Introduction
Salivary gland secretory carcinoma (SC), previously
referred to as mammary analogue SC, is comparable with breast SC
and is typically immunopositive for the S100 protein and
mammaglobin (1). The majority of SC
cases (>90%) exhibit translocation t(12;15)(p13;q25), resulting
in the ETV6::NTRK3 gene fusion. The gene ETS variant 6 (ETV6)
located on the short arm of chromosome 12 encodes an ETS-domain
transcription factor and the neurotrophic tyrosine kinase receptor
3 (NTRK3) located on the long arm of chromosome 5 encodes
tropomyosin receptor kinase C (TRKC). In tumors with an ETV6-NTRK3
fusion, the helix-loop-helix protein dimerization domain of ETV6 is
fused with the kinase domain of TRKC, resulting in constitutive
activation of mitogen-activated protein kinase and phosphorylating
pathways and potent oncogenic transformation (2). Notably, a small number of cases (2-5%)
exhibit ETV6 rearrangement with non-NTRK3 partners (ETV6::X
fusions), including rearranged during transfection (RET), MET and
mastermind like transcriptional coactivator 3 (3,4). RET
is a single-pass receptor tyrosine kinase, and RET fusions, which
are oncogenic drivers in certain tumor types, result in cell
proliferation and survival (5). In
salivary glands, SC with an ETV6::NTRK3 fusion typically presents
as circumscribed, cystic lesions with low-grade features, leading
to a favorable clinical outcome in patients (1,6). In
individuals with the ETV6-X fusion, infiltrative growth and
aggressiveness of the tumor are observed (7,8).
High-grade transformation (HGT), which is associated with
aggressive clinical behaviour, such as cervical lymph node or
distant metastasis, is rarely seen in salivary gland SC (6). ETV6::RET translocation is prone to
occur in the HGT SC, compared with the conventional
