1. Introduction
Osteoarthritis (OA) is the most common degenerative
joint disease, and one of the core factors in its development is
articular cartilage damage (1-2). Due
to the lack of vascular tissue in articular cartilage, if not
treated in time, the damaged cartilage cannot regenerate
spontaneously, thus leading to the occurrence of OA (3,4). At
present, non-surgical methods beneficial to early lesions include
intra-articular injection of sodium hyaluronate, platelet rich
plasma and concentrated cytokines (5-8).
Traditional surgical treatments include subchondral microfracture
and autologous chondrocyte or cartilage transplantation (9-12).
Due to the uncertainty and inconsistency of the results, none of
these methods can achieve completely satisfactory results (13,14).
Therefore, it is urgent to find an improved treatment to restore
the structure and function of damaged cartilage.
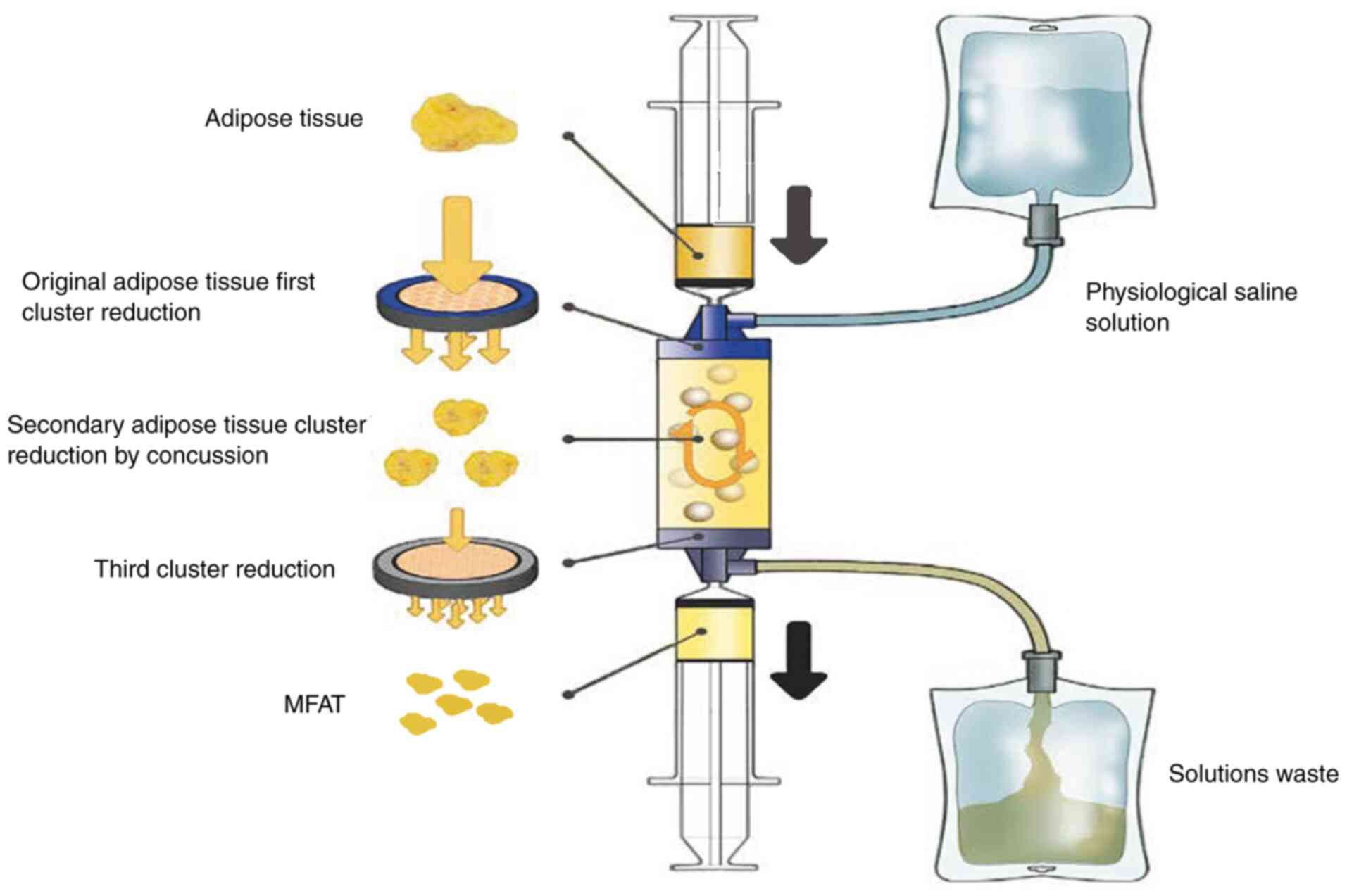
Previous studies have found that through a new type
of fully enclosed device, lipogems®, micro-fragmented
adipose tissue (MFAT) can be obtained by treating adipose tissue
with mild mechanical force (15,16).
