Introduction
Ischemic conditioning intervention therapy
originated for the management of cardiovascular diseases in the
1980s, conferring cardiac protection from a subsequent or ongoing
ischemia-reperfusion injury (IRI) (1). Subsequently, organ protection in brain
and liver diseases has also been investigated, but its apparent
preclinical benefits have not been consistently translated into
clinical practice (2,3). Remote ischemic conditioning (RIC) can
activate ischemia tolerance through transient, non-fatal induction
of remote or limb ischemia by simply inflating a blood pressure
cuff on a leg or arm (4). This
highly attractive treatment strategy is beneficial in terms of
economy, safety, non-invasion and ease of promotion.
RIC, including three types of pre-conditioning
(RIPC, before the ischemic event), per-conditioning (RIPerC, during
the ischemic event) and post-conditioning (RIPostC, after the
ischemic event) based on the timing of induction, has shown promise
in treating several cardiovascular and cerebrovascular diseases
(5,6). RIC protects the liver from IRI via
several mechanisms such as neuro-humoral, mitochondrial autophagy
and exosomal gene mechanisms (4).
Although RIC has a strong short-term hepatoprotective effect
against hepatic ischemia-reperfusion injuries (HIRIs) during
liver-related surgeries, it is neutral in improving long-term
outcomes such as hepatocyte apoptosis index, duration of hospital
stay and survival rate (2). The
potential explanation is interactions with liver-protecting
anesthetics.
Although RIC does not cause harm in patients
undergoing liver-related surgery and has elevated to human trials,
human clinical evidence is limited. Importantly, there are still
several unanswered questions about the optimal timing of
intervention and administration protocols (such as one compared
with two limbs and the number and duration of cycles, among
others). Therefore, the preclinical evidence on RIPreC, RIPerC and
RIPostC in the HIRI models was systematically reviewed and
meta-analyzed in the present review to provide constructive and
helpful information for future works.
Materials and methods
This systematic review was prepared in accordance
with the recommendations of preferred reporting items for
systematic reviews and meta-analyses (PRISMA) to assess the
methodological quality. Preclinical (non-human) studies were
included to compare the effects of RIC on HIRI animal models. This
systematic review has been registered with PROSPERO, registration
number: CRD42023482725.
Search strategy
A comprehensive literature search was conducted on
July 26, 2024 using the PubMed (https://pubmed.ncbi.nlm.nih.gov), OVID (https://ovidsp.ovid.com), Web of Science (https://clarivate.com) and Embase databases
(https://www.embase.com) for articles published
from the inception to July 2024. Search terms comprised various
combinations of ‘remote ischemic conditioning’ or ‘limb ischemic
conditioning’ or ‘remote ischemic treatment’ or ‘remote ischemic
adaptation’ or ‘remote ischemic preconditioning’ or ‘distant
ischemic preconditioning’ or ‘limb ischemic preconditioning’ or
‘remote ischemic perconditioning’ or ‘limb ischemic
perconditioning’ or ‘remote ischemic postconditioning’ or ‘limb
ischemic postconditioning’ or ‘RIC’ or ‘RIP’ or ‘RIPC’ or ‘RPC’ or
‘RIPerC’ or ‘IperC’ or ‘RPostC’, ‘hepatic ischemia-reperfusion’ or
‘liver graft’ or ‘liver transplantation’ or ‘liver resection’ or
‘hepatectomy’. The search terms were adjusted according to
different search engines. There were no language restrictions for
the articles that were included. In addition, the authors manually
searched the references of included studies and other existing
meta-analyses to obtain more eligible studies. A specific search
strategy is presented in Table
SI.
Inclusion and exclusion criteria
The authors CT and AW independently reviewed and
retrieved the full-text articles simultaneously. Different views
were discussed among all authors, and duplicate articles in all
databases were merged. The latest and most complete study was
included when duplicate studies were from the same population.
The inclusion criteria are as follows: i) Any
non-human species, any sex, in the models of hepatic
ischemia-reperfusion; ii) interested intervention was limb RIC
compared with the control group without RIC; iii) controlled
studies with a separate control group; and iv) interested outcomes
were postoperative liver synthetic function and liver
histopathological injury.
The exclusion criteria are as follows: i) Human
subjects, in vitro or computer studies; ii) retrospective or
single-arm studies; iii) case studies, cross-over studies, studies
without a separate control group, editorials, meta-analyses and
reviews; iv) only the abstract of a study was available; and v) no
reports of postoperative aminotransferase levels or data were from
review articles.
Data extraction
The authors CT and AW independently extracted data
from each article. Any disagreements were resolved by the consensus
of a third reviewer (YK). The following information was extracted
from the included articles: First author; year of publication;
country or region of studies; animal model (species, sex, sample
size, the method of ischemic induction, the duration of ischemia);
parameters of RIC (body part, unilateral or bilateral, number of
cycles per treatment, duration of occlusion and release per cycle)
and interested outcomes. The published graphs were enlarged and
measured using Grab software (2) if
the information was unavailable in the text. If data were not
reported or unclear, the reviewers tried to contact the respective
study authors by e-mail (maximum of two attempts). Furthermore, it
should be stated that it was impossible to separate the RIPerC and
RIPostC groups easily; hence, these were combined to form one
group.
Quality assessment
The included animal model studies were assessed
using the risk of bias tool of the SYstematic Review Centre for
Laboratory animal Experimentation (SYRCLE). This assessment was
performed independently by CT and AW. Any disagreements were
resolved by consensus. Categories for the quality investigation
included sequence generation, baseline characteristics, allocation
concealment, random housing, blinding for the performance bias,
random outcome assessment, blinding for the detection bias,
incomplete outcome data, selective outcome data and other sources
of bias. Each category was classified as high, low or uncertain
risk.
The methodological quality of the results was
evaluated using the Grades of Recommendation, Assessment,
Development, and Evaluation (GRADE) guidelines (2). Ultimately, the quality of evidence for
each outcome was rated as high, moderate, low or very low.
Primary and secondary outcomes
The primary outcomes evaluated in this study were
those directly related to liver injury, such as alanine
transaminase (ALT), aspartate transaminase (AST) and liver
histopathology. The secondary outcomes assessed were lactate
dehydrogenase (LDH), tumor necrosis factor-α (TNF-α), interleukin
(IL) -6, IL-10, IL-1β, apoptosis and other possible outcomes.
Statistical analysis
The analysis was performed using the Review Manager
version 5.4 software (The Nordic Cochrane Center; The Cochrane
Collaboration, 2020). The continuous outcomes were reported as
standardized mean difference (SMD) with 95% confidence interval
(CI). The dichotomous outcomes were presented as odds ratio (OR)
with 95%CI, and the random-effects model was used for analysis.
Subgroup meta-regression or sensitivity analyses were then
performed using Stata/MP (version 17.0; StataCorp LLC), and
descriptive analysis was conducted if meta-analysis was
inappropriate. Publication bias was assessed using Egger's linear
regression test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Study characteristics
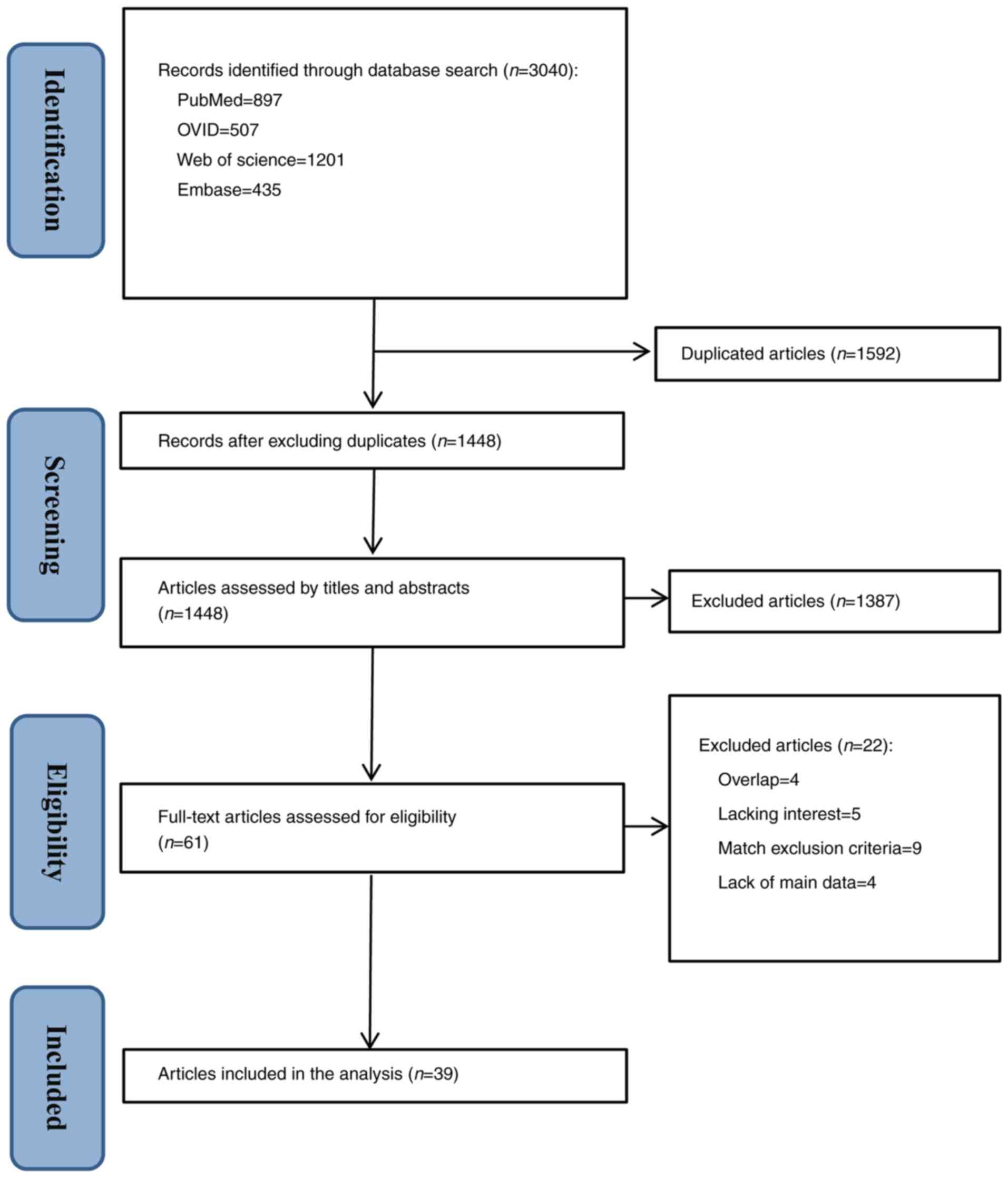
The reviewers initially identified 3,040 relevant
articles, of which 1,592 were duplicates. Excluding duplicates, a
total of 1,448 studies were left for analysis. After analyzing the
titles and abstracts, 1,387 articles that did not fulfil the
criteria were also excluded, and the remaining 61 studies were
selected for a full reading. After reviewing the full texts, 39
animal model studies met the eligibility criteria for data
synthesis (Fig. 1).