ETV6::NTRK3(9). These findings
suggest that the prognosis of patients with SC varies depending on
the fusion partner with ETV6. Of note, in acute myeloid leukemia
(AML), ETV6::AML1 fusions are associated with favorable patient
outcomes, and homeobox HB9::ETV6 fusions are associated with poor
patient outcomes (10).
The recommended treatment for SC ranges from simple
excision to radical resection (1),
and for patients with SC presenting with locally advanced,
recurrent or metastatic disease, precision medical treatment is
required. The administration of NTRK inhibitors shows a favorable
response in SC with ETV6::NTRK3 (11,12),
while SC with ETV6::RET shows no response to the drug and RET
kinase inhibitors may be a promising treatment option (13). Taken together, the confirmation of
the ETV6-partner gene is essential for predicting the prognosis and
selecting an appropriate therapeutic course for SC. The present
study reported a case of salivary gland SC with an ETV6::RET fusion
and aimed to review comparable cases to further elucidate the
clinicopathological features of this disease.
Case report
Case presentation
A 25-year-old male patient presented to Osaka
Medical and Pharmaceutical University Hospital (Osaka, Japan) in
June 2023 with symptoms of pain and firm left preauricular nodules
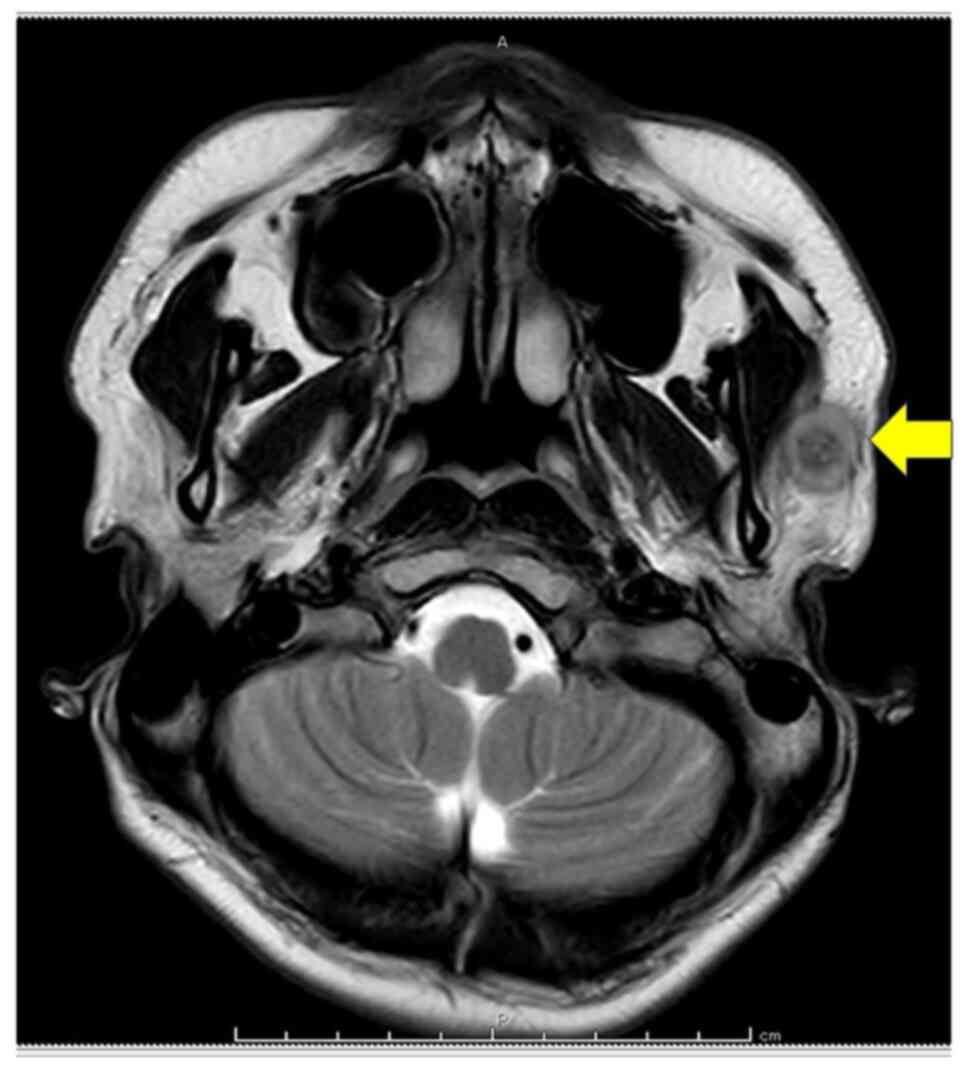
that had persisted for six months. Magnetic resonance imaging of
the neck showed a mass in the lower portion of the left parotid
gland (Fig. 1). Following
superficial parotidectomy and radiotherapy, no medication was
administered and semiannual follow-up was continued. The patient
has remained asymptomatic for one year and three months.
Pathology
Macroscopic examination showed a firm, solid tumor
