Introduction
Parkinson's disease (PD) is a neurodegenerative
disorder that is common in the elderly, ranking second only to
Alzheimer's disease worldwide (1),
with an incidence of ~1% in individuals aged >60 years and ~3%
in individuals aged >80 years in developed countries (2,3). The
pathological features of primary PD include a reduction in dopamine
(DA) neurons in the dense region of the substantia nigra and
degeneration and spherical eosinophilic granule Lewy bodies in the
cytoplasm (4,5). A number of studies have previously
found that mitochondria serve an important role in the pathogenesis
of PD (6,7). Levodopa is a dopamine precursor, the
tablet of which is widely regarded to be the gold standard
treatment for PD worldwide (8). The
current treatment paradigms for PD remain focused on symptomatic
management and has not reached the ideal clinical therapeutic
effects (9,10). However, various neuroprotective
agents, such as hyperoside (11),
walnut oil (12), tribuli fructus
(13) and nutraceuticals (Betanin)
(14), are currently at the
preclinical research phase (11-14).
Although there is currently no cure for PD, pharmacological
interventions, surgical treatments (deep brain stimulation, spinal
cord stimulation for gait symptoms) (15) and other therapeutic approaches, such
as magnetic resonance-guided focused ultrasound (15) and stem cell therapies (16), can alleviate motor (such as resting
tremor, myotonia and bradykinesia) and non-motor (such as sensory
impairments, autonomic nervous system dysfunction and
psychoemotional disorders) symptoms effectively (17).
Although the etiology of PD has not been fully
elucidated, genetic and environmental factors are considered to
serve key roles in its development. Among the environmental
factors, pesticide exposure is an important candidate for PD
pathogenesis, including rotenone (18). Previous studies found that a rat
model treated with rotenone injected intramuscularly could
replicate two signature pathological features of PD, including the
degeneration of substantia nigra DA neurons, formation of Lewy
bodies in surviving substantia nigra neurons and PD-like dyskinesia
(19,20). In addition, paraquat,
6-hydroxydopamine, 1-methyl-4-phenylpyridinium (MPP+)
have been utilized to treat Neuro-2A (N2A) cells for the
construction of in vitro PD models (21,22).
Subsequent studies have demonstrated that rodent models treated
with rotenone exhibit extranigral pathological and non-motor
symptom changes, such as hyposmia, gastrointestinal dysfunction,
sleep disturbances, circadian dysfunction, cognitive decline,
depression and anxiety (19,23,24).
Harpagoside is an iridoid active ingredient that can
be derived from Scrophulariae buergeriana, Scrophularia
striata and Harpagophytum procumbens (HP) (25-27).
It was previously found to have neuroprotective effects. Numerous
studies have previously demonstrated the neuroprotective effects of
plant extracts containing harpagoside against damage in both
cultured cell models and animal models. Radix Scrophulariae,
dried roots of Scrophularia ningpoensis Hemsl, was reported
to exert neuroprotection against cerebral ischemia and reperfusion
injury through the ERK1/2 and p38 MAPK pathways (25). Furthermore, Scrophularia
buergeriana extracts (SBE) demonstrated neuroprotective
properties against glutamate-induced cytotoxicity in SH-SY5Y cell
models through its antioxidant and anti-apoptotic mechanisms
(26). SBE also attenuated
neuroinflammation in BV-2 microglial cells induced by
lipopolysaccharide and promoted neuroprotection in SH-SY5Y
neuroblastoma cells treated with lipopolysaccharide-induced BV-2
conditioned media according to another previous study (27). The application of SBE to SH-SY5Y
cells significantly improved their viability in the presence of
glutamate by promoting the expression of various antioxidant
proteins, such as superoxide dismutase (SOD)1, SOD2 and glutathione
peroxidase-1, in addition to directly augmenting total glutathione
levels (26). In addition, SBE was
observed to reduce DNA damage and diminish the activation of
Bcl-2-associated X protein, cleaved caspase-3 and cleaved
poly[adenosine diphosphate (ADP)-ribose] polymerase (26). SBE also increased Bcl-2 expression
in a glutamate-induced SH-SY5Y cell model through p38 MAPKs
(26). Scrophularia striata
exhibited antioxidant and neuroprotective properties against
neurotoxicity in PC12 cells treated with H2O2
(28). The high-polarity methanolic
fraction of the aerial parts of Scrophularia striata
demonstrated significant neuroprotective activity against
glutamate-induced neurotoxicity in rat cerebellar granule neurons
in a dose-dependent manner in a previous in vitro study
(29). Aerial parts of the plant
were air-dried, powdered and macerated with an 80% ethanol solution
for 3 days with three changes of the solution (29). In addition, harpagide derived from
Scrophularia protected rat cortical neurons against injury
induced by oxygen-glucose deprivation and subsequent reoxygenation
through the reduction of endoplasmic reticulum stress (30). Harpagoside was also effective in
restoring both spatial learning and memory in addition to fear
memory impairments in rats with chronic cerebral hypoperfusion
(31). Additionally, harpagoside
enhanced the activity of Akt whilst inhibiting the activity of
GSK-3β in the hippocampal homogenates of rats with chronic cerebral
hypoperfusion, which are downstream effectors of PTEN (31). Sun et al (32) previously found that harpagoside
alleviated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
(MPTP)/MPP+-induced DA neurodegenerative changes and
movement disorders by enhancing the expression of glial cell
line-derived neurotrophic factor (GDNF) in both cultured
mesencephalic neurons induced by MPP+ and a chronic
MPTP-treated mouse model.
Blocking the GDNF signaling pathway partially,
rather than completely blocking, all of the anti-PD effects of
harpagoside on TH-positive neurons in the cultured mesencephalic
neurons induced by MPP+ (32,33).
Therefore, there may be other pathways involved. Numerous studies
have demonstrated that mitochondria serve an important role in PD
pathogenesis (34,35). Li et al (36) previously found that harpagoside
regulated mitochondrial function with concomitant of diminished
mitochondrial oxidative damage and recovered mitophagy flux through
p53/Parkin-mediated mitophagy in a model of doxorubicin-induced
cardiotoxicity. Consequently, the present study hypothesized that
the mitochondrial pathway may be one of the routes in which
harpagoside can exert its effects on neurons. The present study
investigated the effects of harpagoside on rotenone-induced
mitochondrial damage in N2A cells.
Materials and methods
Cells
N2A cells (Cat. no. TCM29) were obtained from the
The Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences.
