Introduction
Osteoarthritis (OA), among the most common joint
disorders, is characterized by articular cartilage damage and
involves the entirety of joint tissues, leading to the eventual
degeneration, fibrosis and fracture of the articular cartilage and
damage to the complete joint surface (1-3).
The clinical manifestations of OA primarily include pain,
stiffness, hypertrophy and restricted movement, particularly in
weight-bearing joints such as knees and hips (4-6).
Thus, effective pain management is important throughout OA
treatment, with the selection, administration mode and dosage of
medications being critical (7-10).
Topical nonsteroidal anti-inflammatory drugs
(NSAIDs) can inhibit prostaglandin synthesis in the body, thereby
exerting anti-inflammatory and analgesic effects and treating OA
mechanistically (11). Clinical
research has demonstrated that NSAIDs have a superior therapeutic
efficacy in knee OA (12-14).
At present, various commonly used topical formulations are
available. One such formulation called cataplasms are hydrophilic
polymer materials that serve as matrices for transdermal drug
delivery. They possess several advantages, such as high
drug-loading capacity, moisture retention capability,
breathability, and non-allergenic and non-irritating properties.
Common topical NSAIDs in the cataplasm form include loxoprofen
sodium and flurbiprofen (15).
However, head-to-head clinical studies and comparative data
regarding these two drugs are lacking. Thus, the present study
aimed to systematically compare the clinical effectiveness and
related indicators of loxoprofen sodium cataplasm (LSC) and
flurbiprofen cataplasm (FPC) in OA treatment, and to evaluate the
efficacy and safety of LSC in this context.
FPC is a topical NSAIDs that is commonly used for
the treatment of OA. It works by inhibiting cyclooxygenase (COX)
enzymes, thereby reducing the synthesis of prostaglandins, which
are mediators of inflammation and pain (16). The justification for comparing FPC
and LSC in the present study was based on the need for a direct
evaluation of the efficacy and safety profiles of these two
commonly used topical NSAIDs in the treatment of OA. While both
medications are established treatments for OA, there has been a
lack of head-to-head clinical studies that provide comparative data
regarding their effectiveness and safety (17,18).
FPC is widely recognized and used in clinical practice for OA
management (19-21);
by selecting it as the control, the present study aimed to provide
meaningful insights into the relative benefits of LSC. This
comparison is particularly relevant given the increasing prevalence
of OA and the importance of optimizing treatment options to improve
patient outcomes (22,23). Osteoarthritis is a debilitating,
long-lasting condition affecting the structure and function of
synovial joints, affecting >200 million people globally and
having doubled in incidence over the past 50 years. The present
study contributed valuable evidence to inform clinical
decision-making regarding the use of topical NSAIDs in OA
treatment.
The 2-week follow-up was chosen to focus on the
short-term efficacy and safety outcomes, which are critical for
assessing the initial response to treatment in patients with OA.
This duration allowed for the evaluation of immediate pain relief
and functional improvement, which are often the primary concerns
for patients seeking treatment. Additionally, a shorter follow-up
period is common in studies evaluating topical treatments, as it
enables the observation of early adverse effects and therapeutic
responses without the confounding influence of long-term treatment
adherence or cumulative side effects (24,25).
The scientific demand for comparing LSC and FPC
arises from the increasing prevalence of OA and the need for
effective management strategies that minimize systemic side
effects. The two medications are widely used in clinical practice
(17,18), but there is a lack of direct
comparative studies that assess their efficacy and safety in a
controlled environment. By addressing this gap, the present study
provided essential insights that can help clinicians make informed
decisions regarding the choice of topical NSAIDs for OA
treatment.
Materials and methods
Ethical approval
The present study was approved by the Ethics
Committees of The Second Affiliated Hospital of Xi'an Jiaotong
University, Shaanxi, China [ethics approval no. Lun Shen (2023) no.
003 Xi'an, China] and The Second Affiliated Hospital of Nanchang
University, Jiangxi Chian [ethics approval no Lun Shen (2022) no.
16; Nanchang, China] and Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology, Hubei, China [ethics
approval no. Lun Shen (2021) no. (0117)-01; Wuhan, China]. The
participants provided their written informed consent to participate
in the present study.
Subject enrollment. Inclusion and
exclusion criteria
The inclusion criteria were as follows: i)
Participants voluntarily consenting to participate in the study and
providing written informed consent; ii) male and female
participants aged between 20 and 85 years; iii) participants
clinically diagnosed with OA; iv) participants with joint pain
symptoms; and v) participants taking only study-specified/control
medication.
The exclusion criteria were as follows: i) Patients
with bleeding disorders; ii) patients with bronchial asthma; iii)
patients with severe cardiac, hepatic and renal insufficiency, with
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
levels exceeding twice the normal value, and serum creatinine
levels exceeding the normal value. Normal values: <40 U/l for
AST, <50 U/l for ALT, 35-80 µmol/l for serum creatinine; iv)
pregnant and lactating women or women of childbearing age planning
to conceive; v) patients with known hypersensitivity to NSAIDs; and
vi) patients who participated in clinical studies of other drugs
within the past month or had other concurrent diseases or
complications that may affect the assessment of drug efficacy (skin
breakage, infection at the site of drug application or skin
rash).
Discontinuation and withdrawal criteria. The
discontinuation and withdrawal criteria were as follows: i) Adverse
events such as skin sensitization preventing the subject from
continuing treatment; ii) unwillingness to continue treatment; iii)
noncompliance with the study protocol; iv) use of prohibited drugs
during the study; and v) pregnancy.
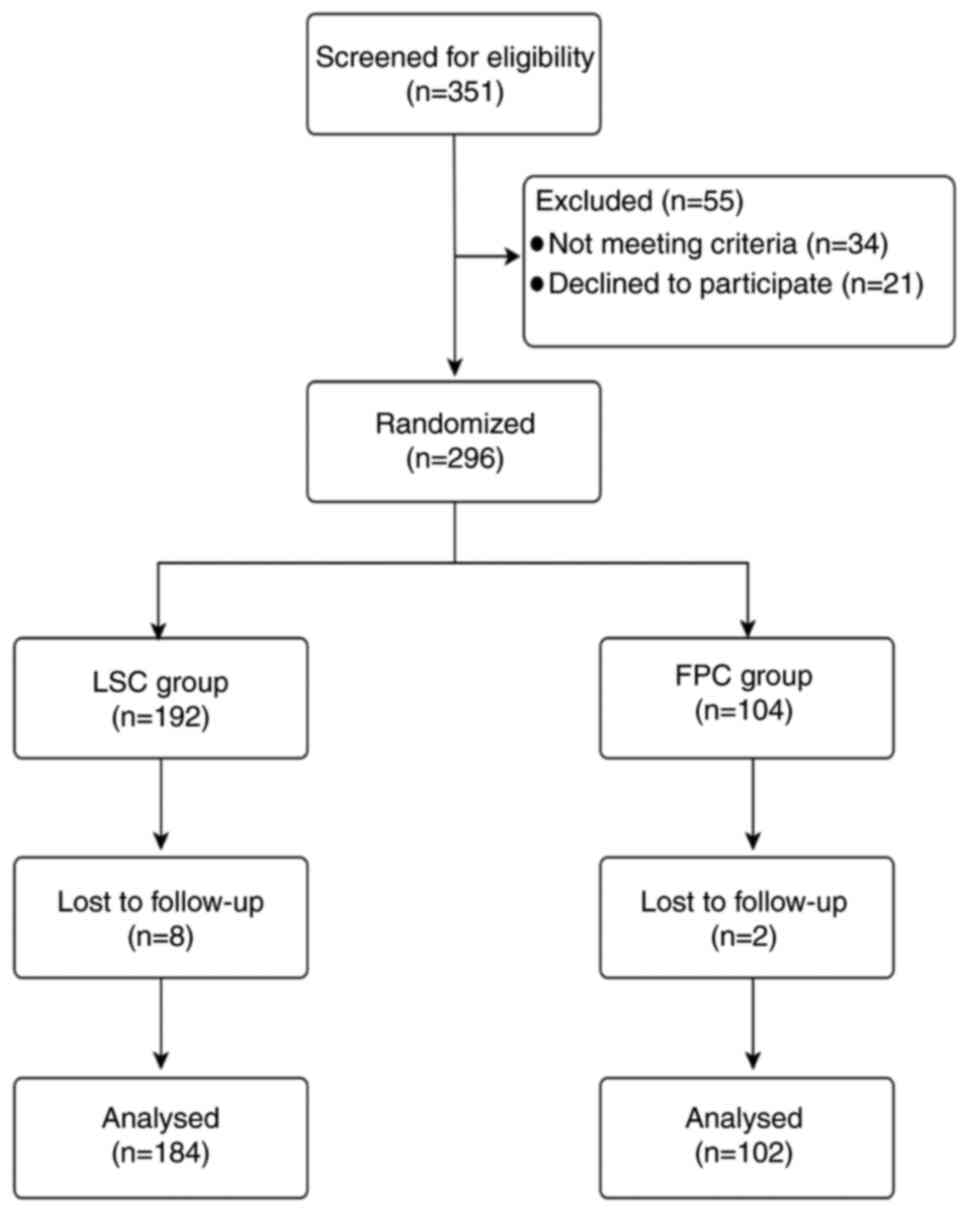
Between June 2023 and April 2024, 351 subjects from
three hospitals were invited to participate. The three hospitals
included The Second Affiliated Hospital of Xi'an Jiaotong
University, the Second Affiliated Hospital of Nanchang University,
and Union Hospital, Tongji Medical College, Huazhong University of
Science and Technology. Among the subjects, 34 were excluded due to
not meeting the inclusion criteria and 21 declined to participate.
