1. Introduction
Glaucoma is the leading cause of irreversible
blindness worldwide. In 2020, ~79.6 million individuals globally
were affected by glaucoma, and this number is projected to exceed
111.8 million by 2040(1). In China,
the incidence of primary angle-closure glaucoma is higher than that
of primary open-angle glaucoma (2),
and the rate of blindness is also greater (3). Primary angle-closure glaucoma is a
complex eye disease that can cause varying degrees of damage to
both the anterior and posterior segments of the eye. The cornea,
part of the anterior segment, is positioned at the very front of
the eyeball. It lacks blood vessels but has the densest
innervation. Its refractive properties allow light to be
transmitted to the retina, and its transparency is essential for
clear vision (4). Therefore,
studying the effect of angle-closure glaucoma on the cornea is
crucial.
With the continuous development of corneal
examination technology, our understanding of corneal morphology and
structural changes in angle-closure glaucoma has deepened.
Ultrasonic thickness measurement is a common method for measuring
corneal thickness, but it is a contact examination. By contrast,
anterior segment optical coherence tomography (AS-OCT) is a
non-contact detection method that conveniently and quickly provides
a new reference for measuring corneal thickness (5). Optical biometry can accurately measure
the biological parameters of the eye, such as the eye axis,
anterior chamber depth, and corneal curvature (6). Specular microscopy, commonly used to
detect corneal endothelium, can qualitatively and quantitatively
examine corneal endothelial cells at high magnification, although
it cannot visually detect the microscopic structure of the
remaining corneal layers (7). The
emergence of in vivo confocal microscopy (IVCM) offers a
non-invasive, sensitive, specific, and reproducible method for
corneal examination (8). IVCM is
frequently used to measure changes in corneal microstructure,
including corneal epithelial cells, nerve, dendritic cells, stromal
cells, and endothelial cells (9-12).
The high resolution and penetrating power of IVCM provide a
significant advantage in evaluating corneal microstructure damage
in angle-closure glaucoma (13).
The increasing number of detection methods also provides valuable
reference indices for observing corneal changes in patients with
angle-closure glaucoma.
2. Effect of primary angle-closure glaucoma
on corneal thickness
Recent studies have demonstrated that central
corneal thickness (CCT) in primary angle-closure glaucoma primarily
undergoes fluctuations due to transient spikes in intraocular
pressure (IOP). These fluctuations primarily result from stromal
and epithelial edema, leading to increased CCT during episodes of
elevated IOP. However, investigations into the impact of historical
spikes in IOP and diverse surgical interventions have indicated
minimal long-term effects on CCT (14,15). A
longitudinal study spanning 5 years, involving 26 patients with
angle-closure glaucoma, revealed no significant alteration in CCT
following laser therapy or trabeculectomy. Moreover, antecedent
episodes of acute intraocular hypertension did not correlate with
notable changes in CCT during extended follow-up periods (15). Niu et al (16) in their comparison of CCT between
healthy individuals and those with a history of acute angle
closure, similarly concluded that prior instances of acute
intraocular hypertension did not induce substantial CCT variations.
Chen et al (17)
corroborated these findings by demonstrating no significant
disparity in CCT among individuals with angle-closure glaucoma,
their fellow unaffected eyes, and normal controls. Sugumaran et
al (18) observed that CCT
averaged 525 µm when IOP ranged from 20 to 40 mmHg, increasing to
528 µm when IOP escalated to 41-60 mmHg. This association
highlighted CCT thickening with rising IOP. Presently, laser
peripheral iridectomy exhibits efficacy in enhancing anterior
chamber depth and volume in patients with angle-closure glaucoma,
consequently reducing the incidence of acute angle closure.
Nonetheless, its impact on CCT remains insignificant (19,20).
Prolonged administration of glaucoma medications may trigger
corneal extracellular matrix degradation, potentially leading to
CCT reduction (15).
3. Effects of primary angle-closure glaucoma
on corneal curvature and astigmatism
The relatively flat small cornea serves as one of
the anatomical foundations for the sudden onset of angle-closure
glaucoma. Often, this type of cornea is accompanied by a lax
ciliary band and an anteriorly positioned lens. Additionally,
individuals with a flat cornea typically exhibit a thicker lens,
collectively contributing to increased crowding within the anterior
chamber (21). Simultaneously, a
reduced posterior corneal curvature poses a higher risk of angular
closure compared with the anterior corneal curvature (22). However, a survey conducted among
patients with primary angle closure disease revealed that the
majority exhibit corneal astigmatism ranging from 0.25 to 1.25D,
similar to individuals of the same age with cataracts. This
suggests that under normal IOP, the disease itself exerts minimal
influence on corneal curvature. Notably, the prevalent type of
astigmatism among patients with angle closure disease is primarily
oblique astigmatism, potentially contributing to decreased visual
acuity (23). Nevertheless, prior
research solely retrospectively observed changes in corneal
curvature and corneal astigmatism in angle-closure diseases with
normal IOP (22). Further
investigations are warranted to elucidate differences in corneal
curvature following acute attacks of angle-closure glaucoma and
their potential impact on the calculation of ocular biological
parameters.
