1. Introduction
Cancer remains a leading cause of morbidity and
mortality globally, with liver cancer, particularly hepatocellular
carcinoma (HCC), accounting for significant incidence and mortality
rates. The Global Cancer Observatory (GLOBOCAN) reported 19.96
million new cancer cases and 9.74 million cancer-related
mortalities in 2022, highlighting the urgent need for improved
early diagnosis, treatment and prevention strategies (1,2).
HCC is the most prevalent form of primary liver
cancer, representing ~75% of cases. Current chemotherapeutic
agents, such as gemcitabine and cisplatin, show limited efficacy
due to the chemoresistance from the cancer, often linked to
overexpression of ATP-binding cassette transporter proteins and
detoxification enzymes in liver cells. This resistance contributes
to low response rates and severe adverse effects from traditional
chemotherapy, including hair loss, nausea and fatigue (3-6).
These are mainly being explained with non-targeted
chemotherapeutics. Hence, reducing the chemotherapeutics-related
adverse side effects has emerged as a crucial issue to improve the
anti-cancer therapeutic efficacy.
At present, pharmaceutical engineering in the field
of medicine has emerged to develop new tumor-targeted drug delivery
systems and multi-purpose drug delivery systems to enhance the
therapeutic efficacy of chemotherapy against cancer (7,8). As
regards the tumor-specific drug delivery systems, a number of
attempts have been tried to create near perfect nanocarriers
against cancer, which may have some specific capabilities such as
tumor targeted without nonspecific cellular invasion, tumor
sensitive without adverse side effects and ability to moderate
multidrug resistance mechanisms (9,10).
Traditional herbal medicine has been recognized as
one of important treatments against liver cancer for years. So far,
a number of herbal plants such as Cinnamomum species
(11), Curcuma species
(12), Artemisia species
(13), Glycyrrhiza uralensis
(14), Equisetum arvense
(15), Rheum undulatum
(16) and Scutellaria
baicalensis (17) have been
shown to have anti-proliferative effects on HCC. However,
traditional herbal medicine has some drawbacks, including direct
anti-proliferative effects on normal and cancer cells and producing
herbal medicine-related adverse side effects such as allergic
reactions, asthmas, rashes, vomiting and diarrhea. In recent years,
traditional herbal medicine has been intensively studied to
investigate its anticancer activity in the field of cancer
pharmacology. It has been considered to develop new drug delivery
systems to improve traditional herbal medicine anticancer efficacy.
A number of biologically active substances such as quercetin
(18) and sesquiterpene lactone
(19) from traditional herbal
medicine have been extensively researched.
The present study aimed to describe the anticancer
mechanisms and inhibition capacity against multidrug resistance
mechanism of quercetin-containing multipurpose nanocarriers and
their targeting potential for HCC.
2. Quercetin
A number of medicinal plants, vegetables and fruits,
including Moringa oleifera, Ficus religuisa,
Styphnolobium japonicum (20), Polygonum hydropiper (21), Polygonum aviculare (22), citrus, apple, broccoli, onions and
chili pepper, produce abundant amount of quercetin as a metabolite
in their life cycle, and it plays important roles in some
physiological processes, growth and developmental stages such as
photosynthesis, antioxidant system and seed germination (23). Quercetin is a flavonoid compound
with a chemical structure of 3, 3', 4', 5, 7-pentahydroxyflavone
(24). In recent years, quercetin
has repeatedly been declared as an effective agent against cancer,
an effective inhibitor of P-glycoprotein (P-gp) and well-known
protector of chemotherapy-exposed cells (25). The quercetin effects on numerous
types of cancers are associated with several mechanisms such as
anti-proliferative effect, inhibition of angiogenesis, reactive
oxygen species (ROS) induction in cancerous cells and apoptosis
induction.
3. Quercetin-related mechanisms against
HCC
Effects on cell growth and
proliferation
A number of studies have attempted to explain the
anticancer mechanism of quercetin against HCC. Anticancer
chemotherapeutics have mainly been designed to inhibit the cancer
cell cycle in their various stages (26). Cell growth and proliferation are
biologically explained by cell cycle staged with growth 1
(G1), synthesis (S), growth 2 (G2) and
mitosis (M), which are regulated via cyclin-dependent kinases
(CDK/cyclin) and signal transduction pathways. The quercetin
mechanism is related to the downregulation of cyclin B1, D, CDK1