Additionally, two subjects in the FPC group and eight in the LSC
group were lost to follow-up (Fig.
1). The LSC group included 83 male and 104 female patients,
with five individuals whose sex was not recorded, totaling 192
individuals, whereas the FPC group comprised 63 male and 41 female
patients. The age of the 192 participants in the LSC group ranged
between 24 and 84 years, with a mean age of 58.46 years; the age of
5 individuals was not included in the analysis. In addition, the
age of the 104 individuals in the FPC group ranged between 35 and
84 years, with a mean age of 59.82 years.
Research methods
The two groups received topical analgesic drugs in
addition to basic treatment for 2 weeks. The basic treatment
provided to all participants included non-pharmacological
interventions such as physical therapy and education on joint
protection techniques. LSC (Hunan Jiudian Pharmaceutical Co., Ltd.;
state drug license H20173272; specifications, 100 mg/patch) was
administered to the LSC group and FPC (Beijing Tide Pharmaceutical
Co., Ltd.; state drug license H20103549; specifications, 40
mg/patch) was administered to the FPC group. LSC was applied
topically to the affected area once daily, with one patch applied
per area for 2 weeks according to the manufacturer's instruction.
FPC was applied twice daily, with one patch applied per area for 2
weeks, according to the manufacturer's instructions. The treatment
duration was 2 weeks and follow-up visits were scheduled at the end
of the second week after the initial treatment.
Assessment. Efficacy assessment
Treatment effectiveness at week 2 was the primary
indicator, and this was assessed using three types of evaluation.
The first evaluation type was based on the Visual Analog Scale
(VAS) score as follows (26): 0, no
pain; 1-3, mild pain; 4-6, moderate pain; 7-10, severe pain; and
10, most severe pain. The efficacy index was defined as follows:
Cured, efficacy index ≥95%; apparent efficacy, efficacy index
between ≥70 and <95%; effective, efficacy index between ≥30 and
<70%; and ineffective, efficacy index <30%. The treatment
effectiveness rate was calculated as (number of cured cases +
number of apparent effective cases + number of effective
cases)/total number of cases x100%.
The second evaluation type was based on Western
Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
(27-29).
The criteria for cured, apparent efficacy and effective were the
same as those for the first evaluation type. For patients with
disease in both knees, the side of the patient with the more severe
disease was evaluated and statistically analyzed. The treatment
effectiveness rate was analyzed as (number of cured cases + number
of apparent effective cases + number of effective cases)/total
number of cases x100%.
The third evaluation type was based on the Lysholm
score (30). The efficacy was
defined as follows: Cured, pain and morning stiffness completely
disappeared, and the Lysholm score improved by ≥30 points;
improvement, improvement in pain, morning stiffness and other
symptoms as well as in the Lysholm score by <30 points but ≥6
points; and ineffective, no improvement in pain, morning stiffness
and other symptoms, with <6-point improvement in the Lysholm
score. The treatment effectiveness rate was assessed as (number of
cured cases + number of improvement cases)/total number of cases
x100%.
Secondary efficacy indicators were changes in the
VAS score, WOMAC global score (0-240), WOMAC knee pain score
(0-50), WOMAC knee stiffness score (0-20), WOMAC knee physical
function score (0-170) and Lysholm score (0-100) (28-30).
Safety assessment. The incidence of general
adverse events, severe adverse events, special adverse events
(including skin itching, skin fever and allergy) and dressing
shedding was documented.
Statistical analysis
The study data were analyzed using SAS version 9.4
(SAS Institute). and descriptive statistics. Continuous variables
are presented as the mean, standard deviation, median, 25th
percentile, 75th percentile, minimum and maximum. Qualitative data
are summarized as frequencies and percentages, with confidence
intervals for overall percentages wherever applicable. Differences
in treatment efficacy between the two groups were assessed using
the χ2 test, or using Fisher's exact test where
appropriate. To compare categorical variables, the χ2
test or Fisher's exact test were used as appropriate. However, for
continuous variables such as data presented as the mean ± standard
deviation and median, descriptive statistics were employed and
appropriate parametric test including independent samples t-test
and paired t-test was used. The 95% confidence interval for the
difference in overall effective rates between the two groups was
calculated using the Newcombe-Wilson method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline data
Between June 2023 and April 2024, 351 subjects from
three hospitals were invited to participate. Among them, 34 were
excluded due to not meeting the inclusion criteria and 21 declined
to participate. Additionally, 2 subjects in the FPC group and 8 in
the LSC group were lost to follow-up (Fig. 1). The LSC group included 83 male and
104 female patients, with 5 individuals whose sex was not recorded,
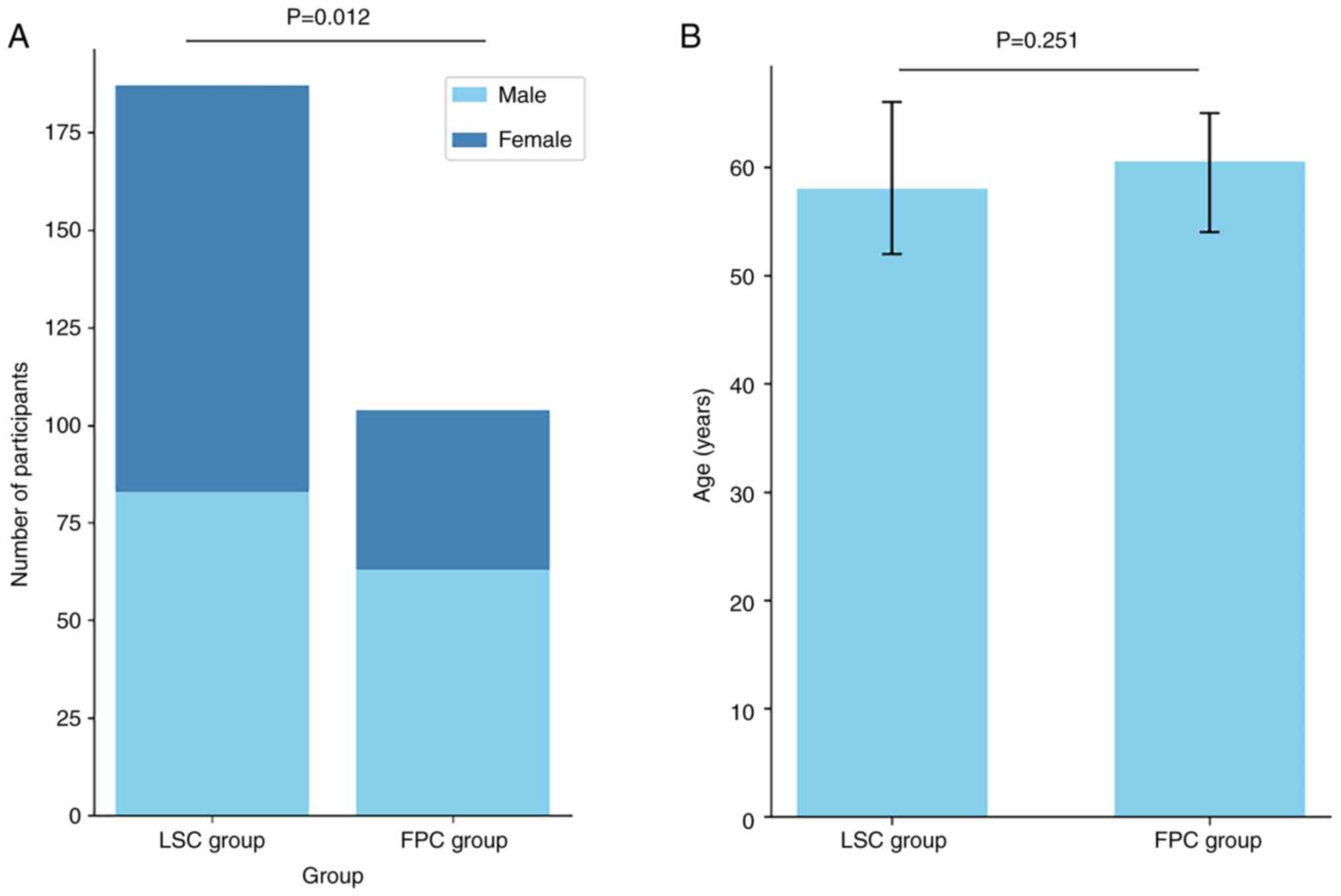
whereas the FPC group comprised 63 male and 41 female patients. A
significant difference was detected between the groups in terms of
sex distribution (P<0.05). The age of the 192 participants in
the LSC group ranged between 24 and 84 years, with a mean age of
58.46 years; the age of 5 individuals was not included in the
analysis. In addition, the age of the 104 individuals in the FPC
group ranged between 35 and 84 years, with a mean age of 59.82
years. The difference in age was not significant (P>0.05;
Table I; Fig. 2).
 | Table IBaseline data. |
Table I
Baseline data.