Main reagents
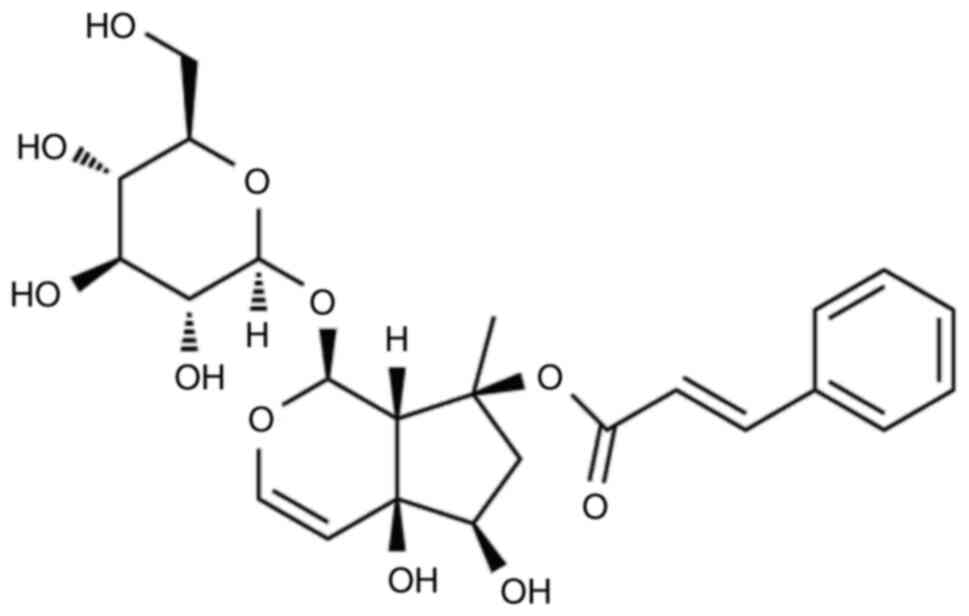
Harpagoside (Fig. 1)
was obtained from Chengdu Pusen Biotechnology Co. Ltd, China.
Rotenone and db-cAMP were obtained from the Sigma-Aldrich; Merk
KGaA. Minimum Essential Medium (MEM), FBS and trypsin were acquired
from Invitrogen; Thermo Fisher Scientific, Inc. The mitochondrial
isolation kit (cat. no. C3601) and caspase 3 detection kits (cat.
no. C1115) were obtained from Beyotime Institute of Biotechnology,
China. Cell Counting Kit-8 (CCK-8) was procured from the Dojindo
Laboratories, Inc. NADH, potassium ferricyanide, Triton X-100 and
HEPES were obtained from Shanghai Aladdin Biochemical Technology
Co., Ltd, China.
Methods. N2A cell culture and
differentiation
N2A cells, a neuroblastoma cell line originally
derived from the spontaneous brain tumor of the mouse neural crest
(37), were cultured in MEM
containing 50 U/ml penicillin, 50 µg/ml streptomycin and 10% FBS.
Depending on the experimental requirements, cells were seeded at a
specific density in 96- or 6-well cell culture plates and incubated
at 37˚C in a 5% CO2 cell culture incubator with
saturated humidity. Since N2A cells exhibit distinct tumorigenic
characteristics and lack certain features of dopaminergic neurons
(37,38), it is necessary to induce their
differentiation (37,39) for PD modelling. According to
previous studies (37,40), N2A cell differentiation was induced
by reducing FBS concentrations in the presence/absence of retinoic
acid or dibutyryl-cAMP (db-cAMP). FBS concentration in the medium
was reduced from 10 to 0.5%, whilst 1 mmol/l of db-cAMP was added
to culture system overnight at 37˚C to obtain differentiated N2A
cells, which were confirmed by the enhanced expression of tyrosine
hydroxylase (TH) as demonstrated by immunocytochemistry (37) and utilized for subsequent
experimental investigations.
Establishment of cell models induced by
rotenone. N2A cells were seeded on a 96-well plate at a density
of 5x104 cells/ml. Each well contained 200 µl cell
suspension. After overnight incubation at 37˚C to allow cell
attachment, cells were treated at 37˚C with 20 nmol/l rotenone for
48 h to establish cell models.
Cell viability assay. Cell viability was
determined using an assay kit, according to the manufacturer's
instructions. After the N2A cells were incubated for 48 h at 37˚C,
10 µl CCK-8 reagent was added to each well and cultured in a 5%
CO2 incubator at 37˚C for 2 h. A measurement wavelength
of 450 nm and a reference wavelength of 620 nm were measured using
a microplate reader. The cell survival rate was calculated using
the following formula: Cell survival=(As-Ab)/(Ac-Ab). In this
formula, As represents the test cell medium, CCK-8 and cytotoxic
substances, Ac represents the control cell culture medium, CCK-8
and no cytotoxic substances. By contrast, Ab represents the blank
well, with no cells or toxic substances but with cell culture
medium and CCK-8. Three independent replicate experiments were
performed.
Effects of harpagoside on rotenone-induced N2A
cells. The experiment was divided into the following five
groups: Control, Model, model with low, medium and high doses of
harpagoside. N2A cells were seeded in a 96-well plate at a density
of 5x104 cells/ml. Each well contained 200 µl cell
suspension. After 12 h incubation at 37˚C, different harpagoside
concentrations (0.1, 1 and 10 µmol/l) were added and incubated at
37˚C for 2 h. Rotenone (20 nmol/l) was then added and incubated at
37˚C for 48 h. The model group received only rotenone for injury
induction, whereas the control group did not receive rotenone or
harpagoside treatment. Cell viability was then assessed using the
CCK-8 reagent.
Effects of harpagoside on rotenone-induced
mitochondrial complex I activity in N2A cells. For cell
culture, N2A cells were seeded at a density of 5x104
cells/ml. After 48 h incubation at 37˚C, 2x107 cells
were collected for mitochondrial preparation. For mitochondrial
preparation, cells were gently suspended in ice-cold PBS,
centrifuged at 600 x g at 4˚C for 5 min and the supernatant was
removed. For pre-treatment, phenylmethyl sulfonyl fluoride was
mixed with the mitochondrial isolation reagent before being added
to the cell pellet. The cells were suspended and incubated on ice
for 15 min, before homogenization, where the cell suspension was
transferred to a glass homogenizer and homogenized ~20 times. The
homogenization efficiency was then determined. After homogenization
for 10 times, 2 µl homogenized solution was added to 30 µl Trypan
Blue staining solution and mixed, before the proportion of
blue-stained cells (positively stained) was observed under a light
microscope. If <50% of the cells were positively stained,
homogenization would be increased five times and observed again. If
≥50% cells were positively stained, homogenization would be
stopped, since excessive homogenization may cause mechanical damage
to mitochondria. The number of homogenizations used for the
subsequent experiments was then recorded. The cell lysate was then
centrifuged at 600 x g and 4˚C for 10 min. The supernatant was
transferred to another centrifuge tube and centrifuged again at
11,000 x g and 4˚C for 10 min. The supernatant was discarded and
the pellet contained isolated mitochondria.
