Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial
carcinoma originating from the mucosa of the nasopharynx (1). Risk factors for NPC include the
consumption of salted fish, preserved and processed foods, tobacco
smoking, betel nut use, genetic changes, and Epstein-Barr virus
infection (1-3).
Although the age-standardized incidence rate (ASIR) of NPC is
generally low worldwide (2.12 per 100,000), certain regions, such
as Hong Kong, Taiwan, and Singapore, report higher ASIRs than the
global average (4,5). Radiotherapy alone or in combination
with chemotherapy, a standard treatment approach, has recently been
shown to improve patient survival rates (6). However, a subset of patients with NPC
still faces poor prognosis due to distant metastases (7). Cisplatin, a commonly used chemotherapy
drug for NPC, has been found to cause resistance in NPC cells
(8,9), which may be one of the reasons for the
adverse effects on some patients.
Plant extracts have shown promise as effective
supplements to cisplatin therapy by enhancing its anticancer
effects on cancer cells (10-14).
For example, fucoidan significantly boosted cisplatin-induced
apoptosis in oral cancer cells by inhibiting the PI3K/AKT pathway
(10). Similarly, resveratrol,
derived from grape peel residue, enhanced cisplatin-induced
apoptosis in human hepatoma cells by inhibiting glutamine
metabolism (11). Additionally,
curcumin increased the antitumor efficacy of cisplatin in bladder
cancer cell lines by modulating the ROS-ERK1/2 pathway (12). These examples underscore the
potential of plant extracts to improve cisplatin-based treatments
by augmenting the apoptotic response in various types of
cancer.
Scutellarein, a flavonoid found in Scutellaria
baicalensis (15), exhibits a
wide range of biological activities, including anti-inflammatory,
antioxidant, and neuroprotective effects (15). Notably, scutellarein also exhibits
significant anticancer properties (16-18).
For instance, it can induce apoptosis in human colon cancer cells
through a ROS-mediated, mitochondria-dependent pathway (16). Additionally, scutellarein was
revealed to promote apoptosis and inhibit proliferation, migration,
and invasion in ovarian cancer cells by targeting the EZH2/FOXO1
signaling pathway (17).
Furthermore, it has been shown to suppress tumor development in
vivo (18). Given these
promising results, the present study aimed to explore whether
scutellarein can further enhance the anticancer effects of
cisplatin in NPC cells.
Materials and methods
Materials
Scutellarein, cisplatin, dimethyl sulfoxide (DMSO),
3-methyladenine (3-MA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
PSC833, and LY294002 were obtained from MilliporeSigma. Fetal
bovine serum (FBS), phosphate-buffered saline (PBS), and RPMI-1640
medium were sourced from Hyclone; Cytiva.
Cell culture
The NPC/HK1 nasopharyngeal carcinoma cell line (cat.
no. iCell-h367), free from mycoplasma contamination, was obtained
from Quantum Biotechnology Co., Ltd., Taiwan, R.O.C. Cells were
maintained in RPMI-1640 medium supplemented with 10% FBS and
cultured at 37˚C with 5% CO2.
MTT assay
The effects of scutellarein and cisplatin on NPC/HK1
cell viability were evaluated using the MTT assay. Cells were
seeded in a 6-well plate at a density of 3x105 cells per
well. Upon reaching ~80% confluence, the cells were treated with
different concentrations of scutellarein (0.3125, 6.25, 12.5 and 25
µM) or cisplatin (4.15, 8.3, 16.6 and 33.2 µM) for 48 h. For
evaluating the combined effects of scutellarein and cisplatin,
cells were exposed to 12.5 µM scutellarein, 4.15 µM cisplatin, or a
combination of both treatments for 48 and 72 h, respectively.
Following treatment, the supernatant was discarded, and 2 ml of MTT
reagent (0.5 mg/ml in PBS) was added to each well. After incubation
at 37˚C with 5% CO2 for 4 h, the supernatants were
removed, and 1 ml of DMSO was added to each well to dissolve the
formazan crystals. Subsequently, 100 µl of the DMSO solution from
each well was transferred to a 96-well plate, and the optical
density was measured at 570 nm using an ELISA reader (BMG LABTECH).
Each assay was conducted in triplicate, and all experiments were
repeated at least twice independently.
Morphological assessment
NPC/HK1 cells (3x105 per well) were
seeded into a 6-well plate. Once the cells reached 80% confluence,
they were treated without (control) or with 12.5 µM scutellarein,
4.15 µM cisplatin, or a combination of both treatments for 48 h.
Cell morphology was subsequently examined and documented using
light microscopy (Olympus CK 40; Olympus Corporation). Apoptosis
was indicated by the presence of plasma membrane blebbing in the
cells (19,20).
ELISA
NPC/HK1 cells were seeded in a 6-well plate at a
density of 3x105 cells per well. Once the cells reached
approximately 80% confluence, they were treated with 12.5 µM
scutellarein, 4.15 µM cisplatin, or their combination for 72 h.
Cytokeratin 18 fragment levels in the cell culture supernatants
were measured using SimpleStep ELISA kit, Human Cytokeratin 18
Fragment (cat. no. ab254515; Abcam), following the manufacturer's
instructions. Each assay was conducted in triplicate, and the
entire experiment was repeated at least twice independently.
Immunoblotting assay
Total protein extraction and immunoblotting were
carried out as previously described (21). Primary antibodies targeting
caspase-8 (cat. no. 9746; 1:1,000), cleaved caspase-8 (cat. no.
9429; 1:1,000), caspase-9 (cat. no. 9502), cleaved caspase-9 (cat.
no. 9505), caspase-7 (cat. no. 9492; 1:1,000), cleaved caspase-7
(cat. no. 9491; 1:1,000), cleaved poly (ADP-ribose) polymerase
(PARP) (cat. no. 9541; 1:1,000), phosphorylated (p)-AKT (cat. no.
9271; 1:1,000), AKT (cat. no. 9272; 1:1,000), Beclin 1 (cat. no.