| Variable | LSC group | FPC group | P-value |
|---|
| No. (missing
values) | 192(5) | 104 (0) | |
| Sex | | | 0.012 |
|
Male
(%) | 83 (44.39) | 63 (60.58) | |
|
Female
(%) | 104 (55.61) | 41 (39.42) | |
| Age, years | | | 0.251 |
|
Mean
(SD) | 58.46 (10.12) | 59.82 (8.77) | |
|
Median (Q1,
Q3) | 58.00 (52.00,
66.00) | 60.50 (54.00,
65.00) | |
|
Min,
max | 24.00, 84.00 | 35.00, 84.00 | |
Clinical outcomes
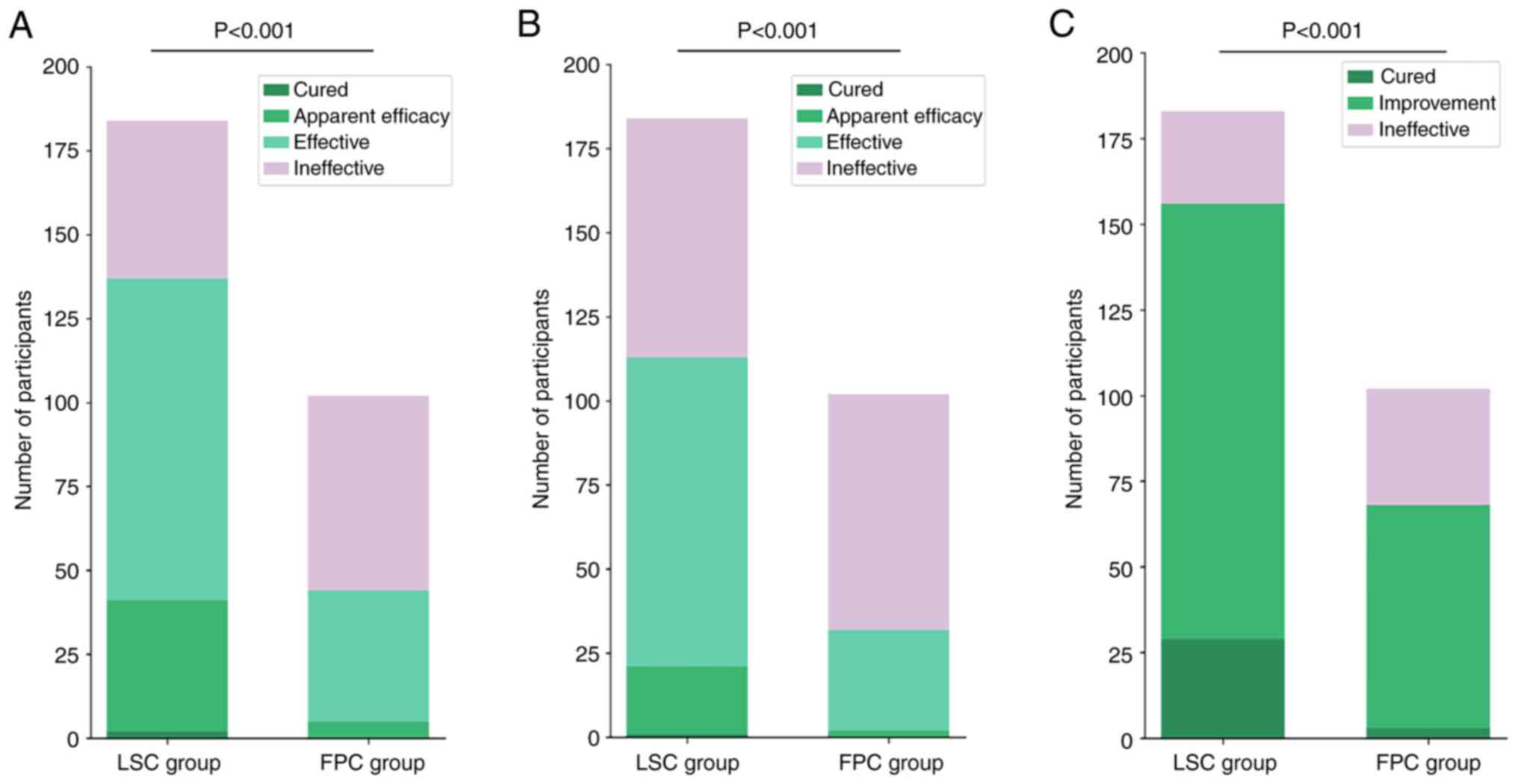
After 2 weeks of treatment, the treatment
effectiveness rate in the LSC group was 74.46% according to the VAS
score, 61.41% according to the WOMAC global score and 85.25%
according to the Lysholm score. By comparison, in the FPC group,
the rates were 43.14, 31.37 and 66.67%, respectively (Table II, Table III and Table IV). Across the primary indicators,
the LSC group achieved significantly higher effectiveness rates
compared with the FPC group (P<0.05; Fig. 3). Newcombe-Wilson analysis confirmed
this advantage, indicating that the lower limit of the 95%
confidence interval exceeded zero for differences in treatment
effectiveness between the LSC and FPC groups, indicating the
superiority of LSC treatment (Table
II, Table III and Table IV).
 | Table IITreatment effectiveness rate (based
on the Visual Analog Scale score) after 2 weeks of treatment. |
Table II
Treatment effectiveness rate (based
on the Visual Analog Scale score) after 2 weeks of treatment.
| Group | Cured, n (%) | Apparent efficacy,
n (%) | Effective, n
(%) | Ineffective, n
(%) | Treatment
effectiveness rate, n (%) | P-value | 95% confidence
interval for the difference in efficacy rates between LSC and FPC
after 2 weeks of treatment (%) |
|---|
| LSC (n=184) | 2 (1.09) | 39 (21.20) | 96 (52.17) | 47 (25.54) | 137 (74.46) | <0.001 | 31.32
(19.51-42.16) |
| FPC (n=102) | 0 (0.00) | 5 (4.90) | 39 (38.24) | 58 (56.86) | 44 (43.14) | | |
 | Table IIITreatment effectiveness rate (based
on Western Ontario and McMaster Universities Osteoarthritis Index
global score) after 2 weeks of treatment. |
Table III
Treatment effectiveness rate (based
on Western Ontario and McMaster Universities Osteoarthritis Index
global score) after 2 weeks of treatment.
| Group | Cured, n (%) | Apparent efficacy,
n (%) | Effective, n
(%) | Ineffective, n
(%) | Treatment
effectiveness rate, n (%) | P-value | 95% confidence
interval for the difference in efficacy rates between LSC and FPC
after 2 weeks of treatment. (%) |
|---|
| LSC (n=184) | 1 (0.54) | 20 (10.87) | 92 (50.00) | 71 (38.59) | 113 (61.41) | <0.001 | 30.04
(18.09-40.64) |
| FPC (n=102) | 0 (0.00) | 2 (1.96) | 30 (29.41) | 70 (68.63) | 32 (31.37) | | |
 | Table IVTreatment effectiveness rate (based
on the Lysholm score) after 2 weeks of treatment. |
Table IV
Treatment effectiveness rate (based
on the Lysholm score) after 2 weeks of treatment.
