Introduction
The use of acid blockers, including vonoprazan (VPZ)
and proton pump inhibitors (PPIs), has risen in response to the
increasing prevalence of gastroesophageal reflux disease (GERD),
which often requires long-term maintenance therapy with VPZ/PPI
(1,2). All-age prevalence of GERD increased by
18.1% between 1990 and 2017(3).
Additionally, the implementation of Helicobacter pylori
eradication therapy, which increases gastric acid secretion,
contributes to the increased prevalence of GERD and, consequently,
an increased consumption of VPZ/PPI. To prevent upper
gastrointestinal bleeding, these acid blockers are persistently
used in combination with low-dose aspirin (LDA) or non-steroidal
anti-inflammatory drugs (NSAIDs). VPZ, a first-class potassium
competitive acid blocker, has been available in Japan since 2015,
and is associated with various lesions, including stardust gastric
mucosal lesions (4).
However, the increase in the prolonged use of acid
blockers has raised concerns among general practitioners regarding
VPZ/PPI-associated gastric mucosal changes, including fundic gland
polyps, gastric hyperplastic polyps, multiple white and flat
elevated lesions, cobblestone-like gastric mucosal lesions and
stardust gastric mucosal lesions (5). Recently, a review introduced ‘web-like
mucus’ as a novel VPZ-associated gastric change (6). Kaneko et al (7) described this mucus as white and
transparent with a spider web-like appearance, which is challenging
to remove even with thorough endoscopic washing. This web-like
mucus may represent a phenotype of excessive mucus production
induced by VPZ use. However, the association between web-like mucus
and long-term acid blocker use remains unclear. The present study
aimed to elucidate the prevalence and associated factors of
web-like mucus in the stomach.
Patients and methods
Study population and design
The present retrospective observational study
included 608 consecutive patients who underwent an
esophagogastroduodenoscopy (EGD) at Shinozaki Medical Clinic
(Utsunomiya, Japan), a local private clinic, between December 2023
and June 2024. All EGD procedures performed using an ultrathin
endoscope (EG-L580NW7; Fujifilm Corporation) were recorded.
Medication histories were obtained from medical records and
personal medication notebooks issued by the Japan Pharmaceutical
Association, ensuring a comprehensive record of the prescribed
medications from other medical facilities. An endoscopist had
reviewed each patients' medication history before performing the
EGD. Acid blockers used included VPZ, PPIs and histamine 2-receptor
antagonists (H2RA). The standard endoscopic report documented the
grade of gastric atrophy using the Kimura-Takemoto system (8), as well as the presence or absence of
fundic gland polyps, gastric hyperplastic polyps, multiple white
and flat elevated lesions, cobblestone-like gastric mucosal
lesions, stardust gastric mucosal lesions and web-like mucus. An
endoscopist meticulously examined these mandatory variables during
the EGD and completed a standardized endoscopic report form
immediately after the procedure. During each EGD session, more than
50 images, including several images of the gastric fundus, were
routinely captured. H. pylori infection status was
determined using serum anti-H. pylori immunoglobulin G
analysis, stool antigen testing or the 13C-urea breath
test. Any history of H. pylori eradication was confirmed
retrospectively through medical records or patient interviews.
