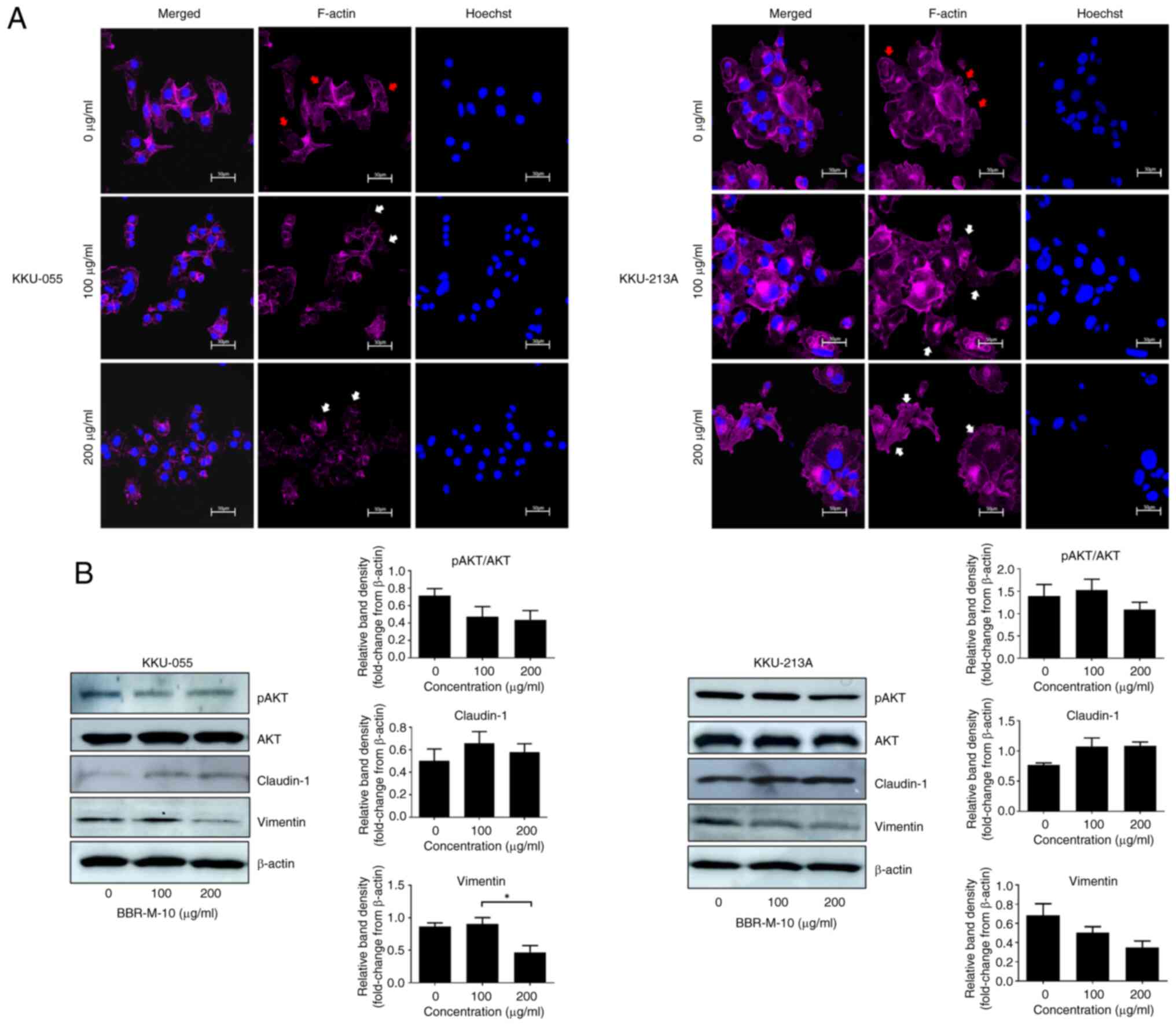
BBR-M-10 on lamellipodium formation, KKU-055 and KKU213A cells were
treated with BBR-M-10 at 0, 100 and 200 µg/ml. The distribution of
filamentous actin (F-actin) and the overall shape of cells were
detected by phalloidin staining and visualized using confocal
microscopy. BBR-M-10 reduced F-actin accumulation at the edges of
both KKU055 and KKU-213 cells, suggesting a decrease in migration
ability (Fig. 4A).
