Introduction
Hepatic encephalopathy (HE) is a general term for a
wide range of neurological or psychiatric conditions, ranging from
preclinical abnormalities to coma, which are suggestive of either
brain dysfunction caused by portal-systemic shunting or liver
failure (1). From an
epidemiological perspective, HE is currently recognized as the most
prevalent cirrhosis consequence, leading to frequent readmissions,
hospital stays, and a higher risk of mortality (2).
Numerous factors contribute to the complex
pathophysiology of HE, including abnormal brain energy metabolism,
increased oxidative stress in astrocytes, changed gut flora,
impaired metabolism of intestinally generated neurotoxins, and
altered blood-brain barrier and neurotransmission. Through several
mechanisms, including increased intracellular osmolality in
astrocytes, impaired transport of amino acids from the blood to the
brain, and abnormal neuronal electrical activity, intestinal
neurotoxin ammonia affects neurological function at multiple sites
(3,4).
HE is classified into three primary categories based
on the underlying cause. Individuals with portosystemic shunting
without intrinsic liver disease develop type B, those with acute
liver failure develop type A and those with cirrhosis are
categorized as type C (5,6). The first-pass hepatic clearance of
neurotoxins generated from the gut, including ammonia, is reduced
during portosystemic shunt. In addition, intestinal glutaminase
activity is elevated, increasing ammonia production in the gut
(7). Polytetrafluoroethylene-coated
stents significantly improve patency and reduce the likelihood of
shunt malfunction. However, HE following portosystemic shunts, such
as the transjugular intrahepatic portosystemic shunt (TIPS),
remains a significant cause of concern (8-10).
According to several previous studies, angiographic
embolization of large shunts in patients with recurrent HE has been
linked to improved neurological symptoms (11-14).
Furthermore, shunt embolization may increase patient survival and
liver function by restoring hepatic blood flow (12). This systematic review and
meta-analysis addresses the effects of shunt embolization on HE
recurrence in patients with major portosystemic shunts.
Materials and methods
Data sources and search strategy
The Preferred Reporting Items for Systematic Reviews
and Meta-Analyses statement approach was used to perform this
systematic review and meta-analysis (15). A medical librarian at the teaching
hospital conducted a comprehensive computerized search of relevant
literature in MEDLINE via PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.google.com/) and Scopus
(https://www.scopus.com/search/form.uri?display=basic)
to find the full-text articles that assessed the effects of shunt
embolization on refractory HE in patients with major portosystemic
shunts from inception until August 2024. Unpublished publications
were examined by searching the World Health Organization
International Clinical Trials Registry Platform (https://www.who.int/clinical-trials-registry-platform).
Auto-alerts were set up to track down recently published documents.
In addition, the reference lists of the papers were manually
searched to find other studies. The search was performed using the
key terms ‘hepatic encephalopathy’, ‘embolization’, and
‘portosystemic shunt’ and the following search strategy:
[TITLE-ABS-KEY (hepatic AND encephalopathy) AND TITLE-ABS-KEY
(embolization) AND TITLE-ABS-KEY (portosystemic AND shunt)] AND
[LIMIT-TO (DOCTYPE, ‘ar’)] AND [LIMIT-TO (LANGUAGE, ‘English’)].
The medical librarian only retrieved the studies and was not
involved in the subsequent steps of the study.
Study selection
Studies that met the following standards were
considered: i) Population: Adults (aged >18 years) with
confirmed liver cirrhosis and a history of HE; ii) interventions:
Embolization, independent of region, frequency, or duration; iii)
comparators: Placebo or other interventions; and iv) the primary
outcomes: The degree of HE or mental state change as determined by
the West-Heaven classification system. This grading system
distinguishes among four clinically distinct HE gradings. Patients
in grade I exhibit inattention and mild personality changes that
are primarily apparent to their family members. The most common
finding in grade II is disorientation for time when combined, for
instance, with inappropriate behavior and lethargy. Patients in
grade III are comatose but react to stimuli. They may also behave
strangely and are confused about where they are and what to do.
Patients in grade IV are unconscious (16); and (5) study design: Clinical trials [both
randomized and non-randomized (prospective or retrospective
cohorts)].
Additionally, and based on this system, there are
two different forms of refractory HE: Recurrent HE and persistent
HE. The hallmark of recurrent HE is recurrent episodes that happen
more than once within 6 months (16). When overt HE recurs, an ongoing
pattern of behavioral changes that persists and coexists with it is
referred to as persistent HE (16).
Grade ≥II was designated as overt HE (16).
The exclusion criteria were as follows: i) Studies
where the population was either pregnant or had congenital or
autoimmune liver diseases; ii) where no specific intervention or
comparator was mentioned; and iii) case series, case reports,
conference proceedings; or studies lacking the necessary data.
Data extraction and quality
assessment
After performing independent searches of all
eligible studies' titles and abstracts to identify any that may be
pertinent, two reviewers (JS and WL) skimmed through the full text
of those studies and collected the necessary data. Disagreements
among reviewers were resolved via discussions with a third reviewer
(JJ). Each chosen study's first author's name, publication year,
trial design, patient age, cirrhosis etiology, HE grades,
Child-Pugh (CP) score or classification, intervention, comparison,
and length of therapy were extracted. When data in articles were
not provided, it was attempted to contact the authors.