4. Effect of primary angle-closure glaucoma
on corneal microstructure
Changes of corneal epithelial
cells
Some patients diagnosed with angle-closure glaucoma,
particularly those with chronic conditions, necessitate prolonged
administrations of anti-glaucoma medication to manage IOP. Notably,
~70% of these medications contain benzalkonium bromide as a
preservative to ensure their long-term stability (24,25).
However, the presence of preservatives can instigate ocular surface
damage, leading to the disruption of microvilli on epithelial cell
surfaces and loss of cell integrity. Prolonged use and increased
dosage of these medications further diminish epithelial cell
vitality and may trigger cell apoptosis (24,26).
Valladales-Restrepo et al (27) observed that prolonged use of
anti-glaucoma medications in patients with angle-closure disease
induced ocular surface damage. However, the utilization of
composite formulations has been found to mitigate eye discomfort
and enhance patient compliance (27). Additionally, Güçlü et al
(28) investigated the impact of
varying drug dosages on corneal epithelial cells in patients with
angle-closure glaucoma. Their findings indicated that anti-glaucoma
drug application resulted in reduced limbal stem cell count and
impaired cell migration, ultimately leading to a decrease in
central corneal epithelial thickness (28). In addition to therapeutic
medications, the extent of angular closure can also inflict damage
on the corneal epithelium. In this study involving patients with
angle-closure glaucoma, individuals with a history of drug
treatment exceeding 1 week were excluded. Results indicated that
with the widening of the angle closure range, there was a
progressive reduction in corneal epithelial cell count, potentially
culminating in limbal stem cell deficiency in severe cases. This
phenomenon primarily stems from diminished nutritional support to
peripheral corneal endothelial cells as the angle closure range
expands. Consequently, dysfunctional corneal endothelial cells lead
to alterations in the corneal limbal microenvironment,
characterized by an increase in corneal inflammatory cells and a
decrease in limbal stem cell count. Therefore, heightened vigilance
for limbal stem cell deficiency is warranted in patients with
advanced disease and multi-directional angular closure (29). Moreover, abrupt increases in IOP
among patients with angle-closure glaucoma can impact epithelial
cells, resulting in cell space widening and epithelial cell
swelling. Severe cases may exhibit large vacuoles between
epithelial cells. Upon decreasing IOP, these changes in epithelial
cells can rapidly revert (13).
However, there is a scarcity of studies examining the long-term
quantity and morphology of corneal epithelial cells following the
onset of intraocular hypertension. Thus, further research is
imperative. Consequently, the protracted and extensive utilization
of anti-glaucoma medications and the broad range of angular closure
may underlie the compromised ocular surface microenvironment in
patients with glaucoma. Meanwhile the enduring effects of acute
intraocular hypertension on corneal epithelium remain enigmatic
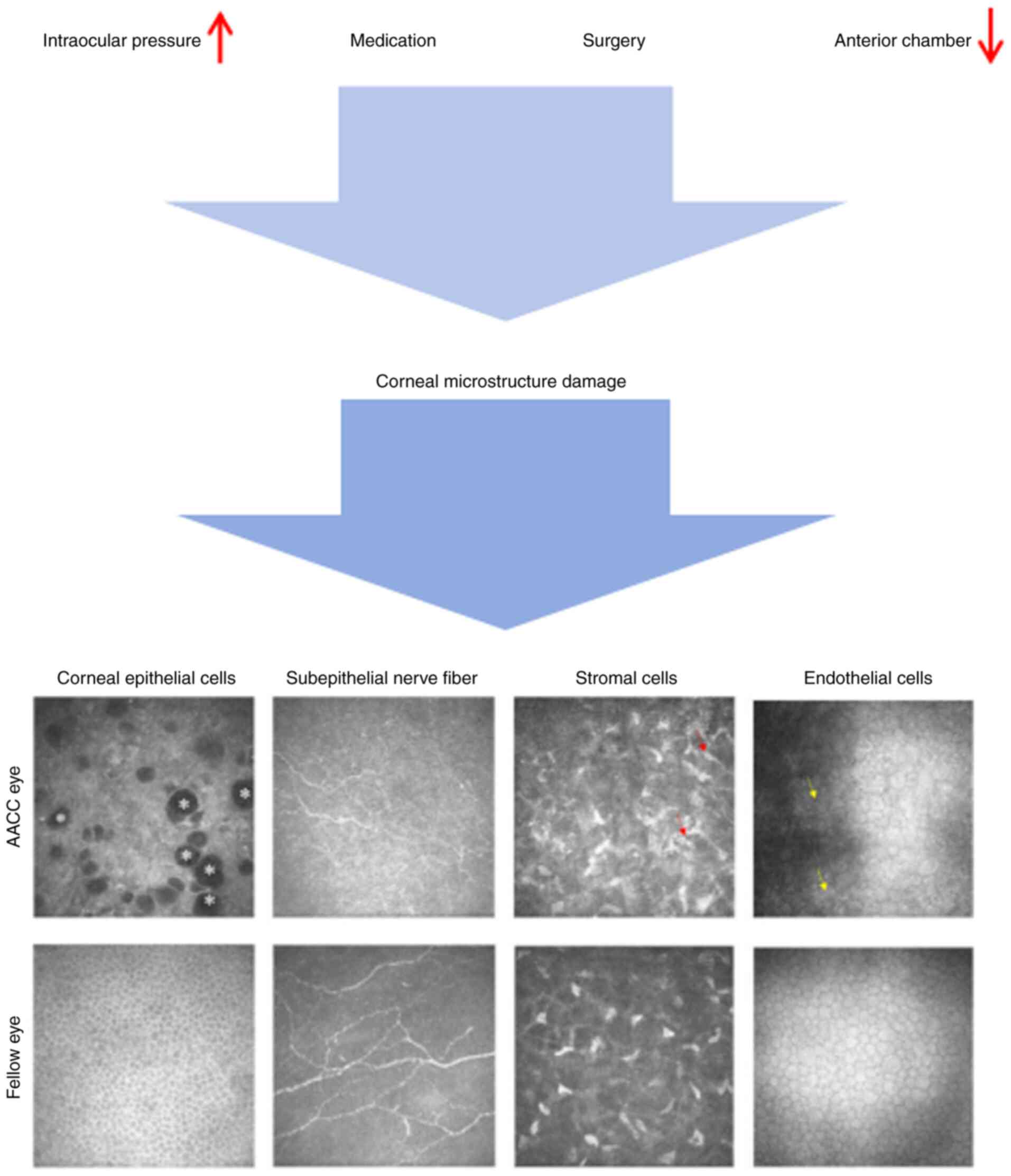
(Fig. 1).
Changes of corneal subepithelial
nerve
Under normal circumstances, corneal nerves play
crucial roles in sensing harmful substances on the corneal surface,
triggering eyelid closure and tear production, and facilitating
corneal repair (30). High IOP can
