Introduction
Sarcopenia, or the age-related loss of muscle mass
and strength, increases the risk of functional decline (1,2) and
dementia (3). As it results in poor
balance, sarcopenia is a major risk factor for falls in
community-dwelling older adults (4-6).
Such falls in the elderly can cause femoral neck and other
fractures, subsequently reducing their ability to carry out
activities with daily living (7,8).
Age-related sarcopenia is accompanied by a regression of myelinated
fibers (MFs) (9,10). Furthermore, electrophysiological
experiments show that a decline in peripheral nerve function
precedes the decline in muscle function during aging (11). These findings suggest that impaired
balance and an increased risk of falls may be due to peripheral
nerve degeneration, in addition to sarcopenia.
Histological studies of lumbar and cervical dorsal
root ganglia (DRG), obtained from 12 human autopsies (age range
60-70 years) of individuals without associated diseases of the
central or peripheral nervous system, have reported a 35% reduction
in the number of neurons relative to those obtained from autopsies
of individuals aged <10 years (12). Additionally, the number of fibers in
the peroneal nerve with diameters >8 µm decreased with the age
of autopsy subjects aged 17-72 years (13). In a study of DRG in the cervical and
lumbar spinal cord of 12-week and 120-week-old rats, the latter
exhibited a 12% decrease in the total number of neurons and a 16%
decrease in the percentage of MFs in cross-sectional scores,
indicating age-related loss of MFs and atrophy (14). Therefore, it appears that selective
atrophy and loss of MFs in peripheral nerves are associated with
aging. In a recent study, the tibial nerves of 20-, 70-, and
97-week-old rats, which had been continuously kept in the same
environment, were examined histologically. MF axon diameter and
myelin sheath thickness in the 97-week-old physically inactive rats
were significantly reduced relative to that in the 20- and
70-week-old rats. However, the 70-week-old rats showed only a mild
decrease in MF axon diameter and myelin sheath thickness relative
to 20-week-old rats, with no other pathological deformities
(15). Furthermore, in a previous
cross-sectional study, which examined the morphology of the tibial
nerve of 24- to 108-week-old mice (physical activity or lack
thereof was unclear), MF morphology remained stable until 48 weeks,
with only slight changes between 48 and 80 weeks. Moreover, the MF
morphology showed significant fiber loss decreased size, and
increased irregular shape from 80 weeks to 108 weeks (9,16).
Based on these histological and morphological findings in rodents,
a marked progression of atrophy and degeneration of axons and
myelin sheaths is predicted between ~70 and 80 weeks of age.
In an electrophysiological study of age-related
changes in human peripheral nerves, action potential amplitudes of
sensory nerves were reduced only in the peroneal (-73%) and median
(-38%) nerves in the older adult group (63-80 years) relative to
the young adult group (21-29 years). By contrast, there was a
marked reduction in conduction velocity and action potential
amplitude in both motor and sensory nerves in the group of
individuals older than 80 years (17). In several aging studies involving
mice, increased myelin sheath thickness, number of MFs with large
diameters, and sensory nerve conduction velocities were observed in
mice up to the age of 48 weeks. This was accompanied by slight
reductions in these parameters in mice aged 48-80 weeks and marked
decrements in mice >80 weeks (9,16,18).
Thus, age-related dysfunction of MFs in peripheral nerves is
associated with histological degeneration and induces a delayed
onset of lower limb muscle activity and reduced action potentials
during postural recovery in response to postural disturbance.
Moreover, biochemical signaling of age-related degeneration in
peripheral nerves is hypothesized to precede the histological and
physiological changes in peripheral nerves associated with
aging.
The ubiquitin-proteasome system (UPS) (19) or autophagy (20,21) is
a major proteolytic mechanism. Defects in these mechanisms are
known to be involved in neurodegeneration. When injured or unfolded
proteins in abnormal conformations accumulate in the endoplasmic
reticulum (ER), the UPS induces either protein repair, degradation,
or apoptosis as a stress avoidance mechanism known as the ER stress
response (22). Within the ER,
damaged and unfolded proteins undergo ubiquitination and are
subsequently returned from the ER to the cytoplasm, where they are
transported to proteasomes for degradation (23). Autophagy also degrades misfolded
protein aggregates in defective cellular organelles and lysosomes,
a process that is part of the ER stress response (20,21).
Under sustained or excessive ER stress that cannot be regulated by
UPS or autophagy, cells eventually undergo apoptosis (24). Thus, UPS, autophagy and apoptosis,
which are all processes of the ER stress response, are important
for processing defective proteins and maintaining protein
homeostasis. Studies in Drosophila show that the expression
of UPS and autophagy-related factors in the proteolytic system
declines, and ubiquitinated proteins (defective proteins)
accumulate in the brain with age (25). In rodent and human cells,
proteolytic function of lysosomes declines with age and subsequent
impairment of autophagy flow exacerbates cellular damage leading to
the development of age-related diseases (26-28).
However, the age at which the decline in UPS and autophagy is
initiated is not clear.
Increased age-related neuronal damage, unfolded
proteins and apoptosis are associated with oxidative stress and
free radical accumulation (29).
Proteasome activity, which protects proteins, DNA and lipids from
oxidative stress in spinal cord neurons, is markedly reduced in
rats after middle age (30).
Additionally, structural defects in axons, myelin and cell bodies
of motoneurons in 80-week-old model mice were associated with
increased oxidative stress and defects observed with normal aging
in 120-week-old wild-type mice (31). Taken together, these findings
suggest that age-related and ER stress-related inhibition of
UPS/autophagy, promotion of apoptosis and increased oxidative
stress promote neurodegeneration. However, the biochemical
mechanisms underlying the regulation of UPS, autophagy and
apoptosis during aging remain unclear.
The present study aimed to determine how the
promotion or inhibition of ER stress-related UPS, autophagy and
apoptotic pathways involved in neurodegeneration is altered during
aging. Specifically, the expression levels of signaling-related
factors of the ER stress response were analyzed in the tibial nerve
of rats aged 20, 50, 70, 90 and 105 weeks.
Materials and methods
Animals
A total of 44 male Wistar rats (mean weight,
330.2±9.7 g) (Japan SLC, Shizuoka, Japan) acquired at 10 weeks of
age were housed continuously until they were 105 weeks old. They
were randomly enrolled into the study at the following age groups:
20 weeks (n=11), 50 weeks (n=12), 70 weeks (n=12), 90 weeks (n=11)
and 105 weeks (n=8). The rats were housed in plastic cages (two
individuals per cage) and kept at a temperature of 22±2˚C with
humidity of 40-60%. A 12/12-h light-dark cycle was maintained with
lights on at 8:00 a.m. and off at 8:00 p.m., and they had access to
food and water ad libitum. The present study was approved by
the Animal Care and Use Committee of Kyoto Tachibana University
(approval no. 19-10; Kyoto, Japan) and was performed in accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (National Research Council,
1996).
Collection and preservation of nerve
and skeletal muscle samples
Randomly selected rats at each week of age were
selected from the five age groups and anesthetized by isoflurane
inhalation (2.0%), followed by intraperitoneal injection of sodium
pentobarbital (15 mg/kg) and heparin (10,000 IU/l) (15). The soleus (Sol) and extensor
digitorum longus (EDL) muscles of the right hindlimb were
extracted. Subsequently, saline was refluxed caudally at 37˚C from
the abdominal aorta, and the nerve segment between the sciatic
L4-L5 isthmus of the right hindlimb and the distal tibial nerve
branching to the medial and lateral plantar nerves was removed
(15). The rats were immediately
euthanized via intraperitoneal administration of 120 mg/kg of
sodium pentobarbital, and nerve samples were immediately immersed
or frozen in isopentane cooled with liquid nitrogen for ~10 sec.
From the aforementioned, Sol, EDL and tibial nerve samples were
taken as part of the terminal procedure and animals were euthanized
after sample collection. The samples were stored at -80˚C until
further use.
Tibial nerve sample preparation and
assay for total protein volume
A portion of the tibial nerve from the distal end of
a frozen peripheral nerve sample of the right hindlimb was cut. It
weighed 10-12 mg. One cut sample was lysed in 500 µl lysis buffer
(cat. no. sc-24948A; RIPA Lysis Buffer System; Santa Cruz
Biotechnology, Inc.) supplemented with 1 mM EDTA and 1 ml protease
inhibitor cocktail and homogenized by sonication under ice-cold
conditions. Lysed samples were centrifuged at 15,000 x g for 15 min
at 4˚C and the total protein content in the supernatant was
quantitated using the Bicinchoninic Acid Protein Assay (cat. no.
TQ300A; Takara Bio, Inc.). The absorbance of each sample was
observed at 560 nm using a microplate reader (Multiskan JX; Thermo
Fisher Scientific, Inc.) to estimate total protein concentration.
Each sample was diluted 2-fold to prepare the sample solution for
electrophoresis and was denatured in sample buffer (0.5 M Tris HCl
(pH 6.8), 20% glycerol, 1% β-mercaptoethanol, 1% SDS and 0.02%
bromophenol blue) by boiling at 95˚C for 5 min and stored at -20˚C
until subsequent assays.