MFAT does not require cell expansion, enzyme treatment or other
major operations, and can maintain an intact stromal vascular niche
(17). In the present review, the
latest research progress of the mechanism of MFAT in the repair of
cartilage injury in OA was discussed, providing a new direction for
the treatment of OA.
2. Overview of MFAT
MFAT can be obtained by treating adipose tissue with
lipogems system (15,16); the adipose tissue is rich in
content, widely sourced and easy to obtain in the body, and MFAT
has strong anti-inflammatory and anti-apoptotic abilities (18-20).
These adipose tissues can release cytokines, extracellular vesicles
and numerous other regulatory information, improve the local
microenvironment of injury, and obviously help to promote the
repair of cartilage injury (21,22)
(Fig. 1).
Advantages of MFAT include the following: i) MFAT is
easy to obtain, rich in sources and does not need to be cultured
in vitro; ii) MFAT is rich in components, including a large
number of pericytes, mesenchymal stem cells (MSCs), growth factors,
exosomes, and a complete three-dimensional biological scaffold with
a highly reductive cell proliferation microenvironment; iii) the
survival rate and homing inhibition rate of MFAT transplantation
were significantly higher than those of MSCs, and the clinical
effect was obvious; iv) the whole acquisition process of MFAT is
completely closed, avoiding contact with air, reducing pollution,
and does not require enzymatic treatment of fat or other additives,
which will not damage the three-dimensional biological scaffold of
MFAT and the microenvironment of cell proliferation, is conducive
to the release of various cytokines, and the obtained end products
can be reinfused (23); v) the
content of MFAT cytokines and exosomes is significantly higher than
that of enzymatic treatment, which can significantly improve the
ability of tissue repair and regeneration, including stem cell
transformation, angiogenesis and ‘homing’ (16); and vi) MFAT is an autologous fat
derivative, which is not from other xenogeneic sources and avoids
the rejection reaction.
In a word, MFAT is rich in multifunctional cells,
MSCs, exosomes and various cytokines, and MFAT has a strong
promoting effect on the repair of tissue damage.
3. MFAT and repair of cartilage damage
OA is a chronic bone and joint disease characterized
by articular cartilage damage and joint inflammation (24), and its main clinical manifestations
are chronic pain and joint movement disorders, which seriously
affect the quality of life of patients. Chondrocytes are the only
cells in articular cartilage; degradation of cartilage
extracellular matrix (ECM), apoptosis of chondrocytes and
production of inflammatory factors are crucial to the pathological
progression of OA (25,26). Therefore, inhibiting ECM
degradation, alleviating chondrocyte apoptosis and inflammatory
response can delay the pathological progression of OA. MFAT can
transmit growth factors, extracellular vesicles and numerous other
regulatory information to the microenvironment around the damaged
cartilage, and promote the repair of cartilage damage (21,22).
A recent study found that 49 patients with knee OA
(Kellgren-Lawrence III-IV) were treated with a single injection of
autologous MFAT and knee arthroscopy; the results showed that
arthroscopic injection of MFAT was a safe and effective method for
the treatment of knee OA, which could significantly improve the
IKDC and KOOS scores, and no major complications occurred in the
2-year follow-up after surgery (27). Another study also showed that the
injection of MFAT can significantly improve the KOOS score and
quality of life of patients, but it needs longer follow-up time to
draw more definite conclusions and expand the indications (28). Ulivi et al (29) performed knee arthroscopy combined
with MFAT in the treatment of knee OA; the follow-up results
demonstrated that the serum biomarkers of cartilage deposition were
significantly increased, indicating that the treatment of MFAT is
effective. Yu et al (30)
performed a single autologous MFAT injection in 20 patients, 40
knees in total, and the postoperative follow-up effect improved
significantly without major complications. Malanga et al
(31) directly injected MFAT into
the knee joint with torn meniscus under ultrasound guidance and
achieved favorable results, which also showed that MFAT was a safe
and potentially effective treatment for patients with degenerative
arthritis and knee pain with torn meniscus. A recent systematic
review demonstrated that MFAT injection is effective in the
treatment of symptomatic knee OA, which can significantly improve
knee pain and function (32).