directly damage corneal nerves. Research indicates that patients
experiencing acute ocular hypertension, such as those with
angle-closure glaucoma, show reduced corneal nerve density and
length in the affected eye compared with the contralateral eye
(13). This reduction is believed
to stem from corneal dystrophy induced by diminished blood flow in
the corneal limbal vascular network due to elevated ocular pressure
(13). Additionally, the neurotoxic
effects of anti-glaucoma drugs on the cornea are noteworthy.
Clinical investigations have demonstrated that both the drugs
themselves and their preservatives can directly harm corneal nerves
(31). Rossi et al (32) in their examination of patients with
angle-closure disease undergoing drug treatment for up to 3 years,
observed a significant decrease in corneal nerve density using
IVCM. Similarly, Agnifili et al (33) reported that an increase in the
number of glaucoma medications was associated with a decrease in
corneal nerve density, increased tortuosity, and noticeable ocular
dryness and discomfort in patients with glaucoma. Injury to the
corneal nerve during surgery is a significant concern. Presently,
the commonly used clear corneal incision in cataract
phacoemulsification combined with angle separation for treating
angle-closure glaucoma results in decreased corneal sensitivity in
38.5% of patients post-surgery, indicating notable damage to
corneal nerves (34). Giannaccare
et al (9) conducted a study
involving 30 patients undergoing unilateral cataract surgery. They
observed a decrease in the density, length and width of corneal
nerve fibers bilaterally 1 month post-surgery compared with
preoperative levels, with a relatively smaller and shorter-lasting
decrease noted in the contralateral eye. Termed sympathetic change,
this phenomenon is primarily attributed to impaired neuroafferent
function leading to the local release of pro-inflammatory
neuropeptides, thereby causing neurogenic inflammation (9). Agnifili et al (35) performed a follow-up study on 38
patients with open-angle glaucoma who had stable IOP control after
trabeculectomy for 6 months. They observed a continuous reduction
in the number of corneal nerve fibers and high reflex points
between nerve fibers in these patients, with the appearance of high
reflex points indicative of inflammatory cell deposition. The use
of steroid eye drops post-surgery demonstrated efficacy in
mitigating corneal inflammation. The reduction in corneal nerve
density observed may be attributed to the inhibitory effect of
mitomycin on corneal cell proliferation, with most of this effect
dissipating within 6 months post-surgery. However, the alterations
in corneal nerve parameters, such as the density, length and
curvature of nerve fibers, following the cessation of drug effects
remain unclear (35). While there
is a lack of specific studies on angle-closure glaucoma, it is
speculated that similar conclusions can be drawn for patients with
this condition. Corneal injury resulting from trabeculectomy may
primarily stem from the influence of intraoperative and
postoperative medications. In treating patients with absolute
angle-closure glaucoma, the extent of nerve damage is contingent
upon the degree and duration of cryopreservation. A study involving
18 dogs revealed a decrease in corneal sensitivity across all
corneal areas by 10 to 42%, 1 week post-surgery, with six dogs
developing corneal ulcers despite avoiding the 3 and 9 o ‘clock
positions during surgery (36).