and CDK2 by activating protein 27 (p27) and protein 21, which
triggers cell cycle arrest in M, G1, S and G2
phases (Fig. 1A) (27,28).
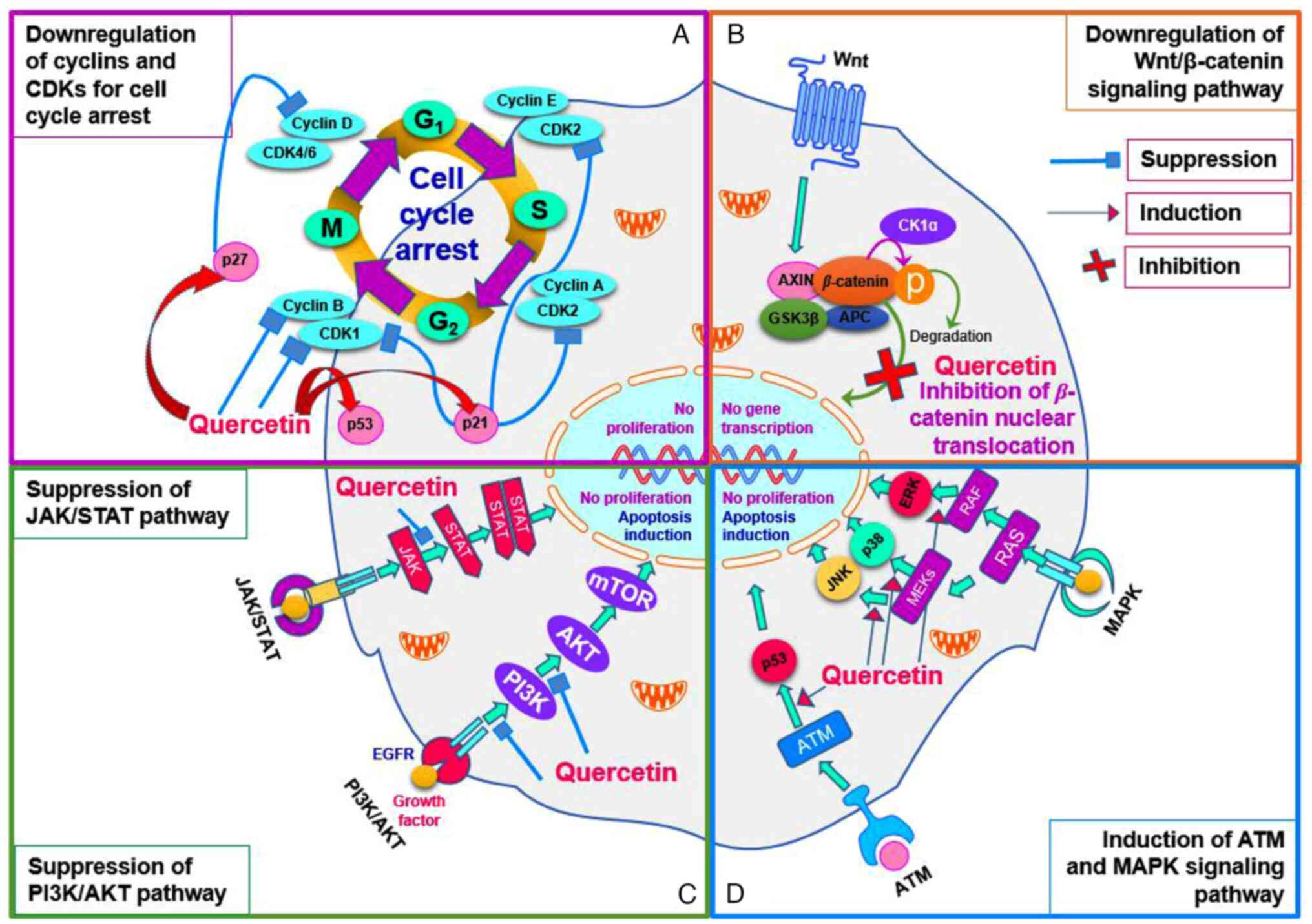 | Figure 1Effects of quercetin on cancer cell
growth and proliferation. (A) Quercetin-triggered cell cycle
arrest. Cell cycle is downregulated by quercetin-related mechanisms
at the all stages. Cyclin B, a mitotic cyclin, is complexed with
CDK1 to regulate the cell growth and mitosis progression
(transition stage of G2 to M). In the cell cycle, cyclin
D is also known as a key regulator in transition stage of M to
G1 phase and is complexed with CDK4/6 to regulate the
cell cycle. CDK2, a serine/threonine protein kinase, plays a
crucial role in the initiation of DNA synthesis in the transition
stage of G1/S. CDK2 also has a role in the transition
stage of S/G2 in which it forms a complex of cyclin
A/CDK2 to phosphorylate CDC6 and E2F1 and then triggers the
S/G2 phase transition. These functional proteins,
including CDK1, CDK2, cyclin B and cyclin D, are suppressed in
quercetin-exposed tumor cells. (B) Quercetin-triggered
downregulation of Wnt/β-catenin signaling pathway. Wnt protein
binds Fz family receptor in the signaling pathway and then it
disrupts the function of destruction complex to inactivate the
GSK3. This mechanism allows the β-catenin to localize in nucleus
for activation of TCF/LEF transcription factors. In the
Wnt/β-catenin signaling pathway, quercetin inhibits the nuclear
translocation of the β-catenin. (C) Quercetin-triggered suppression
of JAK/STAT and PI3K/AKT pathway. In the signaling pathway of
JAK/STAT, a cytokine activates its receptor to trigger the cascade
phosphorylation of its proteins (JAK and STAT proteins), which
plays a crucial role for activating the transcription
process-related genes. In the signaling pathway, the quercetin may
suppress the phosphorylation of STAT by JAKs. As for PI3K/AKT
pathway, it is triggered by EGF and the catalytic domain of EGFR
kinases and then all protein kinases such as PI3K, AKT and mTOR are
phosphorylated to regulate the cell growth and proliferation. This
signaling pathway is also suppressed through quercetin-exposed
mechanisms. (D) Quercetin-triggered induction of ATM and MAPK
pathway. ATM signaling pathway can be induced by EGFR and Mre11
complex and it activates p53 protein for DNA reparation and cell
apoptosis. As for the MAPK pathway, MEK and RAF are induced by
quercetin to activate the JNK, p38 and ERK in the signaling pathway
for apoptosis induction and proliferation suppression. CDK,
cyclin-dependent kinase; CDC6, phosphorylate cell division cycle 6;
E2F1, early region 2 binding factor 1; Fz, frizzled; GSK3, glycogen
synthase kinase 3; TCF/LEF, T cell factor/lymphoid enhancing
factor; JAK/STAT, Janus kinases/signal transducer and activator of
transcription; EGF, epidermal growth factor; ATM, ataxia
telangiectasia mutated kinase; Mre11, mitotic recombination 11;
MEK, mitogen-activated protein kinase kinase; RAF, rapidly
accelerated fibrosarcoma; p38, protein 38; CK1α, casein kinase 1α;
APC, adenomatous polyposis coli. |
Quercetin-related mechanisms against HCC have been
reported to be primarily associated with downregulation of
Wnt/β-catenin signaling pathway (Fig.
1B), enhancing Bcl-2-associated X protein (BAX), caspase
family, extracellular signal-regulated kinase (ERK) and
AMP-activated protein kinase/mammalian target of rapamycin pathway,
suppressing B-cell lymphoma-2 (Bcl-2), epidermal growth factor
receptor, dihydrotestosterone and phosphatidylinositol
3-kinase/protein kinase B pathway (Fig.
1C) (29-31).
Moreover, it is reported that mitogen-activated protein kinase
pathway and ataxia telangiectasia mutated kinase-mediated signaling
pathway are induced due to quercetin exposure (Fig. 1D) (32,33).
Effects on apoptosis induction
A potential role of quercetin has been reported in
the regulation and induction of apoptosis in cancer cells. It is
known that quercetin has the proapoptotic effect which contributes
to the upregulation of tumor protein 53 gene (34,35)
and suppression of Bcl-2 protein (Fig.
2A). Moreover, quercetin has a potential to increase the
expression of proapoptotic proteins such as Bax and cleaved
caspase-3 and 9, which regulate negatively antiapoptotic proteins
such as Bcl-2 and myeloid leukemia 1 (36-38).
This apoptotic pathway highlights the potential of quercetin as a
therapeutic agent in cancer treatment by promoting the elimination
of malignant cells through the activation of intrinsic apoptotic
mechanisms.
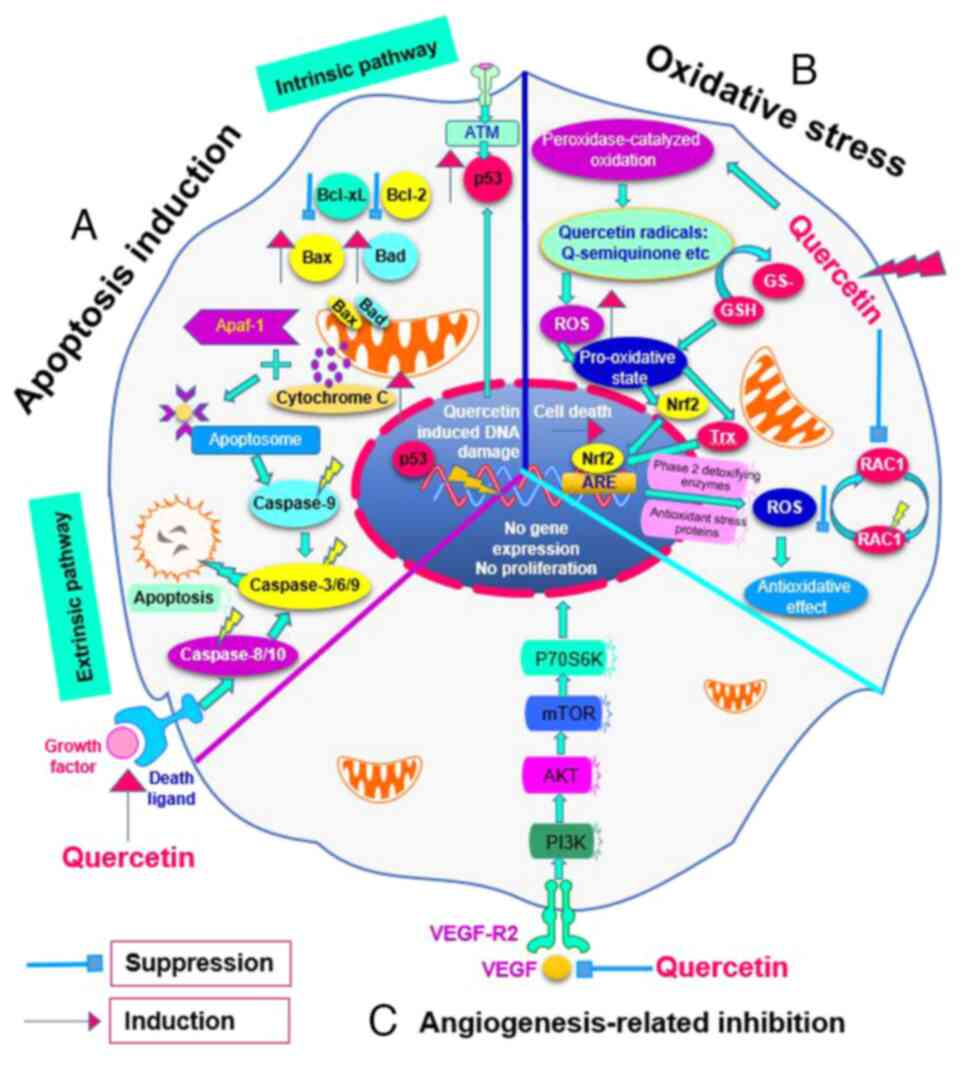 | Figure 2Effects of quercetin on cancer
cellular biomolecules and functions. (A) Apoptosis induction.