Epithelial-to-mesenchymal transition (EMT) is a crucial initiating
step in cancer invasion and metastasis and is modulated via
multiple signaling pathways, including AKT signaling. Several EMT
markers, including claudin-1, Slug and vimentin, are regulated by
this pathway (27). The effect of
BBR-M-10 on CCA cell migration and invasion via EMT was also
investigated. Western blotting analysis demonstrated that BBR-M-10
treatment at 100 and 200 µg/ml markedly increased the protein
expression levels of expression of claudin-1 in KKU-055 (Fig. 4B). BBR-M-10 treatment at 200 µg/ml
significantly decreased vimentin protein expression levels compared
with cells treated with 100 µg/ml BBR-M-10. Additionally, a marked
reduction in the ratio of phosphorylated to non-phosphorylated AKT
(pAKT/AKT) was observed in KKU-055 cells treated with BBR-M-10 at
100 and 200 µg/ml. The protein expression levels of claudin-1 were
markedly increased, whilst the protein expression levels of
vimentin were markedly decreased in KKU-213A cells treated with 100
and 200 µg/ml BBR-M-10 (Fig. 4B). A
marked decrease in the pAKT/AKT ratio was observed in KKU-213A
cells treated with BBR-M-10 at 200 µg/ml only.
BBR-M-10 altered sialylation in
CCA
Aberrant glycosylation is a hallmark of cancer and
is associated with certain behaviors exhibited by cancer cells,
including EMT (28). To investigate
whether BBR-M-10 induced glycosylation changes in CCA cell lines, a
panel of 16 lectins were used to identify differences in glycan
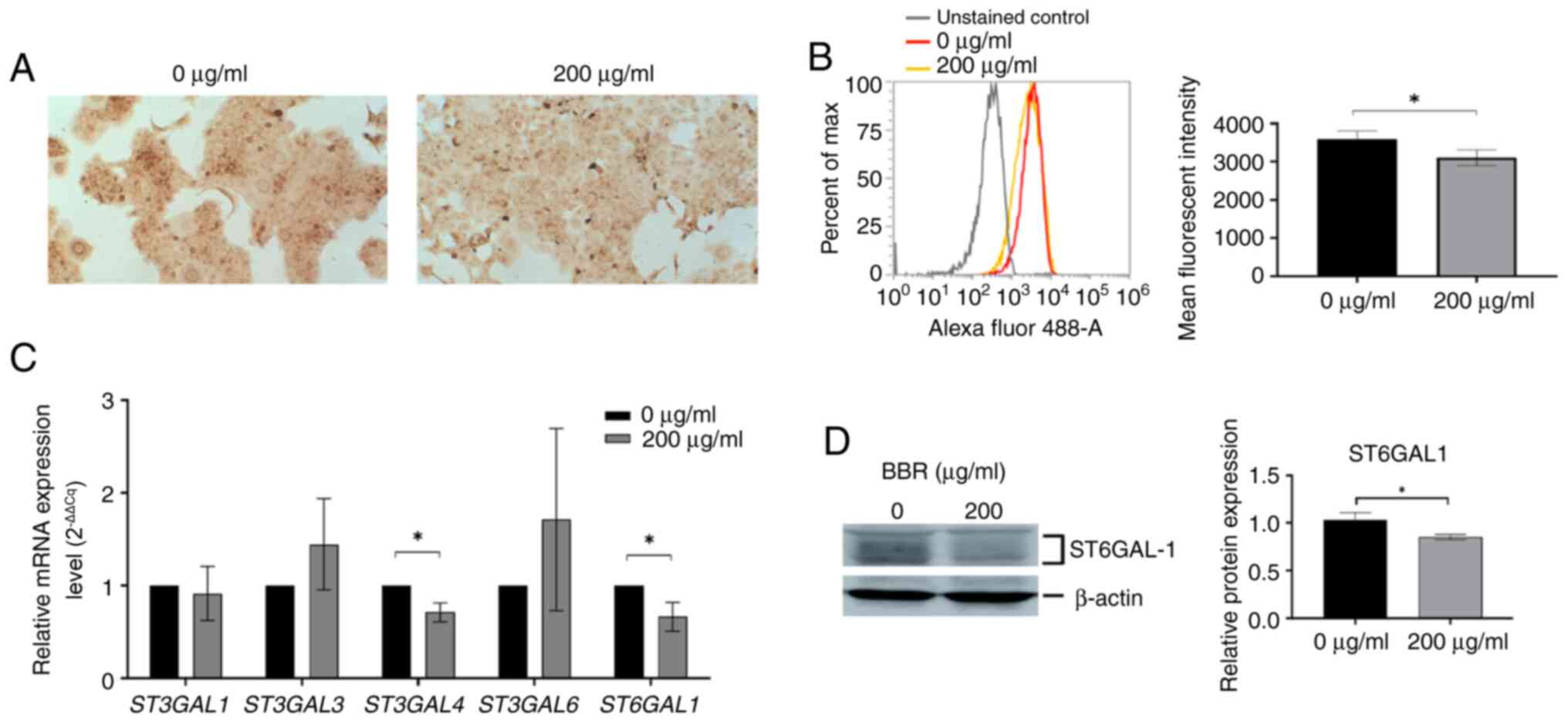
expression between BBR-M-10-treated and control CCA cells (Table II). Lectin cytochemistry
demonstrated that low expression levels of SNA binding
α2,6-sialylated glycan was observed in BBR-M-10-treated KKU-213A
cells compared with the control (Fig.