Changes of corneal dendritic
cells
Normally, dendritic cells are situated between the
corneal epithelium and the stromal layer. In the presence of
corneal inflammation, there is an upsurge in the activation,
quantity and maturity of dendritic cells (37,38).
In patients with angle-closure glaucoma, the aqueous humor harbors
a plethora of inflammatory factors with their expression escalating
alongside IOP. This inflammatory milieu induces a substantial
increase in activated dendritic cells within the corneal
interlayer. As IOP decreases and the disease enters a remission
phase the number of activated dendritic cells may decrease as well
(37,39). Mastropasqua et al (40) conducted pertinent research on the
distribution of augmented dendritic cells in the cornea. They
observed a general increase in dendritic cell count in patients
with glaucoma receiving two or more relevant medications,
particularly concentrated at the corneal limbus compared with the
corneal center, a phenomenon associated with dry eye occurrence
(40). However, pertinent clinical
trials investigating changes in the distribution of corneal
dendritic cells during acute ocular hypertension are lacking. In
conclusion, during acute angle-closure glaucoma, the pronounced
inflammatory response triggers the activation and proliferation of
dendritic cells, underscoring the significance of anti-inflammatory
therapy alongside IOP reduction.
Changes of corneal stromal cells
Corneal stromal cells are highly active,
participating not only in the renewal of the extracellular matrix,
but also in ensuring the uniform passage of light through one or
more stromal cells. It is crucial for maintaining visual acuity
(41). It has been observed that
conditions such as diabetes and dry eye reduce stromal cell density
and alter their morphology (42,43).
However, there is limited research on the impact of angle-closure
glaucoma on stromal cells. Activation of the corneal stroma
indicates inflammation within corneal tissue. During acute
angle-closure glaucoma attacks, the destruction of the
blood-aqueous humor barrier leads to the production of numerous
inflammatory factors in the aqueous humor, triggering stromal cell
activation. This activation is manifested as stromal cell swelling,
enhanced reflectivity, and a more interconnected network (13). Pilocarpine nitrate eye drops, a
first-line treatment for angle-closure glaucoma, can also induce
changes in corneal microstructure. A previous study indicated that
concentrations exceeding 0.625 g/l can result in abnormal stromal
cell morphology and even apoptosis, with damage progressively
worsening over time with prolonged use (44). It is suggested that the
morphological changes of corneal stromal cells can be used as one
of the indices to evaluate the degree of inflammation in
patients.
Effects of primary angle-closure
glaucoma on corneal endothelial cells. Effects of high IOP on
endothelial cells
Recent studies indicate that regardless of the
extent or duration of IOP elevation, there is a decrease in the
number of endothelial cells accompanied by an increase in the
coefficient of variation. This phenomenon is attributed to
endothelial cell dystrophy and pressure imbalances (18,45).
The duration of intraocular hypertension is a crucial factor
influencing endothelial cell loss. Short-term significant increases
in IOP are the primary cause of such loss (46). In an experiment involving rats a
rapid increase in eye pressure within 2 h resulted in irregularly
shaped endothelial cells and decreased cell count (47). In clinical investigations Li et
al (48) conducted a
comprehensive study on the duration of high IOP leading to severe
endothelial cell damage. In this study it was observed that after
the onset of acute angle-closure glaucoma, 12.28% of eyes had an
endothelial cell density (ECD) <1,000/mm2, 30.41% had
an ECD between 1,000-2,000/mm2, and 57.31% had an ECD
>2,000/mm2. The variance in ECD was primarily
attributed to the duration of intraocular hypertension; durations
shorter than 48 h did not significantly affect endothelial cell
count or morphology (48). The
effects of acute intraocular hypertension on endothelial cells have
also been investigated. Tham et al (49) noted a significant decrease in the
number of endothelial cells in patients with chronic angle-closure
glaucoma who had experienced previous episodes of acute intraocular
hypertension, with a reduction of ~11.6%. Yeom et al
(50) conducted measurements
revealing that the ECD in eyes following an acute attack of
angle-closure glaucoma averaged ~1,818±490 cells/mm2,
whereas the ECD of the contralateral eye averaged 2,675±348
cells/mm2. The ECD in eyes experiencing an acute
increase in IOP was significantly lower compared with the
contralateral eye, indicating damage to endothelial cells caused by
acute IOP elevation (50).
Currently, studies demonstrate that high IOP can lead to increased
endothelial cell damage, but there is a lack of research
quantifying the extent of damage corresponding to increased IOP
levels. Generally, higher IOP levels over short durations can
result in further damage to the corneal endothelium.
