Introduction
The WHO predicts that by 2050, the elderly
population in the world will increase in 2020 to 2.1 billion from
1.4 billion individuals, due to increasing healthcare coverage and
life expectancy, and up to 2/3 of this population will live in low-
and middle-income countries (1).
The increase in the elderly changes population structure and
disease distribution, with degenerative and metabolic diseases and
cancers gaining prominence (2). The
physiology of aging must be considered since numerous bodily
functions are significantly impaired and present significant
morbidity. For example, cardiovascular function is known to decline
with increasing age, with fibrosis, reduced contractility and
reduced blood flow, to name a few effects (3,4).
Several studies have focused on promoting ‘healthy aging’ to
promote healthy function despite old age (5-7).
Lifestyle interventions such as physical exercises are well known
to counteract the age-associated decline and promote cardiac
function with increasing age (5,8).
However, up to 1/3 of adults aged ≥45 o lack physical activity.
These sedentary lifestyles cause at least 3.2 million deaths per
year (9,10). Studies from the early 2000s have
already proven strong evidence associating a sedentary lifestyle
with cardiovascular mortality. Currently, a sedentary lifestyle is
associated with a 30% increased risk for all-cause mortality.
Therefore, physical exercise is essential in promoting survival and
function in the aging heart.
Physical exercise has various physiological and
psychological benefits. The physiological benefits include the
improvement of the function of the cardiovascular system and
increasing the heart's resistance to injury (11,12).
This improvement is also contingent on the intensity, type and
duration of the physical exercise, thereby highlighting the
diversity of molecular effects of exercise on the heart. One of the
most used recommendations is by the American Heart Association,
which states that for older adults, a minimum of 150 min of
moderate-intensity physical exercise per week is needed, coupled
with medium-intensity strength training at least two times per week
(13,14). Previous studies have shown that
physical exercises induce physical adaptations of the heart through
the remodeling and growth of cardiomyocytes (15). Although taken to the extreme, this
remodeling may increase the risk of sudden cardiac death. These
so-called physiological cardiac hypertrophies were shown to promote
longevity and confer protective benefits to the heart. However, the
mechanisms by which moderate-intensity physical exercise affects
the aging heart remain unknown.
Autophagy is a cellular homeostasis mechanism
responsible for recycling organelles and proteins, especially
during aging (16). Studies have
found declining levels of autophagy associated with old age,
corresponding with increased levels of damaged organelles and
mitochondria. These accumulations may contribute to the decline in
cardiac function (17,18). Previous studies have also shown that
this pathway interacts with the components of the Hippo pathway
with Yes-associated protein 1 (Yap1) and Taz proving to be a
substrate to autophagy (19,20)
and also influences autophagy activation (21). Yap1 is one of the components of the
Hippo pathway involved in cell regeneration and hypertrophy. Yap1
is a mechanoreceptor whose activation relies on stretch and
mechanical tension (22). Several
studies on skeletal muscles prove the ability of Yap1 to induce
hypertrophy (23,24). Yet, research on cardiomyocytes
revealed that Yap1 induced proliferation instead of cellular
hypertrophy reflecting the complex regulation of this protein
between tissues (25). Studies on
Yap have shown that it influences cardiomyocyte proliferation and
regeneration, protecting against ischemic injury (26). Increased Yap1 activity, either
through inhibition of the Hippo pathway, a known negative regulator
of Yap1 or through gene overexpression, was identified to induce
cardiac overgrowth with increased proliferation and protect against
doxorubicin-induced cytotoxicity (27,28).
Yap1 was also shown to mediate the effects of pathological
hypertrophy caused by mechanical stress overload (29). Therefore, although increasing Yap1
may be able to increase cardiomyocyte growth and proliferation, it
is unknown whether this protein played a role in the physiological
hypertrophy induced by moderate-intensity exercises.
Physiological hypertrophy of the heart is also
related to other functions besides the Yap1 protein. The
distribution of the myosin-heavy chain is also affected in the
aging heart. Several reviews have explored the consequences of
aging in myosin heavy chain (MHC) distributions, which affect
contractile and metabolic functions (30,31).
Autophagy is a well-known physiological process involved in aging,
and its influence may regulate cardiac mass. Several studies have
shown that autophagy influences Yap1 activity, showing their
complex interactions (19,21). Furthermore, autophagy has been
demonstrated to regulate myosin-heavy chain dynamics (32). Singh et al (33) found that the administration of
Rapamycin, or caloric restriction, which induces autophagy
function, lessens the effects of hypertrophic cardiomyopathy caused
by alterations in genes encoding or affecting the MHCs (32-34).
Autophagy is essential in maintaining mitochondrial homeostasis in
the heart and further proving its important role in maintaining
cardiac function. Therefore, in the present study, the molecular
effects of moderate-intensity exercises focusing on the autophagy
process, MHC dynamics and Yap1 activity were explored.
Materials and methods
Animal models
A total of 24 male Wistar rats (Rattus norvegicus)
obtained from PT. Biofarma (Bandung, Indonesia). Animals were
housed from 8th weeks of age and had free access to food and water.
The animals were housed in cages measuring 30x40x60 cm³, lined with
husk bedding, and maintained at a room temperature of 25-27˚C with
a 20-40% humidity level. A 12/12-h light/dark cycle was
implemented, and the bedding was replaced every other day. The
animals were included in the study if they weighed at least 200 g,
could acclimatize, and were healthy during the experiment. The
animals were raised until the 80th week and were allocated to
control and exercise groups. The rats were raised until the 80th
week since it correlates with human in 45 years of age (35). The present study was approved
(approval no. 598/UN6.KEP/EC/202598/UN6.KEP/EC/2022) by the
Research Ethics Committee of Universitas Padjadjaran (Bandung,
Indonesia). Exercise intervention. In the 80th week, the
rats were randomly allocated into two groups (n=12): intervention
and no treatment. Ronny et al (36) and Gunadi et al (37) determined exercise intensity
according to the lactate accumulation levels, and from the
aforementioned studies, the moderate intensity treatment protocol
was derived. The exercise intervention was given using an animal
treadmill at a speed of 20 meters per min for 30 min per day,
repeated five days per week, and lasted 8 weeks. For the
no-treatment (control) group, the rats were kept on the immobile
treadmill. The rats were euthanized using 5% isoflurane for 1 min,
followed by cervical dislocation. After death confirmation, the
heart was isolated and harvested. The organ was weighed, and 500 mg
of it was frozen using liquid nitrogen and stored at -80˚C for RNA
extraction.
