Introduction
Autoimmune hepatitis (AIH) is an inflammatory
disease of the liver resulting from a loss of immune tolerance to
hepatocyte antigens, which is triggered by unknown factors.
Although the mechanisms underlying autoimmune responses in AIH are
not well understood, a combination of genetic and external factors,
such as infection, drugs or herbal medicines, are likely to be
involved. In fact, drugs can cause a variety of phenotypes of liver
injury that demonstrate similar clinical features of AIH and liver
dysfunction, with positive results for autoantibodies (1-5).
In fact, as overlap is often observed between the
clinical and pathological features of idiosyncratic drug-induced
liver injury (DILI) and AIH, distinguishing AIH from DILI based on
the diagnostic criteria commonly used in clinical settings can be
difficult (6-9).
Such cases are often referred to as drug-induced AIH, and have also
been termed drug-induced autoimmune-like hepatitis (DI-ALH),
immune-mediated DILI or DILI with autoimmune features (10-14).
However, the terminology remains poorly defined and controversial.
Recently, the term DI-ALH has been used more frequently to describe
these conditions (15,16).
In terms of pathogenesis, DILI and AIH share
molecular mechanisms, and genetic associations with human leukocyte
antigen variants point to relevant roles of hepatic inflammation
through antigen presentation (4,6,17). In
addition, intrahepatic immunocompetent cells related to
pro-inflammatory conditions and immune regulation are present in
both DILI and AIH (4). Such
findings suggest the existence of common pathways in the molecular
biological mechanisms underlying DILI and AIH pathogeneses. DI-ALH
may thus involve a complex interplay of both mechanisms and clear
separation of these entities is often difficult.
The most commonly reported causative agents in
DI-ALH are interferons, statins, methylprednisolone, adalimumab,
imatinib and diclofenac, but an even wider variety of drugs may be
capable of involvement (14-16,18-21).
The clinical picture also seems to differ depending on the type of
offending agent. In particular, statins are considered to be
associated with a clinical picture more similar to that of AIH
(15).
Contrasting with AIH, which requires long-term
immunosuppressive therapy, DI-ALH often resolves or improves upon
discontinuation of the immunosuppressive drug (1,15,16).
However, the clinical features seem to depend on the type of drug
and the susceptibility of the individual to the drug. An accurate
diagnosis of DI-ALH is therefore necessary to avoid
overmedication.
These unpredictable idiosyncratic or hypersensitive
drug reactions may be involved in a proportion of classical AIH
cases. However, details of the incidence and spectrum of DI-ALH and
other related diseases have yet to be elucidated in detail.
We have already reported several cases of AIH with
unusual clinical courses (22).
These cases were initially diagnosed as DILI, and the liver
dysfunction improved after discontinuation of the causal drugs,
only to relapse later despite no further administration of the drug
and with significantly elevated levels of antinuclear antibody
(ANA) and immunoglobulin (Ig)G. Histological findings were
consistent with AIH. These courses differed from DILI with
subclinical AIH or a second episode of DILI or conventional DI-ALH,
suggesting different patterns of etiology and molecular
pathogeneses. We named this condition DILI-ALH.
Little is known about the frequencies of DILI-ALH
and DI-ALH due to the uncertain clinical definition. Therefore, in
the present study, a detailed retrospective survey was conducted of
medication history at and before the diagnosis of AIH to detect
DI-ALH and DILI-ALH with particular reference to recently proposed
criteria (16). In addition, the
clinicopathological features of DI-ALH and DILI-ALH were compared
to distinguish these entities from idiopathic AIH.
Patients and methods
Patients
The medical records of 57 patients diagnosed with
AIH at Mie Prefectural General Medical Center (Yokkaichi, Japan)
between January 2011 and December 2022 were retrospectively
reviewed. Patients with a history of other liver diseases such as
viral hepatitis (e.g., hepatitis B or C) were excluded. A liver
biopsy was not performed in some patients due to advanced age, poor
general condition, bleeding tendency or a lack of consent, and
these patients were also excluded.
Overall, 42 patients were included in the analysis.
Data on age, sex, body mass index, laboratory data, pathological
data, complications and causative drugs were retrospectively
collected. This study was approved by the Medical Ethics Committee
of Mie Prefectural General Medical Center (approval no.
O-0094).
Evaluation of clinicopathological
findings
The cases of patients diagnosed with AIH were
examined with particular attention paid to the medical history. AIH
was defined using the criteria developed by the International
Autoimmune Hepatitis Group (23).
The Revised AIH Score was used in this study as a commonly used
index both in Japan and worldwide. In patients where drugs are
involved, such as drug-related AIH, the revised AIH score will be 4
points lower, resulting in an apparent lower AIH score (23). A cut-off value of 10 points, as the
cutoff for probable AIH, was therefore used for the pretreatment
AIH score in this analysis.
Although several scoring methods are available for
the diagnosis of DILI, the Roussel Uclaf Causality Assessment
Method (RUCAM) score, which is widely used internationally, was
used in this study (24). The RUCAM
score is a means of assigning points to clinical, biochemical,
serological and radiological features of liver injury, resulting in
an overall assessment score that reflects the likelihood that liver
injury is due to a particular drug.
For the present study, patients with AIH were
classified into three groups: i) De novo AIH; ii) DI-ALH;
and iii) DILI-ALH. De novo AIH was defined as patients with
no history of DILI, a pretreatment AIH score of ≥10 and clinical or
pathological suspicion of AIH. DI-ALH was defined as AIH directly
induced by drugs. To study the influence of drugs in greater
detail, a stricter definition was used with reference to the
definition provided by Björnsson et al (15), as follows: i) Patients without liver
dysfunction before drug administration; ii) no underlying other
liver disease; iii) a temporal association between the start of
drug administration and the onset of AIH; iv) a clinical and
pathological diagnosis of AIH, with a pretreatment AIH score of
≥10; and v) clinically, drugs are involved in the onset of AIH,
with a RUCAM score of ≥6.
