Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disorder that features the generation of numerous autoantibodies
and immune complexes, potentially compromising diverse bodily
systems. B cells are purportedly pivotal in the etiology of SLE
(1), since the abnormal
proliferation, differentiation and maturation of these cells lead
to the secretion of autoantibodies that inflict harm on various
bodily systems. The cytokines B-lymphocyte stimulator (BLyS) and A
proliferation-inducing ligand (APRIL) occupy pivotal positions in
the intricate process of B-cell maturation and differentiation
(2).
Telitacicept is an important biological agent that
can effectively suppress the proliferation and differentiation of B
cells and plasma cells, by specifically targeting both BLyS and
APRIL. Furthermore, it exhibits the potential to dampen the
activity of long-lived plasma cells (3). Telitacicept was approved in March 2021
in China for the treatment of SLE (4), but it has not been widely accepted due
to its high cost.
Thyroid carcinoma is the most common malignancy of
the endocrine system, exhibiting a gradual increase in its
incidence rate in high-income countries to certain middle-income
countries. Globally, in 2020, the estimated number of new cases of
thyroid cancer was ~449,000 in women and ~137,000 in men,
corresponding to age-standardised incidence rates of ~10.1 per
100,000 women and ~3.1 per 100,000 men. In each sex, incidence
rates are five times greater in high and very high Human
Development Index countries than in low and medium Human
Development Index countries (5).
Previous meta-analyses and observational studies have shown that
the risk of thyroid carcinoma is increased in patients with SLE
(6-8).
The present case documents a 28-year-old female patient who
developed papillary thyroid carcinoma (PTC) 1 year after being