Quercetin promotes the upregulation of pro-survival proteins such
as p53, Bcl-2 and Bcl-xl while simultaneously downregulating
pro-apoptotic proteins such as Bax and Bad. This regulatory shift
creates a favorable environment for apoptosis. When quercetin
induces this expression pattern, it leads to the release of
cytochrome c from the mitochondria into the cytoplasm, which
is crucial for apoptosome formation. The formation of an apoptosome
activates a cascade of caspase enzymes, including caspase-3, -6 and
-9. The activation of these caspases is the final step that
initiates the execution phase of apoptosis, leading to programmed
cell death. (B) Oxidative stress. Internalized quercetin can be
converted into quercetin radicals such as quercetin-semiquinone and
quercetin-quinone due to peroxidase-catalyzed oxidation in the
cytoplasm, which produces the pro-oxidative state in the cell.
Then, it activates the Nrf2 and Trx in the cytoplasm and the
activation of the ARE pathway to trigger antioxidant enzyme and
protein synthesis. (C) Angiogenesis-related inhibition. VEGF and
VEGF-R2 are activated to upregulate the PI3K/Akt signaling
pathways, which promotes the endothelial cell proliferation and
migration during cancer cell development. Quercetin reduces the
availability of these potent angiogenetic factors. Nrf2, nuclear
factor (erythroid-derived 2)-like 2; Trx, thioredoxin; ARE,
antioxidant response element; ROS, reactive oxygen species; GSH,
glutathione. |
Effects on oxidative stress
Another mechanism of action of quercetin against HCC
is inhibition of oxidative stress which plays a significant role in
fighting cancer cells (Fig. 2B).
The mechanism of the quercetin is mainly attributed to the
upregulated expression of the antioxidant response element pathway,
nuclear factor (erythroid-derived 2) gene and thioredoxin system.
The upregulation of these mechanisms is triggered by quercetin
radicals-mediated pro-oxidative state due to peroxidase-catalyzed
oxidation in the cytoplasm (39-41).
Quercetin can reduce the ROS level in the cytoplasm
by inhibiting the p66hc-mediated Ras-related C3 botulinum toxin
substrate 1 activation to decrease the cell migration and
proliferation (42).
Quercetin can undergo oxidation, leading to
formation of reactive intermediates, specifically
quercetin-semiquinone and quercetin-quinone. These radicals can
interact with cellular components, contributing to an increase in
overall ROS levels within cancer cells (43).
Notably, it has been reported that quercetin
generates ROS in HepG2 by overexpressing the p53-indcible gene 3
and its product of oxidoreductase. The oxidoreductase enzyme
catalyzes the quercetin and generate ROS with HepG2 cell lines
(44).
4. Angiogenesis-related inhibition
The progression stage of HCC is also associated with
the angiogenesis and metastasis. Thus, the angiogenesis inhibition
may be a targeting option for HCC treatment. The mechanism of
quercetin against angiogenesis is related to the vascular
endothelial growth factor receptor (VEGF-R) 2 targeting, which
regulates AKT/mTOR/P70S6K signaling pathway (Fig. 2C) (45). Igura et al (46) first reported in 2001 that quercetin
inhibited angiogenesis and then could be used as an efficient
antitumor drug (47).
5. Quercetin nanocarriers for HCC
In recent years, several nanocarriers for quercetin
have been designed to use its benefits for HCC treatment (Table SI).
Ren et al (48) synthesized quercetin nanoparticles by
using gold nanoparticles, quercetin solution and poly
(DL-lactide-co-glycolide) PLGA to treat liver cancer. As a result,
the quercetin nanoparticles efficiently suppressed liver cancer
cell growth and colony formation by upregulating p27 and
downregulating c-Myc, cyclin-D1, CDK1, matrix metallopeptidase 7
and β-catenin within the cancer cells. Another possible mechanism
was also revealed in that the quercetin nanoparticle markedly
induced the apoptosis of liver cancer cells via activating
cytochrome c/caspase signaling. The Akt/ERK1/2 signaling
pathway was also inactivated by the quercetin nanoparticles, which
suppressed liver cancer cell growth. It was also reported that the
quercetin nanoparticles suppressed the transcriptional factor AP-2
alpha/human telomerase reverse transcriptase signaling pathway
within the liver cancer cells. These results showed that the
quercetin nanoparticles can be a multipurpose nanocarrier against
liver cancer cells (48).
Guan et al (49) designed a quercetin-loaded
nanoparticle by using poly (lactic-co-glycolic
acid)-d-α-tocopheryl polyethylene glycol 1000 succinate (PLGA and
TPGS1000) for the targeted treatment of liver cancer. Their
research results proved that the quercetin by liver cancer cell
uptake can be increased by using PLGA and TPGS1000 polymers and the
cytotoxicity of liver cancer was efficiently increased by the
designed nanoparticles. Also, they observed that quercetin
nanoparticles markedly promoted cell apoptosis. The study suggested
that quercetin nanoparticles had a higher suppressive effect on
tumor growth by inducing the cell apoptosis and can be a potential
tool to treat liver cancer.
Quercetin-loaded superparamagnetic polymeric
micelles were prepared by a film hydration method to enhance the
treatment efficacy against HCC and improve the disease monitoring.
The designed quercetin-loaded micelles increased the cytotoxicity
and apoptosis for the liver cancer cells. The main mechanism of
action of the quercetin-loaded superparamagnetic polymeric micelles
was explained by the higher accumulation of the quercetin
nanoparticles within the targeted liver cancer cells due to the
extrinsic magnetic field. Hence, Srisa-nga et al (50) suggested that co-delivery of
quercetin and superparamagnetic iron oxide nanoparticles may
increase the therapeutic efficacy against HCC and decrease the side
effects-related to the chemotherapeutics.
A targeted lipid coated nanoparticle for
hepatocellular carcinoma treatment was designed by using RGD
peptide to conduct a co-delivery of sorafenib and quercetin
(51). The RGD peptide has
extensively applied as a targeting agent for cancer treatment due
to its capability of targeting the tumor-associated blood vessels.
As a result of the study, the liver cancer cell uptake of
nanoparticles was increased up to 71.5% and the cytotoxicity was
detected higher than control groups. Thus, it was suggested that
RGD peptide modification may be more significant for liver cancer
targeting and the combination of quercetin and sorafenib was more
effective against HCC. Sorafenib is recognized as an effective
multikinase inhibitor which effectively suppresses tumor cell
proliferation and growth by downregulating VEGFR and
platelet-derived growth factor receptor signaling pathways
(52). It has now been reported
that HCC cells are becoming resistant for sorafenib (53). Therefore, the sorafenib combination
therapy with quercetin and an active targeting agent might be a
more powerful therapeutic tool against HCC.
