Introduction
Interstitial lung disease (ILD) is a heterogeneous
diffuse lung disease characterized by varying degrees of alveolar
and interstitial damage. Pulmonary hypertension (PH) is a
significant cause of dyspnea, increased oxygen consumption and
elevated mortality risk in patients with ILD (1). Previous studies have indicated that
3-86% of patients with ILD suffer from PH, with ~30-50% having
moderate-to-severe PH (Ms-PH) (2).
The severity of idiopathic pulmonary fibrosis (IPF) influences the
prevalence of PH in patients with IPF, with a prevalence rate of
8-15% at diagnosis (3,4), 35-44% prior to lung transplantation
evaluation (5,6), and 86% during lung transplantation
(7). The early clinical
manifestations of PH associated with ILD (PH-ILD) are often
non-specific and obvious, while the late stages of the disease
manifest significant changes, impacting the quality of life of
patients. Therefore, analyzing the clinical characteristics of
patients with PH-ILD and identifying risk factors for Ms-PH, as
well as searching for simple and low-cost biological markers
related to PH-ILD, are particularly important for early diagnosis
and treatment.
Currently, PH is defined as having the mPAP ≥25
mmHg, as measured via right heart catheterization (RHC) at sea
level and in the resting state (8).
RHC demonstrates high accuracy in measuring PH, yet its
invasiveness and potential risk of infection have prevented it from
being adopted as a routine screening method for PH in clinical
practice. Echocardiography is the most commonly used method for
screening PH in the clinical practice and can be used to detect
suspected or confirmed PH caused by various factors, observe
abnormal changes in both the left and right heart, estimate
hemodynamic parameters, and provide important reference value for
disease assessment. Multiple studies have demonstrated a strong
correlation between pulmonary artery systolic pressure (PASP)
measured by echocardiography and mean pulmonary artery pressure
(mPAP) measured by a RHC (9). It
has been reported that a PASP of 35 or 36 mmHg, as measured by
echocardiography, can serve as a normal resting value, at which
point non-invasive diagnosis of PH exhibits favorable sensitivity
and specificity (10). Due to the
long duration and insidious onset of ILD, by the time patients
exhibit noticeable clinical symptoms, they are often in the
advanced stages of the disease, accompanied by Ms-PH, which leads
to numerous patients not receiving timely diagnosis and missing the
optimal treatment opportunities. Therefore, it is of great
significance to analyze the clinical characteristics of patients
with PH-ILD and use simple and easy indicators in clinical practice
to assess whether patients with ILD have Ms-PH as early as
possible.
Patients and methods
Study population
A retrospective analysis was conducted on the
clinical characteristics and risk factors of patients with ILD with
complete clinical data who sought treatment at the Third Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China) from January
2017 to December 2021. All data were collected from the medical
records of the Third Affiliated Hospital of Sun Yat-sen University.
Inclusion criteria were as follows: patients must meet the
diagnosis of ILD, be aged ≥18 years, and have complete clinical
data. For patients who were hospitalized multiple times during the
study period, the first hospitalization was considered. Individuals
who met any of the following criteria were excluded: <18 years
of age; suffering from congenital heart disease, rheumatic heart
disease, left heart failure or chronic thromboembolic disease;
having other chronic lung diseases, including chronic obstructive
pulmonary disease (COPD), asthma, pulmonary infection; being
afflicted with liver cirrhosis, portal hypertension, chronic renal
failure; having idiopathic PH. Ultimately, 226 patients were
included in the study.
The present research protocol was approved (approval
no. SL-II2024-004-01) by the Institutional Review Board of the
Third Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China). Informed consent was waived because of the retrospective
nature of the study. All data are sourced from the Third Affiliated
Hospital of Sun Yat-sen University.
Calculation of sample size
Sample size is fundamental to a study, and a
sufficient sample size ensures a reliable foundation for research
findings. The present study employed a retrospective analysis to
investigate the research content, primarily comprising two parts:
One involves descriptive analysis of clinical characteristics,
while the other explores the risk factors for moderate and severe
PH. The sample size was calculated based on an analysis of
influencing factors. Specifically, the sample size requirement for
multi-factor analysis is at least 5 to 10-fold the number of
independent variables (11,12). In the current analysis, it was
considered whether moderate or severe PH occurred simultaneously as
the dependent variable. Incorporating previous research (13), at least 10-15 independent variables
were included. A logistic model was utilized for multivariate
analysis. Based on 10-fold the number of independent variables, the
sample size for the present study was we determined, resulting in
an estimated minimum of 150 cases.
Diagnostic criteria
Based on the diagnostic criteria for idiopathic
interstitial pneumonia established by experts from the American
Thoracic Society/European Respiratory Society in 2013(14), the main diagnostic criteria include
the presence of the following respiratory symptoms or signs,
accompanied by significant interstitial changes in the lungs
confirmed by chest computed tomography (CT): Ground-glass
opacities, interlobular septal thickening, linear shadows,
reticular shadows and tracking bronchiectasis. Two experienced
radiologists independently reviewed the CT images to determine the
consistency of the observations. If there was a disagreement
between the two opinions, a third radiologist was invited to
independently review the images and make a diagnosis. The diagnosis
of various connective tissue disease (CTD) conforms to the
corresponding guidelines (15-19).
The patient was placed in the left decubitus
position with calm breathing. Experienced color Doppler ultrasound
physicians conducted color Doppler ultrasound examinations on the
patient (model E9; Cytiva), utilizing M5S probes to assess the
patient's cardiac structure, with probe frequencies ranging from
1.7 to 3.4 MHz. The PASP was determined using the tricuspid
regurgitation velocity method, while the maximum tricuspid
regurgitation velocity was measured in the apical four-chamber
view. Continuous Doppler sampling lines were employed to document
the tricuspid regurgitation spectrum, and the maximum regurgitation
velocity was subsequently measured. The machine automatically
computes the pressure difference between the right ventricle and
right atrium. The velocity of the tricuspid regurgitation jet could
only be measured when the complete flow pattern of tricuspid
regurgitation was present and the peak velocity was clearly
visible. During sinus rhythm, each echocardiogram indicator was
measured three times and the average value was calculated, though
not necessarily continuously. When assessing right atrial systolic
pressure, right atrial pressure was estimated based on tricuspid
regurgitation velocity. Specifically, when there was mild tricuspid
regurgitation and the right atrial diameter was normal or slightly
enlarged, the estimated right atrial pressure was set at 5 mmHg.
For moderate tricuspid regurgitation and moderate enlargement of
the right atrial diameter, the estimated right atrial pressure
stands at 10 mmHg. In cases of severe tricuspid regurgitation and
extreme enlargement of the right atrial diameter, the estimated
right atrial pressure rises to 15 mmHg. The modified Bernoulli
equation was used to calculate PASP: PASP (mmHg)=right ventricular
systolic pressure=tricuspid valve pressure difference (4v2,
v=maximum tricuspid valve reflux velocity) + right atrial pressure
(RAP) (20). According to the PASP
value, a necessary condition for diagnosing PH is PASP ≥35 mmHg
(10). Patients were categorized
into three groups based on PASP: Non-PH group (PASP <35 mmHg),
Mild-PH group (PASP 35-49 mmHg) and Ms-PH group (PASP ≥50 mmHg)
(21).
Clinical data
Baseline data encompassed age, sex, smoking,
drinking, diabetes and hypertension. Using a conversion formula,
the doses of methylprednisolone (4 mg) and prednisone (5 mg) were
uniformly adjusted to match the dose of methylprednisolone.
Patients were categorized into three groups based on the hormone
dose cited in (22): No hormone
group, low-dose group (≤0.5 mg/kg/d) and sufficient dose group (1-2
mg/kg/d). Immunosuppressants included cyclophosphamide,
methotrexate, mycophenolate mofetil, leflunomide,
hydroxychloroquine, azathioprine, cyclosporine. Tripterygium
wilfordii and iguratimod were also included. Anti-fibrosis
drugs encompassed nintedanib and pirfenidone. Therapies for PH
encompassed endothelin receptor antagonists, such as bosentan and
axentan.