| Group | Cured, n (%) | Improvement, n
(%) | Ineffective, n
(%) | Treatment
effectiveness rate, n (%) | P-value | 95% confidence
interval for the difference in efficacy rates between LSC and FPC
after 2 weeks of treatment. (%) |
|---|
| LSC (n=184) | 29 (15.85) | 127 (69.40) | 27 (14.75) | 156 (85.25) | <0.001 | 18.58
(8.34-29.15) |
| FPC (n=102) | 3 (2.94) | 65 (63.73) | 34 (33.33) | 68 (66.67) | | |
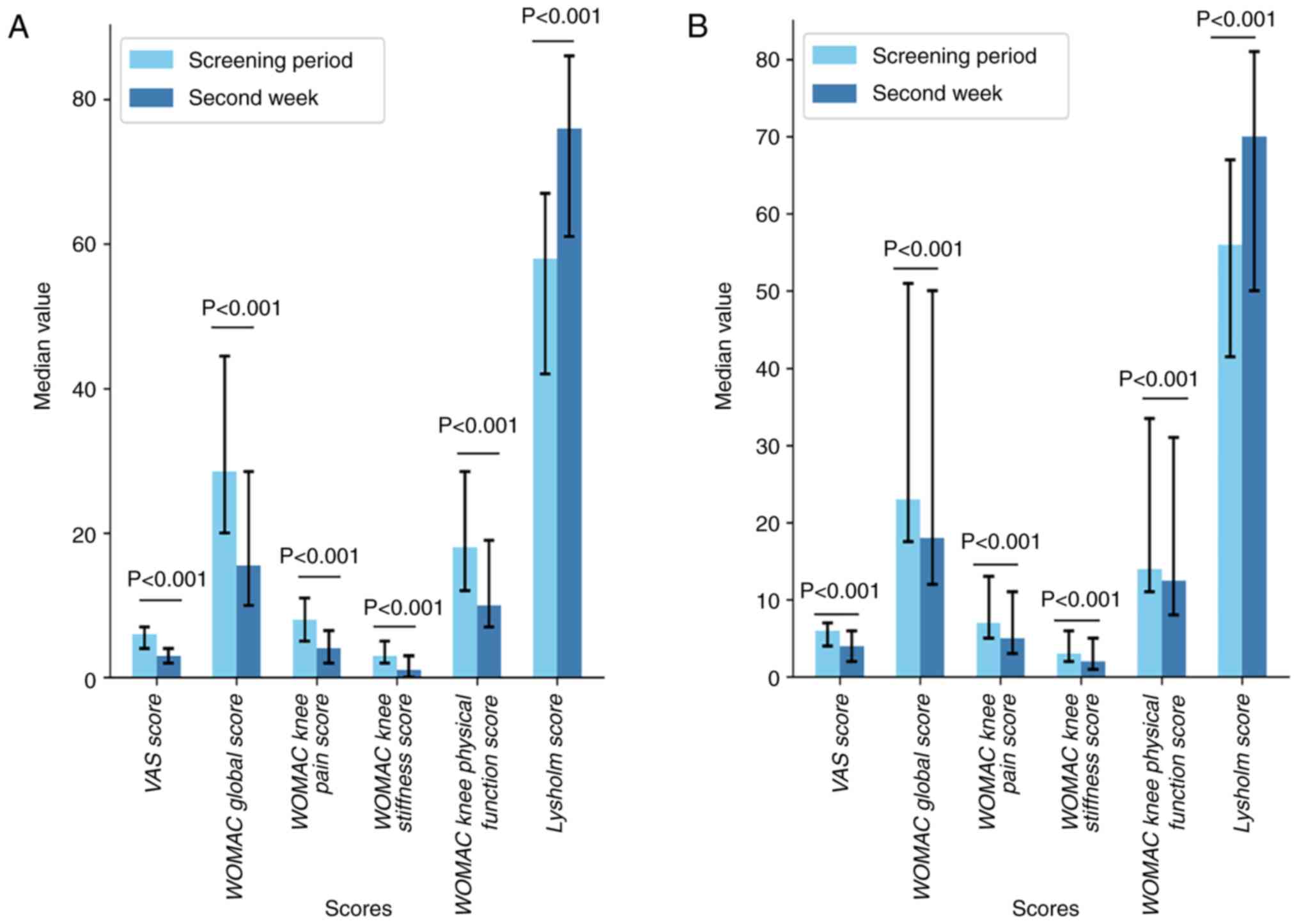
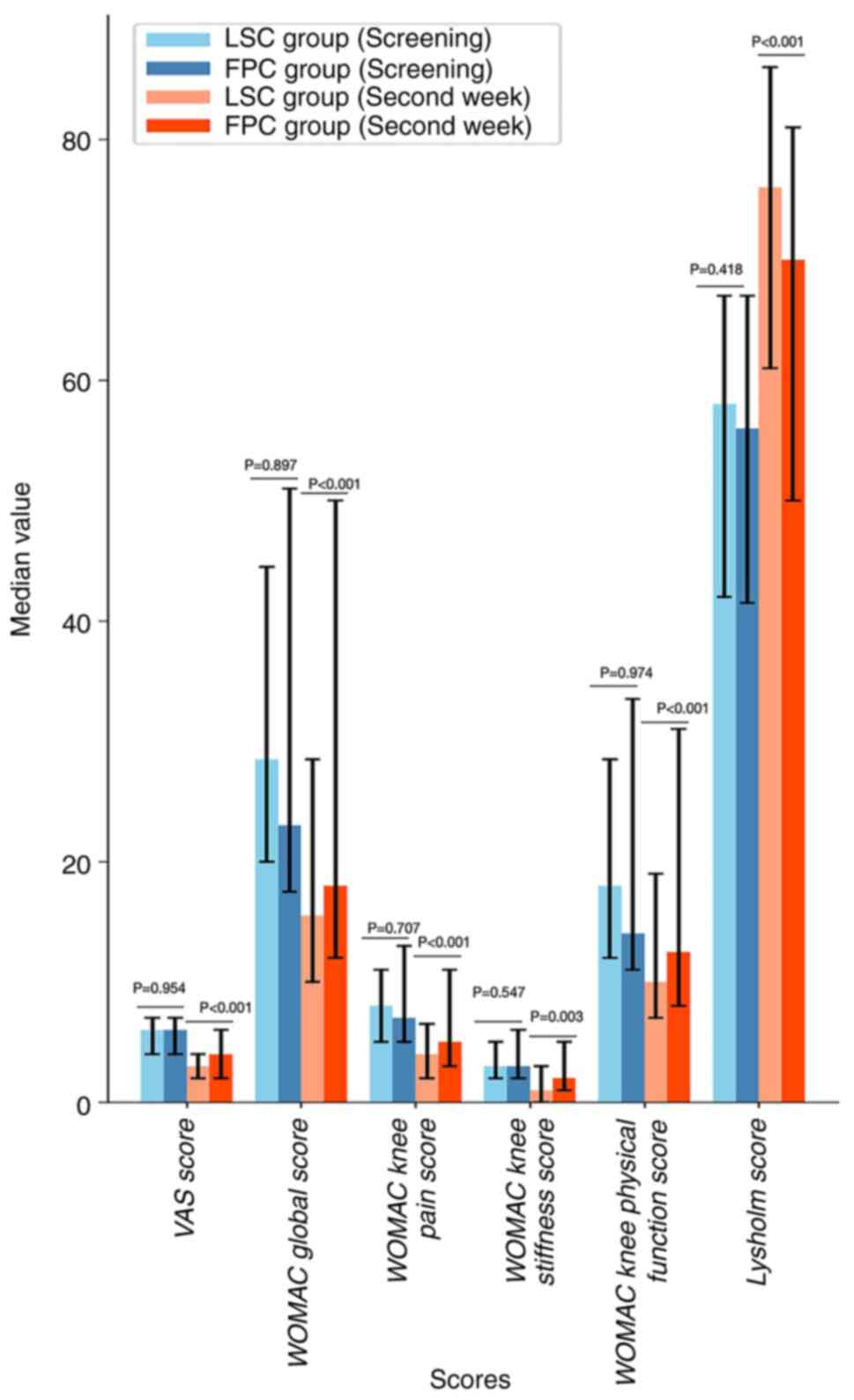
During the screening period, no significant
differences were observed between the two groups in terms of the
VAS score, WOMAC global score (encompassing pain, stiffness and
physical function scores) and Lysholm score (P>0.05; Table VII). After 2 weeks of treatment,
both the LSC and FPC groups showed reductions in the WOMAC global
score (including scores for knee pain, stiffness and physical
function) and VAS score compared with scores at the screening
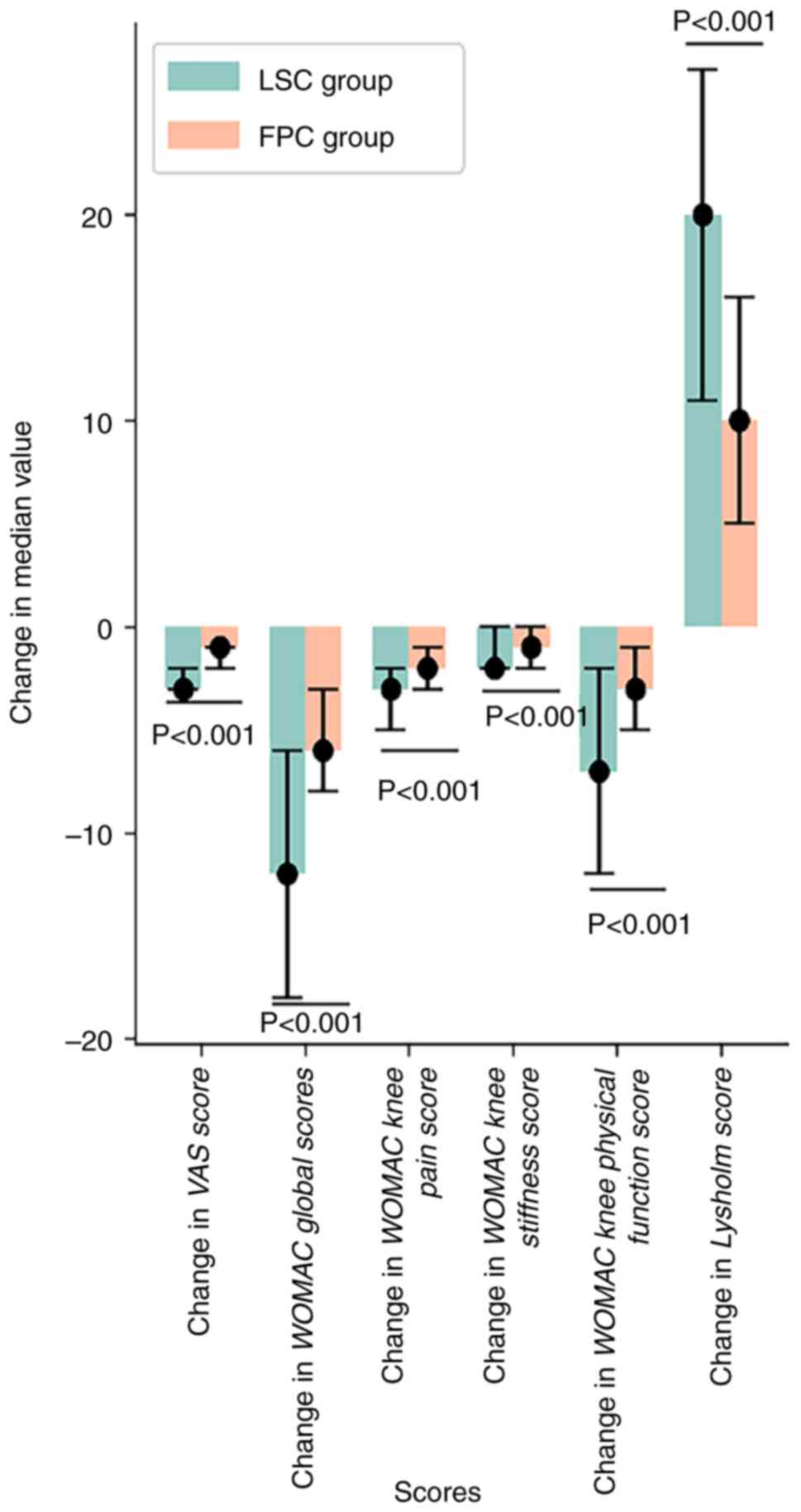
period, with their Lysholm score increasing (P<0.05; Tables V and VI; Fig.