The rat's body weight, organ weight and tibia length
were measured. Several of these measurements were also normalized
with body weight or tibia length (38). For histopathological examination,
500 mg of the heart muscle originating from the left ventricle was
fixed using 10% neutral-buffered formalin at room temperature for
72 h. Histopathological sections with a thickness of 4 µm were made
and stained with hematoxylin for 5 min, followed by Eosin for 2
min, both conducted at room temperature before being evaluated by a
pathologist. H&E staining was chosen since it provides a
balanced visualization of tissue structure, cellular morphology,
and fibrosis assessment. This provided a thorough evaluation of all
the parameters needed. The review was performed on five fields per
sample with an Olympus CX21 light microscope at x100 magnification.
The assessment criteria were cardiomyocyte hypertrophy, myofiber
disarray and focal fibrosis (Table
I).
 | Table IHistological assessment criteria used
in this research. |
Table I
Histological assessment criteria used
in this research.
| Cardiomyocyte
hypertrophy | Myofiber
disarray | Focal fibrosis |
|---|
| 0: No
hypertrophy | 0: No myofiber
disarray | 0: No focal
fibrosis |
| 1: Cardiomyocyte
diameter is 3-4 RBCs large. | 1: Disarray in
1-25% of heart muscle | 1: 1-5 focal
fibrosis |
| 2: Cardiomyocyte
diameter is 4-5 RBCs large. | 2: Disarray in
26-50% of heart muscle | 2: 6-10 focal
fibrosis |
| 3: Cardiomyocyte
diameter is >5 RBCs large. | 3: Disarray in
>50% of heart muscle | 3: >10 focal
fibrosis |
RNA extraction was performed using TRIsure (cat. no.
BIO-38032; Bioline), and RNA purity ratios were examined using
spectrophotometry at 260/280 nm. A semiquantitative PCR was carried
out using the Bioline one-step RT-PCR kit (cat. no. BIO-72005;
Bioline), with GAPDH as a housekeeping gene. The primer sequences
are included in Table I. A 10%
agarose gel electrophoresis was carried out and stained using
SYBRSafe (cat. no. S33102; Invitrogen; Thermo Fisher Scientific,
Inc.). The gel was visualized using the BluePad Detection System
(BP001CU; Bio-Helix Co., Ltd.) and quantified using ImageJ software
version 1.46r (National Institutes of Health). The primer pairs
used for PCR in the present study are shown in Table II.
 | Table IIPrimer pairs used in the present
study. |
Table II
Primer pairs used in the present
study.
| Gene name | Primer sequence
(5'-3') | Base pairs | Annealing (˚C) |
|---|
| Myh6 | F:
GAGCAGGAGCTGATCGAGAC | 151 | 60 |
| | R:
CCTCTGCGTTCCTACACTCC | | |
| Myh7 | F:
GCGGACATTGCCGAGTCCCAG | 133 | 59.5 |
| | R:
GCTCCAGGTCTCAGGGCTTCACA | | |
| Yap | F:
GATCCCTGATGATGTACCACTGCC | 101 | 57 |
| | R:
GCCATGTTGTTGTCTGATCGTTGTG | | |
| Taz | F:
CATGGCGGAAAAAGATCCTCC | 242 | 57 |
| | R:
GTCGGTCACGTCATAGGACTG | | |
| PIK3ca | F:
ACCTCAGGCTTGAAGAGTGTCG | 137 | 59 |
| | R:
CCGTAAGTCGTCGCCATTTTTA | | |
| mTOR | F:
CTGATGTCATTTATTGGCACAAA | 170 | 57 |
| | R:
CAGGGACTCAGAACACAAATGC | | |
| Lc3 | F:
GGTCCAGTTGTGCCTTTATTGA | 153 | 59.5 |
| | R:
GTGTGTGGGTTGTGTACGTCG | | |
| p62 | F:
CTAGGCATCGAGGTTGACATT | 116 | 56 |
| | R:
CTTGGCTGAGTACCACTCTTATC | | |
| GAPDH | F:
GTTACCAGGGCTGCCTTCTC | 177 | 61 |
| | R:
GATGGTGATGGGTTTCCCGT | | |
Statistical analysis
The data was presented as the mean ± standard
deviation (SD) or median (min-max). The normality test was
conducted using the Shapiro-Wilk test; Levene's test was used to
determine the homogeneity of variance. Statistical analysis was
performed using the independent t-test for normally distributed
data or the Mann-Whitney U test for non-parametric data. SPSS V.20
software (IBM Corp.) was used for analysis.
Results
Chronic moderate-intensity physical
exercise causes cardiac muscle hypertrophy in old rats
The heart weight between the control and exercise
groups was compared to ascertain the relationship between physical
exercise and cardiac muscle. The heart weight between the control
and exercise groups was significantly different, with a higher
weight in the exercise group, as shown in Table III. The difference was still
significant even after normalization with the body weight or tibia
length of rats. Therefore, chronic moderate-intensity physical
exercise was shown to be able to significantly increase heart
weight, reflecting the hypertrophy process caused by exercise.
 | Table IIIGross characteristics of control vs.
intervention groups. |
Table III
Gross characteristics of control vs.
intervention groups.