diagnosed with SLE. Following surgical removal of the tumor, the
patient was administered with telitacicept for treatment. To the
best our knowledge, the present case was the first reporting the
instance of a patient suffering from both SLE and PTC undergoing
postoperative telitacicept treatment.
Case report
The patient, a 28-year-old female, was diagnosed
with SLE at Department of Rheumatology and Immunology, People's
Hospital of Longhua (Shenzhen, China) in June 2021, having
experienced systemic edema for >1 month and hair loss for a
week. After undergoing comprehensive examinations, the patient was
diagnosed with lupus-related hematological damage, which was
evidenced by leukopenia, thrombocytopenia, autoimmune hemolytic
anemia and polyserositis accompanied by pericardial and pleural
effusion. Additional complications included lupus nephritis and
secondary antiphospholipid syndrome. After treatment with high-dose
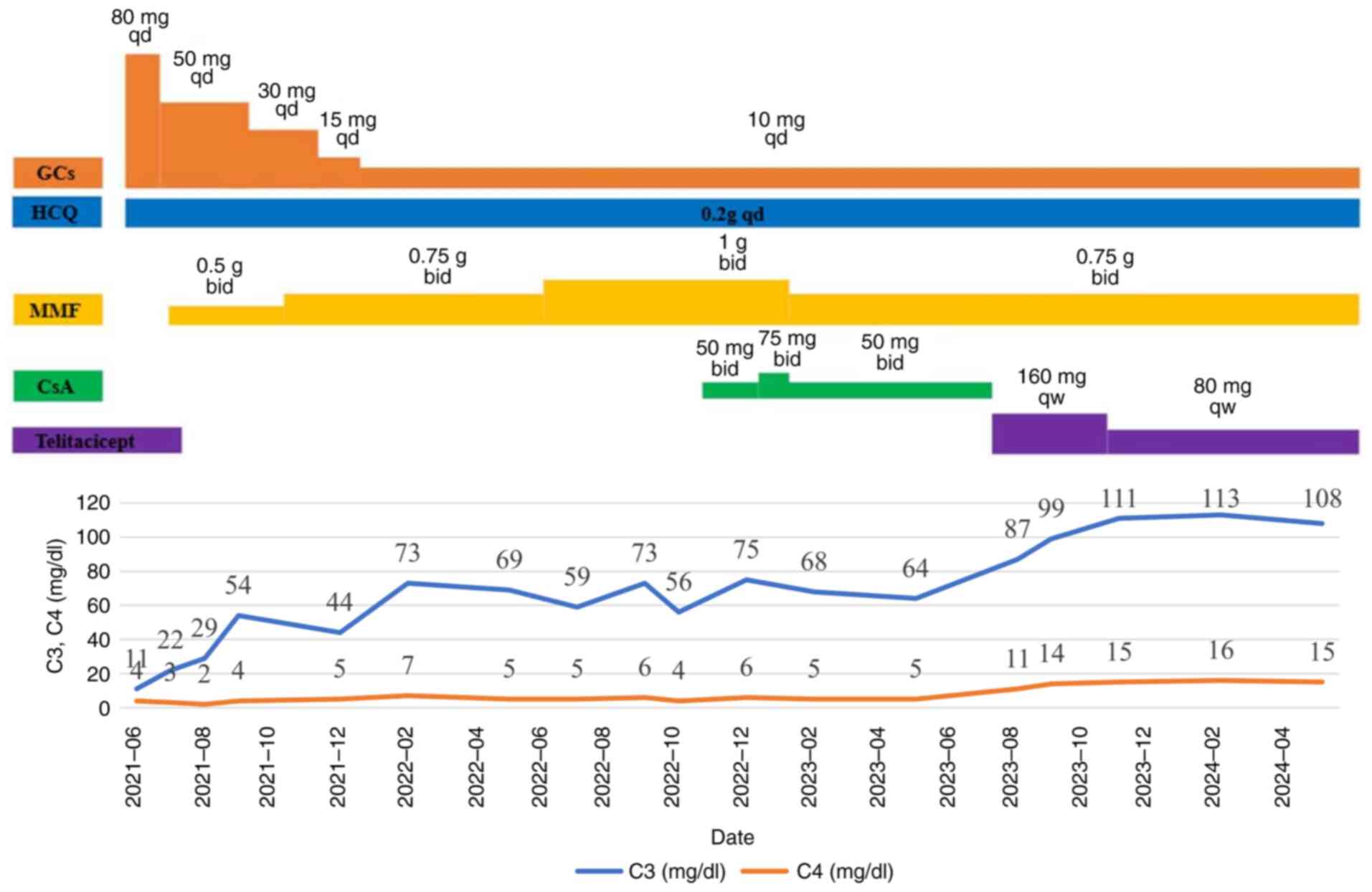
steroids, mycophenolate mofetil (MMF) and cyclosporine A (CsA)
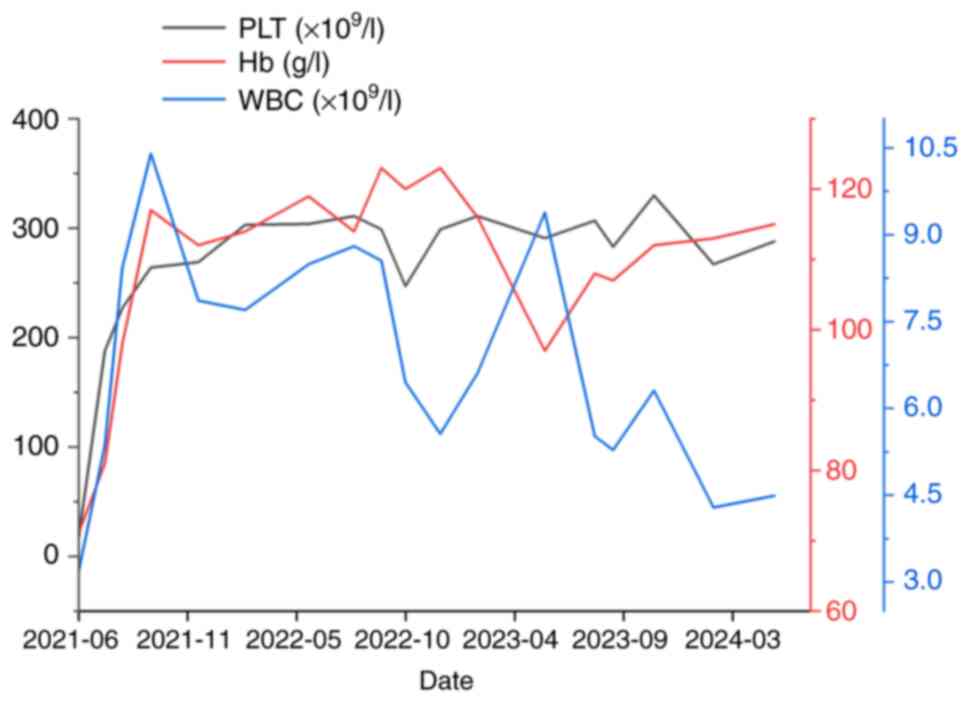
(Fig. 1), the patient's white blood
cells, platelets, hemoglobin, urinary protein and polyserositis
normalized (Fig. 2). Complement 3
(C3) levels improved but could not be maintained within the normal
range for a prolonged period, whilst C4 levels remained unchanged
(Fig. 1). During the course of
treatment (Fig. 1), the patient
developed herpes zoster, which improved after reducing the dosage
of MMF and CsA and administering antiviral treatment with
famciclovir. The dosage of MMF was adjusted from twice a day, 0.75
g each time to once a day, 0.5 g. The dosage of CsA has also been
adjusted from twice a day (bid), 0.05 g each time to once a day
(qd), 0.05 g. Famciclovir is administered three times a day, with
0.25 grams per administration.