4). Furthermore, after 2 weeks of treatment, the LSC group
exhibited a lower WOMAC global score and VAS score and higher
Lysholm score compared with the FPC group (P<0.05), indicating
that the therapeutic effect of LSC was superior to that of FPC
(Tables VII and VIII; Figs.
5 and 6).
 | Table VIISecondary indicators in the LSC and
FPC groups. |
Table VII
Secondary indicators in the LSC and
FPC groups.
| Score | Period | LSC group | FPC group | P-value |
|---|
| VAS score | Beginning of
screening period | | | 0.954 |
| |
n | 192 | 104 | |
| |
Mean
(SD) | 5.78 (1.77) | 5.79 (1.77) | |
| |
Median (Q1,
Q3) | 6.00 (4.00,
7.00) | 6.00 (4.00,
7.00) | |
| |
Min,
max | 2.00, 10.00 | 2.00, 9.00 | |
| | Second week | | | <0.001 |
| |
n | 184 | 102 | |
| |
Mean
(SD) | 3.14 (1.87) | 4.26 (2.12) | |
| |
Median (Q1,
Q3) | 3.00 (2.00,
4.00) | 4.00 (2.00,
6.00) | |
| |
Min,
max | 0.00, 8.00 | 1.00, 8.00 | |
| WOMAC global
scores | Beginning of
screening period | | | 0.897 |
| |
n | 192 | 104 | |
| |
Mean
(SD) | 33.21 (17.60) | 33.51 (21.76) | |
| |
Median (Q1,
Q3) | 28.50 (20.00,
44.50) | 23.00 (17.50,
51.00) | |
| |
Min,
max | 2.00, 75.00 | 10.00, 75.00 | |
| | Second week | | | <0.001 |
| |
n | 184 | 102 | |
| |
Mean
(SD) | 20.47 (14.49) | 28.00 (21.27) | |
| |
Median (Q1,
Q3) | 15.50 (10.00,
28.50) | 18.00 (12.00,
50.00) | |
| |
Min,
max | 2.00, 73.00 | 4.00, 69.00 | |
| WOMAC knee pain
score | Beginning of
screening period | | | 0.707 |
| |
n | 192 | 104 | |
| |
Mean
(SD) | 8.46 (4.26) | 8.66 (4.85) | |
| |
Median (Q1,
Q3) | 8.00 (5.00,
11.00) | 7.00 (5.00,
13.00) | |
| |
Min,
max | 2.00, 19.00 | 3.00, 18.00 | |
| | Second week | | | <0.001 |
| |
n | 184 | 102 | |
| |
Mean
(SD) | 4.77 (3.65) | 6.80 (5.18) | |
| |
Median (Q1,
Q3) | 4.00 (2.00,
6.50) | 5.00 (3.00,
11.00) | |
| |
Min,
max | 0.00, 18.00 | 1.00, 18.00 | |
| WOMAC knee
stiffness score | Beginning of
screening period | | | 0.547 |
| |
n | 192 | 104 | |
| |
Mean
(SD) | 3.43 (1.84) | 3.58 (2.36) | |
| |
Median (Q1,
Q3) | 3.00 (2.00,
5.00) | 3.00 (2.00,
6.00) | |
| |
Min,
max | 0.00, 8.00 | 0.00, 8.00 | |
| | Second week | | | 0.003 |
| |
n | 184 | 102 | |
| |
Mean
(SD) | 1.84 (1.76) | 2.55 (2.25) | |
| |
Median (Q1,
Q3) | 1.00 (0.00,
3.00) | 2.00 (1.00,
5.00) | |
| |
Min,
max | 0.00, 7.00 | 0.00, 7.00 | |
| WOMAC knee physical
function score | Beginning of
screening period | | | 0.974 |
| |
n | 192 | 104 | |
| |
Mean
(SD) | 21.32 (12.27) | 21.27 (14.97) | |
| |
Median (Q1,
Q3) | 18.0 (12.00,
28.50) | 14.0 (11.00,
33.50) | |
| |
Min,
max | 0.00, 50.00 | 3.00, 50.00 | |
| | Second week | | | <0.001 |
| |
n | 184 | 102 | |
| |
Mean
(SD) | 13.86 (9.72) | 18.65 (14.16) | |
| |
Median (Q1,
Q3) | 10.00 (7.00,
19.00) | 12.50 (8.00,
31.00) | |
| |
Min,
max | 1.00, 48.00 | 2.00, 45.00 | |
| Lysholm score | Beginning of
screening period | | | 0.418 |
| |
n | 192 | 104 | |
| |
Mean
(SD) | 53.60 (17.98) | 51.66 (22.32) | |
| |
Median (Q1,
Q3) | 58.0 (42.00,
67.00) | 56.0 (41.50,
67.00) | |
| |
Min,
max | 7.00, 90.00 | 7.00, 88.00 | |
| | Second week | | | <0.001 |
| |
n | 184 | 102 | |
| |
Mean
(SD) | 72.27 (17.48) | 62.86 (22.97) | |
| |
Median (Q1,
Q3) | 76.0 (61.00,
86.00) | 70.0 (50.00,
81.00) | |
| |
Min,
max | 19.00, 99.00 | 15.00, 91.00 | |
 | Table VComparison of secondary indicators in
the loxoprofen sodium cataplasm group. |
Table V
Comparison of secondary indicators in
the loxoprofen sodium cataplasm group.
| Scores | Screening period
(n=192) | Second week
(n=184) | P-value |
|---|
| VAS score | | | <0.001 |
|
Mean
(SD) | 5.78 (1.77) | 3.14 (1.87) | |
|
Median (Q1,
Q3) | 6.00 (4.00,
7.00) | 3.00 (2.00,
4.00) | |
|
Min,
max | 2.00, 10.00 | 0.00, 8.00 | |
| WOMAC global
scores | | | <0.001 |
|
Mean
(SD) | 33.21 (17.60) | 20.47 (14.49) | |
|
Median (Q1,
Q3) | 28.5 (20.00,
44.50) | 15.5 (10.00,
28.50) | |
|
Min,
max | 2.00, 75.00 | 2.00, 73.00 | |
| WOMAC knee pain
score (missing values) | 192 (0) | 184(8) | <0.001 |
|
Mean
(SD) | 8.46 (4.26) | 4.77 (3.65) | |
|
Median (Q1,
Q3) | 8.00 (5.00,
11.00) | 4.00 (2.00,
6.50) | |
|
Min,
max | 2.00, 19.00 | 0.00, 18.00 | |
| WOMAC knee
stiffness score | | | <0.001 |
|
Mean
(SD) | 3.43 (1.84) | 1.84 (1.76) | |
|
Median (Q1,
Q3) | 3.00 (2.00,
5.00) | 1.00 (0.00,
3.00) | |
|
Min,
max | 0.00, 8.00 | 0.00, 7.00 | |
| WOMAC knee physical
function score | | | <0.001 |
|
Mean
(SD) | 21.32 (12.27) | 13.86 (9.72) | |
|
Median (Q1,
Q3) | 18.0 (12.00,
28.50) | 10.00 (7.00,
19.00) | |
|
Min,
max | 0.00, 50.00 | 1.00, 48.00 | |
| Lysholm score | | | <0.001 |
|
Mean
(SD) | 53.60 (17.98) | 72.27 (17.48) | |
|
Median (Q1,
Q3) | 58.0 (42.00,
67.00) | 76.0 (61.00,
86.00) | |
|
Min,
max | 7.00, 90.00 | 19.00, 99.00 | |
 | Table VIComparison of secondary indicators in
the flurbiprofen cataplasm group. |
Table VI
Comparison of secondary indicators in
the flurbiprofen cataplasm group.
| Scores | Screening period
(n=104) | Second week
(n=102) | P-value |
|---|
| VAS score | | | <0.001 |
|
Mean
(SD) | 5.79 (1.77) | 4.26 (2.12) | |
|
Median (Q1,
Q3) | 6.00 (4.00,
7.00) | 4.00 (2.00,
6.00) | |
|
Min,
max | 2.00, 9.00 | 1.00, 8.00 | |
| WOMAC global
scores | | | <0.001 |
|
Mean
(SD) | 33.51 (21.76) | 28.00 (21.27) | |
|
Median (Q1,
Q3) | 23.0 (17.50,
51.00) | 18.0 (12.00,
50.00) | |
|
Min,
max | 10.00, 75.00 | 4.00, 69.00 | |
| WOMAC knee pain
score | | | <0.001 |
|
Mean
(SD) | 8.66 (4.85) | 6.80 (5.18) | |
|
Median (Q1,
Q3) | 7.00 (5.00,
13.00) | 5.00 (3.00,
11.00) | |
|
Min,
max | 3.00, 18.00 | 1.00, 18.00 | |
| WOMAC knee
stiffness score | | | <0.001 |
|
Mean
(SD) | 3.58 (2.36) | 2.55 (2.25) | |
|
Median (Q1,
Q3) | 3.00 (2.00,
6.00) | 2.00 (1.00,
5.00) | |
|
Min,
max | 0.00, 8.00 | 0.00, 7.00 | |
| WOMAC knee physical
function score | | | <0.001 |
|
Mean
(SD) | 21.27 (14.97) | 18.65 (14.16) | |
|
Median (Q1,
Q3) | 14.00 (11.00,
33.50) | 12.50 (8.00,
31.00) | |
|
Min,
max | 3.00, 50.00 | 2.00, 45.00 | |
| Lysholm score | | | <0.001 |
|
Mean
(SD) | 51.66 (22.32) | 62.86 (22.97) | |
|
Median (Q1,
Q3) | 56.00 (41.50,
67.00) | 70.00 (50.00,
81.00) | |
|
Min,
max | 7.00, 88.00 | 15.00, 91.00 | |
 | Table VIIIChanges in secondary indicators
relative to baseline in the LSC and FPC groups. |
Table VIII
Changes in secondary indicators
relative to baseline in the LSC and FPC groups.