3738; 1:1,000), multidrug resistance protein 1 (MDR1) (cat. no.
13342; 1:1,000), and GAPDH (cat. no. 97166; 1:5,000) were purchased
from Cell Signaling Technology, Inc. Secondary antibodies
conjugated with horseradish peroxidase, including goat anti-rabbit
IgG (cat. no. 111-035-144; 1:5,000) and goat anti-mouse IgG (cat.
no. 111-035-146; 1:5,000), were obtained from Jackson
ImmunoResearch, Inc.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was conducted using SPSS software
(version 17.0; SPSS, Inc.). The one-way analysis of variance
followed by Tukey's post hoc test was used for comparisons among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Scutellarein enhances
cisplatin-induced viability inhibition in NPC/HK1 cells
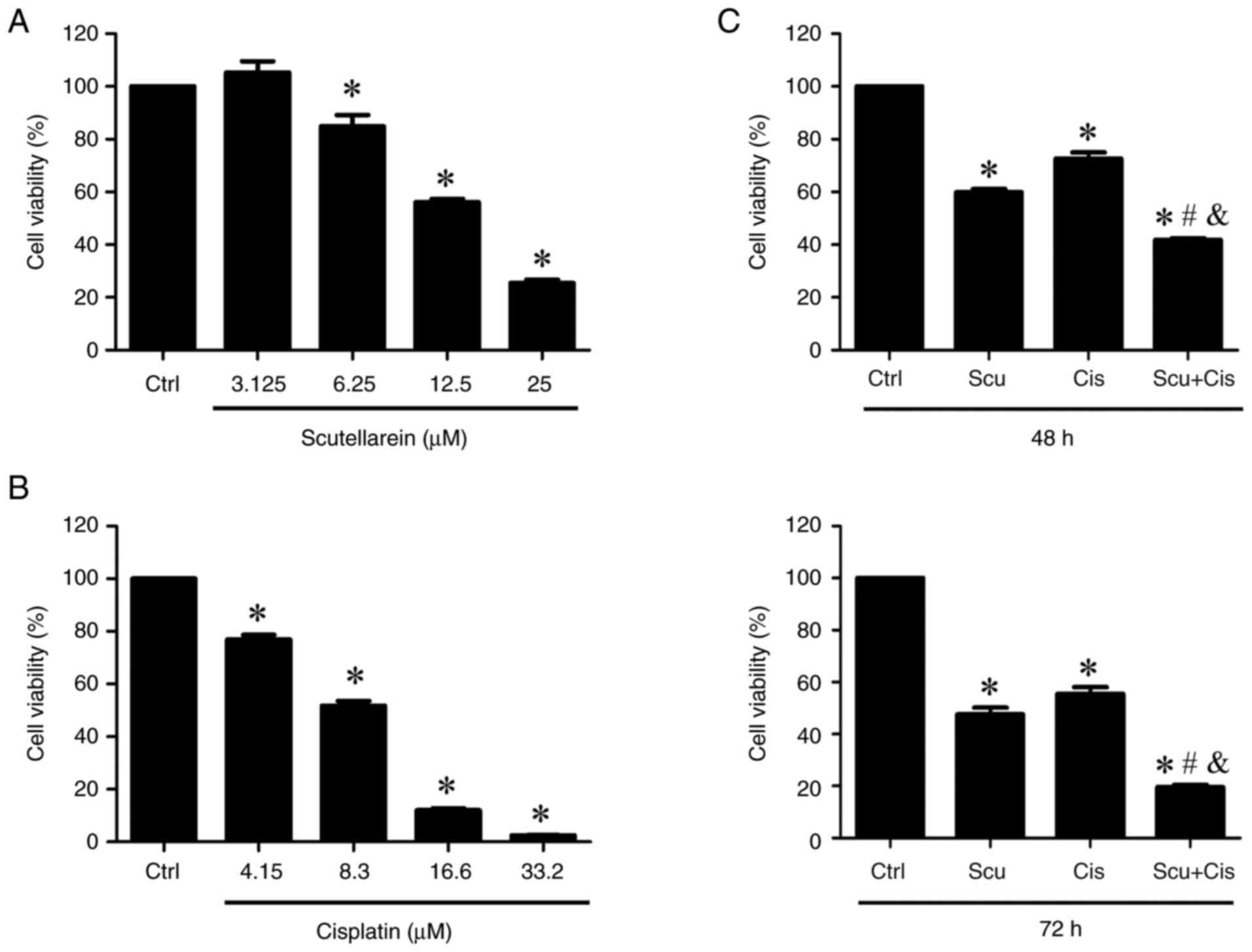
The effects of scutellarein and cisplatin on NPC/HK1
cell viability were first assessed. The cells were treated with
various concentrations of scutellarein and cisplatin, respectively.
Cell viability was examined using the MTT assay. Scutellarein
significantly reduced cell viability at concentrations of 6.25,
12.5, and 25 µM as revealed in Fig.
1A. In addition, cisplatin markedly decreased cell viability at
concentrations of 4.15, 8.3, 16.6, and 33.2 µM as shown in Fig. 1B. The IC50 values for
scutellarein and cisplatin were revealed to be 23.5 and 12.8 µM,
respectively. Subsequently, the combined effects of scutellarein
and cisplatin (12.5 µM scutellarein plus 4.15 µM cisplatin) on
NPC/HK1 cell viability were examined. The results of the MTT assay
revealed that the combination of scutellarein and cisplatin
significantly reduced cell viability more effectively than
scutellarein or cisplatin alone (Fig.
1C), suggesting that scutellarein enhances the efficacy of
cisplatin in inhibiting the viability of NPC/HK1 cells.
Scutellarein enhances
cisplatin-induced apoptotic effects in NPC/HK1 cells
Next, it was investigated whether the combined
treatment of scutellarein and cisplatin enhances apoptotic effects.