Web-like mucus was defined as a mucus pattern in the stomach
resembling a spider web or net-like appearance, extending from the
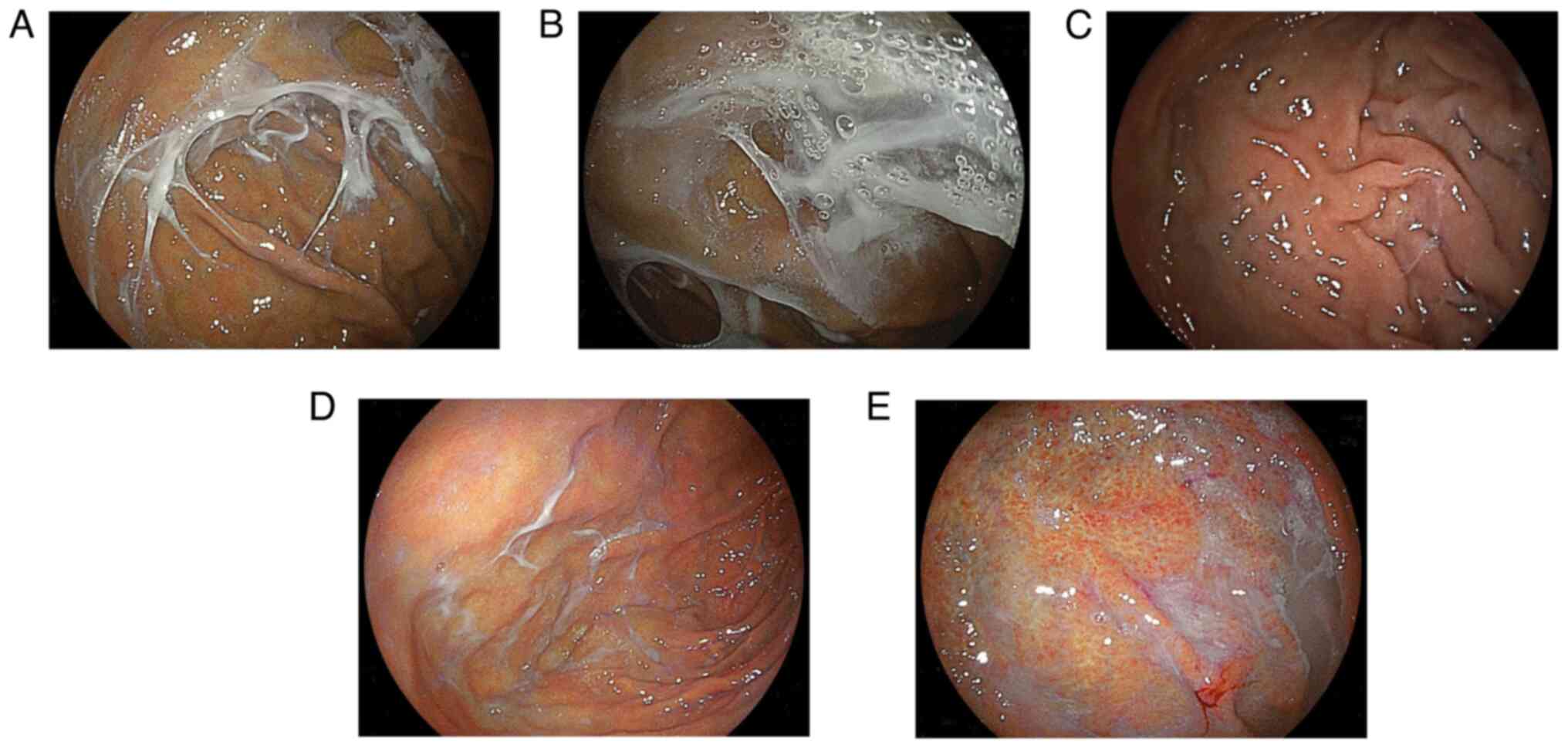
fundus to the greater curvature of the upper body (Fig. 1). This change was diagnosed solely
based on endoscopic findings. The main difference between web-like
mucus and general mucus adhesion is that web-like mucus forms a
solid adhesion resistant to thorough washing with a syringe or
water jet during endoscopy. The duration of VPZ therapy was defined
as the interval between the start date of VPZ and the date of the
EGD.
Patients were excluded from the current
retrospective analysis based on the following criteria: i) A
current H. pylori infection; ii) the current use of acid
blockers for <1 year; iii) a history of esophageal or gastric
surgery; and iv) concurrent use of LDA and/or NSAIDs. Consequently,
61 patients were excluded and the remaining 547 patients were
analyzed. These patients were categorized into four groups: Control
(no acid blocker intake; n=308), VPZ (n=167), PPI (n=49) and H2RA
(n=23) groups. This study protocol was approved by the
Institutional Review Board of Shinozaki Medical Clinic (approval
no. 31-R001), and written informed consent was obtained from all
patients.