Western blotting
Proteins were separated by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis. For electrophoresis,
20 µl of sample solution (protein 1.60 µg/µl) was loaded into each
well and electrophoresis was performed at 200 V for 80 min. The
separated proteins were transferred onto a polyvinylidene fluoride
(PVDF) membrane using a wet blotter (cat. no. 1704070JA; Criterion
Blotter with Plate Electrodes; Bio-Rad Laboratories, Inc.) at 50 V
for 80 min. After blotting, the PVDF membranes were washed with
TBST (25 mM Tris HCl, 0.15 M NaCl, 0.1% Tween-20, pH 7.4) for 3
min; this was followed by washing with deionized water for 3 min
with gentle shaking. PVDF membranes were incubated with Ponceau S
(cat. no. SP-4030; Apro Science Group; https://apro-s.com/) by gentle shaking for 5 min,
followed by washing with deionized water for 2 min to ensure clear
protein staining. Stained PVDF membranes were scanned, and images
were captured using a personal computer. To decolorize protein
staining, the PVDF membranes were incubated with 0.1 M NaOH by
gentle shaking for 30 sec, followed by washing with TBST for 3 min.
The PVDF membranes were incubated with a blocking solution (cat.
no. T7132A; Western BLoT Blocking Buffer; Takara Bio, Inc.)
thereafter for 1 h at room temperature. Subsequently, the membranes
were washed gently with TBST for 5 min. Following this, the
membranes were probed overnight (at 4˚C) with primary antibodies
prepared in blocking solution.
In the present study, focus was addressed on the
age-dependent changes in the expression of proteins related to the
ER stress response in the tibial nerves. In a previous longitudinal
study, histological and morphological analysis of the tibial nerves
at 20, 70 and 97 weeks of age revealed only slight atrophy and
degeneration of axons and myelin sheaths at up to 70 weeks, with a
marked progression of degeneration after 70 weeks (15). Based on this finding, it was
hypothesized in the present study that age-related changes in the
expression of ER stress response-related proteins may be associated
with these histological and morphological changes. However, after
70 weeks, these repair mechanisms are expected to decline, leading
to the accumulation of damaged proteins and subsequent apoptosis.
Specifically, protein repair mechanisms (for example the
ubiquitin-proteasome system) and the ER stress response appear to
be active and suppress these degenerative changes from 20 to 70
weeks of age. However, the function of these repair mechanisms
declines above 70 weeks of age, after which denatured proteins
accumulate and apoptosis is predicted to eventually progress.
Therefore, the present study focused on changes in the expression
of the following proteins associated with the ER stress
response.
Primary antibodies against the following were used:
anti-ER stress-related UPS and autophagy factors, apoptotic
factors, new protein synthesis inhibitors, repair factors and
inflammatory cytokines. The following proteins were employed as
biomarkers: splicing X-box binding protein 1 (XBP1s) for UPS;
Beclin-1 (Becn1) for autophagy; caspase-3 (Casp3) for apoptosis;
eukaryotic initiation factor as novel protein synthesis inhibitor
factor-2α (eIF2α); immunoglobulin heavy chain binding protein or
glucose regulatory protein 78 (BiP/GRP78), protein disulfide
isomerase (PDI) and brain-derived neurotrophic factor (BDNF) for
repair; and interleukin-6 (IL6) as the inflammatory cytokine.
The following primary antibodies were used: XBP1s
rabbit monoclonal antibody (1:1,000; cat. no. 82914; Cell Signaling
Technology, Inc.), eIF2α rabbit monoclonal antibody (1:1,000; cat.
no. 9079; biotinylated; Cell Signaling Technology, Inc.), Becn1
rabbit polyclonal antibody (1:1,000; cat. no. 11306-1-AP;
Proteintech Group, Inc.), Casp3 rabbit polyclonal antibody (1:500;
cat. no. GTX110543; GeneTex, Inc.), BiP/GRP78 rabbit polyclonal
antibody (1:1,000; cat. no.11587-1-AP; Proteintech Group, Inc.),
PDI rabbit polyclonal antibody (1:1,000; cat. no. 2446; Cell
Signaling Technology, Inc.), BDNF mouse monoclonal antibody
(1:1,000; cat. no. ab203573; Abcam) and IL6 rabbit monoclonal
antibody (1:1,000; cat. no. ab259341; Abcam). After probing with
the primary antibodies, PVDF membranes were washed three times (10
min/time) with TBST. They were subsequently incubated for 1 h at
room temperature with the following secondary antibodies: goat
anti-rabbit IgG HRP-conjugated antibody (cat. no. SA 00001-2;
Proteintech Group, Inc.) at a dilution of 1:8,000 in blocking
buffer for XBP1s, Casp3, PDI and IL6; and at 1:10,000 for Becn1;
streptavidin HRP-conjugated antibody (cat. no. 3999; Cell Signaling
Technology, Inc.) at a dilution of 1:10,000 in blocking buffer for
eIF2α; and goat anti-mouse IgG HRP-conjugated antibody (cat. no.
SA00001-1; Proteintech Group, Inc.) at a dilution of 1:8,000 in
blocking buffer for BDNF. Then, the PVDF membranes were washed
three times (10 min/time) with TBST. Following this, the membranes
were sealed within a plastic wrap containing chemiluminescence
detection reagent (cat. no. RPN2232; Amersham ECL Prime; Cytiva)
and allowed to react for 30 sec for the detection of target
proteins. The chemiluminescence images of each target protein were
acquired using a chemiluminescence imaging system (LumiCube;
Liponics; http://www.lipopartners.jp/index.html) and captured on
a personal computer using EOS Utility software (version 2.14; Canon
Inc.) with an exposure time of 20 sec. Images captured on the
personal computer were converted from 16 to 8 bit using ImageJ
software (version 1.53; National Institutes of Health) and
inverted. Luminescent bands corresponding to target proteins were
marked with rectangles, and their chemiluminescence signal
intensities were calculated as the optical density (OD).
Additionally, the OD values of the target proteins were calculated
as relative values by dividing them by the OD value for all the
proteins observed in the Ponceau S staining image (32,33).
Statistical analysis
Physical characteristics (body weight, Sol and EDL
weights) data for the five groups of rats are indicated as the mean
± standard error. Relative amounts of each target protein in each
group were determined by setting the median value of the
20-week-old group as 1. Relative amounts of target proteins are
shown as medians and interquartile ranges; comparisons of physical
characteristics of the five groups of rats were made using one-way
analysis of variance (ANOVA). Comparisons of the relative amounts
of each target protein in the five groups were made using the
Kruskal-Wallis test. Multiple comparisons using Tukey-Kramer post
hoc test and Steel-Dwass test were performed when significant
effects were identified by one-way ANOVA and Kruskal-Wallis tests,
respectively. All statistical analyses were performed using R
version 4.0.0 (R Foundation for Statistical Computing). P<0.05
was considered to indicate a statistically significant
difference.
Results
Physical characteristics
The physical characteristics of the five
experimental groups of rats in the present study are compared in
Table I. ANOVA showed that body
mass was affected by age (P<0.01). Subsequent results of
multiple comparisons showed that the body masses of the four older
groups of rats were significantly higher than those of the
20-week-old rats (P<0.01 for all four groups). Sol muscle masses
of the five groups indicated no differences by age. The EDL muscle
mass of the five groups was affected by age (P<0.01). Both the
50- and 70-week-old groups of rats had significantly greater mass
than those in the 20-, 90- and 105-week-old groups (P<0.01).
 | Table IPhysical characteristics of rats aged
20, 50, 70, 90, and 105 weeks. |
Table I
Physical characteristics of rats aged
20, 50, 70, 90, and 105 weeks.
| | Groups | |
|---|
| Parameters | 20-week-old
(n=11) | 50-week-old
(n=12) | 70-week-old
(n=12) | 90-week-old
(n=11) | 105-week-old
(n=8) | F | P-value in
ANOVA |
|---|
| Body mass (g) | 456.6±7.1 |
561.5±8.6a |
602.9±12.2a |
563.0±32.8a |
599.6±39.0a | F (4, 49) 7.76 | P<0.01 |
| Sol muscle mass
(mg) | 168.7±6.6 | 190.3±8.7 | 183.9±10.2 | 180.3±8.8 | 180.6±7.3 | F (4, 49) 0.86 | Not
significant |
| EDL muscle mass
(mg) | 210.5±6.2 |
244.8±5.7a |
248.0±5.8a |
205.1±7.2b,c |
198.8±10.3b,c | F (4, 49) 11.5 | P<0.01 |
Comparison of the relative amounts of
ER stress-related degradation factors by age
Relative expression of XBP1s differed significantly
among the five age groups of the rats (χ2=24.4, df=4,
P<0.01) (Fig. 1A). XBP1s
expression in the 105-week-old group (0.28, 0.15-0.31) was
significantly lower than that in the 20- (1, 0.88-1.64)
(P<0.01), 50- (2.5, 1.06-3.33) (P<0.01), 70- (1.22,
0.95-1.42) (P<0.01) and 90-week-old groups (1.1, 0.78-1.54)
(P<0.01) (Fig. 1A).