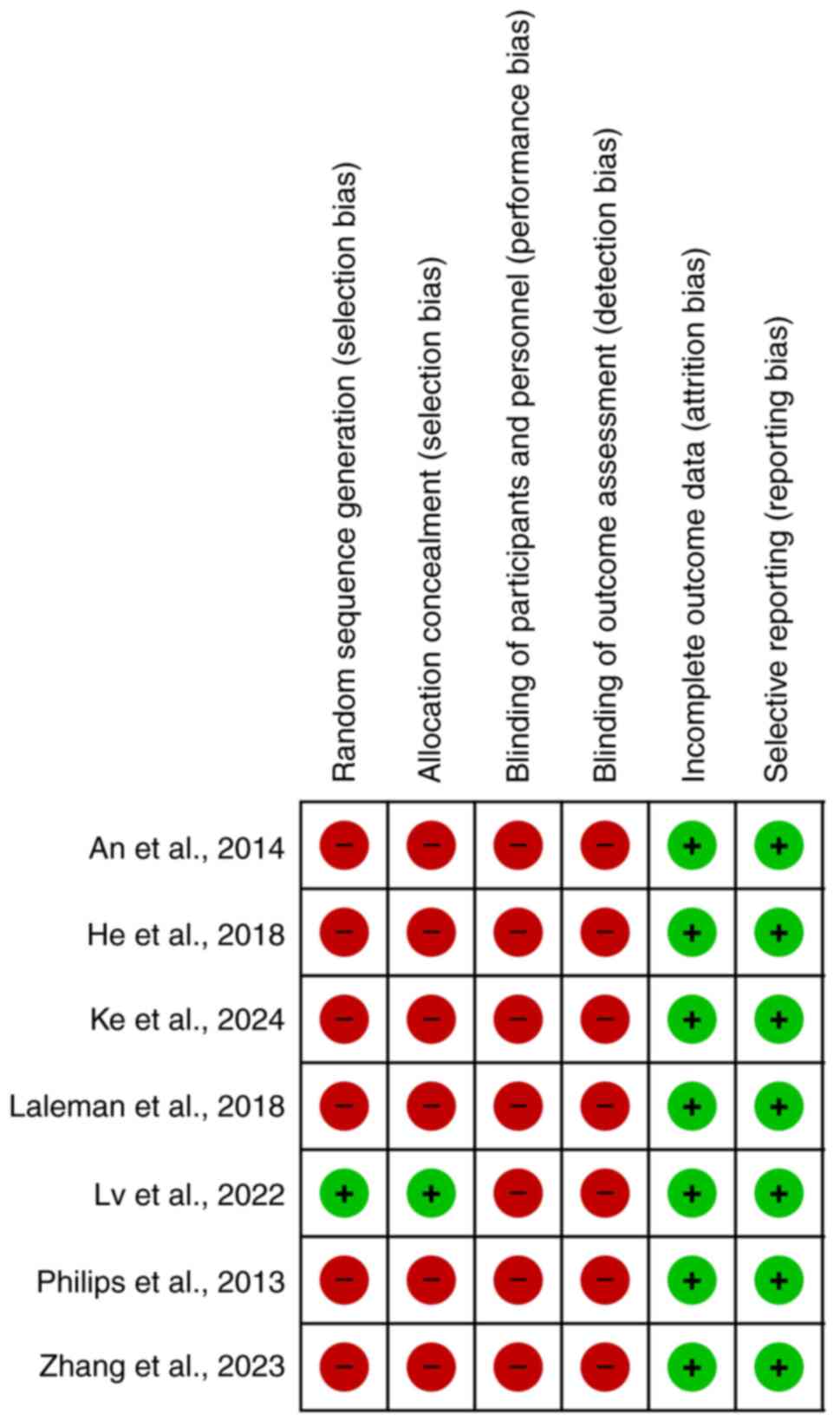
Using the Cochrane Risk of Bias Tool (17), the methodological quality of the
studies was assessed. It was determined whether an article had a
low, uncertain, or high quality for each item. The following areas
were covered in this assessment: Blinding of allocations, random
sequence generation, selective reporting, inadequate outcome data,
blinded outcome evaluation, blinding of participants and staff, and
other risks of bias. For a study to be categorized as having a low
risk of bias, it must have had a low grade across all domains. The
experiment's overall risk of bias was classified as unclear
whenever at least one area had the label ‘uncertain’. If at least
one domain demonstrated a high risk of bias, the trial's overall
risk was likewise considered high.
Statistical analysis
The data were analyzed using the Comprehensive
Meta-Analysis Software program, version 2.0 (Biostat). Dichotomous
factors were compared using odds ratios (OR). 95% confidence
intervals (CI) were provided for each reported outcome. The pooled
OR and 95% CI were estimated using the Mantel-Haenszel method for
dichotomous data. Data heterogeneity was evaluated using the
I2 test and was deemed high if
I2 was >50%. The random-effects model was used
to analyze the data due to their high heterogeneity
(I2>50%). Random-effects models are
advantageous when there is significant heterogeneity across
studies, as they provide a more realistic and generalizable
estimate of the overall effect size while accounting for the
variability in the underlying true effects (17). Trim and fill, Begg's analysis
Egger's regression, and funnel plot were employed to evaluate
publication bias in this body of literature. A funnel plot visually
represents the effect sizes of individual studies against their
precision, ideally forming a symmetrical shape; asymmetry suggests
potential bias, often due to smaller studies with non-significant
results being unpublished. The trim-and-fill method addresses this
issue by first trimming away studies that contribute to asymmetry
and then filling in hypothetical missing studies to restore
symmetry, thereby providing an adjusted overall effect estimate.
Furthermore, Egger's regression test assesses funnel plot asymmetry
by performing a weighted regression of the effect sizes on their
standard errors. A significant intercept in this regression
indicates potential publication bias, suggesting that smaller
studies with non-significant results are missing from the analysis.
Conversely, Begg's analysis, specifically the Begg and Mazumdar
rank correlation test, evaluates the correlation between the ranks
of effect sizes and their variances. A significant correlation
suggests that smaller studies report different results than larger
ones, which can also indicate publication bias (18).
Leave-one-out sensitivity analysis is a robust
technique used in meta-analysis to evaluate the influence of
individual studies on the overall effect size. This method
systematically excludes one study at a time from the analysis and
recalculates the overall effect size and heterogeneity for each
iteration. By doing so, it is identified whether any single study
disproportionately affects the results. Significant changes in the
effect size or a marked decrease in heterogeneity upon exclusion
indicate that the omitted study may be an influential outlier
(19). P<0.05 was utilized as
the threshold for statistical significance.