Statistical analysis
Categorical data were compared using either the
χ2 test or the Fisher's exact tests for expected counts
of <5. Continuous data were compared using the Mann-Whitney U
test. Factors for multivariate analysis using a logistic regression
model were selected based on their clinical significance in
univariate analysis. The aforementioned statistical analyses were
performed using Statflex version 7.0 (Artech Co., Ltd.). The trend
in the duration of VPZ use and the prevalence of web-like mucus was
evaluated using the Cochran-Armitage trend test with BellCurve for
Excel software (Social Survey Research Information Co., Ltd.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Prevalence of web-like mucus
The baseline characteristics of the 547 patients are
presented in Table I. The mean age
of the cases was 64.2 years (standard deviation, 14.3; range, 16-88
years), with 44% of the patients being male. Approximately one-half
of the patients (47%) had a history of H. pylori
eradication. For most patients, the indication for EGD was
screening. Open-type gastric atrophy was less common among patients
with web-like mucus compared with that in patients without this
finding (P=0.02). Hiatal hernia, gastric hyperplastic polyps and
stardust gastric mucosal lesions were significantly more frequent
in the web-like mucus group compared with those in the no web-like
mucus group. The mean duration of VPZ therapy in the VPZ group
(n=167) was 4.3±2.1 years. The overall prevalence of web-like mucus
was 6% (33/547), with 97% (32/33) of these patients using VPZ.
Specifically, 19% (32/167) of VPZ users exhibited web-like mucus.
At the time of EGD, the daily doses of VPZ administered were either
10 mg (n=147) or 20 mg (n=20). The prevalence of web-like mucus was
18% (26/147 patients) in the 10-mg group and 30% (6/20 patients) in
the 20-mg group. There was no significant difference in the
prevalence of web-like mucus between the two dosage groups
(P=0.189).
 | Table ICharacteristics of the 547 included
patients. |
Table I
Characteristics of the 547 included
patients.
| Characteristic | Web-like mucus
(n=33) | No web-like mucus
(n=514) | P-value |
|---|
| Mean age ± SD,
years | 65±15 | 64±14 | 0.487 |
| Sex, male, n (%) | 13 (39.4) | 229 (45.5) | 0.563 |
| History of H.
pylori eradication, n (%) | 13 (39.4) | 242 (47.1) | 0.390 |
| Indication, n
(%) | | |
0.094a |
|
Screening | 32 (96.9) | 468 (91.1) | |
|
Symptoms | 0 (0.0) | 41 (7.9) | |
|
Abnormal
gastrointestinal series | 1 (3.0) | 2 (0.3) | |
|
Anemia | 0 (0.0) | 3 (0.5) | |
| Hiatal hernia, n
(%) | 6 (18.2) | 35 (6.8) | 0.016 |
| Gastric atrophy, n
(%) | | |
0.037a |
|
No
atrophy | 16 (48.5) | 220 (42.8) | |
|
Closed-type | 13 (39.4) | 134 (26.1) | |
|
Open-type | 4 (12.1) | 160 (31.1) | |
| Gastric ulcer scar, n
(%) | 0 (0.0) | 18 (3.5) | 0.274 |
| Duodenal ulcer scar,
n (%) | 3 (9.1) | 23 (4.5) |
0.201a |
| Fundic gland polyp, n
(%) | 13 (39.4) | 142 (27.6) | 0.145 |
| Gastric hyperplastic
polyp, n (%) | 6 (18.2) | 24 (4.7) |
0.006a |
| Multiple white and
flat elevated lesions, n (%) | 5 (15.2) | 117 (22.7) | 0.308 |
| Cobblestone-like
gastric mucosal lesions, n (%) | 3 (9.1) | 35 (6.8) |
0.492a |
| Stardust gastric
mucosal lesions, n (%) | 19 (57.5) | 102 (19.8) | <0.001 |
| Acid blocker, >1
year, n (%) | | |
<0.001a |
|
None | 1 (3.0) | 307 (59.7) | |
|
Vonoprazan | 32 (97.0) | 135 (26.2) | |
|
Proton pump
inhibitor | 0 (0.0) | 49 (9.5) | |
|
Histamine-2
receptor antagonist | 0 (0.0) | 23 (4.5) | |
Factors associated with web-like
mucus
To minimize confounding variables, a multivariate
analysis was performed to identify factors associated with web-like
mucus (Table II). VPZ use was
identified as a significant positive factor (P<0.001), while
open-type gastric atrophy and multiple white and flat elevated
lesions were identified as significant negative factors (P=0.019),
after adjusting for potential confounders.