5A). Additionally, flow cytometry analysis confirmed that
surface SNA binding α2,6-sialylated glycan showed a significant
decrease in expression levels in BBR-M-10-treated KKU-213 cells
(Fig. 5B). Next, it was further
investigated whether BBR-M-10 altered the expression of
sialyltransferase genes, including α2,3 sialyltransferase genes
ST3GAL1, 2, 3, 4 and 6 and α2,6 sialyltransferase gene
ST6GAL1. Gene expression level analysis demonstrated that
the mRNA expression levels of ST3GAL4 and ST6GAL1 were
significantly reduced after BBR-M-10 treatment in KKU-213 cells
compared with the control cells (Fig.
5C).
Moreover, the protein expression level of ST6GAL1
was significantly decreased after BBR-M-10 treatment in KKU-213
cells (Fig. 5D). The altered
expression of α2,3-sialylated glycans in both CCA cell lines was
not detected via MAL II Lectin staining after BBR-M-10 treatment.
Therefore, the protein expression levels of ST3GAL4 were not
included in the present study. Taken together, these findings
suggest that BBR-M-10 may affect CCA progression via the reduction
of glycoprotein sialylation.
Discussion
Cancer is a disease characterized by certain
cellular properties, including increased proliferation, migration,
invasion, metastasis and drug resistance (29). Despite the approval of various
chemotherapies for cancer treatment, success in improving the
survival of patients with metastatic cancer remains limited
(30). In liver fluke-associated
CCA, cell migration to the lymph nodes and distant metastasis are
crucial factors affecting poor prognosis and shortened survival in
patients with CCA (31,32). Therefore, appropriate therapeutic
drugs are needed to prevent and treat CCA. The present study
focused on the potential role of BBR-M-10 in reducing CCA
progression. These results demonstrated that BBR-M-10 did not cause
significant cytotoxic effects in either CCA cell lines or normal
cells. This result is consistent with previous studies on
hepatocellular carcinoma, breast cancer and prostate cancer
(10,33,34).
The cytotoxicity of BBR-M-10 in the immortal MMNK-1 cholangiocyte
cell line was also performed. A decrease in the viability of MMNK-1
and CCA cells was observed at high doses of BBR-M-10, whereas high
doses of BBR-M-10 were less toxic to IMR-90 cells. This may be due
to the immortality of MMNK-1 with retroviral vector encoding simian
virus 40 large T and human telomerase, whereas IMR-90 cells are
derived from a human embryo with a normal karyotype and have a
finite lifespan (25,35). Thus, IMR-90 cells were selected as
the control cell line in the present study instead of MMNK-1 cells,
because they are a human-derived cell line and are well
characterized. Additionally, IMR-90 cells have not undergone
genetic modifications that could affect their behavior.
Intuyod et al (20) anthocyanin complex (AC) nanoparticles
serve a role in CCA development and progression, as evidenced by
anti-inflammatory and anti-fibrotic effects of AC nanoparticles in
an O. viverrini-infected hamster model and the anticancer
activity of AC nanoparticle in CCA cell lines. BBR-M-10 exhibits a
chemoprotective effect on CCA via the reduction of reactive oxygen
species and the anti-metastatic effect of BBR-M-10 in CCA cell
lines has been previously reported (7). Anthocyanins exhibit a wide variety of
biological activities. This may be due to the types of anthocyanins
in the extract used. BBR-M-10 is composed of C3G and P3G, whereas
the AC nanoparticles were prepared from cyanidin, delphinidin,
turmeric extract, caffeic acid and piperine. Intuyod et al
showed that AC nanoparticles mainly had an effect on CCA cell
death. This may be due to the growth inhibition caused by
delphinidin or turmeric extract that has been previously reported
in various types of cancers including CCA (36,37).
Taken together, BBR-M-10 significantly decreased the migration and
invasion of CCA cell lines via reduced lamellipodium formation and
EMT. Thus, BBR-M-10 treatment might represent an alternative
treatment for metastatic CCA.
EMT facilitates metastasis in various types of
cancers (38,39). EMT triggers a phenotypic shift in
epithelial cancer cells, transforming them into mesenchymal cells,
ultimately leading to cancer cell metastasis (38). Mesenchymal cells exhibit loss of
cell-to-cell interactions regulated by a decrease in epithelial
cell markers and an increase in the expression of mesenchymal cell
markers (40). Numerous studies
have highlighted the effects of anthocyanins on cancer cell
invasion. Anthocyanins derived from plants and fruits exhibit
anti-metastatic effects in various cancers, including
hepatocellular carcinoma and breast and prostate cancers (10,33,34).