On December, 2022, a thyroid ultrasound (Fig. S1) revealed a solid nodule near the
isthmus of the left lobe of the thyroid gland, classified as
American College of Radiology Thyroid Imaging Reporting and Data
System (ACRTI-RADS) (9) grade 4,
along with a mixed cystic-solid nodule in the right lobe,
classified as ACRTI-RADS grade 2. Thyroid puncture pathology
confirmed the diagnosis of PTC in the left lobe (data not shown).
In May 2023, a total left thyroidectomy, isthmectomy, subtotal
right thyroidectomy and lymph node dissection in the central region
of the left neck were performed at Department of Thyroid and Breast
Surgery, People's Hospital of Longhua (Shenzhen, China). The
specimens were sectioned into book-leaf-like shapes every 3 mm, and
all suspected lesions visible to the naked eye were sampled. All
specimens were fixed with 4% neutral formaldehyde, routinely
embedded in paraffin, sliced at 4 µm, stained with H&E
according to standard procedures and observed under a light
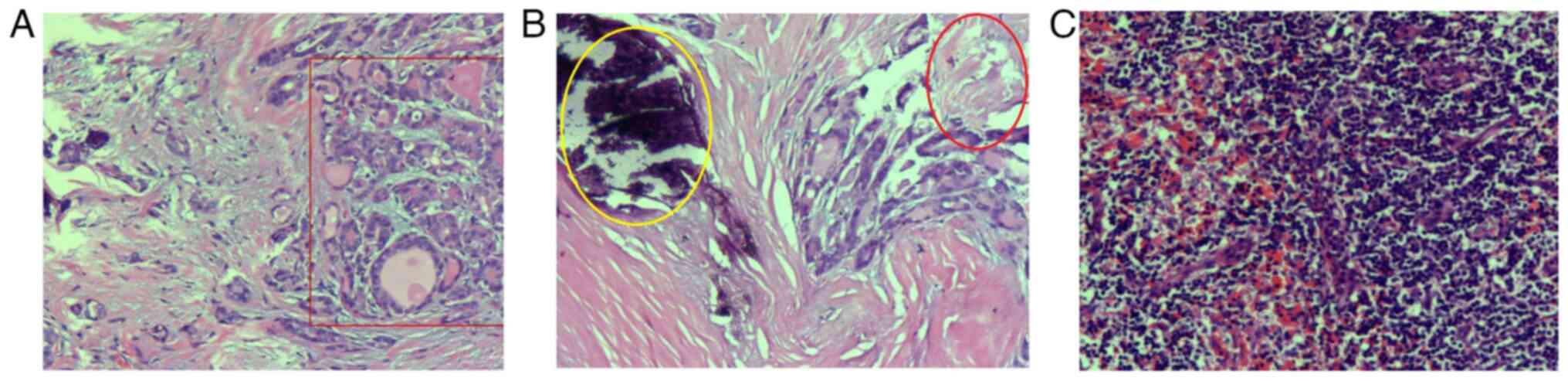
microscope. Fig. 3A shows that the
thyroid follicular epithelium exhibits papillary and branching
proliferation, featuring a fibrovascular core. The surface is lined
with a single layer of tumor cells. The cell nuclei are large,
oval, densely packed and exhibit a ground-glass appearance. Nuclear
grooves and small clear nucleoli can be seen (red rectangular
shape). Left thyroid and isthmus are consistent with PTC. The final
diagnosis was small PTC in the left lobe (pT1N0M0, stage I;
Fig. 3) (10). Postoperative blood examination
revealed a significant decrease in thyroid-stimulating hormone
levels, therefore, oral administration of levothyroxine sodium
tablets at a dose of 100 µg qd was required. Owing to the failure
of conventional drug therapy to successfully restore the patient's
complement levels, the treatment plan has been adjusted accordingly
since July 2023. Next, CsA was discontinued, and treatment with
prednisone at a dosage of 10 mg qd, MMF at 0.75 g bid and
hydroxychloroquine (HCQ) at 0.2 g qd was continued. In addition,
the patient also received a subcutaneous injection of 160 mg of
telitacicept once a week (4). After
1 month, the C3 and C4 levels returned to normal. Due to the
recovery of the patient's C3 and C4 levels and the relatively high
price of telitacicept, the dosage of telitacicept for subcutaneous
injection was reduced to 80 mg once a week starting from September
2023 (Fig. 1).
During the medication period, regular follow-up
visits were made to the Department of Thyroid and Breast Surgery,
People's Hospital of Longhua (Shenzhen, China), where thyroid
function and ultrasound examinations were conducted at half-year
intervals. Thyroid stimulating hormone gradually normalized (data
not shown) and no thyroid nodules or enlarged lymph nodes were
observed.
In December 2023, there was one episode of cough,
nasal congestion and yellow nasal discharge, suggestive of acute