Influence of the shallow anterior
chamber on endothelial cells
The shallow anterior chamber contributes to the
reduction in the number of endothelial cells. Verma et al
(51) investigated 529 patients
diagnosed with primary angle-closure glaucoma and noted that even
in the absence of acute intraocular hypertension, patients with
angle-closure disease exhibited diminished corneal endothelial cell
counts, reduced hexagonal cell percentage, and increased
coefficient of variation (51).
Additionally, the follow-up study of eyes in remission from acute
angle-closure glaucoma and normal eyes 3 months post-cataract
surgery, by Yeom et al (50), revealed that although normal eyes
initially displayed higher ECD counts before surgery, there was no
significant difference in the percentage decline of ECD between the
two groups post-surgery. This suggests that past episodes of acute
intraocular hypertension did not exacerbate endothelial cell damage
following cataract surgery. The diminished ECD observed in patients
with angle-closure glaucoma may be attributed to a history of acute
attacks and a shallow anterior chamber (50). This finding was corroborated by Imai
et al (52).
Effects of YAG laser therapy on
endothelial cells
Laser therapy represents a significant treatment
modality for patients with primary angle closure glaucoma (53). However, there have been variations
in the perceived impact of YAG laser therapy on endothelial cells
compared to historical perspectives. Previous research suggested
that the energy emitted by YAG laser and the consequent
inflammatory response could result in increased heterogeneity,
elevated coefficient of variation, and decreased cell density of
corneal endothelial cells (54,55).
Nevertheless, a previous study indicated no substantial disparity
in the number and morphology of ECD in treated eyes compared with
contralateral eyes within a 72-month period (56). Ono et al (57) conducted a 7-year follow-up study on
patients post-YAG treatment and observed that the annual reduction
rate of ECD in treated patients mirrored that of healthy
individuals of similar age suggesting that age-related changes may
predominantly contribute to ECD reduction, with laser treatment
posing no significant harm to endothelial cells (57). In essence, YAG laser treatment does
not induce severe damage to the cornea.
5. Conclusion
In summary, glaucoma represents an irreversible and
sight-threatening ocular disease. Early detection and effective
control of IOP stand as paramount steps in preventing further optic
nerve damage. Nevertheless, with ongoing research in glaucoma,
treatment strategies continue to evolve, aiming not only to
alleviate patient discomfort but also to enhance long-term quality
of life. During acute attacks of angle-closure glaucoma, patients
often experience corneal edema, exacerbated by delayed repair
during and after surgery, prolonged wound healing, and the extended
use of anti-glaucoma medications (Table
I). These factors increase susceptibility to symptoms such as
eye redness, dryness and discomfort, significantly impacting the
lives of patients. Therefore, alongside IOP reduction, attention
must also be directed towards monitoring corneal structural
changes. Unlike the past reliance solely on corneal endoscopy for
assessing corneal endothelial cell changes, confocal microscopy
offers a precise means to identify corneal microstructure damage.
This provides an objective framework for understanding the
underlying causes of sustained visual impairment and facilitates
early detection of ocular surface damage severity. While confocal
microscopy yields valuable insights into corneal microstructural
changes, further investigation utilizing long-term, large-scale
samples is necessary to elucidate the effects of high IOP on
corneal parameters and the long-term alterations in angle-closure
glaucoma.
 | Table IAlteration of the cornea in primary
angle-closure glaucoma. |
Table I
Alteration of the cornea in primary
angle-closure glaucoma.
| A, Corneal
macrostructure |
|---|
| Name | Functions | Influencing
factor(s) | Mechanism(s) | (Refs.) |
|---|
| Thickness | N/A | IOP | Stromal and
epithelial edema | (14,15) |
| | | Medications | Corneal
extracellular matrix degradation | (15) |
| Curvature | N/A | IOP | N/A | (23) |
| B, Corneal
microstructure |
| Name | Functions | Influencing
factor(s) | Mechanism(s) | (Refs.) |
| Epithelial
cells | Barrier | IOP | Cell space widening
and epithelial cell swelling | (13) |
| | | Medications | Disruption of
microvilli | (24,26) |
| | | Extent of angle
closure | Diminished
nutritional support | (29) |
| Subepithelial
nerves | Facilitate corneal
repair | IOP | Diminished blood
flow in the corneal limbal vascular network | (13) |
| | | Medications | Direct injury | (31) |
| | | Surgery | Direct injury and
damage brought on by drugs | (9,35) |
| Dendritic
cells | Inflammatory
responses | IOP | Inflammatory
milieu | (37,39) |
| Stromal cells | Maintaining visual
acuity | IOP | Destruction of the
blood-aqueous humor barrier | (13) |
| Endothelial
cells | Barrier | IOP | Endothelial cell
dystrophy and pressure imbalances | (18,45) |
| | | Shallow anterior
chamber | Cornea iris
contact | (51) |
| | | YAG laser | Age-related
changes | (57) |
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Projects of
Medical Science Research of Health Commission of Hebei Province,
China (grant no. 20220830).