Results
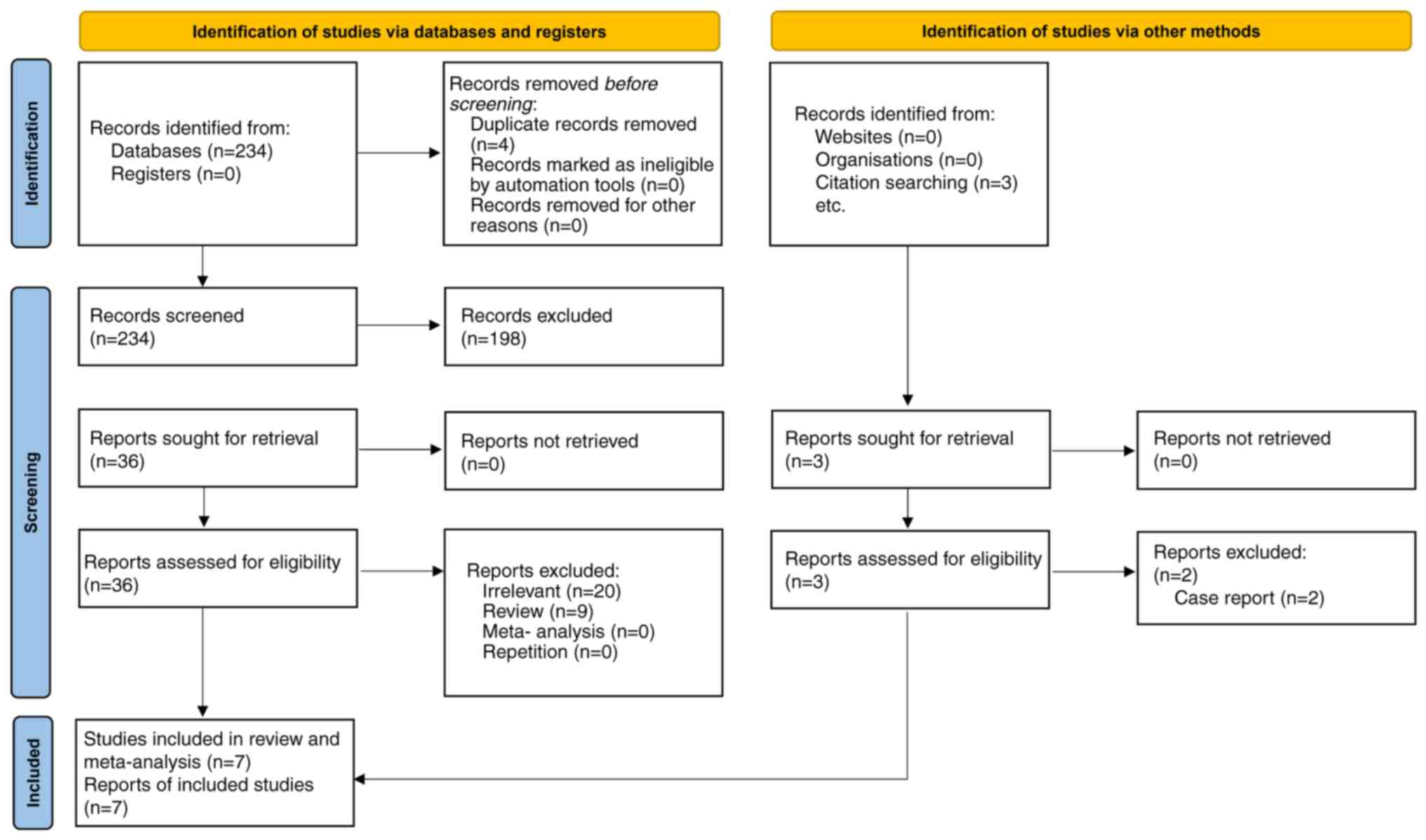
Study selection
During the initial search of the mentioned
databases, 234 studies were found. After four duplicate articles
were excluded, 230 papers were evaluated based on their titles and
abstracts. Accordingly, 128 publications (case reports, case
series, correspondences, review articles, in vitro
experiments or animal studies) were excluded. Thus, 36 publications
were retained for full-text examination. Following a thorough
assessment, three descriptive studies lacking comparative analysis
were excluded.
Furthermore, 29 other papers were excluded for
failing to disclose pertinent data. Thus, six studies fulfilled the
inclusion and not the exclusion criteria (11-14,20,21).
Another study was identified in a manual search and added (22). Hence, seven studies were included in
this meta-analysis. Only one study was a randomized clinical trial
(RCT) (14), while the remaining
studies were retrospective cohort studies. A total of 254 patients
were included in the present meta-analysis. The literature review
process is depicted in Fig. 1.
Table I presents the
characteristics of the included studies.
 | Table ICharacteristics of included studies
in the meta-analysis. |
Table I
Characteristics of included studies
in the meta-analysis.