Fasting venous blood was collected within 24 h after
admission and subjected to routine blood testing using a Sysmex
XE-5000 fully automatic hematology analyzer. The routine blood
parameters encompassed white blood cell count, red blood cell (RBC)
count, hemoglobin level, platelet (PLT) count, neutrophil count,
lymphocyte count, PLT-to-lymphocyte ratio, neutrophil-to-lymphocyte
ratio, red blood cell distribution width (RDW) and PLT distribution
width (PDW).
The Hitachi 7180 fully automatic biochemical
analyzer is utilized to measure the levels of albumin, blood urea
nitrogen, uric acid (UA), serum creatinine, lactate dehydrogenase
(LDH). The latex agglutination method is employed to detect
C-reactive protein (CRP) levels. Additionally, the erythrocyte
sedimentation rate was determined using an Italian ALIfax text1
fully automatic sedimentation instrument.
Using the Roche E601 fully automated
electrochemiluminescence immunoassay, serum autoantibodies were
detected, including immunoglobulin (Ig) G, IgA, IgM and rheumatic
antibodies [antinuclear antibodies (ANAs) and extractable nuclear
antigen (ENA)]. These antibodies included anti-double stranded DNA
antibody (anti-dsDNA), anti-Sjogren's syndrome antigen A,
anti-Sjogren's syndrome antigen B, anti-Smith antibodies,
anti-kinetochore antibodies, anti-perinuclear anti-neutrophil
cytoplasmic antibodies, anti-cytoplasmic anti-neutrophil
cytoplasmic antibody, anti-myeloperoxidase anti-neutrophil
cytoplasmic antibody and anti-scleroderma-70 antibody.
Echocardiography
After the patient was positioned in the left lateral
position and allowed to breathe calmly, our ultrasound technician
used Doppler echocardiography (model E9; Cytiva) to measure the
main pulmonary artery width, PAP and cardiac function parameters,
mainly including the left ventricular diameter (LVD), left atrial
diameter (LAD), right ventricular dimension (RVD), right atrial
long diameter, right atrial transverse diameter, main pulmonary
artery and left ventricular ejection fraction (LVEF).
Pulmonary function test
The experienced pulmonary function technician from
the Third Affiliated Hospital of Sun Yat-sen University utilized
the pulmonary function tester to assess pulmonary function. All
examinations were conducted by professional technicians in the
pulmonary function room, and patients were advised to avoid using
bronchodilators for 24 h prior to the examination. Indicators such
as forced vital capacity as percentage of predicted value
(FEV1/FVC%), forced expiratory volume in 1 sec (FEV1)/forced volume
vital capacity (FVC) ratio (FVC% pred), diffusing capacity for
carbon monoxide (DLCO), carbon monoxide diffusion capacity as
percentage of predicted value (DLCO% pred), diffusing capacity for
carbon monoxide to alveolar volume ratio (DLCO/VA% pred), total
lung volume (TLC) and residual volume (RV) were collected.
Statistical analysis
Statistical analysis was performed using SPSS
version 25 software (IBM Corp.). Continuous data with a normal
distribution is expressed as the mean ± standard deviation (SD).
The measurement data with skewed distribution are expressed by
median and quartile M (P25, P75). Data with a normal distribution
should be compared by analysis of variance (ANOVA), all data
underwent post hoc analysis using the Least Significant Difference
test. Kruskal-Wallis test is reserved for non-parametric
distributions. The counting data were expressed as a constituent
ratio, and the comparison between groups was performed using the
χ2 test or Fisher's exact probability method. Pearson
correlation analysis was used for normal distribution data, and
Spearman correlation analysis was used for skewed distribution data
and rank data. Logistic regression analysis was employed to perform
both univariate and multivariate analyses on the factors
influencing Ms-PH in patients with ILD. Receiver operating
characteristic (ROC) curve was plotted to evaluate the predictive
value of biomarkers for Ms-PH in occurrence of patients with ILD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics and
treatment
Based on the inclusion and exclusion criteria, a
data of 226 patients with ILD confirmed by chest CT or lung biopsy
were included, among whom 91 patients had ILD accompanied by PH,
constituting 40.27% of the total population. The non-PH group
comprised 135 cases, including 35 men and 100 women aged from 27-89
years, with an average age of 51.69±12.77 years. The Mild-PH group
had 72 cases, consisting of 17 men and 55 women, aged from 23 to 78
years, with an average age of 53.67±13.16 years. The Ms-PH group
included 19 cases, with 7 men and 12 women aged from 40-80 years,
averaging 59.21±11.66 years. The age difference among these three
groups was statistically significant (P=0.05), whereas no
significant differences were observed in sex, smoking, drinking,
diabetes, hypertension and ILD subtypes (P>0.05). A total of 45
patients underwent oxygen therapy, 194 received hormone therapy,
and 29 were treated with pH-targeted drugs. Significant differences
were noted in the use of oxygen therapy, hormone therapy and
pH-targeted drug therapy among these three patient groups
(P<0.05). A comparison of baseline characteristics and
treatments is shown in Table I.
 | Table IComparison of baseline
characteristics and treatment of three groups. |
Table I
Comparison of baseline
characteristics and treatment of three groups.
|
Characteristics | Non-PH (n=135) | Mild-PH (n=72) | Ms-PH (n=19) | P-value |
|---|
| Age | 51.69±12.77 | 53.67±13.16 | 59.21±11.66 | 0.05 |
| Sex
(male/female) | 35/100 | 17/55 | 7/12 | 0.504 |
| Smoking, n (%) | 17 (12.6) | 8 (11.1) | 5 (26.3) | 0.207 |
| Drinking, n
(%) | 3 (2.2) | 2 (2.8) | 2 (10.5) | 0.195 |
| Diabetes, n
(%) | 10 (7.4) | 3 (4.2) | 3 (15.8) | 0.208 |
| Hypertension, n
(%) | 15 (11.1) | 10 (13.9) | 3 (15.8) | 0.758 |
| ILD subtypes, n
(%) | | | | 0.065 |
| Idiopathic
pulmonary fibrosis | 9 (6.7) | 6 (8.3) | 5 (26.3) | |
| Connective tissue
disease-related-ILD | 123 (91.1) | 66 (91.7) | 14 (73.7) | |
| Sarcoidosis | 3 (2.2) | 0 (0) | 0 (0) | |
| Oxygen, n (%) | 24 (17.8) | 13 (18.1) | 8
(42.1)a,b | 0.009 |
| Glucocorticoid, n
(%) | | | | 0.002 |
| ≤0.5 mg/kg/d | 104 (77.0) | 51 (70.8) | 8 (42.1) | |
| 1-2 mg/kg/d | 15 (11.1) | 15 (20.8) | 1 (5.3) | |
| Immunosuppressant,
n (%) | | | | 0.249 |
|
Cyclophosphamide | 44 (32.6) | 33 (45.8) | 9 (47.4) | |
| Methotrexate | 11 (8.1) | 5 (6.9) | 1 (5.2) | |
| Mycophenolate
mofetil | 20 (14.8) | 11 (15.3) | 2 (10.5) | |
| Leflunomide | 8 (5.9) | 4 (5.6) | 1 (5.3) | |
|
Hydroxychloroquine | 66 (48.9) | 33 (45.8) | 6 (31.6) | |
| Azathioprine | 7 (5.2) | 3 (4.2) | 0 (0) | |
| Cyclosporine | 2 (1.5) | 0 (0) | 0 (0) | |
| Tripterygium
Wilfordii | 9 (6.7) | 4 (5.6) | 2 (10.5) | |
| Iguratimod | 10 (7.4) | 2 (2.8) | 3 (15.8) | |
| PH drugs, n
(%) | | | | 0. 024 |
| Bosentan | 12 (8.9) | 5 (6.9) | 4 (21.1) | |
| Axentan | 14 (10.4) | 5 (6.9) | 6 (31.6) | |
| Combined with PH
drug therapy, n (%) | 5 (3.7) | 2 (2.8) | 3 (15.8) | 0.075 |
| Antifibrotic
therapy, n (%) | | | | 0.312 |
| Nintedanib | 8 (5.9) | 3 (4.2) | 0 (0) | |
| Pirfenidone | 35 (25.9) | 19 (26.4) | 9 (47.4) | |
Blood and immunological
indicators
Serological comparison revealed that the mean RDW in
the non-PH group was 0.135 (0.127, 0.148), in the Mild-PH group it
was 0.141 (0.132, 0.150), and in the Ms-PH group, it was 0.152
(0.134, 0.164). Significant differences were observed among these
three groups (P=0.007). Similarly, the mean PLT volume (MPV) in the
non-PH group was 10 (9.4, 10.6), in the mild PH group it was 10.1
(9.6, 10.8), and in the Ms-PH group, it was 10.8 (10.1, 12.6), with
statistically significant differences (P=0.005). Additionally,
significant differences were noted in PDW, UA, CRP and LDH among
the three groups (P<0.05). No statistically significant
differences were observed in the other indicators among the three
groups (P>0.05). Statistical analysis revealed significant
differences existed in the levels of immunological indicators, such
as IgG, IgA and C4, among the three groups (P<0.05). However, no
statistically significant difference was observed in IgM and C3
levels among the three groups (P>0.05). A comparison of
serological indicators among these three groups is presented in
Table II.