All 39 included articles/studies (101 animal
experiment records) were published between 2006 and 2023 and
conducted in 10 countries (China, n=15; United Kingdom, n=5;
Brazil, n=3; Korea, n=3; Hungary, n=3; Switzerland, n=3; Germany,
n=2; Canada, n=2; Sweden, n=2; and Turkey, n=1). In total, 32 of
101 animal experiments studied Sprague Dawley rats (n=337), 12 used
Wistar rats (n=178), 16 tested Lewis rats (n=144), 28 examined
C57BL/6 mice (n=270), five used wild-type mice (n=82) and eight
studied New Zealand white rabbits (n=50). To induce HIRI, various
animal models, such as orthotopic liver transplantation (OLT), 70%
liver I/R, total hepatic ischemia (THI), hemorrhagic
shock-resuscitation (HSR) I/R or hindlimb I/R, were used. RIC was
primarily performed by occluding the femoral vascular bundle,
femoral artery or hind limbs. Table
I summarizes the experimental characteristics of all the
involved studies (7-45).
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| First author/s,
year | Country | Species | Model (min) | Method of RIC | Number and duration
of cycles | Time of
administration | Time of
assessmenta | Interested
outcomes | (Refs.) |
|---|
| Mukkala et
al, 2023 | Canada | M, wild-type
mice | HSR-I/R (150) | Right hind limb,
non-invasive | RIPC, 4x5/5
min | RIPC, 30 min
pre-HSR | 2 h | ALT, liver
histopathology (liver necrosis area) TNF-α, IL-6, IL-10 | (7) |
| Li et al,
2023 | China | M, Sprague Dawley
rats | OLT (75) | Hind limbs,
non-invasive | RIPerC, 3x5/5
min | RIPerC, at the
onset of the recipient anhepatic phase | 3 h | ALT, AST, liver
histopathology (Suzuki classification) | (8) |
| Zhou et al,
2021 (ex1-5) | China | M, Sprague Dawley
rats | HSR-I/R (60) | Left hind limb
(femoral artery), invasive | Ex1-5, RIPC, 3x5/5
min | RIPC, 30 min
pre-HSR | 0, 2, 6, 12, and 24
h | ALT, AST TNF-α,
IL-1β | (9) |
| Niu et al,
2020 (ex1 and 2) | China | M, Sprague Dawley
rats | Hindlimb I/R
(240) | Hind limbs,
noninvasive | Ex1, RIPC, 3x5/5
min Ex2, RIPostC, 3x1/1 min | RIPC, 30 min
pre-ischemia RIPostC, at reperfusion | 0 h | ALT, AST LDH,
TNF-α, IL-1β, IL-10, apoptosis index | (10) |
| Choi et al,
2020 | Korea | M, Sprague Dawley
rats | 70% liver I/R
(30) | Unilateral hind
limb, non-invasive | RIPC, 3x5 /5
min | RIPC, 30 min
pre-ischemia | 2 h | ALT, AST, liver
histopathology (3-point scale) TNF-α | (11) |
| Koh et al,
2019 | Korea | M, C57BL/6
mice | 70% liver I/R
(30) | Hind limbs,
noninvasive | RIPC, 4x3 /3
min | RIPC, 24 min
pre-ischemia | 6 h | ALT, AST, liver
histopathology (Suzuki classification) TNF-α, IL-6, IL-10 | (12) |
| Emontzpohl et
al, 2019 (ex1-8) | Germany | M, Lewis rats | OLT (480) | Hind limbs
(Infrarenal aorta), invasive | Ex1-4, RIPC, 4x5/5
min Ex5-8, RIPostC, 4x5/5 min | RIPC, 40 min
pre-ischemia RIPostC, after graft reperfusion | 1, 3, 24, and 168
h | ALT, AST, liver
histopathology (Suzuki classification) | (13) |
| Li et al,
2018 (ex1-3) | China | M, C57BL/6
mice | OLT
(235.7±18.3) | Hind limbs (femoral
vascular bundle), invasive | Ex1-3, RIPerC,
3x5/5 min | RIPerC, during the
time from anesthetisation to graft reperfusion | 2, 24, and 72
h | ALT, AST, liver
histopathology (Suzuki classification) TNF-α, apoptosis index | (14) |
| Kambakamba et
al, 2018 (ex1-12) | Switzerland | M, C57BL/6
mice | Ex1-6, Standard
hepatectomy (68%) Ex7-12, Extended hepatectomy (86%) | Right hind limb
(femoral vessels), invasive | RIPC, 3x5/5
min | RIPC, 30 min
pre-ischemia | 0, 24, 48, 72, 96,
and 168 h | ALT, AST | (15) |
| Gomes et al,
2018 | Brazil | M & F, weaning
Wistar rats | THI (60) | Right hind limb
(femoral vessels), invasive | RIPC, 3x5/5
min | RIPC, 30 min
pre-THI | 24 h | ALT, AST, liver
histopathology (Scheuer scores) | (16) |
| Gao et al,
2018 (ex1 and 2) | China | Ex1, M, Sprague
Dawley rats Ex2, M, Wistar rats | 70% liver I/R
(60) | Right hind limb
(femoral artery), invasive | RIPostC, 1x5/5
min | RIPostC, 60 min
post-ischemia | 6 h | ALT, AST TNF-α,
IL-1β, apoptosis index | (17) |
| Czigany et
al, 2018 (ex1-8) | Germany | M, Lewis rats | OLT (480) | Hind limbs
(Infrarenal aorta), invasive | Ex1-4, RIPC, 4x5/5
min Ex5-8, RIPostC, 4x5/5 min | RIPC, 40 min
pre-ischemia RIPostC, after graft reperfusion | 1, 3, 24, and 168
h | ALT, AST, liver
histopathology (4-point scale) LDH, IL-10 | (18) |
| Liang et al,
2017 | China | M, Sprague Dawley
rats | OLT (45) | Hind limbs,
non-invasive | RIPerC, 3x5/5
min | RIPerC, at the
onset of the recipient anhepatic phase | 3 h | ALT, AST, liver
histopathology (Suzuki classification) | (19) |
| He et al,
2017 | China | M, Sprague Dawley
rats | OLT (75) | Hind limbs,
non-invasive | RIPerC, 3x5/5
min | RIPerC, at the
onset of the recipient anhepatic phase | 3 h | ALT, AST | (20) |
| Ruan et al,
2016 | China | M, Sprague Dawley
rats | 70% liver I/R
(60) | Hind limbs,
non-invasive | RIPC, 3x10/10
min | RIPC, 60 min
pre-ischemia | 6 h | ALT, AST, liver
histopathology (Suzuki classification) TNF-α, IL-6, IL-10 | (21) |
| Park et al,
2016 (ex1 and 2) | Korea | M, Sprague Dawley
rats | 70% liver I/R
(30) | Right hind limb
(femoral artery), invasive | Ex1, RIPC, 3x5/5
min Ex2, RIPerC, 3x5/5 min | RIPC, 30 min
pre-ischemia RIPerC, post-ischemia | 24 h | ALT, AST | (22) |
| Limani et
al, 2016 | Switzerland | M, C57BL/6
mice | 70% liver I/R
(60) | Hind limbs (femoral
vascular bundle), invasive | RIPC, 3x5/5
min | RIPC, 30 min
pre-ischemia | 6 h | ALT, AST, liver
histopathology (liver necrosis area) | (23) |
| Li et al,
2016 (ex1-3) | China | M, C57BL/6
mice | OLT (120) | Hind limbs (femoral
vascular bundle), invasive | Ex1-3, RIPC, 4x6/6
min | RIPC, 48 min
pre-ischemia | 2, 24, and 72
h | ALT, liver
histopathology (Suzuki classification) TNF-α, apoptosis index | (24) |
| Jia et al,
2015 (ex1-3) | China | M, Sprague Dawley
rats | OLT (75) | Hind limbs,