| Variable | LSC group | FPC group | P-value |
|---|
| No. (missing
values) | 192(8) | 104(2) | |
| Change of VAS
score | | | |
|
Mean
(SD) | -2.66 (1.53) | -1.56 (1.01) | <0.001 |
|
Median (Q1,
Q3) | -3.00 (-3.00,
-2.00) | -1.00 (-2.00,
-1.00) | |
|
Min,
max | -7.00, 2.00 | -6.00, 0.00 | |
| Change of WOMAC
global scores | | | |
|
Mean
(SD) | -13.29 (10.60) | -5.78 (3.93) | <0.001 |
|
Median (Q1,
Q3) | -12.00 (-18.00,
-6.00) | -6.00 (-8.00,
-3.00) | |
|
Min,
max | -53.00, 12.00 | -20.00, 7.00 | |
| Change of WOMAC
knee pain score | | | |
|
Mean
(SD) | -3.80 (2.75) | -1.94 (1.39) | <0.001 |
|
Median (Q1,
Q3) | -3.00 (-5.00,
-2.00) | -2.00 (-3.00,
-1.00) | |
|
Min,
max | -12.00, 3.00 | -8.00, 1.00 | |
| Change of WOMAC
knee stiffness score | | | |
|
Mean
(SD) | -1.64 (1.39) | -1.04 (0.89) | <0.001 |
|
Median (Q1,
Q3) | -2.00 (-2.00,
-1.00) | -1.00 (-2.00,
0.00) | |
|
Min,
max | -7.00, 3.00 | -3.00, 1.00 | |
| Change of WOMAC
knee physical function score | | | |
|
Mean
(SD) | -7.85 (7.59) | -2.80 (2.93) | <0.001 |
|
Median (Q1,
Q3) | -7.00 (-12.00,
-2.00) | -3.00 (-5.00,
-1.00) | |
|
Min,
max | -36.00, 11.00 | -12.00, 6.00 | |
| Change of Lysholm
score | | | |
|
Mean
(SD) | 18.76 (12.21) | 11.28 (8.79) | <0.001 |
|
Median (Q1,
Q3) | 20.00 (11.00,
27.00) | 10.00 (5.00,
16.00) | |
|
Min,
max | -41.00, 57.00 | -6.00, 39.00 | |
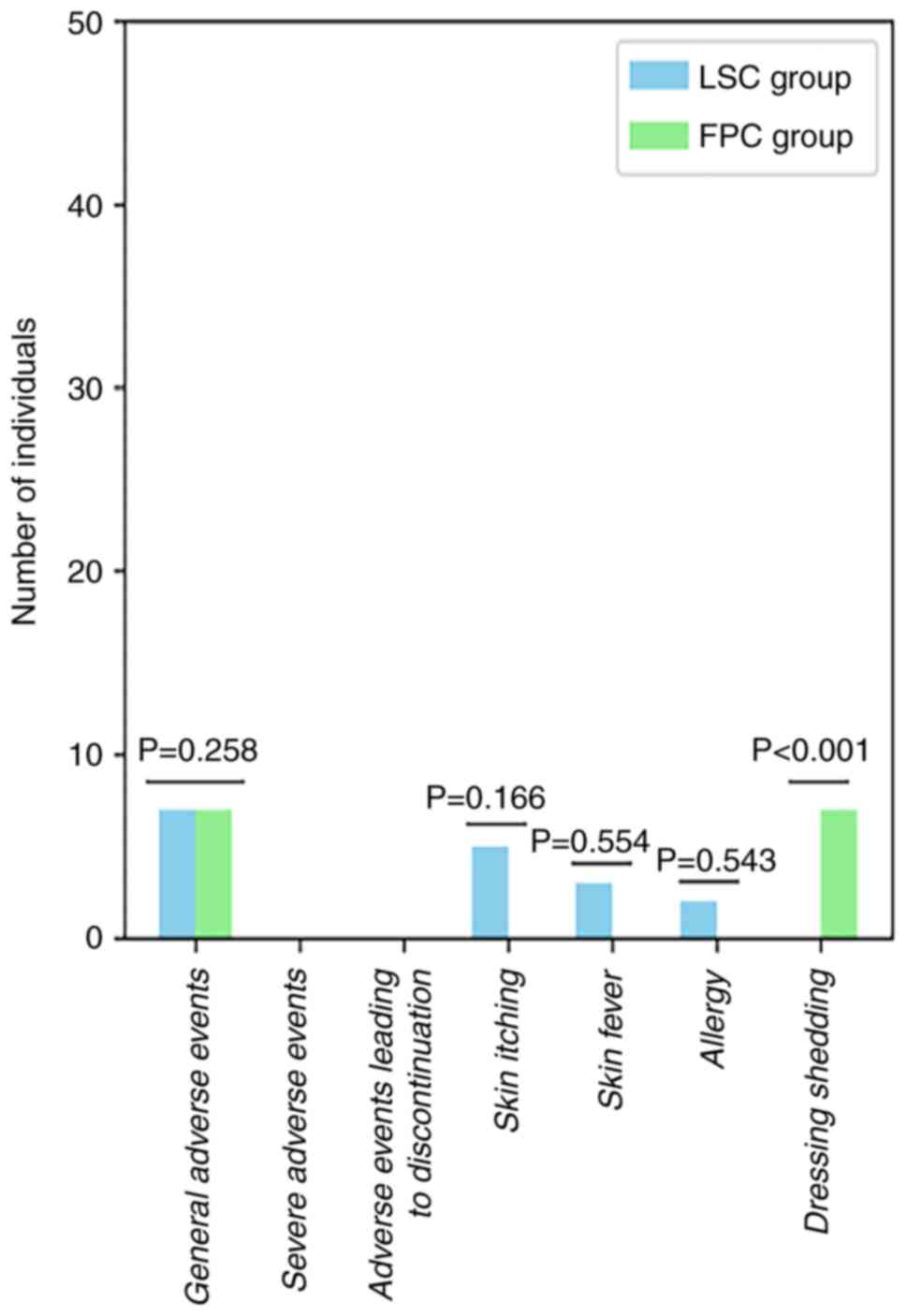
Safety
The FPC group experienced a higher rate of adverse
events (P>0.05) and dressing shedding (P<0.05) compared with
the LSC group, while the LSC group had more specific adverse events
than the FPC group, including skin itching, skin fever and allergy
(P>0.05; Table IX; Fig. 7).
 | Table IXIncidence of adverse events in the
LSC and FPC groups. |
Table IX
Incidence of adverse events in the
LSC and FPC groups.
| | LSC group | FPC group | |
|---|
| Adverse events | Times | Number of
individuals (%) | Times | Number of
individuals (%) | P-value |
|---|
| General adverse
events | 10 | 7 (3.65) | 20 | 7 (6.73) | 0.258 |
| Severe adverse
events | 0 | 0 (0.00) | 0 | 0 (0.00) | - |
| Adverse events
leading to discontinuation | 0 | 0 (0.00) | 0 | 0 (0.00) | - |
| Specific adverse
events | | | | | |
|
Skin
itching | 5 | 5 (2.60) | 0 | 0 (0.00) | 0.166 |
|
Skin
fever | 3 | 3 (1.56) | 0 | 0 (0.00) | 0.554 |
|
Allergy | 2 | 2 (1.04) | 0 | 0 (0.00) | 0.543 |
| Dressing
shedding | 0 | 0 (0.00) | 20 | 7 (6.73) | <0.001 |
Discussion
OA is a degenerative joint disease that markedly
impairs the quality of life of patients, and is primarily
characterized by joint pain, pressure sensitivity, restricted
movement and joint deformity, which together contribute to
decreased bodily function and diminished quality of life (31,32).
The prevalence of OA has been increasing with aging and obesity
trends; from 1990-2019 >50% of individuals aged >65 years in
China suffer from OA, a leading cause of pain and disability among
the elderly (33-35).
The American College of Rheumatology, Arthritis Foundation,
Osteoarthritis Research Society International and European Society
for Clinical Economics of Osteoporosis all recommend NSAIDs as a
cornerstone of pharmacological treatment and pain management for OA
(12,36-38).
However, prolonged or frequent oral NSAIDs use can lead to
tolerance and safety concerns, particularly in the elderly and
those with comorbidities (39,40).
Furthermore, NSAIDs overuse is associated with adverse
gastrointestinal, cardiovascular, renal and hepatic events
(41,42). Topical NSAIDs formulations, which
minimize systemic exposure, are thus recommended for managing OA
symptoms in middle-aged and elderly patients (40).