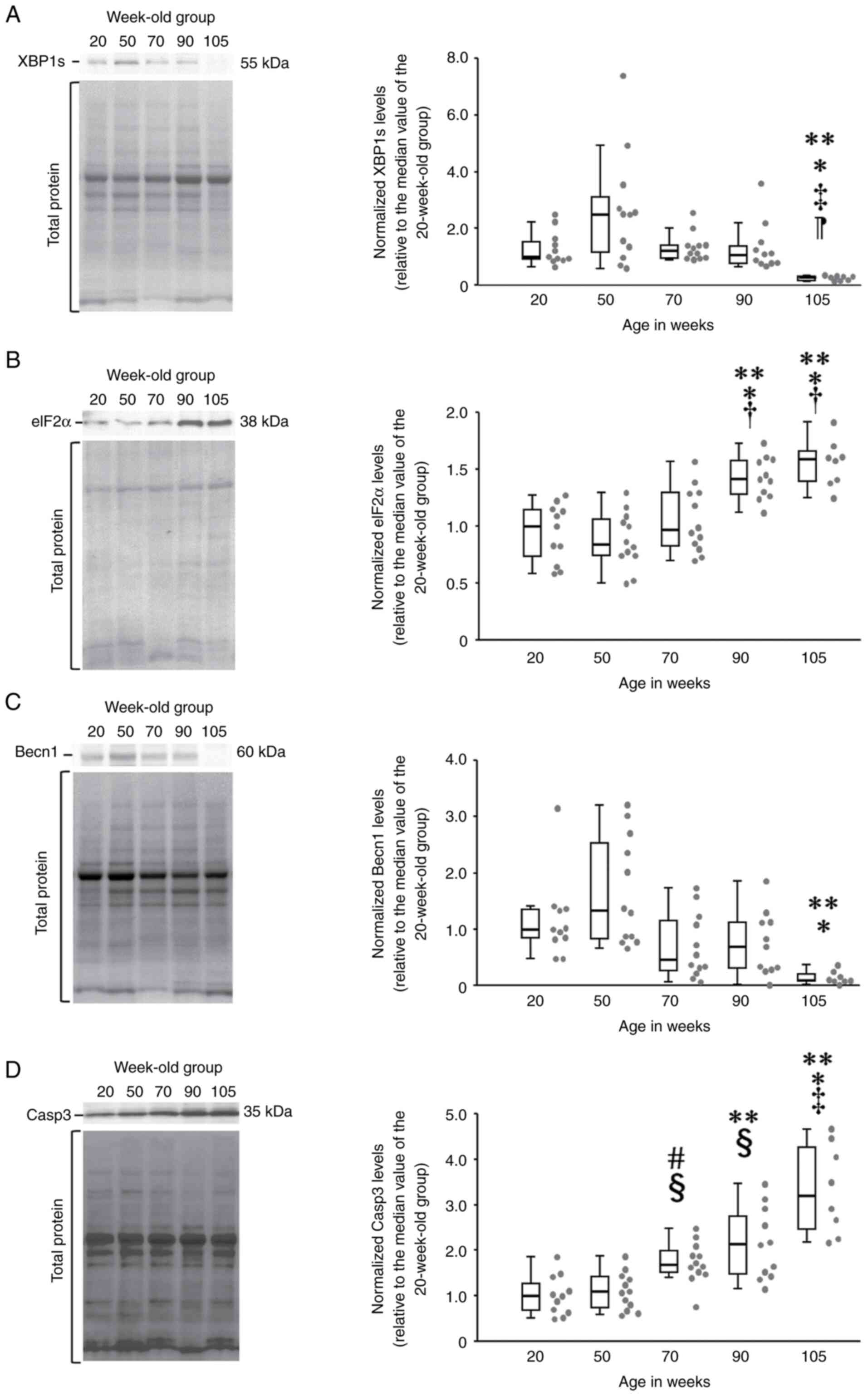 | Figure 1Comparison of relative concentrations
of ER stress-related degradation proteins in aging rats. (A) In
multiple comparisons (Steel-Dwass test), relative expression of
XBP1s was significantly lower in rats in the 70-week-old and older
groups (boxplot diagram, right). In the chemiluminescence image of
XBP1s (left), the signal band of 50-week-old rats was the darkest,
while those for rats older than 70 weeks were thinner. (B) Relative
eIF2α expression in the Steel-Dwass test: rats in the 90- and
105-week-old groups showed significantly greater expression
relative to those in other groups (boxplot diagram, right).
Chemiluminescence images of eIF2α (left) showed that the signal
bands of 90- and 105-week-old rats were significantly darker than
those of the other three groups. (C) Relative Becn1 expression in
the Steel-Dwass test: rats in the 105-week-old group showed
significantly lower expression relative to those in other groups
(boxplot diagram, right). The signal band of the 105-week-old rats
was thinner than those of the other 4 groups, indicating lower
relative expression. (D) Relative Casp3 expression in the
Steel-Dwass test: relative Casp3 expression was significantly
higher in rats in the 70-week-old and older groups (boxplot
diagram, right). In the chemiluminescence image for Casp3 (left),
the signal band gradually darkened with increasing age. The gray
dots on the right side of the box-and-whisker plots indicate the
dot plot for each group. **P<0.01 vs. 20-week-old
group; *P<0.01 vs. 50-week-old group;
‡P<0.01 vs. 70-week-old group; ¶P<0.01
vs. 90-week-old group; #P<0.05 vs. 20-week-old group;
§P<0.05 vs. 50-week-old group; and
†P<0.05 vs. 70-week-old group. ER, endoplasmic
reticulum; XBP1s, X-box binding protein 1; eIF2α, eukaryotic
initiation factor as novel protein synthesis inhibitor factor-2α;
Becn1, Beclin-1; Casp3, caspase. |
Relative expression of eIF2α differed significantly
among the five age groups of the rats (χ2 =30.3, df=4,
P<0.01) (Fig. 1B). eIF2α
expression in the 105-week-old group (1.59, 1.39-1.68) was
significantly higher than that in the 20- (1, 0.64-1.15)
(P<0.01), 50- (0.84, 0.74-1.08) (P<0.01) and 70-week-old
groups (0.97, 0.81-1.29) (P<0.05) (Fig. 1B). eIF2α expression in the
90-week-old group (1.43, 1.27-1.59) was significantly higher than
that in the 20- (P<0.01), 50- (P<0.01) and 70-week-old
(P<0.05) groups (Fig. 1B).
Relative expression of Becn1 differed significantly
among the five age groups of the rats (χ2=23.6, df=4,
P<0.01) (Fig. 1C). Becn1
expression in the 105-week-old group (0.1, 0.08-0.23) was
significantly lower than that in the 20- (1, 0.83-1.37) (P<0.01)
and 50-week-old groups (1.34, 0.80-2.62) (P<0.01) (Fig. 1C). Relative expression of Becn1 in
the 70- (0.46, 0.25-1.2) (P<0.1) and 90-week-old (0.7,
0.29-1.13) (P<0.1) groups also tended to be lower than that in
the 50-week-old group and higher than that in the 105-week-old
group.
Relative expression of Casp3 differed significantly
among the five age groups of the rats (χ2=32.8, df=4,
P<0.01) (Fig. 1D). Casp3
expression in the 105-week-old group (3.21, 2.36-4.37) was
significantly higher than that in the 20- (1, 0.64-1.43)
(P<0.01), 50- (1.09, 0.71-1.43) (P<0.01), and 70-week-old
groups (1.69, 1.49-2.05) (P<0.01) (Fig. 1D). In addition, Casp3 expression in
the 90-week-old group (2.13, 1.44-2.93) was significantly higher
than that in the 20- (P<0.01) and 50-week-old groups (P<0.01)
(Fig. 1D). Furthermore, Casp3
expression in the 70-week-old group was significantly higher than
that in the 20- (P<0.05) and 50-week-old groups (P<0.05)
(Fig. 1D).
Comparison of relative amounts of ER
stress-related repair factors and inflammatory cytokines by
age
Relative expression of BiP/GRP78 differed
significantly among the five age groups of the rats
(χ2=18.9, df=4, P<0.01) (Fig. 2A). BiP/GRP78 expression in the
105-week-old group (0.65, 0.2-1.02) was significantly lower than
that in the 50- (1.2, 1.03-1.33) (P<0.05) and 70-week-old groups
(1.11, 0.98-1.28) (P<0.05) (Fig.
2A). In addition, BiP/GRP78 expression in the 90-week-old group
(0.88, 0.82-1.02) was also significantly lower than those in the
50-week-old group (P<0.01) (Fig.
2A).