 | Table IIComparison of blood and immunological
indicators of three groups. |
Table II
Comparison of blood and immunological
indicators of three groups.
| Variable | Non-PH (n=135) | Mild-PH (n=72) | Ms-PH (n=19) | P-value |
|---|
| White blood cells
(109/l) | 6.17 (4.88,
8.65) | 7.15 (5.55,
9.25) | 6.85 (5.78,
10.22) | 0.278 |
| Hemoglobin
(g/l) | 124 (114, 133) | 123 (110, 132) | 124 (114, 140) | 0.619 |
| Red blood cell
distribution width | 0.135 (0.127,
0.148) | 0.141 (0.132,
0.150) | 0.152 (0.134,
0.164)a,b | 0.007 |
| Platelet
distribution width | 10.8 (9.8,
12.0) | 11.3 (10.0,
12.4) | 11.9 (10.9,
13.1) | 0.033 |
| Mean platelet
volume (fl) | 10 (9.4, 10.6) | 10.1 (9.6,
10.8) | 10.8 (10.1,
12.6) | 0.005 |
| Neutrophils
(109/l) | 3.95 (3.03,
6.05) | 4.40 (3.25,
6.93) | 4.52 (3.55,
6.64) | 0.238 |
| Lymphocytes
(109/l) | 1.45 (1.05,
1.96) | 1.51 (0.98,
1.98) | 1.20 (0.94,
1.74) | 0.730 |
| Platelets
(109/l) | 237 (203, 298) | 257 (213, 314) | 246 (185, 328) | 0.359 |
|
Neutrophil-to-lymphocyte ratio | 2.92 (2.00,
4.16) | 3.08 (1.84,
5.75) | 3.42 (2.44,
6.71) | 0.354 |
|
Platelet-to-lymphocyte ratio | 168.38 (118.89,
230.85) | 172.22 (121.42,
280.79) | 176.19 (129.07,
333.33) | 0.520 |
| Albumin (g/l) | 37.14±4.88 | 36.32±5.34 | 34.62±4.92 | 0.101 |
| Erythrocyte
sedimentation rate (mm/h) | 29 (16, 54) | 31 (14, 60) | 40 (35, 74) | 0.141 |
| C-reactive protein
(mg/l) | 3.55 (1.10,
14.29) | 3.45 (1.80,
21.20) | 16.80 (1.90,
31.15) | 0.040 |
| Lactate
dehydrogenase (U/l) | 221 (182, 285) | 274 (205,
353)a | 295 (241,
309)b | <0.0001 |
| Uric acid
(µmol/l) | 321 (255, 372) | 321.5 (282,
391) | 408 (347,
441)a,b | 0.002 |
| Serum creatinine
(µmol/l) | 57 (49, 65) | 57 (48, 69) | 66 (51, 101) | 0.161 |
| Immunoglobulin G
(g/l) | 14.31 (11.36,
17.39) | 16.13 (11.92,
18.20) | 21.00 (16.29,
25.66)a,b | <0.0001 |
| Immunoglobulin A
(g/l) | 2.62 (1.87,
3.46) | 2.37 (1.96,
2.87) | 3.42 (2.85,
4.06)a,b | 0.010 |
| Immunoglobulin M
(g/l) | 1.29 (0.95,
1.88) | 1.14 (0.83,
1.73) | 1.46 (1.09,
1.75) | 0.394 |
| Complement 3
(g/l) | 1.19±0.21 | 1.17±0.25 | 1.17±0.35 | 0.279 |
| Complement 4
(g/l) | 0.24±0.07 | 0.22±0.08 |
0.19±0.08b | 0.027 |
Rheumatoid antibodies
All patients with CTD-ILD underwent rheumatic
antibody testing. There was no statistically significant difference
in the ANA positivity rate or anti-dsDNA antibody level among the
three groups (P>0.05). In the anti-ENA antibody profile, a
statistically significant difference was observed in anti-U1
ribonucleoprotein (U1RNP) antibody among the three groups
(P=0.009), whereas no statistically significant difference was
found in the positivity rates of other rheumatoid antibodies among
the three groups (P>0.05). The detailed results are included in
Table SI.
Echocardiographic parameters
Significant differences were observed in RVD, right
atrial long diameter, right atrial short diameter and main
pulmonary artery width among the three groups (P<0.05). However,
no significant differences were found in the LVD, LAD and LVEF%
among the three groups (P>0.05). The specific results are shown
in Table III.
 | Table IIIComparison of echocardiographic
parameters of three groups. |
Table III
Comparison of echocardiographic
parameters of three groups.
| Variable | Non-PH (n=135) | Mild-PH (n=72) | Ms-PH (n=19) | P-value |
|---|
| Pulmonary artery
systolic pressure (mmHg) | 28 (25, 31) | 38 (36, 43) | 55 (55, 64) | <0.0001 |
| Left ventricular
diameter (mm) | 44 (42, 46) | 44 (41, 46) | 44 (40, 48) | 0.891 |
| Right ventricular
diameter (mm) | 20 (19, 22) | 21 (19, 23) | 24 (22,
26)a,b | <0.0001 |
| Left atrial
diameter (mm) | 30 (27, 32) | 30 (28, 33) | 32 (29, 33) | 0.071 |
| Right atrial long
diameter (mm) | 40 (38, 43) | 41 (39, 43) | 45 (43,
55)a,b | <0.0001 |
| Right atrial short
diameter (mm) | 30 (28, 32) | 31 (30, 33) | 36 (31,
42)a,b | <0.0001 |
| Main pulmonary
artery width (mm) | 21 (20, 23) | 23 (21,
24)a | 25 (24,
27)a,b | <0.0001 |
| Left ventricular
ejection fraction (%) | 68 (64, 70) | 69 (64, 73) | 67 (62, 73) | 0.258 |
Pulmonary function tests
Among the three groups, 84 cases completed the
pulmonary function examination, with 50 cases in the non-PH group,
25 cases in the Mild-PH group, and 9 cases in the Ms-PH group.
Pulmonary function tests revealed restrictive ventilatory
dysfunction in 55 patients and normal results in 29 patients. No
significant difference was observed in the types of lung function
impairment among the three groups (P>0.05). Statistical analysis
indicated significant differences in the FVC% pred, DLCO, DLCO%
pred, and DLCO/VA% pred values among the three groups (P<0.05).