Morphological analysis revealed that this combination reduced the
number of attached cells and increased the number of cells
exhibiting membrane blebbing, a hallmark of apoptosis (19,20)
(Fig. 2A and B), compared with treatment with
scutellarein or cisplatin alone. The expression of several key
apoptotic protein markers, including cleaved caspase-8, cleaved
caspase-7, and cleaved PARP (22,23),
were also examined using an immunoblotting assay. The results
showed that treatment with scutellarein or cisplatin alone slightly
increased the levels of these apoptosis markers compared with the
control (Fig. 2C). By contrast, the
combined treatment markedly elevated the expression of cleaved
caspase-8, cleaved caspase-7, and cleaved PARP (Fig. 2C). Additionally, the release of
cytokeratin 18 fragments, another indicator of apoptosis (24), was assessed using ELISA. As revealed
in Fig. 2D, treatment with
scutellarein or cisplatin alone led to a slight increase in
cytokeratin 18 fragment release compared with the control. By
contrast, the combination of scutellarein and cisplatin
significantly enhanced the release of cytokeratin 18 fragments from
NPC/HK1 cells compared with either treatment alone. These results
indicated that scutellarein enhances the apoptotic effects induced
by cisplatin in NPC/HK1 cells.
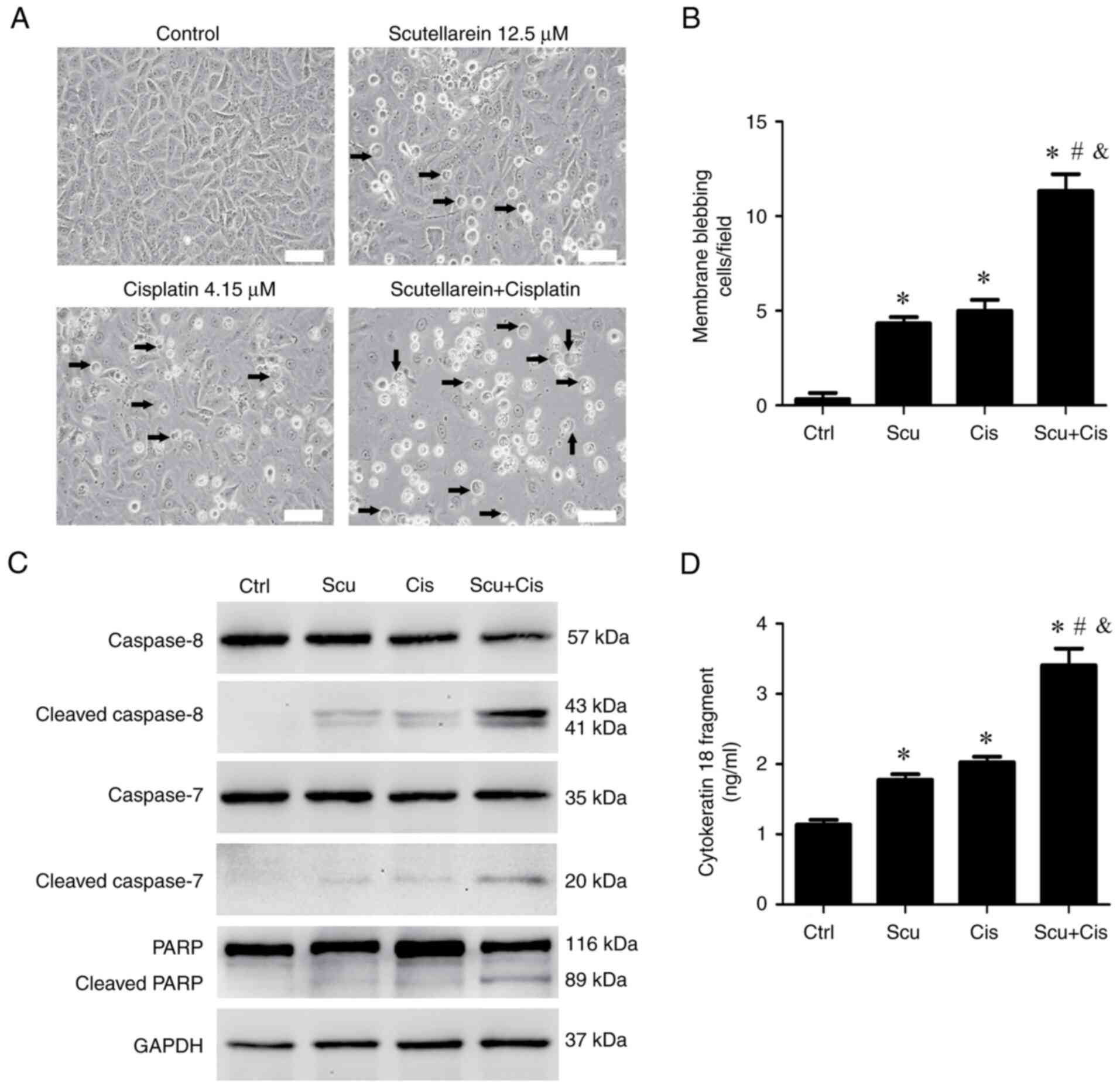 | Figure 2Scutellarein enhances
cisplatin-induced apoptotic effects in NPC/HK1 cells. NPC/HK1 cells
were treated without (Ctrl) or with 12.5 µM scutellarein, 4.15 µM
cisplatin, or their combination for 48 h. (A) The microscopic
observations of membrane blebbing cells. Cell morphology was
observed using light microscopy (scale bar, 100 µm; arrowheads
indicate the membrane-blebbing cells). (B) The average numbers of
membrane blebbing cells per field were evaluated. (C) Protein
expression of cleaved caspase-8, cleaved caspase-7, PARP, and
cleaved PARP was examined using an immunoblotting assay. (D)
NPC/HK1 cells were treated without (Ctrl) or with 12.5 µM
scutellarein, 4.15 µM cisplatin, or their combination for 72 h.
Cytokeratin 18 fragment levels in the cell culture supernatants
were measured using ELISA. Statistical significance was indicated
as follows: *P<0.05 vs. Ctrl; #P<0.05
vs. Cis alone; and &P<0.05 vs. Scu alone; n=3.