Quercetin encapsulated biodegradable plasmonic
nanoparticles were obtained by preparing liposomes and loading
quercetin into the liposomes. Gold coating was conducted to obtain
the gold-coated liposomes. As a consequence of the photothermal
therapy, the obtained quercetin-loaded and gold-coated liposomes
had an efficient effect in inducing the apoptotic death of cancer
cells by suppressing heat shock protein 70 (Hsp70) expression and
also had a marked effect to trigger the disorganization of the
microtubules network and DNA damage. A combination therapy of
quercetin and photothermal therapy was a significant against cancer
(54).
Varshosaz et al (55) fabricated a solid lipid nanoparticle
with loaded quercetin and checked the cellular uptake of the
nanoparticles. HepG2 cells markedly internalized the
quercetin-loaded solid lipid nanoparticles compared with control
cells. The viability of HepG2 cells was also significantly
decreased. The research team suggested that all sterol-contained
solid lipid nanoparticles had good inhibitory effects for cancer
cells.
6. Quercetin effect on reversing multidrug
resistance for anticancer treatment
Although anticancer drugs have efficient therapeutic
effects by suppressing the growth or killing the cancer cells, a
number of drawbacks or adverse effects triggered by the anticancer
drugs have been reported and cause treatment failure.
Anticancer drug resistance in HCC is one of critical
issues in clinical medicine for several reasons, such as limited
treatment options and mechanisms of anticancer drug resistance. HCC
has historically had limited treatment options, including surgery,
locoregional and systemic therapies (56). Main chemotherapeutics against liver
cancer are gemcitabine, cisplatin, doxorubicin, etoposide and
methotrexate and their treatment response rate is comparably low
due to drug resistance mechanisms (5,57,58).
Anticancer drug resistance leads to treatment failure and therefore
is becoming imperative to discuss and address. HCC can exhibit
various mechanisms of resistance to anticancer drugs, including
alterations in drug metabolism, changes in tumor microenvironment
and genetic mutations in genes which encodes the transporter
proteins and detoxification enzymes (59).
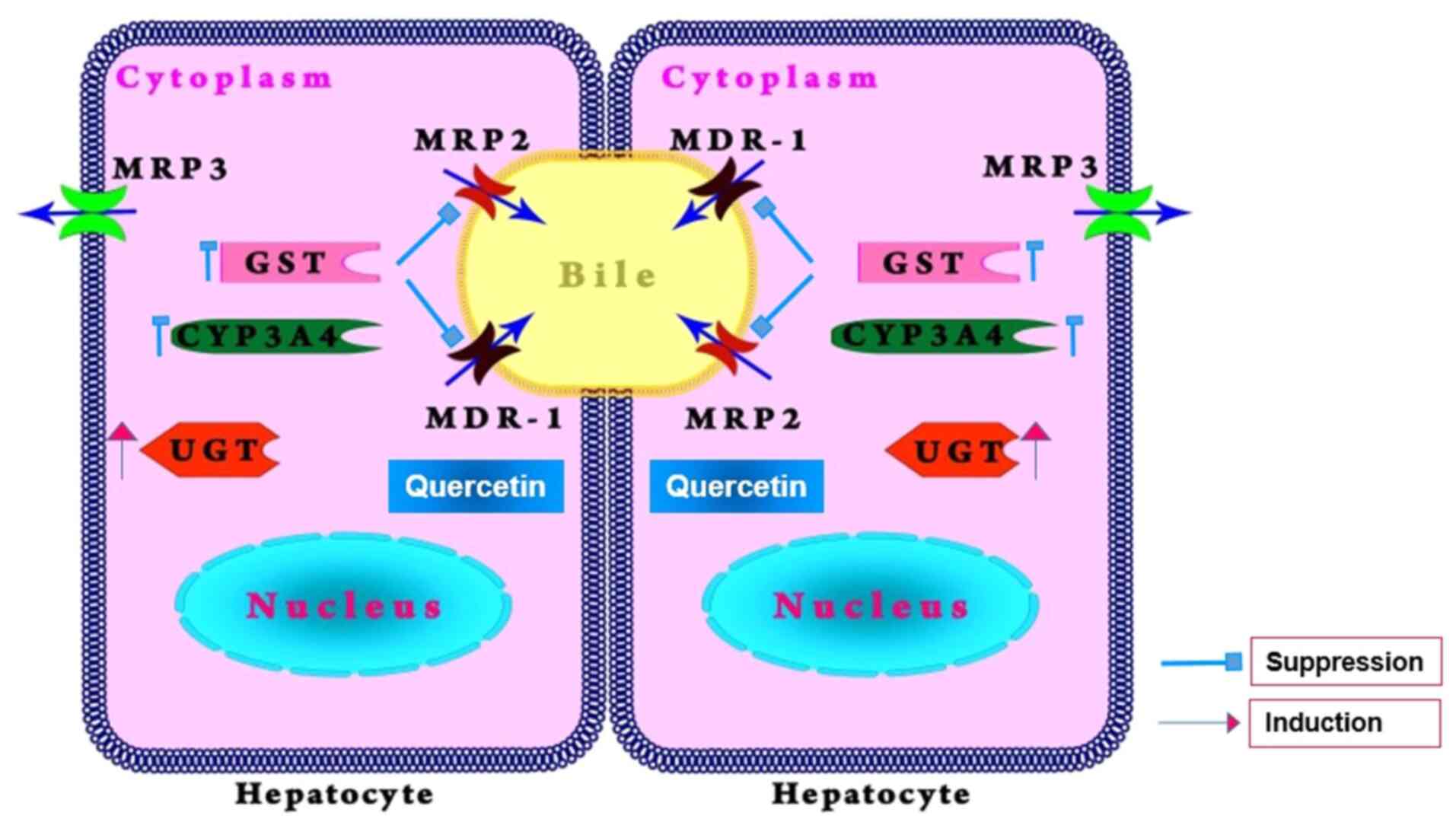
During chemotherapy, the cancer cells may gain the
capability to reduce the efficacy of chemotherapeutics by
overexpressing P-gp and other proteins on the membrane of the
cancer cells (Fig. 3) (60-62).
One of frequent causes to trigger the multidrug
resistance of the anticancer drugs is P-gp (MDR1) which is encoded
by the ATP-binding cassette sub family B member 1 (ABCB1) gene
(63) and has the ability to efflux
the anticancer drugs from the tumor cells. To reverse the
P-gp-associated multidrug resistance of the cancer cells, a number
of attempts have been conducted in last two decades and several
inhibitors of the P-gp efflux pump discovered, such as amiodarone,
verapamil, diosmin sesquiterpene, quercetin, naringin, biochanin
and silymarin (64-68).
Multidrug resistance-associated protein 2 (MRP2) is
another efflux transporter, primarily in the removal of organic
anions and drug conjugates from cells. It is responsible for
anticancer drug resistance (69).
Quercetin has been shown to modulate MRP2 activity, especially
suppressing the efflux mechanism of the transporter in cancer
cells. Therefore, it leads to an increased accumulation of
anticancer drugs inside cancer cells, which could enhance their
anticancer effects (69).
Glutathione S-transferase (GST) is a family of
enzymes involved in the detoxification process of chemical
compounds. The enzyme binds to drugs and xenobiotics via
glutathione and facilitates drug excretion. Quercetin may suppress
the expression and activity of the enzyme, which can decrease the
detoxification processes of chemotherapeutics and their efficacy
(70). The mechanism of quercetin
on GST enzyme may be attributed to suppressing GST activation
through competitive or non-competitive inhibition (71).
Cytochrome P450 3A4 (CYP3A4) is a major enzyme in
the liver which metabolizes numerous drugs, including a number of
chemotherapeutics. It has been reported that quercetin can suppress
its anticancer drug efficacy (72).