Compared with the non-PH group, both FVC%pred and DLCO% pred values
decreased significantly in the mild-PH group (P<0.05). The mean
values of DLCO% pred and DLCO/VA% pred in the Ms-PH group were
found to be lower compared with the non-PH group, with a
statistically significant difference (P<0.05). However, there
were no statistically significant differences in FEV1/FVC%, TLC, or
RV among the three groups (P>0.05). The comparison of lung
function test results among the three groups is presented in
Table IV.
 | Table IVComparison of pulmonary function test
of three groups. |
Table IV
Comparison of pulmonary function test
of three groups.
| Variable | Non-PH (n=50) | Mild-PH (n=25) | Ms-PH (n=9) | P-value |
|---|
| Types, n (%) | | | | 0.143 |
| Normal | 21(42) | 7(28) | 1 (11.1) | |
| Restrictive | 29(58) | 18(72) | 8 (88.9) | |
| Forced vital
capacity as percentage of predicted value, % | 78.40±19.19 |
68.23±15.72a | 66.75±14.73 | 0.033 |
| Forced expiratory
volume in 1 sec/forced volume vital capacity, % | 104.43±8.50 | 103.24±8.29 | 103.04±6.90 | 0.797 |
| Diffusing capacity
for carbon monoxide (mmol/min/Kpa/l) | 5.11 (3.96,
6.05) | 4.12 (3.80,
4.80) | 3.26 (2.16,
4.44) | 0.002 |
| Carbon monoxide
diffusion capacity as percentage of predicted value, % | 66.62±18.14 |
54.31±13.42a |
43.97±12.89a,b | 0.008 |
| Diffusing capacity
for carbon monoxide to Alveolar Volume Ratio, % | 83.68±18.14 | 76.02±15.90 |
65.41±12.99a,b | 0.203 |
| Total lung
capacity, l | 3.92±0.96 | 3.75±0.93 | 3.32±0.74 | 0.438 |
| Residual volume,
l | 1.72±0.41 | 1.79±0.44 | 1.59±0.30 | 0.143 |
Risk factors for ILD associated with
Ms-PH. Univariate analysis
To explore the risk factors for patients with ILD
with Ms-PH, patients with ILD were divided into two groups based on
the value of PASP. The non-PH group and mild-PH group were combined
to form the nmPH group, while the remaining patients formed Ms-PH
group. Using Ms-PH as the dependent variable and age, sex, smoking,
drinking, diabetes, hypertension, treatment, immunology, rheumatic
antibody and serological indicators as independent variables,
logistic regression analysis was conducted to analyze risk factors.
The results of univariate regression analysis revealed
statistically significant differences (P<0.05) in 12 variables
were between the nmPH group and Ms-PH group. The results are shown
in Table V.
 | Table VUnivariate analysis of interstitial
lung disease complicated with moderate to severe PH. |
Table V
Univariate analysis of interstitial
lung disease complicated with moderate to severe PH.
| | 95% confidence
interval |
|---|
| Variable | Regression
coefficient | P-value | Odds ratio | Lower limit | Upper limit |
|---|
| Age | 0.042 | 0.030 | 1.043 | 1.004 | 1.083 |
| Sex (Female) | 0.553 | 0.270 | 1.739 | 0.650 | 4.650 |
| Smoking | -0.956 | 0.090 | 0.385 | 0.128 | 1.159 |
| Drinking | 1.559 | 0.074 | 4.753 | 0.857 | 26.355 |
| Diabetes | -1.029 | 0.137 | 0.357 | 0.092 | 1.385 |
| Hypertension | 0.311 | 0.640 | 1.365 | 0.371 | 5.019 |
| Anti-nuclear
antibodies | 0.069 | 0.906 | 1.071 | 0.339 | 3.386 |
| Anti-double
stranded DNA antibodies | 1.084 | 0.344 | 2.956 | 0.313 | 27.948 |
| Anti-Ro52 | -0.405 | 0.458 | 0.667 | 0.229 | 1.943 |
| Anti-Ro60 | -0.703 | 0.280 | 0.495 | 0.138 | 1.773 |
| Anti-U1
ribonucleoprotein | 1.163 | 0.061 | 3.345 | 1.042 | 9.827 |
| Anti-scleroderma-70
antibody | -1.477 | 0.042 | 3.199 | 0.030 | 1.765 |
| Oxygen | -1.344 | 0.007 | 0.261 | 0.097 | 0.697 |
| Glucocorticoid | -1.713 | 0.001 | 0.180 | 0.066 | 0.493 |
|
Immunosuppressor | -0.911 | 0.105 | 0.402 | 0.134 | 1.209 |
| PH-targeting
drugs | 1.275 | 0.013 | 3.580 | 1.302 | 9,844 |
| Combined with PH
targeted drug | 1.678 | 0.023 | 5.357 | 1.263 | 22.728 |
| Antifibrotic
drugs | -0.699 | 0.148 | 0.497 | 0.193 | 1.283 |
| White blood cells
(109) | 0.028 | 0.734 | 1.029 | 0.875 | 1.210 |
| Red blood cell
distribution width | 24.264 | 0.015 |
3.451x1010 | 117.761 |
1.011x1019 |
| Platelet
distribution width | 0.190 | 0.069 | 1.209 | 0.986 | 1.483 |
| Mean platelet
volume (fl) | 0.740 | 0.000 | 2.095 | 1.434 | 3.062 |
|
Neutrophil-to-lymphocyte ratio | 0.024 | 0.738 | 1.025 | 0.889 | 1.181 |
|
Platelet-to-lymphocyte ratio | 0.000 | 0.761 | 1.000 | 0.997 | 1.004 |
| Uric acid
(µmol/l) | 0.009 | 0.000 | 1.009 | 1.004 | 1.013 |
| Lactate
dehydrogenase (U/l) | 0.001 | 0.126 | 1.001 | 1.000 | 1.003 |
| C-reactive protein
(mg/l) | 0.009 | 0.287 | 1.009 | 0.992 | 1.027 |
| Erythrocyte
sedimentation rate (mm/h) | 0.013 | 0.084 | 1.013 | 0.998 | 1.027 |
| Serum creatinine
(µmol/l) | 0.009 | 0.069 | 1.009 | 0.999 | 1.019 |
| Ιmmunoglobulin G
(g/l) | 0.116 | 0.000 | 1.123 | 1.061 | 1.190 |
| Ιmmunoglobulin A
(g/l) | 0.334 | 0.019 | 1.396 | 1.056 | 1.845 |
| Ιmmunoglobulin M
(g/l) | 0.015 | 0.962 | 1.015 | 0.561 | 1.836 |
| Complement 3
(g/l) | -1.882 | 0.051 | 0.152 | 0.023 | 1.008 |
| Complement 4
(g/l) | -7.793 | 0.020 | 0.000 | 0.000 | 0.294 |
Multivariate analysis. Variables with
P<0.05 from Table V were
included in the multivariate logistic regression analysis. The
results indicated that age, RDW, MPV and IgG levels were
independent risk factors for the occurrence of Ms-PH in patients
with ILD, with the detailed results presented in Table VI.
 | Table VIMultifactor analysis of interstitial
lung disease complicated with moderate and severe PH. |
Table VI
Multifactor analysis of interstitial
lung disease complicated with moderate and severe PH.