non-invasive | Ex1, RIPerC1, 3x1/1
min Ex2, RIPerC2, 3x5/5 min Ex3, RIPerC3, 3x10/10 min | RIPerC, at the
onset of the recipient anhepatic phase | 3 h | ALT, AST, liver
histopathology (Suzuki classification) | (25) |
| Guimarães et
al, 2015 (ex1 and 2) | Brazil | M, Sprague Dawley
rats | 70% liver I/R
(45) | Right hind limb
(femoral vascular bundle), invasive | Ex1 and 2, RIPC,
6x4/4 min | RIPC, 48 min
pre-ischemia | 1 and 3 h | ALT, liver
histopathology (Suzuki classification) IL-6, IL-10 | (26) |
| Czigany et
al, 2015 | Hungary | M, Wistar rats | 70% liver I/R
(60) | Left hind limb
(femoral artery), invasive | RIPerC, 4x5/5
min | RIPerC, 20 min
post-ischemia | 24 h | ALT, AST, liver
histopathology (liver necrosis area) | (27) |
| Wang et al,
2014 (ex1-4) | China | M, wild-type
mice | 70% liver I/R
(45) | Right hind limb
(femoral vascular bundle), invasive | Ex1-4, RIPC, 6x4/4
min | RIPC, 48 min
pre-ischemia | 2, 6, 12, and 24
h | ALT, AST, liver
histopathology (Suzuki classification) | (28) |
| Uysal et al,
2014 | Turkey | M, Wistar albino
rats | 70% liver I/R
(30) | Left hind limb,
non-invasive | RIPC, 3x10/10
min | RIPC, 60 min
pre-ischemia | 4 h | ALT, AST, liver
histopathology (3-point scale) | (29) |
| Oberkofler et
al, 2014 (ex1-3) | Switzerland | M & F, C57B1/6
mice | 70% liver I/R
(60) | Hind limbs (femoral
vascular bundle), invasive | Ex1-3, RIPC, 4x5/5
min | RIPC, 40 min
pre-ischemia | 3, 6, and 24 h | ALT, AST, liver
histopathology (liver necrosis area) | (30) |
| Garab et al,
2014 (ex1-3) | Hungary | M, Sprague Dawley
rats | 70% liver I/R
(60) | Right hind limb
(femoral artery), invasive | Ex1-3, RIPC,
2x10/10 min | RIPC, 40 min
pre-ischemia | 1, 2, and 3 h | ALT, AST LDH,
TNF-α | (31) |
| Costa et al,
2014 | Brazil | M, Wistar rats | 70% liver I/R
(60) | Left hind limb,
non-invasive | RIPerC, 4x5/5
min | RIPerC, 20 min
post-ischemia | 2 h | ALT, AST | (32) |
| Wang et al,
2013 (ex1-4) | China | M, Sprague Dawley
rats | OLT (60) | Hind limbs,
non-invasive | Ex1-4, RIPC, 4x5/5
min | RIPC, 40 min
pre-ischemia | 2, 6, 12, and 24
h | ALT | (33) |
| Czigány et
al, 2013 (ex1-3) | Hungary | M, Wistar rats | 70% liver I/R
(60) | Hind limbs
(Infrarenal aorta), invasive | Ex1-3, RIPerC,
4x5/5 min | RIPerC, 20 min
post-ischemia | 1, 6, and 24 h | ALT, AST, liver
histopathology (Suzuki classification) TNF-α | (34) |
| Tapuria et
al, 2012 | United Kingdom | M, Sprague Dawley
rats | 70% liver I/R
(45) | Unilateral hind
limb, non-invasive | RIPC, 4x5/5
min | RIPC, 40 min
pre-ischemia | 24 h | ALT, AST | (35) |
| Kanoria et
al, 2012 | United Kingdom | M, New Zealand
white rabbits | THI (25) | Right hind limb,
non-invasive | RIPC, 3x10/10
min | RIPC, 65 min
pre-THI | 2 h | ALT LDH | (36) |
| Cao et al,
2012 (ex1-4) | China | M, New Zealand
white rabbits | 70% liver I/R
(25) | Hind limbs (femoral
vascular bundle), invasive | Ex1-4, RIPC, 2x5/10
min | RIPC, 24 h
pre-ischemia | 0.5, 1, 2, and 3
h | ALT | (37) |
| Björnsson et
al, 2012 (ex1-3) | Sweden | M, Sprague Dawley
rats | 70% liver I/R
(60) | Right hind limb,
non-invasive | Ex1-3, RIPC,
1x10/10 min | RIPC, 20 min
pre-ischemia | 0, 1, and 4 h | ALT, AST | (38) |
| Abu-Amara et
al, 2011 | United Kingdom | M, C57BL/6
mice | 70% liver I/R
(40) | Right hind limb,
non-invasive | RIPC, 6x4/4
min | RIPC, 48 min
pre-ischemia | 2 h | ALT, AST, liver
histopathology (4-point scale) | (39) |
| Wang (1) et
al, 2010 (ex1-4) | Canada | F, C57BL/6
mice | 70% liver I/R
(60) | Right hind limb,
non-invasive | Ex1-4, RIPC,
1x10/10 min | RIPC, 20 min
pre-ischemia | 0.5, 1, 2, and 3
h | ALT TNF-α | (40) |
| Wang (2) et
al, 2010 | China | M, Wistar rats | Hindlimb I/R
(240) | Hind limbs,
non-invasive | RIPC, 4x5/5
min | RIPC, 40 min
pre-ischemia | 4 h | ALT, AST | (41) |
| Tapuria et
al, 2009 | United Kingdom | M, Sprague Dawley
rats | 70% liver I/R
(45) | Right hind limb,
non-invasive | RIPC, 4x5/5
min | RIPC, 40 min
pre-ischemia | 3 h | ALT, liver
histopathology (Suzuki classification) | (42) |
| Lai et al,
2006 | China | F, Wistar rats | 70% liver I/R
(45) | Right hind limb
(femoral artery), invasive | RIPC, 4x10/10
min | RIPC, before liver
ischemia | 4 h | ALT | (43) |
| Kanoria et
al, 2006 (ex1-3) | United Kingdom | M, New Zealand
white rabbits | THI (25) | Right hind limb,
non-invasive | Ex1-3, RIPC,
3x10/10 min | RIPC, 65 min
pre-THI | 0.5, 1, and 2
h | ALT, AST LDH | (44) |
| Gustafsson et
al, 2006 (ex1 and 2) | Sweden | F, Wistar rats | THI (60) | Right hind limb
(femoral artery), invasive | Ex1 and 2, RIPC,
1x10/15 min | RIPC, 25 min
pre-THI | 0 and 1 h | ALT | (45) |
Quality assessment
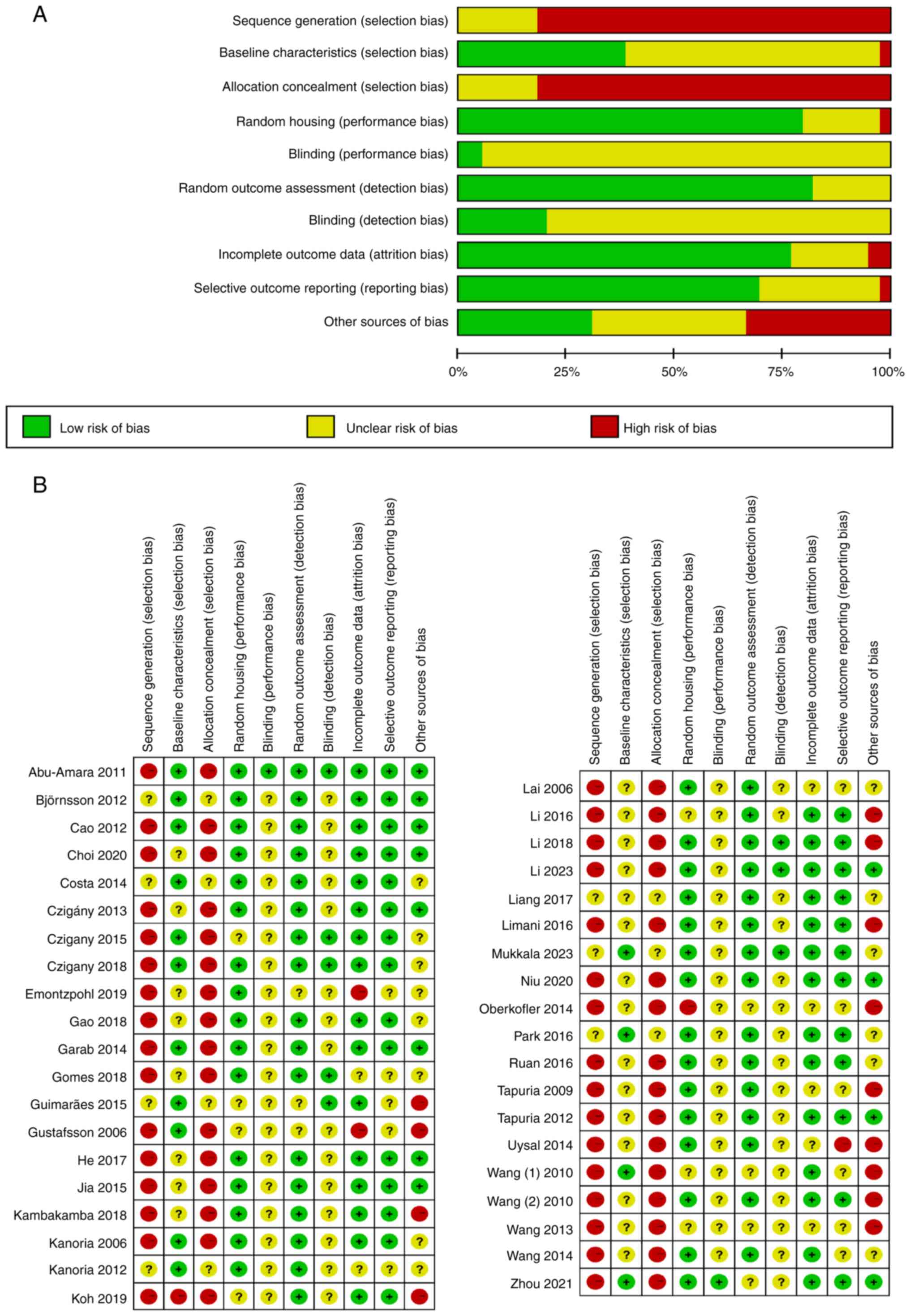
The SYRCLE's risk of bias tool was used to evaluate
the risk of bias in the included animal experiments studies
(Fig. 2). In quality assessment, 15
studies (7,9,18,22,26,27,31,32,36-40,44,45)
were analyzed with the same baseline characteristics among groups,
but none of them conducted random sequence generation or allocation
concealment. A total of 30 studies (7-12,14,15,17-28,31,32,34,35,37-41,44)
had complete outcome data and they also demonstrated a low risk of
attrition and reporting biases. Furthermore, blind bias could not
be evaluated in most studies because of the characteristics of
animal experiments.
Effects of all RIC studies
RIC was significantly effective against all the
primary outcomes (ALT, AST and liver histopathology; all
P<0.00001), although there were significant statistical
heterogeneities: I2=79% in the ALT change, 76% in
the AST change, and 71% in the liver histopathology change
(Table II; Fig. S1, Fig.
S2 and Fig. S3). Notably,
RIPerC/RIPostC exhibited greater effects on ALT and AST changes
[ALT SMD (95%CI): RIPC -1.97 (-2.40, -1.55) vs. -2.78 (-3.77,
-1.78); P=0.142; AST SMD (95%CI): RIPC -1.45 (-1.90, -0.99) vs.
