Introduction
Myelodysplastic syndrome (MDS) refers to various
bone marrow ailments that include aberrant blood cell development
and function (1). MDS arises from
genetic mutations in stem cells that produce blood cells in the
bone marrow (2). These mutations
decrease proliferation of stem cells, resulting in an insufficient
number of normal blood cells. As a result, people with MDS, which
primarily affects older adults (up to 70 years), typically have
decreased blood flow, which leads to fatigue, weakness, shortness
of breath and increased susceptibility to infections (3-5).
MDS has been also referred to as pre-leukemia, due to its
involvement in progression of acute myeloid leukemia (AML)
(2,6).
MDS is caused by dysregulated apoptosis in the
hematopoietic compartment, leading to cell death and cytopenia
(7). MDS has also been identified
as a heterogeneous set of cell maturation malignancies and
deregulated innate and inflammatory immune response (8) caused by several genomic events
(9). Typically, cytopenia on
standard peripheral blood analysis indicates a predisposition to
MDS. Anaemia (low haemoglobin and haematocrit values),
thrombocytopenia and leukopenia, which are easily diagnosed by a
standard complete blood analysis, are indications of
myelodysplastic syndromes (10). To
ascertain the cellularity and aspirate morphology of bone marrow
cells, biopsy is required, and identification of bone marrow blasts
(immature blood cells) is key for risk assessment (2). Numerous internationally recognized
scoring systems are used to categorize MDS. The most widely used
systems are the original International Prognostic Scoring System
(IPSS) (11) and Revised IPSS
(IPSS-R) (12). Broadly, people
with MDS are divided into lower- and higher-risk MDS, according to
karyotype, number of blasts and neutrophils and the number of blood
platelets (13).
Depending on number of abnormal cells in bone
marrow, the proportion of blasts in both blood and bone marrow and
the presence of cytogenetic abnormality, the World Health
Organization (WHO) classifies MDS into different categories:
Refractory anemia (RA) is the most common form of MDS. The
resulting low red blood cell count in RA leads to anemia,
characterized by weakness, fatigue and shortness of breath.
Refractory cytopenia with multilineage dysplasia (RCMD) is another
prevalent subtype affecting multiple blood cell lineages, including
red and white blood cells and platelets; this subtype accounts for
30% of cases of MDS (5,14). Further subtypes of MDS are RA with
excess blasts (RAEB), which has a greater quantity of blasts in the
bone marrow and can progress to AML, and MDS with isolated del (5q)
(14).
MDS is more prevalent at older age but can sometimes
occur in children and young adults (14,15).
MDS accounting for less than 5% of hematopoietic neoplasia in
childhood (15-17).
Age-associated increases in oxidative stress, which interfere with
normal tissue function, are caused by a breakdown of antioxidant
defense systems and reactive oxygen species (ROS) generation
(18,19). Numerous hematological neoplasms,
including MDS, are known to be affected by oxidative stress, and
there is growing evidence for this in the literature (20-22).
The role of ROS in carcinogenesis may be concentration- and
time-dependent (23); disruption in
ROS levels can cause cell death or accelerate the aging process and
age-associated disorders (24).
Decreased ROS levels are more likely to accelerate the growth of a
tumor via enhanced cancer cell proliferation (23). Hence, controlling ROS levels is key
since they serve a critical role in the evolution of tumors and the
emergence of tumor-associated pathologies.
The role of oxidative stress in the pathogenesis and
development of MDS and AML is achieved via mechanisms such as the
accumulation of aberrant and immature blood cells in bone marrow,
leading to a greater generation of ROS (25). In a study with 97 patients with MDS,
a high level of ROS production in bone marrow cells of patients
with MDS and AML was observed (26), which is attributed to iron overload
due to required red blood cell transfusion (27). Furthermore, multiple effects on
blood cells, including DNA damage, apoptosis (programmed cell
death) and impaired differentiation have been observed in patients
with MDS (21,28). These effects lead to further
accumulation of abnormal blood cells, worsening of anemia and
progression to AML (25). For
example, when compared with normal cells, the red blood cells and
platelets of people with low-risk MDS show lower reduced
glutathione (GSH) and elevated ROS levels, respectively (25).
Identification of oxidative stress-related
biomarkers is key. The present study aimed to assess the endogenous
antioxidant defense system in patients with MDS at diagnosis,
without having received any drug therapy or following a specific
diet, by measuring redox biomarkers [GSH, catalase (CAT) activity,
total antioxidant capacity (TAC) and levels of lipid peroxidation
through thiobarbituric acid reactive substances (TBARS) and protein
(carbonyl) oxidation. Identifying the redox status of these
patients may facilitate future study into the treatment of various
disorders, such as MDS.
Materials and methods
Study design
A total of 50 adult patients (28 male and 22
females, median age 75 years, age range 45-89 years) diagnosed with
MDS from January 2020 to January 2022 were enrolled with a median
follow-up of 29 months (range 20-44 months). Patients were
classified as those with refractory anemia (RA, 16 males and 16
females with a median age of 75 years), patients with refractory
anemia with excess blasts (RAEB, 4 males and 2 female with a median
age of 75 years) and patients with refractory cytopenia with
multilineage dysplasia (RCMD, 8 males and 6 females with a median
age of 75 years). Diagnosis and stratification of MDS was based on
WHO criteria (29). An additional
50 age- and sex-matched healthy volunteers (27 males and 23 female;
median age 71 years, range 63-80 years) served as an internal
control. The samples from two groups were collected at the same
time period. Participants were hospitalized or visited the
outpatient clinic of the Hematology Department of the General
University Hospital of Larissa (Larissa, Greece).
All experimental procedures were approved by the
Bioethics Committee of the University of Thessaly (approval no.
47/02.12.2019) and were carried out in conformity with the Helsinki
Declaration. The inclusion criteria for the MDS group were as
follows: i) Diagnosis of MDS syndrome and ii) consent to
participate in the study. The inclusion criteria for healthy
individuals were as follows: i) not suffering from any chronic
disease, ii) not taking any medication and iii) belonging to the
same age group as patients with MDS. If MDS patients have any other
form of malignancy except MDS, and if the healthy volunteers had
any disease (chronic or autoimmune), they were not included in the
study. All participants were mentally competent and provided
written informed consent prior to sample acquisition.
Sample acquisition
A total of 4 ml peripheral blood was drawn from each
participant, collected in EDTA tubes, maintained on ice and
processed within 4 h. All samples were collected at the time of
diagnosis before any treatment. Participants were asked to abstain
from alcohol and tobacco use for ≥5 days prior to the sample
acquisition.