| | 95% confidence
interval |
|---|
| Variable | Regression
coefficient | P-value | Odds ratio | Lower limit | Upper limit |
|---|
| Age | 0.077 | 0.034 | 1.080 | 1.006 | 1.159 |
| Oxygen | 1.564 | 0.084 | 4.779 | 0.810 | 28.196 |
| glucocorticoid | -1.828 | 0.081 | 0.161 | 0.021 | 1.257 |
| PH-targeted drug
therapy | 1.926 | 0.116 | 6.859 | 0.623 | 75.556 |
| Combined with PH
targeted drug therapy | 2.483 | 0.084 | 11.980 | 0.714 | 201.079 |
| Red blood cell
distribution width | 39.996 | 0.022 |
2.345x1017 | 309.548 |
1.777x1032 |
| Mean platelet
volume (fl) | 0.830 | 0.009 | 2.294 | 1.232 | 4.274 |
| Uric acid
(µmol/l) | 0.006 | 0.069 | 1.006 | 1.000 | 1.257 |
| Immunoglobulin G
(g/l) | 0.152 | 0.008 | 1.164 | 1.040 | 1.303 |
| Immunoglobulin A
(g/l) | 0.230 | 0.423 | 1.259 | 0.717 | 2.210 |
| Complement 4
(g/l) | -8.464 | 0.213 | 0.000 | 0.000 | 128.127 |
| Constant | -20.704 | 0.001 | 0.000 | | |
Diagnostic efficacy of RDW and MPV for
patients with ILD with Ms-PH
As shown in Table
VI, RDW and the MPV were found to be independent risk factors
for Ms-PH in patients with ILD. To explore the diagnostic value of
RDW and MPV in patients with Ms-PH, SPSS software was utilized to
analyze the area under the curve (AUC), with the sensitivity as the
y-axis and 1-specificity as the x-axis. The optimal cutoff value
was determined based on the Youden index (sensitivity +
specificity-1). ROC curve analysis revealed that the optimal cutoff
value for RDW in predicting Ms-PH in ILD is 0.323, with an AUC of
0.672, a 95% confidence interval (CI) ranging from 0.547 to 0.796,
and sensitivity and specificity of 0.579 and 0.744, respectively
(P=0.013). For MPV, the optimal cutoff value for predicting Ms-PH
in ILD is 0.378, with an AUC of 0.714, a 95% CI ranging from 0.583
to 0.845, and sensitivity and specificity of 0.842 and 0.536,
respectively (P=0.002). When RDW and MPV were combined, the AUC
increases compared with when they are diagnosed separately
(AUC=0.78, P<0.0001), enhancing the diagnostic efficacy in
predicting Ms-PH in ILD. Detailed results are presented in Table VII and Fig. 1.
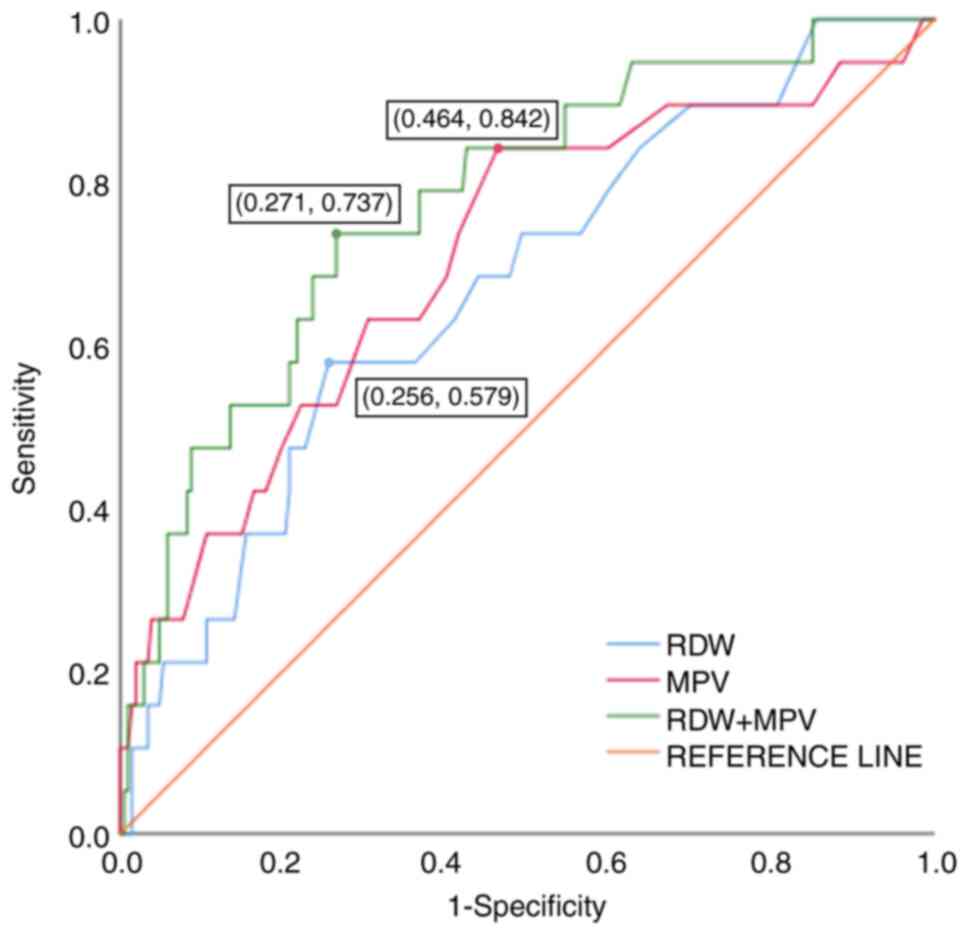 | Figure 1ROC curve analysis of RDW, the MPV
alone and RDW combined with the MPV in patients with ILD with
Ms-PH. In the ROC curve analysis, the optimal cut-off value for RDW
predicting Ms-PH among patients with ILD was determined to be
0.323, with an AUC of 0.672 (95% CI: 0.547 ~ 0.796). For MPV, the
AUC stood at 0.714 (95% CI: 0.583~0.845), the optimal cutoff value
was 0.378, the sensitivity was 84.2%, and the specificity was
53.6%. When RDW and MPV were combined for diagnostic use, the
diagnostic performance surpassed that achieved by using either
indicator alone, with an AUC of 0.780. Furthermore, the areas under
the RDW, MPV and RDW-MPV combination curves were found to be
statistically significant (P<0.05). ROC, receiver operating
characteristic; RDW, red blood cell distribution width; MPV, mean
platelet volume; ILD, interstitial lung disease; Ms-PH, moderate-to
severe pulmonary hypertension; CI, confidence interval. |
 | Table VIIDiagnostic efficiency of RDW, MPV and
their combination in interstitial lung disease complicated with
moderate-to-severe pulmonary hypertension. |
Table VII
Diagnostic efficiency of RDW, MPV and
their combination in interstitial lung disease complicated with
moderate-to-severe pulmonary hypertension.
| Variable | Area under the
curve | P-value | 95% confidence
interval | Sensitivity | Specificity | Optimal cutoff
value |
|---|
| Single index | | | | | | |
| RDW | 0.672 | 0.013 | (0.547, 0.796) | 0.579 | 0.744 | 0.323 |
| MPV (fl) | 0.714 | 0.002 | (0.583, 0.845) | 0.842 | 0.536 | 0.378 |
| Combined index | | | | | | |
| RDW + MPV | 0.780 | <0.0001 | (0.671, 0.889) | 0.737 | 0.729 | 0.466 |
Discussion
The incidence of PH-ILD was ~40.27% in the present
study. Most patients with PH-ILD were middle-aged or elderly.
Currently, the possible pathogenesis of PH-ILD encompasses the
following aspects:
Pulmonary interstitial fibrosis. Progressive
pulmonary fibrosis in patients with ILD can lead to a decrease in
the surface area and vascular density of pulmonary capillaries,
compressing the capillaries and causing vascular occlusion.
Simultaneously, persistent chronic inflammation can result in
medial vascular hypertrophy, obstructive intimal hyperplasia and
fibrosis, further narrowing the pulmonary vascular bed, increasing
pulmonary vascular resistance, and continuously elevating PAP
(23). Research indicates that IPF
is associated with the inflammatory expression of the
senescence-associated secretory phenotype (SASP), encompassing
biological factors such as transforming growth factor beta, tumor
necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). SASP
promotes the progression of fibrosis, with the TNF-α component of
SASP being considered a significant mediator of age-related
fibrosis in IPF (24).