Ctrl, control; Scu, scutellarein; Cis, cisplatin. |
Inhibition of autophagy does not
affect the anticancer effects induced by cisplatin in NPC/HK1
cells
Autophagy is a cellular mechanism that degrades and
recycles damaged organelles and proteins (25,26).
This process is essential in regulating cancer cell survival or
death in response to anticancer treatments (27,28).
For example, inhibition of autophagy by autophagy inhibitors can
enhance anticancer agent-induced apoptotic effects (10). Therefore, it was investigated
whether scutellarein affects autophagy and consequently influences
the anticancer effects of cisplatin in NPC/HK1 cells. Beclin 1 and
autophagy related 3 (Atg3) are well-established autophagy markers
(29). The immunoblotting results
demonstrated that cisplatin markedly increased the protein
expression of Beclin 1 and Atg3 (Fig.
3A), suggesting that cisplatin induces autophagy in NPC/HK1
cells. However, scutellarein treatment markedly inhibited the
cisplatin-induced expression of Beclin 1 and Atg3 (Fig. 3A), suggesting that autophagic
effects induced by cisplatin were suppressed by scutellarein. To
evaluate whether autophagy inhibition could potentiate the
anticancer effects of cisplatin, NPC/HK1 cells were treated with
3-MA, an autophagy inhibitor. Then, cell viability and apoptotic
effects were assessed using MTT and ELISA assays, respectively. As
revealed in Fig. 3B and C, co-treatment with cisplatin and 3-MA did
not significantly alter cell viability (Fig. 3B) or cytokeratin 18 fragment levels
compared with cisplatin treatment alone (Fig. 3C). These results suggest that
autophagy inhibition may not contribute to the anticancer effects
of scutellarein-enhanced cisplatin in NPC/HK1 cells.
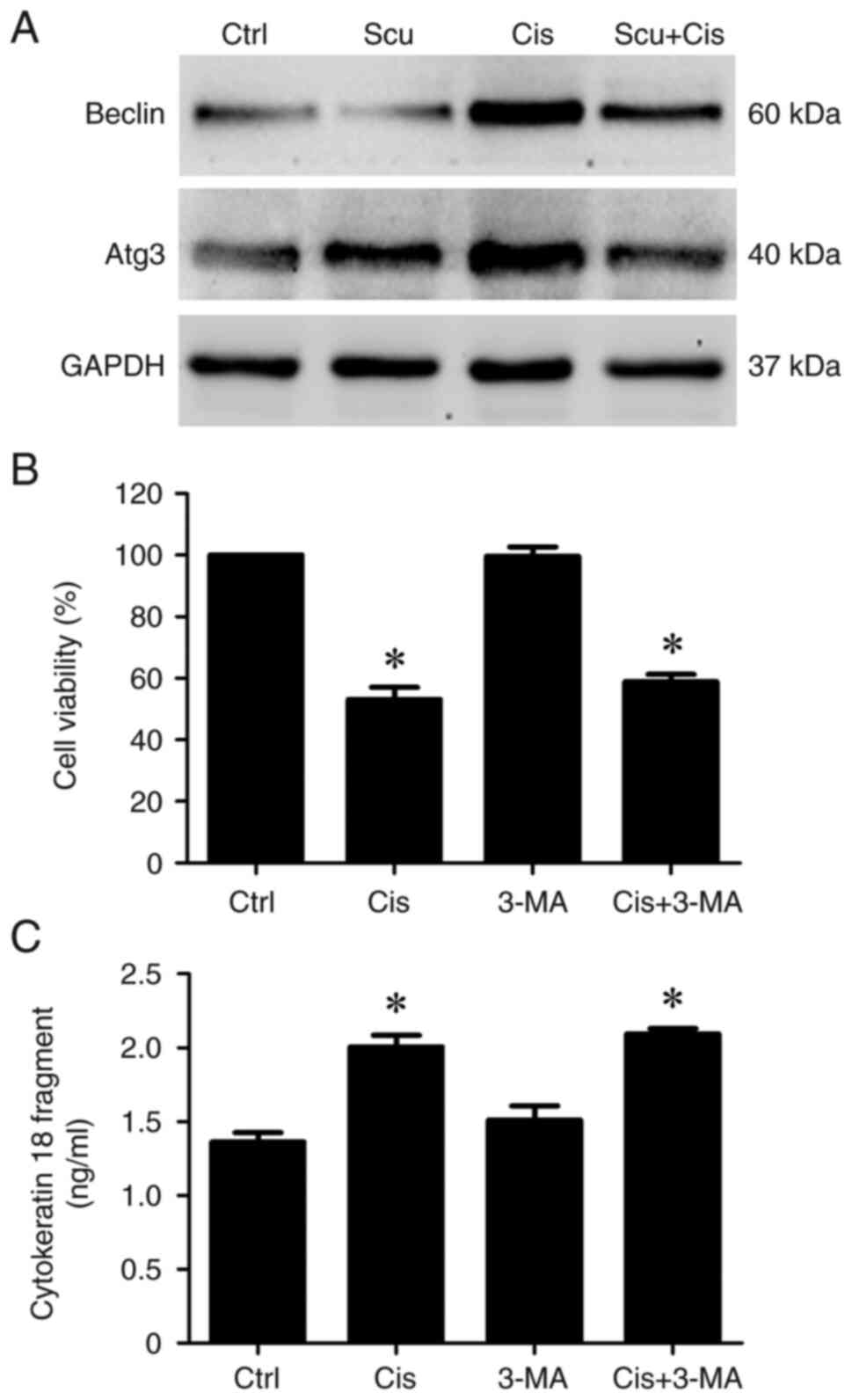 | Figure 3Inhibition of autophagy does not
affect the anticancer effects induced by cisplatin in NPC/HK1
cells. (A) NPC/HK1 cells were treated without (Ctrl) or with 12.5
µM scutellarein, 4.15 µM cisplatin, or their combination for 48 h.