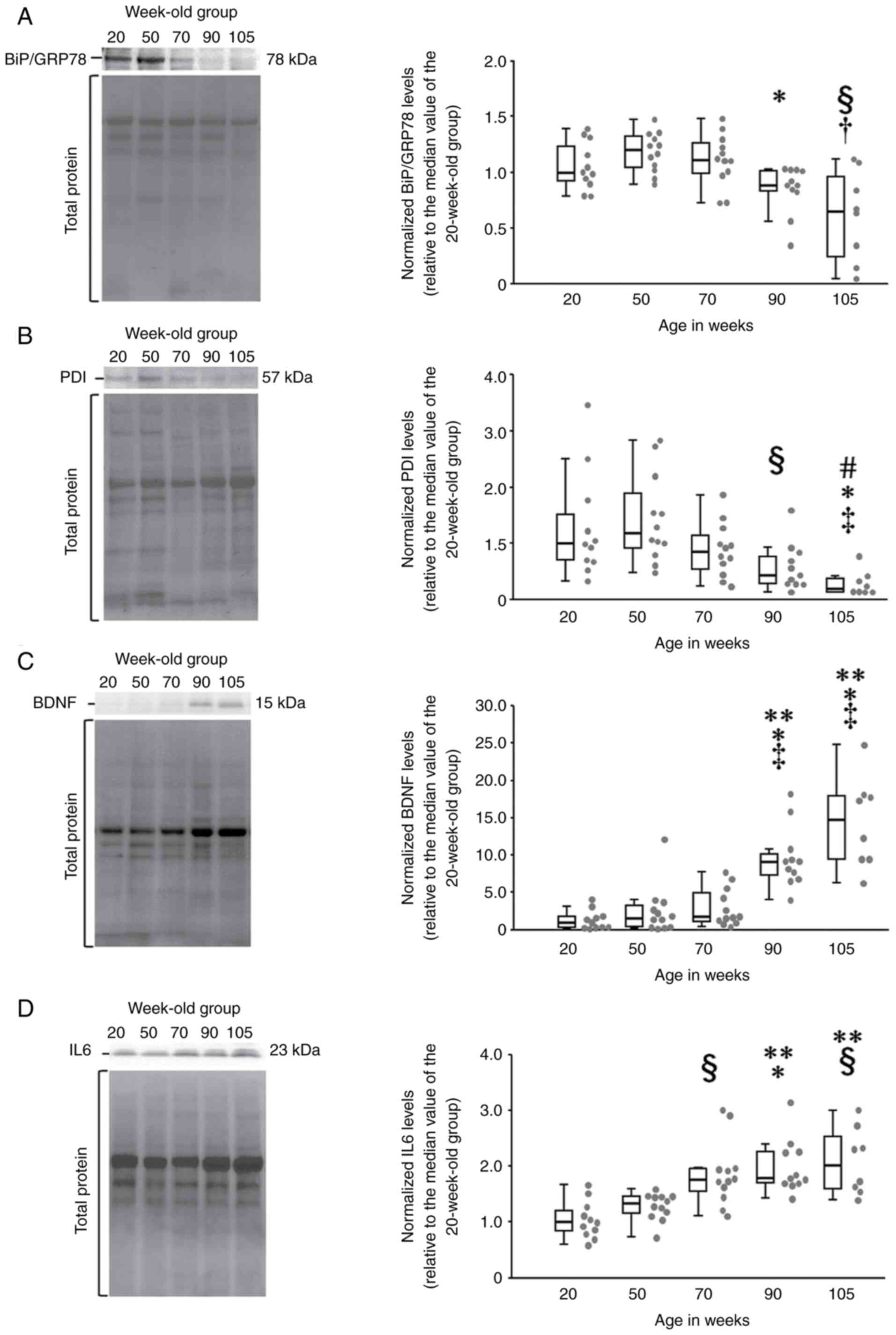 | Figure 2Comparison of the relative expression
of ER stress-related repair proteins and the inflammatory cytokine
IL6 in aging rats. (A) Multiple comparisons of the relative
expression of BiP/GRP78 using the Steel-Dwass test showed
significant reductions in rats in the 90- and 105-week-old groups
(box plot diagram, right). In addition, the chemiluminescence image
of BiP/GRP78 (left) shows that the signal bands of the 90- and
105-week-old rats are thinner than those of rats of other ages. (B)
Relative PDI expression in the Steel-Dwass test: rats in the 90-
and 105-week-old groups showed significantly lower expression
relative to rats in the other groups (box plot diagram, right). (C)
Relative BDNF expression in the Steel-Dwass test: rats in the 90-
and 105-week-old groups had significantly higher expression
relative to those in other groups (boxplot diagram, right). In the
chemiluminescence image of BDNF (left), the signal bands of the 90-
and 105-week-old rats are significantly darker than those of the
other three age groups, indicating higher relative expression. (D)
Relative IL6 expression in the Steel-Dwass test: rats after 70
weeks of age showed significantly higher IL6 expression (box plot
diagram, right). Moreover, the chemiluminescence image of IL6
(left) shows that the signal band became progressively darker with
increasing age. The gray dots on the right side of the
box-and-whisker plots indicate the dot plot for each group.
**P<0.01 vs. 20-week-old group; *P<0.01
vs. 50-week-old group; ‡P<0.01 vs. 70-week-old group;
#P<0.05 vs. 20-week-old group; §P<0.05
vs. 50-week-old group; and †P<0.05 vs. 70-week-old
group. ER, endoplasmic reticulum; IL, interleukin; BiP/GRP78,
immunoglobulin heavy chain binding protein or glucose regulatory
protein 78; PDI, protein disulfide isomerase; BDNF, brain-derived
neurotrophic factor. |
Relative expression of PDI differed significantly
among the five age groups of the rats (χ2=22.6, df=4,
P<0.01) (Fig. 2B). PDI
expression in the 90-week-old group (0.43, 0.28-0.84) was
significantly lower than that in the 50-week-old group (1.18,
0.85-2.05) (P<0.05) (Fig. 2B).
PDI expression in the 105-week-old group (0.19, 0.14-0.40) was also
significantly lower than that in the 20- (1, 0.68-1.79)
(P<0.05), 50- (P<0.01) and 70-week-old groups (0.86,
0.50-1.22) (P<0.05) (Fig.
2B).
Relative expression of BDNF differed significantly
among the five age groups of the rats (χ2=33.7, df=4,
P<0.01) (Fig. 2C). BDNF
expression in the 105-week-old group (14.8, 9.46-18.03) was
significantly higher than that in the 20- (1, 0.32-1.85)
(P<0.01) and 50-week-old groups (1.61, 0.36-3.46) (P<0.01)
(Fig. 2C). BDNF expression in the
90-week-old group (9.17, 6.81-10.83) was also significantly higher
than that in the 20- (P<0.01) and 50-week-old groups (P<0.01)
(Fig. 2C).
Relative expression of IL6 differed significantly
among the five age groups of the rats (χ²=21.5, df=4, P<0.01)
(Fig. 2D). IL6 expression in the
105-week-old group rats (1.69, 1.47-1.91) was significantly higher
than that in the 20- (1, 0.8-1.28) (P<0.05) and 50-week-old
groups (1.34, 1.14-1.46) (P<0.05) (Fig. 2D). In addition, IL6 expression in
the 90-week-old group (1.7, 1.43-1.76) was significantly higher
than that in the 20- (P<0.01) and 50-week-old groups (P<0.05)
(Fig. 2D). Furthermore, IL6
expression in the 70-week-old group (1.6, 1.28-1.91) was
significantly higher than that in the 20-week-old group (P<0.05)
(Fig. 2D).
Discussion
In the present study, changes in the expression
levels of degradation factors (associated with UPS, autophagy and
apoptosis), repair factors, and the inflammatory cytokine IL6
induced by the ER stress response in aging rats, were examined to
detect signs of age-related neurodegeneration. Among the physical
characteristics, body mass of the rats in the four groups aged 50
weeks and older increased more than that of the 20-week-old rats. A
previous study examining the physical parameters of aging male
Wistar rats (n=402) housed in a controlled environment reported a
rapid increase in body mass until the 69th week (mean body mass 629
g), with a gradual increase or maintenance until the 134th week
(mean body mass 660 g), and a decrease thereafter (34). Similar to this finding, rats in the
present study showed a rapid increase in mean body mass up to the
age of 70 weeks, which was maintained thereafter. However, the body
mass after 70 weeks (mean 588 g from 70 to 105 weeks of age) was
~70 g less than the mean body mass in the previous study (660 g).
The body mass of rats is affected by several factors, such as
handling, type and quality of food, density of animals in the cage,
temperature, humidity, light regime and opportunities for active
movement (34). Therefore, the
difference between the body masses of the rats in the study by
Nistiar et al (34) and the
present study may have been influenced by several factors during
the rearing process.
Comparisons of the Sol muscle masses for the five
groups showed no differences with age. However, the 20-, 90- and
105-week-old rats had noticeably lower EDL masses than the 50- and
70-week-old rats. In experiments examining the pathophysiological
characteristics of sarcopenia in young, adult, middle-aged and aged
rats, typical signs such as shortening of single contractions,
reduced relaxation rate, and impaired mobilization of motor units
at stimulus frequencies above 50-60 Hz, were especially observed in
the fast twitch muscle-dominant plantaris muscles of aged rats
(35). Furthermore, mild signs of
sarcopenia have been observed in predominantly slow twitch Sol
muscles (35). In addition,
sarcopenic muscles have altered myofibers, with a reduced type II/
I fiber ratio and decreased muscle mass and strength with age
(36,37). These findings indicate that type II
fibers are more susceptible to atrophy in muscles, and a
predominantly fast-twitch component and is more likely to occur
specifically in sarcopenia. In the present study, the Sol muscle, a
slow-twitch dominant configuration, showed no loss in mass after 50
weeks of age, while the EDL, a fast-twitch dominant configuration,
showed a marked loss in mass in age groups beyond 70 weeks, that
is, at 90 and 105 weeks. The EDL mass of rats in the 90-week-old
group was significantly less than that of rats in the 70-week-old
group, suggesting that EDL mass decreased gradually and
progressively after 70 weeks. In support of this theory, a
transition from type IIb to type I fibers was observed in the
fast-twitch dominant plantaris muscles of middle-aged rats (~58-67
weeks old), without muscle atrophy in the aforementioned
experiments by Tamaki et al (35). This indicates that qualitative
decline had begun even in middle-aged rats without typical
sarcopenia symptoms as follows: shortening and relaxing velocity of
twitch, impaired recruitment of motor units at high stimulus
frequencies, and easy fatigability of the neuromuscular junction.