DILI-ALH, which was newly proposed in the present
study, was defined as a case in which the patient had a history of
DILI and showed initial improvement of liver injury, indicated by
ALT returning to normal or falling to less than one-half of its
peak level, after discontinuation of the suspected causal drug,
followed by exacerbation leading to the diagnosis of AIH. DILI-ALH
was considered to differ from DI-ALH in the lack of a direct
trigger for the onset of AIH. DILI-ALH was defined as follows: i)
Patients who were clinically diagnosed with DILI showing a RUCAM
score of ≥6 and an AIH score of ≤9 at the time of initial liver
injury, with no apparent cause of liver injury other than
medication; ii) improvement of initial liver injury with drug
discontinuation; and iii) recurrence of liver injury with no
apparent drug-induced cause. AIH was clinically diagnosed when the
RUCAM score was ≤5 and the AIH score was ≥10.
For de novo AIH and DI-ALH, blood test data
were collected at the first visit. For DILI-ALH, data were
collected at the time of the DILI diagnosis and at subsequent
visits due to worsening liver injury. The following variables were
obtained by chart review: Total bilirubin (T-Bil), aspartate
aminotransferase (AST) and alanine aminotransferase (ALT) levels,
alkaline phosphatase (ALP) ratio (ALP real value/upper normal
limit) (adopted due to multiple different methods of measuring
ALP), γ-glutamyltransferase level, prothrombin time (PT),
peripheral blood eosinophil count, IgG titer, ANA-positive rate and
anti-mitochondrial antibody (AMA)-positive rate. Pre-treatment AIH
score was used in this study.
Liver biopsy specimens and the report for each
individual were reviewed anonymously by the pathologist and the
following data were collected: Inflammation grade (A0-A3) and
fibrosis grade (F0-F4) based on the new Inuyama classification
(25), lobular inflammation,
interface hepatitis, rosette formation and infiltrating cells,
steatosis, cholestasis and bile duct injury. Inflammation grades
were as follows: A0, no necro-inflammatory reaction; A1, mild
necro-inflammatory reaction; A2, moderate inflammatory reaction;
and A3, severe necro-inflammatory reaction. Fibrosis grades were as
follows: F0, no fibrosis; F1, fibrous portal expansion; F2,
bridging fibrosis (mild fibrosis); F3, bridging fibrosis with
lobular distortion (severe fibrosis); and F4, cirrhosis (25).
Statistical analysis
Categorical variables are presented as numbers and
percentages, while continuous variables are presented as medians
and interquartile ranges. Differences between groups were
determined using Fisher's exact test for categorical variables and
using the Kruskal-Wallis rank-sum test with the Steel-Dwass
non-parametric post hoc test for continuous variables. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using EZR version 1.52
(Jichi Medical University), a graphical user interface for R
version 4.02 (The R Foundation for Statistical Computing; R Core
Team). More specifically, EZR is a modified version of R Commander
designed to provide statistical functions commonly used in
biostatistics (26).
Results
Patient characteristics of
DILI-ALH
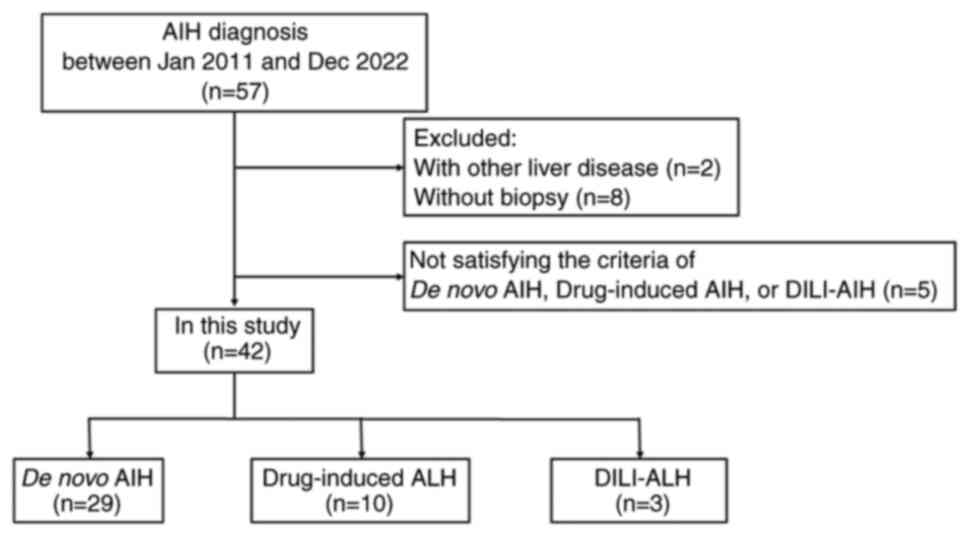
Of the 57 patients diagnosed with AIH at Mie General
Medical Center, 2 patients were excluded due to hepatitis B. A
total of 42 patients who met the selection criteria were included
in the final analysis, including 29 patients with de novo
ALH, 10 patients with DI-ALH and 3 patients with DILI-ALH (Fig. 1).
The suspected causative drugs for the DI-ALH and
DILI-ALH groups are shown in Table
I. The most common causative drugs for DI-ALH were statins in 3
cases and dietary supplements in 2 cases. The most common causative
drugs for DILI-ALH were health foods/supplements in 2 cases and
Tiaramide in 1 case. Of these 3 patients, patient 1 took a dietary
supplement consisting primarily of docosahexaenoic acid,
eicosapentaenoic acid, docosapentaenoic acid and nattokinase during
a 3-month period. Patient 2 had been taking tiaramide
hydrochloroide for ~1 year, but stopped after being diagnosed with
DILI. Patient 3 was diagnosed with DILI in November after taking a
supplement containing zinc, maca, arginine and suppon powder as the
main ingredients. Although statins and health foods/supplements,
including herbs, were the most common causative drugs in this
study, a variety of drugs were suspected to be involved in DI-ALH
and DILI-ALH.
 | Table IDrugs suspected to be responsible for
DI-ALH and DILI-ALH in this study. |
Table I
Drugs suspected to be responsible for
DI-ALH and DILI-ALH in this study.
