1. Introduction
Garlic (Allium sativum) is a species of
bulbous plant, and has been globally used as a culinary and
medicinal herb for centuries. The medicinal properties of garlic
have been extensively studied, with a focus on its bioactive
sulfur-containing compounds, such as allicin, ajoene, diallyl
sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS),
dimethyl trisulfide (DMTS), S-allyl-cysteine (SAC) and
S-propargyl-cysteine (SPRC). These compounds are considered
to contribute to garlic's various therapeutic effects, including
anti-inflammatory, antimicrobial, antioxidant and anticancer
activities (1-4).
Garlic oil, powder and extract are available as supplements for
culinary uses, natural pesticides and medicinal purposes. Aged
garlic extract (AGE), a processed form of garlic produced by aging
garlic in an ethanol-water mixture for >10 months, has gained
attention for its ability to enhance immune function and reduce the
risk of chronic diseases (2,3).
Furthermore, AGE is rich in beneficial organic sulfur compounds,
which provide numerous health advantages. Numerous previous studies
have shown that AGE has tumor-suppressive effects on various cancer
cells, and its use has shown benefit in patients (5-9).
Pancreatic cancer is one of the most aggressive and lethal forms of
cancer, characterized by a poor prognosis and high mortality with
<10% of patients surviving at five-years (10,11).
The development of pancreatic cancer involves complex interactions
between genetic and environmental factors, making it a challenging
disease to prevent, diagnose and treat. Pancreatic ductal
adenocarcinoma (PDAC) is the most common form of pancreatic cancer,
accounting for >90% of all pancreatic malignancies. Genetics and
lifestyle factors such as smoking, alcohol consumption and obesity
are known as risk factors of PDAC; however the great majority of
PDAC cases develop in individuals without known risk factors
(10-12).
Of note, increasing evidence points to bacteria as possible
contributors to PDAC development (11,12).
Recent clinical and experimental investigations have demonstrated
the presence of an intratumor microbiome in PDAC and revealed
oncological actions of some species of bacteria (11-17).
Accumulated studies have investigated the potential role of garlic,
AGE, and their bioactive compounds in the prevention and treatment
of PDAC and related conditions, including pancreatitis and
periodontitis (1,4,8,18,19).
In the present review, it was aimed to summarize and discuss the
potential beneficial effects of garlic and its compounds on PDAC,
and to highlight the possible role of tumor-associated bacteria in
these effects.
2. Effects of garlic, AGE and garlic-derived
sulfur compounds on pancreatic cancer
Natural agents derived from plants are intensely
studied for the development of supplements, antibiotics and
anticancer drugs. Among them, garlic is used for a food source as
well as in traditional medicine. Garlic and AGE contain multiple
pharmacologically-active sulfur compounds and have great potential
to prevent and treat various types of cancers including PDAC
(1,5-9).
Numerous volatile and non-volatile garlic compounds were previously
reported (1,20,21). A
case-control study conducted in the San Francisco Bay area has
reported that garlic and onion consumption correlated with lower
odds of developing pancreatic cancer, suggesting that these
vegetables may help in preventing the disease (19). A randomized double-blind clinical
trial by Ishikawa et al (8)
has reported that daily AGE intake with capsules containing 500 mg
AGE for 3 months increased both the number and activity of NK cells
in patients with advanced liver, pancreatic, or colon cancer.
Although not focused on pancreatic cancer, a randomized
intervention trial included the use of AGE as part of a blinded and
placebo-controlled study. After a follow-up period of over 22
years, the study concluded that this supplementation significantly
reduced the risk of death in patients with gastric cancer (9).
Apoptosis is a form of programmed cell death that
plays a vital role in maintaining cellular homeostasis by
triggering a series of biochemical events. Decreased expression of
anti-apoptosis genes, including B cell lymphoma 2 (Bcl-2), and
elevated expression of pro-apoptosis factors such as caspase-3 and
Bcl-2 associated X-protein (Bax), are commonly observed phenomena
in the apoptotic process. Additionally, other factors such as p21,
p53 and cyclins are well-documented contributors to this process.
In cancer, survival-related genes are often dysregulated, and the
evasion of apoptosis is a hallmark allowing malignant cells to
survive and proliferate uncontrollably (21-25).
The induction of apoptosis through cell cycle arrest and the
regulation of its associated molecules is a critical strategy for
controlling cancer progression, as it efficiently promotes the
elimination of cancer cells. Various substances found in dietary
natural products, including garlic, have been shown to modulate the
expression of common genes involved in cancer cell survival, and
induce apoptosis and cell cycle regulation in cancer cells
(1,26-32).
The activity of garlic and its compounds has been evaluated in
several in vitro studies (Table
I).
 | Table IPotential therapeutic effects of
garlic and its organosulfur compounds on pancreatic cancer. |
Table I
Potential therapeutic effects of
garlic and its organosulfur compounds on pancreatic cancer.
| First author/s,
year | Materials
tested | Target | Therapeutic
effects | (Refs.) |
|---|
| Ishikawa et
al, 2006 | AGE | Advanced cancer
patients with liver, pancreatic, or colon cancer | Increase the number
and activity of NK cells | (8) |
| Lan et al,
2013 | Garlic oil | AsPC-1, MiaPaCa-2,
and Panc-1 | Inhibit cell
proliferation, induce cell cycle arrest and apoptosis | (26) |
| Chhabria et
al, 2015 | Allicin | MiaPaCa-2 | Reduce cell
viability, induce cell cycle arrest and apoptosis | (27) |
| Wang et al,
2013 | Allicin | BxPC3 | Inhibit tumor
growth in a mouse xenograft model | (28) |
| Saini et al,
2017 | DADS | MiaPaCa-2 | Reduced cell
viability | (29) |
| Ma et al,
2014 | DATS | Capan-2 | Reduce cell
viability, induce cell cycle arrest and apoptosis | (30) |
| Lee et al,
2019 | Ajoene | Panc-1 | Reduce cell
viability, induce cell cycle arrest | (31) |
| Wang et al,
2015 | SPRC | Panc-1 | Reduce cell
viability, induce cell cycle arrest and apoptosis, inhibit tumor
growth in a mouse xenograft model | (32) |
Garlic oil
Treatment with garlic oil inhibited the
proliferation of several PDAC cell lines, including AsPC-1, Panc-1
and MiaPaCa-2, and showed pro-apoptotic effects in a dose-dependent
manner. Early stage apoptosis was observed in AsPC-1 cells by
transmission electron microscopy. Moreover, flow cytometric
analysis revealed that the cell cycle of AsPC-1 cells was arrested
at G2/M phase (26).