| | Control [mean ±
SD/median (min-max)] | Intervention [mean
± SD/median (min-max)] | P-value |
|---|
| Heart weight
(g) | 1.15
(0.92-1.68) | 1.65
(1.22-1.92) | 0.01 |
| Body weight
(g) | 362.5
(347-407) | 315.5
(233-372) | 0.001 |
| Tibia length
(cm) | 4.5 (4-5.3) | 4.55 (4-4.9) | 0.843 |
| Heart to body
weight ratio | 0.003±0.001 | 0.01±0.001 | <0.001 |
| Heart to tibia
length ratio | 0.28±0.07 | 0.36±0.05 | 0.003 |
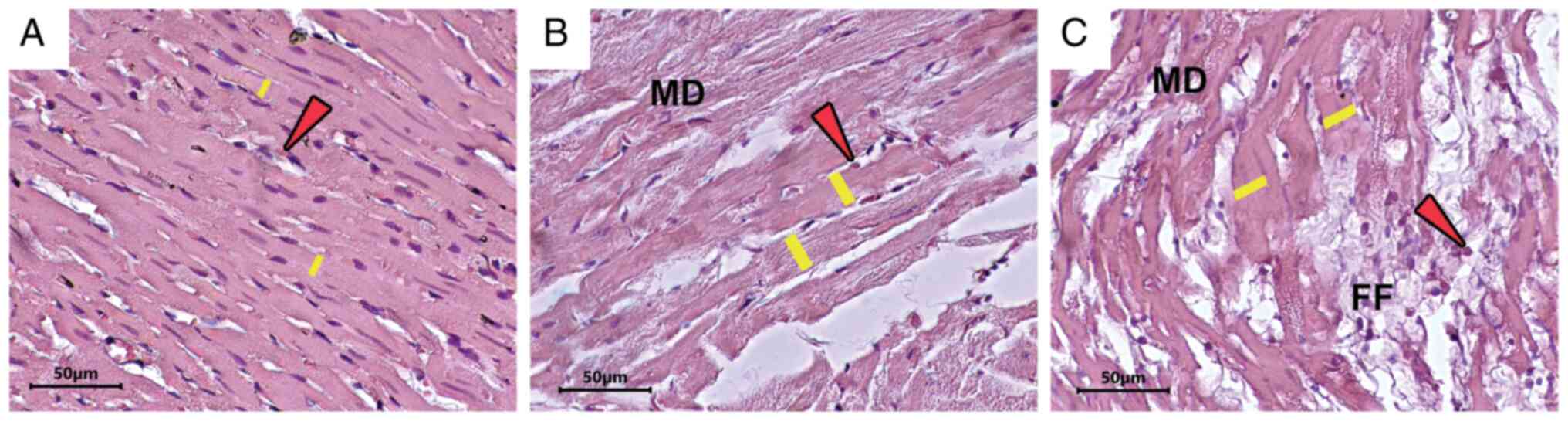
Hypertrophy caused by exercise is not
associated with myofiber disarray and focal fibrosis
The histological appearances of cardiomyocytes were
quantified by scoring system (37),
which compared three main changes i.e., cardiomyocyte hypertrophy,
myofiber disarray and focal fibrosis (Fig. 1). However, the authors differed from
the earlier planned criteria and the assessment requirements were
combined to only yes/no for all histological criteria. The main
difference among the three quantified changes is in cardiomyocyte
hypertrophy, with the exercise group showing significantly higher
hypertrophy than the control group. No significant differences were
found for myofiber disarray and focal fibrosis (Table IV).
 | Table IVComparison of histological
characteristics. |
Table IV
Comparison of histological
characteristics.
| | Control (n=12) | Intervention (n
=12) | P-value |
|---|
| Any Cardiomyocyte
Hypertrophya | 1 (8%) | 7 (58%) | 0.014 |
| Any Myofiber
Disarraya | 1 (8%) | 1 (8%) | 1 |
| Any Focal
Fibrosisa | 0 (0%) | 1 (8%) | 1 |
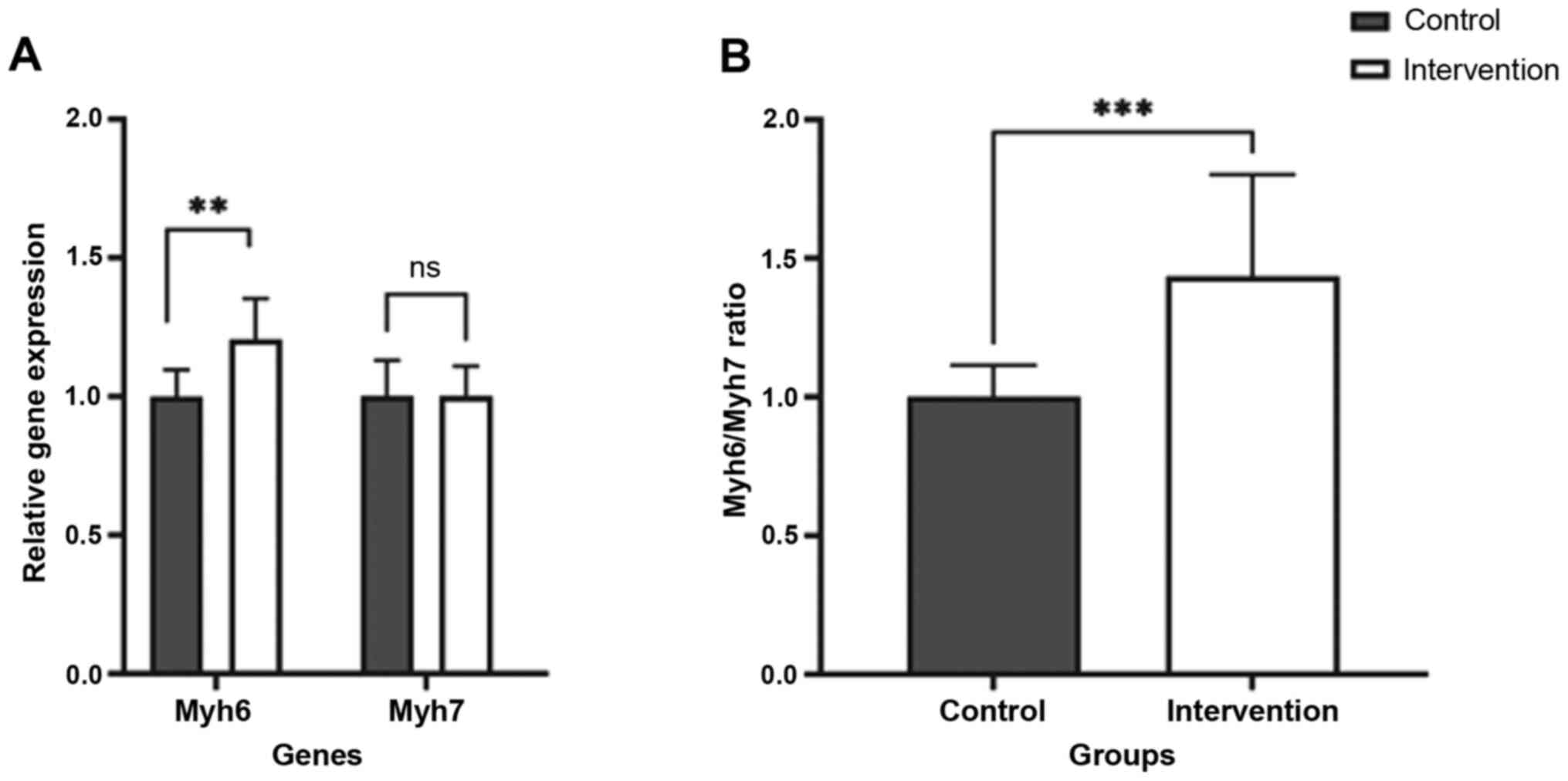
Chronic moderate-intensity physical
exercise causes a significant increase in Myh6 but not Myh7
Two MHC gene isoforms, Myh6 and Myh7, corresponding
to α-MHC and β-MHC isoforms, were quantified using conventional PCR
(Fig. 2). There was a significant
increase in the relative expression of Myh6, with 1.2-fold higher
expression in the intervention group (Table V). No significant increase was found
in the relative expression of Myh7 corresponding to increased α-MHC
expression with no significant changes in β-MHC expression. The
ratio between the expression of these two genes also showed
significant results with a difference of 1.27-fold increase in the
intervention group (P<0.001). The results revealed that
moderate-intensity physical activity caused an increase in cardiac
muscle mass with MHC isoform distribution consistent with
physiological hypertrophy.