Additionally, another study revealed that long-term exposure to
TNF-α elevates the levels of reactive oxygen species (ROS) within B
cells and intensifies the activation of nuclear factor kappa B.
This exposure further triggers the aging of lung endothelial cells
and fibroblasts, activates the SASP pathway, and ultimately leads
to interstitial remodeling and the formation of PH (24,25),
suggesting that elderly patients with ILD are more susceptible to
developing PH.
Pulmonary vasoconstriction. Hypoxemia in
patients with ILD can increase the production of ROS, which can
enhance the concentration of calcium ions in pulmonary artery
smooth muscle cells and induce vascular constriction. Additionally,
pulmonary fibrosis can render alveoli more susceptible to collapse,
thereby aggravating alveolar hypoxia and further promoting hypoxic
pulmonary vasoconstriction. Persistent pulmonary vasoconstriction
can elevate pulmonary vascular resistance and trigger the
development of pulmonary arterial hypertension (24).
Abnormal phenotype of vascular endothelial
cells. IL-6 stimulation induces and increases p53 protein
expression and ROS levels (26,27).
P53 is involved in cell cycle arrest and is expressed in both
pulmonary artery endothelial cells (PAECs) and pulmonary artery
smooth muscle cells (PASMCs). The expression of P53 in PASMCs
induced by hypoxia is lower than that in PAECs. Additionally, it
stimulates the proliferation of PASMCs, promotes Ca2+
entry into PASMCs, and leads to pulmonary vascular remodeling
(28,29). P21 and P16 are also involved in the
process of cell cycle arrest, and cells expressing P16 and P21 have
been detected in plasma lesions and vascular endothelial cells
(30,31). These changes reduce the production
of nitric oxide and prostaglandins in the microenvironment, which
leads to endothelial cell aging. PASMCs and PAECs produce
pro-inflammatory and pro-fibrotic fluids, which are involved in
pulmonary artery remodeling (32).
The treatment of patients with PH-ILD includes basic
and etiological therapies. The primary focus of basic treatment is
oxygen therapy, which aids in alleviating hypoxia. The present
study revealed that the proportion of patients with Ms-PH requiring
oxygen therapy was significantly higher compared with those with
non-PH and mild PH, with a statistically significant difference
(P<0.05), aligning with findings from foreign studies (33). Conversely, another domestic study
indicated no significant difference (P>0.05) between patients
with IPF with PH who received hormone therapy and those without,
contradicting the results of the present study (34). This discrepancy could be attributed
to the larger sample size of patients with CTD-ILD included in the
present study.
RDW is one of the commonly used blood analysis
indicators in clinical practice to assess changes in RBC volume,
evaluate RBC morphology, and diagnose anemia. Due to its advantages
such as easy accessibility and straightforward operation, it has
been widely used in clinical settings in recent years and can be
used as a predictor of poor prognosis for various diseases such as
heart failure, COPD (35), and
community-acquired pneumonia (36).
Foreign studies suggest that RDW is an independent factor affecting
COPD-related PH, and RDW levels can be used to assess disease
severity and prognosis (37).
Numerous studies have shown that RDW is associated with adverse
outcomes in idiopathic PH and chronic thromboembolic PH (38,39).
Ozgul et al (37) discovered
that RDW possesses clinical utility in predicting PASP and
assessing lung function among patients with COPD-related PH. In the
present study, the RDW level among patients with Ms-PH was
significantly higher compared with the non-PH group (P<0.05) and
mild-PH group (P<0.05). This finding is similar to the study
conducted by Yang et al (40), which revealed an increase in RDW is
associated with the severity of PH and a poor prognosis of the
disease. Another study revealed that elevated RDW was an important
diagnostic indicator for PH (95% CI: 2.866-13.698, P=0.013), and
ROC curve suggested that an RDW ≥13.05% represents the optimal
cutoff value, achieving a sensitivity of 82.1% and a specificity of
71.4% (41) which was aligned with
the results of the present study. However, the primary distinction
between the current study and prior research lies in the research
subjects and their grouping. Previous studies investigated whether
patients with ILD exhibit clinical characteristics of PH, whereas
the present study delves deeper into analyzing the risk factors
associated with Ms-PH in patients with ILD. Currently, the
mechanism behind RDW and the progression of pulmonary arterial
hypertension remain unclear. One potential explanation is that
patients with chronic lung disease often experience hypoxia and
inflammation in their respiratory system. It has been previously
indicated that ineffective erythropoiesis, oxidative stress,
thrombosis, inflammation, endothelial dysfunction and other factors
can all impact RDW levels (42).
The increase in RDW may reflect an increase in immature
reticulocytes. A decrease in erythrocyte deformability may
stimulate PLT aggregation, leading to vascular damage and decreased
blood viscosity. Changes in erythrocyte size, decreased
deformability and increased adhesion may contribute to thrombosis
(43). Pulmonary
ischemia-reperfusion is accompanied by hypoxemia, promoting
vascular remodeling, fibroblast proliferation and intraluminal
microthrombosis, ultimately leading to increased pulmonary vascular
resistance and elevated PAP (44).
MPV is an indicator of the average volume of PT in
the body, commonly used to evaluate PLT function. Research on MPV
in patients with PH-ILD was limited, whereas there was a wealth of
studies on patients with other chronic lung diseases, such as COPD.
According to the multivariate logistic regression analysis
conducted by Huang (13), an
elevated MPV has predictive value for the severity of PH-ILD, which
aligned with our research findings. It is currently considered that
MPV contributes to the development of PH-ILD via various
mechanisms:
Impaired function and structure of pulmonary
vascular endothelium. PLT activation promotes adhesion and
aggregation, increasing the likelihood of thrombosis and elevating
pulmonary vascular resistance. Furthermore, the increased release
of thromboxane A2 and endothelin-1 stimulates bronchial and
pulmonary vascular smooth muscle contraction, reduces the
production of vasodilator nitric oxide, exacerbates airway stenosis
and pulmonary vascular resistance, ultimately leading to the
development of PH (45).
Chronic inflammation. inflammation triggers
the release of inflammatory cytokines, such as IL-6, which affects
the production of megakaryocytes and increases PLT volume and
reactivity (46). Additionally,
inflammation can induce PLT activation, adhesion and aggregation,
thereby increasing pulmonary vascular resistance.
Immunoglobulin is an immunocompetent molecule
synthesized and secreted by plasma cells. It is involved in immune
regulation. IgG is the most abundant and important immunoglobulin
secreted by the body, often indicating that the body is in a state
of re-immune response, especially to infectious agents such as
bacteria and viruses. The mechanism by which IgG promotes PH
formation may be related to its immunomodulatory role in the body.
IgG participates in the entire systemic inflammatory response and
local inflammation in the pulmonary artery. In a study on chronic
thromboembolic PH, it was found that low galactosylation of IgG is
positively correlated with circulating inflammatory cytokines,
while normal galactosylation of IgG is negatively correlated with
these inflammatory cytokines. This finding suggests that the
increase in proinflammatory immune response mediated by IgG
galactosylation may play a role in the occurrence and progression
of PH (47). Complement is a
glycoprotein containing enzymatic activity. The levels of IgG and
IgA in the Ms-PH group were higher than those in the non-PH group
and mild PH group, while the level of C4 was lower, with
statistical significance (P<0.05). Multivariate logistic
regression analysis suggested that elevated IgG was an independent
risk factor for Ms-PH in patients with ILD, which was consistent
with the research of Lei et al (48). However, the aforementioned research
primarily focused on patients with systemic lupus erythematosus
complicated by PH, and there was a scarcity of literature on IgG
and ILD-PH. The present study targets patients with ILD, including
those with CTD-ILD, to explore the impact of IgG on ILD-PH. It
addresses the limitation of previous studies that did not consider
PH in CTD without ILD.