Allicin
Allicin, which is a naturally occurring product from
garlic, is known as one of the major organosulfur compounds present
in garlic and is responsible for its pungent smell. Chhabria et
al (27) conjugated alliinase
to a monoclonal antibody against carbohydrate antigen 19-9
(CA19-9), a widely used PDAC biomarker. The conjugate generates
allicin in situ following the addition of alliin, and
thereby reduces PDAC cell viability via induced oxidative stress,
cell cycle arrest at the G1 phase, caspase-3 and p21 protein
expression, DNA fragmentation and apoptosis. Furthermore, in
situ-generated allicin increased p21 gene expression at the
mRNA level, acetylation of histone H3 lysine 14 and phosphorylation
of histone H3 serine 10, and reduced mono-methylation of histone H3
lysine 9(27). Another study
demonstrated the combined effects of allicin and recombinant
interleukin-2 (rIL-2) on a murine subcutaneous xenograft model
established with BxPC-3 pancreatic cancer cells. While allicin
treatment exhihited significant antitumor effects, the combination
further inhibited the growth of tumors and improved the survival
rate of mice when compared with treatment with allicin or rIL-2
alone. This outcome was attributed to the induction of tumor cell
apoptosis, activation of CD4+ T, CD8+ T and
NK cells, and increased levels of interferon gamma (28).
DADS
DADS is a bioactive compound present in garlic. A
study by Saini et al (29)
demonstrated the cytotoxicity of DADS and its synthetic derivatives
on pancreatic cancer MiaPaCa-2 cells. Among the tested DADS
analogs, Bis[3-(3-fluorophenyl)prop-2-ene]disulfide was the most
potent compound. In the pancreatic cancer cells, it upregulated Bax
and concurrently reduced Bcl-2 protein levels, activated caspase-3,
and induced apoptosis by G2/M phase arrest via DNA damage. The
apoptotic process was also associated with checkpoint kinase-1
phosphorylation, upregulated levels of inactivated cell division
cycle 25C and phosphorylation of Cdc2(29).
DATS
DATS, a biologically active garlic compound,
decreases the viability of PDAC cells and induces the activation of
apoptosis through increased cell cycle arrest at the G2/M phase.
This polysulfide increases the protein levels of Bax, Fas, p21, p53
and cyclin B1, whereas it decreases Akt, cyclin D1, MDM2 and Bcl-2
expression in pancreatic cancer Capan-2 cells. The DATS treatment
was identified to regulate gene transcription in pancreatic cancer
cells since the mRNA levels of Bax, Fas and cyclin D1 were
upregulated, whereas Akt and Bcl-2 mRNA levels were downregulated
by DATS treatment (30). In
addition, the pro-apoptotic effects of DATS in various other
cancers have been reported (23).
Ajoene
Ajoene is an organosulfur compound found in garlic,
and it exists as a mixture of two stereoisomers: Z-ajoene
and E-ajoene. Z-ajoene reduces PDAC cell viability,
induces cell cycle arrest at the G2/M phase, and reduces
transcriptional activity and protein level of glioma-associated
oncogene (Gli), which is a transcription factor mediating the
Hedgehog pathway. In addition, Z-ajoene downregulates the
protein expressions of Gli1, Gli2, Ptch, and forkhead box protein
M1 (FoxM1), a cell cycle regulator of G1/S and G2/M transitions and
a known Gli-target protein. By contrast, this sulfur compound does
not disrupt Akt protein level. As a consequence, Z-ajoene
reduces the levels of cell cycle-related proteins including c-myc,
cyclin B1 and survivin, all of which are controlled by
FoxM1(31).
SPRC
SPRC, a structural analog of SAC, reduces the
viability of PDAC cells, triggers cell cycle arrest during the G2/M
phase, and induces apoptosis. Moreover, it inhibits tumor growth in
Panc-1 mouse xenograft model by activating the c-Jun N-terminal
kinase signaling pathway (32).
Therefore, garlic-derived products can exert direct
anticancer effects on PDAC cells through modulating common
molecular pathways. In addition, the exploration of
survival-associated genes regulated by garlic is important to
deepen our understanding of garlic's biological roles in cancer.
Such insights can contribute to enhancing the precision of cancer
therapies, thereby potentially improving clinical outcomes for
patients.
Garlic products may also prevent PDAC by lowering
the severity of conditions linked to PDAC development (Table II). Pancreatitis, characterized by
inflammation of the pancreas, can increase precancerous lesions
that eventually initiate progression to PDAC in the presence of
oncogenic mutations (10-12,33).
The effects of SPRC, a structural analog of SAC, were studied in a
mouse model of acute pancreatitis (AP) induced by cerulein, a
factor known to promote PDAC development synergistically with
oncogenic Kras (33,34). Treatment of mice with SPRC for 3 h
before AP induction significantly reduced inflammation and
pro-inflammatory cytokines in the pancreas and lungs, along with
increased anti-inflammatory cytokines. The protective effects of
SPRC were attributed to its slow release of endogenous hydrogen
sulfide (34). The
anti-inflammatory actions of DADS were also determined in mice with
cerulein-induced AP. Intraperitoneal administration of DADS
significantly reduced pancreatic and pulmonary inflammation by
decreasing serum amylase levels, myeloperoxidase activity, and
histological damage in the pancreas and lungs. Additionally, DADS
inhibited cerulein-induced IκB degradation and subsequent nuclear
factor-kappa B (NF-κB) translocation (35). The same research group performed
further investigation to improve understanding of the molecular
mechanisms underlying the effects of DADS on cerulein-induced
pancreatitis by focusing on the peroxisome proliferator-activated
receptor gamma pathway. It was revealed that DADS attenuated tumor
necrosis factor-alpha, cystathionine-gamma-lyase, signal transducer
and activator of transcription 3 and NF-кB activation, and
increased suppressor of cytokine signaling 3 expression (36). In addition, a recent investigation
showed that DMTS, an additional organosulfur compound from garlic,
reduced the pancreatic infiltration of leukocytes and cellular
damage in mice with AP (37).
During AP, DMTS upregulated the level of pancreatic HSP72, a
stress-induced protective chaperone whose overexpression attenuates
NF-κB activation, enabling accelerated recovery from
cerulein-induced tissue injury (37,38).
In summary, cumulative evidence supports a potential therapeutic
role for garlic and its products in pancreatic cancer.
 | Table IIEffects of AGE and garlic compounds
on conditions linked to pancreatic ductal adenocarcinoma
development. |
Table II
Effects of AGE and garlic compounds
on conditions linked to pancreatic ductal adenocarcinoma
development.
| First author/s,
year | Materials
tested | Target | Therapeutic
effects | (Refs.) |
|---|
| Zini et al,
2020 | AGE | Adult volunteers
with mild to moderate periodontitis | Reduce the level of
probing pocket depth | (18) |
| Sidhapuriwala et
al, 2012 | SPRC | Cerulein-induced AP
in male Swiss mouse | Reduce inflammation
in the pancreas and lungs | (34) |
| Mathan Kumar and
Tamizhselvi, 2020 | DADS | Cerulein-induced AP
in male Swiss mouse | Attenuate severity
of pancreatic and pulmonary inflammation | (35) |
| Marimuthu et
al, 2022 | DADS | Cerulein-induced AP
in male Swiss mouse | Reduce inflammation
in the pancreas and lungs through the pancreatic and pulmonary
PPAR-γ activation | (36) |
| Orján et al,
2023 | DMTS | Cerulein- or
ethanol- palmitoleic acid-induced AP in male FVB/N mouse | Attenuate severity
of inflammation | (37) |
| | DMTS |
L-ornithine-HCl-induced AP in male Wister
rat | Attenuate severity
of inflammation | |
3. Effects of garlic extract and
garlic-derived sulfur compounds on cancer chemoresistance
Chemoresistance is a major obstacle in the effective
treatment of cancers, and contributes to poor patient prognosis in
PDAC (39,40). The mechanisms behind chemoresistance
are highly complex, often involving alterations in cell survival
pathways, efflux pumps and changes in the tumor microenvironment
(39-41).