Loxoprofen sodium, a novel NSAIDs and propionic acid
derivative, exerts its pharmacological effects by inhibiting COX
enzymes, thereby blocking the conversion of arachidonic acid to
prostaglandins. This inhibition leads to anti-inflammatory,
analgesic and antipyretic effects (43). The control group in the present
study was selected based on the common clinical practice of using
FPC as a standard treatment for OA. Flurbiprofen is a
well-established topical NSAID with documented efficacy and safety
in managing OA symptoms (44). By
comparing LSC against the established FPC treatment, the present
study aimed to provide a meaningful evaluation of the efficacy and
safety profile of LSC. The selection of FPC as a control enabled a
direct comparison of treatment outcomes, thereby enhancing the
clinical relevance of the findings.
After 2 weeks of treatment, the LSC group had
treatment effectiveness rates of 74.46 (VAS score), 61.41 (WOMAC
global score) and 85.25% (Lysholm score). In comparison, the FPC
group achieved rates of 43.14, 31.37 and 66.67%, respectively.
Across the primary indicators, the LSC group exhibited
significantly higher effectiveness (P<0.05). Additionally, after
two weeks of treatment, the LSC group had a lower WOMAC knee pain
score and VAS score than the FPC group (P<0.05), indicating more
effective pain management. The LSC group exhibited a lower WOMAC
knee stiffness score and improved physiological function score in
addition to a higher Lysholm score compared with the FPC group
(P<0.05), suggesting superior knee function recovery. This
enhanced performance may be attributed to the rapid absorption and
distribution of loxoprofen sodium and its active metabolites,
making it one of the fastest-acting NSAIDs available (45). Of note, the LSC group was
administered a higher daily dose of loxoprofen sodium (100 mg)
compared with that administered to the FPC group (flurbiprofen; 80
mg), LSC can more effectively alleviate knee joint pain in
patients, which contributes to significant early symptomatic
improvement in patients.
After 2 weeks of treatment, the FPC group exhibited
a higher rate of dressing shedding compared with the LSC group
(P<0.05), which may be attributed to FPC being applied twice as
often as LSC. However, there was no significant difference between
the LSC and FPC groups with respect to general adverse events, skin
itching, skin fever or allergies (P>0.05). Despite the LSC group
receiving a daily dose of 100 mg of loxoprofen sodium compared with
the FPC group receiving 80 mg daily dose of flurbiprofen, the
incidence of adverse events did not differ markedly, indicating
comparable safety profiles for the two treatment options.
The baseline data indicated a disparity in terms of
sex between the LSC and FPC groups, probably attributable to the
small sample size. In a randomized controlled trial, comparability
between the two groups is essential and inherent to the study
design. The LSC group contained a higher proportion of women, who,
according to a previous study, may experience heightened pain
sensitivity in arthritic conditions (46). Nevertheless, the LSC group exhibited
superior outcomes after 2 weeks of treatment, suggesting that LSC
exhibited more effective short-term results than FPC. LSC and FPC
both belong to the class of NSAIDs and exert their analgesic and
anti-inflammatory effects primarily through the inhibition of COX
enzymes (40,44). LSC selectively inhibits COX-1 and
COX-2, leading to a reduction in the synthesis of prostaglandins,
which are mediators of pain and inflammation (43). By contrast, FPC also inhibits both
COX-1 and COX-2 but may have a slightly different affinity for
these enzymes, which can influence its overall efficacy and side
effect profile (44). The
differences in the mechanism of action may contribute to variations
in the onset and duration of analgesic effects between the two
drugs (47). The pharmacokinetic
profiles of LSC and FPC differ in several aspects. LSC is known for
its rapid absorption and distribution, which allows for a quicker
onset of analgesic effects. It is primarily metabolized in the
liver and its metabolites have a longer half-life, providing
sustained therapeutic action. By contrast, FPC has a slower
absorption rate and may take a longer time to reach the peak plasma
concentration. Additionally, the pharmacokinetics of FPC can be
influenced by factors such as formulation and skin permeability,
which may affect its overall efficacy in topical applications
(45). These pharmacokinetic
differences may serve a role in the observed variations in
treatment effectiveness and safety profiles between LSC and FPC in
the management of OA.
A previous study obtained results that the analgesic
effect of FPC in the treatment of OA is superior to that of LSC,
which contradicts the findings of the present study (47), which may be attributed to the
current study assessing short-term efficacy (2 weeks), while the
aforementioned study evaluated long-term efficacy (4 weeks). Thus,
although LSC was associated with improved initial results, FPC
showed greater effectiveness with longer use. The current study
emphasized early pain relief and functional recovery, whereas the
other study focused on sustained pain relief and long-term safety.
Therefore, the opposing conclusions may have resulted from the
different time points of efficacy evaluation.
The present study has several limitations. First,
the sample size was small, limiting the ability to draw more
broadly applicable conclusions from the results. Second, the brief
observation period was insufficient to evaluate long-term efficacy
and potential gastrointestinal and cardiovascular adverse events.
Third, other biomarkers that could also be relevant for assessing
safety in both animal and human use were not analyzed. These
include liver function tests (such as ALT and AST levels), renal
function tests (such as serum creatinine level) and
gastrointestinal biomarkers (such as fecal occult blood).
Additionally, inflammatory markers (such as C-reactive protein) and
pain biomarkers (such as substance P level) may provide additional
insights into the safety profile of the treatments. Future studies
should consider incorporating these additional biomarkers to
enhance the comprehensiveness of safety assessments. Finally, the
absence of blinding may have introduced bias. In the future, a
larger sample size will be employed to ensure the accuracy of the
study, the follow-up period will be extended to clarify the
long-term efficacy and side effects of the drug, and blinding shall
be performed to reduce bias.
In conclusion, OA could be effectively treated with
both LSC and FPC. However, LSC had a higher short-term efficacy,
lower dressing removal rate and improved effect on the knee joints
of patients with OA compared with FPC. Thus, LSC is a safe and
effective treatment for OA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key Program of
Shaanxi Province (grant no. 2023-YBSF-102).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All the authors contributed substantially to the
conception and design of the study. YL and ZL conceived and
designed the work, collected original data, wrote and edited the
manuscript and confirmed the authenticity of the raw data. GG, SL,
XG and QL collected original data. XJ, PY and RT directed the
conception and design of the work. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of The Second Affiliated Hospital of Xi'an Jiaotong
University, Shaanxi, China [ethics approval nos. Lun Shen (2023)
no. 003 and Lun Shen (2022) no. 16; Xi'an, China] and Union
Hospital, Tongji Medical College, Huazhong University of Science
and Technology, Hubei, China [ethics approval no. Lun Shen (2021)
no. (0117)-01; Wuhan, China]. The patients/participants provided
their written informed consent to participate in the present study.
The present study was registered at Chinese Clinical Trial Register
(chictr.org.cn; ChiCTR2300072504). Date of
registration: June 15, 2023.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bennell KL, Paterson KL, Metcalf BR, Duong
V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A,
et al: Effect of intra-articular platelet-rich plasma vs placebo
injection on pain and medial tibial cartilage volume in patients
with knee osteoarthritis: The RESTORE Randomized Clinical Trial.
JAMA. 326:2021–2030. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Katz JN, Arant KR and Loeser RF: Diagnosis
and treatment of hip and knee osteoarthritis. A review. JAMA.
325:568–578. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duong V, Oo WM, Ding C, Culvenor AG and
Hunter DJ: Evaluation and treatment of knee pain: A review. JAMA.
330(1568)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neogi T and Colloca L: Placebo effects in
osteoarthritis: Implications for treatment and drug development.
Nat Rev Rheumatol. 19:613–626. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duan X, Zhao Y, Zhang J, Kong N, Cao R,
Guan H, Li Y, Wang K, Yang P and Tian R: Prediction of early
functional outcomes in patients after robotic-assisted total knee
arthroplasty: A nomogram prediction model. Int J Surg.
109:3107–3116. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tian R, Duan X, Kong N, Li X, Wang J, Tian
H, Shi Z, Yan S, Lyu J, Wang K and Yang P: Robotic-assisted total
knee arthroplasty is more advantageous for knees with severe
deformity: A randomized controlled trial study design. Int J Surg.
109:287–296. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geenen R, Overman CL, Christensen R,
åsenlöf P, Capela S, Huisinga KL, Husebø MEP, Köke AJA, Paskins Z,
Pitsillidou IA, et al: Eular recommendations for the health
professional's approach to pain management in inflammatory
arthritis and osteoarthritis. Ann Rheum Dis. 77:797–807.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bichsel D, Liechti FD, Schlapbach JM and
Wertli MM: Cross-sectional analysis of recommendations for the
treatment of hip and knee osteoarthritis in clinical guidelines.
Arch Phys Med Rehabil. 103:559–569.e5. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Geraghty T, Obeidat AM, Ishihara S, Wood
MJ, Li J, Lopes EBP, Scanzello CR, Griffin TM, Malfait AM and
Miller RE: Age-associated changes in knee
osteoarthritis,pain-related behaviors, and dorsal root ganglia
immunophenotyping of male and female mice. Arthritis Rheumatol.
75:1770–1780. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hunter DJ, Mclachlan AJ, Carroll PR,
Wakefield TAN and Stosic R: Health literacy and appropriateness of
self-care and pain management in osteoarthritis: An understanding
of the patient's perspective. Arthritis Care Res (Hoboken).