For the measurement of mitochondrial complex I
activity, the experiment was divided into four groups: Control,
Model, model with low and high doses of harpagoside. The final
harpagoside concentrations were 0.1 and 1 µmol/l in the model group
treated with low- and high-dose harpagoside, respectively. Buffer
for mitochondrial complex I activity measurement contained NADH
(0.17 mmol/l), potassium ferricyanide (0.6 mmol/l) and Triton X-100
(1 ml/l), dissolved in PBS (pH 7.4). The mitochondrial complex I
activity measurement reaction system contained 35 µl detection
buffer, 5 µl mitochondrial lysate, 5 µl ubiquinone solution and 5
µl rotenone. The addition of mitochondria served as the starting
time for the reaction, followed by incubation at 30˚C in a water
bath for 10 min. The absorbance of NADH was measured at 340 nm
using a spectrophotometer. A standard curve was simultaneously
obtained. The unit definition for mitochondrial complex I activity
is the number of nmol NADH oxidized per min per mg mitochondrial
proteins. The levels of mitochondrial proteins were determined by
BCA protein assay kit.
Effect of harpagoside on rotenone-induced
mitochondrial swelling in N2A cells. For cell culture, the
experiment was divided into the following six groups: Control,
Model and model containing 0.01, 0.1, 1 and 10 µmol/l harpagoside.
N2A cells were seeded at a density of 1x106 cells/ml in
a six-well plate at 2 ml/well. After 12 h of incubation at 37˚C,
harpagoside was added at final concentrations of 0.01, 0.1, 1 and
10 µmol/l. After 2 h of incubation at 37˚C, rotenone (20 nmol/l)
was added and the cells were cultured for 9 h at 37˚C.
To detect mitochondrial swelling, cells were
collected to prepare mitochondrial samples using a buffer solution
for swelling detection. Solutions A and B were then used. Solution
A contained 125 mmol/l sucrose, 65 mmol/l KCl, 10 mmol/l HEPES/KOH
(pH 7.4) and 5 mmol/l potassium succinate. Solution B contained 10
mmol/l CaCl2. The reaction system for this detection
consisted of the ratios of Solution A: Solution B: samples=50:10:40
µl, which was utilized at room temperature. Absorbance was then
measured at 540 nm, starting with adding CaCl2 and
readings were obtained every 2 min for 10 min. Mitochondrial
swelling was estimated as a decrease in absorbance within 10
min.
Effect of harpagoside on rotenone-induced caspase
3 activity in N2A cells. The experiment was divided into the
following four groups: Control, Model, Model treated with low and
high doses of harpagoside. Harpagoside concentrations were 1 and 10
µM in the low-dose and high-dose groups, respectively. A density of
2x105 cells/ml N2A cells was seeded into six-well
plates. After 12 h of incubation at 37˚C, harpagoside was
administered at predetermined concentrations. After 2 h of
incubation at 37˚C, rotenone (20 nmol/l) was added and the cells
were cultured for 24 h at 37˚C.
For sample processing, N2A cells were collected
using a cell scraper and centrifuged at 600 x g and 4˚C for 5 min.
The cells were washed with PBS and centrifuged under identical
conditions to obtain cell pellets. Subsequently, 100 µl cell lysis
buffer (cat. no. C1115-1; contained within the kit) obtained from
Beyotime Institute of Biotechnology, was added to cell pellets. The
cell pellets were then resuspended, lysed in an ice bath for 15 min
and centrifuged at 16,000 x g and 4˚C for 10 min. The supernatant
was analyzed to measure the caspase 3 activity using the caspase-3
activity assay. The Caspase 3 activity detection kit is based on
the ability of caspase 3 to catalyze the substrate
acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA), resulting in
yellow p-nitroaniline (pNA) production. Consequently, the enzymatic
activity of caspase 3 was quantified by measuring the absorbance,
since pNA exhibited strong absorption at ~405 nm. The reaction
system setup included adding detection buffer (80 µl), sample to be
tested (10 µl) and substrate Ac-DEVD-pNA (2 mmol/l; also added as
10 µl), resulting in a total volume of 100 µl. First, the detection
buffer was added, before the sample was introduced to ensure proper
mixing without bubbles before adding Ac-DEVD-pNA. The mixture was
incubated at 37˚C for 120 min. The blank control was substituted
with a detection buffer for the sample. Second, the absorbance was
measured at 405 nm. The absorbance of pNA catalyzed by caspase 3 in
the sample was calculated by subtracting the absorbance of the
blank control from that of the sample. Third, caspase-3 enzyme
activity was calculated as the quantity of enzyme required to
catalyze 1 nmol Ac-DEVD-pNA to pNA per h at 37˚C, defined as one
unit of enzyme activity.
Statistical analysis
Statistical analysis was performed using the SPSS
(version 18.0; SPSS, Inc.). Data are presented as mean ± standard
error of the mean. Multiple groups were compared using a one-way
analysis of variance. If there was statistical significance,
pairwise comparisons were conducted using the Tukey method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protective effect of harpagoside on
rotenone-induced N2A cells
Fig. 2 presents the
effect of harpagoside on rotenone-induced N2A cells. The cell
survival rate, one of the key indicators of cell viability, was
1.000±0.039 in the control group, which was decreased by 65.8% to
0.342±0.042 (P<0.05) after rotenone (20 nmol/l) treatment.
Intervention with harpagoside (10 µmol/l) significantly increased
the cell survival rate, to 0.738±0.030 (P<0.05), compared that
in the model group. However, cell survival rates did not differ
significantly between the model group and those treated with low
and medium doses of harpagoside (0.1 or 1 µmol/l).