-2.13 (-2.91, -1.34); P=0.142], and RIPC exerted a greater effect
on liver histopathology change [SMD (95%CI): RIPC -2.68 (-3.67,
-1.69) vs. -1.58 (-2.24, -0.92); P=0.070]; however, there was no
interactions between the two groups in the meta-regression
analysis. RIC was the greatest magnitude effective if the duration
of each cycle was 4-6 min or the total duration of limb ischemia
was 10-25 min. ALT and AST changes significantly interacted with
the assessment times, with two peaks occurring 2-3 or 6-12 h
post-reperfusion lasting up to 72 h. The efficacy of RIC on ALT
change was greatest in the New Zealand white rabbits model with
species interaction (P=0.041), but this animal model was much fewer
(n=150). In addition, there were no interactions between liver
histopathology and species, the HIRI model, the operation of RIC,
the number of limbs occluded, the number of cycles, the duration of
each cycle, the total duration of limb ischemia, or classification
methods of liver histopathology score in the meta-regression
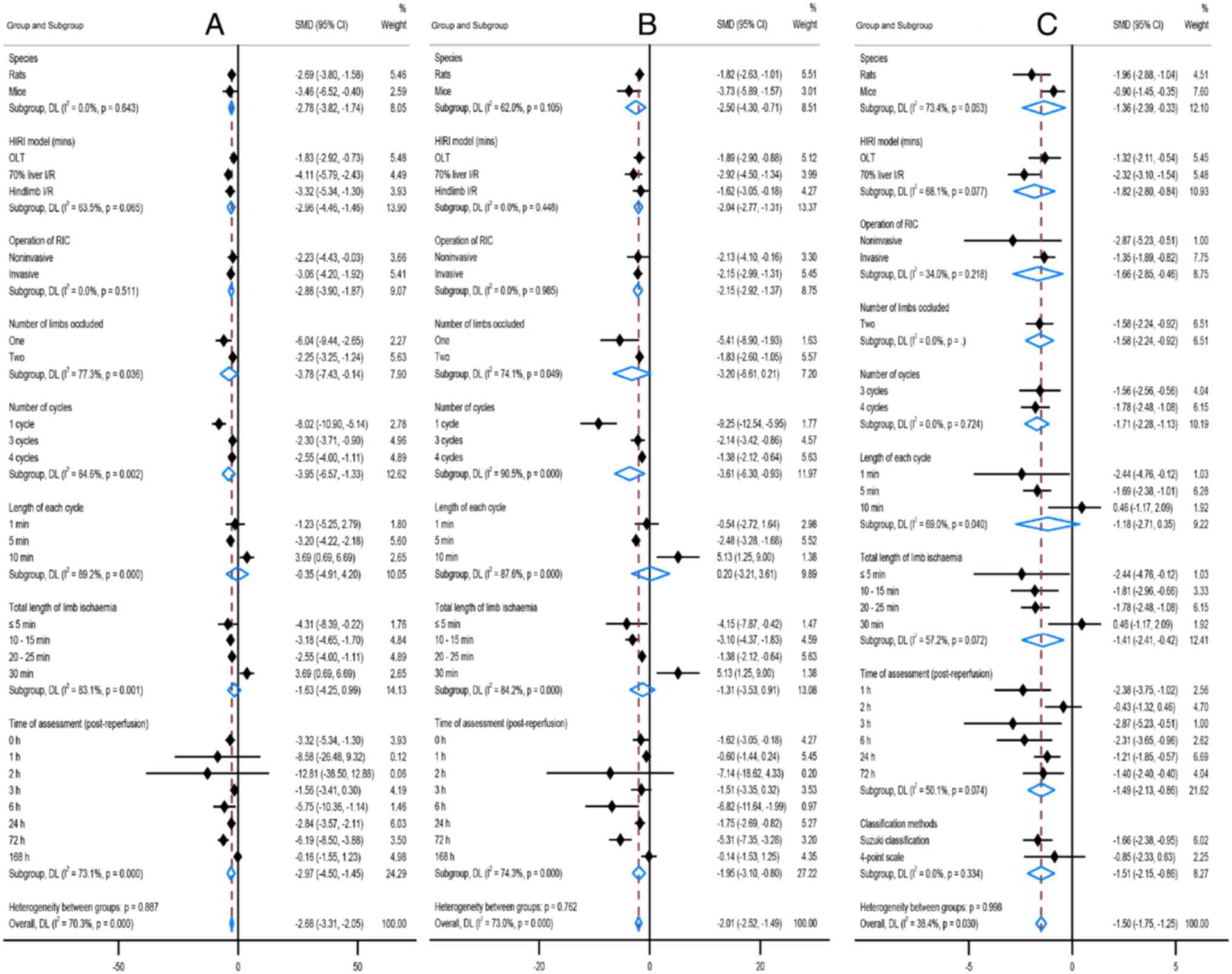
analysis (all P>0.05). The details are shown in Table II and Fig. 3.
 | Table IITreatment effects of different
factors based on primary outcomes. |
Table II
Treatment effects of different
factors based on primary outcomes.
| | ALT | AST | Liver
histopathology (scores) |
|---|
| Overall
effect/factors | No. of experiments
(animals) | SMD (95% CI) | P-value | No. of experiments
(animals) | SMD (95% CI) | P-value | No. of experiments
(animals) | SMD (95% CI) | P-value |
|---|
| Overall effect | 89 (1,087) | -2.15 (-2.54,
-1.75) | <0.00001 | 67(797) | -1.66 (-2.06,
-1.27) | <0.00001 | 28(350) | -2.10 (-2.69,
-1.52) | <0.00001 |
| Time of
administration | | | | | | | | | |
|
RIPC | 66(811) | -1.97 (-2.40,
-1.55) | <0.00001 | 44(521) | -1.45 (-1.90,
-0.99) | <0.00001 | 15(178) | -2.68 (-3.67,
-1.69) | <0.00001 |
|
RIPerC and
RIPostC | 23(276) | -2.78 (-3.77,
-1.78) | <0.00001 | 23(276) | -2.13 (-2.91,
-1.34) | <0.00001 | 13(172) | -1.58 (-2.24,
-0.92) | <0.00001 |
| Species | | | | | | | | | |
|
All
rats | 54(605) | -1.99 (-2.51,
-1.46) | <0.00001 | 44(493) | -1.44 (-1.92,
-0.96) | <0.0001 | 19(218) | -2.23 (-3.06,
-1.41) | <0.00001 |
|
Sprague
Dawley rats | 32(337) | -1.82 (-2.51,
-1.13) | <0.00001 | 25(277) | -1.34 (-2.08,
-0.61) | 0.0003 | 10(104) | -3.02 (-4.86,
-1.18) | 0.001 |
|
Wistar
rats | 12(178) | -2.94 (-4.23,
-1.65) | <0.00001 | 9(126) | -1.93 (-3.04,
-0.81) | 0.0007 | 5(78) | -1.97 (-2.54,
-1.39) | <0.00001 |
|
Lewis
rats | 10(90) | -1.48 (-2.37,
-0.58) | 0.001 | 10(90) | -1.24 (-1.79,
-0.69) | <0.0001 | 4(36) | -1.66 (-2.99,
-0.34) | 0.01 |
|
All
mice | 27(332) | -2.08 (-2.79,
-1.36) | <0.00001 | 20(262) | -2.34 (-3.16,
-1.52) | <0.0001 | 9(132) | -1.89 (-2.69,
-1.09) | <0.00001 |
|
C57BL/6
mice | 22(270) | -1.85 (-2.56,
-1.13) | <0.00001 | 15(200) | -2.24 (-3.17,
-1.31) | <0.00001 | 8(122) | -1.77 (-2.59,
-0.95) | <0.0001 |
|
Wild-type
mice | 5(62) | -3.21 (-6.00,
-0.43) | 0.02 | 5(62) | -2.91 (-4.94,
-0.87) | 0.005 | 1(10) | -3.14 (-5.30,
-0.98) | 0.004 |
|
New Zealand
white rabbits | 8(150) | -3.11 (-3.85,
-2.38) | <0.00001 | 3(42) | -1.15 (-2.31,
0.01) | 0.05 | | | |
| HIRI model
(min) | | | | | | | | | |
|
All OLT | 26(260) | -2.26 (-3.04,
-1.47) | <0.00001 | 19(206) | -1.84 (-2.62,
-1.05) | <0.00001 | 15(172) | -2.05 (-2.85,
-1.24) | <0.00001 |
|
OLT
(45) | 1(12) | -4.70 (-7.26,
-2.14) | 0.0003 | 1(12) | -4.00 (-6.26,
-1.74) | 0.0005 | 1(12) | -8.71 (-13.12,
-4.31) | 0.0001 |
|
OLT
(60) | 4(24) | -4.06 (-6.21,
-1.92) | 0.0002 | | | | | | |
|
OLT
(75) | 5(44) | -0.84 (-3.11,
1.43) | 0.47 | 5(44) | -1.02 (-3.58,
1.54) | 0.43 | 4(34) | -1.78 (-3.66,
0.10) | 0.06 |
|
OLT
(120) | 3(30) | -3.49 (-4.84,
-2.13) | <0.00001 | | | | 3(30) | -4.18 (-5.73,
-2.63) | <0.00001 |
|
OLT
(235.7±18.3) | 3(60) | -3.46 (-6.52,
-0.40) | 0.03 | 3(60) | -3.73 (-5.89,
-1.57) | 0.0007 | 3(60) | -0.90 (-1.45,
-0.35) | 0.001 |
|
OLT
(480) | 10(90) | -1.48 (-2.37,
-0.58) | 0.001 | 10(90) | -1.24 (-1.79,
-0.69) | <0.0001 | 4(36) | -1.66 (-2.99,
-0.34) | 0.01 |
|
All 70%
liver I/R | 41(539) | -2.11 (-2.69,
-1.54) | <0.00001 | 29(365) | -1.82 (-2.51,
-1.13) | <0.00001 | 12(162) | -2.29 (-3.25,
-1.32) | <0.00001 |
|
70%
liver I/R (25) | 4(96) | -2.90 (-3.50,
-2.29) | <0.00001 | | | | | | |
|
70%
liver I/R (30) | 5(61) | -1.71 (-3.53,
0.12) | 0.07 | 5(61) | -1.51 (-2.94,
-0.08) | 0.04 | 3(44) | -2.52 (-3.91,
-1.13) | 0.0004 |
|
70%
liver I/R (40) | 1(12) | -2.40 (-4.02,
-0.77) | 0.004 | 1(12) | -2.10 (-3.63,
-0.57) | 0.007 | 1(12) | -1.06 (-2.31,
0.18) | 0.09 |
|
70%
liver I/R (45) | 9(100) | -1.96 (-3.21,
-0.71) | 0.002 | 5(52) | -2.02 (-3.85,
-0.20) | 0.03 | 4(45) | -2.90 (-6.76,
0.95) | 0.14 |
|
70%
liver I/R (60) | 22(270) | -2.13 (-2.99,
-1.27) | <0.00001 | 18(240) | -1.91 (-2.88,
-0.95) | <0.0001 | 4(60) | -2.32 (-3.02,
-1.62) | <0.00001 |
|
All THI | 7(110) | -2.86 (-3.96,
-1.75) | <0.00001 | 4(58) | -1.06 (-1.84,
-0.27) | 0.008 | 1(16) | -1.27 (-2.37,
-0.16) | 0.02 |
|
THI
(25) | 4(54) | -3.91 (-5.92,
-1.91) | 0.0001 | 3(42) | -1.15 (-2.31,
0.01) | 0.05 | | | |
|
THI
(60) | 3(56) | -2.04 (-3.20,
-0.89) | 0.0005 | 1(16) | -0.96 (-2.01,
0.09) | 0.07 | 1(16) | -1.27 (-2.37,
-0.16) | 0.02 |
| Hindlimb I/R
(240) | 3(36) | -3.49 (-4.69,
-2.29) | <0.00001 | 3(36) | -1.99 (-2.89,
-1.09) | <0.0001 | | | |
|
All
HSR-I/R | 6(72) | -1.70 (-3.17,
-0.22) | 0.02 | 6(72) | -1.38 (-2.54,
-0.21) | 0.02 | | | |
|
HSR-I/R
(60) | 5(50) | -1.00 (-2.02,
0.01) | 0.05 | 5(50) | -0.99 (-2.08,
0.10) | 0.08 | | | |
|
HSR-I/R
(150) | 1(22) | -5.38 (-7.32,
-3.44) | <0.00001 | 1(22) | -2.93 (-4.20,
-1.66) | <0.00001 | | | |
| Operation of
RIC | | | | | | | | | |
|
Non-invasive | 33(382) | -2.21 (-2.99,
-1.42) | <0.00001 | 23(294) | -1.51 (-2.30,
-0.72) | 0.0002 | 11(126) | -2.42 (-3.39,