Availability of data and materials
Not applicable.
Authors' contributions
YW wrote and subsequently revised the article. LY
and YQ collected related articles. FF proposed the writing
directions and reviewed articles. All authors read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kang JM and Tanna AP: Glaucoma. Med Clin
North Am. 105:493–510. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He M, Jiang Y, Huang S, Chang DS, Munoz B,
Aung T, Foster PJ and Friedman DS: Laser peripheral iridotomy for
the prevention of angle closure: A single-centre, randomised
controlled trial. Lancet. 393:1609–1618. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
George R, Panda S and Vijaya L: Blindness
in glaucoma: primary open-angle glaucoma versus primary
angle-closure glaucoma-a meta-analysis. Eye (Lond). 36:2099–2105.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tuck H, Park M, Carnell M, Machet J,
Richardson A, Jukic M and Di Girolamo N: Neuronal-epithelial cell
alignment: A determinant of health and disease status of the
cornea. Ocul Surf. 21:257–270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li EY, Mohamed S, Leung CK, Rao SK, Cheng
AC, Cheung CY and Lam DS: Agreement among 3 methods to measure
corneal thickness: Ultrasound pachymetry, Orbscan II, and Visante
anterior segment optical coherence tomography. Ophthalmology.
114:1842–1847. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Heath MT, Mulpuri L, Kimiagarov E, Patel
RP, Murphy DA, Levine H, Tonk RS, Cooke DL and Riaz KM: Intraocular
lens power calculations in keratoconus eyes comparing keratometry,
total keratometry, and newer formulae. Am J Ophthalmol.
253:206–214. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sugar A: Clinical specular microscopy.
Surv Ophthalmol. 24:21–32. 1979.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Badian RA, Ekman L, Pripp AH, Utheim TP,
Englund E, Dahlin LB, Rolandsson O and Lagali N: Comparison of
novel wide-field in vivo corneal confocal microscopy with skin
biopsy for assessing peripheral neuropathy in type 2 diabetes.
Diabetes. 72:908–917. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Giannaccare G, Bernabei F, Pellegrini M,
Guaraldi F, Turchi F, Torrazza C, Senni C, Scotto R, Sindaco D, Di
Cello L, et al: Bilateral morphometric analysis of corneal
sub-basal nerve plexus in patients undergoing unilateral cataract
surgery: A preliminary in vivo confocal microscopy study. Br J
Ophthalmol. 105:174–179. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ren X, Chou Y, Wang Y, Jing D, Chen Y and
Li X: The utility of oral vitamin B1 and mecobalamin to improve
corneal nerves in dry eye disease: An in vivo confocal microscopy
study. Nutrients. 14(3750)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Misra SL, Slater JA, McGhee CNJ, Pradhan M
and Braatvedt GD: Corneal confocal microscopy in type 1 diabetes
mellitus: A six-year longitudinal study. Transl Vis Sci Technol.
11(17)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Posarelli M, Chirapapaisan C, Muller R,
Abbouda A, Pondelis N, Cruzat A, Cavalcanti BM, Cox SM, Jamali A,
Pavan-Langston D and Hamrah P: Corneal nerve regeneration is
affected by scar location in herpes simplex keratitis: A
longitudinal in vivo confocal microscopy study. Ocul Surf.
28:42–52. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang W, Yang X, Yao Q, Xu Q, Liu W and Liu
J: Corneal confocal microscopic characteristics of acute
angle-closure crisis. BMC Ophthalmol. 22(21)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sosuan GMN and Yap-Veloso MIR: Central
corneal thickness among filipino patients in an ambulatory eye
surgery center using anterior segment optical coherence tomography.
Clin Ophthalmol. 15:2653–2664. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park HM, Choi J, Lee WJ and Uhm KB: Rate
of central corneal thickness changes in primary angle closure eyes:
Long-term follow-up results. BMC Ophthalmol. 21(145)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Niu WR, Dong CQ, Zhang X, Feng YF and Yuan
F: Ocular biometric characteristics of chinese with history of
acute angle closure. J Ophthalmol. 2018(5835791)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen MJ, Liu CJ, Cheng CY and Lee SM:
Corneal status in primary angle-closure glaucoma with a history of
acute attack. J Glaucoma. 21:12–16. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sugumaran A, Devasena MA, Thomas M and
Periyathambi D: A cross sectional study on evaluating the corneal
endothelial cell density and central corneal thickness in eyes with
primary glaucoma. J Family Med Prim Care. 11:4650–4654.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Radhakrishnan S, Chen PP, Junk AK,
Nouri-Mahdavi K and Chen TC: Laser peripheral iridotomy in primary
angle closure: A report by the American academy of ophthalmology.
Ophthalmology. 125:1110–1120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Unterlauft JD, Yafai Y and Wiedemann P:
Changes of anterior chamber architecture induced by laser
peripheral iridotomy in acute angle closure crisis. Int Ophthalmol.