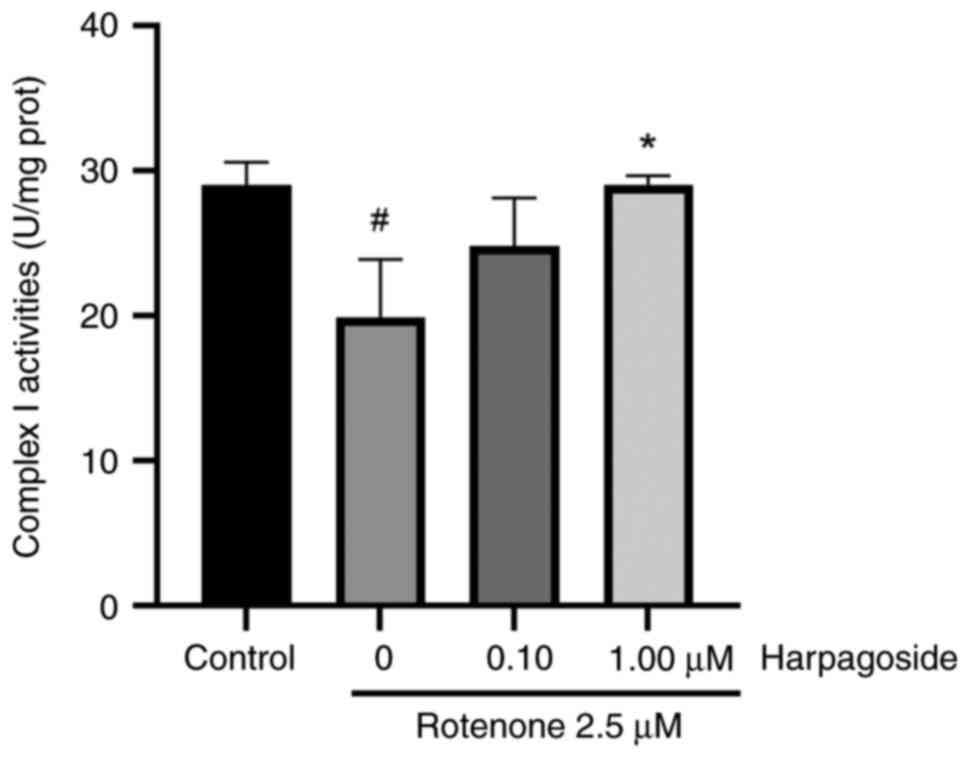
Effect of harpagoside on mitochondrial
complex I activity in rotenone-induced N2A cells
Fig. 3 illustrates
the effect of harpagoside on mitochondrial complex I activity in
rotenone-treated N2A cells. Rotenone (2.5 µmol/l) significantly
inhibited mitochondrial complex I enzyme activity, decreasing from
29.04±0.92 in the control group to 19.93±2.31 U/mg in the model
group, resulting in a 31.37% decrease (P<0.05). Harpagoside (1
µmol/l) significantly reversed the rotenone-induced inhibitory
effects on complex I, increasing its enzyme activity from
19.93±2.31 to 29.03±0.38 U/mg (P<0.05). However, treatment with
lower doses of harpagoside (0.1 µmol/l) did not significantly
affect the rotenone-induced inhibition of complex I.
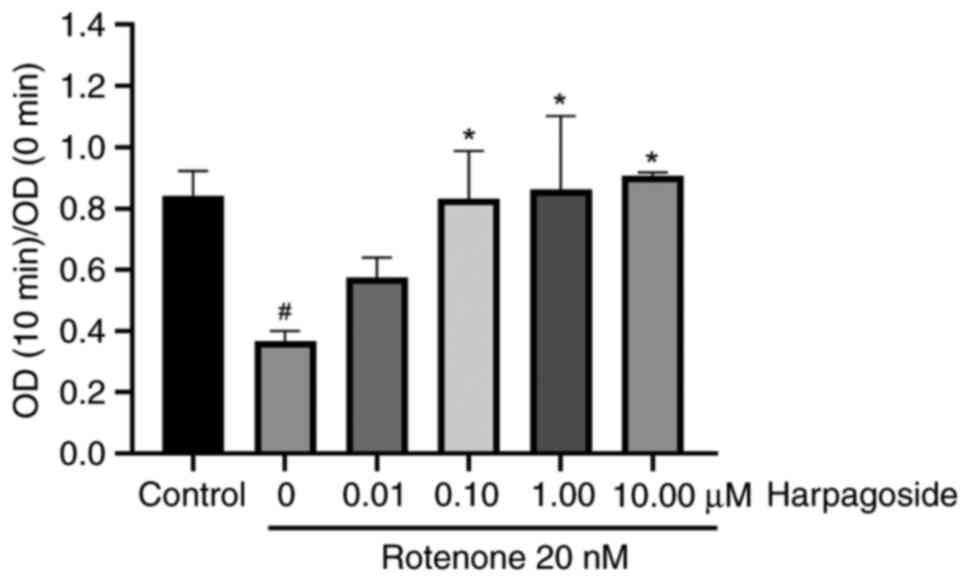
Effect of harpagoside on
rotenone-induced mitochondrial swelling in N2A cells
Fig. 4 presents the
effects of harpagoside on rotenone-induced mitochondrial swelling
in N2A cells. CaCl2 solution was added to the
mitochondrial swelling detection system for the mitochondrial
swelling detection experiment. If the permeability of the
mitochondria was strong, the absorbance at 540 nm would then be
gradually decreased with a larger magnitude. The detection interval
was 10 min and the degree of mitochondrial swelling was expressed
as optical density (OD; 10 min)/OD (0 min) at the end of the
measurement. A smaller ratio indicated a greater degree of
swelling. In the control group, the OD (10 min)/OD (0 min) was
0.842±0.046, whilst treatment with rotenone (20 nmol/l) for 9 h
resulted in a decreased ratio of 0.366±0.019 (P<0.05).
Pre-treatment with harpagoside (0.1, 1 or 10 µmol/l) for 2 h
significantly increased the rotenone-induced mitochondrial OD (10
min)/OD (0 min) from 0.366±0.019 to 0.831±0.090, 0.861±0.139 and
0.907±0.006, respectively (all P<0.05, respectively).
Harpagoside (0.01 µmol/l) did not affect the degree of
rotenone-induced mitochondrial swelling.
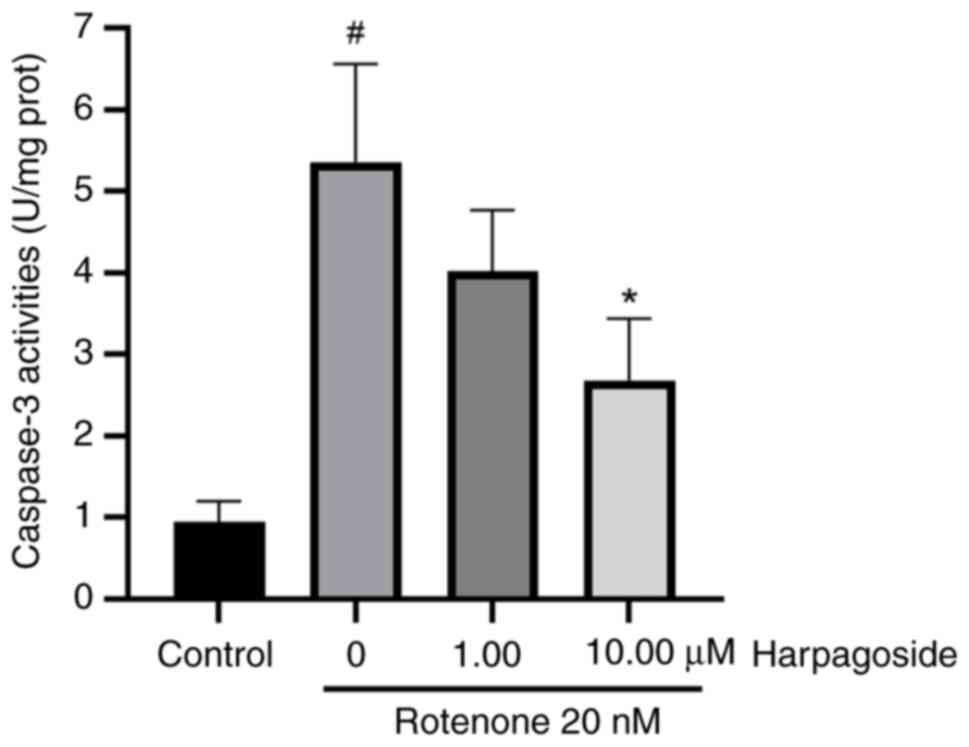
Effect of harpagoside on
rotenone-induced caspase 3 activity in N2A cells
Fig. 5 presents the
effect of harpagoside on rotenone-induced caspase 3 activity in N2A
cells. The caspase 3 enzyme activity was 0.95±0.15 U/mg in the
control group. However, treatment with rotenone for 24 h
significantly increased caspase 3 activity to 5.36±0.69 U/mg
(P<0.05). Pre-treatment with harpagoside (10 µmol/l) for 2 h
significantly inhibited rotenone-induced caspase 3 activation
(P<0.05), reducing its enzyme activity to 2.68±0.44 U/mg. After
intervention with harpagoside (1 µmol/l), the caspase 3 enzyme
activity was 4.02±0.43 U/mg, but no significant difference compared
with that in the model group could be found.