 | Table VThe expression of Myh6 and Myh7 in
control vs. intervention groups. |
Table V
The expression of Myh6 and Myh7 in
control vs. intervention groups.
| | Control (median
(min-max)/mean ± sd) | Intervention
(median (min-max)/mean ± sd) | P-value |
|---|
| Myh6 | 1±0.097 | 1.204±0.148 | 0.001 |
| Myh7 | 1±0.130 | 1±0.11 | 0.992 |
|
Myh6/Myh7a | 0.97
(0.85-1.26) | 1.27
(1.15-2.623) | <0.001 |
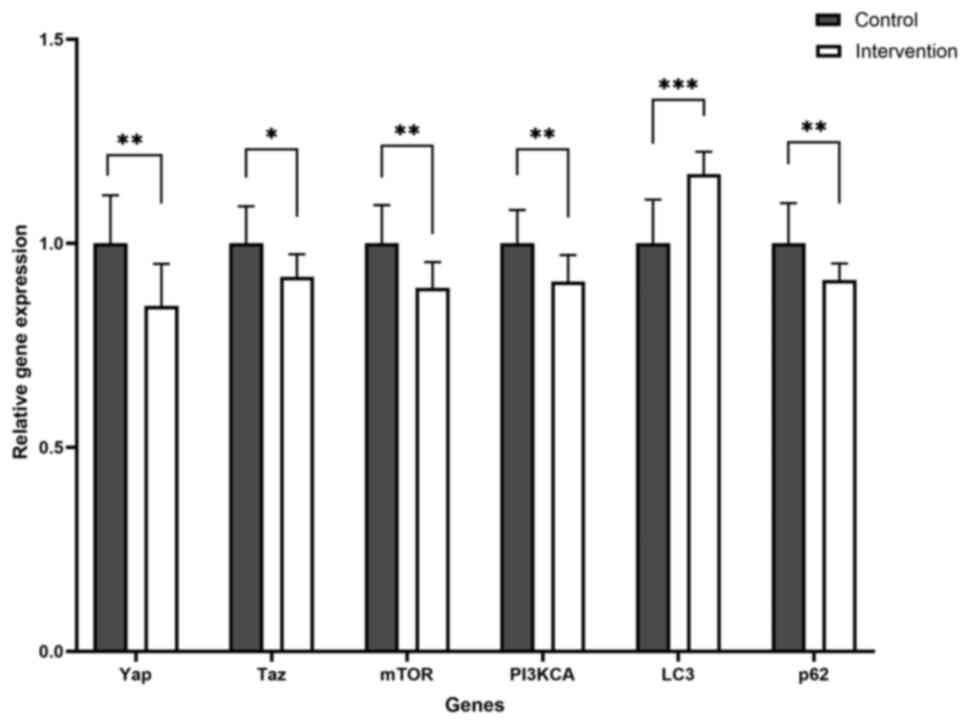
Yap and Taz are downregulated due to
chronic moderate-intensity physical exercise
The expression of Yap and Taz, effectors of the
Hippo pathway, was measured. Semiquantitative measurement
demonstrated a significant underexpression of both genes after
chronic moderate-intensity physical exercise. Yap and Taz were
significantly less expressed in the intervention compared with the
control group, with 0.8 and 0.9-fold lower expression levels,
respectively (Table VI).
 | Table VIComparison of Yap/Taz and
autophagy-related genes. |
Table VI
Comparison of Yap/Taz and
autophagy-related genes.
| | Control (median
(min-max)/mean ± SD) | Intervention
(median (min-max)/mean ± SD) | P-value |
|---|
| Yapa | 0.98
(0.89-1.34) | 0.85
(0.67-0.96) | 0.001 |
| Taz | 1±0.09 | 0.92±0.05 | 0.014 |
| mTOR | 1±0.09 | 0.89±0.06 | 0.003 |
| PI3KCA | 1±0.08 | 0.91±0.06 | 0.005 |
| Lc3 | 1±0.11 | 1.17±0.05 | <0.001 |
| P62a | 0.99
(0.84-1.20) | 0.92
(0.83-0.96) | 0.005 |
Lc3 and p62 gene expression reveals
that exercise potentially increased autophagy activity
In the present study, autophagy pathways were
evaluated by measuring Lc3 expression, which is involved in
autophagosome formation, and p62, which is constantly degraded by
autophagy.
The intervention group exhibited a significantly
higher expression of Lc3, coupled with a significantly lower
expression of p62 (Fig. 3). This
expression pattern suggests potential alterations in autophagy
activity in the intervention group compared with the control group.
Additionally, genes upstream of the autophagy pathway were
examined, specifically the mTOR and PI3KCA genes. Both genes were
also significantly underexpressed after chronic moderate-intensity
physical exercise. This finding suggested that exercise may cause
higher autophagy activity by influencing its upstream regulators,
specifically downregulating mTOR and PI3K. However, further
analysis using protein-level assays is needed to determine whether
these changes reflect an overall increase in autophagy.
Discussion
Research exploring the effects of exercise on the
heart were conducted. It was found that even in aged rats, exercise
can still cause an increase in heart weight. A previous study in
skeletal muscle showed that aging caused a blunted hypertrophic
response to resistance training (39). However, in the heart, several
evidence point out that ventricular hypertrophy is one of the
physiological changes (40).
Ventricular hypertrophy in aging is associated with cardiac
fibrosis and lower heart function, signs of pathological
hypertrophy (41). These changes
cause hypertrophy as a mechanism for compensation to maintain body
perfusion. This research shows that exercise can still induce a
hypertrophic response in cardiac muscle even during aging, as
demonstrated by the increased cardiac weight. Even after
normalization, a significant difference in the body weight and
tibia length was still found to ensure that the increased weight
was not due to body size differences. However, further exploration
of microscopic appearances and gene expression levels is needed to
ascertain whether it is physiological or pathological.
Several studies have found that α-MHC content is
decreased during aging, similar to cardiac changes caused by
overload or heart failure (42,43).