Another systematic review also showed that MFAT can relieve pain
and improve motor function in patients with knee OA in a short
time, and this method is also effective and safe (33). Bisicchia et al (34) found that for symptomatic patients
with focal cartilage injury of the knee joint, the operation of
injecting MFAT plus micro fracture was more effective than the
operation of micro fracture alone.
Desando et al (35) found that MFAT could induce CD-163
wound healing macrophages to migrate to injured cartilage by
injecting MFAT into rabbit OA model, indicating that MFAT can
directly mediate cartilage tissue repair response and promote
cartilage repair. Filardo et al (36) identified that MFAT can reduce
synovial inflammatory response in rabbit OA model and play a
protective role on cartilage. A 24-month follow-up study showed
that a single intra-articular injection of autologous MFAT could
significantly increase the content of glycosaminoglycan in
articular cartilage, which indicated that MFAT could promote the
synthesis of cartilage matrix and delay the progression of OA
(37). Bosetti et al
(38) showed that MFAT can induce
chondrocyte proliferation and ECM production through paracrine, and
provide cells that can regenerate or repair damaged or missing
cartilage at the site of injury. Xu et al (39) through the study of rat cartilage
injury model, found that MFAT significantly promoted the migration
of chondrocytes; through histological evaluation, MFAT treatment
produced tissues similar to normal cartilage, including regular
tissue surface, a large amount of hyaline cartilage, complete
subchondral bone reconstruction and the formation of corresponding
type I, II and VI collagens. The aforementioned study demonstrated
the promoting effect of MFAT on the repair of osteochondral
defects. Ceserani et al (40) demonstrated that MFAT can induce
vascular stabilization and inhibit inflammatory response through
paracrine action, thus delaying the process of cartilage
damage.
These studies have shown that MFAT can inhibit the
inflammatory response and promote the repair of cartilage damage by
transmitting growth factors, extracellular vesicles and numerous
other regulatory information to the microenvironment surrounding
the damaged cartilage, or through paracrine effects. Therefore, it
is particularly important to further study the specific mechanism
and related signaling pathways of MFAT in cartilage damage
repair.
4. Conclusions and perspectives
OA is one of the most common degenerative joint
diseases, and pain and activity limitation are its main symptoms;
OA seriously affects the quality of life of patients and brings
heavy economic burden to families and society (41). Although OA has been extensively
studied, its pathogenesis remains to be fully elucidated, thus OA
cannot be completely cured. MFAT plays an important role in the
repair of cartilage injury in OA, but its specific mechanism of
action and related signaling pathways are not yet fully understood,
thus it requires further study. In general, the present review aims
to delay the progression of OA and improve the quality of life of
patients by elucidating the role of MFAT in the repair of cartilage
damage and the reduction of inflammatory response in OA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the General program
of Natural Science Foundation of Shandong (grant no.
ZR202210220019), the Shandong medical and health development plan
(grant no. 202204070236) and the China University industry research
innovation fund Huatong Guokang medical research special project in
2023 (grant no. 2023HT050).
Availability of data and materials
Not applicable.
Authors' contributions
JW and YS drafted the manuscript and revised the
manuscript. HL, CW, YG and XJ contributed to manuscript conception.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guermazi A, Hayashi D, Roemer FW, Niu J,
Quinn EK, Crema MD, Nevitt MC, Torner J, Lewis CE and Felson DT:
Brief report: Partial- and full-thickness focal cartilage defects
contribute equally to development of new cartilage damage in knee
osteoarthritis: The multicenter osteoarthritis study. Arthritis
Rheumatol. 69:560–564. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gomoll AH and Minas T: The quality of
healing: Articular cartilage. Wound Repair Regen. 22 (Suppl
1):S30–S38. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Armiento AR, Alini M and Stoddart MJ:
Articular fibrocartilage - Why does hyaline cartilage fail to
repair? Adv Drug Deliv Rev. 146:289–305. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jones IA, Togashi R, Wilson ML, Heckmann N
and Vangsness CT Jr: Intra-articular treatment options for knee
osteoarthritis. Nat Rev Rheumatol. 15:77–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Quilliot J, Couderc M, Giraud C, Soubrier
M and Mathieu S: Efficacy of intra-articular hyaluronic acid
injection in knee osteoarthritis in everyday life. Semin Arthritis
Rheum. 49:e10–e11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sundman EA, Cole BJ, Karas V, Della Valle
C, Tetreault MW, Mohammed HO and Fortier LA: The anti-inflammatory
and matrix restorative mechanisms of platelet-rich plasma in
osteoarthritis. Am J Sports Med. 42:35–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ludin A, Sela JJ, Schroeder A, Samuni Y,
Nitzan DW and Amir G: Injection of vascular endothelial growth
factor into knee joints induces osteoarthritis in mice.