Protein expression of Beclin 1, Atg3, and GAPDH was examined using
an immunoblotting assay. (B) NPC/HK1 cells were treated without
(Ctrl) or with 4.15 µM cisplatin, 10 µM 3-MA, or their combination
for 72 h. Cell viability was assessed using the MTT assay. (C)
Cytokeratin 18 fragment levels in the cell culture supernatant were
measured using ELISA. Statistical significance was indicated as
follows: *P<0.05 vs. Ctrl; n=3. Ctrl, control; Scu,
scutellarein; Cis, cisplatin; Atg3, autophagy related 3; 3-MA,
3-methyladenine. |
Scutellarein enhances
cisplatin-induced inhibition of cell viability and release of
cytokeratin 18 fragments by inhibiting the PI3K/AKT-MDR1 pathway in
NPC/HK1 cells
The PI3K/AKT pathway is crucial in advancing cancer
progression and mediating cisplatin resistance (29-31).
Activation of PI3K/AKT has been reported to upregulate the
expression of MDR1 (32,33), a membrane protein that plays a
crucial role in reducing the effectiveness of cisplatin (34,35).
The immunoblotting results revealed that cisplatin treatment
markedly induced the expression of p-AKT (PI3K/AKT activation) and
MDR1 compared with the control (Fig.
4A). Co-treatment with scutellarein and cisplatin markedly
reduced p-AKT and MDR1 expression compared with cisplatin alone
(Fig. 4A), suggesting that
scutellarein can inhibit cisplatin-induced PI3K/AKT activation and
MDR1 expression. To assess whether MDR1 is a downstream target of
the PI3K/AKT pathway in NPC/HK1 cells, LY294002, a PI3K/AKT
inhibitor, was used. Immunoblotting analysis revealed that LY294002
effectively blocked cisplatin-induced AKT activation and MDR1
expression (Fig. 4B). Furthermore,
the results obtained using ELISA showed that LY294002 also enhanced
the cisplatin-induced release of cytokeratin 18 fragments (Fig. 4B). These findings indicated that the
PI3K/AKT pathway regulates MDR1 expression, which may be involved
in the resistance of cisplatin-induced apoptosis. Subsequently, the
role of MDR1 in cisplatin-induced anticancer effects was
investigated in NPC/HK1 cells. NPC/HK1 cells were co-treated with
cisplatin and PSC833, an MDR1 inhibitor. Then, the expression of
MDR1, cell viability, and cytokeratin 18 fragment levels were
measured using immunoblotting, MTT assay, and ELISA, respectively.
The results demonstrated that PSC833 not only reduced
cisplatin-induced MDR1 expression (Fig.
4C) but also enhanced cisplatin-induced inhibition of cell
viability (Fig. 4C) and increased
the release of cytokeratin 18 fragments (Fig. 4C). These findings indicated that
scutellarein may enhance the anticancer efficacy of cisplatin by
inhibiting the PI3K/AKT-MDR1 pathway in NPC/HK1 cells.
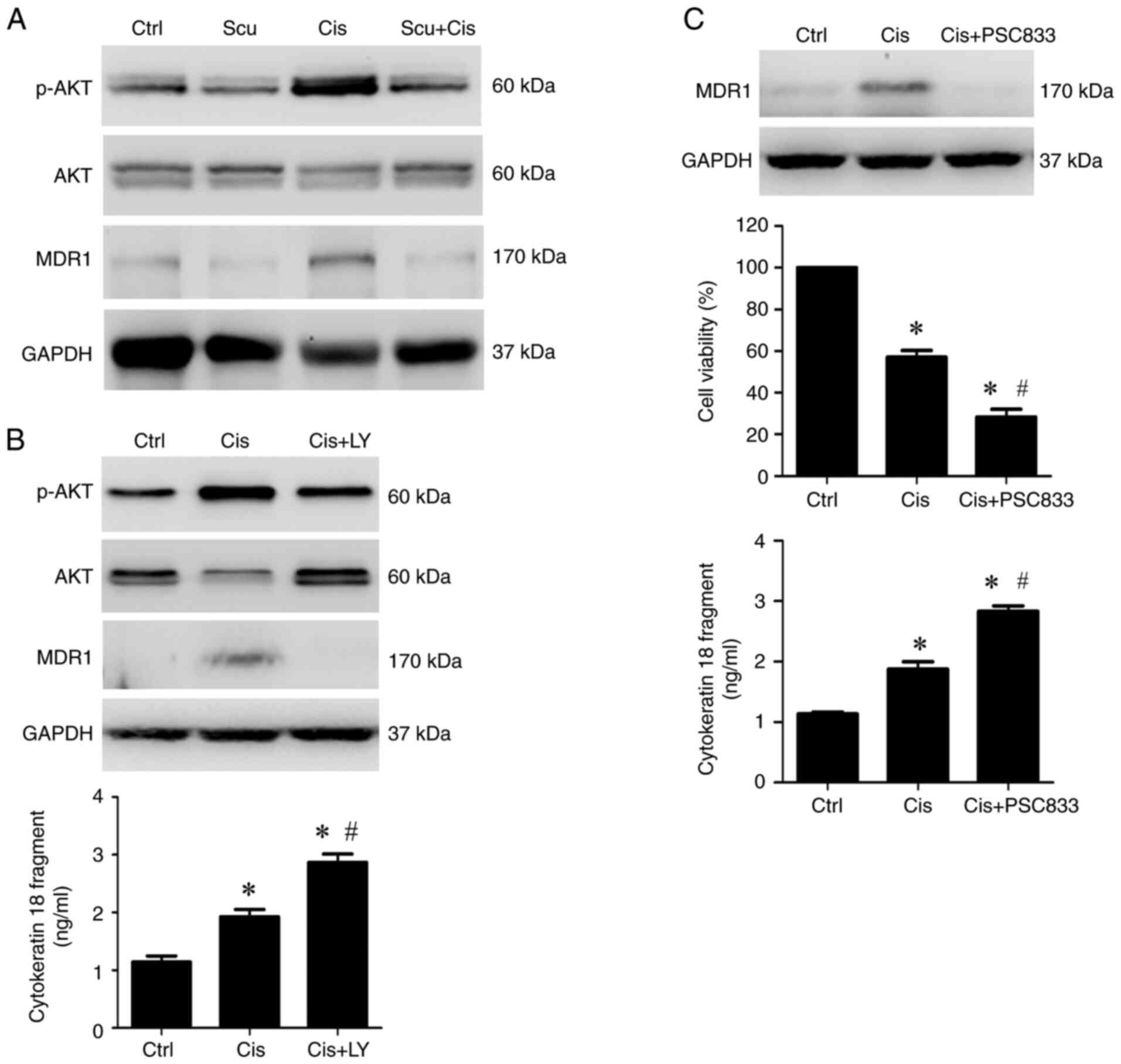 | Figure 4Scutellarein enhances
cisplatin-induced inhibition of cell viability and release of
cytokeratin 18 fragments by inhibiting the PI3K/AKT-MDR1 pathway in
NPC/HK1 cells. (A) NPC/HK1 cells were treated without (Ctrl) or
with 12.5 µM scutellarein, 4.15 µM cisplatin, or their combination
for 48 h. Protein expression of p-AKT, AKT, MDR1, and GAPDH was
examined using an immunoblotting assay. (B) NPC/HK1 cells were
treated without (Ctrl) or with 4.15 µM cisplatin, or 4.15 µM
cisplatin plus 10 µM LY294002 for 48 h. Upper panel, protein
expression of p-AKT, AKT, MDR1, and GAPDH was examined using an
immunoblotting assay. Lower panel, cytokeratin 18 fragment levels
in the cell culture supernatant were measured by ELISA. (C) NPC/HK1
cells were treated without (Ctrl) or with 4.15 µM cisplatin, or
4.15 µM cisplatin plus 10 µM PSC833 for 48 h. Upper panel, protein
expression of MDR1 was examined using an immunoblotting assay.
Middle panel, cell viability was assessed using the MTT assay.
Lower panel, cytokeratin 18 fragment levels in the cell culture