Based on accumulated research findings, garlic and its derived
bioactive compounds may improve therapeutic efficacy and
potentially overcome chemoresistance through modulating multiple
cellular pathways and inducing cell death (1,7,21,23).
Several studies examined the effects of garlic, and garlic
derivatives, on resistance to chemotherapeutic agents including
those indicated in PDAC treatment, and the potential benefit of
combining garlic or its compounds with these agents (Table III).
 | Table IIIEffects of garlic, and garlic
derivatives, on resistance to chemotherapeutic agents including
those indicated in pancreatic ductal adenocarcinoma treatment, and
the potential benefit of combining garlic or its compounds with
these agents. |
Table III
Effects of garlic, and garlic
derivatives, on resistance to chemotherapeutic agents including
those indicated in pancreatic ductal adenocarcinoma treatment, and
the potential benefit of combining garlic or its compounds with
these agents.
| First author/s,
year | Materials
tested | Chemotherapeutic
agent | Therapeutic
effects | (Refs.) |
|---|
| Perez-Ortiz et
al, 2020 | Garlic extract | 5-FU and
Oxaliplatin | Enhance cytotoxic
effects | (43) |
| Horie et al,
2001 | AGE | 5-FU | Prevent intestinal
damage caused by 5-FU | (44) |
| Petrovic et
al, 2018 | Garlic extract | Gemcitabine | Enhance cytotoxic
effects | (45) |
| Zou et al,
2016 | Allicin | 5-FU | Enhance cytotoxic
effects, induce apoptosis, increase intracellular ROS production,
reduce mitochondrial membrane potential, activate caspase-3 and
PARP, downregulate Bcl-2 | (46) |
| Khakbaz et
al, 2021 | Allicin | 5-FU | Enhance cytotoxic
effects, induce apoptosis, decrease P-gp and CD44 protein
level | (47) |
| Tigu et al,
2020 | Allicin | 5-FU | Enhance cytotoxic
effects | (48) |
| Gao et al,
2024 | Allicin | Paclitaxel
(Taxol) | Enhance cytotoxic
effects, inhibit CTSB and P-gp activity, inhibit growth of tumor
nodules in an orthotopic A549/Taxol nude mice model | (49) |
| Su et al,
2024 | DADS | 5-FU | Enhance cytotoxic
effects | (50) |
| Hassan, 2004 | Ajoene | Cytarabine and
Fludarabine | Enhance cytotoxic
effects, activate caspase-3, inhibit Bcl-2 | (52) |
Garlic extract
5-Fluorouracil (5-FU), a widely used pyrimidine
nucleoside analogue for managing PDAC, disrupts DNA synthesis by
the inhibition of thymidylate synthase activity leading to
apoptotic events. A recent study exhibited that white and black
garlic extract in combination with 5-FU increases the effects of
5-FU to Caco-2 cells if compared with 5-FU alone (42). Perez-Ortiz et al (43) examined combined treatment of garlic
extract with either 5-FU or Oxaliplatin, a platinum-based
anticancer agent that is utilized in the treatment of PDAC, against
colon cancer cells. The results showed that garlic extract enhances
the cytotoxicity of each drug (43). A study conducted by Horie et
al (44) revealed that a
standard laboratory diet plus AGE reduces orally administered
5-FU-induced intestinal damage in rats. Gemcitabine is a
deoxycytidine analogue extensively used as a first-line
chemotherapy for PDAC treatment; however, resistance to gemcitabine
is common and significantly limits drug efficacy (39,40).
Combining garlic extract with gemcitabine enhanced the chemotherapy
cytotoxic effect on breast cancer cells (45), suggesting a potential role for
garlic or its bioactive compounds in the management of gemcitabine
resistant pancreatic cancer.
Allicin
It was reported that allicin can enhance the
anticancer effects of 5-FU across different types of cancer cells
(46-48).
Moreover, allicin has been recently found to reverse resistance to
the anticancer drug Paclitaxel in non-small cell lung cancer cells
by inhibiting Cathepsin B activity and P-glycoprotein, a
transmembrane transporter that functions as a drug efflux pump
(49).
DADS
It was recently reported that DADS enhances the
cytotoxic effects of 5-FU on gastric cancer cells (50). Other studies reported that DADS and
DATS also contribute to chemosensitivity across multiple drugs
(23,51).
Ajoene
Ajoene was reported to augment the therapeutic
effects of cytarabine and fludarabine in resistant myeloid leukemia
cells, by enhancing caspase-3 activation and Bcl-2 inhibition
(52).
In addition to their therapeutic potential, garlic
and its components may mitigate the side effects of anticancer
drugs by alleviating tissue damage, modulating immunocytes, and
potentially allowing for lower dosage of the agents (44,53,54).
Taken together, these studies lay the foundation for using garlic
or its derivatives as adjunctive treatments to overcome
chemoresistance and improve the clinical management of patients
with PDAC.
4. Pancreatic cancer cells harbor
intracellular bacteria
Several previous studies have revealed the presence
of a diverse microbiome within human PDAC tissues (13,14,55-57).
Geller et al (55) found the
presence of Gammaproteobacteria in PDAC tissue specimens
obtained from patients who exhibit resistance to gemcitabine
treatment. Similarly, Riquelme et al (56) conducted an analysis of the PDAC
microbiome, and found a distinct intra-tumoral microbiome signature
in long-term survivors, comprising Pseudoxanthomonas,
Streptomyces, Saccharopolyspora and Bacillus
clausii. Additionally, a multinational research group
investigated the oral and gut microbiomes of patients with PDAC,
and found 4 enriched species (Streptococcus anginosus,
Streptococcus oralis, Veillonella parvula and
Veillonella atypica) and a depleted Faecalibacterium
prausnitzii in the gut signatures of patients with PDAC across
3 countries, Japan, Spain and Germany. Notably, these 4 microbial
species are known to reside in the oral cavity (58). These emerging findings suggest the
potential impact of the microbiome on cancer biology, including its
effects on cell phenotype, immune responses, tumor progression and
treatment outcomes. However, of note, several bacterial species
known to colonize the oral cavity have been identified in PDAC
tissues (59). These include
species associated with periodontitis, a condition reported to
increase PDAC risk (11,12). Periodontitis is a prevalent
inflammatory disease that affects the gingival tissue and alveolar
bone, and is generally initiated by a range of pro-inflammatory
factors produced by the host in response to a dysbiotic microbiome
(4,60,61).
Epidemiological studies have shown that oral pathogens, such as
Porphyromonas gingivalis (P. gingivalis),
Aggregatibacter actinomycetemcomitans (A.
actinomycetemcomitans) and Fusobacterium species are
linked to increased risk of pancreatic cancer (60,62-64).