Among the inhibitors of P-gp, quercetin has
attracted more attention from researchers to suppress the growth of
cancer cells by blocking the cellular signaling pathway and reverse
the multidrug resistance during the chemotherapy (73). The mechanism of the action of
quercetin to inhibit P-gp is related to blocking the function of
P-gp efflux pump by binding the interfaces of intracellular helices
and nucleotide-binding domains (74).
7. Quercetin nanocarriers for P-gp
inhibition
So far, quercetin has been used as a promising
inhibitor for the design of nanocarriers with the ability to reduce
anticancer drug resistance in HCC treatment (Table SII).
In a study, quercetin-chitosan micelles were
prepared by the ultrasound method and doxorubicin was entrapped
into the micelles. Then, in vitro cytotoxicity, cellular
uptake and inhibition of P-gp efflux were tested for the
DOX-entrapped quercetin-chitosan micelles. Quercetin and
quercetin-chitosan micelles had comparably effective inhibition of
the P-gp efflux pump and produced a marked long-lasting
cytotoxicity on cancer cells. These results suggested that the
quercetin-chitosan micelles were a promising multipurpose
nanocarrier for oral delivery of anticancer drugs (75).
Guo et al (76) designed a polymeric nanoparticle to
attenuate the drug resistance mechanisms during cancer therapy by
co-delivering paclitaxel and quercetin. The polymeric nanoparticle
was prepared by a sonication method and characterized by checking
its cellular uptake, in vivo biodistribution and antitumor
efficacy. P-gp inhibition assay for the nanoparticle was conducted
to evaluate its effect on drug resistance mechanism of anticancer
drugs. As a result of the polymeric nanoparticle, an enhanced
tumor-suppressing effect was observed due to decrease of P-gp
expression in the cancer cells.
In another study, a polyethylene glycolated liposome
was developed to conduct a codelivery of adriamycin and quercetin
to reverse cancer therapy-related drug resistance. Adriamycin is
known to be a substrate of P-gp and effluxes from the tumor cells
during anticancer therapy. In the study, film-ultrasound technique
with ammonium sulfate transmembrane gradient method was used to
prepare the adriamycin and quercetin co-loaded liposome and
pharmacokinetic study, cell toxicity study and pharmacodynamic
study were also conducted. The relative tumor volume was decreased
and P-gp was effectively suppressed (77).
An amphiphilic carboxymethyl chitosan-quercetin
conjugate has been obtained by using a self-assembly technique to
improve the oral delivery of paclitaxel. Paclitaxel is known as a
substrate of P-gp and therefore leads to therapeutic failure due to
chemotherapeutic efflux mechanism in the tumor cells. For this
purpose, quercetin was applied as an inhibitor of P-gp to increase
the capability of therapeutic efficacy of the drug. In this study,
the antitumor efficacy of the nanocarrier was investigated in in
vivo xenograft model and the inhibition of P-gp was evaluated
by western blot analysis. As a result, paclitaxel-loaded
carboxymethyl chitosan-quercetin polymeric micelles displayed an
improved downregulation effect on P-gp (78).
Another team developed quercetin-loaded benzoylated
methoxy-poly (ethylene glycol)-b-oligo (ε-caprolactone) polymeric
micelles to overcome the drug resistance issues for cancer therapy.
They used P-gp overexpressed leukemia cells (K562/ADR) and checked
the effect of quercetin on P-gp function to reverse the multidrug
resistance. According to the results, quercetin-loaded micelles
were effective in reducing the overexpression of P-gp on K562/ADR
cells (79).
Another nanomicelle was reported in 2018. Chitosan,
quercetin and citraconic anhydride was used to design a
pH-responsive nanomicelle to enhance the intracellular
bioavailability of doxorubicin for cancer treatment. In the study,
a self-assembly technique was used to obtain the nanomicelle and
P-gp inhibition assay was performed for quercetin function on the
efflux pump. The nanomicelle had an improved inhibitory effect on
the P-gp efflux pump and obvious enhancement of suppressing effect
on MCF-7/ADR cells (80).
Multiwalled carbon nanotubes-based nanoconstructs
have been designed to enhance the therapeutic efficacy of drugs
against multidrug resistant cancers. Using this nanocarrier, a
combination of N-desmethyl tamoxifen and quercetin was delivered
into MDA-MB-231 cells and indirectly checked the inhibition of
P-gp. In this study, an increased biodistribution, biocompatibility
and marked cellular uptake of the drug-loaded nanocarriers was
observed. These results were explained by quercetin-related P-gp
inhibition (81).
Qian et al (82) designed a core-shell micelle to
deliver paclitaxel for treatment of multi drug resistant breast
cancer. In this study, quercetin was also chosen as an inhibitor of
P-gp. The characteristics and antitumor efficacy of the micelles
were checked and the effect of quercetin on P-gp efflux pump was
evaluated in MDA-MB-231/MDR1 cells. According to the results, the
micelles enhanced the cellular uptake of antitumor drug by
downregulating P-gp expression in MDA-MB-231/MDR1 cells.
8. Challenges and considerations
Major drawbacks of the anticancer therapeutics such
as adverse effects and multidrug resistance cause failure in cancer
treatment (83). A number of
different strategies have been employed to overcome the drawbacks
for effective cancer treatment. Herbal drug-based nanomedicine has
recently gained attention for cancer treatment due to its
biosafety, biodegradability, promising therapeutic efficacy and
ability of combating anticancer drug resistance (84).
The field of pharmaceutical engineering has produced
more data to develop a favorable therapeutic tool for cancer
therapy (85).
Targeted drug delivery systems, which are methods of
delivering drugs to specific parts of a diseased body, have been
extensively researched in cancer nanotechnology. They have several
benefits as drug delivery systems. They not only significantly
improve the specificity of nanocarriers to tumor cells or diseased
organs but also reduce the side effects of anticancer drugs in
comparison with traditional chemotherapy (86). As for liver targeted delivery,
receptor-mediated drug delivery systems such as asialoglycoprotein
receptor (ASGPR), folate receptor and retinol binding protein
receptor-mediated targeted delivery systems have been widely
recognized as the most effective tools in treating HCC (87,88).
Hepatic carcinoma cells are able to recognize galactose and
N-acetylgalactosamine-terminated glycoproteins through ASGPR
located on their surfaces. In recent years, a number of researches
have been confirming that galactosylated nanoparticles (NPs)
exhibit high hepatocyte specificity in vitro as well as
in vivo for anticancer drug delivery (89).
At present, nanomedicines provide a great
contribution to delivering high concentrations of chemotherapeutic
drugs and MDR inhibitors to tumor cells, in which biodegradable,
biocompatible nanomaterials and target-specific ligands play more
important roles.
Quercetin is now a well-known anticancer agent with
many purposes but it needs further improvement on its pharmacology
and clinical applications (90).
There are a number of drawbacks of quercetin-related chemotherapy
including less absorption in gastrointestinal tract, easy
metabolization by enzymes, instability, poor solubility, low
hydrophobicity and lack of specificity to the target site (91).
To overcome these challenges, researchers have been
developing quercetin-loaded nanocarriers, which can enhance the
therapeutic potential of quercetin, especially against
drug-resistant HCC. Quercetin-loaded nanocarriers have enhanced
bioavailability, targeted delivery and the possibility of
overcoming drug resistance. Quercetin-loaded nanocarriers can
improve the solubility and stability of quercetin, increasing its
bioavailability in the bloodstream and allowing for more effective
concentrations to reach tumor sites. Quercetin-loaded nanocarriers
can be designed to target cancer cells specifically, minimizing
damage to healthy tissues and enhancing the therapeutic effect on
HCC cells. This targeting can reduce the overall side effects of
treatment. Quercetin has been shown to modulate various pathways
involved in drug resistance, such as inhibition of drug efflux
pumps, induction of apoptosis and modulation of signaling pathways.