supernatant were measured by ELISA. Statistical significance was
indicated as follows: *P<0.05 vs. Ctrl; and
#P<0.05 vs. Cis alone; n=3. p-, phosphorylated; MDR1,
multidrug resistance protein 1; Ctrl, control; Scu, scutellarein;
Cis, cisplatin; LY, LY294002. |
Discussion
Cisplatin is commonly used in conjunction with
radiotherapy in the treatment of NPC (36). However, resistance to cisplatin can
develop in some NPC cells (8,9),
potentially leading to a poor prognosis for these patients.
Previous studies have demonstrated that certain plant extracts,
such as fucoidan and curcumin, can enhance the anticancer effects
of cisplatin (10,12). The present study investigated
whether scutellarein, an extract from Scutellaria
baicalensis, can similarly improve the efficacy of cisplatin.
The MTT assay demonstrated that scutellarein significantly reduced
cell viability at concentrations of 6.25, 12.5, and 25 µM (Fig. 1A), reflecting its inherent cytotoxic
properties. Cisplatin also reduced cell viability in a
dose-dependent manner, with effective concentrations ranging from
4.15 to 33.2 µM (Fig. 1B). Notably,
when scutellarein was combined with cisplatin at 12.5 and 4.15 µM,
respectively, the combination led to a more substantial reduction
in cell viability compared with cisplatin alone (Fig. 1C). This indicates that scutellarein
not only has significant anticancer activity on its own but also
enhances the cytotoxic effects of cisplatin. These findings suggest
that incorporating scutellarein into cisplatin-based treatments
could improve therapeutic outcomes for NPC/HK1 cells by boosting
the overall efficacy of cisplatin in reducing cancer cell
viability.
Scutellarein can increase the number of membrane
blebbing cells induced by cisplatin (Fig. 2B) and enhance the cisplatin-induced
caspase-8 and caspase-7 activation, PARP cleavage (Fig. 2C), and cytokeratin 18 fragment
release (Fig. 2D), suggesting that
scutellarein may promote the apoptotic effects of cisplatin in
NPC/HK1 cells. Apoptosis can be triggered through extrinsic and
intrinsic pathways (22), with
caspase-8 and caspase-9 as the initiator caspases for these
pathways, respectively (22). The
findings of the present study suggest that scutellarein primarily
facilitates cisplatin-induced apoptosis through the extrinsic
pathway, as indicated by its predominant activation of caspase-8
(Fig. 2C) rather than caspase-9
(data not shown). Caspase-7, an effector caspase-activated
downstream of caspase-8 (22,23),
plays a critical role in the cleavage of PARP and cytokeratin 18
(22,23,37).
The observed increases in PARP cleavage and cytokeratin 18 fragment
release suggest a possible role for the involvement of the
caspase-8/caspase-7 axis in the enhancement by scutellarein of
cisplatin-induced apoptosis.
Autophagy, an essential cellular mechanism for
degrading and recycling damaged organelles and proteins (25,26),
is crucial in regulating cancer cell survival or death in response
to anticancer agents (27,28). The present study investigated
whether scutellarein influences autophagy and thereby affects the
anticancer efficacy of cisplatin in NPC/HK1 cells. It was observed
that cisplatin treatment markedly elevated the levels of autophagy
markers Beclin 1 and Atg3 (Fig.
3A), indicating an induction of autophagy. Notably,
scutellarein inhibited the cisplatin-induced expression of these
autophagic markers (Fig. 3A),
suggesting that scutellarein disrupts the autophagic response
triggered by cisplatin. To assess whether inhibition of autophagy
could enhance the anticancer effects of cisplatin, NPC/HK1 cells
were co-treated with cisplatin and 3-MA, an autophagy inhibitor.
However, the MTT and ELISA assays revealed no significant changes
in cell viability (Fig. 3B) or
cytokeratin 18 fragment levels (Fig.