P. gingivalis is an anaerobe closely associated with the
progression of periodontitis, and it has been recently demonstrated
that P. gingivalis translocates to the pancreas from the
oral cavity in mice, where it induces acinar-to-ductal metaplasia,
a precursor lesion to neoplasia (14,15).
Moreover, repetitive administration of P. gingivalis to mice
expressing oncogenic Kras in the pancreas accelerated pancreatic
intraepithelial neoplasia progression to PDAC (15). P. gingivalis was also shown
to promote the growth of pancreatic cancer in vivo in
xenograft models (14,65). In addition, a study reported the
detection rate for Fusobacterium species in pancreatic
cancer tissue to be 8.8%, and found higher mortality in the
Fusobacterium-positive group (64). A study by Udayasuryan et al
(16) found that Fusobacterium
nucleatum (F. nucleatum) infection elicited normal
pancreatic and PDAC cells to secrete cytokines including
granulocyte macrophage colony stimulating factor and C-X-C motif
chemokine ligand 1 (CXCL1). Conditioned medium from infected cells
promoted non-infected cell proliferation, and motility of
non-infected and infected PDAC cells (16). Moreover, an independent study has
reported that intracellular F. nucleatum promoted PDAC
progression via the CXCL1 and C-X-C motif chemokine receptor 2
axis, and F. nucleatum positive patients with PDAC had
larger tumor size and worse survival rate (17). Collectively, PDAC harbors a
microbiome including oral pathogens, and emerging evidence
indicates that these bacteria may contribute to the initiation and
progression of PDAC. Although the effects of garlic or its
compounds on the PDAC microbiome are as yet unknown, these findings
suggest that targeting tumor bacteria is an attractive potential
therapeutic strategy in PDAC prevention and treatment.
5. Antimicrobial activity of garlic, AGE and
organosulfur compounds
Garlic and its derived products have antimicrobial
properties on a wide spectrum of bacteria and fungi, suggesting
that treatment with garlic products may impact PDAC indirectly
through effects on the microbes associated with this cancer
(Table IV) (4,66-78).
Three cases will be considered to illustrate this point. First, in
both human and mouse models of PDAC, a unique fungal community
inhabits the pancreas (79-83).
Malassezia species, including Malassezia globosa,
were enriched in PDAC samples and associated with a poorer survival
rate (79,80). Garlic extracts strongly inhibit the
growth of Malassezia species in a dose dependent manner and
with activity comparative to a known antifungal compound,
ketoconazole (66). Second, garlic
products were shown to be active against Helicobacter pylori
(H. pylori), a spiral shaped bacterium that resides in the
stomach lining and is a well-established risk factor for chronic
gastritis, duodenal and gastric ulcers, and gastric cancer
(84). The presence of H.
pylori was also determined in the oral cavity and pancreas, and
its prevalence was reported to have positive association with
pancreatitis and periodontitis (84-88).
Moreover, H. pylori infection is considered to increase risk
of PDAC although the evidence remains debated (89-92).
Garlic and garlic extract are bactericidal against H. pylori
(68-70).
In addition, ajoenes isolated from oil-macerated garlic extract and
allicin inhibited H. pylori proliferation (68). In vivo, AGE alleviated H.
pylori-induced gastritis, indicating that garlic extract could
serve as an effective agent in preventing an inflammatory disorder
caused by H. pylori infection (71). Thus, garlic activity against this
pathogen may impact PDAC risk. Finally, evidence that garlic
products are active against oral anaerobic bacterial species
associated with periodontal disease may also link garlic's
antimicrobial activity to inhibition of the progression of PDAC
(75-78).
Bachrach et al (75)
reported inhibitory effects of allicin on the periopathogenic
species A. actinomycetemcomitans, F. nucleatum and
P. gingivalis. It was also demonstrated that allicin can
reduce the activity of P. gingivalis proteases, known as
major virulence factors of this bacterium (75). In addition, pharmacological studies
have shown that the aqueous extract of garlic inhibited the
proliferation of P. gingivalis, F. nucleatum
and A. actinomycetemcomitans, and blocked the proteolytic
activity of P. gingivalis proteases (76,77).
The garlic compound DAS also inhibits the proliferation and biofilm
formation of A. actinomycetemcomitans (78). Finally, a randomized controlled
double-blind study has reported that daily intake of an AGE product
containing 300 mg of AGE powder for 18 months reduced the level of
probing pocket depth, indicating that AGE can prevent and improve
the progression of periodontitis (18). Taken together, garlic products such
as AGE have great potential to modulate microbial and inflammatory
contributors to PDAC, and thereby reduce the risk of PDAC
development.
 | Table IVPotential antimicrobial effects of
garlic, its extract, and their organosulfur compounds on
microorganisms related to pancreatic ductal adenocarcinoma. |
Table IV
Potential antimicrobial effects of
garlic, its extract, and their organosulfur compounds on
microorganisms related to pancreatic ductal adenocarcinoma.
| First author/s,
year | Materials
tested | Target | Antimicrobial
effects | (Refs.) |
|---|
| Shams-Ghahfarokhi
et al, 2006 | Aqueous extracts of
garlic | Malassezia
furfur and other Malassezia species | Inhibit fungal
growth | (66) |
| Ohta et al,
1999 | Ajoenes (isolated
from oil-macerated garlic extract), Allicin | H.
pylori | Inhibit bacterial
growth | (68) |
| Cellini et
al, 1996 | Aqueous garlic
extract | H.
pylori | Inhibit bacterial
growth | (69) |
| O'Gara et
al, 2000 | Garlic oil, garlic
powder, Allicin, and their diallyl sulfur components | H.
pylori | Inhibit bacterial
growth | (70) |
| Iimuro et
al, 2002 | AGE | H.
pylori-induced gastritis in male Mongolian gerbils | Alleviate gastric
inflammation | (71) |
| Bachrach et
al, 2011 | Allicin | A.
actinomycetemcomitans and F. nucleatum | Inhibit bacterial
growth | (75) |
| | Allicin | P.
gingivalis | Reduce protease
activity of P. gingivalis | |
| Bakri and Douglas,
2005 | Aqueous extract of
garlic | A.
actinomycetemcomitans, F. nucleatum and P.
gingivalis | Inhibit bacterial
growth, reduce protease activity of P. gingivalis | (76) |
| Shetty et
al, 2013 | Ethanolic and
aqueous extracts of garlic | P.
gingivalis and A. actinomycetemcomitans | Inhibit bacterial
growth, reduce protease activity of P. gingivalis | (77) |
| Velliyagounder
et al, 2012 | Garlic extract and
Allicin | A.
actinomycetemcomitans | Inhibit bacterial
growth | (78) |
| | DAS | A.
actinomycetemcomitans | Inhibit bacterial
growth and biofilm formation | |
6. Conclusion and future perspectives
PDAC remains among the most difficult of cancers and
there is an urgent need for improvements in early diagnosis and
effective therapy. Garlic, along with its derived compounds and
products, holds great potential to contribute to the prevention and
treatment of PDAC by exerting direct effects on tumor growth and
indirect effects on tumor-associated microbes (summarized in
Fig. 1). However, to explore the
full potential of garlic in managing PDAC, further mechanistic
studies, and pharmacological experiments aimed at optimizing the
delivery of bioactive compounds to the pancreas, are necessary. The
antimicrobial potential of garlic against intratumor microbes, and
in particular intracellular bacteria, is an exciting area for
exploration, along with garlic's contribution to the balance of
reactive oxygen species in PDAC, and to the tumor immune
microenvironment. These studies will determine how to integrate
garlic products in the future treatment toolbox for PDAC.