The positive rate of anti-U1RNP antibody
demonstrated significant differences among the three groups
(P<0.05), which was similar to the results of a previous study
(48). However, the rates of
positivity for other antibodies were not significantly different
among the three groups, which was similar to the results of the
study by Jin et al (49).
Chang et al (50) observed the differences in RVD, right
atrial long diameter and right atrial short between the mild PH
group and the non-PH group, which one was consistent with the
present results. This may suggest that the disease onset is early,
resulting in minimal damage to the lung structure and compensatory
changes in lung function and cardiac structure. Conversely, another
study revealed early right ventricular diastolic and systolic
myocardial dysfunction in patients with IPF-PH, contradicting our
results (51). Further prospective
studies are warranted to elucidate the relationship between cardiac
structure and PH-ILD.
Upon analyzing the differences in FVC% pred, DLCO,
DLCO% pred and DLCO/VA% pred among patients who underwent pulmonary
function tests, it was observed that the lung function of patients
with Ms-PH decreased compared with that of patients with non-PH and
mild-PH (P<0.05). The results were similar to the results of the
study by Oliveira et al (52), suggesting a correlation between the
severity of patients' lung function and PASP.
There are certain limitations to the present study.
Firstly, this was a single-center study, and variations across
different regions and ethnic groups may exert influence on the
results. Secondly, due to the retrospective nature of the study,
clinicians may introduce certain biases during the information
collection process. Thirdly, due to the study being retrospective,
it is not known exactly how long numerous patients used the drug
before measuring PASP, which is a shortcoming. Therefore,
prospective or randomized controlled studies are required to
strictly control for confounding factors and further validate the
diagnostic value of RDW, MPV and their combination in the diagnosis
of PH-ILD.
In conclusion, middle-aged and elderly patients with
ILD should be vigilant about the occurrence of PH. By combining
clinical manifestations, rheumatic antibodies, cardiac color
ultrasound and pulmonary function tests, early diagnosis and
treatment of PH-ILD can be facilitated. Elevated RDW, MPV and IgG
levels serve as independent risk factors for patients with Ms-PH
among patients with ILD, and these indicators may assist in
assessing the severity of PH-ILD. Moreover, the combination of RDW
and MPV offers certain predictive value for ILD complicated by
Ms-PH.
Supplementary Material
Comparison of rheumatoid antibodies of
three groups.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Guangdong
Provincial Science and Technology Plan Project (grant no.
2017A020215177).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BQW designed the experiments and revised the
manuscript. YXL collected and analyzed the data, and wrote the
manuscript. YXL and BQW confirm the authenticity of all the raw
data. Both authors revised the work critically for intellectual
content, read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
SL-II2024-004-0) by the Ethics Committee of the Third Affiliated
Hospital of Sun Yat-sen University (Guangzhou, China). Informed
consent was waived because of the retrospective nature of the
study. The study was performed in accordance with the Declaration
of Helsinki. All methods were carried out in accordance with the
relevant guidelines and regulations for ethics approval.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nathan SD, Barbera JA, Gaine SP, Harari S,
Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke-Zaba J,
Provencher S, et al: Pulmonary hypertension in chronic lung disease
and hypoxia. Eur Respir J. 53(1801914)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
King CS and Shlobin OA: The trouble with
group 3 pulmonary hypertension in interstitial lung disease:
Dilemmas in diagnosis and the conundrum of treatment. Chest.
158:1651–1664. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hamada K, Nagai S, Tanaka S, Handa T,
Shigematsu M, Nagao T, Mishima M, Kitaichi M and Izumi T:
Significance of pulmonary arterial pressure and diffusion capacity
of the lung as prognosticator in patients with idiopathic pulmonary
fibrosis. Chest. 131:650–656. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kimura M, Taniguchi H, Kondoh Y, Kimura T,
Kataoka K..Nishiyama O, Aso H, Sakamoto K and Hasegawa Y: Pulmonary
hypertension as a prognostic indicator at the initial evaluation in
idiopathic pulmonary fibrosis. Respiration. 85:456–463.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nathan SD, Shlobin OA, Ahmad S, Koch J,
Barnett SD, Ad N, Burton N and Leslie K: Serial development of
pulmonary hypertension in patients with idiopathic pulmonary
fibrosis. Respiration. 76:288–294. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Minai OA, Santacruz JF, Alster JM, Budev
MM and McCarthy K: Impact of pulmonary hemodynamics on 6-min walk
test in idiopathic pulmonary fibrosis. Respir Med. 106:1613–1621.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nathan SD, Shlobin OA, Barnett SD, Saggar
R, Belperio JA, Ross DJ, Ahmad S, Saggar R, Libre E, Lynch JP III
and Zisman DA: Right ventricular systolic pressure by
echocardiography as a predictor of pulmonary hypertension in
idiopathic pulmonary fibrosis. Respir Med. 102:1305–1310.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Walter K: Pulmonary hypertension. JAMA.
326(1116)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lv GJ, Li AL, Tao XC, Zhai YN, Zhang Y,
Lei JP, Gao Q, Xie WM and Zhai ZG: The accuracy and influencing
factors of Doppler echocardiography in estimating pulmonary artery
systolic pressure: comparison with right heart catheterization: A
retrospective cross-sectional study. BMC Med Imaging.
22(91)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American society of
echocardiography endorsed by the European association of
echocardiography, a registered branch of the European society of
cardiology, and the Canadian society of Echocardiography. J Am Soc
Echocardiogr. 23:685–713; quiz 786-8. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kendall M: Multivariate analysis. Charles
Griffin, London, 1975.
|
|
12
|
Li N: Current status and influencing
factors of feeding intolerance in patients with severe nervous
system diseases (unpublished thesis). Lanzhou University, 2021.
|
|
13
|
Huang YK: Clinical characteristics and
risk factors of pulmonary hypertension secondary to connective
tissue disease-related interstitial lung disease (unpublished
thesis). Health Science Center of Nanchang University, 2023.
|
|
14
|
Travis WD, Costabel U, Hansell DM, King TE
Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU,
et al: ATS/ERS Committee on idiopathic interstitial pneumonias. An
official American thoracic society/European respiratory society
statement: Update of the international multidisciplinary
classification of the idiopathic interstitial pneumonias. Am J
Respir Crit Care Med. 188:733–748. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kay J and Upchurch KS: ACR/EULAR 2010
rheumatoid arthritis classification criteria. Rheumatology
(Oxford). 51 (Suppl 6):vi5–vi9. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aringer M, Costenbader K, Daikh D, Brinks
R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen
DL, et al: 2019 European league against rheumatism/American college
of rheumatology classification criteria for systemic lupus
erythematosus. Ann Rheum Dis. 78:1151–1159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van den Hoogen F, Khanna D, Fransen J,
Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP,
Medsger TA Jr, Carreira PE, et al: 2013 classification criteria for
systemic sclerosis: An American college of Rheumatology/European
league against rheumatism collaborative initiative. Ann Rheum Dis.
72:1747–1755. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shiboski CH, Shiboski SC, Seror R,
Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H,
Vitali C, Bowman SJ, et al: 2016 American college of
Rheumatology/European league against rheumatism classification
criteria for primary sjögren's syndrome: A consensus and
data-driven methodology involving three International patient
cohorts. Arthritis Rheumatol. 69:35–45. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (first of two parts). N Engl J Med. 292:344–347.
1975.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsujimoto Y, Kumasawa J, Shimizu S, Nakano
Y, Kataoka Y, Tsujimoto H, Kono M, Okabayashi S, Imura H and Mizuta
T: Doppler trans-thoracic echocardiography for detection of
pulmonary hypertension in adults. Cochrane Database Syst Rev.
