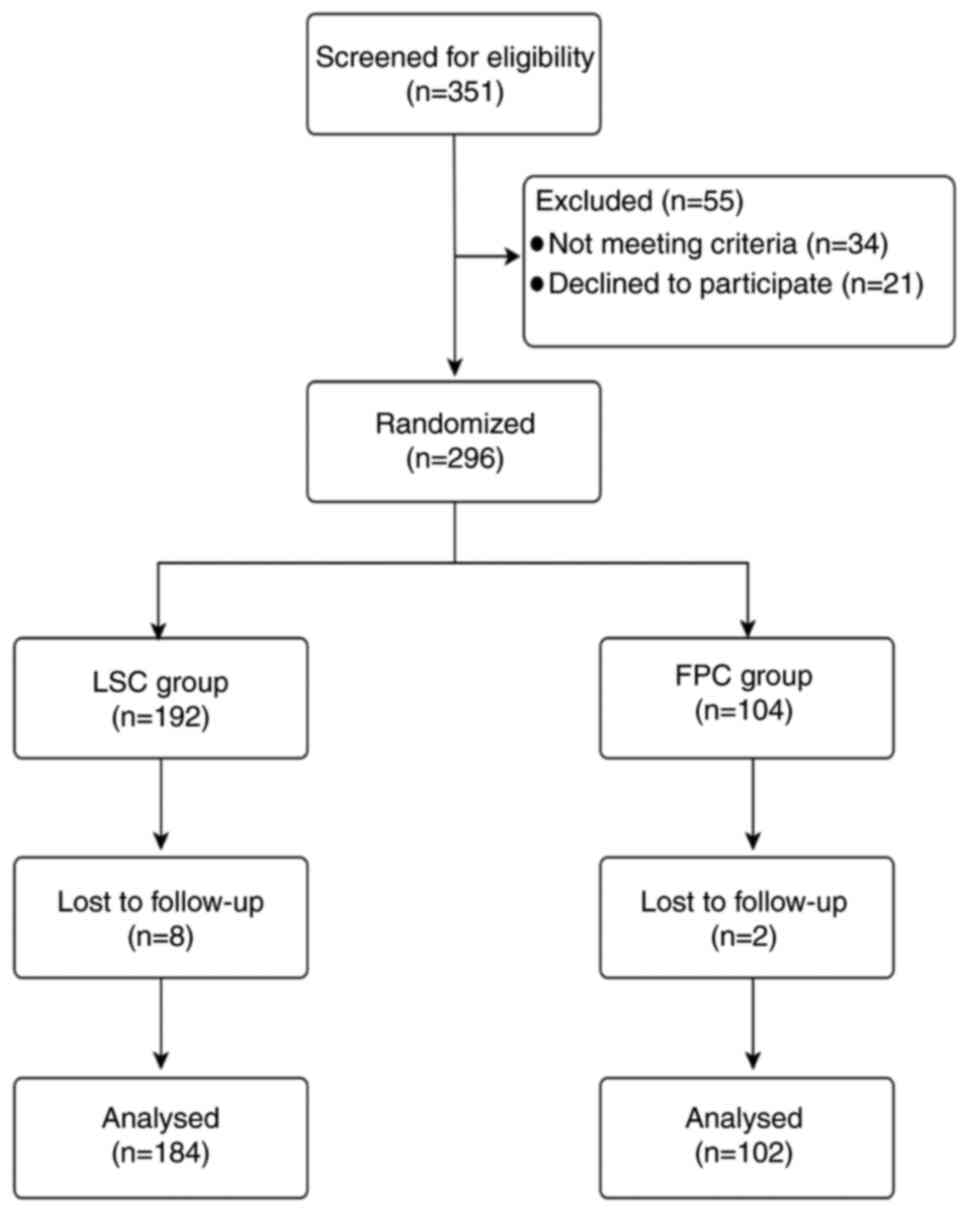
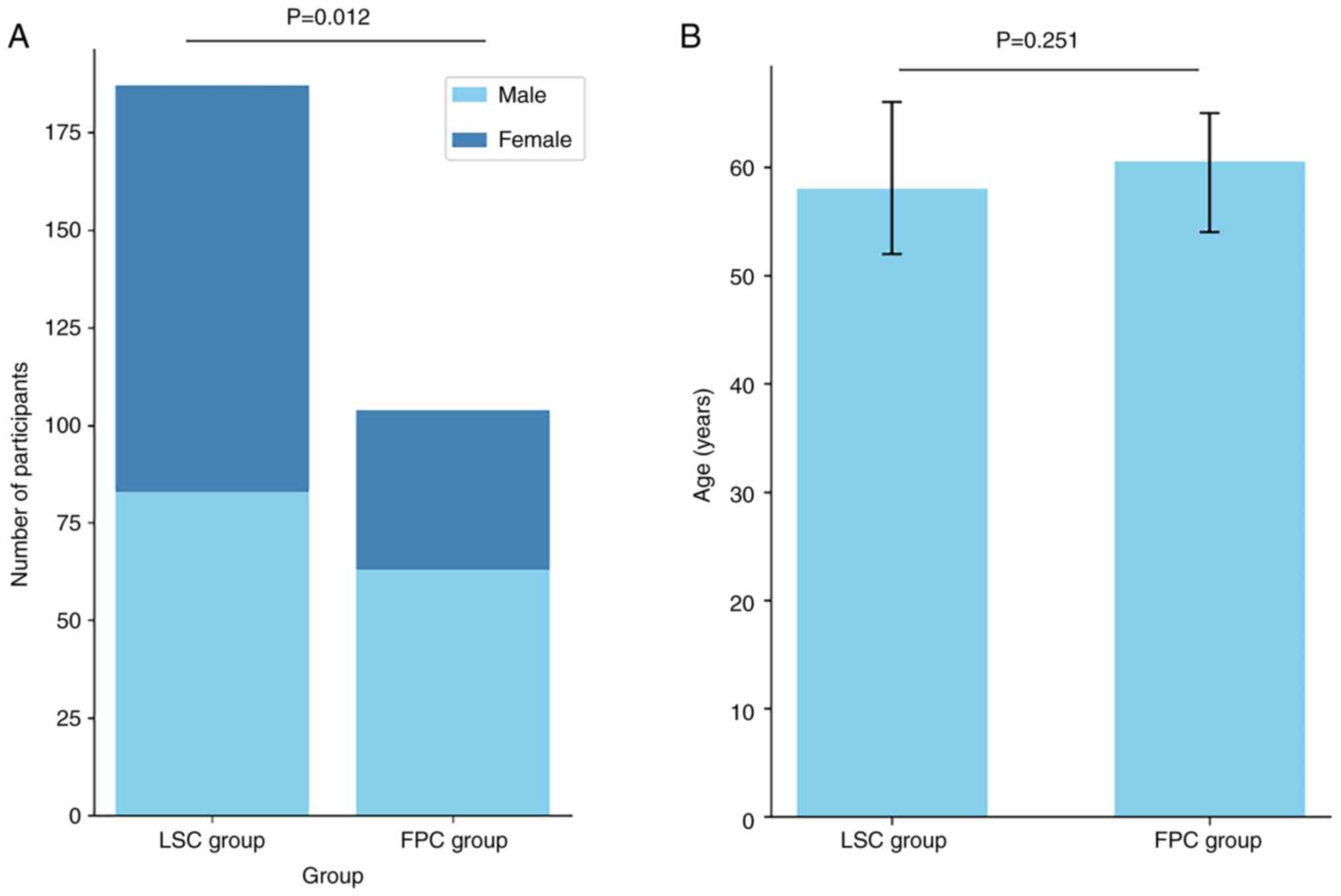
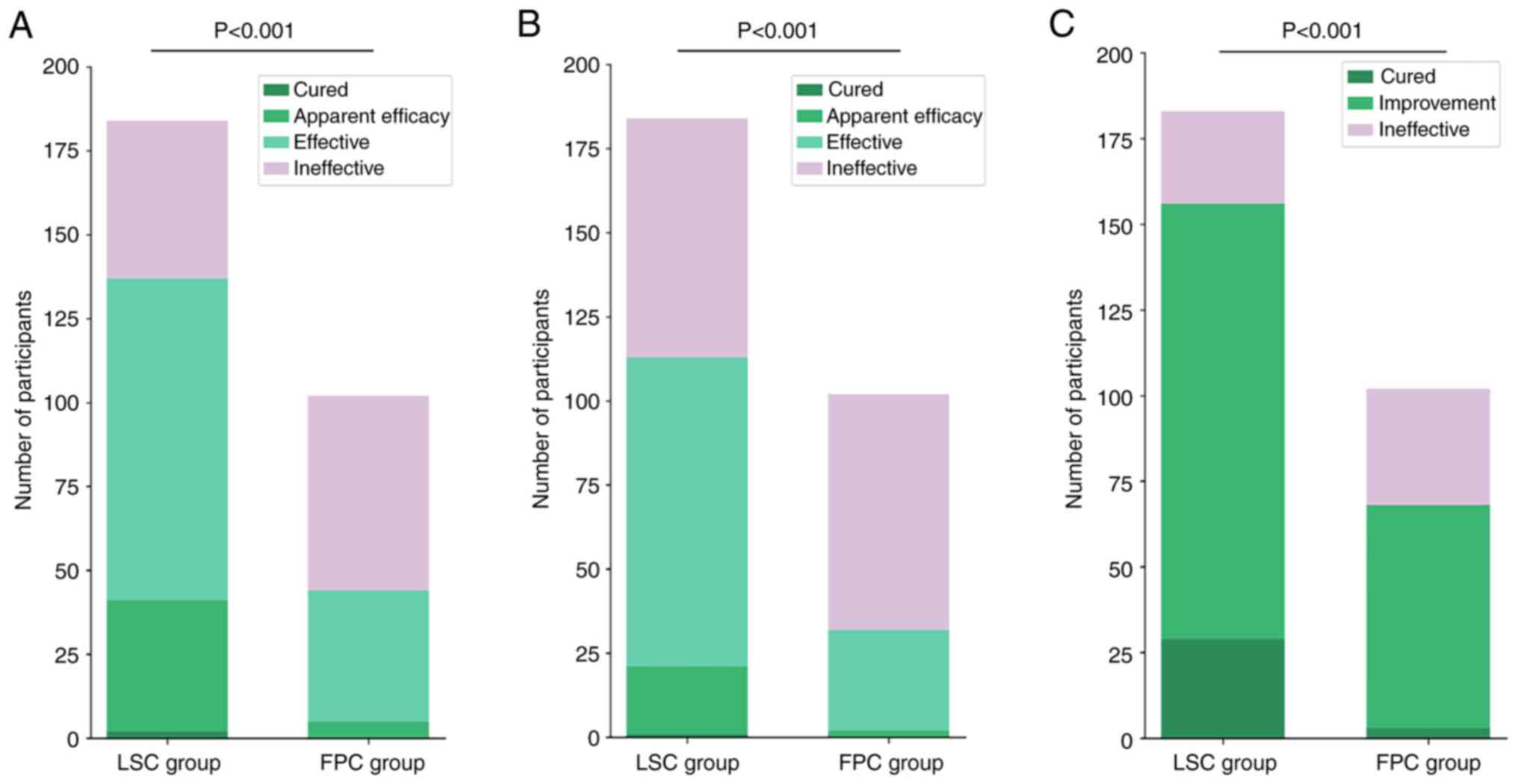
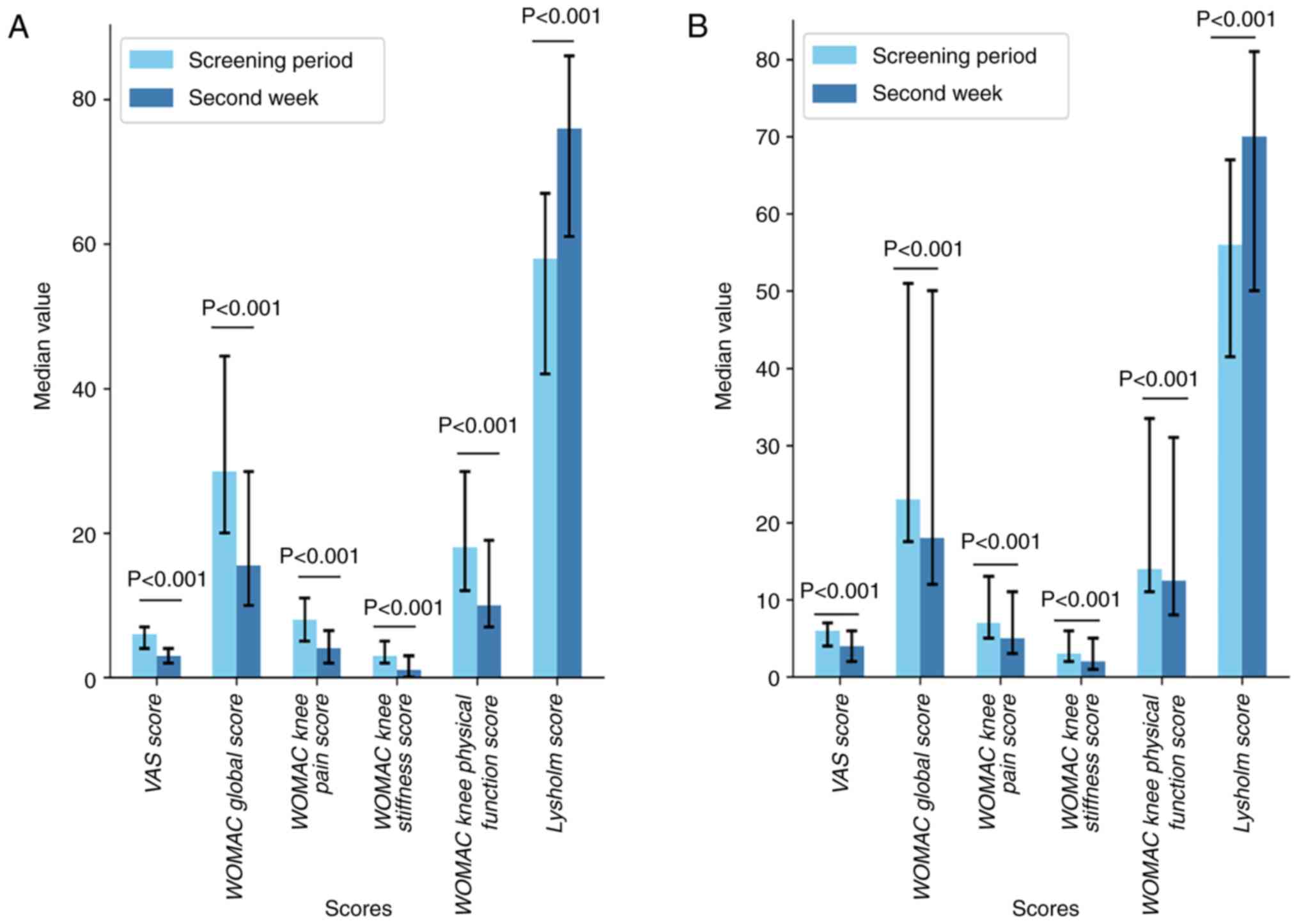
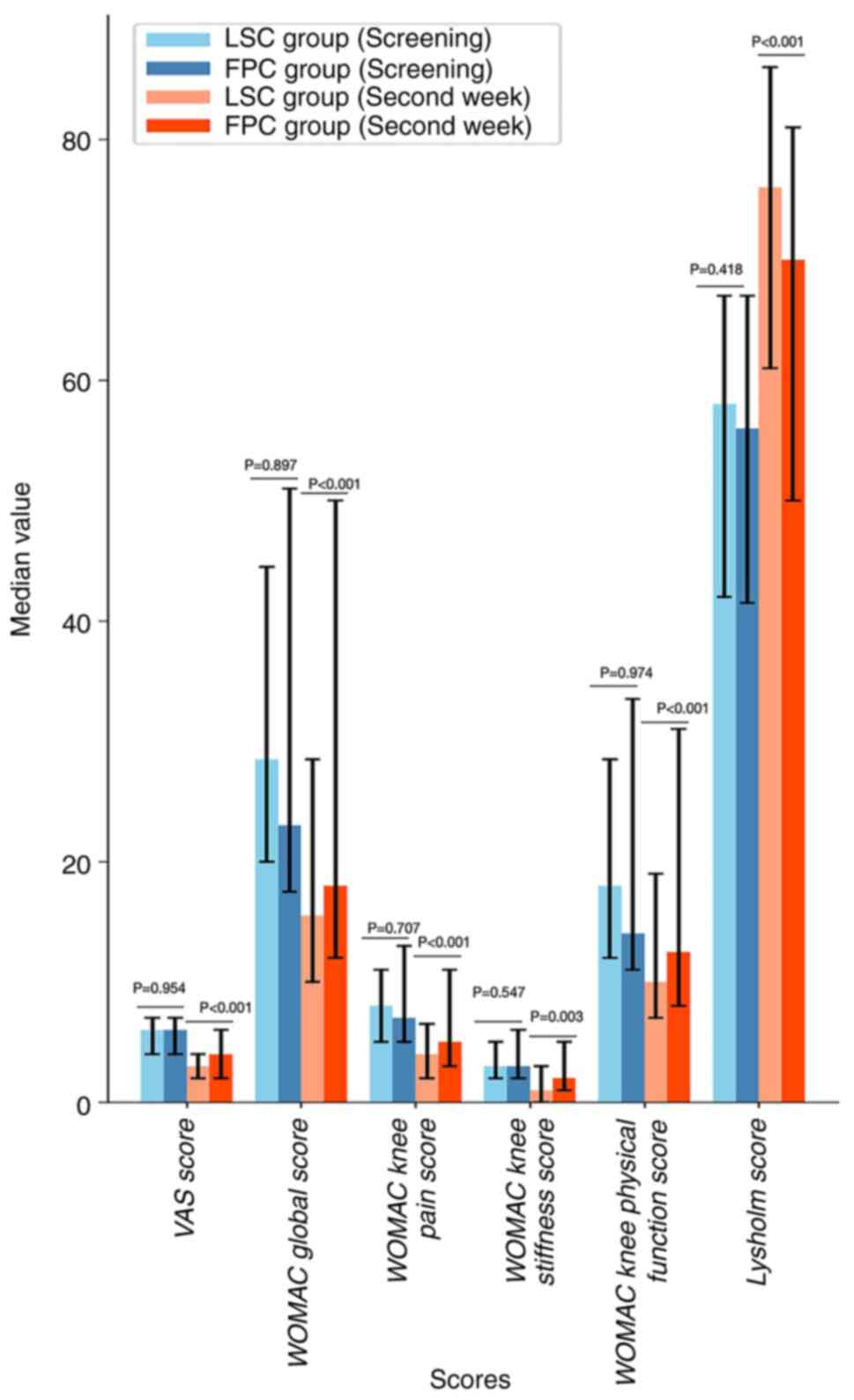
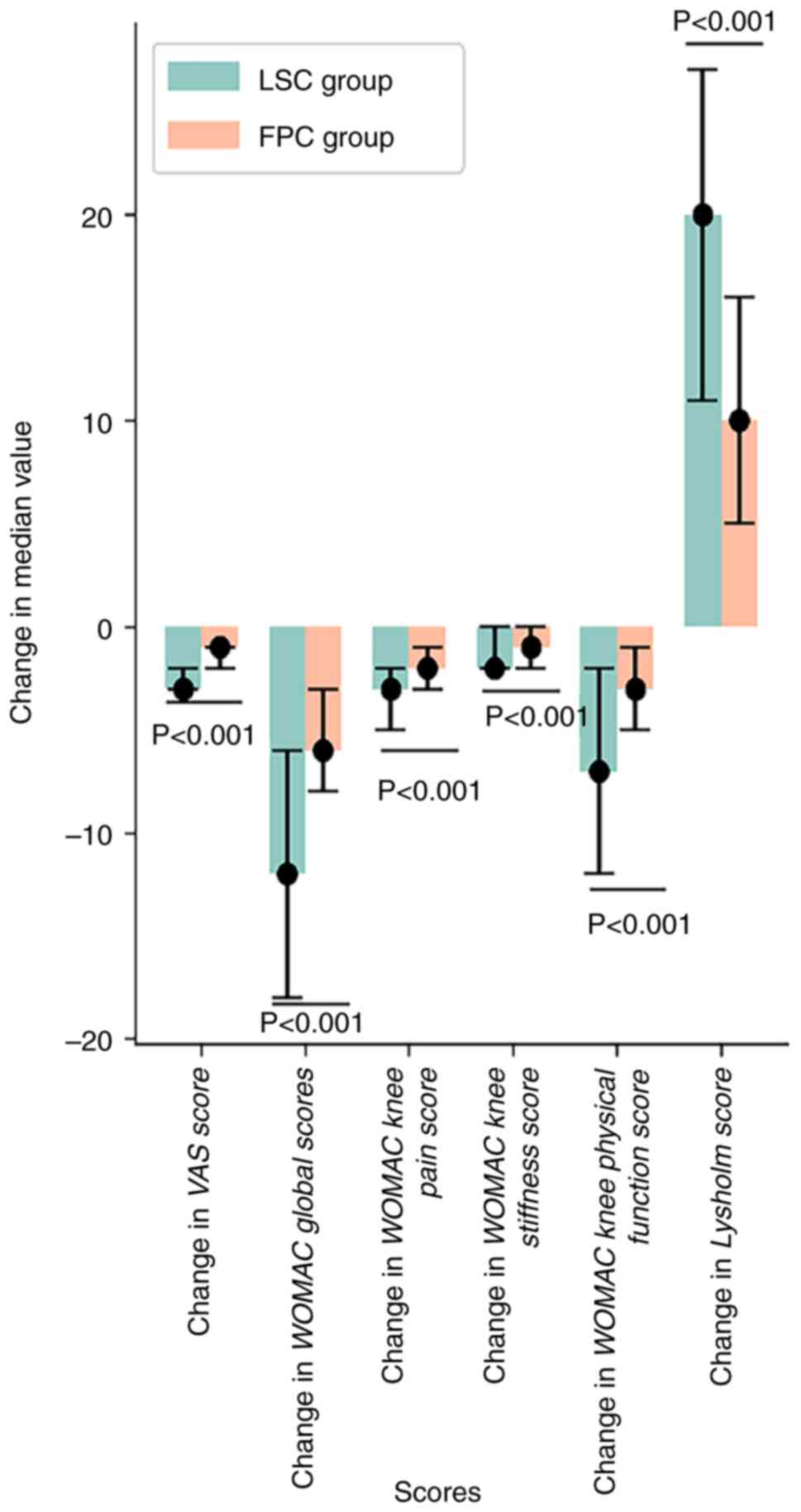
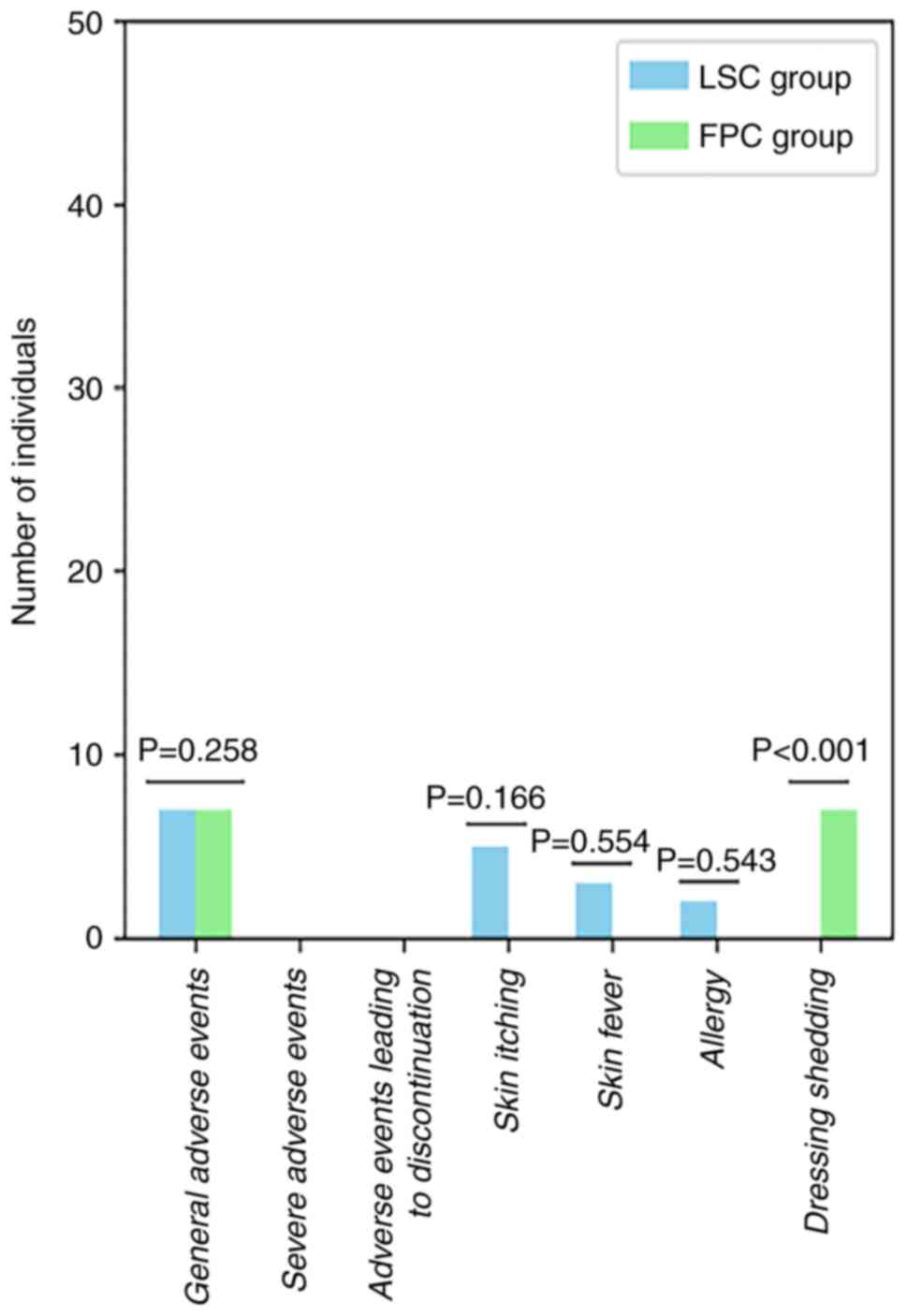
|
1
|
Bennell KL, Paterson KL, Metcalf BR, Duong
V, Eyles J, Kasza J, Wang Y, Cicuttini F, Buchbinder R, Forbes A,
et al: Effect of intra-articular platelet-rich plasma vs placebo