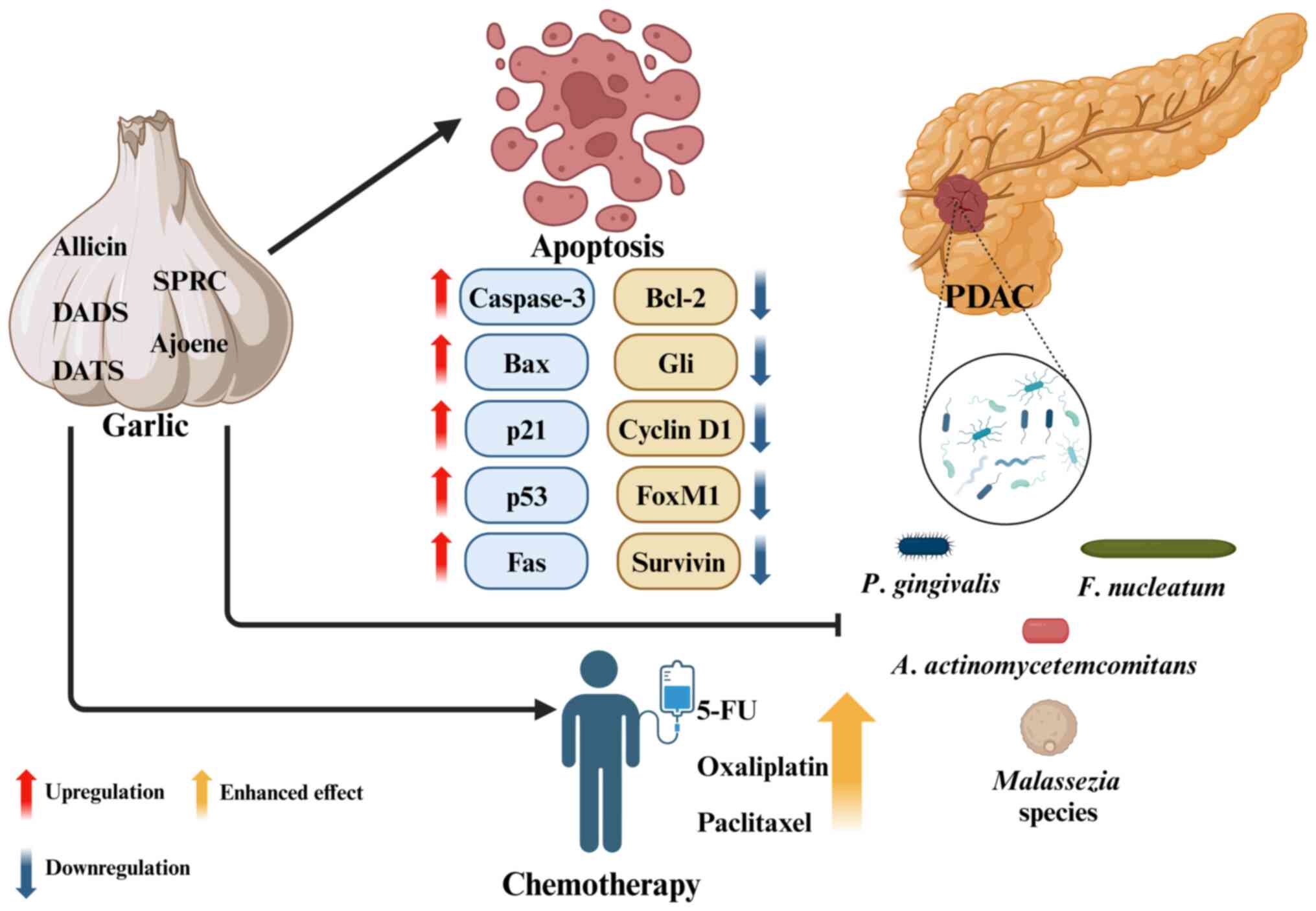 | Figure 1Schematic diagram of the potential
therapeutic effects of garlic and its organosulfur compounds on
PDAC. Garlic components inhibit PDAC cell proliferation by
modulating survival-related molecules and inducing apoptosis. They
also enhance the efficacy of chemotherapeutic agents, such as 5-FU,
Oxaliplatin and Paclitaxel. PDAC tissue harbors microorganisms,
including oral bacteria and fungi such as Malassezia
species. The antimicrobial activity of garlic products has
significant potential to prevent and treat pancreatic malignancies.
The figure was created using BioRender.com. DADS, diallyl disulfide; DATS, diallyl
trisulfide; SPRC, S-propargyl-cysteine; Bax, Bcl-2
associated X-protein; Bcl-2, B cell lymphoma 2; Gli,
glioma-associated oncogene; FoxM1, forkhead box protein M1; 5-FU,
5-fluorouracil; PDAC, pancreatic ductal adenocarcinoma; P.
gingivalis, Porphyromonas gingivalis; F.
nucleatum, Fusobacterium nucleatum; A.
actinomycetemcomitans, Aggregatibacter
actinomycetemcomitans. |
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Israel Cancer
Research Fund and Wakunaga Pharmaceutical Co., Ltd.
Availability of data and materials
Not applicable.
Authors' contributions
HK was a major contributor in writing the
manuscript. GN revised and provided comments during all stages of
writing the manuscript. Both authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Financial support was received from Wakunaga
Pharmaceutical Co., Ltd.
References
|
1
|
De Greef D, Barton EM, Sandberg EN, Croley
CR, Pumarol J, Wong TL, Das N and Bishayee A: Anticancer potential
of garlic and its bioactive constituents: A systematic and
comprehensive review. Semin Cancer Biol. 73:219–264.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ryu K, Ide N, Matsuura H and Itakura Y: N
alpha-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant compound
identified in aged garlic extract. J Nutr. 131:972S–976S.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsutomo T: Potential benefits of garlic
and other dietary supplements for the management of hypertension.
Exp Ther Med. 19:1479–1484. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ohtani M and Nishimura T: The preventive
and therapeutic application of garlic and other plant ingredients
in the treatment of periodontal diseases. Exp Ther Med.
19:1507–1510. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tanaka S, Haruma K, Yoshihara M, Kajiyama
G, Kira K, Amagase H and Chayama K: Aged garlic extract has
potential suppressive effect on colorectal adenomas in humans. J
Nutr. 136 (Suppl 3):821S–826S. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jikihara H, Qi G, Nozoe K, Hirokawa M,
Sato H, Sugihara Y and Shimamoto F: Aged garlic extract inhibits
1,2-dimethylhydrazine-induced colon tumor development by
suppressing cell proliferation. Oncol Rep. 33:1131–1140.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ohkubo S, Dalla Via L, Grancara S,
Kanamori Y, García-Argáez AN, Canettieri G, Arcari P, Toninello A
and Agostinelli E: The antioxidant, aged garlic extract, exerts
cytotoxic effects on wild-type and multidrug-resistant human cancer
cells by altering mitochondrial permeability. Int J Oncol.