Blood separation
Samples were centrifuged for 10 min at 1,380 x g at
4˚C. The plasma supernatant was separated into aliquots for
measuring TAC, TBARS and protein carbonyl levels. The rest of the
packed erythrocytes were lysed in distilled water (1:1 v/v),
agitated and centrifuged at 4,000 x g for 15 min at 4˚C to produce
red blood cell lysate foranalysis of GSH levels and CAT
activity.
Using a commercial kit (Hemoglobin Drabkin, Dutch
Diagnostics; cat. No. 553-820), hemoglobin concentration was
determined. A total of 5 µl erythrocyte lysate was combined with 1
ml working hemoglobin reagent (reagent R1, pH 7.3). An incubation
for 10 min at RT in the absence of light was performed, and the
optical density (OD) of samples was measured at 540 nm. In each
experiment, 1 ml R1 was used as blank.
Redox biomarker assessment. Biomarkers
related to antioxidant capacity
GSH. GSH concentration was assessed as
previously described (30). A total
of 400 µl red blood cell lysate was added to 400 µl 5%
trichloroacetic acid (TCA), vigorously agitated and centrifuged at
14,500 x g for 5 min at 5˚C. The supernatant was collected and 90
µl 5% TCA was added again to each tube, vigorously agitated,
centrifuged at same conditions and clear supernatant was collected.
A total of 20 µl supernatant was mixed with 660 µl sodium-potassium
phosphate buffer (67 mM, pH 8.0) and 330 µl 5,5'-dithiobis-2
nitrobenzoate (1 mM), samples were incubated for 15 min in the
absence of light at room temperature and OD was estimated at 412
nm.
CAT activity. Activity of CAT was measured as
previously described (19,21). A total of 4 µl red blood cell lysate
(diluted in distilled water, 1:10) was added to 2,991 ml
sodium-potassium phosphate buffer (67 mM, pH 7.4). Following 15 min
incubation at 38˚C, 5 µl 30% H2O2 was added.
The conversion of H2O2 into H2O
and O2, which causes an alteration in absorbance, was
read at 240 nm for 125 sec. CAT activity was calculated based on
the molar extinction coefficient of H2O2
(43.6/M/cm) (31).
TAC. TAC was determined as previously
described (32). A total of 20
plasma, 480 phosphate buffer (10 mM; pH 7.4) and 500 µl
2,2-diphenyl-1-picrylhydrazyl radical (0.1 mM) solution was
incubated in the absence of light at room temperature for 60 min.
Samples were centrifuged at 15,000 x g for 5 min at RT and the OD
was estimated at 517 nm.
Biomarkers related to oxidative damage
TBARS. TBARS levels were assessed, as
previously described (33). A total
of 100 µl plasma and 1 ml mixture of 35% TCA and Tris-HCl (pH 7.41)
(1:1) was incubated for 10 min at room temperature. Then, 1 ml
Na2SO4 (2 M) and TBA (55 mM) was added at
95˚C for 45 min. An ice bath was used to cool samples for 4 min at
4˚C, 1 ml 70% TCA was added . Then, 1.5 ml tubes were filled with 1
ml sample and a centrifugation at 11,200 x g for 3 min in RT
performed. The OD was identified at 530 nm. TBARS concentration was
calculated using the molar extinction coefficient of
malondialdehyde (MDA; 155x103/M/cm) (31).
Protein carbonyls. Protein carbonylation was
assessed as previously described (31). A total of 100 µl 20% TCA and plasma
(1:1) was vortexed vigorously and incubated at 4˚C for 15 min,
followed by centrifugation at 15,000 x g at 5˚C for 5 min.
Supernatant was discarded and the sediment was resuspended in 500
µl 2,4-dinitrophenylhydrazine (DNPH; 10 mM), diluted in 2.5 M HCl.
Samples suspended in 500 µl 2.5 M HCl were used as a blank.
Following 1 h incubation at room temperature with vortexing every
15 min for 5 sec in the absence of light. Supernatant was thrown
away, and a resuspension of the sediments with 1 ml 10% TCA was
performed. Samples were centrifuged again under the aforementioned
conditions (15,000 x g at 5˚C for 5 min) and sediment was washed
three times with 1 ml ethanol-ethyl acetate mixture (1:1 v/v).
After resuspending the pellets, samples were centrifuged (15,000 x
g at 5˚C for 5 min). Pellets were reconstituted in 1 ml urea (5 M;
pH 2.3), after the final wash with ethanol-ethyl acetate mixture
(1:1 v/v), vortexed for 5 sec and incubated at 37˚C for 15 min.
Following centrifuging (15,000 x g, 5˚C, 3 min), OD of the mixtures
was calculated at 375 nm. The molar extinction coefficient of DNPH
(22x103/M/cm) served to calculate the protein carbonyl
concentration (31).
Chemicals and equipment
Commercial kits for Hb determination, TCA,
H2O2, solution, DPPH and DNPH were provided
by Sigma-Aldrich (Merck KGaA) and DTNB, TBA,
Na2SO4, urea, ethanol and ethyl acetate were
provided by Thermo Fisher Scientific, Inc. All spectrophotometrical
assays were measured by U-1500 Ultra Violet-Visible
spectrophotometer (Hitachi Corporation).
Statistical analysis
All statistical analyses were conducted with
GraphPad Prism, version 8.0.1 (GraphPad Software, Inc.; Dotmatics).
Normality of distribution was assessed with Shapiro-Wilk test. For
parametric measures, the unpaired t test was used to compare two
groups and one-way ANOVA, with Dunn's post hoc test was used for
multiple comparisons between control group and different MDS
subtypes. Data are presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference. Each
assay was performed in triplicates.
Results
Lower hemoglobin levels and platelet
count in patients with MDS patients
Patients with MDS had statistically significant
lower hemoglobin levels and platelet count compared with controls.