Similarly, no loss of muscle mass was observed in the middle-aged
rats (70 weeks old) in the present study, but it is possible that a
decline in quality had begun and was progressing gradually.
The expression of XBP1s, which promotes UPS and
autophagy, was significantly decreased in rats in the 105-week-old
group relative to those in the other four groups, with no
significant differences between the other four groups. Focusing on
median values, rats in the 50-week-old group (median 2.5) showed a
peak in XBP1s expression, followed by slight decreases at 70
(median 1.22) and 90 (median 1.1) weeks, and a significant decrease
at 105 weeks (median 0.28). Effect sizes (Cohen's d) between the
50- and 70-week-old groups and between the 50- and 90-week-old
groups (0.88 and 0.82, respectively) were large. This indicated
that XBP1s expression peaked at 50 weeks and decreased with aging.
XBP1s increases the levels of degraded proteins to promote UPS when
unfolded proteins accumulate in the ER (38,39).
Additionally, the accumulation of unfolded proteins in the ER also
induces the expression of ER chaperones that correct the
conformation of aberrant proteins (40). These findings suggest that degraded
proteins accumulate in the tibial nerve of the 50-week-old rats in
this study and actively induce both UPS degradation and repair by
ER chaperones; this is accompanied by increased expression of XBP1s
for their processing. In a histological analysis of the tibial
nerve in six groups of mice (6, 9, 12, 16 and 22 months of age and
a mixed age of 27 + 33 months), myelin sheath thickness of MFs
peaked at 12 months (48 weeks) and decreased thereafter, while axon
perimeter length peaked at 16 months (64 weeks) and decreased
thereafter (16). This suggests
that the myelin sheath began to atrophy at as early as 48 weeks in
the aforementioned study. Similarly, myelin sheath degeneration may
have been initiated in the tibial nerves of 50-week-old rats in the
present study, and XBP1s expression may have increased to
facilitate the repair and degradation of degenerated proteins. By
contrast, XBP1s expression levels decreased after 70 weeks of age.
Reduced myelin clearance, axonal regeneration and macrophage
recruitment, and delayed motor recovery have been reported after
sciatic nerve injury to XBP1-deficient mice in the nervous system
(41). Mouse with knockout of ER
stress sensor IRE1 (Inositol requiring 1), which is upstream of
XBP1s (42), show accelerated
age-related cognitive decline. Reduced XBP1s expression in the
peripheral and central nervous system impairs the ER stress
response and accelerates neurodegeneration. In the present study,
neurodegeneration may have been initiated between 50 and 70 weeks
of age, as XBP1s expression decreased after 70 weeks.
Expression of eIF2α increased markedly in the 90-
and 105-week-old rats relative to those in the other three groups.
Increased and/or activated eIF2α upregulates the translation of
certain mRNAs, such as activating transcription factor 4, which
regulates apoptosis-related gene expression, while inhibiting
overall protein translation as an ER stress response (43). Furthermore, eIF2α activation depends
on the activation of PKR-like ER kinase, a stress sensor located on
the ER membrane that is activated when degraded proteins accumulate
in the ER (42,44). Similar to these findings, the marked
increase in eIF2α expression after 90 weeks of age in the present
study suggests that inhibition of new protein biosynthesis and
promotion of apoptosis may have increased. There was no difference
between eIF2α expression in rats in the 50- and 70-week-old groups,
but a moderate effect size (Cohen's d=0.64) was observed (data not
shown). This suggested that ER stress may begin to increase
gradually between 50 and 70 weeks of age. In agreement with this,
Casp3, which acts downstream of apoptosis, showed a marked increase
after 70 weeks of age (Fig. 1D),
suggesting that accumulation of denatured proteins and degradation
failure may progress between 50 and 70 weeks of age.
Becn1 expression was significantly reduced in the
105-week-old rats relative to that in the 20- and 50-week-old rats.
Becn1 expression in the 70- and 90-week-old rats also tended to be
lower than that in the 50-week-old rats and higher than that in the
105-week-old rats. Becn1 is associated with autophagy induction
(45), and decreased Becn1
expression suppresses ER stress-induced autophagy (46). In mouse (45) and human (46) models of Alzheimer's disease, Becn1
expression was shown to decrease with age. While these findings may
not be directly related to peripheral nerve tissues, reduced Becn1
expression in tibial nerves after 50 weeks of age suggests that
autophagy progressively declines, resulting in the gradual
accumulation of degenerative proteins. Experiments involving 293
cells with Becn1 knockdown have been shown to upregulate c-Jun
N-terminal kinase (JNK1) protein expression, associated with
apoptosis induction (46).
Consistent with this finding, Becn1 expression in the present study
decreased after 50 weeks of age, while the apoptosis-related factor
Casp3 progressively increased (Fig.
1D), suggesting that aging promotes apoptosis.
Casp3 expression was markedly increased in rats in
the 70-week and older age groups relative to those in the younger
groups and progressively increased after 70 weeks of age. Apoptosis
is induced when the degradation of denatured proteins by UPS and
autophagy is reduced, resulting in prolonged accumulation of
denatured proteins in the ER. ER stress-related apoptosis signaling
is mainly mediated by the activation of the JNK1(47), nuclear factor
kappa-light-chain-enhancer of activated B-cells (NF-κB) (47,48)
and Caspase 12 (Casp12) pathways (47,49,50)
via the activity of IRE1 receptors present on the ER membrane. In
addition, apoptosis is induced by the activation of the growth
arrest and leucine zipper transcription factor, DNA damage
inducible gene 153 (GADD153, also called CHOP) pathway (48) via the activities of activating
transcription factor 6 and protein kinase RNA-like ER kinase
receptors. Casp3 functions downstream of the caspase 12 pathway
aforementioned, where calpain activity associated with increased ER
stress converts inactive Pro-Casp12 to active Casp12. This is
subsequently transferred from Caspase 9 activity to Casp3 activity
(47,49-51).
Increased Casp3 expression in the present study is expected to have
been initiated between 50 and 70 weeks of age and appears to be
associated with changes in XBP1s, eIF2α and Becn1 expression
levels. Besides the caspase pathway, the aforementioned, JNK1
pathway may also be promoted. Activation of the NF-κB and JNK1
pathways induces the transcription of inflammatory response-related
molecules (52). Expression of IL6,
an inflammatory cytokine assessed in the present study, showed a
marked increase in rats after 70 weeks of age. In cultured
astrocytes, treatment with the ER stress inducers thapsigargin or
tunicamycin resulted in a marked increase in IL6 expression via the
activation of the JNK1 pathway (53). These findings indicate that the
activation of apoptotic signaling simultaneously promotes the
expression of inflammatory agents. Thus, Casp3 and IL6 expression
may have been similarly upregulated from 50 and 70 weeks of age in
the present study.
BiP/GRP78 and PDI expression decreased progressively
after 70 weeks of age. Under ER stress, BiP/GRP78 binds to unfolded
proteins to prevent optimum refolding and aggregate formation, and
PDI catalyzes disulfide bond formation and isomerization of
secreted proteins (54-56).
Besides its role in refolding denatured proteins (molecular
chaperone), GRP78 promotes PDI to maintain the thiol groups of
denatured proteins in a disulfide-eligible form (57). Furthermore, PDI receives electrons
from the denatured protein substrate and reduces the S-S bond to a
thiol group, thereby oxidizing the thiol to S-S bond in the target
protein (58). Thus, both BiP/GRP78
and PDI cooperate to repair misfolded proteins. Furthermore, GRP78
binds to the IRE1 receptor and remains inactivated when not under
ER stress. However, the accumulation of denatured proteins causes
GRP78 to dissociate from the IRE1 receptor and activate IRE1.
Activated IRE1 induces the generation of XBP1s, which promote PDI
transcription (40,42). Therefore, the expression of PDI and
XBP1s may regulate the signaling cascade. Consistent with this,
XBP1s expression was observed to decrease in the tibial nerves of
rats in the present study after 70 weeks of age (Fig. 1A). This suggests that age-related
dysfunction of IRE1 signaling is more likely to occur after 70
weeks of age (or between 50 and 70 weeks of age), with both XBP1s
and PDI expression showing similar declines.
BDNF expression increased progressively in rats
after 70 weeks of age. BDNF promotes MF repair and preservation
(59,60) and is known to be involved in the
inhibition of ER stress-induced apoptosis (61,62).
BDNF expression trends in the present study suggest that
neurodegeneration increases markedly after 70 weeks of age,
indicating increased nerve fiber repair. Similarly, a histological
study of age-related changes in rat tibial nerves reported a marked
progression of axonal and myelin sheath degeneration after 70 weeks
of age (15). Therefore, BDNF
expression may increase to promote repair after 70 weeks of age,
when histological manifestations of degeneration are apparent. With
respect to neurodegeneration, reactive oxygen species (ROS)-induced
oxidative stress is associated with peripheral nerve degeneration
and related diseases (63,64). ROS are formed in mitochondria during
adenosine triphosphate production and cause damage to
carbohydrates, lipids, proteins and nucleic acids (65,66).
Increased cell apoptotic activity with aging is associated with
increased neural concentrations of ROS (67,68).
After 70 weeks of age, tibial nerve fibers are exacerbated by
oxidative stress-associated damage, which may promote apoptosis.
Conversely, BDNF inhibits the Casp12 pathway (apoptosis induction)
via the activation of PI3K (61).