injection on pain and medial tibial cartilage volume in patients
with knee osteoarthritis: The RESTORE Randomized Clinical Trial.
JAMA. 326:2021–2030. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Katz JN, Arant KR and Loeser RF: Diagnosis
and treatment of hip and knee osteoarthritis. A review. JAMA.
325:568–578. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duong V, Oo WM, Ding C, Culvenor AG and
Hunter DJ: Evaluation and treatment of knee pain: A review. JAMA.
330(1568)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Neogi T and Colloca L: Placebo effects in
osteoarthritis: Implications for treatment and drug development.
Nat Rev Rheumatol. 19:613–626. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duan X, Zhao Y, Zhang J, Kong N, Cao R,
Guan H, Li Y, Wang K, Yang P and Tian R: Prediction of early
functional outcomes in patients after robotic-assisted total knee
arthroplasty: A nomogram prediction model. Int J Surg.
109:3107–3116. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tian R, Duan X, Kong N, Li X, Wang J, Tian
H, Shi Z, Yan S, Lyu J, Wang K and Yang P: Robotic-assisted total
knee arthroplasty is more advantageous for knees with severe
deformity: A randomized controlled trial study design. Int J Surg.
109:287–296. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geenen R, Overman CL, Christensen R,
åsenlöf P, Capela S, Huisinga KL, Husebø MEP, Köke AJA, Paskins Z,
Pitsillidou IA, et al: Eular recommendations for the health
professional's approach to pain management in inflammatory
arthritis and osteoarthritis. Ann Rheum Dis. 77:797–807.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bichsel D, Liechti FD, Schlapbach JM and
Wertli MM: Cross-sectional analysis of recommendations for the
treatment of hip and knee osteoarthritis in clinical guidelines.
Arch Phys Med Rehabil. 103:559–569.e5. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Geraghty T, Obeidat AM, Ishihara S, Wood
MJ, Li J, Lopes EBP, Scanzello CR, Griffin TM, Malfait AM and
Miller RE: Age-associated changes in knee
osteoarthritis,pain-related behaviors, and dorsal root ganglia
immunophenotyping of male and female mice. Arthritis Rheumatol.