In the present study, it was validated that even during aging,
chronic-moderate-intensity physical exercises can still induce
physiological hypertrophy of the heart. The findings of the present
study demonstrated that physical exercise caused a preferential
increase of alpha myosin over beta myosin, as shown by the higher
expression of alpha myosin and the ratio between both myosin
isoforms. The increase in alpha myosin is consistent with
physiological hypertrophy, which was found with a higher alpha
myosin content (41). The α-MHC
isoform has the highest myosin ATPase activity and contractile
speed, with several research showing that a higher combination of
this protein is associated with increased contractile velocity
(42). Targeting α-MHC has been the
focus of several research studies, and it has been found that
overexpression of α-MHC allowed a modest improvement in ventricular
function after myocardial infarction (44), and interventions aiming at this
protein also alleviated heart failure (45). Therefore, the present findings
extended previous findings of increased α-MHC in cardiac muscle
after exercise and that the increase of α-MHC still occurs in the
context of aging.
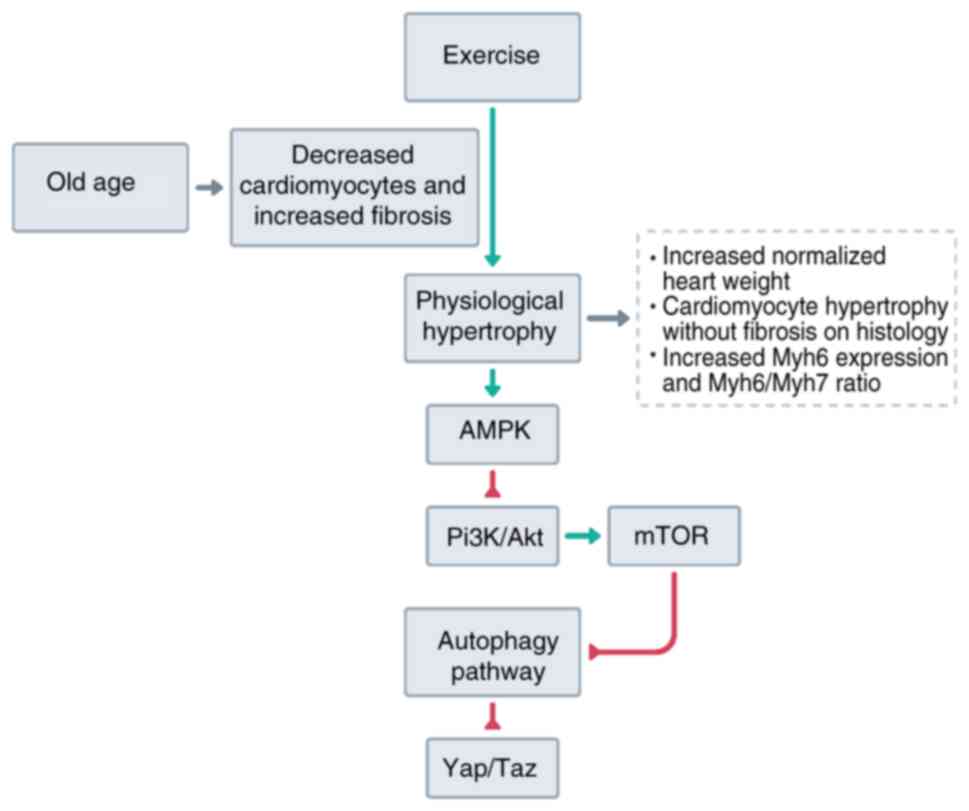
The increase in alpha myosin contrasts with
hypertrophy caused by cardiac overload. It has been previously
reported that chronic pressure overload preferentially increases
beta myosin (43). Beta myosin has
a lower ATPase activity but is more economically efficient in the
contractile force it generates. Therefore, the increased force
needed for heart function will cause a preferential increase of
this isoform. The role of beta myosin is probably better explained
by mutations of this gene, which is one of the known causes of
hypertrophic cardiomyopathy (46,47).
Mutations cause up to a 30% reduction in contractile speed and
force generation (46,48), which causes compensatory hypertrophy
to generate enough force for heart function. In the present study,
it was found that chronic moderate-intensity exercise in old rats
did not increase the β-MHC gene expression, supporting the role of
exercise in restoring cardiac function even during old age
(Fig. 4).
Microscopy further supports the role of exercise in
causing physiological hypertrophy. In pathological hypertrophy, a
significant amount of fibrosis usually occurs in the cardiac tissue
(41). These were considered to be
due to several factors: Chronic injury, mismatch between vascular
and cardiomyocyte growth, imbalances between pro- and antifibrotic
proteins, intense exercises and aging (49-53).
Although exercises were known to reduce cardiac fibrosis (49,54,55),
it is unknown whether the same effects can be observed on the aging
heart, especially considering the lower functional capacity of aged
hearts. The current research found that exercise did not cause an
increase in cardiac tissue fibrosis compared with control.
Additionally, since there is no evidence of fibrosis, a sign of
pathological hypertrophy (41), it
can be further supported that the hypertrophy caused by exercise,
even during old age, is consistent with features of physiological
hypertrophy.
Yap/Taz is the effector of the Hippo pathway, which
was previously known to cause skeletal muscle hypertrophy. Yap/Taz
was activated by several mechanoreceptors and stretch, causing
hypertrophy in skeletal muscles (22). Therefore, during exercise, which
causes physical stress on the heart muscle, it was initially
postulated that Yap/Taz expression would increase. The authors'
assumption was supported by previous studies showing the essential
function of Yap in cardiomyocyte function after cardiac injury and
embryonic development (56,57). However, the present study identified
that their expression is reduced in the exercise group compared
with the control. Therefore, it is considered that although the
cardiac muscle experienced hypertrophy, it is not due to increased
expression of Yap and Taz. Since cardiac muscle hypertrophy
involves the enlargement of cardiomyocytes instead of proliferation
(41), perhaps the role of Yap and
Taz is not that pronounced in cardiac muscle. The current findings
are consistent with previous studies that showed Yap is necessary
for cardiomyocyte proliferation (25,56,58).
Since physical exercise causes hypertrophy through enlargement, the
effect of exercise on Yap/Taz is negligible.
Autophagy is a pathway that has been previously
researched as a beneficial process in the heart induced by exercise
(59-62).
However, this function gradually decreases during aging (63), and it was explored whether exercise
can still induce autophagy in the aged heart. Therefore, expression
of genes involved in autophagy functions such as Lc3 and p62, was
determined. The intervention group exhibited an expression pattern
consistent with increased autophagy function with high Lc3, markers
of autophagosome formation, and low p62, a specific autophagy
substrate. However, due to resource constraints, further
protein-level analysis which is necessary to confirm the present
findings, could not be conducted. The suggested increase in
autophagy is supported by previous studies reporting that exercise
is one of the main factors inducing autophagy (61,62).