As BDNF in the present study increased progressively after 70 weeks
of age, it may have a role in promoting nerve fiber repair and
inhibiting apoptosis. However, it is contradictory that the
expression of Casp3, which functions downstream of the apoptotic
pathway (Casp12 cascade), increased at ~70 weeks of age.
Considering the aforementioned histological progression of tibial
nerve MF degeneration after 70 weeks of age (15), the promotion of apoptosis may be
dominant over the promotion of repair by BDNF, resulting in an
imbalance between these two antagonistic pathways.
Regarding IL6 expression, the 90- and 105-week-old
rats showed a significant increase relative to those in the 50- and
20-week-old groups. There was no significant difference between the
70- and 50-week-old groups, but the effect size (Cohen's d=0.98)
(data not shown) was large, indicating a substantial increase at 70
weeks and thereafter. IL6 induces inflammatory responses that
induce neutrophil and macrophage migration under ER stress
conditions, and its biosynthesis is ROS-inducible (69). It is also known that XBP1 is a
regulator of IL6 production, which is essential for plasma cell
differentiation, survival and immunoglobulin synthesis, in addition
to unfolding protein repair and UPS induction (70,71).
These findings suggest that IL6 expression is upregulated as XBP1
expression increases under ER stress. The present study reveals
contradictory results: the expression of spliced XBP1 mRNAs (XBP1s)
decreased after 70 weeks of age, whereas IL6 expression increased
after 70 weeks of age. As discussed for Casp3 earlier, promotion of
the JNK1 pathway may be involved in the regulation of IL6
expression (52). Therefore, it is
suggested that increased IL6 expression after 70 weeks in the
present study was not affected by the regulation of XBP1 (or XBP1s)
expression and was caused by the promotion of apoptosis through
increased Casp3 expression. Transient injection of IL6 in rats
decreased myofibrillar protein by 17% and total muscle protein by
9%. A standard deviation increase in IL6 expression levels in older
patients decreased grip strength by 1.12-2.37 kg, suggesting that
increased IL6 expression may also cause atrophy of peripheral nerve
fiber axons and myelin sheaths (72,73).
Mitochondrial dysfunction is also associated with increased
apoptosis with aging. Age-related mitochondrial dysfunction leads
to increased ROS, resulting in increased accumulation of
degenerative proteins and DNA damage (74). This could be a reason for the
tendency of degenerated proteins to accumulate after 70 weeks in
the present study. Hence, it is suggested that the promotion of
apoptosis by the accumulation of denatured proteins induces
increased IL6 expression. Furthermore, both BDNF and IL6 expression
increased progressively after 50 weeks. Previous studies suggested
that BDNF and IL6 expression levels increase in acute
neuroinflammation (75,76). However, in chronic inflammation, IL6
expression is maintained or increased, whereas BDNF expression
levels are reduced (77), which is
not in agreement with the results of the present study. Although
there is little direct evidence to resolve this discrepancy, there
are factors to consider. For instance, BDNF levels were reported to
be low when the ratio of inflammatory cytokines to the
anti-inflammatory cytokine IL6 in the prefrontal cortex was
significantly higher than normal (77). That is, anti-inflammatory cytokines
may be involved in the increased expression of BDNF. Therefore, it
may be necessary to consider the expression of anti-inflammatory
cytokines in addition to the inflammatory cytokine IL6 in the
present study.
Oxidative stress, including the generation of ROS
and free radicals, is widely recognized as an important contributor
to age-related neuronal degeneration and death (29,68).
In spinal neurons, for instance, the activity of proteasomes-a
critical defense mechanism that mitigates oxidative damage to DNA,
proteins and lipids-declines significantly with advancing age. This
decrease in proteasome function has been reported to be associated
with motor neuron degeneration in aging rats (30). Similarly, in animal models
characterized by increased oxidative stress, motor dysfunction
develops early, and structural damage to axons, myelin sheaths, and
neuronal cell bodies is observed by middle age. This accelerated
degeneration is similar to the changes associated with normal aging
but occurs at an earlier stage (31). In the present study, the rats were
subjected to a lifetime of physical inactivity, which may have
resulted in a marked increase in ROS production as early as 70
weeks of age and after. ROS also interact with the ER stress
pathway, creating a feedback loop that exacerbates cellular damage.
ROS production is associated with the activation of ER stress via
pathways such as the UPR, which amplifies oxidative stress by
disrupting redox homeostasis in the ER lumen (78). This bidirectional relationship
suggests that oxidative and ER stress act synergistically to
promote age-related neuronal apoptosis and inflammation. Given the
interplay between oxidative stress and neuroprotective mechanisms,
it is plausible that increased ROS levels contribute to the
progressive regression of neurons in the central and peripheral
nervous systems. Moreover, the impact of reduced antioxidant
defenses, such as proteasome activity, likely extends to motor MFs
in peripheral nerves, exacerbating age-related degenerative
changes.
Overall, it was observed in the present study that
expression levels of ER stress-related degradation proteins XBP1s,
eIF2α, Becn1 and Casp3 begin to change between 50 and 70 weeks of
age in rats. XBP1s and Becn1, which are associated with UPS and
autophagy in response to ER stress, respectively, are significantly
reduced after 50 weeks of age. This suggests a decline in their
degradation systems. Hence, it is likely that after 50 weeks of
age, new protein synthesis was suppressed (increased eIF2α
expression) and apoptosis was promoted (increased Casp3 expression)
along with the accumulation of denatured proteins. Additionally,
expression levels of ER stress-related repair proteins BiP/GRP78,
PDI, BDNF and IL6 changed between 50 and 70 weeks of age. BiP/GRP78
expression remained unchanged between 50 and 70 weeks of age,
whereas that of PDI decreased. Furthermore, after 70 weeks of age,
both BiP/GRP78 and PDI expression decreased remarkably with age.
Thus, the repair function begins to decline between 50 and 70 weeks
of age and continues to decline with age. Previous studies have
shown that oxidative stress and free radicals may be associated
with age-related neuronal regression (29,68).
The activity of proteasomes, which protect proteins, DNA and lipids
in spinal cord neurons from oxidative stress, was markedly reduced
in rats after 12 months (48 weeks), mediating motor neuron
degeneration (30). Presumably, the
regression of spinal cord neurons after ~50 weeks involves reduced
activity of neuroprotective factors such as proteasomes, oxidative
stress and increased free radicals, which may also affect
peripheral nerve MFs. However, BDNF gradually increased after 50
weeks of age, compensating for the decline in refolding function.
Apoptosis (Casp3) was also promoted at the same time after 70 weeks
of age, suggesting that apoptosis may become predominant with
aging. Mitochondrial dysfunction occurred between 50 and 70 weeks
of age, resulting in increased oxidative stress, accumulation of
denatured proteins, increased IL6 expression and apoptosis.
There are certain limitations to the present study.
First, it was not possible to have homogeneity in the numbers of
rats in each age group. This is because after 90 weeks, a few more
rats than expected succumbed before reaching 105 weeks of age,
resulting in a few less rats in the 105-week-old group, relative to
the other groups. The possibility that the bias introduced due to
this heterogeneity in sample size may have reduced the power to
detect variance and between-group differences, cannot be denied.
Therefore, to resolve this problem as much as possible, effect size
tests were also performed as appropriate. Second, male rats were
exclusively used to minimize the confounding effects of hormonal
fluctuations associated with the estrous cycle in females. Previous
studies suggest that hormonal factors, such as estrogen, may play a
neuroprotective role in female rats, potentially mitigating
age-related neurodegeneration and associated inflammation (79,80).
The absence of such protective effects in male rats might have
amplified the observed changes in the present study. Therefore, it
is plausible that female rats might exhibit different patterns of
neurodegeneration and inflammatory responses with aging. Future
research should address these limitations by including rats of both
sexes to fully explore the interplay between sex hormones and
age-related changes. Third, focus was addressed on changes in the
expression levels of ER stress-related degradation proteins, repair
proteins, and the inflammatory cytokine IL6 with aging. However,
further determination of ER stress by assessing the concentrations
of ROS and the total amount of degenerated proteins affecting
peripheral nerve degeneration was not performed. Because the
present study did not directly assess ROS levels, future research
should explore this relationship in detail to allow for an improved
understanding of the combined effects of oxidative stress and ER
stress on peripheral nerve degeneration during aging. The inclusion
of ROS measurements in subsequent experiments will provide valuable
insights into the molecular pathways underlying these phenomena.
Additionally, histological and morphological analysis of the aging
tibial nerve was not performed. Biochemical, histological and
morphological changes associated with aging may not develop
simultaneously. Rather, histological changes may be delayed.
Therefore, the differences in the time scales between biochemical,
histological and morphological changes should be clarified in
future studies and applied to prevention and treatment strategies.
Finally, it is important to acknowledge the limitations of the
present study regarding the use of Casp3 and IL6 as biomarkers for
apoptosis and induction of inflammation, respectively. These
markers are not specific to ER stress and may be affected by other
aging-related pathways. This limitation may affect the specificity
of the current findings on ER stress-related apoptosis and
inflammation. Future studies will address these limitations by
incorporating biomarkers such as family with sequence similarity
134, member B (FAM134B) and tumor necrosis factor-α (TNF-α), which
are specific biomarkers in the induction of ER stress
response-related apoptosis and inflammation, respectively. FAM134B,
a member of the reticulon family, has been identified as a crucial
mediator of ER-phagy, a selective autophagic process that regulates
ER turnover and maintains ER homeostasis. Dysregulation of FAM134B
has been implicated in neurodegenerative diseases and peripheral
neuropathy due to its role in ER stress-induced apoptosis (81,82).