 | Table IIFactors associated with web-like
mucus. |
Table II
Factors associated with web-like
mucus.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age, >60
years | 1.127 | 0.524-2.421 | 0.759 | | | |
| Male | 0.809 | 0.394-1.661 | 0.563 | | | |
| History of H.
pylori eradication | 0.731 | 0.356-1.500 | 0.392 | | | |
| Hiatal hernia | 3.041 | 1.178-7.855 | 0.021 | 1.567 | 0.512-4.791 | 0.430 |
| Open-type gastric
atrophy | 0.305 | 0.106-0.883 | 0.028 | 0.252 | 0.082-0.773 | 0.015 |
| Fundic gland
polyp | 1.703 | 0.825-3.514 | 0.149 | | | |
| Gastric
hyperplastic polyp | 4.537 | 1.712-12.027 | 0.002 | 1.781 | 0.561-5.649 | 0.327 |
| Multiple white and
flat elevated lesions | 0.606 | 0.229-1.604 | 0.313 | 0.285 | 0.099-0.820 | 0.019 |
| Cobblestone-like
gastric mucosal lesion | 1.369 | 0.398-4.708 | 0.618 | | | |
| Stardust gastric
mucosal lesions | 5.482 | 2.659-11.302 | <0.001 | 0.884 | 0.367-2.129 | 0.783 |
| Vonoprazan use | 89.837 | 12.158-663.827 | <0.001 | 119.420 | 15.091-945.033 | <0.001 |
Presence or absence of web-like mucus
before starting VPZ
A back-to-back analysis of the EGD findings before
the initiation of VPZ was conducted among the 32 patients with
web-like mucus who were undergoing long-term VPZ therapy. EGD
images of the gastric fundus before starting treatment with VPZ
were obtained for all patients, and none of these patients had
exhibited web-like mucus before starting VPZ (0%) (Fig. 1C).
Duration of VPZ therapy and prevalence
of web-like mucus
The association between the duration of VPZ therapy