Osteoarthritis Cartilage. 21:491–497. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ruta DJ, Villarreal AD and Richardson DR:
Orthopedic surgical options for joint cartilage repair and
restoration. Phys Med Rehabil Clin N Am. 27:1019–1042.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schinhan M, Gruber M, Dorotka R, Pilz M,
Stelzeneder D, Chiari C, Rössler N, Windhager R and Nehrer S:
Matrix-associated autologous chondrocyte transplantation in a
compartmentalized early stage of osteoarthritis. Osteoarthritis
Cartilage. 21:217–225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gudas R, Gudaitė A, Mickevičius T,
Masiulis N, Simonaitytė R, Cekanauskas E and Skurvydas A:
Comparison of osteochondral autologous transplantation,
microfracture, or debridement techniques in articular cartilage
lesions associated with anterior cruciate ligament injury: A
prospective study with a 3-year follow-up. Arthroscopy. 29:89–97.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang BJ, Hu JC and Athanasiou KA:
Cell-based tissue engineering strategies used in the clinical
repair of articular cartilage. Biomaterials. 98:1–22.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Simon TM and Jackson DW: Articular
cartilage: Injury pathways and treatment options. Sports Med
Arthrosc Rev. 26:31–39. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee WY and Wang B: Cartilage repair by
mesenchymal stem cells: Clinical trial update and perspectives. J
Orthop Translat. 9:76–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tremolada C, Colombo V and Ventura C:
Adipose tissue and mesenchymal stem cells: State of the Art and
lipogems® technology development. Curr Stem Cell Rep. 2:304–312.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tremolada C, Ricordi C, Caplan AI and
Ventura C: Mesenchymal stem cells in lipogems, a reverse story:
From clinical practice to basic science. Methods Mol Biol.
1416:109–122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kaewsuwan S, Song SY, Kim JH and Sung JH:
Mimicking the functional niche of adipose-derived stem cells for
regenerative medicine. Expert Opin Biol Ther. 12:1575–1588.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang J, Liu Y, Chen Y, Yuan L, Liu H,
Wang J, Liu Q and Zhang Y: Adipose-derived stem cells: Current
applications and future directions in the regeneration of multiple
tissues. Stem Cells Int. 2020(8810813)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Im GI: Bone marrow-derived stem/stromal
cells and adipose tissue-derived stem/stromal cells: Their
comparative efficacies and synergistic effects. J Biomed Mater Res
A. 105:2640–2648. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zheng CX, Sui BD, Liu N, Hu CH, He T,
Zhang XY, Zhao P, Chen J, Xuan K and Jin Y: Adipose mesenchymal
stem cells from osteoporotic donors preserve functionality and
modulate systemic inflammatory microenvironment in osteoporotic
cytotherapy. Sci Rep. 8(5215)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kunze KN, Burnett RA, Wright-Chisem J,
Frank RM and Chahla J: Adipose-derived mesenchymal stem cell
treatments and available formulations. Curr Rev Musculoskelet Med.
13:264–280. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao
H and Gao J: Transplantation of human mesenchymal stem-cell-derived
exosomes immobilized in an adhesive hydrogel for effective
treatment of spinal cord injury. Nano Lett. 20:4298–4305.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bianchi F, Maioli M, Leonardi E, Olivi E,
Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M,
Tremolada C and Ventura C: A new nonenzymatic method and device to
obtain a fat tissue derivative highly enriched in pericyte-like
elements by mild mechanical forces from human lipoaspirates. Cell
Transplant. 22:2063–2077. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Taruc-Uy RL and Lynch SA: Diagnosis and
treatment of osteoarthritis. Prim Care. 40:821–836, vii.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aigner T, Söder S, Gebhard PM, McAlinden A
and Haag J: Mechanisms of disease: Role of chondrocytes in the
pathogenesis of osteoarthritis-structure, chaos and senescence. Nat
Clin Pract Rheumatol. 3:391–399. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Le LT, Swingler TE and Clark IM: Review:
the role of microRNAs in osteoarthritis and chondrogenesis.