53:1257–1268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ishikawa H, Saeki T, Otani T, Suzuki T,
Shimozuma K, Nishino H, Fukuda S and Morimoto K: Aged garlic
extract prevents a decline of NK cell number and activity in
patients with advanced cancer. J Nutr. 136 (Suppl 3):816S–820S.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L,
Zhang Y, Guo Y, Zhou T, Li JY, Shen L, et al: Effects of
Helicobacter pylori treatment and vitamin and garlic
supplementation on gastric cancer incidence and mortality:
Follow-up of a randomized intervention trial. BMJ.
366(l5016)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rawla P, Sunkara T and Gaduputi V:
Epidemiology of pancreatic cancer: Global trends, etiology and risk
factors. World J Oncol. 10:10–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Michaud DS: Role of bacterial infections
in pancreatic cancer. Carcinogenesis. 34:2193–2197. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thomas RM and Jobin C: Microbiota in
pancreatic health and disease: The next frontier in microbiome
research. Nat Rev Gastroenterol Hepatol. 17:53–64. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abe S, Masuda A, Matsumoto T, Inoue J,
Toyama H, Sakai A, Kobayashi T, Tanaka T, Tsujimae M, Yamakawa K,
et al: Impact of intratumoral microbiome on tumor immunity and
prognosis in human pancreatic ductal adenocarcinoma. J
Gastroenterol. 59:250–262. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan Q, Ma X, Yang B, Liu Y, Xie Y, Wang X,
Yuan W and Ma J: Periodontitis pathogen Porphyromonas
gingivalis promotes pancreatic tumorigenesis via neutrophil
elastase from tumor-associated neutrophils. Gut Microbes.
14(2073785)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saba E, Farhat M, Daoud A, Khashan A,
Forkush E, Menahem NH, Makkawi H, Pandi P, Angabo S, Kawasaki H, et
al: Oral bacteria accelerate pancreatic cancer development in mice.
Gut. 73:770–786. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Udayasuryan B, Ahmad RN, Nguyen TTD, Umaña
A, Roberts LDM, Sobol P, Jones SD, Munson JM, Slade DJ and
Verbridge SS: Fusobacterium nucleatum induces proliferation
and migration in pancreatic cancer cells through host autocrine and
paracrine signaling. Sci Signal. 15(eabn4948)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hayashi M, Ikenaga N, Nakata K, Luo H,
Zhong PS, Date S, Oyama K, Higashijima N, Kubo A, Iwamoto C, et al:
Intratumor Fusobacterium nucleatum promotes the progression
of pancreatic cancer via the CXCL1-CXCR2 axis. Cancer Sci.
114:3666–3678. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zini A, Mann J, Mazor S and Vered Y:
Beneficial effect of aged garlic extract on periodontitis: A
randomized controlled double-blind clinical study. J Clin Biochem
Nutr. 67:297–301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chan JM, Wang F and Holly EA: Vegetable
and fruit intake and pancreatic cancer in a population-based
case-control study in the San Francisco bay area. Cancer Epidemiol
Biomarkers Prev. 14:2093–2097. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Abe K, Hori Y and Myoda T: Volatile
compounds of fresh and processed garlic. Exp Ther Med.
19:1585–1593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trio PZ, You S, He X, He J, Sakao K and
Hou DX: Chemopreventive functions and molecular mechanisms of
garlic organosulfur compounds. Food Funct. 5:833–844.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh P, Patel M, Bhowmik D, Kumari N,
Prajapati SK and Gupta R: Identification of common biomarkers
affecting patient survival in cancers. World Acad Sci J.
6(53)2024.
|
|
23
|
Lu L, Gao Z, Song J, Jin L and Liang Z:
The potential of diallyl trisulfide for cancer prevention and
treatment, with mechanism insights. Front Cell Dev Biol.
12(1450836)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sarhadi VK and Armengol G: Molecular
biomarkers in cancer. Biomolecules. 12(1021)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sugiura R, Satoh R and Takasaki T: ERK: A
double-edged sword in cancer. ERK-dependent apoptosis as a
potential therapeutic strategy for cancer. Cells.
10(2509)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lan XY, Sun HY, Liu JJ, Lin Y, Zhu ZY, Han
X, Sun X, Li XR, Zhang HC and Tang ZY: Effects of garlic oil on
pancreatic cancer cells. Asian Pac J Cancer Prev. 14:5905–5910.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chhabria SV, Akbarsha MA, Li AP, Kharkar
PS and Desai KB: In situ allicin generation using targeted
alliinase delivery for inhibition of MIA PaCa-2 cells via
epigenetic changes, oxidative stress and cyclin-dependent kinase
inhibitor (CDKI) expression. Apoptosis. 20:1388–1409.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang CJ, Wang C, Han J, Wang YK, Tang L,
Shen DW, Zhao Y, Xu RH and Zhang H: Effect of combined treatment
with recombinant interleukin-2 and allicin on pancreatic cancer.
Mol Biol Rep. 40:6579–6585. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Saini V, Manral A, Arora R, Meena P,
Gusain S, Saluja D and Tiwari M: Novel synthetic analogs of diallyl
disulfide triggers cell cycle arrest and apoptosis via ROS
generation in MIA PaCa-2 cells. Pharmacol Rep. 69:813–821.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma HB, Huang S, Yin XR, Zhang Y and Di ZL:
Apoptotic pathway induced by diallyl trisulfide in pancreatic
cancer cells. World J Gastroenterol. 20:193–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee HJ, Jeong JH and Ryu JH:
Anti-pancreatic cancer activity of Z-ajoene from garlic: An
inhibitor of the Hedgehog/Gli/FoxM1 axis. J Funct Foods.
56:102–109. 2019.
|
|
32
|
Wang W, Cheng J and Zhu Y: The JNK
signaling pathway is a novel molecular target for
S-propargyl-L-cysteine, a naturally-occurring garlic derivatives:
Link to its anticancer activity in pancreatic cancer in vitro and
in vivo. Curr Cancer Drug Targets. 15:613–623. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Murtaugh LC and Keefe MD: Regeneration and
repair of the exocrine pancreas. Annu Rev Physiol. 77:229–249.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sidhapuriwala JN, Hegde A, Ang AD, Zhu YZ
and Bhatia M: Effects of S-propargyl-cysteine (SPRC) in
caerulein-induced acute pancreatitis in mice. PLoS One.