| Drug | n |
|---|
| DI-AIH | |
|
Statin | 3 |
|
Cefcapene
pivoxil | 1 |
|
Benzbromarone | 1 |
|
Febuxostat | 1 |
|
Esomeprazole
magnesium | 1 |
|
Herbal
medicine (Hochu-ekkito) | 1 |
|
Health
foods/supplements | 2 |
| DILI-AIH | |
|
Tiaramide
hydrochloride | 1 |
|
Health
foods/supplements | 2 |
DILI-ALH was newly defined as a case in which the
patient had a history of DILI and initially showed improvement of
liver injury after discontinuing the drug in question, but later
worsened and was diagnosed with AIH. The present study included 3
such cases. The characteristics of these cases are shown in
Table II and the clinical course
of each case is shown in Fig. 2. A
clear precipitating drug was identified in the first episode of
each case and DILI was suspected based on scoring, but the
diagnosis of AIH was not met at that time. With the second episode,
liver injury occurred without any obvious drug trigger and AIH was
diagnosed.
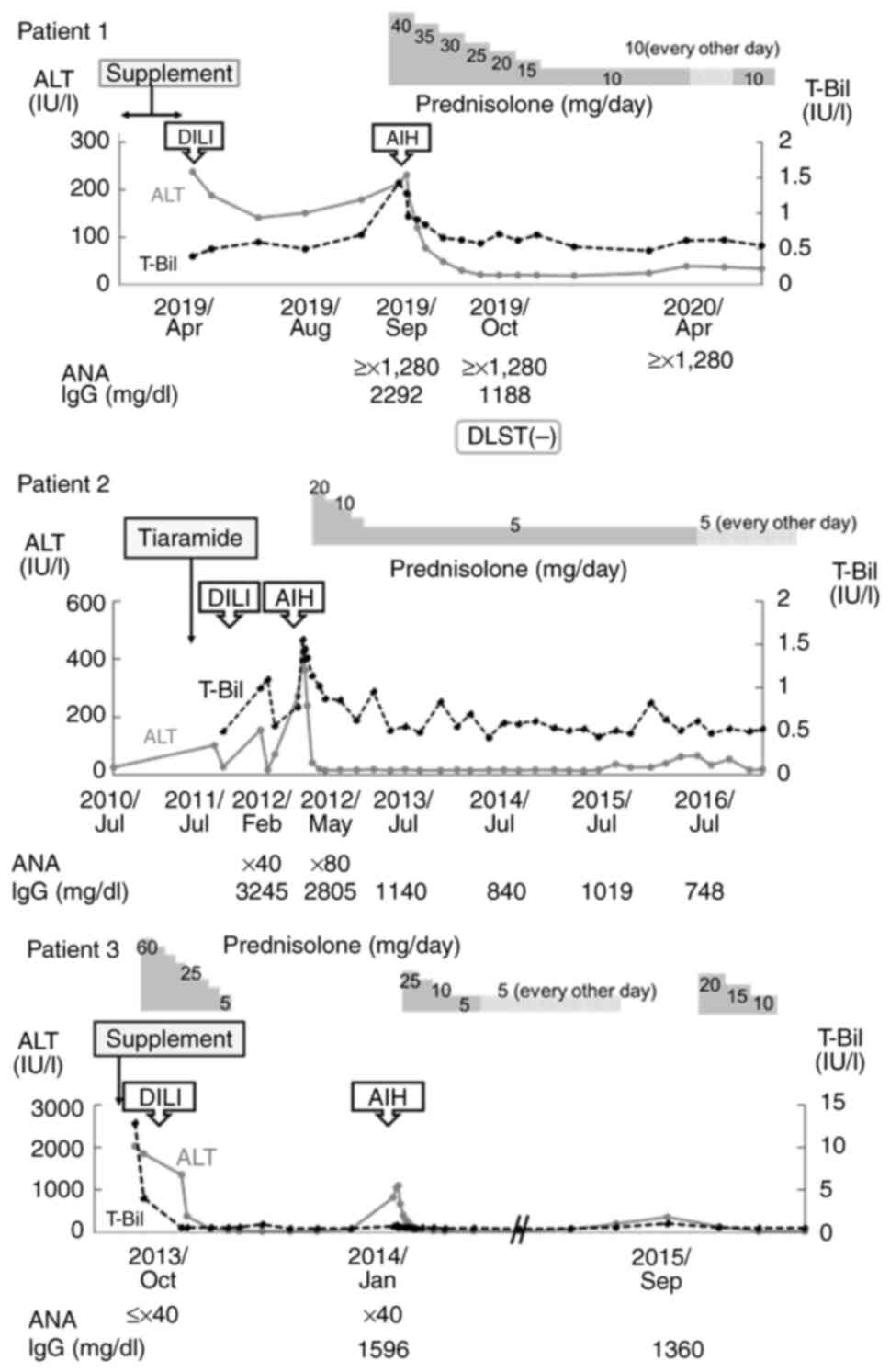 | Figure 2Short-term clinical courses of 3
patients with DILI-ALH. Changes to ALT and T-Bil in each patient
along with ANA, IgG, diagnoses of DILI and AIH, and suspected
causative drugs. DILI-ALH, autoimmune-like hepatitis following
drug-induced liver injury; AIH, autoimmune hepatitis; T-Bil, total
bilirubin; ALT, alanine aminotransferase; IgG, immunoglobulin G;
ANA, antinuclear antibody; DLST, drug-induced lymphocyte
stimulation. |
 | Table IIRUCAM scores and revised AIH scores
in patients with DILI-ALH. |
Table II
RUCAM scores and revised AIH scores
in patients with DILI-ALH.