Autophagy confers several benefits, such as allowing clearance of
damaged mitochondria, which prevents cardiomyocyte injury and
apoptosis (61). Interestingly,
lower autophagy has been associated with cardiomyocyte hypertrophy.
However, this hypertrophy is associated with pathological changes
such as lower contractility (64)
and increased fibrosis (65). A
study by Yan et al (66)
demonstrated the essential role of autophagy in mediating the
beneficial effects of exercise. Without autophagy, exercise caused
an increase in fibrosis, impaired mitochondrial biogenesis and
fetal gene reprogramming. Therefore, a balanced autophagy level is
needed to ensure physiological hypertrophy of the cardiomyocytes.
Additionally, the increased autophagy found may explain the lower
expression of Yap/Taz in our research. Several studies have found
Yap as a substrate for autophagy (21). However, further research is needed
to ascertain this conclusion.
The gene expression pattern suggesting increased
autophagy activity also occurs with decreased expression of
upstream regulators of autophagy, specifically PI3K and mTOR. These
findings are counterintuitive since both proteins were usually
upregulated during resistance exercises in skeletal muscles,
considering their function in activating protein synthesis
(67). mTOR is notorious for
enhancing protein synthesis and was found to be essential for
muscle hypertrophy (68). However,
previous research used acute resistance exercise (67,69,70);
in the present study, the authors opted for chronic exercise on
aged animal models. The current results are supported by studies in
rats, which found chronic exercise caused the downregulation of
mTOR and PI3K phosphorylation in the brain (71) and the downregulation of mTORC1
activity in the skeletal muscle (70). These findings perhaps reflected the
lower anabolic signaling in chronic exercise. Therefore, the main
benefit of chronic exercise lies in the ability of autophagy to
maintain mitochondrial health and prevent myocardial injury in the
heart. This effect is still observed even in aging animal models.
Future studies should focus on the expression of protein levels and
explore the impact on female rats to account for hormonal
differences.
However, several limitations are apparent in the
present study. The results are based on rats with significantly
different distributions of MHC isoforms, and other mechanisms might
exist in humans. It is acknowledged that only the left ventricle
was examined in the current study since it plays a critical role in
systemic circulation, and its hypertrophic changes are more likely
to be reflective of overall heart function in response to exercise.
Additionally, specific markers of fibrosis were not investigated
using Masson's Trichrome or Picrosirius red staining, which would
allow for clearer visualization of collagen fibers and a more
accurate assessment of fibrotic changes. Gene expression data were
only measured, and thus post-translational modifications may alter
the results of the present study. Nevertheless, it can be concluded
that even in old age, exercise remains a potent and viable
intervention in increasing heart mass and potentially delays the
decline in function associated with aging.
In conclusion, the current research has shown that
chronic moderate-intensity exercise can induce hypertrophic
response in the heart of old rats. This hypertrophic response is
consistent with features of physiological hypertrophy with minimal
fibrosis and increased α-MHC isoforms and ratio in the intervention
group. Additionally, this hypertrophy is not dependent on Yap/Taz
expression. Hypertrophy is associated with low anabolic signaling
through the PI3KCA and mTOR expression but with gene expression
patterns implicating high autophagy function, suggesting that
autophagy function may be more critical during regular exercise
compared with anabolic signaling.
Acknowledgements
The authors acknowledge Dr Ardo Sanjaya from
Maranatha Christian University for writing and technical assistance
in proofreading the manuscript.
Funding
Funding: The present study was supported by the Fundamental
Research Grant 2024 (grant no. 3986/UN6.3.1/PT.00/2024) from
Universitas Padjadjaran and the Internal Research Grant (grant no.
005/PEG-PRJ/SL-YPTKM/UKM/X/2020) from Maranatha Christian
University.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL, VV, DKJ, and RL conceptualized the present
study. YL, VV, JWG and RL designed the methodology. YL, TL, JWG,
KDMW and DKJ performed experiments and statistical analysis. YL,
TL, and JWG created the original draft of the manuscript. YL, VV,
DKJ, and RL produced the final version of the manuscript. All
authors read and approved the final version of the manuscript. YL,
JWG and RL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
598/UN6.KEP/EC/2022) by the Research Ethics Committee of
Universitas Padjadjaran (Bandung, Indonesia).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steverson M: Ageing and health. World
Health Organization, Geneva, 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=At%20this%20time%20the%20share,2050%20to%20reach%20426%20million.
Accessed April 18, 2024.
|
|
2
|
Marengoni A, Angleman S, Melis R,
Mangialasche F, Karp A, Garmen A, Meinow B and Fratiglioni L: Aging
with multimorbidity: A systematic review of the literature. Ageing
Res Rev. 10:430–439. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
North BJ and Sinclair DA: The Intersection
between aging and cardiovascular disease. Circ Res. 110:1097–1108.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Paneni F, Diaz Cañestro C, Libby P,
Lüscher TF and Camici GG: The aging cardiovascular system:
Understanding it at the cellular and clinical levels. J Am Coll
Cardiol. 69:1952–1967. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eckstrom E, Neukam S, Kalin L and Wright
J: Physical activity and healthy aging. Clin Geriatr Med.
36:671–683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tracy E, Rowe G and LeBlanc AJ: Cardiac
tissue remodeling in healthy aging: The road to pathology. Am J
Physiol Cell Physiol. 319:C166–C182. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nakou ES, Parthenakis FI, Kallergis EM,
Marketou ME, Nakos KS and Vardas PE: Healthy aging and myocardium:
A complicated process with various effects in cardiac structure and
physiology. Int J Cardiol. 209:167–175. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park JH, Moon JH, Kim HJ, Kong MH and Oh
YH: Sedentary lifestyle: Overview of updated evidence of potential
health risks. Korean J Fam Med. 41:365–373. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Taylor L: Third of adults are not getting
enough physical activity, says WHO. BMJ. 385(q1428)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu L, Li T, He W, Cao D, Wu C and Qin L:
Prevalence of sufficient physical activity among general adult
population and sub-populations with chronic conditions or
disability in the USA. Eur J Public Health. 33:891–896.
2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wei X, Liu X and Rosenzweig A: What do we
know about the cardiac benefits of exercise? Trends Cardiovasc Med.
25:529–536. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Platt C, Houstis N and Rosenzweig A: Using
exercise to measure and modify cardiac function. Cell Metab.