As for the synergistic effect of quercetin, it can be used in
combination with other anticancer drugs and quercetin can enhance
their effectiveness and help overcome resistance mechanisms.
Hence, quercetin-supported nanocarriers with good
therapeutic efficacy and cancer-targeting ability, as well as
capacity of attenuating multidrug resistance have emerged as a
promising approach to enhance the bioavailability and therapeutic
efficacy of quercetin for cancer therapy. In further studies,
addressing these aforementioned weaknesses of quercetin is now a
major challenge to develop an effective nanocarrier with quercetin
against HCC.
9. Conclusion
Based on these well-studied considerations, the
present review demonstrated that quercetin has multiple antitumor
properties. There are some limitations, but advances in
pharmaceutical engineering may mitigate these. Consequently,
comprehensive research is essential to develop effective
nanocarriers for quercetin targeting HCC.
Supplementary Material
Summary of quercetin nanoparticles
designed for hepatocellular carcinoma treatment.
Summary of quercetin nanocarriers
designed for P-gp inhibition.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by an internal grant
from Science Technology Foundation, Mongolian National University
of Medical Sciences, Ulaanbaatar, Mongolia (grant no.
STF-MNUMS-200138).
Availability of data and materials
Not applicable.
Authors' contributions
AT performed the conception and design of the review
article and provided administrative support. TB collected the
information and performed the interpretation. The two authors read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J and Arnold
M: The global landscape of esophageal squamous cell carcinoma and
esophageal adenocarcinoma incidence and mortality in 2020 and
projections to 2040: New estimates from GLOBOCAN 2020.
Gastroenterology. 163:649–658.e2. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sayiner M, Golabi P and Younossi ZM:
Disease burden of hepatocellular carcinoma: A global Dig Dis. Sci.
64:910–917. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Joyce H, McCann A, Clynes M and Larkin A:
Influence of multidrug resistance and drug transport proteins on
chemotherapy drug metabolism. Expert Opin Drug Metab Toxicol.
11:795–809. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lundqvist EÅ, Fujiwara K and Seoud M:
Principles of chemotherapy. Int J Gynaecol Obstet. 131 (Suppl
2):S146–S149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu H, Wei M, Xu Y, Li Y, Zhai X, Su P, Ma
Q and Zhang H: PDA-based drug delivery nanosystems: A potential
approach for glioma treatment. Int J Nanomedicine. 17:3751–3775.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ding Y, Li W, Zhang F, Liu Z, Zanjanizadeh
Ezazi N, Liu D and Santos HA: Electrospun fibrous architectures for
drug delivery, tissue engineering and cancer therapy. Adv Funct
Mater. 29(1802852)2019.
|
|
9
|
Ali I, Nadeem Lone M, Suhail M, Danish
Mukhtar S and Asnin L: Advances in nanocarriers for anticancer
drugs delivery. Curr Med Chem. 23:2159–2187. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Y, Su J, Liu G, Chen J, Zhang X, Zhang
R, Jiang M and Qiu M: Advances of blood cell-based drug delivery
systems. Eur J Pharm Sci. 96:115–128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Upadhyay RK: Therapeutic and
pharmaceutical potential of Cinnamomum tamala. Res Rev Pharm
Pharm Sci. 6:18–28. 2017.
|
|
12
|
Sultana S, Munir N, Mahmood Z, Riaz M,
Akram M, Rebezov M, Kuderinova N, Moldabayeva Z, Shariati MA, Rauf
A and Rengasamy KRR: Molecular targets for the management of cancer
using Curcuma longa Linn. phytoconstituents: A review.
Biomed Pharmacother. 135(111078)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taleghani A, Emami SA and Tayarani-Najaran
Z: Artemisia: A promising plant for the treatment of cancer.
Bioorg Med Chem. 28(115180)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ji S, Li Z, Song W, Wang Y, Liang W, Li K,
Tang S, Wang Q, Qiao X, Zhou D, et al: Bioactive constituents of
Glycyrrhiza uralensis (Licorice): Discovery of the effective
components of a traditional herbal medicine. J Nat Prod.
79:281–292. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Makia R, Al-Sammarrae K, Al-Halbosiy M and
Al-Mashhadani M: In vitro cytotoxic activity of total flavonoid
from Equisetum arvense extract. Rep Biochem Mol Biol.
11:487–492. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee D, Park S, Choi S, Kim SH and Kang KS:
In vitro estrogenic and breast cancer inhibitory activities of
chemical constituents isolated from Rheum undulatum L.
Molecules. 23(1215)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheng CS, Chen J, Tan HY, Wang N, Chen Z
and Feng Y: Scutellaria baicalensis and cancer treatment:
Recent progress and perspectives in biomedical and clinical
studies. Am J Chin Med. 46:25–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Men K, Duan X, Wei XW, Gou ML, Huang MJ,
Chen LJ, Qian ZY and Wei YQ: Nanoparticle-delivered quercetin for
cancer therapy. Anticancer Agents Med Chem. 14:826–832.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
An T, Yin H, Lu Y and Liu F: The emerging
potential of parthenolide nanoformulations in tumor therapy. Drug
Des Devel Ther. 16:1255–1272. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dang X, Cho S, Wang H, Seok WJ, Ha JH and
Kim IH: Quercetin extracted from Sophora japonica flower improves
growth performance, nutrient digestibility, cecal microbiota, organ
indexes, and breast quality in broiler chicks. Anim Biosci.
35:577–586. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lekar AV, Borisenko SN, Vetrova EV,
Sushkova SN and Borisenko NI: Extraction of quercetin from
Polygonum hydropiper L. by subcritical water. Am J Agric
Biol Sci. 9:1–5. 2014.
|
|
22
|
Yang HH, Hwangbo K, Zheng MS, Cho JH, Son
JK, Kim HY, Baek SH, Choi HC, Park SY and Kim JR:
Quercetin-3-O-β-D-glucuronide isolated from Polygonum
aviculare inhibits cellular senescence in human primary cells.
Arch Pharm Res. 37:1219–1233. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Anand David AV, Arulmoli R and Parasuraman
S: Overviews of Biological importance of quercetin: A bioactive
flavonoid. Pharmacogn Rev. 10:84–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Michala AS and Pritsa A: Quercetin: A
molecule of great biochemical and clinical value and its beneficial
effect on diabetes and cancer. Diseases. 10(37)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang SY, Duan KM, Li Y, Mei Y, Sheng H,
Liu H, Mei X, Ouyang W, Zhou HH and Liu ZQ: Effect of quercetin on
P-glycoprotein transport ability in Chinese healthy subjects. Eur J
Clin Nutr. 67:390–394. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Davoodvandi A, Shabani Varkani M, Clark
CCT and Jafarnejad S: Quercetin as an anticancer agent: Focus on
esophageal cancer. J Food Biochem. 44(e13374)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vafadar A, Shabaninejad Z, Movahedpour A,
Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A,
Hajighadimi S, Moradizarmehri S, et al: Quercetin and cancer: New
insights into its therapeutic effects on ovarian cancer cells. Cell
Biosci. 10(31)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jeong JH, An JY, Kwon YT, Rhee JG and Lee
YJ: Effects of low dose quercetin: Cancer cell-specific inhibition
of cell cycle progression. J Cell Biochem. 106:73–82.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ganthala PD, Alavala S, Chella N,
andugulapati SB, Bathini NB and Sistla R: Co-encapsulated
nanoparticles of erlotinib and quercetin for targeting lung cancer
through nuclear EGFR and PI3K/AKT inhibition. Colloids Surf B
Biointerfaces. 211(112305)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Safi A, Heidarian E and Ahmadi R:
Quercetin synergistically enhances the anticancer efficacy of
docetaxel through induction of apoptosis and modulation of
PI3K/AKT, MAPK/ERK and JAK/STAT3 signaling pathways in MDA-MB-231
breast cancer cell line. Int J Mol Cell Med. 10:11–22.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo T, Wu C, Zhang J, Yu J, Li G, Jiang H,
Zhang X, Yu R and Liu X: Dual blockade of EGFR and PI3K signaling
pathways offers a therapeutic strategy for glioblastoma. Cell
Commun Signal. 21(363)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Y, Han N, Hou P, Zhao FQ and Liu H:
Roles of MAPK and Nrf2 signaling pathways in quercetin alleviating
redox imbalance induced by hydrogen peroxide in mammary epithelial
cells. Anim Nutr. 1(e1)2024.