There was no significant variation in white blood cell count
(Table I).
 | Table IBlood parameters. |
Table I
Blood parameters.
| Parameter | Control group | MDS group |
|---|
| Hemoglobin,
g/l | 13.70±0.16 |
11.50±0.23a |
| Platelet count,
x109/l | 240.80±8.34 |
188.30±17.30b |
| White blood cell
count, x109/l | 5.50±0.19 | 9.07±2.00 |
Classification of MDS subtype
Patients with MDS were classified into the
appropriate categories, according to WHO (Table II): 32 patients had RA), 6 out of
50 patients have refractory anemia with excess blasts (RAEB) and 12
have refractory cytopenia with multilineage dysplasia (RCMD).
 | Table IIClassification of patients with
MDS. |
Table II
Classification of patients with
MDS.
| MDS subtype | Number of
patients |
|---|
| Refractory
anemia | 32 |
| Refractory anemia
with excess blasts | 56 |
| Refractory
cytopenia with multilineage dysplasia | 12 |
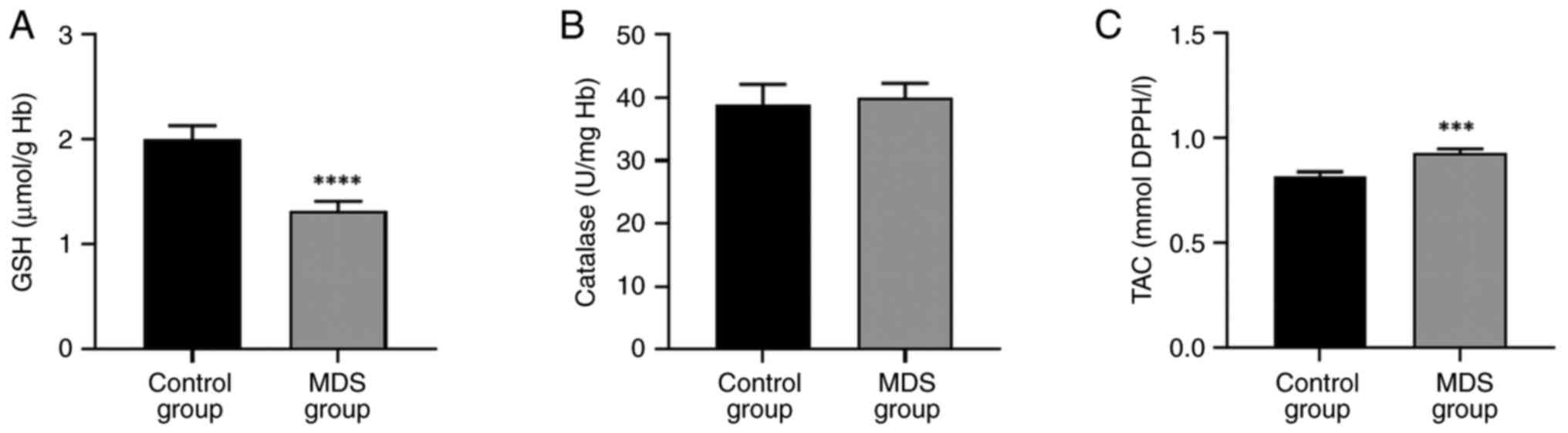
Decreased GSH and increased TAC in
patients with MDS
A significant decrease in GSH levels was observed in
patients with MDS while TAC significantly increased compared with
the control group (Fig. 1A and
C). Catalase activity remained
unaffected in patients with MDS compared with controls (Fig. 1B).
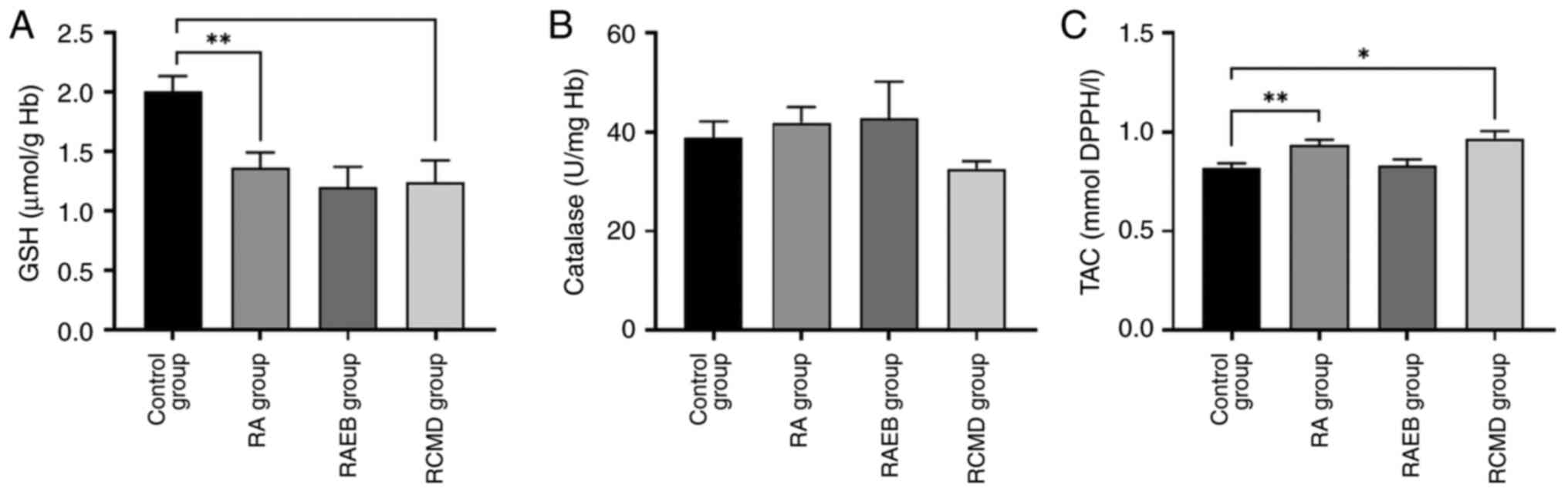
A significant decrease in GSH levels was observed
between control and RA and RCMD group (Fig. 2A). TAC significantly increased in RA
and RCMD compared with control group (Fig. 2C), while CAT activity remained
unaffected (Fig. 2B).