These studies are consistent with our present study, in which
BBR-M-10 significantly reduced the migration and invasion of CCA
cell lines (10,31,34).
The actin cytoskeleton regulates cell structure and motility,
allowing cells to migrate and invade (26). The dynamic polymerization and
depolymerization of F-actin are regulated by actin-binding
proteins, which typically stabilize the polymerization of F-actin
and drive the protrusion of the cell membrane (26,41).
Alteration and accumulation of F-actin at the cell edges or in the
lamellipodium has been reported to contribute to the aggressiveness
of cancer cell invasion through the extracellular matrix in several
cancer types (42,43). The reduction in F-actin in
lamellipodia formation has been associated with a decrease in
cancer invasiveness (44). Previous
studies reported the effects of flavonoids and anthocyanins on the
disruption of F-actin formation in diabetic kidney and prostate
cancer cells (34,45). In the present study, BBR-M-10
reduced F-actin accumulation in CCA cell lines. These findings
suggest that BBR-M-10 prevented CCA cell migration and invasion by
modulating F-actin formation.
Moreover, BBR-M-10 altered the expression of EMT
genes via the upregulation of epithelial markers (claudin-1) and
downregulation of mesenchymal markers (vimentin). This may be due
to the role of vimentin in providing flexibility to cells and
promoting cell motility in various cancers (46,47).
In addition, claudin-1 is recognized as a tight junction protein.
Downregulation of claudin-1 can be associated with invasion in
various cancers, including CCA (40,48).
However, in some cancers, claudin-1 has the opposite role in that
its high expression levels suggests its potential involvement in
the progression of cancers, such as colon cancer (49). The findings of the present study
suggested that BBR-M-10 attenuated CCA cell migration and invasion
via a decrease in the EMT.
The PI3K/AKT pathway has been reported to be a
driver of cancer progression through increasing cell proliferation
and metastasis (50). The increase
in activation of the PI3K/AKT signaling pathway is correlated with
cell growth and metastasis in CCA (51). Additionally, downregulation of
epithelial marker E-cadherin, and upregulation of EMT-related
transcription factors (EMT-TFs), including Snail, Twist and ZEB1 in
CCA tissues were strongly associated with a positive metastasis
status (52). It has been reported
that EMT-TFs, including Snail, Twist and ZEB1, are regulated by the
PI3K/AKT pathway. EMT-TFs induce the expression of mesenchymal
markers, including N-cadherin and vimentin, and suppress the
expression of epithelial markers such as E-cadherin and claudin-1
(53,54). Additionally, inhibition of AKT
activity reduces the expression of EMT-TFs and EMT markers, leading
to decreases in cell migration and invasion (55,56).
In the present study, BBR-M-10 diminished the phosphorylation of
AKT in CCA cell lines, resulting in increased claudin-1 and
decreased vimentin expression levels in CCA cell lines. This
finding is consistent with studies in breast cancer, in which
anthocyanins extracted from cherries reduced invasion of cells via
downregulation of AKT expression (57). Additionally, MK-2206 is an orally
active allosteric Akt inhibitor. The effects of MK2206 on CCA
migration and invasion have been previously reported. MK2206
reduces the phosphorylation of Akt leading to the reduction of CCA
migration and invasion (58). In
the present study, the phosphorylation of AKT upon BBR-M-10
treatment was markedly decreased in both CCA cell lines.
Additionally, Chen et al (59) and Zhou et al (33) demonstrated that black rice
anthocyanins inhibit EMT and metastasis of breast cancer cells by
targeting the RAS/RAF/MAPK pathway or protein tyrosine kinase 2
(FAK) signaling. Therefore, the potential anti-metastatic effect of
BBR-M-10 via RAS/RAF/MAPK pathway or FAK signaling in CCA requires
further study in the future.
Glycosylation, a major post-translational
modification, usually acts as a fine tuner of cellular and
molecular interactions (60).
Glycosylation changes are a hallmark of cancer that serve a
signaling role in several aspects of malignancy, including
proliferation, invasion and metastasis. EMT is a critical step in
metastasis and is associated with glycosylation changes, as
evidenced by N-glycan branching, O-glycan truncation, terminal
sialylation and terminal fucosylation during EMT (61). The present study demonstrated a
decrease in terminal α2,6-sialylated glycans in CCA cell lines via
SNA Lectin staining after BBR-M-10 treatment. However, BBR-M-10
altered the expression levels of sialyltransferases only in
KKU-213A cells and downregulated ST6GAL1 expression, the primary
enzyme responsible for α2,6sialylation. These findings suggested
that BBR-M-10-altered sialylation is cell type-specific, since
KKU-213A has a high invasion capacity. This is consistent with a
previous study that showed that patients with metastatic CCA with
low ST6GAL1 expression levels had a shorter overall survival
compared with patients with metastatic CCA with high ST6GAL1
expression levels (62).