upper respiratory tract infection. Cefuroxime was administered
twice daily, with 0.5 g each time, and compound pseudoephednine
hydrochloride was administered twice daily, with one tablet each
time. After administering both orally for 3 days, the
aforementioned symptoms improved. In late December 2023, a urine
nitrite test yielded positive results (data not shown), but there
were no symptoms of frequent urination, urgency or pain. A complete
urine culture test indicated the presence of Klebsiella
pneumoniae (data not shown). After taking levofloxacin tablets
for 14 days, once a day at a dosage of 0.5 g each time, the urine
nitrite test became negative.
Discussion
The reasons for the comorbidity of SLE and thyroid
carcinoma remain elusive. Antonelli et al (11) previously found that the incidence of
thyroid carcinoma in patients with SLE is significantly elevated
compared with that in age-matched normal controls, particularly in
those with evidence of thyroid autoimmunity. In another study,
Okosieme et al (12)
discovered that patients with autoimmune thyroid disease and
thyroid carcinoma exhibit similar yet distinct thyroid globulin
antigen epitopes compared with those with positive anti-thyroid
globulin antibodies, suggesting that autoimmune mechanisms may
serve a role in the development of thyroid carcinoma. In addition,
SLE and thyroid carcinoma are more prevalent in women. Previous
studies indicate a significant increase in estrogen receptor
expression within thyroid carcinoma tissues and in the peripheral
blood of patients with SLE (13-15).
Estrogen may exert a significant role in the pathogenesis of both
conditions, though the exact mechanism remains elusive. Therefore,
further research is necessary to determine whether there is an
association between the comorbidity of these two conditions.
Although inheritance, hormones and the environment
have been identified as risk factors for developing SLE (16,17),
abnormal activation of the immune system is the core mechanism,
characterized by excessive B-cell responses and the production of
autoantibodies (18). Telitacicept
is a TACI-Fc fusion protein that, by competitively inhibiting the
TACI site, neutralizes the activity of BLyS and APRIL, thereby
inhibiting the development and survival of plasma cells and mature
B cells. It not only inhibits the transformation of immature B
lymphocytes into mature B lymphocytes, but can also prevent the
transition of mature B cells into plasma cells. In addition, it can
affect the secretion of autoantibodies from abnormal plasma cells,
thereby controlling disease progression (19). BLyS binds to B-cell activating
factor receptor 3 and functions through the
alternative/noncanonical NF-κB signaling pathway, effectively
blocking its activity (3). The
application of telitacicept in the treatment of SLE has resulted in
a higher response rate of the Systemic Lupus Erythematosus Disease
Activity Index, significantly reducing immunoglobulin levels and
increasing complement levels (20).