-1.45) | <0.00001 |
|
Invasive | 56(705) | -2.11 (-2.56,
-1.66) | <0.00001 | 44(503) | -1.71 (-2.16,
-1.26) | <0.00001 | 17(224) | -1.93 (-2.66,
-1.19) | <0.00001 |
| Number of limbs
occluded | | | | | | | | | |
|
One
limb | 47(567) | -1.88 (-2.44,
-1.33) | <0.00001 | 36(427) | -1.49 (-2.05,
-0.92) | <0.00001 | 8(98) | -2.33 (-3.88,
-0.78) | 0.003 |
|
Two
limbs | 42(520) | -2.44 (-2.99,
-1.89) | <0.00001 | 31(370) | -1.87 (-2.41,
-1.34) | <0.00001 | 20(252) | -2.03 (-2.63,
-1.44) | <0.00001 |
| Number of
cycles | | | | | | | | | |
|
1 cycle | 11(152) | -1.93 (-3.32,
-0.54) | 0.006 | 5(72) | -2.69 (-6.50,
1.11) | 0.17 | | | |
|
2
cycles | 7(132) | -2.58 (-3.07,
-2.09) | <0.00001 | 3(36) | -1.25 (-2.18,
-0.33) | 0.008 | | | |
|
3
cycles | 33(385) | -1.91 (-2.58,
-1.24) | <0.00001 | 32(373) | -1.71 (-2.29,
-1.12) | <0.00001 | 12(158) | -1.76 (-2.53,
-0.99) | <0.00001 |
|
4
cycles | 31(342) | -2.32 (-2.97,
-1.67) | <0.00001 | 22(264) | -1.53 (-2.04,
-1.02) | <0.00001 | 12(146) | -2.41 (-3.06,
-1.76) | <0.00001 |
|
6
cycles | 7(76) | -2.73 (-4.54,
-0.92) | 0.003 | 5(52) | -2.62 (-4.58,
-0.67) | 0.009 | 4(46) | -2.04 (-5.00,
0.93) | 0.18 |
| Duration of each
cycle | | | | | | | | | |
|
1 min | 2(20) | -1.23 (-5.25,
2.79) | 0.55 | 2(20) | -0.54 (-2.72,
1.64) | 0.62 | 1(8) | -2.44 (-4.76,
-0.12) | 0.04 |
|
3 min | 1(20) | -1.48 (-2.50,
-0.47) | 0.004 | 1(20) | -1.85 (-2.93,
-0.76) | 0.0008 | 1(20) | -2.11 (-3.25,
-0.97) | 0.0003 |
|
4 min | 7(76) | -2.73 (-4.54,
-0.92) | 0.003 | 5(52) | -2.62 (-4.58,
-0.67) | 0.009 | 4(46) | -2.04 (-5.00,
0.93) | 0.18 |
|
5 min | 56(667) | -2.30 (-2.80,
-1.80) | <0.00001 | 47(545) | -1.95 (-2.42,
-1.48) | <0.00001 | 16(212) | -2.01 (-2.65,
-1.37) | <0.00001 |
|
6 min | 3(50) | -3.49 (-4.84,
-2.13) | <0.00001 | | | | 3(30) | -4.18 (-5.73,
-2.63) | <0.00001 |
|
10 min | 20(264) | -1.58 (-2.43,
-0.73) | 0.0003 | 12(160) | -0.55 (-1.51,
0.42) | 0.26 | 3(34) | -1.30 (-2.92,
0.33) | 0.12 |
| Total duration of
limb ischemia | | | | | | | | | |
|
≤5 min | 4(44) | -4.31 (-8.39,
-0.22) | 0.04 | | | | 1(8) | -2.44 (-4.76,
-0.12) | 0.04 |
|
10-15
min | 38(521) | -1.84 (-2.40,
-1.29) | <0.00001 | 28(345) | -1.89 (-2.61,
-1.17) | <0.00001 | 9(136) | -1.93 (-2.82,
-1.04) | <0.0001 |
|
20-25
min | 39(422) | -2.39 (-2.99,
-1.78) | <0.00001 | 26(296) | -1.61 (-2.14,
-1.09) | <0.00001 | 15(172) | -2.42 (-3.38,
-1.46) | <0.00001 |
|
30 min | 7(88) | -2.02 (-4.06,
0.01) | 0.05 | 6(76) | -0.69 (-1.87,
0.48) | 0.25 | 3(34) | -1.30 (-2.92,
0.33) | 0.12 |
|
40 min | 1(12) | -1.77 (-3.19,
-0.35) | 0.01 | | | | | | |
| Time of
assessmenta | | | | | | | | | |
|
0 h | 5(70) | -1.68 (-3.31,
-0.04) | 0.04 | 4(50) | -0.58 (-1.71,
0.55) | 0.32 | | | |
|
0.5 h | 3(48) | -1.90 (-3.45,
-0.34) | 0.02 | 1(14) | -0.32 (-1.38,
0.73) | 0.55 | | | |
|
1 h | 10(142) | -2.09 (-3.80,
-0.38) | 0.02 | 6(76) | -0.26 (-1.64,
1.13) | 0.72 | 2(28) | -0.22 (-4.48,
4.04) | 0.92 |
|
2 h | 14(182) | -2.58 (-3.70,
-1.45) | <0.00001 | 9(120) | -2.16 (-3.17,
-1.15) | <0.0001 | 5(62) | -2.46 (-4.14,
-0.78) | 0.004 |
|
3 h | 14(156) | -2.00 (-3.03,
-0.96) | 0.0002 | 10(98) | -1.27 (-2.47,
-0.07) | 0.04 | 7(70) | -3.58 (-5.67,
-1.50) | 0.0007 |
|
4 h | 4(54) | -1.66 (-3.68,
0.36) | 0.11 | 3(42) | -1.96 (-4.36,
0.44) | 0.11 | 1(14) | -1.95 (-3.30,
-0.60) | 0.005 |
|
6 h | 10(122) | -3.12 (-4.19,
-2.06) | <0.00001 | 9(116) | -3.17 (-4.46,
-1.89) | <0.00001 | 3(48) | -2.22 (-2.98,
-1.46) | <0.00001 |
|
12 h | 3(26) | -2.90 (-4.72,
-1.08) | 0.002 | 2(20) | -4.70 (-8.21,
-1.20) | 0.009 | | | |
|
24 h | 16(179) | -2.26 (-3.00,
-1.53) | <0.00001 | 14(163) | -1.63 (-2.25,
-1.02) | <0.00001 | 8(98) | -1.62 (-2.30,
-0.94) | <0.00001 |
|
48 h | 2(20) | -1.54 (-7.15,
4.06) | 0.59 | 2(20) | -5.29 (-15.37,
4.79) | 0.30 | | | |
|
72 h | 4(50) | -2.64 (-5.25,
-0.03) | 0.05 | 3(40) | -3.48 (-7.54,
0.58) | 0.09 | 2(30) | -2.87 (-6.29,
0.56) | 0.10 |
|
168 h | 4(38) | -0.16 (-0.81,
0.50) | 0.64 | 4(38) | -0.33 (-1.00,
0.35) | 0.34 | | -2.10 (-2.69,
-1.52) | |
RIC was also significantly effective against several
secondary outcomes such as LDH, TNF-α and apoptosis index (all
P<0.00001) and there were also significant statistical
heterogeneities (I2=75%,
I2=76%, I2=51%, respectively;
Table SII; Fig. S4). Due to the limitations of the
sample size and data integrity, only a meta-regression analysis was
performed by dividing the groups of RIPC and RIPerC/RIPostC, and
there were no interactions between the two groups (P=0.91, P=0.16,
P=0.75, respectively; Fig. S5). In
addition, only four studies (five experiments; 78 animals)
(7,12,21,26)
evaluated IL-6 change, and five studies (13 experiments; 156
animals) (10,12,18,21,26)
evaluated IL-10 change, showing that RIC was ineffective against
IL-6 and IL-10 changes [SMD (95%CI): -1.47 (-3.72, 0.79); P=0.20;
SMD (95% CI): -0.34 (-1.39, 0.71); P=0.52].
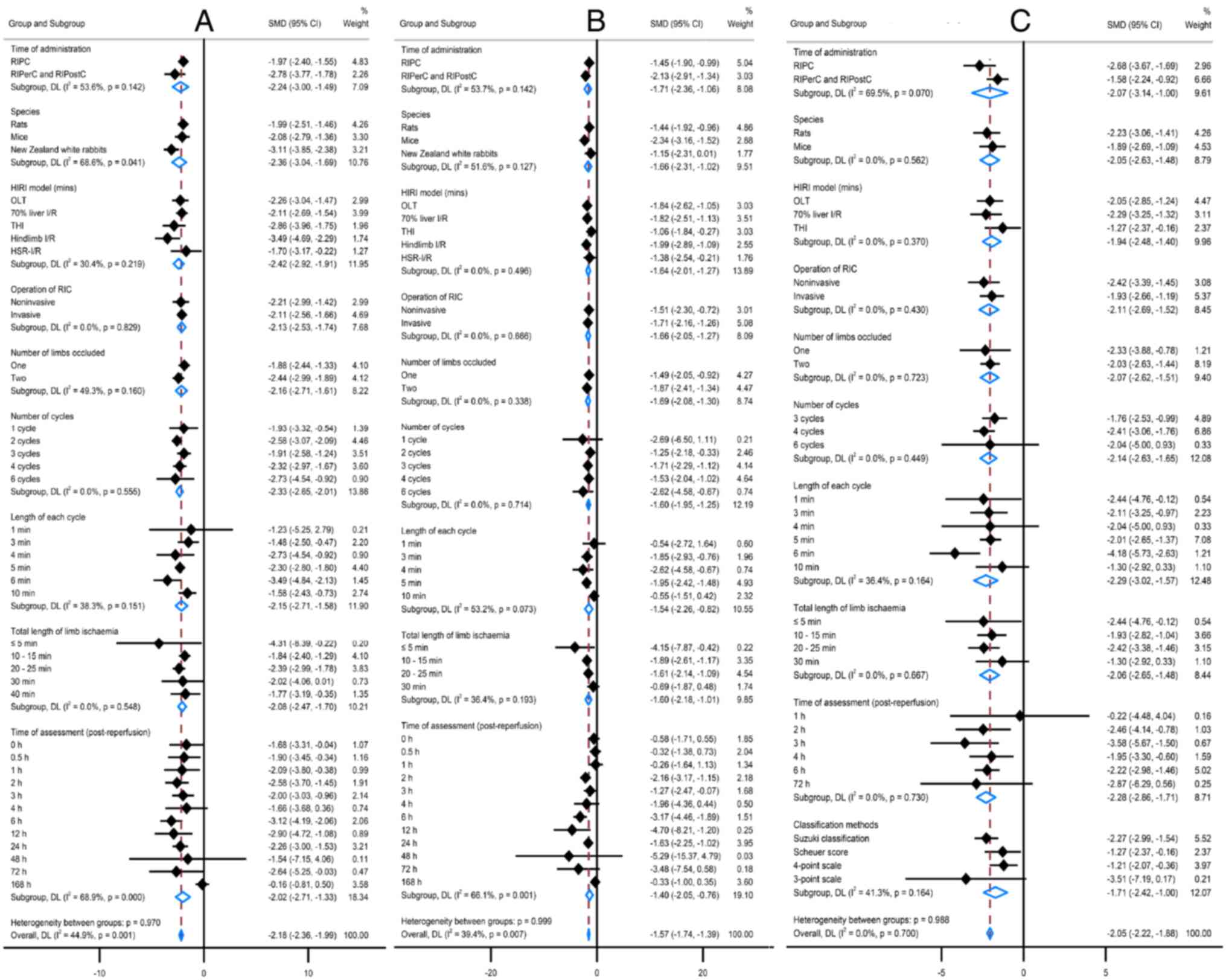
Effects of RIPC studies
The effects of RIPC protocol variables were assessed
against the primary outcomes changes using meta-regression
(Fig. 4). The effects of RIPC on
ALT and AST changes were significant if the duration of each cycle
was 6 min or the total duration of limb ischemia was 10-25 min,
with two peaks occurring at 2-3 or 6-12 h following reperfusion and
lasting up to 72 h. RIPC was most effective against ALT change in
New Zealand white rabbits and had no interactions in rats or mice
(P=0.008). Using one or two limbs of RIPC reduced ALT but using two
limbs was significantly improved than one limb [SMD (95%CI): -2.56
(-3.17, -1.96) vs. -1.16 (-2.17, -1.09); P=0.025]. In addition, the
effect of RIPC on the liver histopathological change was improved
in the OLT model (P=0.017), and there were no interactions between
RIPC and the liver histopathological score classification method
(P=0.156).