Moreover, ST6GAL1 overexpression promotes cell
migration and invasion by activating the PI3K/AKT signaling pathway
(63). Based on the results of the
present study, it could be suggested that BBR-M-10 attenuated AKT
activation, reduced CCA cell migration and invasion through the
downregulation of ST6GAL1 and EMT-related genes. To the best of our
knowledge, the present study was the first to report the effect of
anthocyanins on cancer-associated glycosylation. However, further
studies are needed to elucidate the mechanism of action underlying
BBR-M-10-modulated sialylation.
In conclusion, black rice bran is a valuable source
of anthocyanins with beneficial health effects. In the present
study, BBR-M-10 diminished metastatic phenotypes, including reduced
EMT and sialylation. The AKT pathway may potentially serve a vital
role in this inhibitory effect. These findings suggested that
BBR-M-10 could potentially be used in the future as a treatment for
metastatic CCA.
Supplementary Material
Anti-proliferative effect of BBR-M-10
on immortal cholangiocyte cell line MMNK-1. MMNK-1 cell line was
treated with BBR-M-10 treatment at concentration 0, 39.06, 78.13,
156.25, 312.50, 625.00, 1,250.00, 2,500.00 and 5,000.00
μg/ml. Data were presented as mean ± SD (n=3).
*P<0.05, **P<0.01.
Anti-proliferative effect of BBR-M-10
on IMR-90, MMNK-1, KKU-055 and KKU-213A cells. IMR-90, MMNK-1,
KKU-055, and KKU-213A, cell lines were treated with BBR-M-10
treatment at concentration 0, 39.06, 78.13, 156.25, 312.50, 625.00,
1,250.00, 2,500.00 and 5,000.00 μg/ml. Data were presented
as mean ± SD (n=3). **P<0.01.
Acknowledgements
We thank Mr. Bryan Roderick Hamman, Publication
Clinic, Research Affairs, Faculty of Medicine, Khon Kaen
University, Khon Kaen 40002, Thailand for assistance with the
English language editing of the manuscript. We thank Professor
Sopit Wongkham, Department of Biochemistry, Faculty of Medicine,
Khon Kaen University, Khon Kaen 40002, Thailand for providing the
immortalized human cholangiocyte MMNK-1 cell line.
Funding
Funding: The present work was supported by the Thailand Research
Fund International Research Network grant (grant no. IRN62W0004)
and both the Suranaree University of Technology, Thailand Science
Research and Innovation (TSRI) and National Science, Research and
Innovation Fund (grant no. 179281).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JKC, CT and KT acquired grant resources. CT
conceived and designed the study. SK, SC and CT performed the
experiments. CT and SK analyzed the data. JKC and KT provided
advice and resources. SK drafted the manuscript. CT and JKC revised
the manuscript. JKC assisted with English language editing. All
authors have read and approved the final version of the manuscript.
CT and SK confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tan BL, Norhaizan ME and Chan LC: Rice
bran: From waste to nutritious food ingredients. Nutrients.
15(2503)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wisetkomolmat J, Arjin C, Satsook A,
Seel-Audom M, Ruksiriwanich W, Prom UTC and Sringarm K: Comparative
analysis of nutritional components and phytochemical attributes of
selected Thai rice bran. Front Nutr. 9(833730)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Manzoor A, Kumar Pandey V, Dar AH, Fayaz
U, Dash KK, Shams R, Ahmad S, Bashir I, Fayaz J, Singh P, et al:
Rice bran: Nutritional, phytochemical, and pharmacological profile
and its contribution to human health promotion. Food Chem Adv.
2(100296)2023.
|
|
4
|
Gonçalves AC, Nunes AR, Falcão A, Alves G
and Silva LR: Dietary effects of anthocyanins in human health: a
comprehensive review. Pharmaceuticals (Basel).
14(690)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khoo HE, Azlan A, Tang ST and Lim SM:
Anthocyanidins and anthocyanins: Colored pigments as food,
pharmaceutical ingredients, and the potential health benefits. Food
Nutr Res. 61(1361779)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Sousa Moraes LF, Sun X, Peluzio MDCG
and Zhu MJ: Anthocyanins/anthocyanidins and colorectal cancer: What
is behind the scenes? Crit Rev Food Sci Nutr. 59:59–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khophai S, Chokchaisiri S, Talabnin K,
Ketudat Cairns JR and Talabnin C: Black rice bran-derived
anthocyanins prevent H2O2-induced oxidative stress and DNA damage
in cholangiocytes through activation of the Nrf2-NQO1 axis.