measuring 1.8 cm, which was ill-defined and without encapsulation.
The tissue was fixed in 10% neutral buffered formalin overnight at
room temperature (RT) and embedded in paraffin wax for H&E and
immunohistochemical staining. Sections of 4 µm thickness from the
paraffin block were dewaxed and rehydrated, and they were stained
with hematoxylin for 5 min and eosin for 1 min at RT. The stained
sections were examined under a light microscope (BX53; Olympus
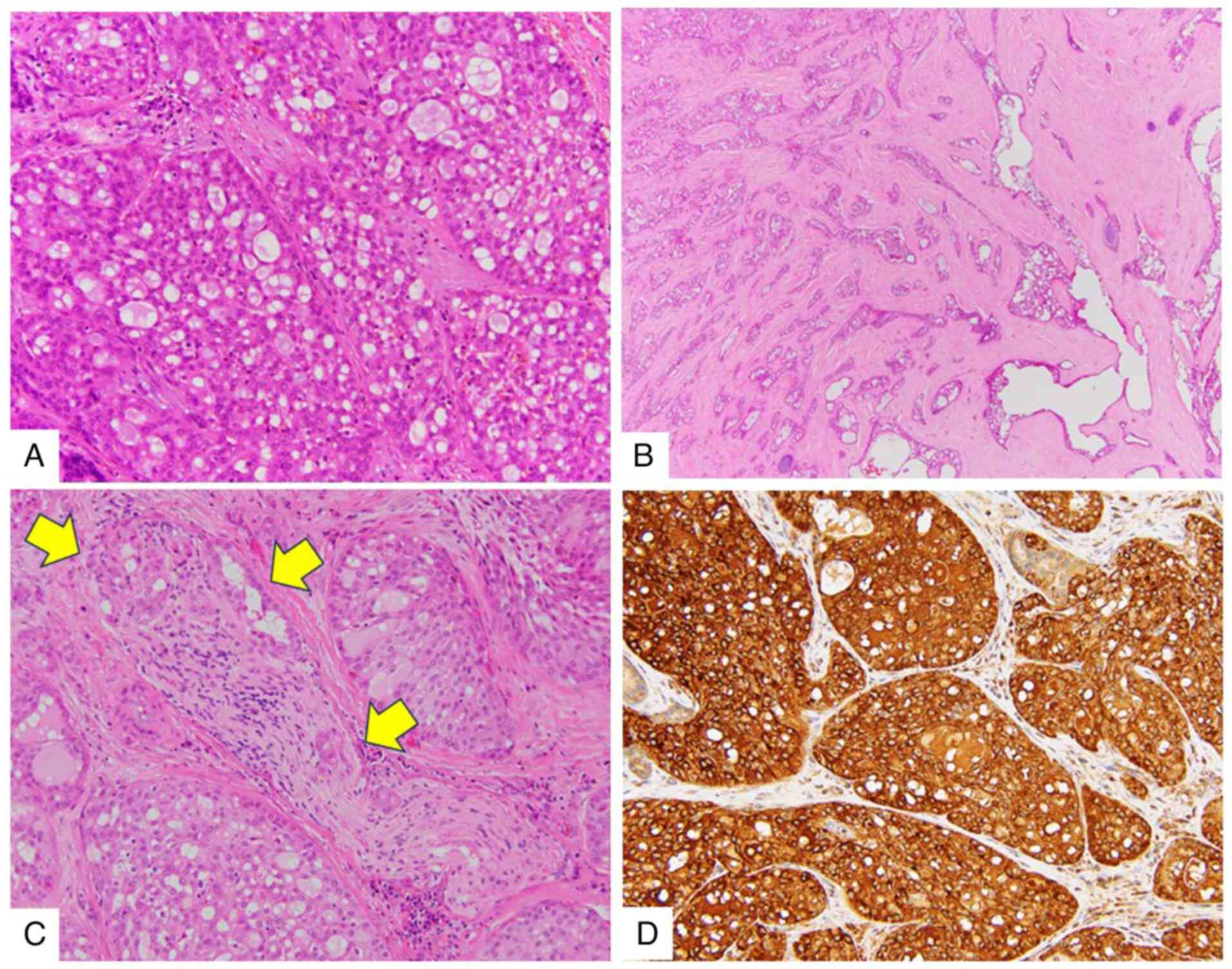
Corp.). Histological analysis showed a lobulated growth pattern,
characterized by microcystic and follicular structures with
colloid-like secretions. In addition, eosinophilic and occasionally
vacuolated cytoplasm were observed (Fig. 2A). Nuclei were oval to round with a
single nucleolus, demonstrating mild pleomorphism. There was no
evidence of mitotic figures or necrosis. Central tumor regions
showed macrocystic and trabecular structures in a prominent
hyalinized sclerotic stroma (Fig.
2B). Tumor infiltration extended into surrounding connective
tissue, muscle and nerve (Fig. 2C).
For immunohistochemical analysis, an automated immunostaining
device, the VENTANA BenchMark ULTRA (Roche Tissue Diagnostics) was
used, and the procedures including antigen retrieval, blocking,
washing and detection using secondary antibodies followed the
manufacturer's instructions. In brief, before staining, to block
endogenous peroxidases, the immunohistochemistry device-dedicated
reagent (UltraView DAB Universal; Roche Tissue Diagnostics) was
used. In the deparaffinization process, EZ prep (Roche Tissue
Diagnostics) was used. The stained sections were observed under a
light microscope (BX53; Olympus Corp.). Immunohistochemistry showed
that tumor cells were positive for S100 protein (cat. no.