3C) compared with cisplatin treatment alone. These results
suggest that blocking autophagy with 3-MA does not improve the
anticancer efficacy of cisplatin in this cell model. Previous
studies have shown that autophagy inhibition can enhance the
anticancer effects of cisplatin in lung and ovarian cancer cells
(27,38). However, in the present study,
autophagy inhibition did not produce the same effect in NPC/HK1
cells. It is suggested that differences in cellular backgrounds may
influence whether autophagy inhibition enhances the anticancer
efficacy of cisplatin. The PI3K/AKT signaling pathway is a
well-established cancer progression driver and a key cisplatin
resistance mediator (30-33).
This activation of this pathway has been shown to upregulate MDR1
(32,33), a membrane protein that diminishes
the effectiveness of cisplatin by exporting it out of the cell. The
immunoblotting results support these findings, demonstrating that
cisplatin treatment leads to a marked increase in p-AKT and MDR1
expression (Fig. 4A), highlighting
the role of this pathway in mediating cisplatin resistance.
Notably, co-treatment with scutellarein and cisplatin resulted in a
marked reduction in both p-AKT and MDR1 levels compared with
cisplatin treatment alone (Fig.
4A), suggesting that scutellarein effectively inhibits PI3K/AKT
activation and subsequent MDR1 expression. Additionally, it was
found that treatment with LY294002, a PI3K/AKT inhibitor, inhibited
AKT phosphorylation and suppressed cisplatin-induced MDR1
expression (Fig. 4B), indicating
that the PI3K/AKT pathway is upstream of MDR1 expression.
Furthermore, a previous study has shown that inhibiting MDR1
expression can increase cisplatin sensitivity in bladder cancer
cells (34). In the present study,
it was also observed that inhibition of MDR1 expression by PSC833
(Fig. 4C), an MDR1 inhibitor, not
only enhanced the ability of cisplatin to inhibit cell viability
(Fig. 4C), but also increased the
release of cytokeratin 18 fragments (Fig. 4C), indicating that inhibition of
MDR1 expression also enhances cisplatin sensitivity in NPC cells.
The present study revealed that scutellarein enhances the
anticancer effects of cisplatin by inhibiting the PI3K/AKT-MDR1
pathway, which may be one of the potential therapeutic strategies
for overcoming cisplatin resistance in NPC.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the National Science and
Technology Council (grant nos. MOST-107-2320-B-471-001 and
MOST-110-2635-B-214-001), Pingtung Veterans General Hospital (grant
nos. PTVGH-E-11305 and PTVGH-11301), E-Da Hospital (grant no.
EDPJ110059), and I-Shou University (grant nos. ISU113-IND012-007
and ISU-113-IUC-12).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The present study was designed by YTW, LCC, HHW, YJC
and YYL. HHW, CXH, CHC, CCT and YL performed all the experiments.
Data were collected and analyzed by YTW, LCC and HHW. YTW, LCC, and
YYL wrote the initial manuscript. YJC and YYL revised the
manuscript. YJC and YYL confirm the authenticity of all the raw
data. The final manuscript was read, reviewed and approved by all
authors, confirming the accuracy and integrity of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jicman Stan D, Niculet E, Lungu M, Onisor
C, Rebegea L, Vesa D, Bezman L, Bujoreanu FC, Sarbu MI, Mihailov R,
et al: Nasopharyngeal carcinoma: A new synthesis of literature data
(Review). Exp Ther Med. 23(136)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang XR, Diehl S, Pfeiffer R, Chen CJ, Hsu
WL, Dosemeci M, Cheng YJ, Sun B, Goldstein AM and Hildesheim A:
Chinese and American Genetic Epidemiology of NPC Study Team.
Evaluation of risk factors for nasopharyngeal carcinoma in
high-risk nasopharyngeal carcinoma families in Taiwan. Cancer
Epidemiol Biomarkers Prev. 14:900–905. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Feng H, Zhou Y, Wang L, Wang Y, Zhou S and
Tian F: Consumption of processed food and risk of nasopharyngeal
carcinoma: A systematic review and meta-analysis. Transl Cancer
Res. 11:872–879. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bai R, Sun J, Xu Y, Sun Z and Zhao X:
Incidence and mortality trends of nasopharynx cancer from 1990 to
2019 in China: An age-period-cohort analysis. BMC Public Health.
22(1351)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu H, Yin X, Mao Y, Chen M, Tang Q and Yan
S: The global burden of nasopharyngeal carcinoma from 2009 to 2019:
An observational study based on the global burden of disease study
2019. Eur Arch Otorhinolaryngol. 279:1519–1533. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blanchard P, Lee A, Marguet S, Leclercq J,
Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al:
Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An
update of the MAC-NPC meta-analysis. Lancet Oncol. 16:645–655.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu J, Zhou Q, Pan Z, Wang Y, Hu L, Chen G,
Wang S and Lyu J: Development and validation of a nomogram for
predicting long-term overall survival in nasopharyngeal carcinoma:
A population-based study. Medicine (Baltimore).
99(e18974)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu P, Tang Y, He J, Qi L, Jiang W and Zhao
S: ARC is highly expressed in nasopharyngeal carcinoma and confers
X-radiation and cisplatin resistance. Oncol Rep. 30:1807–1813.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Song Y, Zhou X, Bai W and Ma X: FBW7
increases drug sensitivity to cisplatin in human nasopharyngeal
carcinoma by downregulating the expression of multidrug
resistance-associated protein. Tumour Biol. 36:4197–4202.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang CH, Chang YC, Hsu CC, Lin CH, Chen
IJ, Wu YT and Lan YY: Fucoidan enhances cisplatin-induced effects
on SCC-25 human oral cancer cells by inhibiting the PI3K/AKT
pathway. Anticancer Res. 43:4015–4022. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu Z, Peng Q, Li Y and Gao Y: Resveratrol
enhances cisplatin-induced apoptosis in human hepatoma cells via
glutamine metabolism inhibition. BMB Rep. 51:474–479.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park BH, Lim JE, Jeon HG, Seo SI, Lee HM,
Choi HY, Jeon SS and Jeong BC: Curcumin potentiates antitumor
activity of cisplatin in bladder cancer cell lines via ROS-mediated
activation of ERK1/2. Oncotarget. 7:63870–63886. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liao XZ, Gao Y, Zhao HW, Zhou M, Chen DL,
Tao LT, Guo W, Sun LL, Gu CY, Chen HR, et al: Cordycepin reverses
cisplatin resistance in non-small cell lung cancer by activating
AMPK and Inhibiting AKT signaling pathway. Front Cell Dev Biol.