| Author(s),
year | Design | No. of
patients | Age, years | CTP score | MELD score | No. of HE
episodes | Maximum HE
grade | Outcome(s) | (Refs.) |
|---|
| An et al,
2016 | Retrospective
cohort | 17 | 62
(56-65.5)a | 9
(8-10)b | 13
(11-15)a | 1-2 (n=9); ≥3
(n=8) | II (n=6); III-IV
(n=11) | The 2-year HE
recurrence rate in the embolization group was considerably lower
than in the control group (39.9 vs. 79.9%, P=0.02) | (12) |
| He et al,
2018 | Retrospective
cohort | 44 | 51.2±11.6 | 7.2±1.9 | 11.2±4.0 | 15 | III-IV (n=5) | The SPSS group had
a higher risk of HE compared with the SPSS + ES groups | (13) |
| Laleman et
al, 2013 | Retrospective
cohort | 37 | 61.0±2.0 | 7.9±0.3 | 13.2±0.9 | - | IV | Improved severity
of the worst HE episode after embolization in three-quarters of the
patients | (11) |
| Lv et al,
2022 | RCT | 27 | 48.0
(43-56.5)a | 7.0
(6.0-8.0)b | 10.0
(8.0-13.0)b | 1 | - | Concurrent large
SPSS embolization decreased the chance for overt HE in cirrhotic
patients receiving TIPS for variceal bleeding without worsening
other complications | (14) |
| Philips et
al, 2013 | Retrospective
cohort | 21 | 56.6±10.6 | 9.6±1.9 | 15.7±4.1 | - | 1-2 (n=15); ≥3
(n=6) | Recurrent and
persistent HE markedly improved in the short (n=20, 1 to 3 months),
intermediate (n=12, 3 to 6 months) and long (n=7, 6 to 9 months)
follow-up | (20) |
| Zhang et al,
2022 | Retrospective
cohort | 13 | 57.5±10.0 | 11.0 | 12.0 | - | - | When treating
SPSS-induced refractory HE, the 6-month mortality decreased
following SESV (in comparison to ES) | (21) |
| Ke et al,
2024 | Retrospective
cohort | 95 | 56.4±9.5 | 9.0±2.0 | 13.0
(11.0-16.0)a | | II (n=30); III-IV
(n=4) | Embolization seems
to be a safe therapeutic approach for individuals with cirrhosis
who have refractory HE linked to large SPSS | (22) |
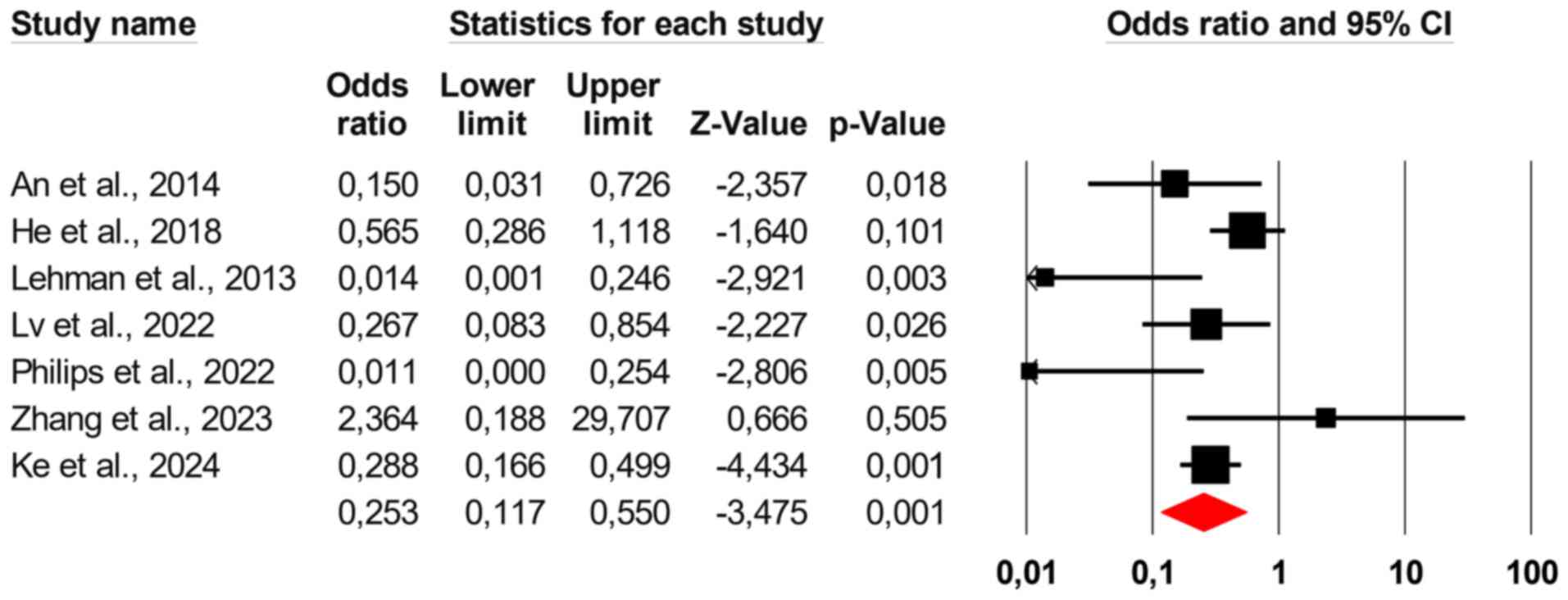
Effect of shunt embolization on
portosystemic refractory HE
It was found that shunt embolization significantly
reduced HE recurrence in patients with portosystemic shunts due to
liver cirrhosis (the pooled OR=0.253, 95% CI: 0.117-0.550; Fig. 2). The overall heterogeneity of the
data was found to be high (I2=60.52% and
P=0.001).
Safety
An et al (12) reported no major procedure-related
complications in the embolization group. Patients with either a
Model for End-stage Liver Disease (MELD) score ≥15 or
hepatocellular carcinoma at baseline experienced all severe
sequelae throughout the follow-up. Two of these six patients had
persistent hepatorenal syndrome and all six patients died within a
year following embolization. These patients complained of either
mild stomach bloating or fever, which all disappeared a week after
embolization with conservative therapy. Laleman et al
(11) noticed one major
complication. This study reported eight early procedure-related
complications, seven minor and symptomatically addressed (two
episodes of self-limiting fever, one contrast-induced nephropathy,
three localized hematomas at the puncture site, and a cutaneous
infection at the puncture site). Throughout the monitoring period,
the authors found no significant worsening of portal hypertension
(11). In the study by Philips
et al (20) (n=21), the
procedure caused the following immediate problems: Two patients
(9.52%) experienced negative outcomes that were specifically
connected to the surgery. One of these patients experienced a local
site hematoma of grade 1 Common Terminology Criteria for Adverse
Events (CTCAE) event, which was treated conservatively; the other
patient, however, experienced events of grade 5 CTCAE
(hemoperitoneum and multiple organ failures), which resulted in
death within 24 h of the procedure (20). Ke et al (22) found no early procedure-related
complications in the embolization group. Throughout the follow-up,
the two groups exhibited no significant difference in the incidence
of long-term complications. Only two patients (or 5.9%) required
additional embolization due to postoperative follow-up imaging
revealing recanalization of the initial embolic shunt (22).