Assessing FAM134B expression would provide a more specific
indication of ER stress-related apoptotic mechanisms in the
peripheral nervous system. Similarly, TNF-α is a pro-inflammatory
cytokine that plays a central role in mediating inflammation during
ER stress. TNF-α is known to be upregulated in response to the UPR
signaling pathway, specifically through the activation of the PERK
and IRE1 pathways, which are key components of ER stress (83). The inclusion of TNF-α as a marker
could enhance the specificity of our evaluation of ER
stress-related inflammation. Moreover, analyzing the expression of
FAM134B and TNF-α will increase the specificity of studies on the
ER stress response in peripheral nerves. Future investigations will
be designed to include FAM134B and TNF-α as specific markers for ER
stress-induced apoptosis and inflammation, thereby addressing the
limitations of our current approach.
In conclusion, in the present study, changes in
expression levels of ER stress-related UPS and autophagy,
apoptosis, repair, and inflammatory cytokine proteins were examined
to demonstrate signs of age-related neurodegeneration in rats. It
was observed that UPS and autophagy declined after 50 weeks of age,
followed by the promotion of apoptosis. Repair function decreased
after 50 weeks of age, whereas the expression of the inflammatory
cytokine IL6 increased after 50 weeks of age. Future research
should examine the inhibitory effects of pre- and post-50-week
regular aerobic exercise (treatment) interventions on age-related
neurodegeneration in terms of the ER stress response.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a Grant-in-Aid for
Scientific Research (C) from the Japan Society for the Promotion of
Science (https://kaken.nii.ac.jp; grant no. 20K19694).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS conceived the study design and planned the study.
MS, KS, SS, AY and KY performed statistical analyses and prepared
figures and tables. MS, WI, KN, SM and RM managed the animal
breeding and conducted the experiments. MS coordinated the
experiments and wrote the manuscript. All authors read and approved
the final version of the manuscript. MS, KS, SS, AY and KY confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Kyoto Tachibana University (approval no.
19-10; Kyoto, Japan) and was performed in accordance with the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health (National Research Council, 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beaudart C, Zaaria M, Pasleau F, Reginster
JY and Bruyère O: Health outcomes of sarcopenia: A systematic
review and meta-analysis. PLoS One. 12(e0169548)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kelley GA and Kelley KS: Is sarcopenia
associated with an increased risk of all-cause mortality and
functional disability? Exp Gerontol. 96:100–103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen X, Cao M, Liu M, Liu S, Zhao Z and
Chen H: Association between sarcopenia and cognitive impairment in
the older people: A Meta-analysis. Eur Geriatr Med. 13:771–787.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang X, Huang P, Dou Q, Wang C, Zhang W,
Yang Y, Wang J, Xie X, Zhou J and Zeng Y: Falls among older adults
with sarcopenia dwelling in nursing home or community: A
meta-analysis. Clin Nutr. 39:33–39. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Landi F, Liperoti R, Russo A, Giovannini
S, Tosato M, Capoluongo E, Bernabei R and Onder G: Sarcopenia as a
risk factor for falls in elderly individuals: Results from the
ilSIRENTE study. Clin Nutr. 31:652–658. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cruz-Jentoft AJ, Baeyens JP, Bauer JM,
Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y,
Schneider SM, et al: Sarcopenia: European consensus on definition
and diagnosis: Report of the European Working Group on Sarcopenia
in Older People. Age Ageing. 39:412–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Su YC, Chang SF and Tsai HC: The
relationship between sarcopenia and injury events: A systematic
review and Meta-analysis of 98,754 Older Adults. J Clin Med.
11(6474)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yeung SSY, Reijnierse EM, Pham VK,
Trappenburg MC, Lim WK, Meskers CGM and Maier AB: Sarcopenia and
its association with falls and fractures in older adults: A
systematic review and meta-analysis. J Cachexia Sarcopenia Muscle.
10:485–500. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Verdú E, Ceballos D, Vilches JJ and
Navarro X: Influence of aging on peripheral nerve function and
regeneration. J Peripher Nerv Syst. 5:191–208. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Coletti C, Acosta GF, Keslacy S and
Coletti D: Exercise-mediated reinnervation of skeletal muscle in
elderly people: An update. Eur J Transl Myol. 32:1–11.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu
AL and Xu XZ: Functional aging in the nervous system contributes to
age-dependent motor activity decline in C. elegans. Cell Metab.
18:392–402. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagashima K and Oota K: A
histopathological study of the human spinal ganglia. 1. Normal
variations in aging. Acta Pathol Jpn. 24:333–344. 1974.PubMed/NCBI View Article : Google Scholar
|
|
13
|
O'Sullivan DJ and Swallow M: The fibre
size and content of the radial and sural nerves. J Neurol Neurosurg
Psychiatry. 31:464–470. 1968.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bergman E and Ulfhake B: Loss of primary
sensory neurons in the very old rat: Neuron number estimates using
the disector method and confocal optical sectioning. J Comp Neurol.
396:211–222. 1998.PubMed/NCBI
|
|
15
|
Sakita M, Murakami S, Nonaka K, Sakamoto
R, Saito T, Isobe W and Kumagai S: Different patterns in
age-related morphometric alteration of myelinated fibers and
capillaries of the tibial nerve: A longitudinal study in normal
rats. J Anat. 236:1101–1111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ceballos D, Cuadras J, Verdu E and Navarro
X: Morphometric and ultrastructural changes with ageing in mouse
peripheral nerve. J Anat. 195:563–576. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bouche P, Cattelin F, Saint-Jean O, Léger
JM, Queslati S, Guez D, Moulonguet A, Brault Y, Aquino JP and
Simunek P: Clinical and electrophysiological study of the
peripheral nervous system in the elderly. J Neurol. 240:263–268.
1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Verdú E, Butí M and Navarro X: Functional
changes of the peripheral nervous system with aging in the mouse.
Neurobiol Aging. 17:73–77. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ciechanover A: Intracellular protein
degradation: From a vague idea thru the lysosome and the
ubiquitin-proteasome system and onto human diseases and drug
targeting. Cell Death Differ. 12:1178–1190. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aman Y, Schmauck-Medina T, Hansen M,
Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen
T and Tavernarakis N: Autophagy in healthy aging and disease. Nat
Aging. 1:634–650. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rajawat YS, Hilioti Z and Bossis I: Aging:
Central role for autophagy and the lysosomal degradative system.
Ageing Res Rev. 8:199–213. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Amm I, Sommer T and Wolf DH: Protein
quality control and elimination of protein waste: The role of the
ubiquitin-proteasome system. Biochim Biophys Acta. 1843:182–196.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo J, Huang X, Dou L, Yan M, Shen T, Tang
W and Li J: Aging and aging-related diseases: From molecular
mechanisms to interventions and treatments. Signal Transduct Target
Ther. 7(391)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Perner C and Krüger E: Endoplasmic
reticulum stress and its role in homeostasis and immunity of
central and peripheral neurons. Front Immunol.
13(859703)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shinno K, Miura Y, Iijima KM, Suzuki E and
Ando K: Axonal distribution of mitochondria maintains neuronal
autophagy during aging via eIF2β. bioRxiv.
2024(576435)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cuervo AM and Dice JF: How do
intracellular proteolytic systems change with age? Front Biosci.
3:d25–d43. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Hughes AL and Gottschling DE: An early age
increase in vacuolar pH limits mitochondrial function and lifespan
in yeast. Nature. 492:261–265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Leidal AM, Levine B and Debnath J:
Autophagy and the cell biology of age-related disease. Nat Cell
Biol. 20:1338–1348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thrasivoulou C, Soubeyre V, Ridha H,
Giuliani D, Giaroni C, Michael GJ, Saffrey MJ and Cowen T: Reactive
oxygen species, dietary restriction and neurotrophic factors in
age-related loss of myenteric neurons. Aging Cell. 5:247–257.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Keller JN, Huang FF and Markesbery WR:
Decreased levels of proteasome activity and proteasome expression
in aging spinal cord. Neuroscience. 98:149–156. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sims-Robinson C, Hur J, Hayes JM, Dauch
JR, Keller PJ, Brooks SV and Feldman EL: The role of oxidative
stress in nervous system aging. PLoS One. 8(e68011)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eaton SL, Roche SL, Llavero Hurtado M,
Oldknow KJ, Farquharson C, Gillingwater TH and Wishart TM: Total
protein analysis as a reliable loading control for quantitative
fluorescent Western blotting. PLoS One. 8(e72457)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Moritz CP: Tubulin or not tubulin: Heading
toward total protein staining as loading control in western blots.
Proteomics: 17, 2017 doi: 10.1002/pmic.201600189.
|
|
34
|
Nistiar F, Racz O, Lukacinova A, Hubkova
B, Novakova J, Lovasova E and Sedlakova E: Age dependency on some
physiological and biochemical parameters of male Wistar rats in
controlled environment. J Environ Sci Health A Tox Hazard Subst
Environ Eng. 47:1224–1233. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tamaki T, Hirata M and Uchiyama Y:
Qualitative alteration of peripheral motor system begins prior to
appearance of typical sarcopenia syndrome in middle-aged rats.