Arthritis Rheum. 65:1963–1974. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Giorgini A, Selleri F, Zambianchi F,
Cataldo G, Francioni E and Catani F: Autologous micro-fragmented
adipose tissue associated with arthroscopy in moderate-severe knee
osteoarthritis: Outcome at two year follow-up. BMC Musculoskelet
Disord. 23(963)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cattaneo G, De Caro A, Napoli F, Chiapale
D, Trada P and Camera A: Micro-fragmented adipose tissue injection
associated with arthroscopic procedures in patients with
symptomatic knee osteoarthritis. BMC Musculoskelet Disord.
19(176)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ulivi M, Meroni V, Viganò M, Colombini A,
Lombardo MDM, Rossi N, Orlandini L, Messina C, Sconfienza LM,
Peretti GM, et al: Micro-fragmented adipose tissue (mFAT)
associated with arthroscopic debridement provides functional
improvement in knee osteoarthritis: A randomized controlled trial.
Knee Surg Sports Traumatol Arthrosc. 31:3079–3090. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu Y, Lu Q, Li S, Liu M, Sun H, Li L, Han
K and Liu P: Intra-articular injection of autologous
micro-fragmented adipose tissue for the treatment of knee
osteoarthritis: A prospective interventional study. J Pers Med.
13(504)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Malanga GA, Chirichella PS, Hogaboom NS
and Capella T: Clinical evaluation of micro-fragmented adipose
tissue as a treatment option for patients with meniscus tears with
osteoarthritis: A prospective pilot study. Int Orthop. 45:473–480.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hohmann E, Keough N, Frank RM and Rodeo S:
Micro-fragmented adipose tissue demonstrates comparable clinical
efficacy to other orthobiologic injections in treating symptomatic
knee osteoarthritis: A systematic review of level I to IV clinical
studies. Arthroscopy. 41:418–441.e14. 2025.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shoukrie SI, Venugopal S, Dhanoa RK,
Selvaraj R, Selvamani TY, Zahra A, Malla J, Hamouda RK and Hamid
PF: Safety and efficacy of injecting mesenchymal stem cells into a
human knee joint to treat osteoarthritis: A systematic review.
Cureus. 14(e24823)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bisicchia S, Bernardi G, Pagnotta SM and
Tudisco C: Micro-fragmented stromal-vascular fraction plus
microfractures provides better clinical results than microfractures
alone in symptomatic focal chondral lesions of the knee. Knee Surg
Sports Traumatol Arthrosc. 28:1876–1884. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Desando G, Bartolotti I, Martini L,
Giavaresi G, Nicoli Aldini N, Fini M, Roffi A, Perdisa F, Filardo
G, Kon E and Grigolo B: Regenerative features of adipose tissue for
osteoarthritis treatment in a rabbit model: Enzymatic Digestion
versus mechanical disruption. Int J Mol Sci.
20(2636)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Filardo G, Tschon M, Perdisa F, Brogini S,
Cavallo C, Desando G, Giavaresi G, Grigolo B, Martini L, Nicoli
Aldini N, et al: Micro-fragmentation is a valid alternative to cell
expansion and enzymatic digestion of adipose tissue for the
treatment of knee osteoarthritis: A comparative preclinical study.
Knee Surg Sports Traumatol Arthrosc. 30:773–781. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Borić I, Hudetz D, Rod E, Jeleč Ž,
Vrdoljak T, Skelin A, Polašek O, Plečko M, Trbojević-Akmačić I,
Lauc G and Primorac D: A 24-month follow-up study of the effect of
intra-articular injection of autologous microfragmented fat tissue
on proteoglycan synthesis in patients with knee osteoarthritis.
Genes (Basel). 10(1051)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bosetti M, Borrone A, Follenzi A,
Messaggio F, Tremolada C and Cannas M: Human lipoaspirate as
autologous injectable active scaffold for one-step repair of
cartilage defects. Cell Transplant. 25:1043–1056. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu T, Yu X, Yang Q, Liu X, Fang J and Dai
X: Autologous micro-fragmented adipose tissue as stem cell-based
natural scaffold for cartilage defect repair. Cell Transplant.
28:1709–1720. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ceserani V, Ferri A, Berenzi A, Benetti A,
Ciusani E, Pascucci L, Bazzucchi C, Coccè V, Bonomi A, Pessina A,
et al: Angiogenic and anti-inflammatory properties of
micro-fragmented fat tissue and its derived mesenchymal stromal
cells. Vasc Cell. 8(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|