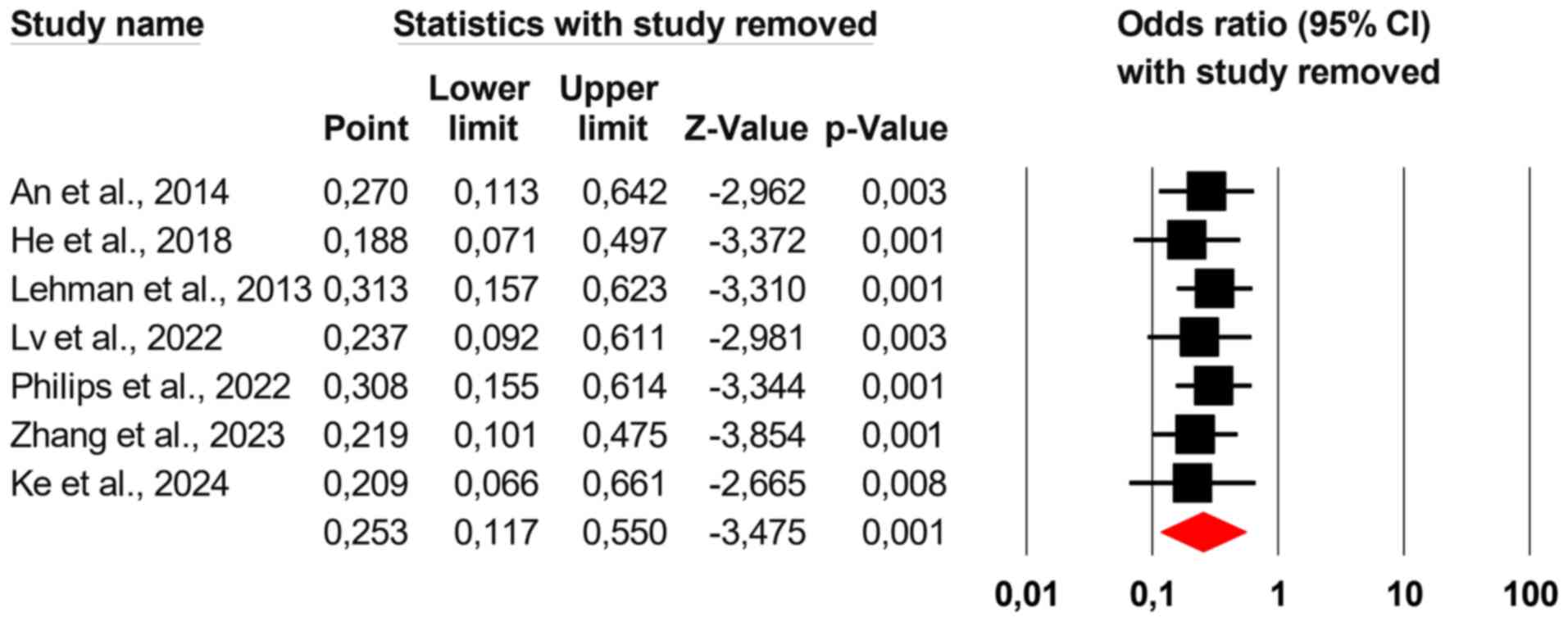
Sensitivity analysis
To test the robustness of the findings, an iterative
leave-one-out sensitivity analysis was used, removing one study at
a time and recalculating the overall OR. This analysis showed
consistent results, proving that leaving out any one study would
not significantly change the study's overall conclusions.
Consequently, it is improbable that a single study will have a
major impact on the OR in either direction (Fig. 3).
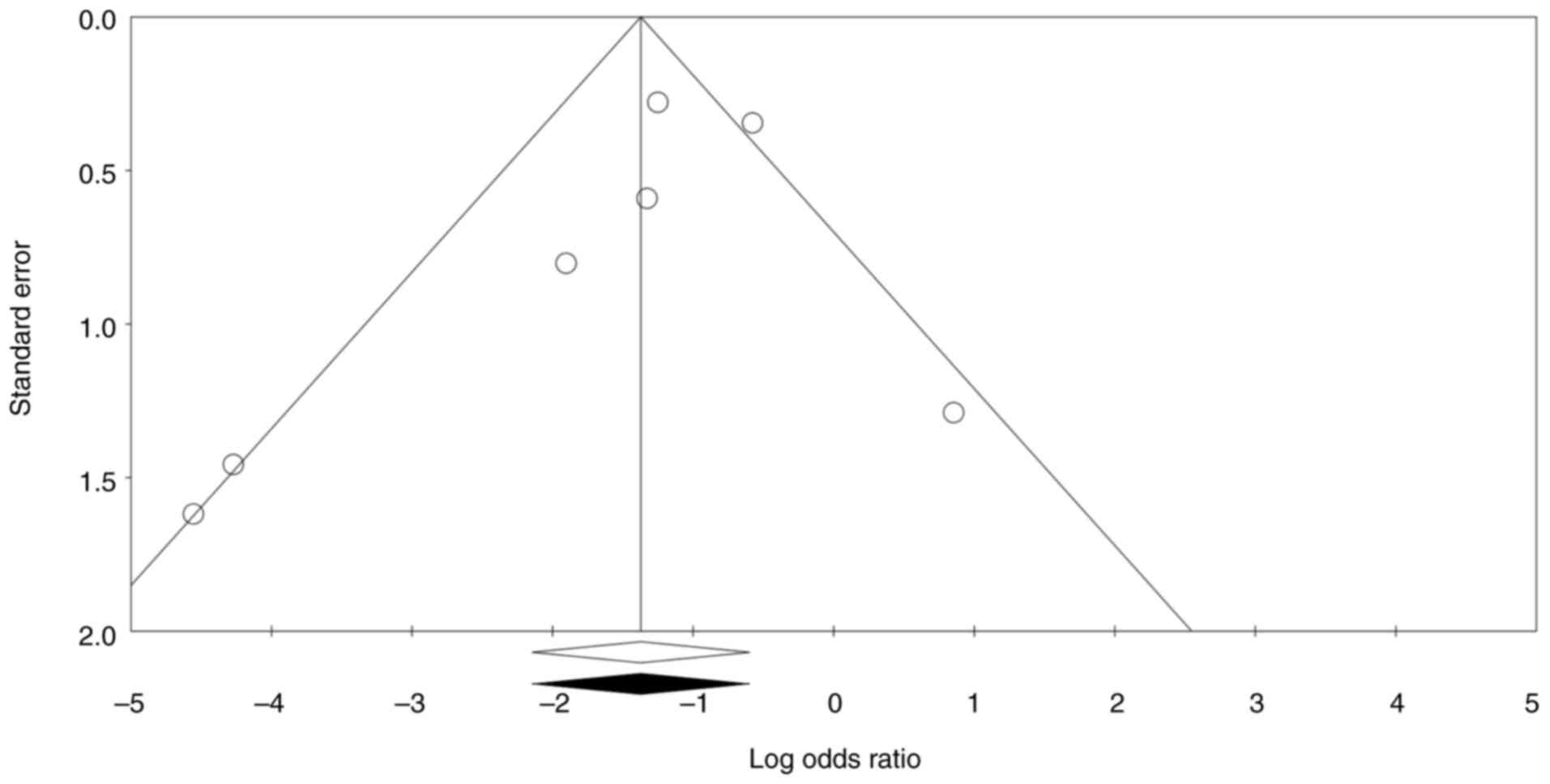
Publication bias and study
quality
Publication bias was assessed using funnel plot and
trim and fill analysis. A funnel plot plots the effect size of
individual studies against a measure of their precision, typically
the standard error. If the funnel plot is asymmetrical, it may
indicate potential publication bias, where studies with
non-significant results are less likely to be published. This can
lead to overestimating the intervention effect in the meta-analysis
(23). The funnel plot was
reasonably symmetrical and no study was trimmed to either side of
the mean.