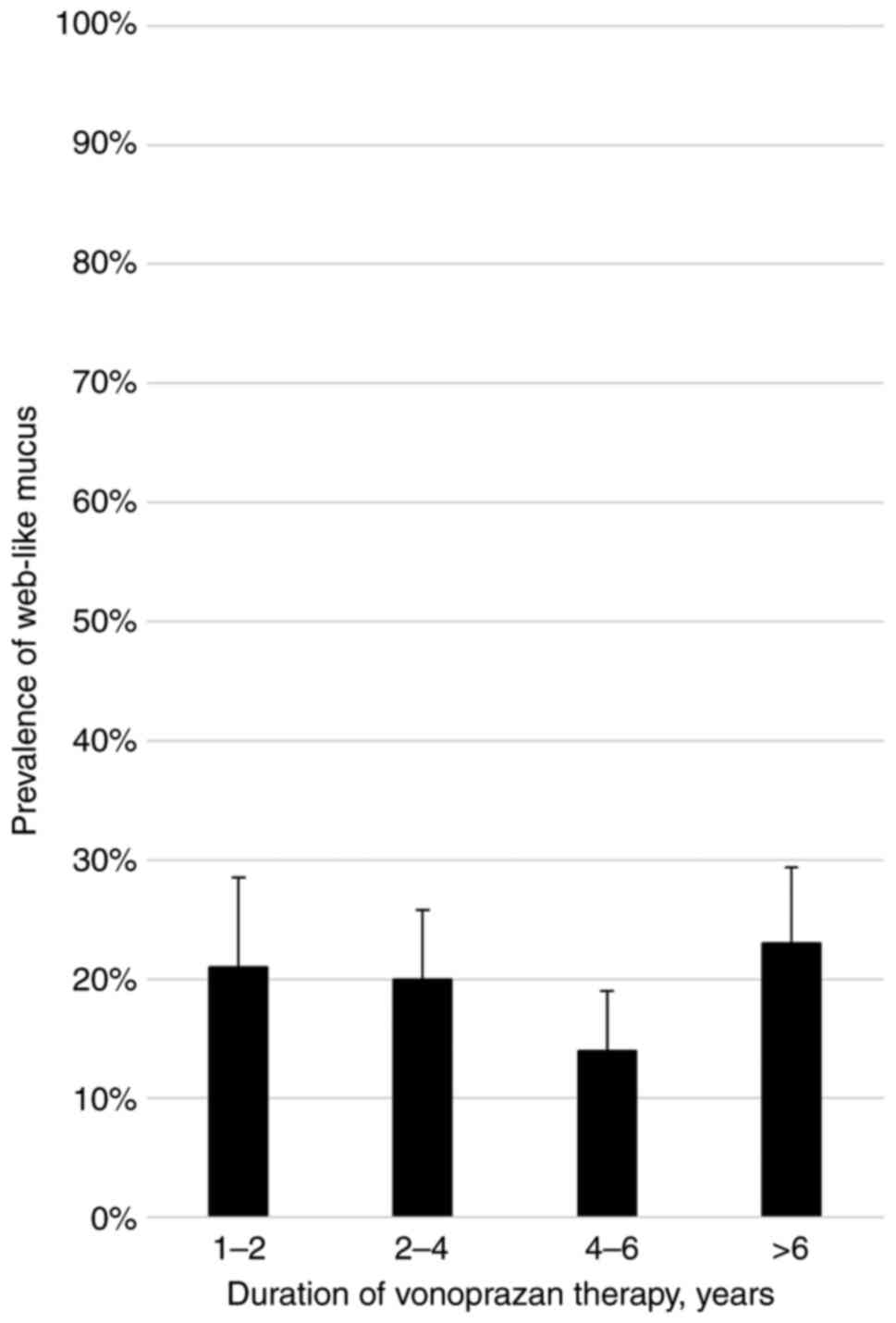
and the prevalence of web-like mucus was evaluated (Fig. 2). No significant increase in
prevalence was observed over time. The Cochran-Armitage trend test
indicated no significant association between the duration of VPZ
therapy and the prevalence of web-like mucus (P=0.884).
Discussion
The present retrospective observational study
demonstrated a significant association between web-like mucus and
the use of VPZ, a finding supported by the multivariate analysis.
Severe gastric atrophy and multiple white and flat elevated lesions
were identified as significant negative factors for web-like mucus.
In back-to-back analyses, no instances of web-like mucus were
observed before initiating VPZ therapy. Furthermore, the duration
of VPZ therapy did not influence the prevalence of web-like
mucus.
The relationship between mucin production and acid
blockers has been explored in previous studies. A double-blind
study involving asymptomatic volunteers found that rabeprazole
increased gastric mucin by 41%, while pentagastrin increased it by
160% (9). Additionally, rabeprazole
and pentagastrin administration elevated gastric juice viscosity
(9). Web-like mucus may develop due
to excessive mucin (mucus glycoprotein) production stimulated by
gastrin or prostaglandin, potentially exacerbating by acid
blockers. Although both VPZ and PPIs induce hypergastrinemia, VPZ
induces significantly higher levels than PPIs (10). Further studies are needed to
investigate the effects of VPZ/PPI on the development of web-like
mucus.