75:1770–1780. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hunter DJ, Mclachlan AJ, Carroll PR,
Wakefield TAN and Stosic R: Health literacy and appropriateness of
self-care and pain management in osteoarthritis: An understanding
of the patient's perspective. Arthritis Care Res (Hoboken).
75:848–859. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Greig SL and Garnock-Jones KP: Loxoprofen:
A review in pain and inflammation. Clin Drug Investig. 36:771–781.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bannuru RR, Osani MC, Vaysbrot EE, Arden
NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott
JH, Bhandari M, et al: OARSI guidelines for the non-surgical
management of knee, hip, and polyarticular osteoarthritis.
Osteoarthritis Cartilage. 27:1578–1589. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Arden NK, Perry TA, Bannuru RR, Bruyère O,
Cooper C, Haugen IK, Hochberg MC, McAlindon TE, Mobasheri A and
Reginster JY: Non-surgical management of knee osteoarthritis:
Comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol.
17:59–66. 2019.
|
|
14
|
Weng Q, Goh S, Wu J, Persson MSM, Wei J,
Sarmanova A, Li X, Hall M, Doherty M, Jiang T, et al: Comparative
efficacy of exercise therapy and oral non-steroidal
anti-inflammatory drugs and paracetamol for knee or hip
osteoarthritis: A network meta-analysis of randomised controlled
trials. Br J Sports Med. 57:990–996. 2023.
|
|
15
|
Chen GY, Zhou CQ, Li H and Mao XZ:
Efficacy and safety of topical nsaids combined with physiotherapy
for frozen shoulder: A randomized controlled trial. Eur Rev Med
Pharmacol Sci. 28:3761–3770. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Voilley N: Acid-sensing ion channels
(ASICs): New targets for the analgesic effects of non-steroid
anti-inflammatory drugs (NSAIDs). Curr Drug Targets Inflamm
Allergy. 3:71–79. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li P, Li H, Shu X, Wu M, Liu J, Hao T, Cui
H and Zheng L: Intra-articular delivery of flurbiprofen sustained
release thermogel: Improved therapeutic outcome of collagenase
II-induced rat knee osteoarthritis. Drug Deliv. 27:1034–1043.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mu R, Bao CD, Chen ZW, Zheng Y, Wang GC,
Zhao DB, Hu SX, Li YJ, Shao ZW, Zhang ZY, et al: Efficacy and
safety of loxoprofen hydrogel patch versus loxoprofen tablet in
patients with knee osteoarthritis: A randomized controlled
non-inferiority trial. Clin Rheumatol. 35:165–173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iguchi M, Takahashi T and Takeshita K:
Effectiveness and adherence rate of s-flurbiprofen plaster for the
pain management of patients with moderate and end-stage knee
osteoarthritis. Cureus. 15(e44556)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin T, Liu Z, Ji W and Zhang P: Effects of
knee debridement with flurbiprofen on knee function, inflammatory
levels, and bone metabolism activity in patients with knee
osteoarthritis. Comput Math Methods Med.
2022(8031360)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tomatsu K, Yasuda S, Fuady A, Matsumoto H
and Sumariyono : Efficacy and safety of s-flurbiprofen
plaster in knee osteoarthritis patients: A 2-week randomized
controlled phase III clinical trial compared to diclofenac gel. Int
J Rheum Dis. 25:563–570. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pereira D, Ramos E and Branco J:
Osteoarthritis. Acta Med Port. 28:99–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wathier M, Lakin BA, Cooper BG, Bansal PN,
Bendele AM, Entezari V, Suzuki H, Snyder BD and Grinstaff MW: A
synthetic polymeric biolubricant imparts chondroprotection in a rat
meniscal tear model. Biomaterials. 182:13–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin JB, Poh S and Panitch A: Controlled
release of anti-inflammatory peptides from reducible
thermosensitive nanoparticles suppresses cartilage inflammation.
Nanomedicine. 12:2095–2100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Garriga C, Sánchez-Santos MT, Judge A,
Hart D, Spector T, Cooper C and Arden NK: Predicting incident
radiographic knee osteoarthritis in middle-aged women within four
years: The importance of knee-level prognostic factors. Arthritis
Care Res (Hoboken). 72:88–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jones IA, Togashi R, Wilson ML, Heckmann N
and Vangsness CT Jr: Intra-articular treatment options for knee
osteoarthritis. Nat Rev Rheumatol. 15:77–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Boer CG, Radjabzadeh D, Medina-Gomez C,
Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram
MA, et al: Intestinal microbiome composition and its relation to
joint pain and inflammation. Nat Commun. 10(4881)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Belk JW, Lim JJ, Keeter C, Mcculloch PC,
Houck DA, Mccarty EC, Frank RM and Kraeutler MJ: Patients with knee
osteoarthritis who receive platelet-rich plasma or bone marrow
aspirate concentrate injections have better outcomes than patients
who receive hyaluronic acid: Systematic review and meta-analysis.