Discussion
The pathogenesis of PD is complex. Oxidative stress,
mitochondrial dysfunction and abnormal protein overexpression and
aggregation have all been documented to be involved in the
degeneration and deletion of substantia nigra DA neurons and the
reduction of striatum DA levels (41,42).
In particular, mitochondria-dependent apoptosis is a key factor in
PD-related DA neurodegeneration. Age-related mitochondrial changes,
including those in mitochondrial DNA, are strongly associated with
PD (43). Mitochondrial energy
disturbances and changes in mitochondrial distribution in neurons
are also important factors in PD pathogenesis (44,45).
Previous PD genomics studies have revealed that mitochondrial
dysfunctions caused by bioenergetic defects, mutations in
mitochondrial DNA, nuclear DNA gene mutations linked to
mitochondria, are important aspects of the pathogenesis of PD
(46,47). A number of familial PD-related
pathogenic proteins can interact directly or indirectly with
mitochondria (48,49). The proteins encoded by several
PD-related genes, including α-synuclein, Parkin, PTEN-induced
kinase 1, protein deglycase, leucine-rich repeat kinase 2 and
serotonin receptor 2A, can be localized to the mitochondria. The
aggregation of these proteins may cause mitochondrial DNA damage
and dysfunction (50). It has been
previously proposed that the decreased activity of complex I in the
mitochondrial respiratory chain in patients with PD and neurotoxin
rotenone injury can cause abnormal energy production function,
oxidative stress and mitochondria-dependent apoptosis in rat models
of PD (46,51).
N2A cells have been extensively utilized to
investigate neuronal differentiation, axonal growth and associated
signaling pathways (37,52). A notable feature of this cell type
is their capacity to differentiate into neurons within a matter of
days (37,52). Although various treatments, such as
serum deprivation, retinoids and bone morphogenetic proteins, can
be used to generate N2A neurons, only db-cAMP can significantly
promote the formation of DA neurons (37). Treatment of N2A cells with 1-4
mmol/l dbcAMP resulted in extensive differentiation and neurite
outgrowth (53). Both TH and DA
levels were previously demonstrated to significantly elevate in the
presence of db-cAMP, as demonstrated by Western blotting (WB),
immunocytochemistry and high-performance liquid chromatography
(HPLC) (37). Specifically, WB
analysis revealed that TH was endogenously expressed in N2A cells
at detectable levels, where it was significantly (>2.5-fold)
enhanced upon treatment with db-cAMP (37). The immunohistochemistry results
indicated that strong TH expression was noted in individual N2A
cells, which formed large colonies upon db-cAMP treatment for 3
days (37). The HPLC detection
results revealed that N2A cells expressed significant levels of DA,
which was further augmented upon db-cAMP treatment (37). N2A cells has been extensively used
to study PD (54,55). N2A exhibited greater sensitivity to
the lethal effects of MPP+ compared with human SH-SY5Y
cells with a half lethal concentration (LC50) ~10X lower
and rat PC-12 cells (with a LC50 ~2X lower) (52). Rotenone is prevalently utilized as a
trigger to induce neurodegenerative alterations in both in
vitro and in vivo experimental models of PD (56,57).
Intracellular calcium, rather than oxidative stress, constitutes a
major factor for rotenone-induced apoptosis in neuronal cells
(58).
A previous study has found that the cell viability
of N2A cells is decreased after treatment with 10 nmol/l deguelin
for 24-72 h in a time-dependant manner or after the treatment of
0.01-1 µmol/l deguelin for 48 h in a dose-dependant manner, with
IC50 of 16 nmol/l (59).
Rotenine is a structural analogue of degulin in terms of its
chemical composition (60). The
cell survival rate was found to be 1.000±0.039 in the control group
and decreased by 65.8% to 0.342±0.042 after rotenone (20 nmol/l)
treatment. Elevated concentrations of rotenone resulted in
increased cell death (59). After
the pre-experiment of cell viability, the concentration of rotenone
(20 nmol/l) was established in the present cellular experiments.
Significant inhibition of mitochondrial complex I activities by 5
µmol/l rotenone in SK-N-MC cells was reported by Sherer et
al (61). In the experiment of
the mitochondrial complex I assay, 2.5 µmol/l rotenone was
introduced to isolated mitochondria instead of to N2A cells. In all
the experiments apart from the mitochondrial complex I assay, 20
nmol/l rotenone was administered to N2A cells. The concentration of
rotenone utilized in the mitochondrial complex I assay was
significantly higher compared with that employed in the other
assays. This discrepancy arises from the fact that, in the
mitochondrial complex I assay, rotenone acted on the mitochondria
isolated from N2A cells for 10 min. By contrast, during the other
experiments, rotenone continued to exert its effects on N2A cells
over a duration of 48 h.
The secondary root extract of HP is rich in
bioactive iridoid glycosides, specifically known as harpagoside
(62). Extracts of HP have
demonstrated a concentration-dependent inhibition of lipid
peroxidation in brain homogenates induced by different pro-oxidants
(Fe2+ or sodium nitroprusside) (63). In addition, the ethyl acetate
fraction of HP exhibited the most pronounced antioxidant effects by
either reducing lipid peroxidation and cellular damage or restoring
thiol levels and catalase activity in brain cortical slices induced
by different pro-oxidants (63). HP
extracts have also been reported to upregulate brain-derived
neurotrophic factor gene expression and downregulating TNF-α gene
expression in rat cortex synaptosomes treated with amyloid
β-peptide (64). Furthermore, the
extracts mitigated amyloid β-peptide-induced stimulation of
malondialdehyde and 3-hydroxykynurenine levels and attenuated the
reduction in DA, norepinephrine and serotonin concentrations in the
rat cortex treated with amyloid β-peptide (64). Harpagoside was demonstrated to be
effective in providing promotion of axonal outgrowth in primary
spinal cord neurons under reactive oxygen species-insulting
conditions induced by ferrous sulfate (65). In addition, previous studies have
found that harpagoside (0.1-10 µmol/l) isolated from
Scrophulariae can protect against glutamate-induced cortical
neuronal injury in rats (66),
improve memory in mice injured by scopolamine and exhibit
antioxidant activity (67).