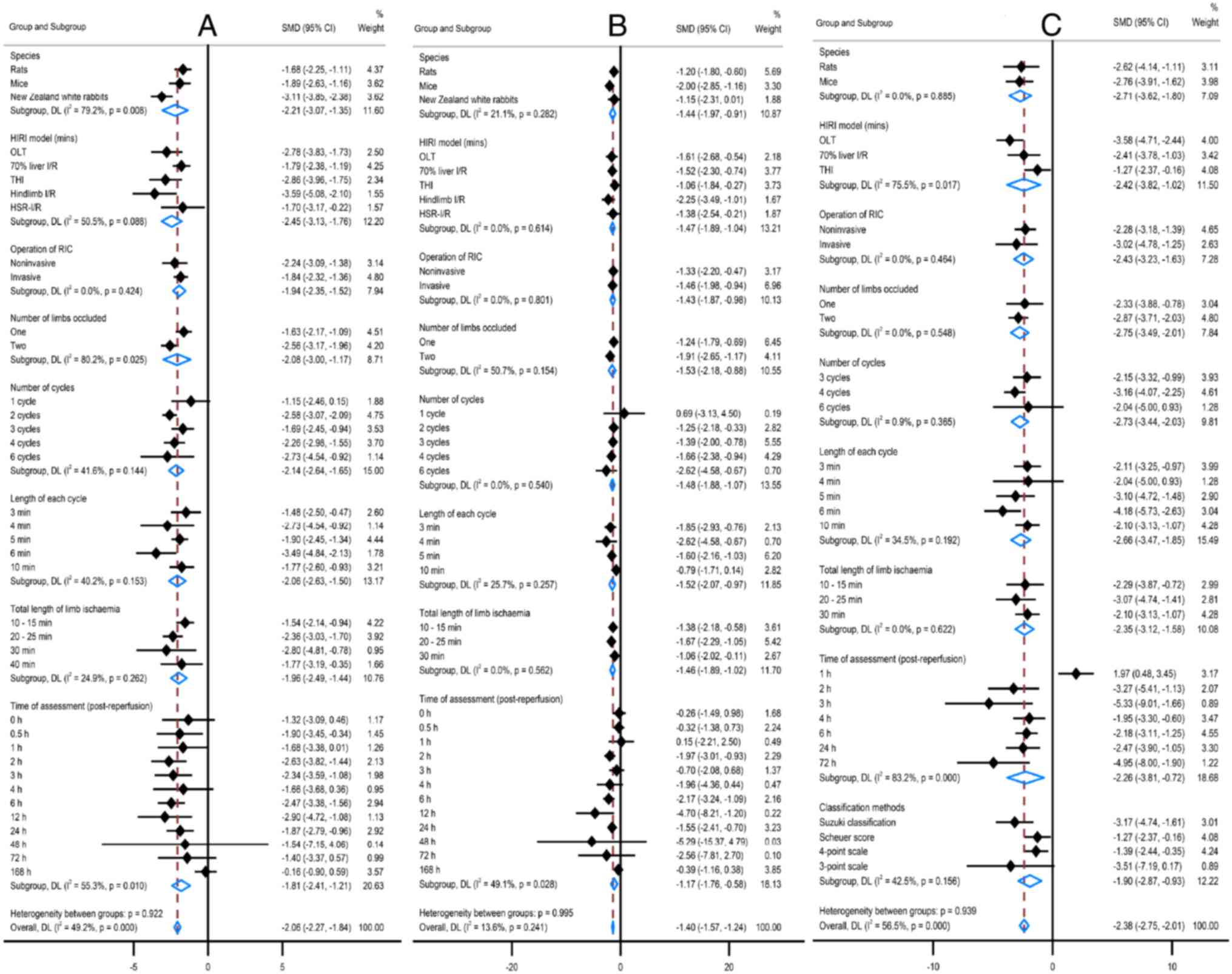
Effects of RIPerC/RIPostC studies
The effects of RIPerC/RIPostC protocol variables
were assessed against the primary outcomes changes using
meta-regression (Fig. 5). Similar
to the RIPC protocol, the protective effects against ALT and AST
changes revealed two peaks, but the first peak occurred earlier
(1-3 h post-reperfusion). The efficacies of RIPerC/RIPostC on ALT
and AST interacted with the RIC cycles, the most significant at one
cycle of RIC (P=0.002; P<0.001), but this animal model is much
fewer (n=24). Using one or two limbs of RIPerC/RIPostC reduced ALT
and AST, but using one limb was a significant improvement on two
limbs [ALT SMD (95%CI): -6.04 (-9.44, -2.65) vs. -2.25 (-3.25,
-1.24), P=0.036; AST SMD (95%CI): -5.41 (-8.90, -1.93) vs. -1.83
(-2.60, -1.05), P=0.049]. Notably, the protection was ineffective
if the duration of each cycle was 10 min or the total duration of
the limb occlusion was 30 min.
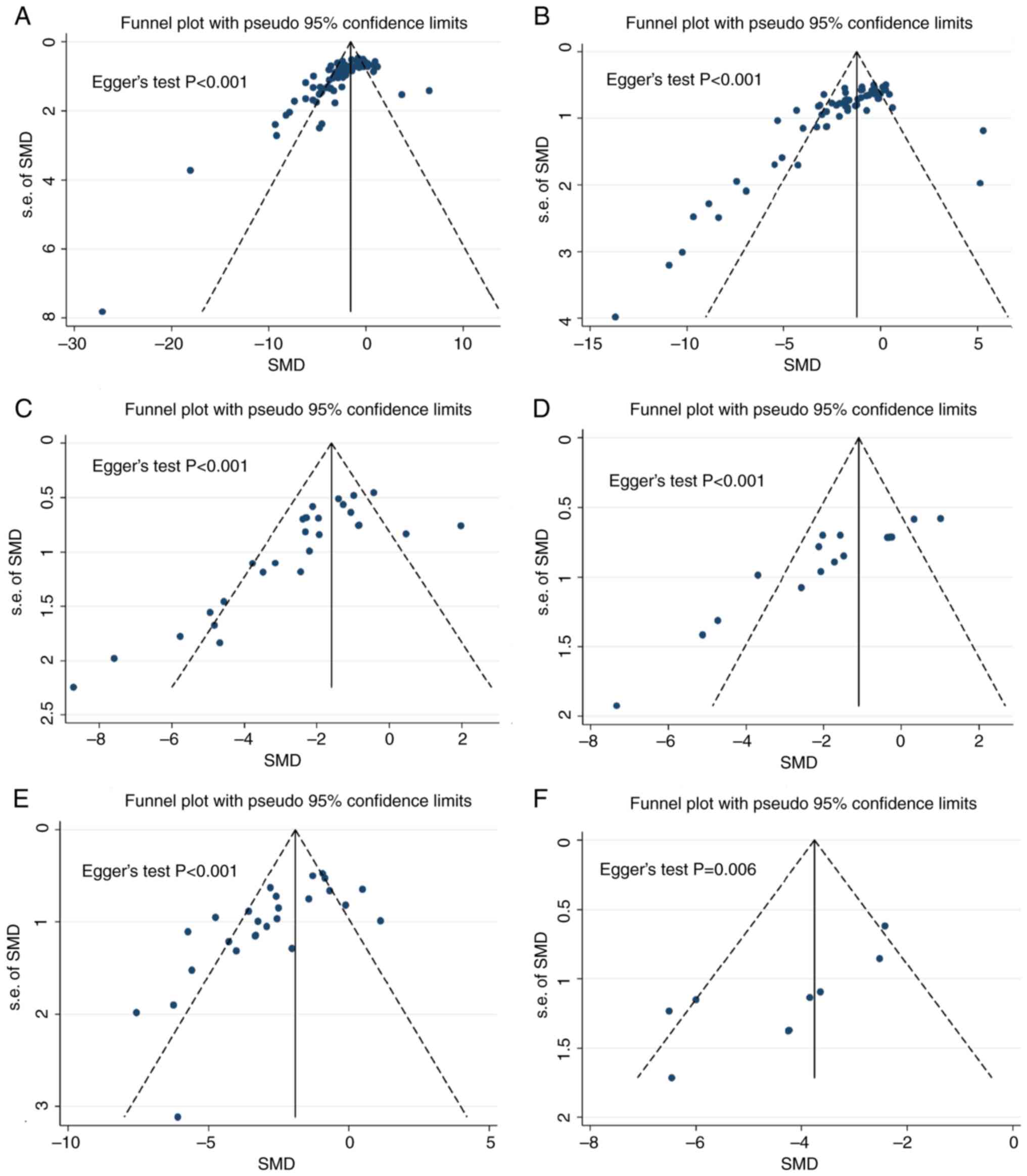
Publication bias
Notably, Begg's funnel plot (Fig. 6) shows the asymmetric patterns in
included studies, suggesting a possible publication bias in this
meta-analysis (All Begg's statistics P<0.05). Further analyzing
the sources of statistical heterogeneity revealed the presence of
interactions with respect to country. The protective effects of RIC
on ALT and AST changes were highest in Brazil and the impact on
liver histopathology was highest in Korea (Fig. S6).