To date, only two biological agents have been
approved for the treatment of SLE, namely belimumab (21) and telitacicept (4). There have been multiple reports
(20,22-28)
whereby patients with SLE without a history of carcinoma have
significantly elevated complement levels after treatment with
telitacicept. The present case showed a significant increase in
complement levels after the addition of telitacicept, similar to
the treatment effects observed in previously reported cases of
patients with SLE without concurrent cancer treated with
telitacicept (20,22-28).
To the best of our knowledge, no previous study has
documented the use of these agents in patients with a history of
carcinoma who also suffer from SLE. However, biological agents used
to treat other rheumatic diseases, such as rheumatoid arthritis
(RA), psoriasis (PS), inflammatory bowel disease (IBD) and
ankylosing spondylitis (AS) have been marketed earlier (29). In addition, there have been reports
on the use of biological agents in patients with a history of
carcinoma who also suffer from these rheumatic diseases. A Danish
population-based cohort study previously showed that among patients
with RA with a history of carcinoma, treatment with biological
disease-modifying antirheumatic drugs was not associated with an
increased risk of a second malignant neoplasm (30). Furthermore, two large prospective
studies, one originating from the British Society for Rheumatology
Biologics (31) and the other from
the German RA Observation of Biologic Therapy registry (32), have been conducted to investigate
the utilization of biological drugs in patients diagnosed with RA.
However, neither studies reported any increases in carcinoma
recurrence in patients with a previous history of malignancy and
under biological treatments. Another recent study has suggested
that the administration of biologics targeting TNF-α, IL-17, IL-23
and IL-12 for the treatment of PS in patients with a prior history
of carcinoma appears to be a safe approach (33). A multicenter real-life experience
added evidence to the safety of secukinumab in patients with PS
with a personal history of carcinoma (34). Secukinumab, as the world's first
approved fully human IL-17A inhibitor, can specifically neutralize
IL-17A from multiple sources and inhibit its pro-inflammatory
activity (35). Initially,
secukinumab was approved by the United States Food and Drug
Administration (FDA) for the treatment of patients with moderate to
severe plaque psoriasis, AS, and active psoriatic arthritis (PsA)
(36). A previous meta-analysis
included 34 studies (17 IBD, 14 RA, 2 PS and 1 AS), consisting of
24,328 patients and 85,784 person-years of follow-up. Rates of
carcinoma recurrence were found to be similar among individuals not
on immunosuppression, receiving anti-TNFs, immunomodulators and
combination immunosuppression (37).
Whilst several existing guidelines recommend
avoiding immunosuppression for 5 years after index carcinoma
(38-42),
a number of studies have indicated it may be safe to initiate these
agents earlier than 5 years in certain patients (37,43).
The majority of thyroid carcinoma mortality cases are from the
non-papillary histological subtypes, whereas PTC has a 5-year
survival rate of >90% (44). In
addition, thyroid carcinoma deaths have fallen by 50% in women and
by 33% in men over the past 40 years (45). Considering the present SLE case with
hypocomplementemia and PTC who underwent surgery, after the
treatment with MMF and CsA failed to achieve satisfactory results,
the patient was effectively treated with telitacicept. During the
postoperative follow-up, no tumor recurrence or lymph node
metastasis was observed. Furthermore, the safety data derived from
patients suffering from RA, PS, IBD and AS cannot be universally
applied to patients with SLE due to various reasons, primarily
because each disease exhibits a unique pathogenic mechanism.
In conclusion, to the best of our knowledge, the
present case was the first to report the use of telitacicept for
the successful treatment of a patient with SLE with post-surgical
PTC, providing a potential therapeutic option for SLE with a prior
history of carcinoma. However, the present case is only based on a
single case and therefore the conclusion drawn may not be broadly
representative and cannot be directly generalized to a broader
patient population. Therefore, further research, particularly
prospective randomized controlled trials, are warranted to verify
the present findings and ensure patient safety.