7(e32574)2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mathan Kumar M and Tamizhselvi R:
Protective effect of diallyl disulfide against cerulein-induced
acute pancreatitis and associated lung injury in mice. Int
Immunopharmacol. 80(106136)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Marimuthu MK, Moorthy A and Ramasamy T:
Diallyl disulfide attenuates STAT3 and NF-κB pathway through PPAR-γ
activation in cerulein-induced acute pancreatitis and associated
lung injury in mice. Inflammation. 45:45–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Orján EM, Kormányos ES, Fűr GM, Dombi A,
Bálint ER, Balla Z, Balog BA, Dágó A, Totonji A, Bátai ZI, et al:
The anti-inflammatory effect of dimethyl trisulfide in experimental
acute pancreatitis. Sci Rep. 13(16813)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lunova M, Zizer E, Kucukoglu O, Schwarz C,
Dillmann WH, Wagner M and Strnad P: Hsp72 overexpression
accelerates the recovery from caerulein-induced pancreatitis. PLoS
One. 7(e39972)2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hamada S, Masamune A and Shimosegawa T:
Novel therapeutic strategies targeting tumor-stromal interactions
in pancreatic cancer. Front Physiol. 4(331)2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Saiki Y, Hirota S and Horii A: Attempts to
remodel the pathways of gemcitabine metabolism: Recent approaches
to overcoming tumours with acquired chemoresistance. Cancer drug
Resist. 3:819–831. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Patel M, Singh P, Gandupalli L and Gupta
R: Identification and evaluation of survival-associated common
chemoresistant genes in cancer. Biomed Biotechnol Res J. 8:320–327.
2024.
|
|
42
|
Ozalp Unal D and Sel T: Investigation of
antiproliferative effects of combinations of white and black garlic
extracts with 5-fluorouracil (5-FU) on caco-2 colorectal
adenocarcinoma cells. Mol Nutr Food Res.
68(e2300820)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Perez-Ortiz JM, Galan-Moya EM, de la
Cruz-Morcillo MA, Rodriguez JF, Gracia I, Garcia MT and
Redondo-Calvo FJ: Cost effective use of a thiosulfinate-enriched
Allium sativum extract in combination with chemotherapy in
colon cancer. Int J Mol Sci. 21(2766)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Horie T, Awazu S, Itakura Y and Fuwa T:
Alleviation by garlic of antitumor drug-induced damage to the
intestine. J Nutr. 131 (3s):1071S–1074S. 2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Petrovic V, Nepal A, Olaisen C, Bachke S,
Hira J, Søgaard CK, Røst LM, Misund K, Andreassen T, Melø TM, et
al: Anti-cancer potential of homemade fresh garlic extract is
related to increased endoplasmic reticulum stress. Nutrients.
10(450)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zou X, Liang J, Sun J, Hu X, Lei L, Wu D
and Liu L: Allicin sensitizes hepatocellular cancer cells to
anti-tumor activity of 5-fluorouracil through ROS-mediated
mitochondrial pathway. J Pharmacol Sci. 131:233–240.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Khakbaz P, Panahizadeh R, Vatankhah MA and
Najafzadeh N: Allicin reduces 5-fluorouracil-resistance in gastric
cancer cells through modulating MDR1, DKK1, and WNT5A expression.
Drug Res (Stuttg). 71:448–454. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Țigu AB, Toma VA, Moț AC, Jurj A, Moldovan
CS, Fischer-Fodor E, Berindan-Neagoe I and Pârvu M: The synergistic
antitumor effect of 5-fluorouracil combined with allicin against
lung and colorectal carcinoma cells. Molecules.
25(1947)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gao X, Xu C, Santhanam RK, Zhang Y and
Zhao Q: Allicin: A natural weapon against Taxol resistance in
non-small cell lung cancer through cathepsin B inhibition and
lysosomal-autophagy disruption. Food Front. 1–14. 2024.
|
|
50
|
Su J, Xia H, He H, Tang H, Zhou J, Xun Y,
Liu F, Su B and Su Q: Diallyl disulfide antagonizes DJ-1 mediated
proliferation, epithelial-mesenchymal transition, and
chemoresistance in gastric cancer cells. Environ Toxicol.
39:4105–4119. 2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Malla R, Marni R, Chakraborty A and Kamal
MA: Diallyl disulfide and diallyl trisulfide in garlic as novel
therapeutic agents to overcome drug resistance in breast cancer. J
Pharm Anal. 12:221–231. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hassan HT: Ajoene (natural garlic
compound): A new anti-leukaemia agent for AML therapy. Leuk Res.
28:667–671. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Raisuddin S, Ahmad S, Fatima M and Dabeer
S: Toxicity of anticancer drugs and its prevention with special
reference to role of garlic constituents. Ann Phytomed. 7:13–26.
2018.
|
|
54
|
Zhang QY, Wang FX, Jia KK and Kong LD:
Natural product interventions for chemotherapy and
radiotherapy-induced side effects. Front Pharmacol.
9(1253)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Geller LT, Barzily-Rokni M, Danino T,
Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee
K, et al: Potential role of intratumor bacteria in mediating tumor
resistance to the chemotherapeutic drug gemcitabine. Science.
357:1156–1160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Riquelme E, Zhang Y, Zhang L, Montiel M,
Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et
al: Tumor microbiome diversity and composition influence pancreatic
cancer outcomes. Cell. 178:795–806.e12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Del Castillo E, Meier R, Chung M, Koestler
DC, Chen T, Paster BJ, Charpentier KP, Kelsey KT, Izard J and
Michaud DS: The microbiomes of pancreatic and duodenum tissue
overlap and are highly subject specific but differ between
pancreatic cancer and noncancer subjects. Cancer Epidemiol
Biomarkers Prev. 28:370–383. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nagata N, Nishijima S, Kojima Y, Hisada Y,
Imbe K, Miyoshi-Akiyama T, Suda W, Kimura M, Aoki R, Sekine K, et
al: Metagenomic identification of microbial signatures predicting
pancreatic cancer from a multinational study. Gastroenterology.
163:222–238. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pushalkar S, Hundeyin M, Daley D,
Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres
LE, et al: The pancreatic cancer microbiome promotes oncogenesis by
induction of innate and adaptive immune suppression. Cancer Discov.
8:403–416. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Maisonneuve P, Amar S and Lowenfels AB:
Periodontal disease, edentulism, and pancreatic cancer: A
meta-analysis. Ann Oncol. 28:985–995. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kawasaki H and Amano H: Anti-inflammatory
role of microRNA-429 in human gingival epithelial cells-inhibition
of IL-8 production through direct binding to IKKβ mRNA. Mol Med
Rep. 24(581)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Michaud DS, Izard J, Wilhelm-Benartzi CS,
You DH, Grote VA, Tjønneland A, Dahm CC, Overvad K, Jenab M,
Fedirko V, et al: Plasma antibodies to oral bacteria and risk of
pancreatic cancer in a large European prospective cohort study.
Gut. 62:1764–1770. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fan X, Alekseyenko AV, Wu J, Peters BA,
Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R,
Miller G, et al: Human oral microbiome and prospective risk for
pancreatic cancer: A population-based nested case-control study.
Gut. 67:120–127. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga
Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, et
al: Association of Fusobacterium species in pancreatic
cancer tissues with molecular features and prognosis. Oncotarget.
6:7209–7220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Gnanasekaran J, Gallimidi AB, Saba E,
Pandi K, Berchoer LE, Hermano E, Angabo S, Makkawi H, Khashan A,
Daoud A, et al: Intracellular Porphyromonas gingivalis
promotes the tumorigenic behavior of pancreatic carcinoma cells.