35:549–556. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shon K, Sung KR and Yoon JY: Implications
of the relationship between refractive error and biometry in the
pathogenesis of primary angle closure. Invest Ophthalmol Vis Sci.
62(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang B, Cao K, Wang Z, Zhang Y, Congdon N
and Wang T: Analyzing anatomical factors contributing to angle
closure based on anterior segment optical coherence tomography
imaging. Curr Eye Res. 47:256–261. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zuo C, Gong R, Chen W, Chen C, Su J, Wei
K, Gao X, Lin M and Ge J: Investigation of corneal astigmatism in
chinese patients with primary angle closure disease. J Glaucoma.
27:1131–1135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Goldstein MH, Silva FQ, Blender N, Tran T
and Vantipalli S: Ocular benzalkonium chloride exposure: Problems
and solutions. Eye (Lond). 36:361–368. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hedengran A and Kolko M: The molecular
aspect of anti-glaucomatous eye drops - are we harming our
patients? Mol Aspects Med. 93(101195)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thacker M, Sahoo A, Reddy AA, Bokara KK,
Singh S, Basu S and Singh V: Benzalkonium chloride-induced dry eye
disease animal models: Current understanding and potential for
translational research. Indian J Ophthalmol. 71:1256–1262.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Valladales-Restrepo LF, Oyuela-Gutiérrez
MC, Delgado-Araujo AC and Machado-Alba JE: Use pattern of
ophthalmic antiglaucoma agents with and without preservatives: A
cross-sectional study. Pharmaceuticals (Basel).
16(753)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Güçlü H, Çınar AK, Çınar AC, Akaray İ,
Şambel Aykutlu M, Sakallıoğlu AK and Gürlü V: Corneal epithelium
and limbal region alterations due to glaucoma medications evaluated
by anterior segment optic coherence tomography: A case-control
study. Cutan Ocul Toxicol. 40:85–94. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mao J, Wang Y, Gao Y, Wan S, Jiang W, Pan
Y, Yan Y, Cong Y, Shi X, Huang L and Yang Y: Correlation between
anterior chamber angle status and limbal stem cell deficiency in
primary angle-closure glaucoma. Am J Ophthalmol. 262:178–185.
2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu FX, Lee PSY, Yang L, Gao N, Zhang Y,
Ljubimov AV, Yang E, Zhou Q and Xie L: The impact of sensory
neuropathy and inflammation on epithelial wound healing in diabetic
corneas. Prog Retin Eye Res. 89(101039)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Patel S, Hwang J, Mehra D and Galor A:
Corneal nerve abnormalities in ocular and systemic diseases. Exp
Eye Res. 202(108284)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rossi GCM, Scudeller L, Lumini C, Mirabile
AV, Picasso E, Bettio F, Pasinetti GM and Bianchi PE: An in vivo
confocal, prospective, masked, 36 months study on glaucoma patients
medically treated with preservative-free or preserved monotherapy.
Sci Rep. 9(4282)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Agnifili L, Brescia L, Villani E,
D'Onofrio G, Figus M, Oddone F, Nucci P and Mastropasqua R: In vivo
confocal microscopy of the corneal sub-basal nerve plexus in
medically controlled glaucoma. Microsc Microanal. 1–8.
2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
34
|
Graae Jensen P, Gundersen M, Nilsen C,
Gundersen KG, Potvin R, Gazerani P, Chen X, Utheim TP and Utheim
ØA: Prevalence of dry eye disease among individuals scheduled for
cataract surgery in a norwegian cataract clinic. Clin Ophthalmol.
17:1233–1243. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Agnifili L, Brescia L, Oddone F, Sacchi M,
D'Ugo E, Di Marzio G, Perna F, Costagliola C and Mastropasqua R:
The ocular surface after successful glaucoma filtration surgery: A
clinical, in vivo confocal microscopy, and immune-cytology study.
Sci Rep. 9(11299)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sebbag L, Crabtree EE, Sapienza JS, Kim K
and Rodriguez E: Corneal hypoesthesia, aqueous tear deficiency, and
neurotrophic keratopathy following micropulse transscleral
cyclophotocoagulation in dogs. Vet Ophthalmol. 23:171–180.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Downie LE, Zhang X, Wu M, Karunaratne S,
Loi JK, Senthil K, Arshad S, Bertram K, Cunningham AL, Carnt N, et
al: Redefining the human corneal immune compartment using dynamic
intravital imaging. Proc Natl Acad Sci USA.
120(e2217795120)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen Q, Wang L, Zhang Y, Xu X, Wei Z,
Zhang Z, Wei Y, Pang J, Guo X, Cao K and Liang Q: Corneal
epithelial dendritic cells: An objective indicator for ocular
surface inflammation in patients with obstructive meibomian gland
dysfunction? Ocul Immunol Inflamm. 32:79–88. 2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
He W, Xu F, Chen L, Huang W, Jiang L, Tang
F, Yan W, Zhong S, Shen C, Huang H, et al: Association of
high-mobility group box-1 with inflammationrelated cytokines in the
aqueous humor with acute primary angle-closure eyes. Curr Mol Med.