5(CD012809)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Q and Lu J: Analysis of the clinical
value of echocardiography and chest CT in the diagnosis of COPD
complicated with pulmonary hypertension. Chin J CT MRI. 18:88–90.
2020.(In Chinese).
|
|
22
|
Cheng H, Yu Z, Yan CL, Yang HD, Gao C and
Wen HY: Long-term efficacy and low adverse events of
methylprednisolone pulses combined to low-dose glucocorticoids for
systemic sclerosis: A retrospective clinical study of 10 years'
follow-up. J Inflamm Res. 15:4421–4433. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Panagiotou M, Church AC, Johnson MK and
Peacock AJ: Pulmonary vascular and cardiac impairment in
interstitial lung disease. Eur Respir Rev.
26(160053)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schafer MJ, White TA, Iijima K, Haak AJ,
Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y,
et al: Cellular senescence mediates fibrotic pulmonary disease. Nat
Commun. 8(14532)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khan SY, Awad EM, Oszwald A, Mayr M, Yin
X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P and Breuss JM:
Premature senescence of endothelial cells upon chronic exposure to
TNFα can be prevented by N-acetyl cysteine and plumericin. Sci Rep.
7(39501)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kojima H, Kunimoto H, Inoue T and Nakajima
K: The STAT3-IGFBP5 axis is critical for IL-6/gp130-induced
premature senescence in human fibroblasts. Cell Cycle. 11:730–739.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu D, Zeng F, Han L, Wang J, Yin Z, Lv L,
Guo L, Wang D, Xu Y and Zhou H: The synergistic action of phosphate
and interleukin-6 enhances senescence-associated calcification in
vascular smooth muscle cells depending on p53. Mech Ageing Dev.
182(111124)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Z, Yang K, Zheng Q, Zhang C, Tang H,
Babicheva A, Jiang Q, Li M, Chen Y, Carr SG, et al: Divergent
changes of p53 in pulmonary arterial endothelial and smooth muscle
cells involved in the development of pulmonary hypertension. Am J
Physiol Lung Cell Mol Physiol. 316:L216–L228. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wakasugi T, Shimizu I, Yoshida Y, Hayashi
Y, Ikegami R, Suda M, Katsuumi G, Nakao M, Hoyano M, Kashimura T,
et al: Role of smooth muscle cell p53 in pulmonary arterial
hypertension. PLoS One. 14(e0212889)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
van der Feen DE, Bossers GPL, Hagdorn QAJ,
Moonen JR, Kurakula K, Szulcek R, Chappell J, Vallania F, Donato M,
Kok K, et al: Cellular senescence impairs the reversibility of
pulmonary arterial hypertension. Sci Transl Med.
12(eaaw4974)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Noureddine H, Gary-Bobo G, Alifano M,
Marcos E, Saker M, Vienney N, Amsellem V, Maitre B, Chaouat A,
Chouaid C, et al: Pulmonary artery smooth muscle cell senescence is
a pathogenic mechanism for pulmonary hypertension in chronic lung
disease. Circ Res. 109:543–553. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Roger I, Milara J, Belhadj N and Cortijo
J: Senescence alterations in pulmonary hypertension. Cells.
10(3456)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alhamad EH, Cal JG, Alrajhi NN and Alharbi
WM: Predictors of mortality in patients with interstitial lung
disease-associated pulmonary hypertension. J Clin Med.
9(3828)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang H, Li J, Zhang Y and Li M: Effects
of pulmonary hypertension on physiological indexes in patients with
idiopathic pulmonary fibrosis. Clin Med Res Pract. 2:22–23,32.
2017.(In Chinese).
|
|
35
|
Marvisi M, Mancini C, Balzarini L and
Ramponi S: Red cell distribution width: A new parameter for
predicting the risk of exacerbation in COPD patients. Int J Clin
Pract. 75(e14468)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ren Q, Liu H, Wang Y, Dai D, Tian Z, Jiao
G and Liu X: The role of red blood cell distribution width in the
severity and prognosis of community-acquired pneumonia. Can Respir
J. 2021(8024024)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ozgul G, Seyhan EC, Özgül MA and Günlüoğlu
MZ: Red blood cell distribution width in patients with chronic
obstructive pulmonary disease and healthy subjects. Arch
Bronconeumol. 53:107–113. 2017.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
38
|
Rhodes CJ, Wharton J, Howard LS, Gibbs JS
and Wilkins MR: Red cell distribution width outperforms other
potential circulating biomarkers in predicting survival in
idiopathic pulmonary arterial hypertension. Heart. 97:1054–1060.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Förhécz Z, Gombos T, Borgulya G, Pozsonyi
Z, Prohászka Z and Jánoskuti L: Red cell distribution width in
heart failure: Prediction of clinical events and relationship with
markers of ineffective erythropoiesis, inflammation, renal
function, and nutritional state. Am Heart J. 158:659–666.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang J, Liu C, Li L, Tu X and Lu Z: Red
blood cell distribution width predicts pulmonary hypertension
secondary to chronic obstructive pulmonary disease. Can Respir J.
2019(3853454)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang W, Liu J, Yang YH, Zhai ZG, Wang C
and Wang J: Red cell distribution width is increased in chronic
thromboembolic pulmonary hypertension. Clin Respir J. 10:54–60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yıldız A, Kaya H, Ertaş F, Oylumlu M,
Bilik MZ, Yüksel M, Polat N, Akil MA, Atılgan Z and Ulgen MS:
Association between neutrophil to lymphocyte ratio and pulmonary
arterial hypertension. Turk Kardiyol Dern Ars. 41:604–609.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rezende SM, Lijfering WM, Rosendaal FR and
Cannegieter SC: Hematologic variables and venous thrombosis: Red
cell distribution width and blood monocyte count are associated
with an increased risk. Haematologica. 99:194–200. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lechartier B and Humbert M: Pulmonary
arterial hypertension in systemic sclerosis. Presse Med.
50(104062)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Humbert M, Morrell NW, Archer SL, Stenmark
KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel NF and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43 (12 Suppl
S):13S–24S. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Harrison S, Vavken P, Kevy S, Jacobson M,
Zurakowski D and Murray MM: Platelet activation by collagen
provides sustained release of anabolic cytokines. Am J Sports Med.
39:729–734. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang ZJ, Wang HF, Lian TY, Zhou YP, Xu
XQ, Guo F, Wei YP, Li JY, Sun K, Liu C, et al: Human plasma IgG
N-Glycome profiles reveal a proinflammatory phenotype in chronic
thromboembolic pulmonary hypertension. Hypertension. 80:1929–1939.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lei Y, Zhang X, Feng Y, Wang J and Luo R:
Risk factors of pulmonary arterial hypertension in patients with
systemic lupus erythematosus. Cardiol Young. 31:1619–1624.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jin Y, Guo G, Wang C and Jiang B:
Association of red cell distribution width with pulmonary arterial
hypertension in patients with mixed connective tissue disease. BMC
Pulm Med. 23(299)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chang B, Wigley FM, White B and Wise RA:
Scleroderma patients with combined pulmonary hypertension and
interstitial lung disease. J Rheumatol. 30:2398–2405.
2003.PubMed/NCBI
|
|
51
|
D'Andrea A, Stanziola A, Di Palma E,
Martino M, D'Alto M, Dellegrottaglie S, Cocchia R, Riegler L,
Betancourt Cordido MV, Lanza M, et al: Right ventricular structure
and function in idiopathic pulmonary fibrosis with or without
pulmonary hypertension. Echocardiography. 33:57–65. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Oliveira RKF, Waxman AB, Hoover PJ,
Dellaripa PF and Systrom DM: Pulmonary vascular and right
ventricular burden during exercise in interstitial lung disease.
Chest. 158:350–358. 2020.PubMed/NCBI View Article : Google Scholar
|