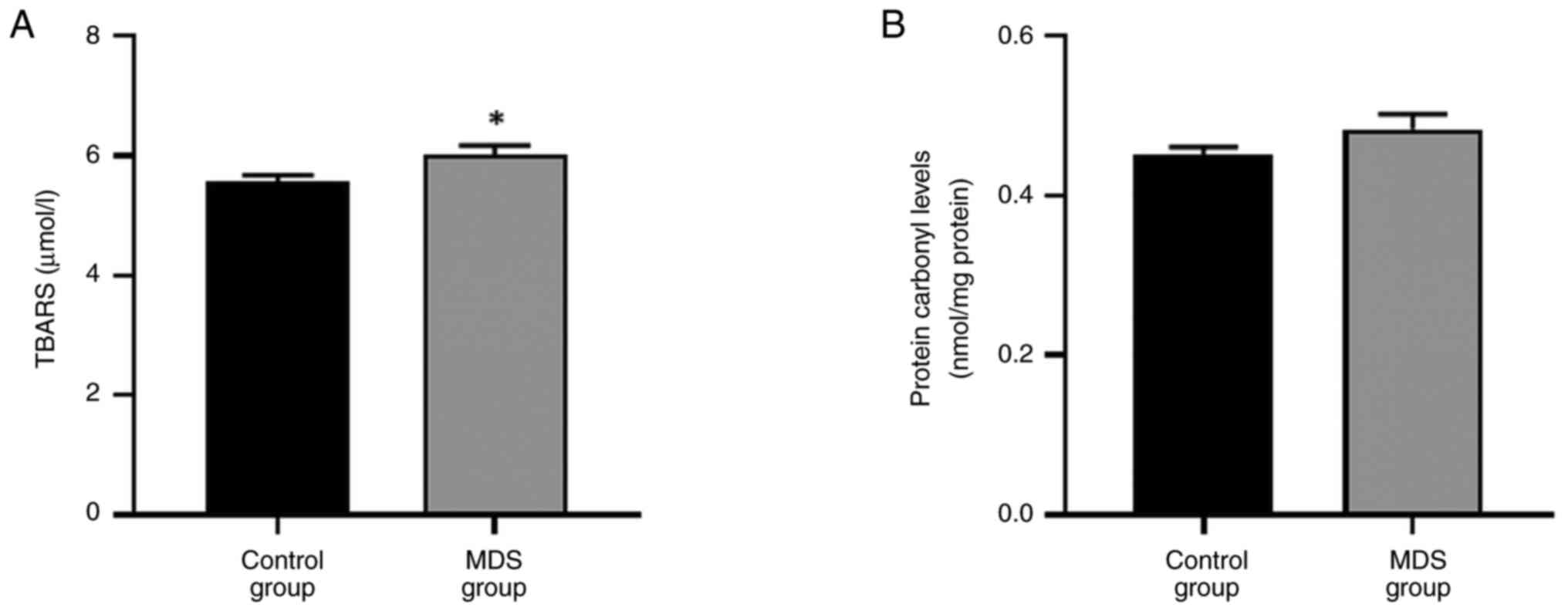
Increased TBARS levels in patients
with MDS
A statistically significant increase in the levels
of lipid peroxidation, indicated by TBARS, was observed in patients
with MDS compared with controls (Fig.
3A), while no statistically significant change in protein
carbonyl levels was observed (Fig.
3B).
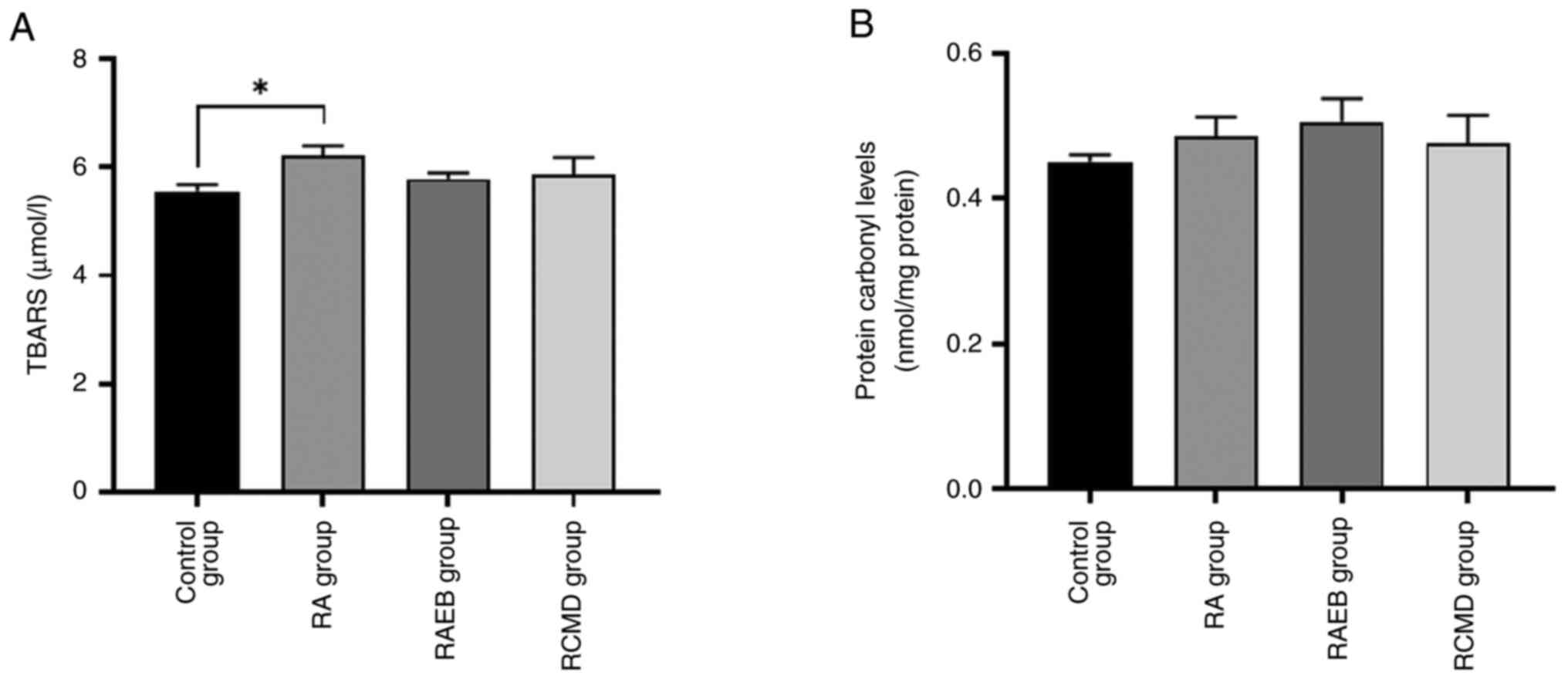
A significant increase in lipid peroxidation was
observed between control and RA group (Fig. 4A), while no changes in protein
carbonyl levels were observed (Fig.
4B).