Discussion
HIRI is a complex pathophysiological process, which
is essentially a series of inflammatory cascade reactions triggered
by the release of a large number of inflammatory mediators
following the activation of hepatic Kupffer cells, hepatic
sinusoidal endothelial cells and hepatic stellate cells, which may
lead to the postoperative liver dysfunction and partial residual
liver cell death, or even an increase of perioperative mortality
(46,47). Various strategies such as
pharmacological modifiers, physiological scavengers and physical
processes have been investigated to avoid the adverse effects of
HIRI following liver transplantation and liver resection (4,48). RIC
is one of these novel strategies, which can induce nonlethal stress
to the remote organs through transient ischemia, resulting in local
and systemic tolerance to IRI. There is too little clinical
evidence; however, the role of RIC in animal HIRI models has been
extensively studied. To the best of the authors' knowledge, there
have been no systematic reviews and meta-analyses related to this
topic.
The reviewers carefully set the primary outcomes to
ensure that this systematic review and meta-analysis of preclinical
studies can be applied to the clinics. Although there is still a
lack of a reliable endpoint that can effectively predict the
outcome of patients undergoing surgery, most ongoing HIRI clinical
trials focus on the markers of liver injury (ALT and AST) as
primary outcomes (2). Considering
this clinical setting, the present review briefly analyzed the
efficacy of RIC on HIRI in preclinical studies with ALT, AST and
liver histopathology as the primary outcomes and LDH, TNF-α, and
apoptosis index as secondary outcomes. The present systematic
review and meta-analysis of 39 articles (101 animal experiments,
1,061 animals) confirmed that RIC (RIPC or RIPerC/RIPostC) had a
powerful effect on improving ALT, AST and liver histopathology
changes in the preclinical liver I/R models. In all RIPerC/RIPostC
studies, ALT and AST changes appeared to be more efficacious than
RIPC in both rats and mice, in 70% of liver I/R models and using
one or two limbs and using invasive or non-invasive operations.
However, RIPC appeared to have a more potent effect on liver
histopathology change.
Significant statistical heterogeneities were present
in both RIPC and RIPerC/RIPostC groups and the meta-regression
subgroup analysis helped to explore the effects of these two
protocol variables on the changes in primary outcomes. Significant
interactions with species in RIC experiments showed that RIPC was
most effective in New Zealand white rabbits. However, the
RIPerC/RIPostC group lacked the New Zealand white rabbits' trial,
and both groups were equally effective in rats and mice. This
interspecies difference has raised concerns about treatment failure
when moving into clinical trials. A recent study (2) evaluating the beneficial effects and
applicability of RIPC in hepatectomy demonstrates that RIPC has
some short-term liver protection against HIRI during hepatectomy
but has limited improvement in clinical outcomes.
RIPerC/RIPostC studies revealed significant
interactions with the total duration of limb ischemia (defined as
the dose of RIC) and RIC was ineffective when the dose was 30 min.
The 40-min dose was not tested in the RIPerC/RIPostC group, but the
effect began to wane in the RIPC group. However, RIPC studies
showed no significant interactions with doses, suggesting the
existence of a dose therapeutic window with an optimal period
between 10 and 25 min. The number of limbs used for RIC may
somewhat reflect the dose. However, the present review showed that
RIPC was a significant improvement, compared with unilateral,
regarding ALT change on both sides, whereas RIPerC/RIPostC was the
opposite. Frustratingly, the reviewers gained no reasonable
explanation and heterogeneity or publication bias could not be
ruled out. In addition, none of the included studies involved a
repeat dose regimen (RIPC+RIPerC/RIPostC), and whether this
provides added benefit warrants further verification.
The mechanisms of RIC are still being explored and
may involve various inflammatory mediators, receptors, gene
expression and other links. Some studies have demonstrated that RIC
has two protective time windows (49-51).
The first protective time window (also known as the classical
protective window) occurs immediately after RIC and has a strong
effect. It lasts for 2-3 h and possibly relates to altering
endogenous substances (such as adenosine, bradykinin and nitric
oxide) produced during RIC. The second window of protection occurs
several hours after RIC, and the effect is weak, lasting 72-96 h.
This second window of protection may be related to the cell
signaling pathway and gene regulation after releasing endogenous
substances. In the present review, the two peak levels of RIC
action confirmed the protective time window effect of RIC, and the
first peak of RIPerC/RIPostC appeared earlier, indicating that it
may take effect more quickly.
Although the present meta-analysis provided rigorous
information on RIC's efficacy for HIRI, there were still some
limitations, suggesting that the results of all outcomes should be
interpreted with caution, needing more well-designed preclinical
and clinical studies. First, the majority of articles included in
this review studied healthy young male rodent models, which may not
accurately reflect clinical scenarios involving comorbidities. By
contrast, most patients were middle-aged and elderly with one or
more comorbidities that may inhibit the effects of RIC, such as
hypertension, diabetes, hepatitis B, fatty liver or cirrhosis.
Second, anesthesia during RIC implementation is another concern, as
propofol or sevoflurane has been reported to have liver protective
effects (52,53). All current clinical studies have
been conducted under propofol anesthesia or propofol combined with
inhalation anesthesia, which may interfere with the effects of RIC
and is also a hot topic of debate. Third, for the countries where
animal experiments were conducted in this study, the sources of
heterogeneity were significant. There was also a substantial risk
of publication bias, with a worrying tendency to overinterpret
positive results. Fourth, as shown in Table II, the number of experiments on
some models and species is small, and some experiments have limited
data, so it is difficult to fully consider all animal models and
species differences in meta-analysis. Therefore, some species or
models were simply merged, such as ‘All rats’ and ‘All OLT’ in
Table II and Fig. 3. However, from the statistical data
of the present review, these will not affect the main results of
this study. Fifth, the optimal frequency and repeated dosing
effects remain unclear. RIPC combined with RIPerC/RIPostC or a
daily repetition protocol would be a promising exploration.
Overall, the present review demonstrated promising preclinical
evidence for RIC in HIRI, but its clinical translation requires
addressing these limitations. However, it remains a comprehensive
review and probably the most accurate preclinical evidence of the
literatures to date.
In summary, RIC significantly alleviated HIRI in the
experimental models. RIPerC/RIPostC acted more quickly and affected
ALT and AST changes, whereas RIPC significantly affected liver
histopathology. RIC has a dose therapeutic window and the best
period is 10-25 min. However, given the significant statistical
heterogeneities and risk of publication bias, future studies using
repeated doses in animal models with comorbidities will generate
innovative ideas for its therapeutic applications.
Supplementary Material
Forest plot for ALT measurements. ALT,
alanine transaminase; RIC, remote ischemic conditioning.
Forest plot for AST measurements. AST,
aspartate transaminase; RIC, remote ischemic conditioning.
Forest plot for liver histopathology
measurements. RIC, remote ischemic conditioning.
Forest plots for secondary outcomes.
Changes in (A) LDH, (B) TNF-α and (C) apoptosis index. LDH, lactate
dehydrogenase; TNF-α, tumor necrosis factor-α; RIC, remote ischemic
conditioning.
Subgroup analysis of secondary
outcomes. Changes in (A) LDH, (B) TNF-α and (C) apoptosis index.
LDH, lactate dehydrogenase; TNF-α, tumor necrosis factor-α; RIC,
remote ischemic conditioning.
Subgroup analyses of all RIC studies
by country. Changes in (A) ALT, (B) AST and (C) liver
histopathology. RIC, remote ischemic conditioning; ALT, alanine
transaminase; AST, aspartate transaminase.
The search strategy.
Treatment effects of different factors
based on secondary outcomes.
Acknowledgements
Not applicable.
Funding
Funding: The present systematic review was funded by the Natural
Science Foundation of Yongchuan District, Chongqing, China (Ycstc;
grant no. 2020nb0229).
Availability of data and materials
Not applicable.
Authors' contributions
CT was responsible for conceptualization, funding
acquisition, data curation and writing the original draft. AW was
responsible for validation, data curation, software, supervision,
writing, reviewing and editing. YK was responsible for
conceptualization, validation, writing, reviewing and editing. Data
authentication is not applicable
Ethics approval and consent to
participate
The present review was exempted from an ethical
opinion by the Ethics Committee of Yongchuan Hospital of Chongqing
Medical University as it was a systematic review of the
literature.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tian C, Wang A, Huang H and Chen Y:
Effects of remote ischemic preconditioning in hepatectomy: A
systematic review and meta-analysis. BMC Anesthesiol.
24(118)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Q, Guo J, Chen HS, Blauenfeldt RA, Hess
DC, Pico F, Khatri P, Campbell BCV, Feng X, Abdalkader M, et al:
Remote ischemic conditioning with medical management or reperfusion
therapy for acute ischemic stroke: A systematic review and
meta-analysis. Neurology. 102(e207983)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stankiewicz R and Grąt M: Direct, remote
and combined ischemic conditioning in liver surgery. World J
Hepatol. 13:533–542. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weir P, Maguire R, O'Sullivan SE and
England TJ: A meta-analysis of remote ischaemic conditioning in
experimental stroke. J Cereb Blood Flow Metab. 41:3–13.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao W, Hausenloy DJ, Hess DC, Yellon DM
and Ji X: Remote ischemic conditioning: Challenges and
opportunities. Stroke. 54:2204–2207. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mukkala AN, Petrut R, Goldfarb R, Beroncal
EL, Leung CH, Khan Z, Ailenberg M, Jerkic M, Andreazza AC, Rhind
SG, et al: Augmented Parkin-dependent mitophagy underlies the
hepatoprotective effect of remote ischemic conditioning used prior
to hemorrhagic shock. Mitochondrion. 70:20–30. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li JH, Jia JJ, He N, Zhou XL, Qiao YB, Xie
HY, Zhou L and Zheng SS: Exosome is involved in liver graft
protection after remote ischemia reperfusion conditioning.
Hepatobiliary Pancreat Dis Int. 22:498–503. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou H, Li L, Sun H, Li H, Wu Y, Zhang X
and Zhang J: Remote ischemic preconditioning attenuates hepatic
ischemia/reperfusion injury after hemorrhagic shock by increasing
autophagy. Int J Med Sci. 18:873–882. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Niu Q, Sun W, Chen Q, Long Y, Cao W, Wen
S, Li A, Dong F and Shi H: Protective effects of ischemic
postconditioning on livers in rats with limb ischemia-reperfusion
via glycogen synthase kinase 3 beta (GSK-3β)/Fyn/Nuclear
receptor-erythroid-2-related factor (Nrf2) pathway. Med Sci Monit.
26(e923049)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choi EK, Jung H, Jeon S, Lim JA, Lee J,
Kim H, Hong SW, Jang MH, Lim DG and Kwak KH: Role of remote
ischemic preconditioning in hepatic ischemic reperfusion injury.
Dose Response. 18(1559325820946923)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koh WU, Kim J, Lee J, Song GW, Hwang GS,
Tak E and Song JG: Remote ischemic preconditioning and diazoxide
protect from hepatic ischemic reperfusion injury by inhibiting
HMGB1-induced TLR4/MyD88/NF-kappaB signaling. Int J Mol Sci.