| Characteristic | Patient 1 | Patient 2 | Patient 3 |
|---|
| Age, years | 66 | 68 | 40 |
| Sex | Male | Female | Male |
| BMI,
kg/m2 | 20.6 | 14.5 | 27.8 |
| History of
autoimmune disease | None | None | None |
| On first
episode | | | |
|
T-Bil,
g/ml | 0.4 | 1.2 | 12.9 |
|
ALT,
U/l | 238 | 274 | 2031 |
|
IgG,
mg/dl | 1,819 | NA | NA |
|
ANA | + (discrete) | x40
(nucleolar) | NA |
|
Drug
suspected for DILI | Supplement | Tiaramide
hydrochloride | Supplement |
|
Treatment | Discontinued
drug | Discontinued
drug | Discontinued drug
and PSL 0.8 mg/kg/day orally |
| Duration between
first episode and latter episode, months | 6 | 10 | 3 |
| On latter
episode | | | |
|
T-Bil,
g/ml | 1.43 | 0.78 | 0.5 |
|
ALT,
U/l | 213 | 273 | 1,095 |
|
IgG,
mg/dl | 2,292 | 2805 | 1,687 |
|
ANA | ³x1,280 | x80 | Negative |
|
Treatment | PSL0.8 mg/kg/day
orally | PSL 0.6 mg/kg/day
orally | PSL 0.6 mg/kg/day
orally |
| Outcome | No relapse for 40
months | No relapse for 100
months | Relapse during
steroid tapering |
| Scoring | | | |
|
First
episode | | | |
|
RUCAM
score | 6 (probable) | 7 (probable) | 6 (probable) |
|
Revised
AIH score | 5 (unlikely) | 9 (unlikely) | 3 (unlikely) |
|
Latter
episode | | | |
|
RUCAM
score | 4 (possible) | 3 (possible) | 4 (possible) |
|
Revised
AIH score | 16 (probable) | 15 (possible) | 11 (possible) |
In all 3 patients in this study, AIH score at the
time of the first DILI was ≤9, indicating that AIH was unlikely.
However, when liver injury occurred again, the score increased by
≥6, indicating that the diagnosis of AIH was either possible or
probable.
Patient 1 was diagnosed with liver injury after
taking a dietary supplement and showing a RUCAM score of 6
(probable DILI) and an AIH score of 5 (unlikely AIH). The patient
was initially diagnosed with DILI, which only improved with
discontinuation of the supplement. During the follow-up, the
patient was no longer on medication, but 5 months later, a
recurrence of the liver injury occurred. AIH was diagnosed based on
a RUCAM score of 4 (possible DILI) and an AIH score of 16 (definite
AIH). Patients 1 and 2 also showed DILI that improved only with
drug discontinuation, while patient 3 had previously received
steroids as treatment for DILI. All patients showed exacerbation of
liver injury, during steroid tapering in patient 3 and during
follow-up in the other two patients, without resumption of
discontinued medications. Although none of the patients showed any
increase in DILI score for the recurrent liver injury, all
displayed an increase in AIH score of ≥6 points, indicating a
possible or probable diagnosis of AIH. Relapse of liver damage was
observed in only one case, and liver damage worsened during steroid
tapering.
Clinical characteristics of AIH,
DI-ALH and DILI-ALH
The baseline characteristics and laboratory findings
of the patients are shown in Table
III. A total of 29 patients had de novo AIH, 10 patients
had DI-ALH and 3 patients had DILI-ALH. In the de novo AIH
group, patients had significantly fewer subjective symptoms (de
novo AIH vs. DI-ALH, P=0.01; de novo AIH vs. DILI-ALH,
P>0.99; DI-ALH vs. DILI-ALH, P=0.22; Steel-Dwass test). The
de novo AIH group included a greater proportion of women,
but the difference was not significant (de novo AIH vs.
DI-ALH, P=0.33; de novo AIH vs. DILI-ALH, P=0.25; DI-ALH vs.
DILI-ALH, P=0.56; Steel-Dwass test). The proportion of older
patients tended to be higher in the DILI-ALH group than in the
other groups (de novo AIH vs. DI-ALH, P>0.99; de
novo AIH vs. DILI-ALH, P>0.99; DI-ALH vs. DILI-ALH,
P>0.99; Steel-Dwass test). No significant differences in relapse
or complication rates of autoimmune diseases were evident among the
three groups (relapse; de novo AIH vs. DI-ALH, P>0.99;
de novo AIH vs. DILI-ALH, P>0.99; DI-ALH vs. DILI-ALH,
P>0.99, and complication rates of autoimmune diseases; P=0.80,
P>0.99 and P>0.99; Steel-Dwass test).
 | Table IIICharacteristics and laboratory
findings for de novo AIH, DI-ALH and DILI-ALH. |
Table III
Characteristics and laboratory
findings for de novo AIH, DI-ALH and DILI-ALH.