Begg's and Egger's tests are statistical methods
that help determine whether the results of studies are
systematically skewed due to selective publication (23). Begg's test (P=0.229), Egger's test
(P=0.273), and the funnel plot showed no significant risk of
publication bias (Fig. 4). Quality
appraisal showed that all included studies were of low quality,
except the study by Lv et al (14), in which the patients were randomized
into two groups of intervention and control (Fig. 5).
Discussion
From asymptomatic portal hypertension to recurrent
and refractory HE, the portosystemic shunt syndrome comprises a
spectrum of clinical symptoms that, in patients with cirrhosis and
accompanying large portosystemic shunts, eventually lead to
progressive liver failure. The splenorenal, gastrorenal, and
dilated paraumbilical veins are frequently seen in cirrhosis and
can all present with recurrent or refractory HE (24). After ruling out other neurological
conditions, HE in patients with cirrhosis with periods of
persistent cognitive impairments and a poor MELD score may merit
examination for significant spontaneous portosystemic shunt
(25).
Large shunts with a diameter of >8 mm can be
embolized using several different methods. The proposed
portosystemic shunt embolization aims to enhance portal blood flow
to the liver, improving liver metabolism and function and
decreasing brain exposure to neurotoxic compounds (26-28).
The surgical closure of portosystemic shunts has been proven to be
beneficial in correcting chronic persistent hepatic encephalopathy,
but it is also linked with significant mortality (29). It is also a concern that the
embolization surgery worsens portal hypertension (30).
A promising series of five patients with chronic
portosystemic encephalopathy who were chosen for radiological
interventional therapy was initially described by Uflacker et
al (31) in 1987. Two patients
were subjected to direct splenic artery embolization by the
authors, two patients underwent progressive venous shunt occlusion
through a transhepatic route utilizing steel coils and one patient
underwent surgical mesocaval shunt occlusion using a ‘home-made’
big detachable balloon. One patient died as a direct result of
increased portal pressure and subsequent intra-abdominal bleeding.
However, permanent encephalopathy control was achieved in four
patients with a longer life (8-37 months) (31). In another study by An et al
(12) on cirrhotic individuals with
recurrent HE and marginally retained liver function, embolization
of a large spontaneous portosystemic shunt enhanced survival and
liver function and reduced the incidence of HE. He et al
(13) confirmed that a pre-existing
big portosystemic shunt syndrome was linked to a higher risk of HE
among cirrhotic patients with TIPS.
On the other hand, portosystemic shunt embolization
lowered that risk. There was no conclusive link between the
existence or embolization of the portosystemic shunt and death,
shunt malfunction or portosystemic shunt syndrome clinical
recurrence (13). In a multicenter
European cohort study by Laleman et al (11), the efficiency and safety of
embolizing portosystemic shunts were supported, assuming there was
sufficient functional liver reserve. In a randomized clinical trial
by Lv et al (14), it was
found that concurrent large portosystemic shunt embolization
decreased the risk for overt HE without worsening adverse events in
patients with cirrhosis who were receiving TIPS for variceal
bleeding. Therefore, they suggested that concurrent large
portosystemic shunt embolization should be considered to prevent
TIPS-related HE (14). These
findings were replicated in the studies by Philips et al
(20) and Zhang et al
(21). The findings from these
studies were in line with the results of the present meta-analysis,
which showed a decrease in the incidence of HE after shunt
embolization in patients with chronic portosystemic shunts.
While portosystemic shunt embolization has already
been clinically established for numerous years, the present
meta-analysis aims to systematically synthesize the existing
literature, providing a comprehensive overview of the effectiveness
of this intervention across various studies and allowing for a
quantitative assessment of the treatment's effectiveness that may
not be evident from individual reports. The present meta-analysis
confirms the effectiveness of portosystemic shunt embolization and
highlights the variability in methodologies and patient populations
across studies. This variability underscores the need for a
systematic review to evaluate the robustness of the existing
evidence and identify gaps in the current understanding of the
procedure's effectiveness.
Of note, the present study had several limitations
and areas for improvement. First, the embolization methods and
embolic agents used in the included publications varied. The degree
of embolization and choice of embolic agents may impact the
recurrence of HE. The sustained variceal perfusion through
collaterals may be enabled by proximal embolization of the afferent
arteries employing coils alone. As they aid in occluding or causing
thrombosis of the variceal cavity, liquid agents such as
cyanoacrylate and ethanol may be used in distal embolization, which
may be more successful. To avoid the development of new collateral
veins and reperfusion, a combination of coils and liquid agents may
be advised to generate a long-acting embolization of proximal and
peripheral collateral veins (32).