While VPZ/PPI-related gastric mucosal changes are
commonly discussed in relation to hypergastrinemia, several
conditions, such as fundic gland polyps, multiple white and flat
elevated lesions, and cobblestone-like gastric mucosal changes, are
not related to hypergastrinemia (5). Conversely, the present multivariate
analysis did not establish significant associations between these
lesions and web-like mucus, although gastric hyperplastic polyps
and stardust gastric mucosal lesions may be related to
hypergastrinemia. Additionally, the present study identified
open-type gastric atrophy as a negative factor for web-like mucus,
which contradicts our previous findings linking it to
hypergastrinemia during prolonged VPZ therapy (11). In patients with severe gastric
atrophy, the number of mucus-producing cells may be reduced,
leading to diminished mucus production. Although hypergastrinemia
is more predominant among females than males, regardless of the
acid suppression therapy (11,12),
no sex-specific predominance in the prevalence of web-like mucus
was observed in the present study. In addition to hypergastrinemia,
we speculate that VPZ-specific mechanisms, which remain
unidentified, may contribute to the development of web-like
mucus.
The mucus layer of the stomach protects the gastric
mucosa from acid and pepsin (13).
Generally, LDA/NSAIDs reduce mucin production, compromising the
gastric mucus barrier and increasing susceptibility to mucosal
injury (14). This reduction in
gastric mucus is caused by decreased cyclooxygenase and
prostaglandin production due to LDA/NSAIDs. PPIs help reduce upper
gastrointestinal bleeding associated with LDA/NSAIDs by enhancing
the gastric mucosal barrier (15,16).
Importantly, VPZ has demonstrated significantly better efficacy
than that of PPIs in preventing LDA-associated upper
gastrointestinal bleeding (17),
potentially due to its superior enhancement of gastric mucus
production. However, a conclusion cannot be made over whether
web-like mucus represents a harmful change. VPZ is known to have a
protective effect against NSAID-induced gastric mucosal damage,
which we hypothesize may be related to an increased mucosal layer
resulting from hypergastrinemia induced by VPZ. This excessive
mucus production could contribute to the formation of web-like
mucus.
Strong and sustained acid suppression therapy with
VPZ leads to bacterial overgrowth possibly associated with the
development of web-like mucus. PPIs alter the normal microbiota
throughout the gastrointestinal tract, and small intestinal
bacterial overgrowth has been observed in 50% of patients using
PPIs (18). Additionally, studies
have shown that PPI users had significantly higher levels of
non-H. pylori bacteria, including streptococci, in gastric
juice compared with non-PPI users or H2RA users (19,20),
and that streptococcal species are present in patients with
web-like mucus (7). PPI therapy can
independently lead to both bacterial overgrowth and increased
gastric mucus production as a protective mechanism. It is plausible
that bacterial overgrowth due to VPZ therapy may further stimulate
mucus secretion. However, more targeted research is needed to
elucidate this relationship.
The present study offers several novel contributions
compared with previous research on web-like mucus. Firstly, a
back-to-back analysis of endoscopic findings before and after
initiating VPZ therapy, which was not addressed in earlier studies,
clarified that none of the patients exhibited web-like mucus prior
to VPZ administration, which strengthened the causal association
between VPZ use and the development of web-like mucus. Secondly,
the current duration-dependent analysis indicated that the
prevalence of web-like mucus is not associated with the duration of
VPZ use. Thirdly, the mean duration of VPZ therapy was 52 months,
which was significantly longer than the 1-26 months reported in
prior research (7). This extended
duration provides a more comprehensive understanding of the
long-term effects of VPZ. Fourthly, subjects with current H.
pylori infection were excluded to eliminate potential
confounding effects. Fifthly, the prevalence of web-like mucus
among VPZ users and H2RA users was compared, which has not been
previously reported. Lastly, a multivariate analysis was performed
that included representative lesions associated with
potassium-competitive acid blockers and PPIs, providing a more
detailed assessment of factors associated with web-like mucus.