ScienceAsia (In press).
|
|
8
|
Joshua M, Okere C, Sylvester O, Yahaya M,
Precious O, Dluya T, Um JY, Neksumi M, Boyd J, Vincent-Tyndall J,
et al: Disruption of angiogenesis by anthocyanin-rich extracts of
Hibiscus sabdariffa. Int J Sci Eng Res. 8:299–307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang L, Zhou P, Feng R, Luo Z, Li X and
Gao L: Anti-proliferation activities of Oryza sativa L.
anthocyanins-Hohenbuehelia serotina polysaccharides complex after
in vitro gastrointestinal digestion. Food Chem Toxicol.
135(111012)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen PN, Kuo WH, Chiang CL, Chiou HL,
Hsieh YS and Chu SC: Black rice anthocyanins inhibit cancer cells
invasion via repressions of MMPs and u-PA expression. Chem Biol
Interact. 163:218–229. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun W, Zhang ND, Zhang T, Li YN, Xue H,
Cao JL, Hou WS, Liu J, Wang Y and Jin CH: Cyanidin-3-O-glucoside
induces the apoptosis of human gastric cancer MKN-45 cells through
ROS-mediated signaling pathways. Molecules. 28(652)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee J, Shin A, Oh JH and Kim J: Colors of
vegetables and fruits and the risks of colorectal cancer. World J
Gastroenterol. 23:2527–2538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu C, Zeng XT, Liu TZ, Zhang C, Yang ZH,
Li S and Chen XY: Fruits and vegetables intake and risk of bladder
cancer: A PRISMA-compliant systematic review and dose-response
meta-analysis of prospective cohort studies. Medicine (Baltimore).
94(e759)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Larsson SC, Bergkvist L and Wolk A: Fruit
and vegetable consumption and incidence of gastric cancer: A
prospective study. Cancer Epidemiol Biomarkers Prev. 15:1998–2001.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Srivatanakul P, Sriplung H and Deerasamee
S: Epidemiology of liver cancer: An overview. Asian Pac J Cancer
Prev. 5:118–125. 2004.PubMed/NCBI
|
|
16
|
Sripa B and Pairojkul C:
Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol.
24:349–356. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sarcognato S, Sacchi D, Fassan M, Fabris
L, Cadamuro M, Zanus G, Cataldo I, Capelli P, Baciorri F,
Cacciatore M and Guido M: Cholangiocarcinoma. Pathologica.
113:158–169. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Tienderen GS, van Beek MEA, Schurink
IJ, Rosmark O, Roest HP, Tieleman J, Demmers J, Muntz I, Conboy J,
Westergren-Thorsson G, et al: Modelling metastatic colonization of
cholangiocarcinoma organoids in decellularized lung and lymph
nodes. Front Oncol. 12(1101901)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Intuyod K, Priprem A, Limphirat W,
Charoensuk L, Pinlaor P, Pairojkul C, Lertrat K and Pinlaor S:
Anti-inflammatory and anti-periductal fibrosis effects of an
anthocyanin complex in Opisthorchis viverrini-infected
hamsters. Food Chem Toxicol. 74:206–215. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Intuyod K, Priprem A, Pairojkul C,
Hahnvajanawong C, Vaeteewoottacharn K, Pinlaor P and Pinlaor S:
Anthocyanin complex exerts anti-cholangiocarcinoma activities and
improves the efficacy of drug treatment in a gemcitabine-resistant
cell line. Int J Oncol. 52:1715–1726. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sripa B, Seubwai W, Vaeteewoottacharn K,
Sawanyawisuth K, Silsirivanit A, Kaewkong W, Muisuk K, Dana P,
Phoomak C, Lert-Itthiporn W, et al: Functional and genetic
characterization of three cell lines derived from a single tumor of
an Opisthorchis viverrini-associated cholangiocarcinoma
patient. Hum Cell. 33:695–708. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pangestu NS, Chueakwon P, Talabnin K,
Khiaowichit J and Talabnin C: RNF43 overexpression attenuates the
Wnt/β-catenin signalling pathway to suppress tumour progression in
cholangiocarcinoma. Oncol Lett. 22(846)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Talabnin C, Trasaktaweesakul T,
Jaturutthaweechot P, Asavaritikrai P, Kongnawakun D, Silsirivanit
A, Araki N and Talabnin K: Altered O-linked glycosylation in benign
and malignant meningiomas. PeerJ. 12(e16785)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nichols WW, Cristofalo VJ, Toji LH, Greene
AE, Aronson MM, Dwight S, Charpentier R and Hoffman E:
Characterization of a new human diploid cell line-IMR-91. In Vitro.