Discussion
Here, the redox profile of patients with MDS was
evaluated by measuring GSH levels, the decomposition rate of
H2O2 through CAT enzyme activity, TAC, lipid
peroxidation (TBARS) and protein oxidation levels (protein
carbonyls). CAT activity and protein oxidation levels were not
significantly affected in patients with MDS compared with the
control group. TBARS and TAC levels revealed statistically
significant increases, with a concomitant decrease in GSH levels in
patients with MDS compared with the control group. CAT activity and
protein oxidation levels did not exhibit significant differences
between MDS subtypes. TBARS increased significantly in RA group,
TAC increased both in RA and RCMD group compared with control,
while GSH concentration was significantly higher in RA and RCMD
group compared with controls. While the present study indicates
that the most affected MDS subtype is RA, the number of patients
belonging to the other subtypes (RAEB and RCMD) was inadequate to
draw safe conclusions. As a result, further studies with larger
sample sizes are needed.
MDS is a heterogeneous group of acquired clonal
disorders of hematopoietic progenitor cells, characterized by
inefficient hematopoiesis in bone marrow, dysplasia in one or more
myeloid lineages, cytopenia in the peripheral blood and an
increased risk of progression to AML (34-36).
In MDS, bone marrow precursor cells suffer damage and normal
hematopoiesis is blocked. The marrow cannot produce enough cells,
resulting in cytopenia. Bone marrow is the semi-solid tissue inside
the bones. In adults, it is the main hematopoietic organ, producing
~500 billion blood cells/day, which enter the circulation through
the vascular network of the marrow (37). The core chambers of long bones and
bones in the axial skeleton contain bone marrow, which consists of
hematopoietic tissue and adipose cells surrounded by vascular
sinuses within a lattice of cancellous bone, representing ~5% of
body weight in humans. It is the primary lymphoid tissue and
hematopoietic organ, producing erythrocytes, granulocytes,
monocytes, lymphocytes and platelets (38).
According to growing evidence of the literature,
oxidative stress is associated with development and progression of
a wide range of hematological neoplasms (1,20,21).
Development of many hematological disorders, including MDS and
leukemia, is impacted by chronic oxidative stress (28). Leukemic cells (blasts) survive under
oxidative stress and acquire a plethora of mechanisms to shield
themselves from stress, including increase of gene expression
encoding for antioxidant enzymes (20,39).
ROS are critical signaling molecules that are closely involved in
the pathophysiology of many types of disease and have an impact on
carcinogenesis, including in MDS, AML and chronic myeloid leukemia
(28). ROS have been observed to
restrict the self-renewal of hematopoietic stem cells, associated
with MDS and inefficient hematopoiesis (28,40).
Patients with MDS have high levels of ROS
production, leading to oxidative stress, and vice versa (28). Oxidative stress is responsible for
damage to key biomolecules, such as DNA, lipid and protein, and
also contributes to mitochondrial dysfunction (41). Studies have indicated that patients
with MDS present evidence of mitochondrial damage, such as
transcriptional, morphological and functional abnormalities
(42,43). MDS is also associated with mutations
in both nuclear-encoded mitochondrial genes and mitochondrial DNA
(42,44). Anemia and ineffective erythropoiesis
(the main symptoms of MDS), have also been hypothesized to be
associated with MDS due in part to mitochondrial dysfunction
(44). The present study did not
perform any mitochondrial assessment to determine changes in their
function in patients with MDS.
ROS may be involved not only in oxidative damage of
DNA, protein and lipids, leading to cell damage (45), but also in pathogenesis and
resilience to drugs (20). Although
several references report oxidative stress and elevated ROS levels,
they only concentrate on biomarkers related to cellular metabolism
and do not assess the endogenous antioxidant defense system in
patients with MDS (21,28,35).
GSH, TBARS and TAC may impact on the prognosis of MDS, since these
biomarkers are crucial for the redox status estimation (26,46,47).
GSH is the most abundant endogenous, non-enzymatic
antioxidant in the body, so it is a key biomarker for the
estimation of the andioxidant potential in both blood and tissues
(48). One important factor in MDS
is the association between oxidative damage and GSH levels.
Patients with MDS have lower intracellular GSH content and higher
ROS levels in bone marrow cells, indicating oxidative stress
(28). The present findings
revealed that GSH levels were significantly decreased in patients
with MDS compared with the control group. Additionally, GSH levels
were significantly decreased in patients with RA and RMCD.
Mechanisms linking MDS with oxidative stress conditions and
potentially reduced glutathione levels are mitochondrial DNA
mutation, systemic inflammation, bone marrow stromal abnormalities,
and mitochondrial dysfunction (35). The majority of the present patients
were aged 55-86 years, contributing to the hypothesis that
oxidative stress leads to age-associated impairment of normal body
function (24). Key parameters
associated with alterations in GSH levels are its recycling rate
and activity of enzymes involved. Glutathione peroxidase (GPx) and
glutathione reductase (GR), are enzymes that convert GSH to the
oxidized form (GSSG) and vice versa (49). Therefore, the decrease in GSH in MDS
is likely associated with increased activity of GPx, which
contributes to its oxidation, converting it to GSSG, and decreased
activity of GR, which regenerates it. Moreover, in a 2007 study of
14 patients with MDS, the decrease in GSH levels may also have been
due to elevated ROS levels (25):
Decreased GSH levels in erythrocytes and platelets and increased
ROS levels were detected (25).
According to Saigo et al (50), such an increase in ROS production is
due to overaccumulation of iron molecules in patients with MDS.
Conversely, in a recent study by Montes et al (20), it was reported that patients with
MDS have higher levels of GSH compared with healthy individuals. In
the aforementioned study, there was no difference in GSH levels in
patients with early and advanced MDS. The findings of the present
study indicate the inability to maintain adequate levels of GSH, a
key endogenous antioxidant, in MDS. Decreased levels of GSH may be
due to either a decrease in activity of enzymes that reduce it or
higher amounts of endogenous ROS production.
The course of disease and length of time that
patients with MDS survive are influenced by GSH levels. Low GSH
levels cause increased oxidative stress in MDS due to increased ROS
generation. This may worsen survival rate, cause more cellular
damage and accelerate the disease progression (51-53).
Furthermore, oxidative stress is linked to cellular apoptosis and
DNA damage. This contributes to the inefficient hematopoiesis that
characterizes MDS, where bone marrow is unable to produce adequate
levels of healthy red blood cells (26). This inefficiency can lead to more
severe symptoms and complications, decreasing survival time
(51).
As MDS progresses, bone marrow function gradually
declines and there is an increased probability of transformation to
AML. Inadequate GSH levels intensify oxidative stress, which can
accelerate bone marrow dysfunction and raise risk of leukemic
transition (54).