Cancers (Basel). 12(2331)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Shams-Ghahfarokhi M, Shokoohamiri MR,
Amirrajab N, Moghadasi B, Ghajari A, Zeini F, Sadeghi G and
Razzaghi-Abyaneh M: In vitro antifungal activities of Allium cepa,
Allium sativum and ketoconazole against some pathogenic
yeasts and dermatophytes. Fitoterapia. 77:321–323. 2006.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nakamoto M, Kunimura K, Suzuki J and
Kodera Y: Antimicrobial properties of hydrophobic compounds in
garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp
Ther Med. 19:1550–1553. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ohta R, Yamada N, Kaneko H, Ishikawa K,
Fukuda H, Fujino T and Suzuki A: In vitro inhibition of the growth
of Helicobacter pylori by oil-macerated garlic constituents.
Antimicrob Agents Chemother. 43:1811–1812. 1999.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Cellini L, Di Campli E, Masulli M, Di
Bartolomeo S and Allocati N: Inhibition of Helicobacter
pylori by garlic extract (Allium sativum). FEMS Immunol
Med Microbiol. 13:273–277. 1996.PubMed/NCBI View Article : Google Scholar
|
|
70
|
O'Gara EA, Hill DJ and Maslin DJ:
Activities of garlic oil, garlic powder, and their diallyl
constituents against Helicobacter pylori. Appl Environ
Microbiol. 66:2269–2273. 2000.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Iimuro M, Shibata H, Kawamori T, Matsumoto
T, Arakawa T, Sugimura T and Wakabayashi K: Suppressive effects of
garlic extract on Helicobacter pylori-induced gastritis in
Mongolian gerbils. Cancer Lett. 187:61–68. 2002.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Müller A, Eller J, Albrecht F, Prochnow P,
Kuhlmann K, Bandow JE, Slusarenko AJ and Leichert LIO: Allicin
induces thiol stress in bacteria through S-allylmercapto
modification of protein cysteines. J Biol Chem. 291:11477–11490.
2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Petropoulos S, Fernandes Â, Barros L,
Ciric A, Sokovic M and Ferreira ICFR: Antimicrobial and antioxidant
properties of various Greek garlic genotypes. Food Chem. 245:7–12.
2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Fujisawa H, Watanabe K, Suma K, Origuchi
K, Matsufuji H, Seki T and Ariga T: Antibacterial potential of
garlic-derived allicin and its cancellation by sulfhydryl
compounds. Biosci Biotechnol Biochem. 73:1948–1955. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Bachrach G, Jamil A, Naor R, Tal G, Ludmer
Z and Steinberg D: Garlic allicin as a potential agent for
controlling oral pathogens. J Med Food. 14:1338–1343.
2011.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bakri IM and Douglas CWI: Inhibitory
effect of garlic extract on oral bacteria. Arch Oral Biol.
50:645–651. 2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Shetty S, Thomas B, Shetty V, Bhandary R
and Shetty R: An in-vitro evaluation of the efficacy of garlic
extract as an antimicrobial agent on periodontal pathogens: A
microbiological study. Ayu. 34:445–451. 2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Velliyagounder K, Ganeshnarayan K,
Velusamy SK and Fine DH: In vitro efficacy of diallyl sulfides
against the periodontopathogen Aggregatibacter
actinomycetemcomitans. Antimicrob Agents Chemother.
56:2397–2407. 2012.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Aykut B, Pushalkar S, Chen R, Li Q,
Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, et al:
The fungal mycobiome promotes pancreatic oncogenesis via activation
of MBL. Nature. 574264–267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Okuno K, Tokunaga M, Von Hoff D, Kinugasa
Y and Goel A: PDAC Biomarker Working Group. Intratumoral
Malassezia globosa levels predict survival and therapeutic
response to adjuvant chemotherapy in patients with pancreatic
ductal adenocarcinoma. Gastroenterology. 165:502–504.e2.
2023.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Narunsky-Haziza L, Sepich-Poore GD,
Livyatan I, Asraf O, Martino C, Nejman D, Gavert N, Stajich JE,
Amit G, González A, et al: Pan-cancer analyses reveal
cancer-type-specific fungal ecologies and bacteriome interactions.
Cell. 185:3789–3806.e17. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Alam A, Levanduski E, Denz P,
Villavicencio HS, Bhatta M, Alhorebi L, Zhang Y, Gomez EC, Morreale
B, Senchanthisai S, et al: Fungal mycobiome drives IL-33 secretion
and type 2 immunity in pancreatic cancer. Cancer Cell.
40:153–167.e11. 2022.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Brayer KJ, Hanson JA, Cingam S, Martinez
C, Ness SA and Rabinowitz I: The inflammatory response of human
pancreatic cancer samples compared to normal controls. PLoS One.
18(e0284232)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kunovsky L, Dite P, Jabandziev P, Dolina
J, Vaculova J, Blaho M, Bojkova M, Dvorackova J, Uvirova M, Kala Z
and Trna J: Helicobacter pylori infection and other bacteria
in pancreatic cancer and autoimmune pancreatitis. World J
Gastrointest Oncol. 13:835–844. 2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Nilsson HO, Stenram U, Ihse I and Wadstrom
T: Helicobacter species ribosomal DNA in the pancreas, stomach and
duodenum of pancreatic cancer patients. World J Gastroenterol.
12:3038–3043. 2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Lindén SK, Wickström C, Lindell G,
Gilshenan K and Carlstedt I: Four modes of adhesion are used during
Helicobacter pylori binding to human mucins in the oral and
gastric niches. Helicobacter. 13:81–93. 2008.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Umeda M, Kobayashi H, Takeuchi Y, Hayashi
J, Morotome-Hayashi Y, Yano K, Aoki A, Ohkusa T and Ishikawa I:
High prevalence of Helicobacter pylori detected by PCR in
the oral cavities of periodontitis patients. J Periodontol.
74:129–134. 2003.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wei X, Zhao HQ, Ma C, Zhang AB, Feng H,
Zhang D and Liu C: The association between chronic periodontitis
and oral Helicobacter pylori: A meta-analysis. PLoS One.
14(e0225247)2019.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hirabayashi M, Inoue M, Sawada N, Saito E,
Abe SK, Hidaka A, Iwasaki M, Yamaji T, Shimazu T and Tsugane S:
Helicobacter pylori infection, atrophic gastritis, and risk
of pancreatic cancer: A population-based cohort study in a large
Japanese population: The JPHC study. Sci Rep.
9(6099)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Stolzenberg-solomon RZ, Blaser MJ, Limburg
PJ, Perez-Perez G, Taylor RP, Virtamo J and Albanes D:
Helicobacter pylori seropositivity as a risk factor for
pancreatic cancer. J Natl Cancer Inst. 93:937–941. 2001.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Trikudanathan G, Philip A, Dasanu CA and
Baker WL: Association between Helicobacter pylori infection
and pancreatic cancer. A cumulative meta-analysis. JOP. 12:26–31.
2011.PubMed/NCBI
|
|
92
|
Risch HA: Pancreatic cancer:
Helicobacter pylori colonization, N-nitrosamine exposures,
and ABO blood group. Mol Carcinog. 51:109–118. 2012.PubMed/NCBI View Article : Google Scholar
|