8(609285)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu Y, Kong W, Zhao S, Xiong D and Wang Y:
Capsaicin enhances cisplatin-induced anti-metastasis of
nasopharyngeal carcinoma by inhibiting EMT and ERK signaling via
serpin family B member 2. Carcinogenesis. 45:556–568.
2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lai J and Li C: Review on the
pharmacological effects and pharmacokinetics of scutellarein. Arch
Pharm (Weinheim). 357(e2400053)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo F, Yang F and Zhu YH: Scutellarein
from Scutellaria barbata induces apoptosis of human colon cancer
HCT116 cells through the ROS-mediated mitochondria-dependent
pathway. Nat Prod Res. 33:2372–2375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lang X, Chen Z, Yang X, Yan Q, Xu M, Liu
W, He Q, Zhang Y, Cheng W and Zhao W: Scutellarein induces
apoptosis and inhibits proliferation, migration, and invasion in
ovarian cancer via inhibition of EZH2/FOXO1 signaling. J Biochem
Mol Toxicol. 35(e22870)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi X, Chen G and Liu X, Qiu Y, Yang S,
Zhang Y, Fang X, Zhang C and Liu X: Scutellarein inhibits cancer
cell metastasis in vitro and attenuates the development of
fibrosarcoma in vivo. Int J Mol Med. 35:31–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Costigan A, Hollville E and Martin SJ:
Discriminating between apoptosis, necrosis, necroptosis, and
ferroptosis by microscopy and flow cytometry. Curr Protoc.
3(e951)2023.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Hsu M, Wu SY, Chang SS, Su IJ, Tsai CH,
Lai SJ, Shiau AL, Takada K and Chang Y: Epstein-Barr virus lytic
transactivator Zta enhances chemotactic activity through induction
of interleukin-8 in nasopharyngeal carcinoma cells. J Virol.
82:3679–3688. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mishra AP, Salehi B, Sharifi-Rad M,
Pezzani R, Kobarfard F, Sharifi-Rad J and Nigam M: Programmed cell
death, from a cancer perspective: An overview. Mol Diagn Ther.
22:281–295. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ulukaya E, Karaagac E, Ari F, Oral AY,
Adim SB, Tokullugil AH and Evrensel T: Chemotherapy increases
caspase-cleaved cytokeratin 18 in the serum of breast cancer
patients. Radiol Oncol. 45:116–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khandia R, Dadar M, Munjal A, Dhama K,
Karthik K, Tiwari R, Yatoo MI, Iqbal HMN, Singh KP, Joshi SK and
Chaicumpa W: A comprehensive review of autophagy and its various
roles in infectious, non-infectious, and lifestyle diseases:
Current knowledge and prospects for disease prevention, novel drug
design, and therapy. Cells. 8(674)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou Y, Manghwar H, Hu W and Liu F:
Degradation mechanism of autophagy-related proteins and research
progress. Int J Mol Sci. 23(7301)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen J, Zhang L, Zhou H, Wang W, Luo Y,
Yang H and Yi H: Inhibition of autophagy promotes cisplatin-induced
apoptotic cell death through Atg5 and Beclin 1 in A549 human lung
cancer cells. Mol Med Rep. 17:6859–6865. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim A, Yim NH and Ma JY: Samsoeum, a
traditional herbal medicine, elicits apoptotic and autophagic cell
death by inhibiting Akt/mTOR and activating the JNK pathway in
cancer cells. BMC Complement Altern Med. 13(233)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang T, Yu J, Cheng S, Zhang Y, Zhou CH,
Qin J and Luo H: Research progress on the anticancer molecular
mechanism of targets regulating cell autophagy. Pharmacology.
108:224–237. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rascio F, Spadaccino F, Rocchetti MT,
Castellano G, Stallone G, Netti GS and Ranieri E: The pathogenic
role of PI3K/AKT pathway in cancer onset and drug resistance: An
updated review. Cancers (Basel). 13(3949)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Navaei ZN, Khalili-Tanha G, Zangouei AS,
Abbaszadegan MR and Moghbeli M: PI3K/AKT signaling pathway as a
critical regulator of Cisplatin response in tumor cells. Oncol Res.
29:235–250. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z,
Li W, Hu J, Lu C and Liu Y: PI3K/AKT pathway as a key link
modulates the multidrug resistance of cancers. Cell Death Dis.
11(797)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun Y, Guan Z, Liang L, Cheng Y, Zhou J,
Li J and Xu Y: HIF-1α/MDR1 pathway confers chemoresistance to
cisplatin in bladder cancer. Oncol Rep. 35:1549–1556.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He C, Sun Z, Hoffman RM, Yang Z, Jiang Y,
Wang L and Hao Y: P-Glycoprotein overexpression is associated with
cisplatin resistance in human osteosarcoma. Anticancer Res.
39:1711–1718. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen L, Li YC, Hu M, Zhao SJ and Yang QW:
Efficacy and safety of weekly versus triweekly cisplatin concurrent
with radiotherapy in nasopharyngeal carcinoma: A meta-analysis.
Medicine (Baltimore). 101(e31842)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Singh Bhangu J, Macher-Beer A, Schimek V,
Garmroudi B, Tamandl D, Unger LW, Bachleitner-Hofmann T and Oehler
R: Circulating caspase-cleaved cytokeratin 18 correlates with
tumour burden and response to therapy in patients with colorectal
cancer liver metastasis. Clin Chim Acta. 538:53–59. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang J and Wu GS: Role of autophagy in
cisplatin resistance in ovarian cancer cells. J Biol Chem.
289:17163–17173. 2014.PubMed/NCBI View Article : Google Scholar
|