|
Characteristics | De novo AIH
(n=29) | DI-ALH (n=10) | DILI-ALH (n=3) | P-value |
|---|
| Female, n (%) | 25 (86.2) | 6 (60.0) | 1 (33.3) | P=0.33a, P=0.25b, P=0.56c |
| Age, years | 67.0 [54.0,
73.0] | 57.5 [48.5,
67.8] | 66.0 [53.0,
67.0] | P=0.77a, P=0.70b, P=0.96c |
| ≥65 years old | 15 (51.7) | 4 (40.0) | 2 (66.6) |
P>0.99a, P>0.99b, P>0.99c |
| BMI,
kg/m2 | 23.6 [22.2,
26.2] | 25.4 [20.9,
28.7] | 20.6 [17.5,
24.2] | P=0.88a, P=0.56b, P=0.57c |
| Symptoms | 10 (34.5) | 9 (90.0) | 1 (33.3) | P=0.01a,d, P>0.99b, P=0.22c |
| Follow-up period,
months | 39.0 [16.0,
58.0] | 23.5 [18.8,
44.5] | 100.0 [68.5,
104] | P=0.95a, P=0.35b, P=0.15c |
| Autoimmune disease,
n (%) | 2 (6.9) | 2 (20.0) | 0 (0.0) | P=0.80a, P>0.99b, P>0.99c |
| T-Bil, g/ml | 0.91 [0.65,
1.99] | 2.80 [1.33,
7.96] | 0.78 [0.64,
1.43] | P=0.15a, P=0.74b, P=0.15c |
| AST, U/l | 138 [91.0,
514] | 531 [312, 714] | 474 [409, 482] | P=0.17a, P=0.53b, P=0.78c |
| ALT, U/l | 216 [93.0,
537] | 663 [401,
1076] | 273 [243, 684] |
P=0.039a,d,
P=0.58b,
P=0.78c |
| ALP ratio | 0.96 [0.69,
1.22] | 1.11 [0.78,
1.25] | 1.30 [1.22,
1.39] | P=0.94a, P=0.66b, P=0.78c |
| γ-glutamyl
transpeptidase, U/l | 162 [114, 282] | 233 [188, 333] | 208 [155, 285] | P=0.22a, P=0.91b, P=0.94c |
| PT, % | 84 [75, 101] | 87 [75, 103] | 87 [79, 105] | P=0.92a, P=0.89b, P=0.98c |
| Peripheral blood
eosinophil, % | 2.4 [1.6, 4.1] | 3.2 [1.1, 3.5] | 5.1 [4.2, 7.2] | P=0.93a, P=0.17b, P=0.18c |
| IgG, mg/dl | 2,458 [1863,
2972] | 2,059 [1773,
2326] | 2,292 [1990,
2459] | P=0.18a, P=0.81b, P=0.87c |
| ANA positivity, n
(%) | 26 (89.7) | 10 (100.0) | 2 (66.6) |
P>0.99a, P>0.99b, P=0.69c |
| AMA positivity, n
(%) | 7 (24.1) | 1 (10.0) | 0 (0.0) |
P>0.99a, P>0.99b, P>0.99c |
| Revised AIH score
before treatment | 15 [14, 17] | 13 [11, 14] | 12 [11.5,
13.5] |
P=0.040a,d,
P=0.16b,
P=0.99c |
| Steroid therapy, n
(%) | 24 (82.8) | 10 (100.0) | 3 (100.0) | P=0.91a. P>0.99b, P>0.99c |
| Relapse after
steroid tapering, n (%) | 10 (34.5) | 4 (40.0) | 1 (33.3) |
P>0.99a, P>0.99b, P>0.99c |
Blood tests showed that ALT levels were
significantly higher in the DI-ALH group compared with those in the
de novo AIH group (de novo AIH vs. DI-ALH, P=0.039;
de novo AIH vs. DILI-ALH, P=0.58; DI-ALH vs. DILI-ALH,
P=0.78; Steel-Dwass test). No significant differences in T-Bil
(P=0.15, P=0.74 and P=0.15), AST (P=0.17, P=0.53 and P=0.78), ALP
ratio (P=0.94, P=0.66 and P=0.78), γ-glutamyl transferase (P=0.22,
P=0.91 and P=0.94), PT (P=0.92, P=0.89 and P=0.98), ANA-positive
rate (P>0.99, P>0.99 and P=0.69) or AMA-positive rate
(P>0.99, P>0.99 and P>0.99) were observed among the three
groups. Peripheral blood eosinophil percentage (P=0.93, P=0.17 and
P=0.18) tended to be higher in the DILI-ALH group, and IgG titer
(P=0.18, P=0.81 and P=0.87) tended to be higher in the de
novo AIH group, although these results were not statistically
significant. Distinguishing these types of AIH by blood tests was
difficult. Revised AIH scores were lower in DI-ALH, possibly due to
drug subtraction in AIH scoring. Steroids were used for treatment
in 82.8% of the AIH group, 100.0% of the DI-ALH group and 100.0% of
the DILI-ALH group, and the frequencies of recurrent liver injury
were 34.5, 40.0 and 33.3%, respectively. No significant differences
in steroid use or recurrence rates were observed between the groups
(P=0.91, P>0.99 and P>0.99).
Pathological features of AIH, DI-ALH
and DILI-ALH
Table IV shows the
pathological characteristics of AIH, DI-ALH and DILI-ALH. Interface
hepatitis was observed in three groups, and there is no significant
difference in rosettes (de novo AIH vs. DI-ALH, P=0.26;
de novo AIH vs. DILI-ALH, P>0.99; DI-ALH vs. DILI-ALH,
P>0.99; Steel-Dwass test). Lymphocyte infiltration was seen in
all three groups. There are no significant differences in plasma
cells (de novo AIH vs. DI-ALH, P>0.99; de novo AIH
vs. DILI-ALH, P>0.99; DI-ALH vs. DILI-ALH, P>0.99;
Steel-Dwass test), eosinophils (P>0.99; P>0.99 and P>0.99)
and neutrophils infiltration (P>0.99, P>0.99 and P>0.99).
Also, there are no significant differences in steatosis (P>0.99
P>0.99 and P>0.99), hepatocellular cholestasis (P>0.99
P>0.99 and P>0.99), cholangiolar cholestasis (P>0.99
P>0.99 and P>0.99) and bile duct injury (P>0.99 P>0.99
and P>0.99). In the present study, no significant differences
were found among the three groups, but a higher percentage of cases
in the DI-ALH group tended to show lobular inflammation (de
novo AIH vs. DI-ALH, P=0.19; de novo AIH vs. DILI-ALH,
P>0.99; DI-ALH vs. DILI-ALH, P=0.33; Steel-Dwass test). By
contrast, all three cases in the DILI-ALH group showed inflammatory
findings, mainly in the portal area. No significant difference in
the progression of fibrosis was observed among the three
groups.
 | Table IVResults of liver biopsy for patients
with de novo AIH, DI-ALH, DILI-ALH. |
Table IV
Results of liver biopsy for patients
with de novo AIH, DI-ALH, DILI-ALH.