518-110109) (Fig. S1A),
mammaglobin (cat. no. 518-111380) (Fig.
2D) and GATA binding protein 3 (cat. no. 518-111953) (Fig. S1B). The tumor cells were negative
for DOG1 (cat. no. 518-110789). The Ki-67 (cat. no. 518-102456)
labelling index was maximally 8% (Fig.
S1C). All antibodies were from Roche Tissue Diagnostics and
prediluted.
Molecular genetic analysis
Reverse transcription (RT)-PCR was performed using
paraffin-embedded tissue. Total RNA was extracted and the cDNA
samples were then subjected to PCR as previously described
(1,3). The primer sequences are shown in
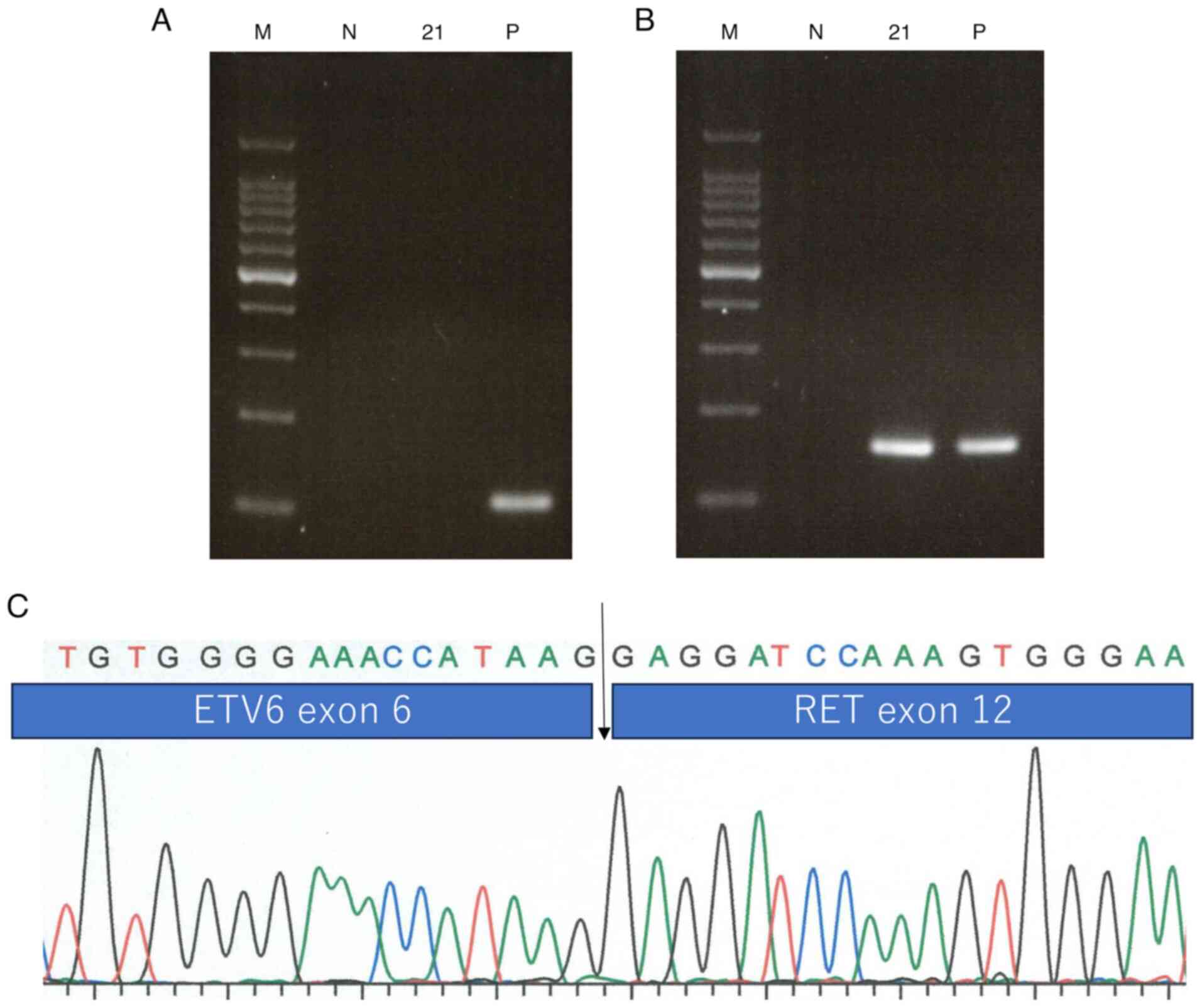
Table I. The RT-PCR results
demonstrated the presence of ETV6::RET fusion and the absence of an
ETV6::NTRK3 fusion (Fig. 3A and
B). The ETV6::RET fusion was
confirmed following direct sequencing of the PCR fragment using a
Big Dye Terminator Sequence kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) (Fig. 3C). The
analysis was performed according to the manufacturer's
instructions.