20(5899)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Emontzpohl C, Stoppe C, Theißen A, Beckers
C, Neumann UP, Lurje G, Ju C, Bernhagen J, Tolba RH and Czigany Z:
The role of macrophage migration inhibitory factor in remote
ischemic conditioning induced hepatoprotection in a rodent model of
liver transplantation. Shock. 52:e124–e134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li DY, Liu WT, Wang GY and Shi XJ: Impact
of combined ischemic preconditioning and remote ischemic
perconditioning on ischemia-reperfusion injury after liver
transplantation. Sci Rep. 8(17979)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kambakamba P, Linecker M, Schneider M,
Kron P, Limani P, Tschuor C, Ungethüm U, Humar B and Clavien PA:
Novel benefits of remote ischemic preconditioning through
VEGF-dependent protection from resection-induced liver failure in
the mouse. Ann Surg. 268:885–893. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gomes PFM, Tannuri ACA, Nogueira TM,
Iuamoto LR, Paes VR, Coelho MCM, Gonçalves JO, Serafini S and
Tannuri U: Remote ischemic preconditioning is efficient in reducing
hepatic ischemia-reperfusion injury in a growing rat model and does
not promote histologic lesions in distant organs. Transplant Proc.
50:3840–3844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao Y, Zhou S, Wang F, Zhou Y, Sheng S, Qi
D, Huang JH, Wu E, Lv Y and Huo X: Hepatoprotective effects of limb
ischemic post-conditioning in hepatic ischemic rat model and liver
cancer patients via PI3K/ERK pathways. Int J Biol Sci.
14:2037–2050. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Czigany Z, Bleilevens C, Beckers C, Stoppe
C, Möhring M, Fülöp A, Szijarto A, Lurje G, Neumann UP and Tolba
RH: Limb remote ischemic conditioning of the recipient protects the
liver in a rat model of arterialized orthotopic liver
transplantation. PLoS One. 13(e0195507)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang RP, Jia JJ, Li JH, He N, Zhou YF,
Jiang L, Bai T, Xie HY, Zhou L and Sun YL: Mitofusin-2 mediated
mitochondrial Ca2+ uptake 1/2 induced liver injury in rat remote
ischemic perconditioning liver transplantation and alpha mouse
liver-12 hypoxia cell line models. World J Gastroenterol.
23:6995–7008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He N, Jia JJ, Li JH, Zhou YF, Lin BY, Peng
YF, Chen JJ, Chen TC, Tong RL, Jiang L, et al: Remote ischemic
perconditioning prevents liver transplantation-induced
ischemia/reperfusion injury in rats: Role of ROS/RNS and eNOS.
World J Gastroenterol. 23:830–841. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ruan W, Liu Q, Chen C, Li S and Xu J: Limb
remote ischemic preconditioning attenuates liver ischemia
reperfusion injury by activating autophagy via modulating PPAR-γ
pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 41:918–928.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Park MS, Joo SH, Kim BS, Lee JW, Kim YI,
Hong MK and Ahn HJ: Remote preconditioning on rat hepatic
ischemia-reperfusion injury downregulated bax and cleaved caspase-3
expression. Transplant Proc. 48:1247–1250. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Limani P, Linecker M, Oberkofler CE,
Barmettler G, Kaech A, Graf R, Humar B and Clavien PA: Remote
ischemic preconditioning: A novel strategy in rescuing older livers
from ischemia-reperfusion injury in a rodent model. Ann Surg.
264:797–803. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li DY, Shi XJ, Li W, Sun XD and Wang GY:
Ischemic preconditioning and remote ischemic preconditioning
provide combined protective effect against ischemia/reperfusion
injury. Life Sci. 150:76–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jia J, Li J, Jiang L, Zhang J, Chen S,
Wang L, Zhou Y, Xie H, Zhou L and Zheng S: Protective effect of
remote limb ischemic perconditioning on the liver grafts of rats
with a novel model. PLoS One. 10(e0121972)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guimarães Filho MA, Cortez E, Garcia-Souza
ÉP, de Melo Soares V, Moura AS, Carvalho L, de Araujo Maya MC and
Pitombo MB: Effect of remote ischemic preconditioning in the
expression of IL-6 and IL-10 in a rat model of liver
ischemia-reperfusion injury. Acta Cir Bras. 30:452–460.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Czigany Z, Turoczi Z, Kleiner D, Lotz G,
Homeyer A, Harsányi L and Szijártó A: Neural elements behind the
hepatoprotection of remote perconditioning. J Surg Res.
193:642–651. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Shen J, Xiong X, Xu Y, Zhang H,
Huang C, Tian Y, Jiao C, Wang X and Li X: Remote ischemic
preconditioning protects against liver ischemia-reperfusion injury
via heme oxygenase-1-induced autophagy. PLoS One.
9(e98834)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Uysal AI, Ocmen E, Akan M, Ozkardesler S,
Ergur BU, Guneli E, Kume T, Koca U and Togrul BU: The effects of
remote ischemic preconditioning and n-Acetylcysteine with remote
ischemic preconditioning in rat hepatic ischemia reperfusion injury
model. Biomed Res Int. 2014(892704)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Oberkofler CE, Limani P, Jang JH,
Rickenbacher A, Lehmann K, Raptis DA, Ungethuem U, Tian Y,
Grabliauskaite K, Humar R, et al: Systemic protection through
remote ischemic preconditioning is spread by platelet-dependent
signaling in mice. Hepatology. 60:1409–1417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Garab D, Fet N, Szabo A, Tolba RH, Boros M
and Hartmann P: Remote ischemic preconditioning differentially
affects NADPH oxidase isoforms during hepatic ischemia-reperfusion.
Life Sci. 105:14–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Costa FL, Yamaki VN, Gonçalves TB, Coelho
JV, Percário S and Brito MV: Combined remote ischemic
perconditioning and local postconditioning on liver
ischemia-reperfusion injury. J Surg Res. 192:98–102.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang M, Shen J, Feng B, Gui L, Chen Q,
Zhang B, Tang J and Li X: Remote ischemic preconditioning promotes
early liver cell proliferation in a rat model of small-for-size
liver transplantation. J Surg Res. 179:e245–e253. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Czigány Z, Turóczi Z, Ónody P, Harsányi L,
Lotz G, Hegedüs V and Szijártó A: Remote ischemic perconditioning
protects the liver from ischemia-reperfusion injury. J Surg Res.
185:605–613. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tapuria N, Junnarkar S, Abu-Amara M,
Fuller B, Seifalian AM and Davidson BR: Modulation of
microcirculatory changes in the late phase of hepatic
ischaemia-reperfusion injury by remote ischaemic preconditioning.
HPB (Oxford). 14:87–97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kanoria S, Glantzounis G, Quaglia A,
Dinesh S, Fusai G, Davidson BR and Seifalian AM: Remote
preconditioning improves hepatic oxygenation after ischaemia
reperfusion injury. Transpl Int. 25:783–791. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cao L, Yuan G, Wang Y, Chang Y, Xu J, Zou
D and Wei L: Limb ischemic preconditioning reduces rabbit hepatic
ischemia-reperfusion injury through inhibition the phosphorylation
of proteins in the mapk signal pathway in the late phase. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 37:591–557. 2012.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
38
|
Björnsson B, Winbladh A, Bojmar L,
Trulsson LM, Olsson H, Sundqvist T, Gullstrand P and Sandström P:
Remote or conventional ischemic preconditioning-local liver
metabolism in rats studied with microdialysis. J Surg Res.
176:55–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Abu-Amara M, Yang SY, Quaglia A, Rowley P,
de Mel A, Tapuria N, Seifalian A, Davidson B and Fuller B: Nitric
oxide is an essential mediator of the protective effects of remote
ischaemic preconditioning in a mouse model of liver
ischaemia/reperfusion injury. Clin Sci (Lond). 121:257–266.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang F, Birch SE, He R, Tawadros P, Szaszi
K, Kapus A and Rotstein OD: Remote ischemic preconditioning by
hindlimb occlusion prevents liver ischemic/reperfusion injury: The
role of high mobility group-box 1. Ann Surg. 251:292–299.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang BQ, Kan YF and Yang QH: The
protective effect of the limb ischemia preconditioning on the
hepatic injury related to NO/ET-1 system in rats. Zhongguo Ying
Yong Sheng Li Xue Za Zhi. 26:376–379. 2010.PubMed/NCBI(In Chinese).
|
|
42
|
Tapuria N, Junnarkar SP, Dutt N, Abu-Amara
M, Fuller B, Seifalian AM and Davidson B: Effect of remote ischemic
preconditioning on hepatic microcirculation and function in a rat
model of hepatic ischemia reperfusion injury. HPB (Oxford).
11:108–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lai IR, Chang KJ, Chen CF and Tsai HW:
Transient limb ischemia induces remote preconditioning in liver
among rats: The protective role of heme oxygenase-1.
Transplantation. 81:1311–1317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kanoria S, Jalan R, Davies NA, Seifalian
AM, Williams R and Davidson BR: Remote ischaemic preconditioning of
the hind limb reduces experimental liver warm ischaemia-reperfusion
injury. Br J Surg. 93:762–768. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gustafsson BI, Friman S, Wallin M, Heiman
J and Delbro DS: Effect of remote preconditioning on mild or severe
ischemia-reperfusion injury to rat liver. Transplant Proc.
38:2708–2709. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hirao H, Nakamura K and Kupiec-Weglinski
JW: Liver ischaemia-reperfusion injury: A new understanding of the
role of innate immunity. Nat Rev Gastroenterol Hepatol. 19:239–256.
2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gracia-Sancho J, Caparrós E,
Fernández-Iglesias A and Francés R: Role of liver sinusoidal
endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol.
18:411–431. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Nemeth N, Peto K, Magyar Z, Klarik Z,
Varga G, Oltean M, Mantas A, Czigany Z and Tolba R: Hemorheological
and microcirculatory factors in liver ischemia-reperfusion
injury-an update on pathophysiology, molecular mechanisms and
protective strategies. Int J Mol Sci. 22(1864)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kharbanda RK, Nielsen TT and Redington AN:
Translation of remote ischaemic preconditioning into clinical
practice. Lancet. 374:1557–1565. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yellon DM and Downey JM: Preconditioning
the myocardium: From cellular physiology to clinical cardiology.
Physiol Rev. 83:1113–1151. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kanoria S, Jalan R, Seifalian AM, Williams
R and Davidson BR: Protocols and mechanisms for remote ischemic
preconditioning: A novel method for reducing ischemia reperfusion
injury. Transplantation. 84:445–458. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Matsumi J and Sato T: Protective effect of
propofol compared with sevoflurane on liver function after
hepatectomy with Pringle maneuver: A randomized clinical trial.
PLoS One. 18(e0290327)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Benoit L, Dieu A, Foguenne M and
Bonaccorsi-Riani E: Experimental and clinical aspects of
sevoflurane preconditioning and postconditioning to alleviate
hepatic ischemia-reperfusion injury: A scoping review. Int J Mol
Sci. 24(2340)2023.PubMed/NCBI View Article : Google Scholar
|