According to Li et al (68),
harpagoside alleviated amyloid-β-induced cognitive disorders in
rats by upregulating brain-derived neurotrophic factor expression
and the MAPK/PI3K signaling pathway. Harpagoside reduced
neuroinflammation-induced neuronal damage by inhibiting the
Toll-like receptor 4/molecule myeloid differentiation factor
88/NF-κB pathway (69,70). In another study, harpagoside
suppressed the overactivation of PTEN induced by chronic cerebral
hypoperfusion. Specifically, harpagoside increased the activity of
Akt and inhibited the activity of GSK-3β, a downstream effector of
PTEN (31). Sun et al
(32) previously found that
harpagoside can alleviate the symptoms of
MPTP/MPP+-induced DA neurodegenerative changes and
movement disorders by increasing GDNF mRNA and GDNF protein
expession levels. Li et al (36) found that harpagoside protected
against doxorubicin-induced cardiotoxicity through
p53/Parkin-mediated mitophagy. Harpagoside influences the
p53/Parkin-mediated cascade involving mitophagy deficiency,
mitochondrial dyshomeostasis and apoptosis through a novel
interaction between p53 and Parkin (36), whereby harpagoside promoted Parkin
translocation to mitochondria and substantially restored
Parkin-mediated mitophagy by inhibiting the binding of p53 and
Parkin. In the present study, harpagoside (10 µmol/l) exerted a
significant protective effect on rotenone-induced N2A cells. When
the harpagoside concentrations were ≥0.1 µmol/l, they significantly
prevented rotenone-induced mitochondrial damage in N2A cells and
relieved rotenone-induced mitochondrial swelling. However,
harpagoside (1 µmol/l) significantly antagonized the
rotenone-induced inhibition of complex I. The harpagoside (10
µmol/l) significantly inhibited rotenone-induced caspase 3
activation.
To conclude, results from the present study suggests
that harpagoside can protect against a rotenone-induced pseudo-PD
cell model through the mitochondrial protection pathway. However,
it must be noted that experiments in the present study were
performed using spectrophotometry, without support using other
research methods or further delving into the impact on other
genes/proteins, including cytochrome c, pro-apoptotic
genes/proteins and caspase 9. In future studies, experimental
investigations into mitochondrial-related signaling pathways should
be performed by incorporating additional detection methods, such as
WB. In addition, whether harpagoside can regulate the mitochondrial
pathway through its influence on oxidative stress and determine
whether harpagoside can exert an impact on mitochondria-dependent
apoptotic signaling should be assessed.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical Science
and Technology Project of Zhejiang Province (grant no.
2019KY730).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZX was the main contributor to writing the
manuscript. JL and ZX designed the experiments and were responsible
for the statistical and data analysis. JL and ZX drafted and
revised the original manuscript. JL and ZX confirmed the
authenticity of all original data and interpreted the results of
the study and gave the final approval for the forthcoming version.
JL and ZX read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tolosa E, Garrido A, Scholz SW and Poewe
W: Challenges in the diagnosis of Parkinson's disease. Lancet
Neurol. 20:385–397. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhou ZD, Yi LX, Wang DQ, Lim TM and Tan
EK: Role of dopamine in the pathophysiology of Parkinson's disease.
Transl Neurodegener. 12(44)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Norel R, Agurto C, Heisig S, Rice JJ,
Zhang H, Ostrand R, Wacnik PW, Ho BK, Ramos VL and Cecchi GA:
Speech-based characterization of dopamine replacement therapy in
people with Parkinson's disease. NPJ Parkinsons Dis.
6(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang X, Wang J, Zeng W, Zhang X, Yang X,
Xu Y, Xu Y and Cao X: Time-dependent alterations in the rat
nigrostriatal system after intrastriatal injection of fibrils
formed by α-Syn and tau fragments. Front Aging Neurosci.
14(1049418)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sosnik R, Fahoum F, Katzir Z, Mirelman A
and Maidan I: Key shifts in frontoparietal network activity in
Parkinson's disease. NPJ Parkinsons Dis. 11(2)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schapira AH: Mitochondria in the aetiology
and pathogenesis of Parkinson's disease. Lancet Neurol. 7:97–109.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schapira AH, Gu M, Taanman JW, Tabrizi SJ,
Seaton T, Cleeter M and Cooper JM: Mitochondria in the etiology and
pathogenesis of Parkinson's disease. Ann Neurol. 44 (3 Suppl
1):S89–S98. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ondo W: IPX066, a mixed
immediate/sustained-release levodopa preparation for Parkinson's
disease. Expert Opin Pharmacother. 15:2081–2085. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Caronni S, Del Sorbo F, Barichella M,
Fothergill-Misbah N, Denne T, Laguna J, Urasa S, Dekker MCJ, Akpalu
A, Sarfo FS, et al: Mucuna pruriens to treat Parkinson's disease in
low-income countries: Recommendations and practical guidelines from
the farmer to clinical trials. Paving the way for future use in
clinical practice. Parkinsonism Relat Disord.
124(106983)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kumari S, Gupta S, Sukhija R, Gurjar S,
Dubey SK and Taliyan R: Neuroprotective potential of Epigenetic
modulators, its regulation and therapeutic approaches for the
management of Parkinson's disease. Eur J Pharmacol.
985(177123)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo F, Qin X, Mao J, Xu Y and Xie J:
Potential protective effects of pungent flavor components in
neurodegenerative diseases. Molecules. 29(5700)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Filaferro M, Avallone R, Rustichelli C and
Vitale G: Characterization of walnut oil and evaluation of its
neuroprotective effects in an in vitro model of Parkinson's
disease. Molecules. 29(5718)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JH, Huh E, Eo H, Kim JS, Kwon Y, Ju
IG, Choi Y, Yoon HJ, Son SR, Jang DS, et al: Tribuli Fructus
alleviates 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine
(MPTP)-induced Parkinson's disease by suppressing neuroinflammation
via JNK signaling. Metab Brain Dis. 40(69)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Natarajan K, Chandrasekaran R, Sundararaj
R, Joseph J and Asaithambi K: Neuroprotective assessment of
nutraceutical (Betanin) in neuroblastoma cell line SHSY-5Y: An
in-vitro and in-silico approach. Neurochem Res.
50(54)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hvingelby VS and Pavese N: Surgical
advances in Parkinson's disease. Curr Neuropharmacol. 22:1033–1046.