Arthroscopy. 39:1714–1734. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stonehouse W, Benassi-Evans B, Bednarz J,
Vincent AD, Hall S and Hill C: Krill oil improved osteoarthritic
knee pain in adults with mild to moderate knee osteoarthritis: A
6-month multicenter, randomized, double-blind, placebo-controlled
trial. Am J Clin Nutr. 116:672–685. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Migliorini F, Schäfer L, Bell A, Weber CD,
Vecchio G and Maffulli N: Meniscectomy is associated with a higher
rate of osteoarthritis compared to meniscal repair following acute
tears: A meta-analysis. Knee Surg Sports Traumatol Arthrosc.
31:5485–5495. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
O'Neill TW and Felson DT: Mechanisms of
osteoarthritis (OA) pain. Curr Osteoporos Rep. 16:611–616.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Perrot S, Trouvin AP and Bouhassira D:
Three dimensions of pain in osteoarthritis: Development and
validation of the osteoarthritis symptom inventory scale. Pain.
164:1566–1577. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Allen KD, Thoma LM and Golightly YM:
Epidemiology of osteoarthritis. Osteoarthritis Cartilage.
30:184–195. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cui A, Li H, Wang D, Zhong J, Chen Y and
Lu H: Global, regional prevalence, incidence and risk factors of
knee osteoarthritis in population-based studies. EClinicalMedicine.
29-30(100587)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Long H, Liu Q, Yin H, Wang K, Diao N,
Zhang Y, Lin J and Guo A: Prevalence trends of site-specific
osteoarthritis from 1990 to 2019: Findings from the global burden
of disease study 2019. Arthritis Rheumatol. 74:1172–1183.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kolasinski SL, Neogi T, Hochberg MC, Oatis
C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D,
et al: 2019 American college of rheumatology/arthritis foundation
guideline for the management of osteoarthritis of the hand, hip,
and knee. Arthritis Care Res (Hoboken). 72:149–162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bruyère O, Honvo G, Veronese N, Arden NK,
Branco J, Curtis EM, Al-Daghri NM, Herrero-Beaumont G,
Martel-Pelletier J, Pelletier JP, et al: An updated algorithm
recommendation for the management of knee osteoarthritis from the
european society for clinical and economic aspects of osteoporosis,
osteoarthritis and musculoskeletal diseases (ESCEO). Semin
Arthritis Rheum. 49:337–350. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mezey GA, Máté Z and Paulik E: Factors
influencing pain management of patients with osteoarthritis: A
cross-sectional study. J Clin Med. 11(1352)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schjerning A, Mcgettigan P and Gislason G:
Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev
Cardiol. 17:574–584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zeng C, Wei J, Persson MSM, Sarmanova A,
Doherty M, Xie D, Wang Y, Li X, Li J, Long H, et al: Relative
efficacy and safety of topical non-steroidal anti-inflammatory
drugs for osteoarthritis: A systematic review and network
meta-analysis of randomised controlled trials and observational
studies. Br J Sports Med. 52:642–650. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Da Costa BR, Pereira TV, Saadat P,
Rudnicki M, Iskander SM, Bodmer NS, Bobos P, Gao L, Kiyomoto HD,
Montezuma T, et al: Effectiveness and safety of non-steroidal
anti-inflammatory drugs and opioid treatment for knee and hip
osteoarthritis: Network meta-analysis. BMJ.
375(n2321)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ozen G, Pedro S and Michaud K: Major
adverse cardiovascular events and mortality with opioids versus
NSAIDs initiation in patients with rheumatoid arthritis. Ann Rheum
Dis. 82:1487–1494. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ji C, Yu Y, Zhang M, Yu W and Dong S:
Loxoprofen sodium alleviates oxidative stress and apoptosis induced
by angiotensin II in human umbilical vein endothelial cells
(HUVECs). Drug Des Devel Ther. 14:5087–5096. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nelson AE: Osteoarthritis year in review
2017: Clinical. Osteoarthritis Cartilage. 26:319–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kien NT, Geiger P, Van Chuong H, Cuong NM,
Van Dinh N, Pho DC, Anh VT and Giang NT: Preemptive analgesia after
lumbar spine surgery by pregabalin and celecoxib: A prospective
study. Drug Des Devel Ther. 13:2145–2152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Walker Taylor JL, Campbell CM, Thorpe RJ
Jr, Whitfield KE, Nkimbeng M and Szanton SL: Pain, racial
discrimination, and depressive symptoms among African American
women. Pain Manag Nurs. 19:79–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li D, Cheng Y, Yuan P, Wu Z, Liu J, Kan J,
Zhang K, Wang Z, Zhang H, Zhang G, et al: Efficacy and safety of
flurbiprofen cataplasms versus loxoprofen sodium cataplasms in knee
osteoarthritis: A randomized controlled trial. Chin Med J (Engl).
136:2187–2194. 2023.PubMed/NCBI View Article : Google Scholar
|