Supplementary Material
Thyroid ultrasound indicated a solid
nodule in the left lobe of the thyroid gland near the isthmus,
which is classified as ACRTI-RADS grade 4. A mixed cystic and solid
nodule was present in the right lobe of the thyroid gland, which
was classified as ACRTI-RADS grade 2. (A) Multiple mixed cystic and
solid nodules are visible in the right lobe of the thyroid, with
the largest one measuring ~3.9x2.7 mm (red rectangular shape). The
internal echoes are uneven, the margins are distinct and the aspect
ratio is <1. (B) CDFI: No significant blood flow signals are
detected within the nodule. (C) In the left lobe of the thyroid
near the isthmus, a low-echo solid nodule is visible, measuring
~4.1x3.1 mm (red diamond shape). The internal echoes are uneven,
with punctate strong echoes visible inside, the margin is blurred
and the aspect ratio is <1. (D) CDFI: No significant blood flow
signals are detected within the nodule. ACRTI-RADS, American
College of Radiology Thyroid Imaging Reporting and Data System;
CDFI, color doppler flow imaging.
Acknowledgements
Not applicable.
Funding
Funding: The present report was supported by the Scientific
Research Projects of Medical and Health Institutions of Longhua
District, Shenzhen (grant no. 2022046).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JT and BL designed and supervised the study. JT, HH
and LT performed data collection and analysis. HH and LT performed
data analysis and interpretation. All authors confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of People's Hospital of Longhua (Shenzhen, China;
approval no. 059A[2024]). The patient provided written informed
consent for the case study to be published.
Patient consent for publication
The patient who participated in the study provided
written informed consent for the publication of any associated data
and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma K, Du W, Wang X, Yuan S, Cai X, Liu D,
Li J and Lu L: Multiple functions of B cells in the pathogenesis of
systemic lupus erythematosus. Int J Mol Sci.
20(6021)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Möckel T, Basta F, Weinmann-Menke J and
Schwarting A: B cell activating factor (BAFF): Structure,
functions, autoimmunity and clinical implications in Systemic Lupus
Erythematosus (SLE). Autoimmun Rev. 20(102736)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shi F, Xue R, Zhou X, Shen P, Wang S and
Yang Y: Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune
disease. Immunopharmacol Immunotoxicol. 43:666–673. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dhillon S: Telitacicept: First approval.
Drugs. 81:1671–1675. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pizzato M, Li M, Vignat J, Laversanne M,
Singh D, La Vecchia C and Vaccarella S: The epidemiological
landscape of thyroid cancer worldwide: GLOBOCAN estimates for
incidence and mortality rates in 2020. Lancet Diabetes Endocrinol.
10:264–272. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang M, Wang Y, Wang Y, Bai Y and Gu D:
Association between systemic lupus erythematosus and cancer
morbidity and mortality: Findings from cohort studies. Front Oncol.
12(860794)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Song L, Wang Y, Zhang J, Song N, Xu X and
Lu Y: The risks of cancer development in systemic lupus
erythematosus (SLE) patients: A systematic review and
meta-analysis. Arthritis Res Ther. 20(270)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bernatsky S, Ramsey-Goldman R, Labrecque
J, Joseph L, Boivin JF, Petri M, Zoma A, Manzi S, Urowitz MB,
Gladman D, et al: Cancer risk in systemic lupus: an updated
international multi-centre cohort study. J Autoimmun. 42:130–135.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tessler FN, Middleton WD and Grant EG:
Thyroid imaging reporting and data system (TI-RADS): A user's
guide. Radiology. 287:29–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Antonelli A, Mosca M, Fallahi P, Neri R,
Ferrari SM, D'Ascanio A, Ghiri E, Carli L, Miccoli P and
Bombardieri S: Thyroid cancer in systemic lupus erythematosus: a
case-control study. J Clin Endocrinol Metab. 95:314–318.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Okosieme OE, Evans C, Moss L, Parkes AB,
Premawardhana LD and Lazarus JH: Thyroglobulin antibodies in serum
of patients with differentiated thyroid cancer: Relationship
between epitope specificities and thyroglobulin recovery. Clin
Chem. 51:729–734. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Inui A, Ogasawara H, Naito T, Sekigawa I,
Takasaki Y, Hayashida Y, Takamori K and Ogawa H: Estrogen receptor
expression by peripheral blood mononuclear cells of patients with
systemic lupus erythematosus. Clin Rheumatol. 26:1675–1678.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zeng Q, Chen G, Vlantis A, Tse G and van
Hasselt C: The contributions of oestrogen receptor isoforms to the
development of papillary and anaplastic thyroid carcinomas. J
Pathol. 214:425–433. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kansakar E, Chang YJ, Mehrabi M and Mittal
V: Expression of estrogen receptor, progesterone receptor, and
vascular endothelial growth factor-A in thyroid cancer. Am Surg.