| Parameter | De novo AIH
(n=29) | DI-ALH (n=10) | DILI-ALH (n=3) | P-value |
|---|
|
Fibrosisa, n | | | | |
|
F0 | 0 | 0 | 0 | |
|
F1 | 14 | 6 | 1 | |
|
F2 | 7 | 2 | 1 | |
|
F3 | 6 | 2 | 1 | |
|
F4 | 2 | 0 | 0 | |
|
Inflammationa, n | | | | |
|
A0 | 1 | 0 | 0 | |
|
A1 | 8 | 1 | 0 | |
|
A2 | 14 | 4 | 2 | |
|
A3 | 6 | 5 | 1 | |
| Lobular
inflammation, n (%) | 16 (55.2) | 9 (90.0) | 1 (33.3) | P=0.19b, P>0.99c, P=0.33d |
| Interface
hepatitis, n (%) | 29 (100.0) | 10 (100.0) | 3 (100.0) | - |
| Rosette, n (%) | 5 (17.2) | 5 (50.0) | 1 (33.3) | P=0.26b, P>0.99c, P>0.99d |
| Lymphocyte
infiltration, n (%) | 29 (100.0) | 10 (100.0) | 3 (100.0) | - |
| Plasma cell
infiltration, n (%) | 28 (96.6) | 9 (90.0) | 3 (100.0) |
P>0.99b, P>0.99c, P>0.99d |
| Eosinophil
infiltration, n (%) | 9 (31.0) | 5 (50.0) | 1 (33.3) |
P>0.99b, P>0.99c, P>0.99d |
| Neutrophil
infiltration, n (%) | 5 (17.2) | 2 (20.0) | 0 (0.0) |
P>0.99b, P>0.99c, P>0.99d |
| Steatosis, n
(%) | 8 (27.6) | 3 (30.0) | 1 (33.3) |
P>0.99b, P>0.99c, P>0.99d |
| Hepatocellular
cholestasis, n (%) | 5 (17.2) | 3 (30.0) | 1 (33.3) |
P>0.99b, P>0.99c, P>0.99d |
| Cholangiolar
cholestasis, n (%) | 2 (6.9) | 0 (0.0) | 0 (0.0) |
P>0.99b, P>0.99c, P=1.00d |
| Bile duct injury, n
(%) | 11 (37.9) | 4 (40.0) | 0 (0.0) |
P>0.99b, P>0.99c, P>0.99d |
Bile duct injury and eosinophilic infiltration,
which are characteristics of DILI, tended to be higher in the
DI-ALH group, but no significant differences were evident.
Overall, pathological findings suggestive of DILI
were not prominent in the DILI-ALH group, and distinguishing DILI
from de novo ALH based on pathological findings alone was
difficult.
Discussion
DILI is known to be able to cause all forms of liver
injury (17). A condition that is
clinically, biochemically, immunologically and histologically
similar to AIH is referred to as DI-ALH. However, distinguishing
DI-ALH from idiopathic AIH is important (2,3,15,16).
This is due to the fact that DI-ALH does not require long-term
immunosuppressive treatment, and some cases have been reported to
resolve spontaneously after discontinuation of the drugs involved.
However, the number of cases of DI-ALH that are drug-related and
which drugs are involved is not well understood.
The present study reviewed in detail the premorbid
drug history of our previous AIH cases (22), identifying three cases with notable
clinical courses. When liver injury occurred, these cases were
diagnosed as DILI based on the scoring systems of AIH and RECUM,
and did not meet the diagnostic criteria for AIH. Liver injury
improved after discontinuing the drugs suspected as triggers. Liver
injury then flared within 3-10 months. At that time, ANA titer and
IgG levels were elevated, and the liver pathology was compatible
with AIH. AIH was therefore considered as the most likely form of
disease in the second episode. This notable clinical course of AIH
after DILI has rarely been reported, although we have previously
reported a case with a similar course (22). The present study showed that similar
cases certainly exist for a certain percentage of cases with
AIH.
A previous study suggested that a second episode of
DILI is more characteristic of AIH (18). However, the second liver injury in
the present cases were considered unlikely to represent DILI, as
the patients had already stopped taking the drug that was
considered to be the trigger. The possibility that AIH was present
at baseline cannot be excluded, but at least at the time of the
first episode, clear demonstration of the presence of AIH using the
AIH scoring system was difficult. The pathological form that
follows such a course and develops AIH was therefore categorized as
DILI-ALH.
The pathogenesis of DILI was considered to mainly
involve an immune response to drugs, their metabolites or their
metabolites when bound to self-proteins (4,8,17). No
major antigens have been identified for AIH, but the possibility of
long-term exposure to self-proteins or environmental, dietary or
bacterial antigens has been suggested (4,9). In
the present case of DILI-ALH, exposure to drug antigens had already
ended after the initial liver injury. We speculate that an
autoimmune response is evoked after discontinuation of the drug
administration in patients or induced in response to some antigen
produced after the liver injury in patients with DILI-ALH.
Previous studies have reported AIH induced by
hepatitis A, hepatitis B or Epstein-Barr virus (27-31).
In some cases, AIH was diagnosed from the liver injury flare
several months after the onset of hepatitis. In those cases,
genetic predispositions and immune responses to asialoglycoprotein
receptors were suggested (27-31).
Taking such factors into account, DILI-ALH could also be caused by
immune induction due to liver damage associated with DILI, rather
than by the drug or its metabolites acting directly as antigens. A
number of suspected drugs were identified in the present study,
including statins and herbal medicines known to cause liver damage
similar to that with AIH. This suggests that certain drug classes
may be involved, rather than specific drugs.
The next question is with regard to how many cases
of AIH are suspected to be drug-related. Previous reports have
shown that DI-ALH is present in 9-17% of cases, representing a
relatively wide range (1,11,14,16,19,32,33).
This may be due in part to the difficulty of making these
diagnoses. Once a diagnosis of AIH is made clinically,
immunosuppressive drugs are administered, generally without
consideration of drug involvement as a trigger. On the other hand,
once DILI is diagnosed, the diagnosis of AIH tends to be delayed
with respect to subsequent liver injury. In the present study, drug
involvement (as DI-ALH) or DILI involvement (as DILI-ALH) was
suspected in 31.0% of cases diagnosed with AIH. This high rate may
be due to the brief, retrospective review of the medication history
for the patient prior to the diagnosis of AIH. In numerous cases,
drug involvement was not considered at the time AIH was actually
diagnosed. This may have been due to the fact that a number of the
patients were receiving ongoing treatment for other conditions and
were therefore receiving pharmacotherapy. However, further studies
are needed to clarify the exact rates of DI-ALH and DILI-ALH using
established diagnostic criteria.