|
|
33
|
Liu W, Chen D, Su J, Zheng R, Kong R, Zhu
B, Dong H and Li Y: Quercetin induced HepG2 cells apoptosis through
ATM/JNK/STAT3 signaling pathways. Biocell. 47:187–194. 2023.
|
|
34
|
Wang ZX, Ma J, Li XY, Wu Y, Shi H, Chen Y,
Lu G, Shen HM, Lu GD and Zhou J: Quercetin induces p53-independent
cancer cell death through lysosome activation by the transcription
factor EB and reactive oxygen species-dependent ferroptosis. Br J
Pharmacol. 178:1133–1148. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chan ST, Yang NC, Huang CS, Liao JW and
Yeh SL: Quercetin enhances the antitumor activity of trichostatin A
through upregulation of p53 protein expression in vitro and in
vivo. PLoS One. 8(e54255)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Granado-Serrano AB, Martín MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee DH, Szczepanski M and Lee YJ: Role of
Bax in quercetin-induced apoptosis in human prostate cancer cells.
Biochem Pharmacol. 75:2345–2355. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang CF, Liu SH, Ho TJ, Lee KI, Fang KM,
Lo WC, Liu JM, Wu CC and Su CC: Quercetin induces tongue squamous
cell carcinoma cell apoptosis via the JNK activation-regulated
ERK/GSK-3α/β-mediated mitochondria-dependent apoptotic signaling
pathway. Oncol Lett. 23(78)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu D, Hu MJ, Wang YQ and Cui YL:
Antioxidant activities of quercetin and its complexes for medicinal
application. Molecules. 24(1123)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee YJ, Lee DM and Lee SH: Nrf2 expression
and apoptosis in quercetin-treated malignant mesothelioma cells.
Mol Cells. 38:416–425. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rashidi Z, Aleyasin A, Eslami M, Nekoonam
S, Zendedel A, Bahramrezaie M and Amidi F: Quercetin protects human
granulosa cells against oxidative stress via thioredoxin system.
Reprod Biol. 19:245–254. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Baba RA, Mir HA, Mokhdomi TA, Bhat HF,
Ahmad A and Khanday FA: Quercetin suppresses ROS production and
migration by specifically targeting Rac1 activation in gliomas.
Front Pharmacol. 15(1318797)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Biswas P, Dey D, Biswas PK, Rahaman TI,
Saha S, Parvez A, Khan DA, Lily NJ, Saha K, Sohel M, et al: A
comprehensive analysis and anti-cancer activities of quercetin in
ROS-mediated cancer and cancer stem cells. Int J Mol Sci.
23(11746)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang Q, Cheng G, Qiu H, Zhu L, Ren Z,
Zhao W, Zhang T and Liu L: The p53-inducible gene 3 involved in
flavonoid-induced cytotoxicity through the reactive oxygen
species-mediated mitochondrial apoptotic pathway in human hepatoma
cells. Food Funct. 6:1518–1525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7(e47516)2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Igura K, Ohta T, Kuroda Y and Kaji K:
Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer
Lett. 171:11–16. 2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Okumo T, Furuta A, Kimura T, Yusa K, Asano
K and Sunagawa M: Inhibition of angiogenic factor productions by
quercetin in vitro and in vivo. Medicines (Basel).
8(22)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ren KW, Li YH, Wu G, Ren JZ, Lu HB, Li ZM
and Han XW: Quercetin nanoparticles display antitumor activity via
proliferation inhibition and apoptosis induction in liver cancer
cells. Int J Oncol. 50:1299–1311. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guan X, Gao M, Xu H, Zhang C, Liu H, Lv L,
Deng S, Gao D and Tian Y: Quercetin-loaded poly (lactic-co-glycolic
acid)-d-α-tocopheryl polyethylene glycol 1000 succinate
nanoparticles for the targeted treatment of liver cancer. Drug
Deliv. 23:3307–3318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Srisa-Nga K, Mankhetkorn S, Okonogi S and
Khonkarn R: Delivery of superparamagnetic polymeric micelles loaded
with quercetin to hepatocellular carcinoma cells. J Pharm Sci.
108:996–1006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang C, Su L, Wu C, Wu J, Zhu C and Yuan
G: RGD peptide targeted lipid-coated nanoparticles for
combinatorial delivery of sorafenib and quercetin against
hepatocellular carcinoma. Drug Dev Ind Pharm. 42:1938–1944.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Deng Q, Huang Y, Zeng J, Li X, Zheng X,
Guo L, Shi J and Bai L: Recent advancements in the small-molecule
drugs for hepatocellular carcinoma (HCC): Structure-activity
relationships, pharmacological activities, and the clinical trials.
Biomed Pharmacother. 179(117343)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: Theoretical basis
and therapeutic aspects. Signal Transduct Target Ther.
5(87)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pradhan A, Kumari A, Srivastava R and
Panda D: Quercetin encapsulated biodegradable plasmonic
nanoparticles for photothermal therapy of hepatocellular carcinoma
cells. ACS Appl Bio Mater. 2:5727–5738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Varshosaz J, Jafarian A, Salehi G and
Zolfaghari B: Comparing different sterol containing solid lipid
nanoparticles for targeted delivery of quercetin in hepatocellular
carcinoma. J Liposome Res. 24:191–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Koulouris A, Tsagkaris C, Spyrou V, Pappa
E, Troullinou A and Nikolaou M: Hepatocellular carcinoma: An
overview of the changing landscape of treatment options. J
Hepatocell Carcinoma. 8:387–401. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. Int J
Mol Sci. 21(3233)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Talib WH, Alsayed AR, Barakat M, Abu-Taha
MI and Mahmod AI: Targeting drug chemo-resistance in cancer using
natural products. Biomedicines. 9(1353)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Al Saihati HA and Rabaan AA: Cellular
resistance mechanisms in cancer and the new approaches to overcome
resistance mechanisms chemotherapy. Saudi Med J. 44:329–344.
2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Halder J, Pradhan D, Kar B, Ghosh G and
Rath G: Nanotherapeutics approaches to overcome
P-glycoprotein-mediated multi-drug resistance in cancer.
Nanomedicine. 40(102494)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Waghray D and Zhang Q: Inhibit or evade
multidrug resistance P-glycoprotein in cancer treatment:
Miniperspective. J Med Chem. 61:5108–5121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li S, Zhao Q, Wang B, Yuan S, Wang X and
Li K: Quercetin reversed MDR in breast cancer cells through
down-regulating P-gp expression and eliminating cancer stem cells
mediated by YB-1 nuclear translocation. Phytother Res.
32:1530–1536. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ganesan M, Kanimozhi G, Pradhapsingh B,
Khan HA, Alhomida AS, Ekhzaimy A, Brindha GR and Prasad NR:
Phytochemicals reverse P-glycoprotein mediated multidrug resistance
via signal transduction pathways. Biomed Pharmacother.