75:785–789; discussion 789. 2009.PubMed/NCBI
|
|
16
|
Lahita RG: The role of sex hormones in
systemic lupus erythematosus. Curr Opin Rheumatol. 11:352–356.
1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cooper GS, Dooley MA, Treadwell EL, St
Clair EW, Parks CG and Gilkeson GS: Hormonal, environmental, and
infectious risk factors for developing systemic lupus
erythematosus. Arthritis Rheum. 41:1714–1724. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kiriakidou M and Ching CL: Systemic lupus
erythematosus. Ann Intern Med. 172:ITC81–ITC96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fan Y, Gao D and Zhang Z: Telitacicept, a
novel humanized, recombinant TACI-Fc fusion protein, for the
treatment of systemic lupus erythematosus. Drugs Today (Barc).
58:23–32. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu D, Li J, Xu D, Merrill JT, van
Vollenhoven RF, Liu Y, Hu J, Li Y, Li F, Huang C, et al:
Telitacicept in patients with active systemic lupus erythematosus:
Results of a phase 2b, randomised, double-blind, placebo-controlled
trial. Ann Rheum Dis. 83:475–487. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Navarra SV, Guzmán RM, Gallacher AE, Hall
S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al:
Efficacy and safety of belimumab in patients with active systemic
lupus erythematosus: A randomised, placebo-controlled, phase 3
trial. Lancet. 377:721–731. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng J, Peng Y, Wu Q, Wu Q, He J and Yuan
G: Efficacy and safety of telitacicept therapy in systemic lupus
erythematosus with hematological involvement. Clin Rheumatol.
43:2229–2236. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang L, Qian G, Zhang H, Li Q, Chen L,
Tang X and Zhao H: Efficacy of telitacicept in a systemic lupus
erythematosus patient with suboptimal response to Belimumab: A case
report. Lupus. 33:172–175. 2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan Q, Ji H, Liu Y, Jia C, Zou L and Yang
H: Refractory lupus hepatitis successfully treated with
telitacicept who failed to belimumab: A case report and literature
review. Lupus. 33:414–419. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ma X, Fu X, Cui B and Lin H: Telitacicept
for recalcitrant cutaneous manifestations of systemic lupus
erythematosus: A case report and review of the literature. Tohoku J
Exp Med. 258:219–223. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li S, Deng S, Wen S, Peng S, Jiang N, Li
B, Chen B, Yuan Y, Wu Q, Tao Y, et al: Telitacicept treatment
refractory lupus nephritis: A case report. Case Rep Nephrol Dial.
14:42–47. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang X, Lin F and Chen H: Efficacy and
safety of telitacicept in patients with lupus nephritis: A
single-center, real-world retrospective study. Clin Exp Nephrol.
28:902–909. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen R, Fu R, Lin Z, Huang C and Huang W:
The efficacy and safety of telitacicept for the treatment of
systemic lupus erythematosus: A real life observational study.