21:237–245. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mastropasqua R, Agnifili L, Fasanella V,
Lappa A, Brescia L, Lanzini M, Oddone F, Perri P and Mastropasqua
L: In vivo distribution of corneal epithelial dendritic cells in
patients with glaucoma. Invest Ophthalmol Vis Sci. 57:5996–6002.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Espana EM and Birk DE: Composition,
structure and function of the corneal stroma. Exp Eye Res.
198(108137)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen D, Wang L, Guo X, Zhang Z, Xu X, Jin
ZB and Liang Q: Evaluation of limbal stem cells in patients with
type 2 diabetes: An in vivo confocal microscopy study. Cornea.
43:67–75. 2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cagini C, Di Lascio G, Torroni G,
Mariniello M, Meschini G, Lupidi M and Messina M: Dry eye and
inflammation of the ocular surface after cataract surgery:
Effectiveness of a tear film substitute based on
trehalose/hyaluronic acid vs hyaluronic acid to resolve signs and
symptoms. J Cataract Refract Surg. 47:1430–1435. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yuan XL, Wen Q, Zhang MY and Fan TJ:
Cytotoxicity of pilocarpine to human corneal stromal cells and its
underlying cytotoxic mechanisms. Int J Ophthalmol. 9:505–511.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Vallabh NA, Kennedy S, Vinciguerra R,
McLean K, Levis H, Borroni D, Romano V and Willoughby CE: Corneal
endothelial cell loss in glaucoma and glaucoma surgery and the
utility of management with descemet membrane endothelial
keratoplasty (DMEK). J Ophthalmol. 2022(1315299)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wenzel DA, Schultheiss C, Druchkiv V,
Hellwinkel OJC, Spitzer MS, Schultheiss M, Casagrande M and
Steinhorst NA: Effect of elevated irrigation bottle height during
cataract surgery on corneal endothelial cells in porcine eyes. BMC
Ophthalmol. 23(211)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li X, Zhang Z, Ye L, Meng J, Zhao Z, Liu Z
and Hu J: Acute ocular hypertension disrupts barrier integrity and
pump function in rat corneal endothelial cells. Sci Rep.
7(6951)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li Z, Fan N, Cheng Y, Xiang F, Pan X, Cao
K, Zhang Y, Zhang Q and Li S: Factors associated with severe
corneal endothelial damage following acute primary angle closure in
Chinese subjects. Graefes Arch Clin Exp Ophthalmol. 261:2927–2934.
2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tham CC, Kwong YY, Lai JS and Lam DS:
Effect of a previous acute angle closure attack on the corneal
endothelial cell density in chronic angle closure glaucoma
patients. J Glaucoma. 15:482–485. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yeom H, Hong EH, Shin YU, Kang MH, Cho HY
and Seong M: Corneal endothelial cell loss after
phacoemulsification in eyes with a prior acute angle-closure
attack. Korean J Ophthalmol. 34:432–438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Verma S, Nongpiur ME, Husain R, Wong TT,
Boey PY, Quek D, Perera SA and Aung T: Characteristics of the
corneal endothelium across the primary angle closure disease
spectrum. Invest Ophthalmol Vis Sci. 59:4525–4530. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Imai K, Sawada H, Hatase T and Fukuchi T:
Iridocorneal contact as a potential cause of corneal decompensation
following laser peripheral iridotomy. Jpn J Ophthalmol. 65:460–471.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yuan Y, Wang W, Xiong R, Zhang J, Li C,
Yang S, Friedman DS, Foster PJ and He M: Fourteen-year outcome of
angle-closure prevention with laser iridotomy in the zhongshan
angle-closure prevention study: Extended follow-up of a randomized
controlled trial. Ophthalmology. 130:786–794. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chen HC, Lee CY, Liu CF, Hsueh YJ, Meir
YJ, Cheng CM and Wu WC: Corneal endothelial changes following early
capsulotomy using neodymium:yttrium-aluminum-garnet laser.
Diagnostics (Basel). 12(150)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Higashihara H, Sotozono C, Yokoi N,
Inatomi T and Kinoshita S: The blood-aqueous barrier breakdown in
eyes with endothelial decompensation after argon laser iridotomy.
Br J Ophthalmol. 95:1032–1034. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liao C, Zhang J, Jiang Y, Huang S, Aung T,
Foster PJ, Friedman D and He M: Long-term effect of YAG laser
iridotomy on corneal endothelium in primary angle closure suspects:
A 72-month randomised controlled study. Br J Ophthalmol.
105:348–353. 2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ono T, Iida M, Sakisaka T, Minami K and
Miyata K: Effect of laser peripheral iridotomy using argon and
neodymium-YAG lasers on corneal endothelial cell density: 7-year
longitudinal evaluation. Jpn J Ophthalmol. 62:216–220.
2018.PubMed/NCBI View Article : Google Scholar
|