 | Table ISequences of primers for PCR. |
Table I
Sequences of primers for PCR.
| ETV6::NTRK3 |
| Forward |
5'-ACCACATCATGGTCTCTGTCTC CC-3' |
| Reverse | 5'-CAGTTCTCGCTTCAGCAC
GATG-3' |
| ETV6::RET |
| Forward |
5'-CGATGGGAGGACAAAGAATC-3' |
| Reverse |
5'-GACCACTTTTCCAAATTCGCC-3' |
Literature review
Literature analysis was performed using PubMed
database (https://pubmed.ncbi.nlm.nih.gov/) using the key words
‘secretory carcinoma’, ‘salivary gland’ and ‘ETV6-RET’. In total,
21 SC cases with ETV6::RET fusion were reviewed, including the case
reported in the present study. Clinical and pathological
characteristics of these cases are detailed in Table II (3,4,9,14-17).
The median patient age at diagnosis was 40 years (range, 14-77
years), with a higher number of cases reported in male patients
(male/female, 15:6). Tumors primarily affected the parotid gland
(14 cases), submandibular glands (6 cases) and lip (1 case), and
tumor sizes averaged 29.0 mm (range, 12-70 mm). Regarding the TNM
classification, 3 cases of T1, 2 cases of T2, 8 cases of T3 and 1
case of T4 were included, and lymph node and distant metastases
were observed in 3 and 2 cases, respectively. Lymphovascular and
neural invasions were seen in 6 and 6 cases, respectively, and 8
cases presented with hyalinized sclerotic stroma.
 | Table IISecretory carcinoma with ETV6::RET
fusion. |
Table II
Secretory carcinoma with ETV6::RET
fusion.
| Case no. | Age/sex | Location | Tumor size, mm | TNM | Meta-stasis | Lymphova scular
invasion | Neural invasion | Peripheral
invasion | Capsule | Thick fibrous
septum | Hyalinized
sclerosis | Cyst | HGT | Necrosis | Follow-up | Outcome | (Refs.) |
|---|
| 1 | 50/M | Lip | 15 | NA | NA | NA | NA | NA | NA | - | - | - | - | - | NA | NA | (3) |
| 2 | 29/M | Parotid | 23 | pT3pN1M0 | Lymph node | + | + | Extraglandular, | - | + | ++ | - | - | - | 4y6mo | Alive, NED | (3) |
| 3 | 31/M | Submandibular | 30 | pT3pN0M0 | - | - | + | muscle | - | - | ++ | ++ | - | - | -4y | Alive, NED | (3) |
| 4 | 77/F | Submandibular | 70 | pT3N0M0 | - | - | - | - | focal | + | + | - | - | - | 3y | DOC | (3) |
| 5 | 51/M | Parotid | 10 | pT3N0M0 | - | - | + | - | - | ++ | ++ | - | - | + | 8mo | Alive, NED | (3) |
| 6 | 20/F | Parotid | 40 | pT3N0M0 | - | - | - | Extraglandular | - | + | + | - | - | - | NA | NA | (3) |
| 7 | 55/M | Parotid | 70 | pT3N0M1 | Bone | + | + | Perivascular | - | + | + | + | - | + | 2y | DOD | (3) |
| 8 | 28/F | Parotid | 12 | pT1N0M0 | - | - | - | Intraglandular | - | + | + | - | - | - | 2y | Alive, NED | (3) |
| 9 | 33/M | Parotid | 17 | pT1pN0 | - | - | - | - | + | - | - | + | - | - | 3y9mo | Alive, NED | (3) |
| 10 | 34/M | Parotid | 19 | pT2N0M0 | - | - | - | - | + | - | - | - | - | - | 4y2mo | Alive, NED | (3) |
| 11 | 42/M | Parotid | 29 | pT3pN0M0 | - | + | - | Muscle | NA | NA | NA | NA | - | NA | 4y | Alive, NED | (4) |
| 12 | 68/M | Submandibular | 15 | NA | NA | - | - | - | NA | NA | NA | NA | - | NA | 21y | Recurrence | (4) |
| 13 | 54/F | Parotid | 30 | M1 | Lung, bone,