The clinical presentations of AIH, DI-ALH and
DILI-ALH were next compared. Female patients made up 86.2% of the
AIH cases, which is compatible with the Japanese national survey of
AIH, whereas the proportions of female patients in the DI-ALH and
DILI-ALH groups were 60.0 and 33.3%, suggesting some differences in
the pathogeneses of these clinical entities (34). In terms of blood biochemistry, ALT
levels were significantly higher in DI-ALH than in de novo
AIH. However, AMA positivity was observed in only one case of
DI-ALH and not at all in the DILI-ALH group. DI-ALH reportedly
tends to have an acute onset and higher liver function test results
compared with AIH (19,35). However, IgG and ANA are not reported
to show significant differences (13,14,32).
Such findings are consistent with the present results, in which no
significant difference with regard to these factors was found in
the DILI-ALH and AIH groups, suggesting that DILI-ALH presents a
clinical picture close to that of AIH.
Histological examination also revealed no
significant differences between the three groups in this study.
However, the DI-ALH group tended to show more cases with an acute
hepatitis pattern and eosinophil infiltration. No previous reports
have described significant histological differences between AIH and
DI-ALH (19,35). However, advanced fibrosis is
reportedly not observed in DI-ALH (1,16,31).
By contrast, in the present study, 3 patients with DI-ALH (27.3%)
displayed a liver fibrosis grade of ≥F3. Although the etiology of
this was not clear, the possibility that AIH was potentially
present cannot be ruled out.
Another factor distinguishing DI-ALH from AIH is
that DI-ALH has been reported to respond to treatment earlier than
AIH and is characterized by the absence of relapse when
immunosuppressive agents are discontinued (16). In the present study, the majority of
patients with AIH and DILI-ALH were treated with steroids, and all
3 patients in the DILI-ALH group required steroids to achieve
recovery. However, the results also showed 17.2% of the AIH group
did not require immunosuppressive drugs. In fact, a Japanese
national study also found that ~20% of patients with AIH did not
receive immunosuppressive therapies such as steroids (34). Distinguishing between the three
groups based on differences in treatment methods or response to
treatment might thus be difficult.
In addition, no significant difference in relapse
rate during steroid tapering was evident between the three groups.
According to previous reports, the absence of recurrence after
long-term follow-up without immunosuppressive therapy is an
important feature of DI-ALH (15,16).
However, in the present case, 40.0% of patients in the DI-ALH group
experienced recurrence. As some drugs, including statins, may
induce a more classical form of AIH, differentiating AIH by the
presence of recurrence may be difficult.
The present study identified a new phenotype after
DILI in which liver damage with the characteristics of AIH appears.
There are some limitations to the study. First, differentiating
this diagnosis from AIH based on clinical, laboratory and
histological examinations in addition to response to steroid
therapy was considered difficult. Second, the total number of cases
in this study was relatively small in order to obtain definite
results. In addition, the cases were considered from a clinical
perspective, so the immunological mechanisms of disease formation
could not be fully elucidated. It will be necessary to accumulate
more cases and further elucidate the pathogenesis.
In conclusion, the present study suggested that
drugs may play a greater role in the pathogenesis of AIH than
currently recognized, and the associations between DILI
immunophenotypes and AIH should be properly classified and
investigated in detail to clarify the exact epidemiologies of
drug-related AIH. The present study revealed a new phenotype,
DILI-ALH, as one phenotype of drug-related ALH. Specific biomarkers
that allow differentiation of phenotypes are also important, and
further studies will be needed to develop guidelines for the
management of these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KI, SF, MK, YS, YN, HM, YN, DS, IM, YY, HI and KS
designed the research and performed data curation. KI, SF, KF and
KS confirm the authenticity of all the raw data, and were
responsible for data collection and statistical analyses. KI and KS
were major contributors to writing the original draft of the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of Mie Prefectural General Medical Center (Yokkaichi,
Japan; no. O-0094). Informed consent was considered to have been
obtained from all patients included in the study via an opt-out
protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Björnsson E, Talwalkar J, Treeprasertsuk
S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M and Lindor K:
Drug-induced autoimmune hepatitis: Clinical characteristics and
prognosis. Hepatology. 51:2040–2048. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liberal R, Longhi MS, Mieli-Vergani G and
Vergani D: Pathogenesis of autoimmune hepatitis. Best Pract Res
Clin Gastroenterol. 25:653–664. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Manns MP, Lohse AW and Vergani D:
Autoimmune hepatitis-update 2015. J Hepatol. 62 (Suppl
1):S100–S111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sebode M, Schulz L and Lohse AW:
‘Autoimmune(-like)’ drug and herb induced liver injury: New
insights into molecular pathogenesis. Int J Mol Sci.
18(1954)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lammert C, Chalasani SN, Atkinson EJ,
McCauley BM and Lazaridis KN: Environmental risk factors are
associated with autoimmune hepatitis. Liver Int. 41:2396–2403.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chalasani NP, Maddur H, Russo MW, Wong RJ
and Reddy KR: Practice Parameters Committee of the American College
of Gastroenterology. ACG clinical guideline: Diagnosis and
management of idiosyncratic drug-induced liver injury. Am J
Gastroenterol. 116:878–898. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
European Association for the Study of the
Liver. EASL clinical practice guidelines: Autoimmune hepatitis. J
Hepatol. 63:971–1004. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
European Association for the Study of the
Liver. Electronic address: easloffice@easloffice.eu; European
Association for the Study of the Liver. EASL clinical practice
guidelines: Drug-induced liver injury. J Hepatol. 70:1222–1261.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mack CL, Adams D, Assis DN, Kerkar N,
Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH and Czaja AJ:
Diagnosis and management of autoimmune hepatitis in adults and
children: 2019 Practice guidance and guidelines from the American
association for the study of liver diseases. Hepatology.
72:671–722. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weiler-Normann C and Schramm C: Drug
induced liver injury and its relationship to autoimmune hepatitis.
J Hepatol. 55:747–749. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Castiella A, Zapata E, Lucena MI and
Andrade RJ: Drug-induced autoimmune liver disease: A diagnostic
dilemma of an increasingly reported disease. World J Hepatol.
6:160–168. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de Boer YS, Kosinski AS, Urban TJ, Zhao Z,
Long N, Chalasani N, Kleiner DE and Hoofnagle JH: Drug-Induced
Liver Injury Network. Features of autoimmune hepatitis in patients
with drug-induced liver injury. Clin Gastroenterol Hepatol.