Second, each included trial had a distinct form of varices or
collateral vessels. The effectiveness of variceal embolization may
thus differ depending on the structure and hemodynamics of the
varices. Third, the papers considered varied in their indications
of variceal embolization. Fourth, even though a thorough literature
search was performed using three databases without any publication
language restrictions, only seven articles were included in the
present meta-analysis. When the number of included studies is low
(e.g., <5), the overall sample size needs to be bigger, limiting
the statistical power to detect effects of interest (33). Low power increases the risk of
false-negative results, where the meta-analysis fails to find an
effect even if one truly exists (34). Fifth, only one study was of good
quality and the remaining studies had a high risk of
quality-related bias. Studies with poor design or execution may
yield results that do not accurately reflect the true effect being
measured, thereby misleading researchers and practitioners about
the efficacy of an intervention (34). Here, the results should be
interpreted with caution. The findings may need to be more
generalizable to broader populations or real-world settings, as the
limitations of the included studies may heavily influence them.
This can lead to erroneous conclusions about the effectiveness of
treatments or interventions in practice (35). Despite the low quality of certain
included studies, the meta-analysis employed robust statistical
methods, including random-effects modeling and assessment of
publication bias through funnel plots and Egger's test. These
methodologies help mitigate the impact of individual study
limitations and provide a more reliable pooled estimate of the
treatment effect. Sixth, in the present study, mostly observational
(and only 1 RCT) studies were included. However, the inclusion of
observational studies can provide valuable insights, particularly
in fields where RCTs are limited or unavailable. Finally, the
majority of the patients had cirrhosis with a viral origin; as a
result, no firm conclusions can be made for individuals with other
chronic liver illnesses. While the limitation in the
generalizability of the present findings is acknowledged, it is
important to note that the pathophysiology of HE is partially
dependent on the specific etiology of cirrhosis. The development of
HE is primarily driven by the presence of portosystemic shunts and
the accumulation of neurotoxins, which can occur in various types
of cirrhosis (36).
In conclusion, the present meta-analysis of seven
trials suggests that shunt embolization following TIPS or other
portosystemic shunts may help reduce the frequency of HE. However,
it is essential to interpret these findings cautiously due to the
limitations associated with the low quality of the included studies
and the small sample size. The variability in embolic agents, types
of varices, and methods of variceal embolization across the studies
further complicates the interpretation of the results. Future
research should focus on conducting higher-quality RCTs that can
provide more definitive conclusions to strengthen the evidence
base. Additionally, exploring the long-term outcomes of shunt
embolization would be valuable in understanding its effectiveness
and safety in HE management.
Acknowledgements
We extend our gratitude to Dr Hongyuan Yang and Dr
Xiaofei Ye, attending physicians at the Cancer Center of the Fifth
Affiliated Hospital of Wenzhou Medical University (Lishui, China),
for their invaluable assistance in the comprehensive literature
search for this study. Their expertise in identifying and accessing
relevant scientific articles significantly contributed to the
foundation of this research. We greatly appreciate their dedication
and support throughout the research process.
Funding
Funding: No funding was received.
Availability of data and materials
The pooled dataset prepared and used in the present
study may be requested from the corresponding author.
Authors' contributions
JS, WL, SY, FW, ZZ and JJ developed the study
protocol. JS and WL were responsible for data collection. SY and FW
analyzed the data. JS and JJ checked and confirmed the authenticity
of the raw data (pertaining to the pooled dataset). All authors
contributed to the writing of the manuscript and read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rose CF, Amodio P, Bajaj JS, Dhiman RK,
Montagnese S, Taylor-Robinson SD, Vilstrup H and Jalan R: Hepatic
encephalopathy: Novel insights into classification, pathophysiology
and therapy. J Hepatol. 73:1526–1547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hirode G, Vittinghoff E and Wong RJ:
Increasing burden of hepatic encephalopathy among hospitalized
adults: An analysis of the 2010-2014 National Inpatient Sample. Dig
Dis Sci. 64:1448–1457. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Felipo V: Hepatic encephalopathy: Effects
of liver failure on brain function. Nat Rev Neurosci. 14:851–858.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Poh Z and Chang PE: A current review of
the diagnostic and treatment strategies of hepatic encephalopathy.
Int J Hepatol. 2012(480309)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Riggio O, Nardelli S, Moscucci F, Pasquale
C, Ridola L and Merli M: Hepatic encephalopathy after transjugular
intrahepatic portosystemic shunt. Clin Liver Dis. 16:133–146.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pereira K, Carrion AF, Martin P, Vaheesan
K, Salsamendi J, Doshi M and Yrizarry JM: Current diagnosis and
management of post-transjugular intrahepatic portosystemic shunt
refractory hepatic encephalopathy. Liver Int. 35:2487–2494.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Romero-Gomez M, Jover M, Diaz-Gomez D, de
Teran LC, Rodrigo R, Camacho I, Echevarria M, Felipo V and Bautista
JD: Phosphate-activated glutaminase activity is enhanced in brain,
intestine and kidneys of rats following portacaval anastomosis.