21:227–236. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nelson ME, Rejeski WJ, Blair SN, Duncan
PW, Judge JO, King AC, Macera CA and Castaneda-Sceppa C: Physical
activity and public health in older adults: Recommendation from the
American college of sports medicine and the American heart
association. Med Sci Sports Exerc. 39:1435–1445. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lane-Cordova AD, Jerome GJ, Paluch AE,
Bustamante EE, LaMonte MJ, Pate RR, Weaver RG, Webber-Ritchey KJ
and Gibbs BB: Committee on Physical Activity of the American Heart
Association Council on Lifestyle and Cardiometabolic Health.
Supporting physical activity in patients and populations during
life events and transitions: A scientific statement from the
American heart association. Circulation. 145(e128)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fulghum K and Hill BG: Metabolic
mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc
Med. 5(127)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aman Y, Schmauck-Medina T, Hansen M,
Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen
T, Tavernarakis N, et al: Autophagy in healthy aging and disease.
Nat Aging. 1:634–650. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shirakabe A, Ikeda Y, Sciarretta S,
Zablocki DK and Sadoshima J: Aging and autophagy in the heart. Circ
Res. 118:1563–1576. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kaushik S, Tasset I, Arias E, Pampliega O,
Wong E, Martinez-Vicente M and Cuervo AM: Autophagy and the
hallmarks of aging. Ageing Res Rev. 72(101468)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sanjaya A, Lesmana R, Goenawan H, Setiawan
I, Sylviana N, Pratiwi YS and Dewi FN: The functional relationship
of Yap/Taz with autophagy functions in sarcopenia associated with
aging. Nutr Healthy Aging. 8:31–39. 2023.
|
|
20
|
Christoper A, Herman H, Abdulah R,
Zulhendri F, Sanjaya A and Lesmana R: Physiological roles of Hippo
signaling pathway and autophagy in dementia. Curr Aging Sci.
16:112–124. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang D, He J, Huang B, Liu S, Zhu H and Xu
T: Emerging role of the Hippo pathway in autophagy. Cell Death Dis.
11(880)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fischer M, Rikeit P, Knaus P and Coirault
C: YAP-mediated mechanotransduction in skeletal muscle. Front
Physiol. 7(41)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Watt KI, Turner BJ, Hagg A, Zhang X, Davey
JR, Qian H, Beyer C, Winbanks CE, Harvey KF and Gregorevic P: The
Hippo pathway effector YAP is a critical regulator of skeletal
muscle fibre size. Nat Commun. 6(6048)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Judson RN, Gray SR, Walker C, Carroll AM,
Itzstein C, Lionikas A, Zammit PS, De Bari C and Wackerhage H:
Constitutive expression of Yes-associated protein (Yap) in adult
skeletal muscle fibres induces muscle atrophy and myopathy. PLoS
One. 8(e59622)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
von Gise A, Lin Z, Schlegelmilch K, Honor
LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD and Pu
WT: YAP1, the nuclear target of Hippo signaling, stimulates heart
growth through cardiomyocyte proliferation but not hypertrophy.
Proc Natl Acad Sci USA. 109:2394–2399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Del Re DP, Yang Y, Nakano N, Cho J, Zhai
P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D and Sadoshima J:
Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte
survival and growth to protect against myocardial ischemic injury.
J Biol Chem. 288:3977–3988. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Heallen T, Zhang M, Wang J,
Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF: Hippo
pathway inhibits Wnt signaling to restrain cardiomyocyte
proliferation and heart size. Science. 332:458–561. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang P, Wang M, Hu Y, Chen J, Cao Y, Liu
C, Wu Z, Shen J, Lu J and Liu P: Isorhapontigenin protects against
doxorubicin-induced cardiotoxicity via increasing YAP1 expression.
Acta Pharm Sin B. 11:680–693. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yue P, Zhang Y, Liu L, Zhou K, Xia S, Peng
M, Yan H, Tang X, Chen Z, Zhang D, et al: Yap1 modulates
cardiomyocyte hypertrophy via impaired mitochondrial biogenesis in
response to chronic mechanical stress overload. Theranostics.
12:7009–7031. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Strait JB and Lakatta EG: Aging-associated
cardiovascular changes and their relationship to heart failure.
Heart Fail Clin. 8:143–164. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sheydina A, Riordon DR and Boheler KR:
Molecular mechanisms of cardiomyocyte aging. Clin Sci (Lond).
121:315–329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zech ATL, Singh SR, Schlossarek S and
Carrier L: Autophagy in cardiomyopathies. Biochim Biophys Acta Mol
Cell Res. 1867(118432)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Singh SR, Zech ATL, Geertz B,
Reischmann-Düsener S, Osinska H, Prondzynski M, Krämer E, Meng Q,
Redwood C, van der Velden J, et al: Activation of autophagy
ameliorates cardiomyopathy in Mybpc3-targeted knockin mice. Circ
Heart Fail. 10(e004140)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gatica D, Chiong M, Lavandero S and
Klionsky DJ: Molecular mechanisms of autophagy in the
cardiovascular system. Circ Res. 116:456–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sengupta P: The laboratory rat: Relating
its age with human's. Int J Prev Med. 4:624–630. 2013.PubMed/NCBI
|
|
36
|
Lesmana R, Iwasaki T, Iizuka Y, Amano I,
Shimokawa N and Koibuchi N: The change in thyroid hormone signaling
by altered training intensity in male rat skeletal muscle. Endocr
J. 63:727–738. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gunadi JW, Tarawan VM, Setiawan I, Lesmana
R, Wahyudianingsih R and Supratman U: Cardiac hypertrophy is
stimulated by altered training intensity and correlates with
autophagy modulation in male Wistar rats. BMC Sports Sci Med
Rehabil. 11(9)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yin FC, Spurgeon HA, Rakusan K, Weisfeldt
ML and Lakatta EG: Use of tibial length to quantify cardiac
hypertrophy: Application in the aging rat. Am J Physiol.
243:H941–H947. 1982.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kirby TJ, Lee JD, England JH, Chaillou T,
Esser KA and McCarthy JJ: Blunted hypertrophic response in aged
skeletal muscle is associated with decreased ribosome biogenesis. J
Appl Physiol (1985). 119:321–327. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dai DF, Chen T, Johnson SC, Szeto H and
Rabinovitch PS: Cardiac aging: From molecular mechanisms to
significance in human health and disease. Antioxid Redox Signal.
16:1492–1526. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shimizu I and Minamino T: Physiological
and pathological cardiac hypertrophy. J Mol Cell Cardiol.