19:797–804. 1983.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Izdebska M, Zielińska W, Grzanka D and
Gagat M: The role of actin dynamics and actin-binding proteins
expression in epithelial-to-mesenchymal transition and its
association with cancer progression and evaluation of possible
therapeutic targets. Biomed Res Int. 2018(4578373)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karimi Roshan M, Soltani A, Soleimani A,
Rezaie Kahkhaie K, Afshari AR and Soukhtanloo M: Role of AKT and
mTOR signaling pathways in the induction of epithelial-mesenchymal
transition (EMT) process. Biochimie. 165:229–234. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lucena MC, Carvalho-Cruz P, Donadio JL,
Oliveira IA, de Queiroz RM, Marinho-Carvalho MM, Sola-Penna M, de
Paula IF, Gondim KC, McComb ME, et al: Epithelial mesenchymal
transition induces aberrant glycosylation through hexosamine
biosynthetic pathway activation. J Biol Chem. 291:12917–12929.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brown JS, Amend SR, Austin RH, Gatenby RA,
Hammarlund EU and Pienta KJ: Updating the definition of cancer. Mol
Cancer Res. 21:1142–1147. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Anderson RL, Balasas T, Callaghan J,
Coombes RC, Evans J, Hall JA, Kinrade S, Jones D, Jones PS, Jones
R, et al: A framework for the development of effective
anti-metastatic agents. Nat Rev Clin Oncol. 16:185–204.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chansitthichok S, Chamnan P, Sarkhampee P,
Lertsawatvicha N, Voravisutthikul P and Wattanarath P: Survival of
patients with cholangiocarcinoma receiving surgical treatment in an
O. viverrini endemic area in Thailand: A retrospective
cohort study. Asian Pac J Cancer Prev. 21:903–909. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sarkhampee P, Ouransatien W,
Lertsawatvicha N, Chansitthichok S and Wattanarath P: Survival
outcome of 736 cholangiocarcinoma patient receiving surgical
treatment in Thailand. HPB. 25 (Suppl 2):S265–S266. 2023.
|
|
33
|
Zhou J, Zhu YF, Chen XY, Han B, Li F, Chen
JY, Peng XL, Luo LP, Chen W and Yu XP: Black rice-derived
anthocyanins inhibit HER-2-positive breast cancer
epithelial-mesenchymal transition-mediated metastasis in
vitro by suppressing FAK signaling. Int J Mol Med.
40:1649–1656. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jongsomchai K, Leardkamolkarn V and
Mahatheeranont S: A rice bran phytochemical, cyanidin 3-glucoside,
inhibits the progression of PC3 prostate cancer cell. Anat Cell
Biol. 53:481–492. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Maruyama M, Kobayashi N, Westerman KA,
Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A,
Stolz DB, et al: Establishment of a highly differentiated
immortalized human cholangiocyte cell line with SV40T and hTERT.
Transplantation. 77:446–451. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Prakobwong S, Gupta SC, Kim JH, Sung B,
Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S and Aggarwal
BB: Curcumin suppresses proliferation and induces apoptosis in
human biliary cancer cells through modulation of multiple cell
signaling pathways. Carcinogenesis. 32:1372–1380. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu A, Zhu Y, Han B, Peng J, Deng X, Chen
W, Du J, Ou Y, Peng X and Yu X: Delphinidin induces cell cycle
arrest and apoptosis in HER-2 positive breast cancer cell lines by
regulating the NF-κB and MAPK signaling pathways. Oncol Lett.
22(832)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15(129)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lai X, Li Q, Wu F, Lin J, Chen J, Zheng H
and Guo L: Epithelial-mesenchymal transition and metabolic
switching in cancer: Lessons from somatic cell reprogramming. Front
Cell Dev Biol. 8(760)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lorente G, Syriani E and Morales M: Actin
filaments at the leading edge of cancer cells are characterized by
a high mobile fraction and turnover regulation by profilin I. PLoS
One. 9(e85817)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen M, Zhu W, Liang Z, Yao S, Zhang X and
Zheng Y: Effect of f-actin organization in lamellipodium on
viscoelasticity and migration of Huh-7 cells under pH
microenvironments using AM-FM atomic force microscopy. Front Phys.
9(674958)2021.
|
|
43
|
Chung WL, Eibauer M, Li W,
Boujemaa-Paterski R, Geiger B and Medalia O: A network of mixed
actin polarity in the leading edge of spreading cells. Commun Biol.
5(1338)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee EJ, Kang MK, Kim YH, Kim DY, Oh H, Kim
SI, Oh SY and Kang YH: Dietary chrysin suppresses formation of
actin cytoskeleton and focal adhesion in AGE-exposed mesangial
cells and diabetic kidney: Role of autophagy. Nutrients.