2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rahimi Darehbagh R, Seyedoshohadaei SA,
Ramezani R and Rezaei N: Stem cell therapies for neurological
disorders: Current progress, challenges, and future perspectives.
Eur J Med Res. 29(386)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang S, Liu H, Lin Y, Liu M, Li Y, Mao H,
Zhang Z, Zhang Y, Ye P, Ding L, et al: Berberine protects against
NLRP3 inflammasome via ameliorating autophagic impairment in
MPTP-induced Parkinson's disease model. Front Pharmacol.
11(618787)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vellingiri B, Chandrasekhar M, Sri Sabari
S, Gopalakrishnan AV, Narayanasamy A, Venkatesan D, Iyer M, Kesari
K and Dey A: Neurotoxicity of pesticides-A link to
neurodegeneration. Ecotoxicol Environ Saf.
243(113972)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Johnson ME and Bobrovskaya L: An update on
the rotenone models of Parkinson's disease: Their ability to
reproduce the features of clinical disease and model
gene-environment interactions. Neurotoxicology. 46:101–116.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Betarbet R, Sherer TB, MacKenzie G,
Garcia-Osuna M, Panov AV and Greenamyre JT: Chronic systemic
pesticide exposure reproduces features of Parkinson's disease. Nat
Neurosci. 3:1301–1306. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Tao X, Zhang W, Chen C, Tao Y, Tao Y, Chen
Z and Zhang G: miR-101a-3p/ROCK2 axis regulates neuronal injury in
Parkinson's disease models. Aging (Albany NY). 16:8732–8746.
2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Menchinskaya E, Chingizova E, Pislyagin E,
Likhatskaya G, Sabutski Y, Pelageev D, Polonik S and Aminin D:
Neuroprotective effect of 1,4-naphthoquinones in an in vitro model
of paraquat and 6-OHDA-induced neurotoxicity. Int J Mol Sci.
22(9933)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Verma A and Goyal A: Plumbagin's healing
effect on motor impairment in rotenone-toxified rodents. Curr
Neurovasc Res: Sep 3, 2024 (Epub ahead of print).
|
|
24
|
Olubodun-Obadun TG, Ishola IO, Folarin OR,
Oladoja FA, Gilbert TT, Aniekwensi IM, Bisiriyu A, Joseph-Iwebi NA,
Adebanjo FO, Olopade JO and Adeyemi OO: Cajanus cajan (L) Millsp
seeds extract prevents rotenone-induced motor- and non-motor
features of Parkinson disease in mice: Insight into mechanisms of
neuroprotection. J Ethnopharmacol. 322(117623)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Meng X, Xie W, Xu Q, Liang T, Xu X, Sun G
and Sun X: Neuroprotective effects of radix scrophulariae on
cerebral ischemia and reperfusion injury via MAPK pathways.
Molecules. 23(2401)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee HJ, Spandidos DA, Tsatsakis A, Margina
D, Izotov BN and Yang SH: Neuroprotective effects of Scrophularia
buergeriana extract against glutamate-induced toxicity in SH-SY5Y
cells. Int J Mol Sci. 43:2144–2152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim HL, Min DE, Lee SK, Choi BK and Lee
DR: Scrophularia buergeriana Extract (Brainon) attenuates
neuroinflammation in BV-2 microglia cells and promotes
neuroprotection in SH-SY5Y neuroblastoma cells. J Med Food.
26:328–341. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Azadmehr A, Oghyanous KA, Hajiaghaee R,
Amirghofran Z and Azadbakht M: Antioxidant and neuroprotective
effects of Scrophularia striata extract against oxidative
stress-induced neurotoxicity. Cell Mol Neurobiol. 33:1135–1141.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Salavati P, Ramezani M, Monsef-Esfahani
HR, Hajiagha R, Parsa M, Tavajohi S and Ostad SN: Neuroprotective
effect of total and sequential extract of scrophularia striata
boiss. in rat cerebellar granule neurons following
glutamate-induced neurotoxicity: An in-vitro study. Iran J Pharm
Res. 12:389–394. 2013.PubMed/NCBI
|
|
30
|
Wang K, Lou Y, Xu H, Zhong X and Huang Z:
Harpagide from Scrophularia protects rat cortical neurons from
oxygen-glucose deprivation and reoxygenation-induced injury by
decreasing endoplasmic reticulum stress. J Ethnopharmacol.
253(112614)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen C, Zhang H, Xu H, Xue R, Zheng Y, Wu
T and Lian Y: Harpagoside rescues the memory impairments in chronic
cerebral hypoperfusion rats by inhibiting PTEN activity. J
Alzheimers Dis. 63:445–455. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun X, Xiong Z, Zhang Y, Meng Y, Xu G, Xia
Z, Li J, Zhang R, Ke Z, Xia Z and Hu Y: Harpagoside attenuates
MPTP/MPP(+) induced dopaminergic neurodegeneration and movement
disorder via elevating glial cell line-derived neurotrophic factor.
J Neurochem. 120:1072–1083. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dinda B, Dinda M, Kulsi G, Chakraborty A
and Dinda S: Therapeutic potentials of plant iridoids in
Alzheimer's and Parkinson's diseases: A review. Eur J Med Chem.
169:185–199. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Shinoda Y, Cheng A, Kawahata I and
Fukunaga K: Epidermal fatty acid-binding protein 5 (FABP5)
involvement in alpha-synuclein-induced mitochondrial injury under
oxidative stress. Biomedicines. 9(110)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He L, Wang J, Yang Y, Li J and Tu H:
Mitochondrial sirtuins in Parkinson's disease. Neurochem Res.
47:1491–1502. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li W, Wang X, Liu T, Zhang Q, Cao J, Jiang
Y, Sun Q, Li C, Wang W and Wang Y: Harpagoside protects against
doxorubicin-induced cardiotoxicity via P53-parkin-mediated
mitophagy. Front Cell Dev Biol. 10(813370)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tremblay RG, Sikorska M, Sandhu JK,
Lanthier P, Ribecco-Lutkiewicz M and Bani-Yaghoub M:
Differentiation of mouse Neuro 2A cells into dopamine neurons. J
Neurosci Methods. 186:60–67. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hergenhahn L, Padutsch N, Azawi S,
Weiskirchen R, Liehr T and Rincic M: Cytogenomic characterization
of murine neuroblastoma cell line neuro-2a and its two derivatives
neuro-2a TR-alpha and Neuro-2a TR-Beta. Cells.
13(1889)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lei C, Wang J, Zhang X, Ge X, Zhao W, Li
X, Jiang W, Ma M, Wang Z, Sun S, et al: The wnt/pyruvate kinase,
muscle axis plays an essential role in the differentiation of mouse
neuroblastoma cells. Neurochem Int. 181(105901)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar M and Katyal A: Data on retinoic
acid and reduced serum concentration induced differentiation of
Neuro-2a neuroblastoma cells. Data Brief. 21:2435–2440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Subramaniam SR and Chesselet MF:
Mitochondrial dysfunction and oxidative stress in Parkinson's
disease. Prog Neurobiol. 106-107:17–32. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen R, Gu X and Wang X: α-Synuclein in
Parkinson's disease and advances in detection. Clin Chim Acta.