The decomposition rate of hydrogen peroxide is
determined by activity of enzymes such as peroxidase and CAT
(55). CAT converts
H2O2, which is a potentially reactive form of
oxygen, into H2O and O2. In the present
study, no significant change in catalase enzyme activity was
observed between healthy individuals and patients with MDS. A
statistical significant difference was also not observed between
the MDS subtypes and the control. However, in a recent study, an
increase in CAT activity was observed in MDS-affected people
overall and when these patients were divided according to the stage
of disease (20).
TAC levels were statistically significantly higher
in patients with MDS, compared with control group. TAC was elevated
in RA and RMCD compared with controls. In a study in 2019, in
patients with a variety of neoplastic diseases, including MDS, a
negative correlation was observed between TAC and lipid
peroxidation levels (22).
TAC represents the capacity of blood plasma to
eliminate free radicals. Every blood component has an antioxidant
effect that contributes differently to TAC, which functions as a
general measure of overall antioxidant status (56). Therefore, TAC may be influenced by
various factors, making it less specific as a biomarker than GSH or
TBARS. Further investigation is needed to validate TAC function as
a prognostic marker in MDS. Proteins and amino acids, under
oxidative stress are damaged, irreversibly generating carbonylated
proteins. Here, no significant variation was observed in protein
oxidation levels in MDS compared with healthy participants. In a
study conducted in Spain involving patients with MDS aged 70 years,
no significant change in protein carbonyl levels was observed
(20). Conversely, in the
aforementioned study, when participants were separated into early
and advanced MDS, patients with early MDS had significantly reduced
protein oxidation levels, suggesting protection from oxidative
stress impacts (20). On the other
hand, in a study with 32 patients with MDS, carbonylated protein
levels were considerably higher in RARS and RMCD group compared
with controls (45).
Lipid peroxidation was statistically significantly
higher in patients with MDS compared with healthy controls.
Polyunsaturated fatty acids are converted into active and unstable
lipid peroxides under oxidative stress. MDA is the product of lipid
oxidation, therefore TBARS are reported as equivalents of MDA,
accordingly (49). Serological and
molecular markers in patients with MDS are associated with an
increased concentration of MDA and therefore elevated levels of
TBARS and lipid peroxidation (35).
In patients with MDS, a significant increase in TBARS levels is
observed with a concomitant elevation in ROS levels and a
significant decrease in TAC (22).
A recent study observed a significant decrease in TBARS levels in
patients with early and advanced MDS (20). However, in the aforementioned study,
a concurrent increase in GSH endogenous levels and rate of hydrogen
peroxide degradation via the activity of CAT was observed in
patients with MDS, suggesting that these patients developed
antioxidant defense mechanisms that could shield them from
oxidative damage associated with MDS (20,35).
Anemia and iron overload, which affect the majority
of patients with MDS, are hypothesized to be negative independent
prognostic factors linked to a higher risk of leukemic
transformation and shorter survival time (46,57).
As aforementioned, lipid peroxidation, which can inhibit
hematopoietic stem cell self-renewal and directly cause DNA damage
and genomic instability, is brought on by an excess of ROS
(38,46). Elevated levels of TBARS, as observed
in the present study, indicate oxidative stress, which can damage
critical biomolecules (49).
It is widely acknowledged that redox status
biomarkers are prognostically important in various types of
neoplastic disease, including MDS (22,51).
Older patients with MDS have higher levels of oxidative stress than
healthy people (22). The findings
of the present study are consistent with those of earlier studies
in which biomarkers (TBARS and protein carbonyls) associated with
oxidative damage were increased, while antioxidant
capacity-associated markers (GSH, CAT and TAC) were either
decreased or unchanged (22,54).
Oxidative stress may be involved in the development of MDS.
Patients with MDS do not have properly developed antioxidant
mechanisms to cope with such pathological conditions (25,50).
Antioxidants could be included everyday diet as a potential remedy;
polyphenol-rich plant extracts prevent DNA damage caused by peroxyl
radicals and enhance the overall redox profile (58). For example, tannic acid, a common
tannin found in red wines, tea and coffee, administerred in male
C3H mice with liver neoplasms results in a decrease in the overall
incidence of hepatic tumors (59).
All biomarkers evaluated in the present study are a
first step to a comprehensive strategy for assessing redox status
of individuals (47). All present
patients with MDS had a mild hematological profile during the time
of their disease prognosis (i.e. low hemoglobin and hematocrit
values). As maintained by WHO, patients diagnosed with
myelodysplastic syndrome who have moderate anaemia are classified
as low-risk and they have prolonged survival and delayed disease
progression. Median follow-up length was 29 months, which is not
long enough to definitively address whether patients with low-risk
myelodisplastic abnormality have prolonged survival and delayed
disease progression. Consequently, the present study did not
determine the association between redox biomarkers (GSH, TAC,
TBARS) and prognosis in patients with MDS.
The present study revealed a disturbance of
oxidative redox homeostasis in patients with MDS compared with
healthy controls, which was substantiated by a decrease in GSH and
increase in TBARS levels. To the best of our knowledge, the present
study is the first to assess redox biomarkers in MDS subtypes and
controls. Subtype analysis revealed that the most affected MDS type
was RA, but more studies with a larger number of patients are
needed to validate this. These observations indicate a possible
manifestation of oxidative damage in the blood of patients with MDS
via the disruption of the antioxidant defense system based on key
antioxidant molecules. The development of lipid peroxidation and
the drop in GSH levels demonstrated this. The increase in TAC may
serve as an adaptive mechanism of antioxidant defense, but without
protecting the patients against MDS, which was expressed through
the promotion of plasma lipid peroxidation levels in MDS group.
Future research is required to define the role that redox
biomarkers serve in the pathogenesis and clinical course of MDS and
whether adding antioxidants, such as vitamin E, ascorbic acid or
iron chelators, to the regular diets of patients with MDS improves
their redox profile. These substances may combat myelodisplastic
disorder via promotion of hematopoiesis that is normally hindered
along with the progression of MDS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NG, GV and DK conceived the study. ZS and SM
analyzed data. ET designed and performed experiments. ET and SM
wrote the manuscript. SM and ZS revised the manuscript. ET and SM
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Bioethics Committee of the University of Thessaly (approval no.
47/02.12.2019) and adhered to the principles of the Helsinki
Declaration. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tria FP IV, Ang DC and Fan G:
Myelodysplastic syndrome: Diagnosis and screening. Diagnostics
(Basel). 12(1581)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Garcia-Manero G, Chien KS and
Montalban-Bravo G: Myelodysplastic syndromes: 2021 Update on
diagnosis, risk stratification and management. Am J Hematol.