pleural+effusion | + | - | - | NA | NA | NA | NA | - | NA | <1y | DOD | (4) |
| 14 | 18/M | Parotid | 20 | pT1 | NA | NA | - | - | NA | NA | NA | + | - | - | 9mo | Alive, NED | (14) |
| 15 | 20/M | Submandibular | 21 | pT2pN0 | - | - | - | - | + | - | - | + | - | - | 1y | Alive, NED | (15) |
| 16 | 14/F | Submandibular | 21 | NA | NA | NA | NA | - | NA | NA | NA | - | - | NA | 1y6mo | Alive, NED | (16) |
| 17 | 34/F | Parotid | 21 | T4aN1M0 | NA | + | NA | Infiltrative | NA | NA | NA | + | - | NA | 4mo | Alive, NED | (17) |
| 18 | 62/M | Parotid | 42 | pN1M1 | lung | + | + | NA | NA | NA | NA | NA | + | + | 72mo | Multiple
recurrence | (9) |
| 19 | 46/M | Parotid | 30 | pN0M0 | - | - | - | NA | NA | NA | NA | NA | + | + | 12mo | Alive, NED | (9) |
| 20 | 51/M | Submandibular | 55 | pN0M0 | - | - | - | NA | NA | NA | NA | NA | + | + | 40mo | Alive, NED | (9) |
| 21 | 25/M | Parotid | 18 | pT3N0M0 | - | - | + | Muscle | - | + | ++ | - | - | - | 1y3mo | Alive, NED | Present case |
Discussion
Salivary gland tumors exhibit certain characteristic
gene abnormalities, including point mutations and fusions. The
majority of salivary gland SC possess the ETV6::NTRK3 fusion,
characterized by circumscribed lesions with a cystic component,
low-grade features and favorable clinical outcomes (1,6). Cases
of SC harboring the ETV6::non-NTRK3 (ETV6::X) fusion, which is
morphologically and immunohistochemically typical for SC, have been
previously reported. SC with an ETV6::X fusion shows a histology of
marked stromal fibrosis and invasive features of perineural or
vascular tumor involvement, and one of these previously reported
cases showed regional lymph node metastasis (7). SC with ETV6::RET fusion demonstrates
three histological growth patterns (3). The first is well-circumscribed and
surrounded by a thick, focally uninterrupted fibrous capsule. The
second is a solid and microcystic growth with a multilobular
structure divided by thin fibrous septa, lacking a capsule or only
partially encapsulated with prominent infiltrating borders. The
third is a prominent hyalinized sclerotic stroma in the center of
the tumor. The present case demonstrated the combination of the
latter two patterns. Recently, a 3-tiered grading system of
salivary gland SC, namely Grade (G)1, G2 and G3, was proposed
(18). This grading system
associates high-grade tumors with a solid architecture, more
prominent hyalinization, infiltrative tumor borders, nuclear
pleomorphism, the presence of perineural and/or lymphovascular
invasion and a Ki-67 proliferative index of >30%, and a high
grade is associated with an unfavorable prognosis. The present case
exhibited the aforementioned features; however, the Ki-67 index was
relatively low, aligning with G2. A review of patients with
ETV6::RET fusion SC (Table II)
showed that 52% of cases included T3 tumors (tumors >4 cm and/or
with extraparenchymal extension) or distant metastasis, and
ETV6::RET fusion SC may be characterized by advanced tumor stage
and high grade, revealing invasive growth, neural and/or
lymphovascular invasion and hyalinized sclerosis.