15:103–112.e2. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lammert C, Zhu C, Lian Y, Raman I, Eckert
G, Li QZ and Chalasani N: Exploratory study of autoantibody
profiling in drug-induced liver injury with an autoimmune
phenotype. Hepatol Commun. 4:1651–1663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan CK, Ho D, Wang LM and Kumar R:
Drug-induced autoimmune hepatitis: A minireview. World J
Gastroenterol. 28:2654–2666. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Björnsson ES, Medina-Caliz I, Andrade RJ
and Lucena MI: Setting up criteria for drug-induced autoimmune-like
hepatitis through a systematic analysis of published reports.
Hepatol Commun. 6:1895–1909. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Andrade RJ, Aithal GP, de Boer YS, Liberal
R, Gerbes A, Regev A, Terziroli Beretta-Piccoli B, Schramm C,
Kleiner DE, De Martin E, et al: Nomenclature, diagnosis and
management of drug-induced autoimmune-like hepatitis (DI-ALH): An
expert opinion meeting report. J Hepatol. 79:853–866.
2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Andrade RJ, Chalasani N, Björnsson ES,
Suzuki A, Kullak-Ublick GA, Watkins PB, Devarbhavi H, Merz M,
Lucena MI, Kaplowitz N and Aithal GP: Drug-induced liver injury.
Nat Rev Dis Primers. 5(58)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lucena MI, Kaplowitz N, Hallal H,
Castiella A, García-Bengoechea M, Otazua P, Berenguer M, Fernandez
MC, Planas R and Andrade RJ: Recurrent drug-induced liver injury
(DILI) with different drugs in the Spanish Registry: The dilemma of
the relationship to autoimmune hepatitis. J Hepatol. 55:820–827.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yeong TT, Lim KH, Goubet S, Parnell N and
Verma S: Natural history and outcomes in drug induced autoimmune
hepatitis. Hepatol Res. 46:E79–E88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Björnsson ES, Stephens C, Atallah E,
Robles-Diaz M, Alvarez-Alvarez I, Gerbes A, Weber S, Stirnimann G,
Kullak-Ublick G, Cortez-Pinto H, et al: A new framework for
advancing in drug-induced liver injury research. The prospective
European DILI registry. Liver Int. 43:115–126. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ueno M, Takabatake H, Kayahara T, Morimoto
Y, Notohara K and Mizuno M: Long-term outcomes of drug-induced
autoimmune-like hepatitis after pulse steroid therapy. Hepatol Res.
53:1073–1083. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sugimoto K, Ito T, Yamamoto N and Shiraki
K: Seven cases of autoimmune hepatitis that developed after
drug-induced liver injury. Hepatology. 54:1892–1893.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alvarez F, Berg PA, Bianchi FB, Bianchi L,
Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet
VJ, et al: International autoimmune hepatitis group report: Review
of criteria for diagnosis of autoimmune hepatitis. J Hepatol.
31:929–938. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Benichou C, Danan G and Flahault A:
Causality assessment of adverse reactions to drugs-II. An original
model for validation of drug causality assessment methods: Case
reports with positive rechallenge. J Clin Epidemiol. 46:1331–1336.
1993.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ichida F, Tsuji T, Omata M, Ichida T,
Inoue K, Kamimura T, Yamada G, Hino K, Yokosuka O and Suzuki H: New
Inuyama classification; new criteria for histological assessment of
chronic hepatitis. Int Hepatol Commun. 6:112–119. 1996.
|
|
26
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huppertz HI, Triechel U, Gassel AM,
Jeschke R and Büschenfelde KHMZ: Autoimmune hepatitis following
hepatitis A virus infection. J Hepatology. 23:204–208.
1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Singh G, Palaniappan S, Rotimi O and
Hamlin PJ: Autoimmune hepatitis triggered by hepatitis A. Gut.
56(304)2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rigopoulou EI, Zachou K, Gatselis N,
Koukoulis GK and Dalekos GN: Autoimmune hepatitis in patients with
chronic HBV and HCV infections: Patterns of clinical
characteristics, disease progression and outcome. Ann Hepatol.
13:127–135. 2013.PubMed/NCBI
|
|
30
|
Pischke S, Gisa A, Suneetha PV, Wiegand
SB, Taubert R, Schlue J, Wursthorn K, Bantel H, Raupach R, Bremer
B, et al: Increased HEV seroprevalence in patients with autoimmune
hepatitis. PLoS One. 9(e85330)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zellos A, Spoulou V, Roma-Giannikou E,
Karentzou O, Dalekos GN and Theodoridou M: Autoimmune hepatitis
type-2 and Epstein-Barr virus infection in a toddler: Art of facts
or an artifact? Ann Hepatol. 12:147–151. 2013.PubMed/NCBI
|
|
32
|
Martínez-Casas OY, Díaz-Ramírez GS,
Marín-Zuluaga JI, Muñoz-Maya O, Santos O, Donado-Gómez JH and
Restrepo-Gutiérrez JC: Differential characteristics in drug-induced
autoimmune hepatitis. JGH Open. 2:97–104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Stephens C, Robles-Diaz M, Medina-Caliz I,
Garcia-Cortes M, Ortega-Alonso A, Sanabria-Cabrera J,
Gonzalez-Jimenez A, Alvarez-Alvarez I, Slim M, Jimenez-Perez M, et
al: Comprehensive analysis and insights gained from long-term
experience of the Spanish DILI registry. J Hepatol. 75:86–97.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Takahashi A, Arinaga-Hino T, Ohira H,
Torimura T, Zeniya M, Abe M, Yoshizawa K, Takaki A, Suzuki Y, Kang
JH, et al: Autoimmune hepatitis in Japan: Trends in a nationwide
survey. J Gastroenterol. 52:631–640. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang H, Fu J, Liu G, Wang L, Yan A and
Wang G: Drug induced autoimmune hepatitis (DIAIH): Pathological and
clinical study. Biomed Res. 28:6028–6034. 2017.
|