75:848–859. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Greig SL and Garnock-Jones KP: Loxoprofen:
A review in pain and inflammation. Clin Drug Investig. 36:771–781.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bannuru RR, Osani MC, Vaysbrot EE, Arden
NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott
JH, Bhandari M, et al: OARSI guidelines for the non-surgical
management of knee, hip, and polyarticular osteoarthritis.
Osteoarthritis Cartilage. 27:1578–1589. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Arden NK, Perry TA, Bannuru RR, Bruyère O,
Cooper C, Haugen IK, Hochberg MC, McAlindon TE, Mobasheri A and
Reginster JY: Non-surgical management of knee osteoarthritis:
Comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol.
17:59–66. 2019.
|
|
14
|
Weng Q, Goh S, Wu J, Persson MSM, Wei J,
Sarmanova A, Li X, Hall M, Doherty M, Jiang T, et al: Comparative
efficacy of exercise therapy and oral non-steroidal
anti-inflammatory drugs and paracetamol for knee or hip
osteoarthritis: A network meta-analysis of randomised controlled
trials. Br J Sports Med. 57:990–996. 2023.
|
|
15
|
Chen GY, Zhou CQ, Li H and Mao XZ:
Efficacy and safety of topical nsaids combined with physiotherapy
for frozen shoulder: A randomized controlled trial. Eur Rev Med
Pharmacol Sci. 28:3761–3770. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Voilley N: Acid-sensing ion channels
(ASICs): New targets for the analgesic effects of non-steroid
anti-inflammatory drugs (NSAIDs). Curr Drug Targets Inflamm
Allergy. 3:71–79. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li P, Li H, Shu X, Wu M, Liu J, Hao T, Cui
H and Zheng L: Intra-articular delivery of flurbiprofen sustained
release thermogel: Improved therapeutic outcome of collagenase
II-induced rat knee osteoarthritis. Drug Deliv. 27:1034–1043.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mu R, Bao CD, Chen ZW, Zheng Y, Wang GC,
Zhao DB, Hu SX, Li YJ, Shao ZW, Zhang ZY, et al: Efficacy and
safety of loxoprofen hydrogel patch versus loxoprofen tablet in
patients with knee osteoarthritis: A randomized controlled
non-inferiority trial. Clin Rheumatol. 35:165–173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iguchi M, Takahashi T and Takeshita K:
Effectiveness and adherence rate of s-flurbiprofen plaster for the
pain management of patients with moderate and end-stage knee
osteoarthritis. Cureus. 15(e44556)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin T, Liu Z, Ji W and Zhang P: Effects of
knee debridement with flurbiprofen on knee function, inflammatory
levels, and bone metabolism activity in patients with knee
osteoarthritis. Comput Math Methods Med.
2022(8031360)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tomatsu K, Yasuda S, Fuady A, Matsumoto H
and Sumariyono : Efficacy and safety of s-flurbiprofen
plaster in knee osteoarthritis patients: A 2-week randomized
controlled phase III clinical trial compared to diclofenac gel. Int
J Rheum Dis. 25:563–570. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pereira D, Ramos E and Branco J:
Osteoarthritis. Acta Med Port. 28:99–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wathier M, Lakin BA, Cooper BG, Bansal PN,
Bendele AM, Entezari V, Suzuki H, Snyder BD and Grinstaff MW: A
synthetic polymeric biolubricant imparts chondroprotection in a rat
meniscal tear model. Biomaterials. 182:13–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin JB, Poh S and Panitch A: Controlled
release of anti-inflammatory peptides from reducible
thermosensitive nanoparticles suppresses cartilage inflammation.
Nanomedicine. 12:2095–2100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Garriga C, Sánchez-Santos MT, Judge A,
Hart D, Spector T, Cooper C and Arden NK: Predicting incident
radiographic knee osteoarthritis in middle-aged women within four
years: The importance of knee-level prognostic factors. Arthritis
Care Res (Hoboken). 72:88–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jones IA, Togashi R, Wilson ML, Heckmann N
and Vangsness CT Jr: Intra-articular treatment options for knee
osteoarthritis. Nat Rev Rheumatol. 15:77–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Boer CG, Radjabzadeh D, Medina-Gomez C,
Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram
MA, et al: Intestinal microbiome composition and its relation to
joint pain and inflammation. Nat Commun. 10(4881)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Belk JW, Lim JJ, Keeter C, Mcculloch PC,
Houck DA, Mccarty EC, Frank RM and Kraeutler MJ: Patients with knee
osteoarthritis who receive platelet-rich plasma or bone marrow
aspirate concentrate injections have better outcomes than patients
who receive hyaluronic acid: Systematic review and meta-analysis.
Arthroscopy. 39:1714–1734. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stonehouse W, Benassi-Evans B, Bednarz J,
Vincent AD, Hall S and Hill C: Krill oil improved osteoarthritic
knee pain in adults with mild to moderate knee osteoarthritis: A
6-month multicenter, randomized, double-blind, placebo-controlled
trial. Am J Clin Nutr. 116:672–685. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Migliorini F, Schäfer L, Bell A, Weber CD,
Vecchio G and Maffulli N: Meniscectomy is associated with a higher
rate of osteoarthritis compared to meniscal repair following acute
tears: A meta-analysis. Knee Surg Sports Traumatol Arthrosc.
31:5485–5495. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
O'Neill TW and Felson DT: Mechanisms of
osteoarthritis (OA) pain. Curr Osteoporos Rep. 16:611–616.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Perrot S, Trouvin AP and Bouhassira D:
Three dimensions of pain in osteoarthritis: Development and
validation of the osteoarthritis symptom inventory scale. Pain.
164:1566–1577. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Allen KD, Thoma LM and Golightly YM:
Epidemiology of osteoarthritis. Osteoarthritis Cartilage.
30:184–195. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cui A, Li H, Wang D, Zhong J, Chen Y and
Lu H: Global, regional prevalence, incidence and risk factors of
knee osteoarthritis in population-based studies. EClinicalMedicine.
29-30(100587)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Long H, Liu Q, Yin H, Wang K, Diao N,
Zhang Y, Lin J and Guo A: Prevalence trends of site-specific
osteoarthritis from 1990 to 2019: Findings from the global burden
of disease study 2019. Arthritis Rheumatol. 74:1172–1183.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kolasinski SL, Neogi T, Hochberg MC, Oatis
C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D,
et al: 2019 American college of rheumatology/arthritis foundation
guideline for the management of osteoarthritis of the hand, hip,
and knee. Arthritis Care Res (Hoboken). 72:149–162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bruyère O, Honvo G, Veronese N, Arden NK,
Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G,
Martel-Pelletier J, Pelletier JP, et al: An updated algorithm
recommendation for the management of knee osteoarthritis from the
european society for clinical and economic aspects of osteoporosis,
osteoarthritis and musculoskeletal diseases (ESCEO). Semin
Arthritis Rheum. 49:337–350. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mezey GA, Máté Z and Paulik E: Factors
influencing pain management of patients with osteoarthritis: A
cross-sectional study. J Clin Med. 11(1352)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schjerning A, Mcgettigan P and Gislason G:
Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev
Cardiol. 17:574–584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zeng C, Wei J, Persson MSM, Sarmanova A,
Doherty M, Xie D, Wang Y, Li X, Li J, Long H, et al: Relative
efficacy and safety of topical non-steroidal anti-inflammatory
drugs for osteoarthritis: A systematic review and network
meta-analysis of randomised controlled trials and observational
studies. Br J Sports Med. 52:642–650. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Da Costa BR, Pereira TV, Saadat P,
Rudnicki M, Iskander SM, Bodmer NS, Bobos P, Gao L, Kiyomoto HD,
Montezuma T, et al: Effectiveness and safety of non-steroidal
anti-inflammatory drugs and opioid treatment for knee and hip
osteoarthritis: Network meta-analysis. BMJ.
375(n2321)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ozen G, Pedro S and Michaud K: Major
adverse cardiovascular events and mortality with opioids versus
NSAIDs initiation in patients with rheumatoid arthritis. Ann Rheum
Dis. 82:1487–1494. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ji C, Yu Y, Zhang M, Yu W and Dong S:
Loxoprofen sodium alleviates oxidative stress and apoptosis induced
by angiotensin II in human umbilical vein endothelial cells
(HUVECs). Drug Des Devel Ther. 14:5087–5096. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nelson AE: Osteoarthritis year in review
2017: Clinical. Osteoarthritis Cartilage. 26:319–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kien NT, Geiger P, Van Chuong H, Cuong NM,
Van Dinh N, Pho DC, Anh VT and Giang NT: Preemptive analgesia after
lumbar spine surgery by pregabalin and celecoxib: A prospective
study. Drug Des Devel Ther. 13:2145–2152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Walker Taylor JL, Campbell CM, Thorpe RJ
Jr, Whitfield KE, Nkimbeng M and Szanton SL: Pain, racial
discrimination, and depressive symptoms among African American
women. Pain Manag Nurs. 19:79–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li D, Cheng Y, Yuan P, Wu Z, Liu J, Kan J,
Zhang K, Wang Z, Zhang H, Zhang G, et al: Efficacy and safety of
flurbiprofen cataplasms versus loxoprofen sodium cataplasms in knee
osteoarthritis: A randomized controlled trial. Chin Med J (Engl).
136:2187–2194. 2023.PubMed/NCBI View Article : Google Scholar
|