139(111632)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Suroowan S, Abdallah HH and Mahomoodally
MF: Herb-drug interactions and toxicity: Underscoring potential
mechanisms and forecasting clinically relevant interactions induced
by common phytoconstituents via data mining and computational
approaches. Food Chem Toxicol. 156(112432)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Palmeira A, Sousa E, Vasconcelos MH and
Pinto MM: Three decades of P-gp inhibitors: Skimming through
several generations and scaffolds. Curr Med Chem. 19:1946–2025.
2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gurunath S, Nanjwade BK and Patil PA: Oral
bioavailability and intestinal absorption of candesartan cilexetil:
Role of naringin as P-glycoprotein inhibitor. Drug Dev Ind Pharm.
41:170–176. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Di Sotto A, Irannejad H, Eufemi M,
Mancinelli R, Abete L, Mammola CL, Altieri F, Mazzanti G and Di
Giacomo S: Potentiation of low-dose doxorubicin cytotoxicity by
affecting P-glycoprotein through caryophyllane sesquiterpenes in
hepG2 cells: An in vitro and in silico study. Int J Mol Sci.
21(633)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dewanjee S, Dua TK, Bhattacharjee N, Das
A, Gangopadhyay M, Khanra R, Joardar S, Riaz M, Feo V and
Zia-Ul-Haq M: Natural products as alternative choices for
P-glycoprotein (P-gp) inhibition. Molecules. 22(871)2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mohos V, Fliszár-Nyúl E, Ungvári O, Kuffa
K, Needs PW, Kroon PA, Telbisz Á, Özvegy-Laczka C and Poór M:
Inhibitory effects of quercetin and its main methyl, sulfate, and
glucuronic acid conjugates on cytochrome p450 enzymes, and on OATP,
BCRP and MRP2 transporters. Nutrients. 12(2306)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Arinç E, Yilmaz D and Bozcaarmutlu A:
Mechanism of inhibition of CYP1A1 and glutathione S-transferase
activities in fish liver by quercetin, resveratrol, naringenin,
hesperidin, and rutin. Nutr Cancer. 67:137–144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
van Zanden JJ, Ben Hamman O, van Iersel
ML, Boeren S, Cnubben NH, Lo Bello M, Vervoort J, van Bladeren PJ
and Rietjens IM: Inhibition of human glutathione S-transferase P1-1
by the flavonoid quercetin. Chem Biol Interact. 145:139–148.
2003.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Choi JS, Piao YJ and Kang KW: Effects of
quercetin on the bioavailability of doxorubicin in rats: role of
CYP3A4 and P-gp inhibition by quercetin. Arch Pharm Res.
34:607–613. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Borska S, Sopel M, Chmielewska M, Zabel M
and Dziegiel P: Quercetin as a potential modulator of
P-glycoprotein expression and function in cells of human pancreatic
carcinoma line resistant to daunorubicin. Molecules. 15:857–870.
2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Singh A, Patel SK, Kumar P, Das KC, Verma
D, Sharma R, Tripathi T, Giri R, Martins N and Garg N: Quercetin
acts as a P-gp modulator via impeding signal transduction from
nucleotide-binding domain to transmembrane domain. J Biomol Struct
Dyn. 40:4507–4515. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mu Y, Fu Y, Li J, Yu X, Li Y, Wang Y, Wu
X, Zhang K, Kong M, Feng C and Chen X: Multifunctional quercetin
conjugated chitosan nano-micelles with P-gp inhibition and
permeation enhancement of anticancer drug. Carbohydr Polym.
203:10–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Guo Y, Liu S, Luo F, Tang D, Yang T, Yang
X and Xie Y: A nanosized codelivery system based on intracellular
stimuli-triggered dual-drug release for multilevel chemotherapy
amplification in drug-resistant breast cancer. Pharmaceutics.
14(422)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yu J, Chen H, Jiang L and Wang J, Dai J
and Wang J: Codelivery of adriamycin and P-gp inhibitor quercetin
using PEGylated liposomes to overcome cancer drug resistance. J
Pharm Sci. 108:1788–1799. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J
and Yao J: Amphiphilic carboxymethyl chitosan-quercetin conjugate
with P-gp inhibitory properties for oral delivery of paclitaxel.
Biomaterials. 35:7654–7665. 2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Khonkarn R, Daowtak K and Okonogi S:
Chemotherapeutic efficacy enhancement in P-gp-overexpressing cancer
cells by flavonoid-loaded polymeric micelles. AAPS PharmSciTech.
21(121)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Mu Y, Wu G, Su C, Dong Y, Zhang K, Li J,
Sun X, Li Y, Chen X and Feng C: pH-sensitive amphiphilic
chitosan-quercetin conjugate for intracellular delivery of
doxorubicin enhancement. Carbohydr Polym.
223(115072)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kumar M, Sharma G, Misra C, Kumar R, Singh
B, Katare OP and Raza K: N-desmethyl tamoxifen and quercetin-loaded
multiwalled CNTs: A synergistic approach to overcome MDR in cancer
cells. Mater Sci Eng C Mater Biol Appl. 89:274–282. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Qian J, Liu S, Yang T, Xiao Y, Sun J, Zhao
J, Zhang Z and Xie Y: Polyethyleneimine-tocopherol hydrogen
succinate/hyaluronic acid-quercetin (PEI-TOS/HA-QU) core-shell
micelles delivering paclitaxel for combinatorial treatment of MDR
breast cancer. J Biomed Nanotechnol. 17:382–398. 2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Dallavalle S, Dobričić V, Lazzarato L,
Gazzano E, Machuqueiro M, Pajeva I, Tsakovska I, Zidar N and
Fruttero R: Improvement of conventional anti-cancer drugs as new
tools against multidrug resistant tumors. Drug Resist Updat.
50(100682)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Deshmukh R, Prajapati M and Harwansh RK:
Management of colorectal cancer using nanocarriers-based drug
delivery for herbal bioactives: Current and emerging approaches.
Curr Pharm Biotechnol. 25:599–622. 2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kenchegowda M, Rahamathulla M, Hani U,
Begum MY, Guruswamy S, Osmani RAM, Gowrav MP, Alshehri S, Ghoneim
MM, Alshlowi A and Gowda DV: Smart nanocarriers as an emerging
platform for cancer therapy: A review. Molecules.
27(146)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Tang L, Li J, Zhao Q, Pan T, Zhong H and
Wang W: Advanced and innovative nano-systems for anticancer
targeted drug delivery. Pharmaceutics. 13(1151)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kumar V, Rahman M, Gahtori P, Al-Abbasi F,
Anwar F and Kim HS: Current status and future directions of
hepatocellular carcinoma-targeted nanoparticles and nanomedicine.
Expert Opin Drug Deliv. 18:673–694. 2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Mishra AK, Pandey M, Dewangan HK, Sl N and
Sahoo PK: A comprehensive review on liver targeting: Emphasis on
nanotechnology-based molecular targets and receptors mediated
approaches. Curr Drug Targets. 23:1381–1405. 2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Dutta R and Mahato RI: Recent advances in
hepatocellular carcinoma therapy. Pharmacol Ther. 173:106–117.
2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Aghababaei F and Hadidi M: Recent advances
in potential health benefits of quercetin. Pharmaceuticals (Basel).
16(1020)2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Manzoor MF, Hussain A, Sameen A, Sahar A,
Khan S, Siddique R, Aadil RM and Xu B: Novel extraction, rapid
assessment and bioavailability improvement of quercetin: A review.
Ultrason Sonochem. 78(105686)2021.PubMed/NCBI View Article : Google Scholar
|