11(127)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Berr AL, Wiese K, Dos Santos G, Koch CM,
Anekalla KR, Kidd M, Davis JM, Cheng Y, Hu YS and Ridge KM:
Vimentin is required for tumor progression and metastasis in a
mouse model of non-small cell lung cancer. Oncogene. 42:2074–2087.
2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Usman S, Waseem NH, Nguyen TKN, Mohsin S,
Jamal A, Teh MT and Waseem A: Vimentin is at the heart of
epithelial mesenchymal transition (EMT) mediated metastasis.
Cancers (Basel). 13(4985)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rao RK and Samak G: Bile duct epithelial
tight junctions and barrier function. Tissue Barriers.
1(e25718)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bhat AA, Syed N, Therachiyil L, Nisar S,
Hashem S, Macha MA, Yadav SK, Krishnankutty R, Muralitharan S,
Al-Naemi H, et al: Claudin-1, A double-edged sword in cancer. Int J
Mol Sci. 21(569)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Maharati A and Moghbeli M: PI3K/AKT
signaling pathway as a critical regulator of epithelial-mesenchymal
transition in colorectal tumor cells. Cell Commun Signal.
21(201)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yothaisong S, Dokduang H, Techasen A,
Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ and
Loilome W: Increased activation of PI3K/AKT signaling pathway is
associated with cholangiocarcinoma metastasis and PI3K/mTOR
inhibition presents a possible therapeutic strategy. Tumour Biol.
34:3637–3648. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Vaquero J, Guedj N, Clapéron A, Nguyen
Ho-Bouldoires TH, Paradis V and Fouassier L: Epithelial-mesenchymal
transition in cholangiocarcinoma: From clinical evidence to
regulatory networks. J Hepatol. 66:424–441. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Suman S, Kurisetty V, Das TP, Vadodkar A,
Ramos G, Lakshmanaswamy R and Damodaran C: Activation of AKT
signaling promotes epithelial-mesenchymal transition and tumor
growth in colorectal cancer cells. Mol Carcinog. 53 (Suppl
1):E151–E160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Moghbeli M: PI3K/AKT pathway as a pivotal
regulator of epithelial-mesenchymal transition in lung tumor cells.
Cancer Cell Int. 24(165)2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen W, Wu S, Zhang G, Wang W and Shi Y:
Effect of AKT inhibition on epithelial-mesenchymal transition and
ZEB1-potentiated radiotherapy in nasopharyngeal carcinoma. Oncol
Lett. 6:1234–1240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lee S, Choi EJ, Cho EJ, Lee YB, Lee JH, Yu
SJ, Yoon JH and Kim YJ: Inhibition of PI3K/Akt signaling suppresses
epithelial-to-mesenchymal transition in hepatocellular carcinoma
through the Snail/GSK-3/beta-catenin pathway. Clin Mol Hepatol.
26:529–539. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Layosa MAA, Lage NN, Chew BP, Atienza L,
Mertens-Talcott S, Talcott S and Noratto GD: Dark sweet cherry
(Prunus avium) phenolics enriched in anthocyanins induced apoptosis
in MDA-MB-453 breast cancer cells through MAPK-dependent signaling
and reduced invasion via Akt and PLCγ-1 downregulation. Nutr
Cancer. 73:1985–1997. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhong W, Tong Y, Li Y, Yuan J, Hu S, Hu T
and Song G: Mesenchymal stem cells in inflammatory microenvironment
potently promote metastatic growth of cholangiocarcinoma via
activating Akt/NF-κB signaling by paracrine CCL5. Oncotarget.
8:73693–73704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chen XY, Zhou J, Luo LP, Han B, Li F, Chen
JY, Zhu YF, Chen W and Yu XP: Black rice anthocyanins suppress
metastasis of breast cancer cells by targeting RAS/RAF/MAPK
pathway. Biomed Res Int. 2015(414250)2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gabius HJ: The sugar code: Why glycans are
so important. Biosystems. 164:102–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pucci M, Malagolini N and Dall'Olio F:
Glycobiology of the epithelial to mesenchymal transition.
Biomedicines. 9(770)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Park DD, Xu G, Park SS, Haigh NE, Phoomak
C, Wongkham S, Maverakis E and Lebrilla CB: Combined analysis of
secreted proteins and glycosylation identifies prognostic features
in cholangiocarcinoma. J Cell Physiol. 239(e31147)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lu J, Isaji T, Im S, Fukuda T, Hashii N,
Takakura D, Kawasaki N and Gu J: β-Galactoside
α2,6-sialyltranferase 1 promotes transforming growth
factor-β-mediated epithelial-mesenchymal transition. J Biol Chem.
289:34627–34641. 2014.PubMed/NCBI View Article : Google Scholar
|