Stardust gastric mucosal lesions, another novel
VPZ-associated gastric mucosal change, were reported by Yoshizaki
et al (4) in 2021. These
lesions, strongly linked to VPZ use, histologically manifest as
periodic acid-Schiff stain-positive mucus pools within dilated
ducts. Similar mechanisms may underlie both intraductal mucus
production and web-like mucus formation due to VPZ administration.
However, the present multivariate analysis did not establish a
significant association between stardust gastric mucosal lesions
and web-like mucus. Therefore, further investigations are warranted
to elucidate the developmental mechanisms of these changes.
There are some limitations to the present study.
Firstly, this was a single-center retrospective observational
study. Although consecutive patients were included, there may be
selection and information biases that could potentially impact the
accuracy of the results. Secondly, a bacterial analysis or
molecular investigation of the web-like mucus was not performed;
therefore, the present study did not explore its potential
pathological mechanisms. Thirdly, web-like mucus was diagnosed
solely based on endoscopic findings. Fourthly, the endoscopist was
not blinded to the medication history and did not evaluate the
gastric mucosa using magnifying endoscopy.
In conclusion, web-like mucus is strongly associated
with VPZ use, with 19% of VPZ users developing this feature after
initiating VPZ therapy; however, the prevalence of web-like mucus
is not associated with the duration of VPZ therapy. Further studies
are needed to clarify the pathophysiology of web-like mucus and its
association with potent acid suppression.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SS and HS were responsible for conception and
design, data collection, data analysis and interpretation, and
drafting and writing the manuscript. HO and TY were responsible for
conception and design, and critical revision of important
intellectual content in the manuscript. HY was responsible for data
analysis and interpretation, and critical revision of important
intellectual content in the manuscript. All authors read and
approved the manuscript.
Ethics of approval statement and patient
consent statement
This study protocol was approved by the
Institutional Review Board of Shinozaki Medical Clinic (approval
no. 31-R001). The Institutional Review Board waived the requirement
for informed consent from participants due to the retrospective
nature of the study.
Patient consent for publication
The Institutional Review Board waived the
requirement for informed consent from participants due to the
retrospective nature of the study.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
AI tools were utilized to enhance the readability
and language of the manuscript. Subsequently, the authors revised
and edited the AI-generated content, taking full responsibility for
the final version of the manuscript.
References
|
1
|
Yamamichi N, Shimamoto T, Takahashi Y,
Takahashi M, Takeuchi C, Wada R and Fujishiro M: Trends in proton
pump inhibitor use, reflux esophagitis, and various upper
gastrointestinal symptoms from 2010 to 2019 in Japan. PLoS One.
17(e0270252)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Iwakiri K, Fujiwara Y, Manabe N, Ihara E,
Kuribayashi S, Akiyama J, Kondo T, Yamashita H, Ishimura N,
Kitasako Y, et al: Evidence-based clinical practice guidelines for
gastroesophageal reflux disease 2021. J Gastroenterol. 57:267–285.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2017 Gastro-oesophageal Reflux Disease
Collaborators. The global, regional, and national burden of
gastro-oesophageal reflux disease in 195 countries and territories,
1990-2017: A systematic analysis for the Global Burden of Disease
Study 2017. Lancet Gastroenterol Hepatol. 5:561–581.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoshizaki T, Morisawa T, Fujinami M,
Matsuda T, Katayama N, Inoue K, Matsumoto M, Ikeoka S, Takagi M,
Sako T, et al: Propensity score matching analysis: Incidence and
risk factors for ‘stardust’ gastric mucosa, a novel gastric finding
potentially induced by vonoprazan. Aliment Pharmacol Ther.