95:1399–1420. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Luskin MR and Abel GA: Management of older
adults with myelodysplastic syndromes (MDS). J Geriatr Oncol.
9:302–307. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abel GA, Kim HT, Hantel A, Steensma DP,
Stone R, Habib A, Ho VT, Wadleigh M, El-Jawahri A, Alyea EP, et al:
Fit older adults with advanced myelodysplastic syndromes: Who is
most likely to benefit from transplant? Leukemia. 35:1166–1175.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta G, Singh R, Kotasthane DS and
Kotasthane VD: Myelodysplastic syndromes/neoplasms: Recent
classification system based on World Health Organization
classification of tumors-international agency for research on
cancer for hematopoietic and lymphoid tissues. J Blood Med.
1:171–182. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dayyani F, Conley AP, Strom SS, Stevenson
W, Cortes JE, Borthakur G, Faderl S, O'Brien S, Pierce S,
Kantarjian H and Garcia-Manero G: Cause of death in patients with
lower-risk myelodysplastic syndrome. Cancer. 116:2174–2179.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Raza A, Gezer S, Mundle S, Gao XZ, Alvi S,
Borok R, Rifkin S, Iftikhar A, Shetty V, Parcharidou A, et al:
Apoptosis in bone marrow biopsy samples involving stromal and
hematopoietic cells in 50 patients with myelodysplastic syndromes.
Blood. 86:268–276. 1995.PubMed/NCBI
|
|
8
|
Gañán-Gómez I, Wei Y, Starczynowski DT,
Colla S, Yang H, Cabrero-Calvo M, Bohannan ZS, Verma A, Steidl U
and Garcia-Manero G: Deregulation of innate immune and inflammatory
signaling in myelodysplastic syndromes. Leukemia. 29:1458–1469.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Papaemmanuil E, Gerstung M, Malcovati L,
Tauro S, Gundem G, Van Loo P, Yoon CJ, Ellis P, Wedge DC,
Pellagatti A, et al: Clinical and biological implications of driver
mutations in myelodysplastic syndromes. Blood. 122:3616–3627, 3699.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sekeres MA and Taylor J: Diagnosis and
treatment of myelodysplastic syndromes: A review. JAMA.
328:872–880. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Greenberg P, Cox C, LeBeau MM, Fenaux P,
Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al:
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 89:2079–2088. 1997.PubMed/NCBI
|
|
12
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scalzulli E, Pepe S, Colafigli G and
Breccia M: Therapeutic strategies in low and high-risk MDS: What
does the future have to offer? Blood Rev. 45(100689)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vardiman JW, Harris NL and Brunning RD:
The World Health Organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wlodarski MW, Sahoo SS and Niemeyer CM:
Monosomy 7 in pediatric myelodysplastic syndromes. Hematol Oncol
Clin North Am. 32:729–743. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schratz KE and DeZern AE: Genetic
predisposition to myelodysplastic syndrome in clinical practice.
Hematol Oncol Clin North Am. 34:333–356. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kennedy AL and Shimamura A: Genetic
predisposition to MDS: Clinical features and clonal evolution.
Blood. 133:1071–1085. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cabello-Verrugio C, Simon F, Trollet C and
Santibañez JF: Oxidative stress in disease and aging: Mechanisms
and therapies 2016. Oxid Med Cell Longev.
2017(4310469)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sies H, Berndt C and Jones DP: Oxidative
stress. Annu Rev Biochem. 86:715–748. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Montes P, Guerra-Librero A, García P,
Cornejo-Calvo ME, López MDS, Haro T, Martínez-Ruiz L, Escames G and
Acuña-Castroviejo D: Effect of 5-azacitidine treatment on redox
status and inflammatory condition in MDS patients. Antioxidants
(Basel). 11(139)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Imbesi S, Musolino C, Allegra A, Saija A,
Morabito F, Calapai G and Gangemi S: Oxidative stress in
oncohematologic diseases: An update. Expert Rev Hematol. 6:317–325.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tsamesidis I, Pantaleo A, Pekou A, Gusani
A, Iliadis S, Makedou K, Manca A, Carruale A, Lymperaki E and Fozza
C: Correlation of oxidative stress biomarkers and hematological
parameters in blood cancer patients from Sardinia, Italy. Int J
Hematol Oncol Stem Cell Res. 13:49–57. 2019.PubMed/NCBI
|
|
23
|
Nikitovic D, Corsini E, Kouretas D,
Tsatsakis A and Tzanakakis G: ROS-major mediators of extracellular
matrix remodeling during tumor progression. Food Chem Toxicol.
61:178–186. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ghoti H, Amer J, Winder A, Rachmilewitz E
and Fibach E: Oxidative stress in red blood cells, platelets and
polymorphonuclear leukocytes from patients with myelodysplastic
syndrome. Eur J Haematol. 79:463–467. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Picou F, Vignon C, Debeissat C, Lachot S,
Kosmider O, Gallay N, Foucault A, Estienne MH, Ravalet N, Bene MC,
et al: Bone marrow oxidative stress and specific antioxidant
signatures in myelodysplastic syndromes. Blood Adv. 3:4271–4279.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim CH and Leitch HA: Iron
overload-induced oxidative stress in myelodysplastic syndromes and
its cellular sequelae. Crit Rev Oncol Hematol.
163(103367)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jing Q, Zhou C, Zhang J, Zhang P, Wu Y,
Zhou J, Tong X, Li Y, Du J and Wang Y: Role of reactive oxygen
species in myelodysplastic syndromes. Cell Mol Biol Lett.
29(53)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hong M and He G: The 2016 revision to the
World Health Organization classification of myelodysplastic
syndromes. J Transl Int Med. 5:139–143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Reddy YN, Murthy SV, Krishna DR and
Prabhakar MC: Role of free radicals and antioxidants in
tuberculosis patients. Indian J Tuberc. 51:213–218. 2004.
|
|
31
|
Veskoukis AS, Kyparos A, Paschalis V and
Nikolaidis MG: Spectrophotometric assays for measuring redox
biomarkers in blood. Biomarkers. 21:208–217. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Janaszewska A and Bartosz G: Assay of
total antioxidant capacity: Comparison of four methods as applied
to human blood plasma. Scand J Clin Lab Invest. 62:231–236.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Spanidis Y, Goutzourelas N, Stagos D,
Mpesios A, Priftis A, Bar-Or D, Spandidos DA, Tsatsakis AM, Leon G
and Kouretas D: Variations in oxidative stress markers in elite
basketball players at the beginning and end of a season. Exp Ther
Med. 11:147–153. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hasserjian RP: Myelodysplastic syndrome
updated. Pathobiology. 86:7–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Farquhar MJ and Bowen DT: Oxidative stress
and the myelodysplastic syndromes. Int J Hematol. 77:342–350.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Avgerinou C, Alamanos Y, Kouraklis A,
Tavernarakis I, Zikos P, Aktypi A, Kaiafas P, Raptis C, Karakantza
M, Zoumbos N and Symeonidis A: Epidemiology of myelodysplastic
syndromes in the area of Southwestern Greece during the period
1990-2009. Achaiki Iatriki. 30:105–124. 2011.
|
|
37
|
Vunjak-Novakovic G, Tandon N, Godier A,
Maidhof R, Marsano A, Martens TP and Radisic M: Challenges in
cardiac tissue engineering. Tissue Eng Part B Rev. 16:169–187.