World J Gastroenterol. 12:2406–2411. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Perarnau JM, Le Gouge A, Nicolas C,
d'Alteroche L, Borentain P, Saliba F, Minello A, Anty R,
Chagneau-Derrode C, Bernard PH, et al: Covered vs. uncovered stents
for transjugular intrahepatic portosystemic shunt: A randomized
controlled trial. J Hepatol. 60:962–968. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bureau C, Garcia-Pagan JC, Otal P,
Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM,
Abraldes JG, Bouchard L, et al: Improved clinical outcome using
polytetrafluoroethylene-coated stents for TIPS: Results of a
randomized study. Gastroenterology. 126:469–475. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Riggio O, Angeloni S and Ridola L: Hepatic
encephalopathy after transjugular intrahepatic portosystemic shunt:
Still a major problem. Hepatology. 51:2237–2238. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Laleman W, Simon-Talero M, Maleux G, Perez
M, Ameloot K, Soriano G, Villalba J, Garcia-Pagan JC, Barrufet M,
Jalan R, et al: Embolization of large spontaneous portosystemic
shunts for refractory hepatic encephalopathy: A multicenter survey
on safety and efficacy. Hepatology. 57:2448–2457. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
An J, Kim KW, Han S, Lee J and Lim YS:
Improvement in survival associated with embolisation of spontaneous
portosystemic shunt in patients with recurrent hepatic
encephalopathy. Aliment Pharmacol Ther. 39:1418–1426.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He C, Lv Y, Wang Z, Guo W, Tie J, Li K,
Niu J, Zuo L, Yu T, Yuan X, et al: Association between non-variceal
spontaneous portosystemic shunt and outcomes after TIPS in
cirrhosis. Dig Liver Dis. 50:1315–1323. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv Y, Chen H, Luo B, Bai W, Li K, Wang Z,
Xia D, Guo W, Wang Q, Li X, et al: Concurrent large spontaneous
portosystemic shunt embolization for the prevention of overt
hepatic encephalopathy after TIPS: A randomized controlled trial.
Hepatology. 76:676–688. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vilstrup H, Amodio P, Bajaj J, Cordoba J,
Ferenci P, Mullen KD, Weissenborn K and Wong P: Hepatic
encephalopathy in chronic liver disease: 2014 practice guideline by
the American association for the study of liver diseases and the
European association for the study of the liver. Hepatology.
60:715–735. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane handbook for
systematic reviews of interventions. 2nd edition. John Wiley &
Sons, Chichester, 2019.
|
|
18
|
Luo C, Marks-Anglin A, Duan R, Lin L, Hong
C, Chu H and Chen Y: Accounting for publication bias using a
bivariate trim and fill meta-analysis procedure. Stat Med.
41:3466–3478. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Greenhouse JB and Iyengar S: Sensitivity
analysis and diagnostics. In: The handbook of research synthesis
and meta-analysis. Cooper H, Hedges LV and Valentine JC (eds). 2nd
edition. Russell Sage Foundation, pp 417-433, 2009.
|
|
20
|
Philips CA, Kumar L and Augustine P: Shunt
occlusion for portosystemic shunt syndrome related refractory
hepatic encephalopathy-A single-center experience in 21 patients
from Kerala. Indian J Gastroenterol. 36:411–419. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Wei B, Wang Z, Tong H and Wu H:
Treatment of refractory hepatic encephalopathy induced by
spontaneous portosystemic shunt: Selective splenic vein
embolization versus shunt embolization. Dig Liver Dis. 55:381–386.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ke Q, He J, Cai L, Lei X, Huang X, Li L,
Liu J and Guo W: Safety and efficacy of interventional embolization
in cirrhotic patients with refractory hepatic encephalopathy
associated with spontaneous portosystemic shunts. Sci Rep.
14(14848)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions. The Cochrane
Collaboration, 2008.
|
|
24
|
Philips CA, Rajesh S, Augustine P,
Padsalgi G and Ahamed R: Portosystemic shunts and refractory
hepatic encephalopathy: Patient selection and current options.
Hepat Med. 11:23–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Simón-Talero M, Roccarina D, Martínez J,
Lampichler K, Baiges A, Low G, Llop E, Praktiknjo M, Maurer MH,
Zipprich A, et al: Association between portosystemic shunts and
increased complications and mortality in patients with cirrhosis.
Gastroenterology. 154:1694–1705.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chikamori F, Kuniyoshi N, Shibuya S and
Takase Y: Transjugular retrograde obliteration for chronic
portosystemic encephalopathy. Abdom Imaging. 25:567–571.
2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cardoso JE, Gautreau C, Jeyaraj PR,
Patrzalek D, Cherruau B, Vaubourdolle M, Legendre C, Wroblewski T
and Houssin D: Augmentation of portal blood flow improves function
of human cirrhotic liver. Hepatology. 19:375–380. 1994.PubMed/NCBI
|
|
28
|
Sakurabayashi S, Sezai S, Yamamoto Y,
Hirano M and Oka H: Embolization of portal-systemic shunts in
cirrhotic patients with chronic recurrent hepatic encephalopathy.
Cardiovasc Intervent Radiol. 20:120–124. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carrión JA, Bellot P, Colmenero J and
Garcia Pagan JC: Large spontaneous splenorenal shunt as a cause of
chronic hepatic encephalopathy. J Hepatol. 40(868)2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arabi M, Almat'hami A, Qadri M and
Albenmousa A: Shunt-Preserving splenic vein embolization using a
vascular plug for treatment of hepatic encephalopathy. J Vasc
Interv Radiol. 26:1416–1419. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Uflacker R, Silva Ade O, d'Albuquerque LA,
Piske RL and Mourão GS: Chronic portosystemic encephalopathy:
Embolization of portosystemic shunts. Radiology. 165:721–725.
1987.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qi X, Liu L, Bai M, Chen H, Wang J, Yang
Z, Han G and Fan D: Transjugular intrahepatic portosystemic shunt
in combination with or without variceal embolization for the
prevention of variceal rebleeding: A meta-analysis. J Gastroenterol
Hepatol. 29:688–696. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pigott T: Advances in meta-analysis.
Springer Science & Business Media, 2012.
|
|
34
|
Flather MD, Farkouh ME, Pogue JM and Yusuf
S: Strengths and limitations of meta-analysis: Larger studies may
be more reliable. Control Clin Trials. 18:568–579; discussion
661-6. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Walker E, Hernandez AV and Kattan MW:
Meta-analysis: Its strengths and limitations. Cleve Clin J Med.
75:431–439. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jagdish RK, Roy A, Kumar K, Premkumar M,
Sharma M, Rao PN, Reddy DN and Kulkarni AV: Pathophysiology and
management of liver cirrhosis: From portal hypertension to
acute-on-chronic liver failure. Front Med (Lausanne).
10(1060073)2023.PubMed/NCBI View Article : Google Scholar
|