529:76–86. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bose A and Beal MF: Mitochondrial
dysfunction in Parkinson's disease. J Neurochem. 139:216–231.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Perez MJ, Baden P and Deleidi M:
Progresses in both basic research and clinical trials of NAD+ in
Parkinson's disease. Mech Ageing Dev. 197(111499)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang Y and Lu B: Mitochondrial
morphogenesis, distribution, and Parkinson disease: Insights from
PINK1. J Neuropathol Exp Neurol. 68:953–963. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vila M, Ramonet D and Perier C:
Mitochondrial alterations in Parkinson's disease: new clues. J
Neurochem. 107:317–328. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Casoli T, Lisa R, Fabbietti P and Conti F:
Analysis of mitochondrial DNA allelic changes in Parkinson's
disease: A preliminary study. Aging Clin Exp Res. 32:345–349.
2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Henchcliffe C and Beal MF: Mitochondrial
biology and oxidative stress in Parkinson disease pathogenesis. Nat
Clin Pract Neurol. 4:600–609. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shen L and Dettmer U: Alpha-synuclein
effects on mitochondrial quality control in Parkinson's disease.
Biomolecules. 14(1649)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang JL, Weissman L, Bohr VA and Mattson
MP: Mitochondrial DNA damage and repair in neurodegenerative
disorders. DNA Repair (Amst). 7:1110–1120. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pathak RU and Davey GP: Complex I and
energy thresholds in the brain. Biochim Biophys Acta. 1777:777–782.
2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mazzio EA, Soliman YI and Soliman KF:
Variable toxicological response to the loss of OXPHOS through
1-methyl-4-phenylpyridinium-induced mitochondrial damage and anoxia
in diverse neural immortal cell lines. Cell Biol Toxicol.
26:527–539. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wong CK, Yeung HY, Mak NK, DiMattia GE,
Chan DK and Wagner GF: Effects of dibutyryl cAMP on stanniocalcin
and stanniocalcin-related protein mRNA expression in neuroblastoma
cells. J Endocrinol. 173:199–209. 2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ferrari E, Cardinale A, Picconi B and
Gardoni F: From cell lines to pluripotent stem cells for modelling
Parkinson's disease. J Neurosci Methods. 340(108741)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
LePage KT, Dickey RW, Gerwick WH, Jester
EL and Murray TF: On the use of neuro-2a neuroblastoma cells versus
intact neurons in primary culture for neurotoxicity studies. Crit
Rev Neurobiol. 17:27–50. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Agafonova I, Chingizova E, Chaikina E,
Menchinskaya E, Kozlovskiy S, Likhatskaya G, Sabutski Y, Polonik S,
Aminin D and Pislyagin E: Protection activity of
1,4-naphthoquinones in rotenone-induced models of neurotoxicity.
Mar Drugs. 22(62)2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Xia N, Madore V, Albalakhi A, Lin S,
Stimpson T, Xu Y, Schwarzschild MA and Bakshi R:
Microglia-dependent neuroprotective effects of 4-octyl itaconate
against rotenone-and MPP+-induced neurotoxicity in Parkinson's
disease. Sci Rep. 13(15539)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Swarnkar S, Goswami P, Kamat PK, Gupta S,
Patro IK, Singh S and Nath C: Rotenone-induced apoptosis and role
of calcium: A study on Neuro-2a cells. Arch Toxicol. 86:1387–1397.
2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xiong Z, Wang S and Lang J: Inhibitory
effects of deguelin on the viability of neuro-2A cells. Chin
Pharmacist. 17:1793–1796. 2014.(In Chinese).
|
|
60
|
Caboni P, Sherer TB, Zhang N, Taylor G, Na
HM, Greenamyre JT and Casida JE: Rotenone, deguelin, their
metabolites, and the rat model of Parkinson's disease. Chem Res
Toxicol. 17:1540–1548. 2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sherer TB, Betarbet R, Testa CM, Seo BB,
Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A and
Greenamyre JT: Mechanism of toxicity in rotenone models of
Parkinson's disease. J Neurosci. 23:10756–10764. 2003.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kondamudi N, Turner MW and McDougal OM:
Harpagoside content in devil's claw extracts. Nat Prod Commun.
11:1215–1216. 2016.PubMed/NCBI
|
|
63
|
Schaffer LF, Peroza LR, Boligon AA,
Athayde ML, Alves SH, Fachinetto R and Wagner C: Harpagophytum
procumbens prevents oxidative stress and loss of cell viability in
vitro. Neurochem Res. 38:2256–2267. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ferrante C, Recinella L, Locatelli M,
Guglielmi P, Secci D, Leporini L, Chiavaroli A, Leone S, Martinotti
S, Brunetti L, et al: Protective effects induced by
microwave-assisted aqueous harpagophytum extract on rat cortex
synaptosomes challenged with amyloid β-peptide. Phytother Res.
31:1257–1264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hong JY, Kim H, Yeo C, Lee J, Jeon WJ, Lee
YJ and Ha IH: Epidural injection of harpagoside for the recovery of
rats with lumbar spinal stenosis. Cells. 12(2281)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kim SR, Lee KY, Koo KA, Sung SH, Lee NG,
Kim J and Kim YC: Four new neuroprotective iridoid glycosides from
Scrophularia buergeriana roots. J Nat Prod. 65:1696–1699.
2002.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Jeong EJ, Lee KY, Kim SH, Sung SH and Kim
YC: Cognitive-enhancing and antioxidant activities of iridoid
glycosides from Scrophularia buergeriana in scopolamine-treated
mice. Eur J Pharmacol. 588:78–84. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Li J, Ding X, Zhang R, Jiang W, Sun X, Xia
Z, Wang X, Wu E, Zhang Y and Hu Y: Harpagoside ameliorates the
amyloid-β-induced cognitive impairment in rats via up-regulating
BDNF expression and MAPK/PI3K pathways. Neuroscience. 303:103–114.
2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Hao RJ, Yu GR, Qin XD and Lu YW:
Harpagoside alleviates neuronal damage caused by neuroinflammation
via suppressing TLR4/MyD88/NF-κB pathway. Chin Tradit Herbal Drugs.
54:4202–4213. 2023.(In Chinese).
|
|
70
|
Huang TH, Tran VH, Duke RK, Tan S,
Chrubasik S, Roufogalis BD and Duke CC: Harpagoside suppresses
lipopolysaccharide-induced iNOS and COX-2 expression through
inhibition of NF-kappa B activation. J Ethnopharmacol. 104:149–155.
2006.PubMed/NCBI View Article : Google Scholar
|