53:94–102. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shinozaki S, Osawa H, Miura Y, Nomoto H,
Sakamoto H, Hayashi Y, Yano T, Despott EJ and Yamamoto H:
Endoscopic findings and outcomes of gastric mucosal changes
relating to potassium-competitive acid blocker and proton pump
inhibitor therapy. DEN Open. 5(e400)2024.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Kubo K, Kimura N and Kato M:
Potassium-competitive acid blocker-associated gastric mucosal
lesions. Clin Endosc. 57:417–423. 2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kaneko H, Sato H, Suzuki Y, Ikeda A,
Kuwashima H, Ikeda R, Sato T, Irie K, Sue S and Maeda S: A novel
characteristic gastric mucus named ‘Web-like Mucus’ potentially
induced by vonoprazan. J Clin Med. 13(4070)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969.
|
|
9
|
Skoczylas T, Sarosiek I, Sostarich S,
McElhinney C, Durham S and Sarosiek J: Significant enhancement of
gastric mucin content after rabeprazole administration: Its
potential clinical significance in acid-related disorders. Dig Dis
Sci. 48:322–328. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kinoshita Y, Haruma K, Yao T, Kushima R,
Akiyama J, Kanoo T, Miyata K, Kusumoto N and Uemura N: Ep54 Final
Observation Results of Vision Trial: A Randomized, Open-Label Study
to Evaluate the Long-Term Safety of Vonoprazan as Maintenance
Treatment in Patients with Erosive Esophagitis. Gastroenterology.
164:S–1202. 2023.
|
|
11
|
Shinozaki S, Osawa H, Miura Y, Hayashi Y,
Sakamoto H, Yano T, Lefor AK and Yamamoto H: Long-term changes in
serum gastrin levels during standard dose vonoprazan therapy. Scand
J Gastroenterol. 57:1412–1416. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shiotani A, Katsumata R, Gouda K,
Fukushima S, Nakato R, Murao T, Ishii M, Fujita M, Matsumoto H and
Sakakibara T: Hypergastrinemia in Long-Term Use of Proton Pump
Inhibitors. Digestion. 97:154–162. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Niv Y and Boltin D: Secreted and
membrane-bound mucins and idiopathic peptic ulcer disease.
Digestion. 86:258–263. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sakai T, Ishihara K, Saigenji K and Hotta
K: Recovery of mucin content in surface layer of rat gastric mucosa
after HCl-aspirin-induced mucosal damage. J Gastroenterol.
32:157–163. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lanas Á, Carrera-Lasfuentes P, Arguedas Y,
García S, Bujanda L, Calvet X, Ponce J, Perez-Aísa Á, Castro M,
Muñoz M, et al: Risk of upper and lower gastrointestinal bleeding
in patients taking nonsteroidal anti-inflammatory drugs,
antiplatelet agents, or anticoagulants. Clin Gastroenterol Hepatol.
13:906–912.e902. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Blandizzi C, Gherardi G, Marveggio C,
Natale G, Carignani D and Del Tacca M: Mechanisms of protection by
omeprazole against experimental gastric mucosal damage in rats.
Digestion. 56:220–229. 1995.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kawai T, Oda K, Funao N, Nishimura A,
Matsumoto Y, Mizokami Y, Ashida K and Sugano K: Vonoprazan prevents
low-dose aspirin-associated ulcer recurrence: Randomised phase 3
study. Gut. 67:1033–1041. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lombardo L, Foti M, Ruggia O and Chiecchio
A: Increased incidence of small intestinal bacterial overgrowth
during proton pump inhibitor therapy. Clin Gastroenterol Hepatol.
8:504–508. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sanduleanu S, Jonkers D, De Bruine A,
Hameeteman W and Stockbrügger RW: Non-Helicobacter pylori bacterial
flora during acid-suppressive therapy: Differential findings in
gastric juice and gastric mucosa. Aliment Pharmacol Ther.
15:379–388. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Minalyan A, Gabrielyan L, Scott D, Jacobs
J and Pisegna JR: The gastric and intestinal microbiome: Role of
proton pump inhibitors. Curr Gastroenterol Rep.
19(42)2017.PubMed/NCBI View Article : Google Scholar
|