2009.
|
|
38
|
Travlos GS: Normal structure, function,
and histology of the bone marrow. Toxicol Pathol. 34:548–565.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuhn V, Diederich L, Keller TCS IV, Kramer
CM, Lückstädt W, Panknin C, Suvorava T, Isakson BE, Kelm M and
Cortese-Krott MM: Red blood cell function and dysfunction: Redox
regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal.
26:718–742. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Visconte V, Tiu RV and Rogers HJ:
Pathogenesis of myelodysplastic syndromes: An overview of molecular
and non-molecular aspects of the disease. Blood Res. 49:216–227.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cui H, Kong Y and Zhang H: Oxidative
stress, mitochondrial dysfunction, and aging. J Signal Transduct.
2012(646354)2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gupta M, Madkaikar M, Rao VB, Mishra A,
Govindaraj P, Thangaraj K and Ghosh K: Mitochondrial DNA variations
in myelodysplastic syndrome. Ann Hematol. 92:871–876.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Filanovsky K, Haran M, Mirkin V, Braester
A, Shvetz O, Stanevsky A, Sigler E, Votinov E, Zaltsman-Amir Y,
Gross A and Shvidel L: Clinical benefit and improvement of
mitochondrial function in low risk myelodysplastic syndrome treated
by combination ultra coenzyme Q10 and L-carnitine. Blood. 130
(Suppl 1)(S1704)2017.
|
|
44
|
Sen T, Jain M, Gram M, Mattebo A, Soneji
S, Walkley CR and Singbrant S: Enhancing mitochondrial function in
vivo rescues MDS-like anemia induced by pRb deficiency. Exp
Hematol. 88:28–41. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hlaváčková A, Štikarová J, Pimková K,
Chrastinová L, Májek P, Kotlín R, Čermák J, Suttnar J and Dyr JE:
Enhanced plasma protein carbonylation in patients with
myelodysplastic syndromes. Free Radic Biol Med. 108:1–7.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
de Souza GF, Barbosa MC, de Jesus Santos
TE, Carvalho TM, de Freitas RM, Martins MR, Gonçalves RP, Pinheiro
RF and Magalhães SM: Increased parameters of oxidative stress and
its relation to transfusion iron overload in patients with
myelodysplastic syndromes. J Clin Pathol. 66:996–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Veskoukis A, Kerasioti E, Priftis A, Kouka
P, Spanidis Y, Makri S and Kouretas D: A battery of translational
biomarkers for the assessment of the in vitro and in vivo
antioxidant action of plant polyphenolic compounds: The biomarker
issue. Curr Opin Toxicol. 13:99–109. 2019.
|
|
48
|
Carvalho AN, Lim JL, Nijland PG, Witte ME
and Van Horssen J: Glutathione in multiple sclerosis: More than
just an antioxidant? Mult Scler. 20:1425–1431. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mylonas C and Kouretas D: Lipid
peroxidation and tissue damage. In Vivo. 13:295–309.
1999.PubMed/NCBI
|
|
50
|
Saigo K, Takenokuchi M, Hiramatsu Y, Tada
H, Hishita T, Takata M, Misawa M, Imoto S and Imashuku S: Oxidative
stress levels in myelodysplastic syndrome patients: Their
relationship to serum ferritin and haemoglobin values. J Int Med
Res. 39:1941–1945. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gonçalves AC, Alves R, Baldeiras I, Jorge
J, Marques B, Paiva A, Oliveiros B, Cortesão E, Nascimento Costa JM
and Sarmento-Ribeiro AB: Oxidative stress parameters can predict
the response to erythropoiesis-stimulating agents in
myelodysplastic syndrome patients. Front Cell Dev Biol.
9(701328)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kaweme NM, Zhou S, Changwe GJ and Zhou F:
The significant role of redox system in myeloid leukemia: From
pathogenesis to therapeutic applications. Biomark Res.
8(63)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Čipak Gašparović A: Free radical research
in cancer. Antioxidants (Basel). 9(157)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gonçalves AC, Cortesão E, Oliveiros B,
Alves V, Espadana AI, Rito L, Magalhães E, Lobão MJ, Pereira A,
Nascimento Costa JM, et al: Oxidative stress and mitochondrial
dysfunction play a role in myelodysplastic syndrome development,
diagnosis, and prognosis: A pilot study. Free Radic Res.
49:1081–1094. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Makri S, Kafantaris I, Stagos D,
Chamokeridou T, Petrotos K, Gerasopoulos K, Mpesios A, Goutzourelas
N, Kokkas S, Goulas P, et al: Novel feed including bioactive
compounds from winery wastes improved broilers' redox status in
blood and tissues of vital organs. Food Chem Toxicol. 102:24–31.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bartosz G: Total antioxidant capacity. Adv
Clin Chem. 37:219–292. 2003.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lyle L and Hirose A: Iron overload in
myelodysplastic syndromes: Pathophysiology, consequences,
diagnosis, and treatment. J Adv Pract Oncol. 9:392–405.
2018.PubMed/NCBI
|
|
58
|
Skenderidis P, Kerasioti E, Karkanta E,
Stagos D, Kouretas D, Petrotos K, Hadjichristodoulou C and Tsakalof
A: Assessment of the antioxidant and antimutagenic activity of
extracts from goji berry of Greek cultivation. Toxicol Rep.
5:251–257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nepka C, Sivridis E, Antonoglou O,
Kortsaris A, Georgellis A, Taitzoglou I, Hytiroglou P,
Papadimitriou C, Zintzaras I and Kouretas D: Chemopreventive
activity of very low dose dietary tannic acid administration in
hepatoma bearing C3H male mice. Cancer Lett. 141:57–62.
1999.PubMed/NCBI View Article : Google Scholar
|