Lupus. 32:94–100. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sarzi-Puttini P, Ceribelli A, Marotto D,
Batticciotto A and Atzeni F: Systemic rheumatic diseases: From
biological agents to small molecules. Autoimmun Rev. 18:583–592.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dreyer L, Cordtz RL, Hansen IMJ,
Kristensen LE, Hetland ML and Mellemkjaer L: Risk of second
malignant neoplasm and mortality in patients with rheumatoid
arthritis treated with biological DMARDs: A danish population-based
cohort study. Ann Rheum Dis. 77:510–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dixon WG, Watson KD, Lunt M, Mercer LK,
Hyrich KL and Symmons DP: British Society For Rheumatology
Biologics Register Control Centre Consortium; British Society for
Rheumatology Biologics Register. Influence of anti-tumor necrosis
factor therapy on cancer incidence in patients with rheumatoid
arthritis who have had a prior malignancy: Results from the British
society for rheumatology biologics register. Arthritis Care Res
(Hoboken). 62:755–763. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Strangfeld A, Hierse F, Rau R, Burmester
GR, Krummel-Lorenz B, Demary W, Listing J and Zink A: Risk of
incident or recurrent malignancies among patients with rheumatoid
arthritis exposed to biologic therapy in the German biologics
register RABBIT. Arthritis Res Ther. 12(R5)2010.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Mastorino L, Dapavo P, Avallone G, Merli
M, Cariti C, Rubatto M, Pala V, Quaglino P and Ribero S: Biologic
treatment for psoriasis in cancer patients: Should they still be
considered forbidden? J Dermatolog Treat. 33:2495–2502.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pellegrini C, Esposito M, Rossi E, Gisondi
P, Piaserico S, Dapavo P, Conti A, Gambardella A, Burlando M,
Narcisi A, et al: Secukinumab in patients with psoriasis and a
personal history of malignancy: A multicenter real-life
observational study. Dermatol Ther (Heidelb). 12:2613–2626.
2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Blauvelt A: Safety of secukinumab in the
treatment of psoriasis. Expert Opin Drug Saf. 15:1413–1420.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Frieder J, Kivelevitch D and Menter A:
Secukinumab: A review of the anti-IL-17A biologic for the treatment
of psoriasis. Ther Adv Chronic Dis. 9:5–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gupta A, Peyrin-Biroulet L and
Ananthakrishnan AN: Risk of cancer recurrence in patients with
immune-mediated diseases with use of immunosuppressive therapies:
An updated systematic review and meta-analysis. Clin Gastroenterol
Hepatol. 22:499–512.e6. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Siegel CA, Finlayson SR, Sands BE and
Tosteson AN: Adverse events do not outweigh benefits of combination
therapy for Crohn's disease in a decision analytic model. Clin
Gastroenterol Hepatol. 10:46–51. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Scott FI, Vajravelu RK, Bewtra M, Mamtani
R, Lee D, Goldberg DS and Lewis JD: The benefit-to-risk balance of
combining infliximab with azathioprine varies with age: A markov
model. Clin Gastroenterol Hepatol. 13:302–309.e11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Buchbinder R, Barber M, Heuzenroeder L,
Wluka AE, Giles G, Hall S, Harkness A, Lewis D, Littlejohn G,
Miller MH, et al: Incidence of melanoma and other malignancies
among rheumatoid arthritis patients treated with methotrexate.
Arthritis Rheum. 59:794–799. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Allegretti JR, Barnes EL and Cameron A:
Are patients with inflammatory bowel disease on chronic
immunosuppressive therapy at increased risk of cervical high-grade
dysplasia/cancer? A meta-analysis. Inflamm Bowel Dis. 21:1089–1097.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bernheim O, Colombel JF, Ullman TA,
Laharie D, Beaugerie L and Itzkowitz SH: The management of
immunosuppression in patients with inflammatory bowel disease and
cancer. Gut. 62:1523–1528. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shelton E, Laharie D, Scott FI, Mamtani R,
Lewis JD, Colombel JF and Ananthakrishnan AN: Cancer recurrence
following immune-suppressive therapies in patients with
immune-mediated diseases: A systematic review and meta-analysis.
Gastroenterology. 151:97–109.e4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Karadaghy OA, Kallogjeri D and Piccirillo
JF: Development of a new clinical severity staging system for
patients with nonmetastatic papillary thyroid carcinoma. JAMA
Otolaryngol Head Neck Surg. 143:1173–1180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Torregrossa L, Poma AM, Macerola E, Rago
T, Vignali P, Romani R, Proietti A, Di Stefano I, Scuotri G,
Ugolini C, et al: The Italian consensus for the classification and
reporting of thyroid cytology: Cytohistologic and molecular
correlations on 37,371 nodules from a single institution. Cancer
Cytopathol. 130:899–912. 2022.PubMed/NCBI View Article : Google Scholar
|