Both NTRK3 and RET, as ETV6 fusion partners, induce
constitutively active chimeric tyrosine kinases. NTRK inhibitors,
such as entrectinib and larotrectinib, are considered effective
treatment options, leading to favorable responses in patients with
ETV6::NTRK3 fusion-positive SC (11,12).
Of note, NTRK fusions are more frequently associated with
microsatellite instability-high cancers compared with RET fusions
(19), meaning that patients with
NTRK fusion may benefit from immune checkpoint inhibitor therapy.
Conversely, RET fusions occur in 1-2% of cases of non-small cell
lung cancer, and in ~20% of cases of papillary thyroid cancer and
thyroid medullary carcinoma. In addition, inhibitors of RET
receptor tyrosine kinase, such as selpercatinib and pralsetinib,
demonstrated efficacy in RET fusion-positive solid tumors other
than lung or thyroid tumors (13).
These aforementioned therapeutic responses were also observed in
salivary gland tumors, including salivary duct carcinoma,
regardless of the tumor type or RET fusion partner (13).
In conclusion, salivary gland SC with ETV6::RET
fusion may be associated with advanced tumor stage and a high
histological grade, exhibiting invasive growth, neural and/or
lymphovascular invasion and stromal hyalinized sclerosis. Detection
of the ETV6 fusion partner is crucial for the prediction of patient
prognosis and the selection of an appropriate therapy.
Supplementary Material
Immunohistochemical findings of the
left parotid gland tumor. (A) S-100-positive tumors cells; (B) GATA
binding protein 3-positive tumor cells; (C) Ki-67 immunostaining
(Ki-67 labelling index was maximally 8%) (magnification,
x100).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AI collected and analyzed data. HK, EY, TN and YH
designed the study and evaluated the pathological findings. TJ, MH
and SH contributed to clinical data acquisition and interpretation.
HK wrote the manuscript. All authors have read and approved the
final manuscript. HK and EY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report, including medical
information and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skálová A, Vanecek T, Sima R, Laco J,
Weinreb I, Peres-Ordonez B, Starek I, Geierova M, Simpson RHW,
Passador-Santos F, et al: Mammary analogue secretory carcinoma of
salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto
undescribed salivary gland tumor entity. Am J Surg Pathol.
34:599–608. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cocco E, Scaltriti M and Drilon A: NTRK
fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin
Oncol. 15:731–747. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Skalova A, Vanecek T, Martinek P, Weinreb
I, Stevens TM, Simpson RHW, Hyrcza M, Rupp NJ, Baneckova M, Michal
M Jr, et al: Molecular profiling of mammary analog secretory
carcinoma revealed a subset of tumors harboring a novel ETV6-RET
translocation. Report of 10 cases. Am J Surg Pathol. 42:234–246.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guilmette J, Dias-Santagata D, Nose V,
Lennerz JK and Sadow PM: Novel gene fusions in secretory carcinoma
of the salivary glands: Enlarging the ETV6 family. Hum Pathol.