Front Aging Neurosci. 6(296)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F,
Zhao L, Shang Y, Ma Y and Ma B: Associated factors of sarcopenia in
community-Dwelling older adults: A systematic review and
Meta-analysis. Nutrients. 13(4291)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cruz-Jentoft AJ and Sayer AA: Sarcopenia.
Lancet. 393:2636–2646. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hollien J and Weissman JS: Decay of
endoplasmic reticulum-localized mRNAs during the unfolded protein
response. Science. 313:104–107. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hollien J, Lin JH, Li H, Stevens N, Walter
P and Weissman JS: Regulated Ire1-dependent decay of messenger RNAs
in mammalian cells. J Cell Biol. 186:323–331. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park SM, Kang TI and So JS: Roles of XBP1s
in transcriptional regulation of target genes. Biomedicines.
9(791)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Oñate M, Catenaccio A, Martínez G,
Armentano D, Parsons G, Kerr B, Hetz C and Court FA: Activation of
the unfolded protein response promotes axonal regeneration after
peripheral nerve injury. Sci Rep. 6(21709)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cabral-Miranda F, Tamburini G, Martinez G,
Ardiles AO, Medinas DB, Gerakis Y, Hung MD, Vidal R, Fuentealba M,
Miedema T, et al: Unfolded protein response IRE1/XBP1 signaling is
required for healthy mammalian brain aging. EMBO J.
41(e111952)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Costa-Mattioli M and Walter P: The
integrated stress response: From mechanism to disease. Science.
368:2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kashiwagi K, Yokoyama T, Nishimoto M,
Takahashi M, Sakamoto A, Yonemochi M, Shirouzu M and Ito T:
Structural basis for eIF2B inhibition in integrated stress
response. Science. 364:495–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rashid HO, Yadav RK, Kim HR and Chae HJ:
ER stress: Autophagy induction, inhibition and selection.
Autophagy. 11:1956–1977. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li J, Ni M, Lee B, Barron E, Hinton DR and
Lee AS: The unfolded protein response regulator GRP78/BiP is
required for endoplasmic reticulum integrity and stress-induced
autophagy in mammalian cells. Cell Death Differ. 15:1460–1471.
2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Smith MI and Deshmukh M: Endoplasmic
reticulum stress-induced apoptosis requires bax for commitment and
Apaf-1 for execution in primary neurons. Cell Death Differ.
14:1011–1019. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kaufman RJ: Stress signaling from the
lumen of the endoplasmic reticulum: Coordination of gene
transcriptional and translational controls. Genes Dev.
13:1211–1233. 1999.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294.
2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
51
|
Rasheva VI and Domingos PM: Cellular
responses to endoplasmic reticulum stress and apoptosis. Apoptosis.
14:996–1007. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chaudhari N, Talwar P, Parimisetty A,
Lefebvre d'Hellencourt C and Ravanan P: A molecular web:
Endoplasmic reticulum stress, inflammation, and oxidative stress.
Front Cell Neurosci. 8(213)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sanchez CL, Sims SG, Nowery JD and Meares
GP: Endoplasmic reticulum stress differentially modulates the IL-6
family of cytokines in murine astrocytes and macrophages. Sci Rep.
9(14931)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Brocchieri L, Conway de Macario E and
Macario AJ: hsp70 genes in the human genome: Conservation and
differentiation patterns predict a wide array of overlapping and
specialized functions. BMC Evol Biol. 8(19)2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ibrahim IM, Abdelmalek DH and Elfiky AA:
GRP78: A cell's response to stress. Life Sci. 226:156–163.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ellgaard L and Ruddock LW: The human
protein disulphide isomerase family: Substrate interactions and
functional properties. EMBO Rep. 6:28–32. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mayer M, Kies U, Kammermeier R and Buchner
J: BiP and PDI cooperate in the oxidative folding of antibodies in
vitro. J Biol Chem. 275:29421–29425. 2000.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Grek C and Townsend DM: Protein disulfide
isomerase superfamily in disease and the regulation of apoptosis.
Endoplasmic Reticulum Stress Dis. 1:4–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Helan M, Aravamudan B, Hartman WR,
Thompson MA, Johnson BD, Pabelick CM and Prakash YS: BDNF secretion
by human pulmonary artery endothelial cells in response to hypoxia.
J Mol Cell Cardiol. 68:89–97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wilhelm JC, Xu M, Cucoranu D, Chmielewski
S, Holmes T, Lau KS, Bassell GJ and English AW: Cooperative roles
of BDNF expression in neurons and Schwann cells are modulated by
exercise to facilitate nerve regeneration. J Neurosci.
32:5002–5009. 2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Shimoke K, Utsumi T, Kishi S, Nishimura M,
Sasaya H, Kudo M and Ikeuchi T: Prevention of endoplasmic reticulum
stress-induced cell death by brain-derived neurotrophic factor in
cultured cerebral cortical neurons. Brain Res. 1028:105–111.
2004.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bhaskar PT and Hay N: The two TORCs and
Akt. Dev Cell. 12:487–502. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Scott TL, Rangaswamy S, Wicker CA and
Izumi T: Repair of oxidative DNA damage and cancer: Recent progress
in DNA base excision repair. Antioxid Redox Signal. 20:708–726.
2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fukui K: Reactive oxygen species induce
neurite degeneration before induction of cell death. J Clin Biochem
Nutr. 59:155–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ma Q: Transcriptional responses to
oxidative stress: Pathological and toxicological implications.
Pharmacol Ther. 125:376–393. 2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hong Y, Boiti A, Vallone D and Foulkes NS:
Reactive oxygen species signaling and oxidative stress:
transcriptional regulation and evolution. Antioxidants (Basel).
13(312)2024.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Shokouhi G, Tubbs RS, Shoja MM, Roshangar
L, Mesgari M, Ghorbanihaghjo A, Ahmadi N, Sheikhzadeh F and Rad JS:
The effects of aerobic exercise training on the age-related lipid
peroxidation, Schwann cell apoptosis and ultrastructural changes in
the sciatic nerve of rats. Life Sci. 82:840–846. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wickens AP: Ageing and the free radical
theory. Respir Physiol. 128:379–391. 2001.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Haddad JJ, Saadé NE and Safieh-Garabedian
B: Redox regulation of TNF-alpha biosynthesis: augmentation by
irreversible inhibition of gamma-glutamylcysteine synthetase and
the involvement of an IkappaB-alpha/NF-kappaB-independent pathway
in alveolar epithelial cells. Cell Signal. 14:211–218.
2002.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Iwakoshi NN, Lee AH, Vallabhajosyula P,
Otipoby KL, Rajewsky K and Glimcher LH: Plasma cell differentiation
and the unfolded protein response intersect at the transcription
factor XBP-1. Nat Immunol. 4:321–329. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
71
|
Reimold AM, Iwakoshi NN, Manis J,
Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D,
Grusby MJ, Alt F and Glimcher LH: Plasma cell differentiation
requires the transcription factor XBP-1. Nature. 412:300–307.
2001.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Haddad F, Zaldivar F, Cooper DM and Adams
GR: IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985).
98:911–917. 2005.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Visser M, Pahor M, Taaffe DR, Goodpaster
BH, Simonsick EM, Newman AB, Nevitt M and Harris TB: Relationship
of interleukin-6 and tumor necrosis factor-alpha with muscle mass
and muscle strength in elderly men and women: The Health ABC Study.
J Gerontol A Biol Sci Med Sci. 57:M326–M332. 2002.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lima T, Li TY, Mottis A and Auwerx J:
Pleiotropic effects of mitochondria in aging. Nat Aging. 2:199–213.
2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Xu S, Ito A, Wang T, Kawai H, Aoyama T and
Kuroki H: Ultrasound therapy of injury site modulates gene and
protein expressions in the dorsal root ganglion in a sciatic nerve
crush injury rat model. Ultrasound Med Biol. 48:2502–2511.
2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Lin G, Zhang H, Sun F, Lu Z,
Reed-Maldonado A, Lee YC, Wang G, Banie L and Lue TF: Brain-derived
neurotrophic factor promotes nerve regeneration by activating the
JAK/STAT pathway in Schwann cells. Transl Androl Urolo. 5:167–175.
2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Xie ZM, Wang XM, Xu N, Wang J, Pan W, Tang
XH, Zhou ZQ, Hashimoto K and Yang JJ: Alterations in the
inflammatory cytokines and brain-derived neurotrophic factor
contribute to depression-like phenotype after spared nerve injury:
Improvement by ketamine. Sci Rep. 7(3124)2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293.
2007.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Shindo S, Chen SH, Gotoh S, Yokobori K, Hu
H, Ray M, Moore R, Nagata K, Martinez J, Hong JS and Negishi M:
Estrogen receptor α phosphorylated at Ser216 confers inflammatory
function to mouse microglia. Cell Commun Signal.
18(117)2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Villa A, Vegeto E, Poletti A and Maggi A:
Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev.
37:372–402. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Khaminets A, Heinrich T, Mari M, Grumati
P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N,
et al: Regulation of endoplasmic reticulum turnover by selective
autophagy. Nature. 522:354–358. 2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Cinque L, De Leonibus C, Iavazzo M,
Krahmer N, Intartaglia D, Salierno FG, De Cegli R, Di Malta C,
Svelto M, Lanzara C, et al: MiT/TFE factors control ER-phagy via
transcriptional regulation of FAM134B. EMBO J.
39(e105696)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008.PubMed/NCBI View Article : Google Scholar
|
