97:245–462. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gupta MP: Factors controlling cardiac
myosin-isoform shift during hypertrophy and heart failure. J Mol
Cell Cardiol. 43:388–403. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Miyata S, Minobe W, Bristow MR and
Leinwand LA: Myosin heavy chain isoform expression in the failing
and nonfailing human heart. Circ Res. 86:386–390. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
James J, Hor K, Moga MA, Martin LA and
Robbins J: Effects of myosin heavy chain manipulation in
experimental heart failure. J Mol Cell Cardiol. 48:999–1006.
2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang N, Zhang Y, Xu J, Wang P, Wu B, Lu
S, Lu X, You S, Huang X, Li M, et al: α-myosin heavy chain
lactylation maintains sarcomeric structure and function and
alleviates the development of heart failure. Cell Res. 33:679–698.
2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Van Driest SL, Jaeger MA, Ommen SR, Will
ML, Gersh BJ, Tajik AJ and Ackerman MJ: Comprehensive analysis of
the beta-myosin heavy chain gene in 389 unrelated patients with
hypertrophic cardiomyopathy. J Am Coll Cardiol. 44:602–610.
2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
McNally EM: Beta-myosin heavy chain gene
mutations in familial hypertrophic cardiomyopathy: The usual
suspect? Circ Res. 90:246–247. 2002.PubMed/NCBI
|
|
48
|
Lankford EB, Epstein ND, Fananapazir L and
Sweeney HL: Abnormal contractile properties of muscle fibers
expressing beta-myosin heavy chain gene mutations in patients with
hypertrophic cardiomyopathy. J Clin Invest. 95:1409–1414.
1995.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang K, Deng Y and Xiao H: Exercise and
cardiac fibrosis. Curr Opin Physiol. 31(100630)2023.
|
|
50
|
Rao Z, Wang S, Bunner WP, Chang Y and Shi
R: Exercise induced right ventricular fibrosis is associated with
myocardial damage and inflammation. Korean Circ J. 48:1014–1024.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lu L, Guo J, Hua Y, Huang K, Magaye R,
Cornell J, Kelly DJ, Reid C, Liew D, Zhou Y, et al: Cardiac
fibrosis in the ageing heart: Contributors and mechanisms. Clin Exp
Pharmacol Physiol. 44 (Suppl 1):S55–S63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Frangogiannis NG: Cardiac fibrosis.
Cardiovasc Res. 117:1450–1488. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lakhan SE and Harle L: Cardiac fibrosis in
the elderly, normotensive athlete: Case report and review of the
literature. Diagn Pathol. 3(12)2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hong Y, Yang AL, Wong JKS, Masodsai K, Lee
SD and Lin YY: Exercise intervention prevents early aged
hypertension-caused cardiac dysfunction through inhibition of
cardiac fibrosis. Aging (Albany NY). 14:4390–4401. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cui X, Wang K, Zhang J and Cao ZB: Aerobic
exercise ameliorates myocardial fibrosis via affecting vitamin D
receptor and transforming growth factor-β1 signaling in vitamin
D-deficient mice. Nutrients. 15(741)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Xin M, Kim Y, Sutherland LB, Murakami M,
Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, et
al: Hippo pathway effector Yap promotes cardiac regeneration. Proc
Natl Acad Sci USA. 110:13839–13844. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Boogerd CJ, Perini I, Kyriakopoulou E, Han
SJ, La P, van der Swaan B, Berkhout JB, Versteeg D,
Monshouwer-Kloots J and van Rooij E: Cardiomyocyte proliferation is
suppressed by ARID1A-mediated YAP inhibition during cardiac
maturation. Nat Commun. 14(4716)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Flinn MA, Link BA and O'Meara CC: Upstream
regulation of the Hippo-Yap pathway in cardiomyocyte regeneration.
Semin Cell Dev Biol. 100:11–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang L, Wang J, Cretoiu D, Li G and Xiao
J: Exercise-mediated regulation of autophagy in the cardiovascular
system. J Sport Health Sci. 9:203–210. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sasaki Y, Ikeda Y, Iwabayashi M, Akasaki Y
and Ohishi M: The impact of autophagy on cardiovascular senescence
and diseases. Int Heart J. 58:666–673. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wu NN, Tian H, Chen P, Wang D, Ren J and
Zhang Y: Physical exercise and selective autophagy: Benefit and
risk on cardiovascular health. Cells. 8(1436)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Dai M and Hillmeister P: Exercise-mediated
autophagy in cardiovascular diseases. Acta Physiol (Oxf).
236(e13890)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Abdellatif M, Sedej S, Carmona-Gutierrez
D, Madeo F and Kroemer G: Autophagy in cardiovascular aging. Circ
Res. 123:803–824. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ott C, Jung T, Brix S, John C, Betz IR,
Foryst-Ludwig A, Deubel S, Kuebler WM, Grune T, Kintscher U and
Grune J: Hypertrophy-reduced autophagy causes cardiac dysfunction
by directly impacting cardiomyocyte contractility. Cells.
10(805)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Taneike M, Yamaguchi O, Nakai A, Hikoso S,
Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, et al:
Inhibition of autophagy in the heart induces age-related
cardiomyopathy. Autophagy. 6:600–606. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yan Z, Kronemberger A, Blomme J, Call JA,
Caster HM, Pereira RO, Zhao H, de Melo VU, Laker RC, Zhang M and
Lira VA: Exercise leads to unfavourable cardiac remodelling and
enhanced metabolic homeostasis in obese mice with cardiac and
skeletal muscle autophagy deficiency. Sci Rep.
7(7894)2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Watson K and Baar K: mTOR and the health
benefits of exercise. Semin Cell Dev Biol. 36:130–139.
2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sciarretta S, Volpe M and Sadoshima J:
Mammalian target of rapamycin signaling in cardiac physiology and
disease. Circ Res. 114:549–564. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Song Z, Moore DR, Hodson N, Ward C, Dent
JR, O'Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff YG, et al:
Resistance exercise initiates mechanistic target of rapamycin
(mTOR) translocation and protein complex co-localisation in human
skeletal muscle. Sci Rep. 7(5028)2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Langer HT, West D, Senden J, Spuler S, van
Loon LJC and Baar K: Myofibrillar protein synthesis rates are
increased in chronically exercised skeletal muscle despite
decreased anabolic signaling. Sci Rep. 12(7553)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Gao L, Liu F and Liu R: The The mechanism
of aerobic exercise regulating the PI3K/Akt-mTOR signaling pathway
intervenes in hippocampal neuronal apoptosis in vascular dementia
rats. Int J Environ Res Public Health. 20(1893)2023.PubMed/NCBI View Article : Google Scholar
|