83:50–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Drilon A, Hu ZI, Lai GGY and Tan DSW:
Targeting RET-driven cancers: Lessons from evolving preclinical and
clinical landscapes. Nat Rev Clin Oncol. 15:151–167.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Skalova A, Bishop JA, Kholova I and Urano
M: Secretory carcinoma. In: WHO Classification of Tumours. 5th
edition. International Agency for Research on Cancer, Lyon,
pp210-212, 2024.
|
|
7
|
Ito Y, Ishibashi K, Masaki A, Fujii K,
Fujiyoshi Y, Hattori H, Kawakita D, Matsumoto M, Miyabe S,
Shimozato K, et al: Mammary analogue secretory carcinoma of
salivary glands: A clinicopathologic and molecular study including
2 cases harboring ETV6-X fusion. Am J Surg Pathol. 39:602–610.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Skálová A, Vanecek T, Simpson RHW, Laco J,
Majewska H, Baneckova M, Steiner P and Michal M: Mammary analogue
secretory carcinoma of salivary glands. Molecular analysis of 25
ETV6 gene rearranged tumors with lack of detection of classical
ETV6-NTRK3 fusion transcript by standard RT-PCR: Report of 4 cases
harboring ETV6-X gene fusion. AmJ Surg Pathol. 40:3–13.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Sun J, Sun B, Zhang C, Tian Z,
Wang L and Li J: The genetic and immune features of salivary gland
secretory carcinoma with high-grade transformation. Oral Dis.
30:4320–4330. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
von Bergh ARM, van Drunen E, van Wering
ER, van Zutven LJCM, Hainmann I, Lönnerholm G, Meijerink JP,
Pieters R and Beverloo HB: High incidence of t(7;12)(q36;p13) in
infant AML but not in infant ALL, with a dismal outcome and ectopic
expression of HLXB9. Genes Chromosomes Cancer. 45:731–739.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yokota T, Yukino H, Doi M and Ohori H:
Real-world experience of tropomyosin receptor kinase inhibition
with entrectinib in ETV6-NTRK3 positive metastatic salivary
secretory carcinoma: A case series. Head Neck. 45:E10–E15.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moriyama E, Nagasu S, Tanaka T,
Shimotsuura Y, Ono T, Umeno H, Akiba J, Kawahara A, Fujita F,
Kawaguchi T and Miwa K: Case report: A case of complete response to
entrectinib in NTRK fusion gene-positive parotid gland cancer.
Front Oncol. 13(1247435)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Subbiah V, Cassier PA, Siena S, Garralda
E, Paz-Ares L, Garrido P, Nadal E, Vuky J, Lopes G, Kalemkerian GP,
et al: Pan-cancer efficacy of pralsetinib in patients with RET
fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat
Med. 28:1640–1645. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Black M, Liu CZ, Onozato M, Iafrate AJ,
Darvishian F, Jour G and Cotzia P: Concurrent identification of
novel EGFR-SEPT14 fusion and ETV6-RET fusion in secretory carcinoma
of the salivary gland. Head Neck Pathol. 14:817–821.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith ME, Surrey LF, Zhang PJ, Weinstein
GS and LiVolsi VA: Molecular identification of an ETV6-RET fusion
in a secretory carcinoma associated with a pleomorphic adenoma.
Oral Surg Oral Med Oral Pathol Oral Radiol. 134:733–738.
2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Salgado CM, Alaggio R, Reyes-Mugica M, Zin
A and de Vito R: Clinicopathologic and molecular characterization
of four cases of pediatric salivary secretory carcinoma (SSC), one
with ETV6-RET fusion. Head Neck Pathol. 15:796–802. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Su YJ, Lee YH, Jin YT and Hsieh MS: Using
pan-TRK and RET immunohistochemistry for the detection of fusion
types of salivary gland secretory carcinoma. Appl Immunohistochem
Mol Morphol. 30:264–272. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Baněčková M, Thompson LDR, Hyrcza MD,
Vaněček T, Agaimy A, Laco J, Simpson RHW, Di Palma S, Stevens TM,
Brcic L, et al: Salivary gland secretory carcinoma:
Clinicopathologic and genetic characteristics of 215 cases and
proposal for a grading system. Am J Surg Pathol. 47:661–677.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang J, Li R, Li J, Yi Y, Liu X, Chen J,
Zhang H, Lu J, Li C, Wu H and Liang Z: Comprehensive analysis of
oncogenic fusions in mismatch repair deficient colorectal
carcinomas by sequential DNA and RNA next generation sequencing. J
Transl Med. 19(433)2021.PubMed/NCBI View Article : Google Scholar
|