Introduction
Angiogenesis, the formation of new blood vessels, is
a crucial process in cancer progression, including lung cancer
(1,2). Tumor cells and stromal cells in the
tumor microenvironment (TME) secrete various molecules that
stimulate endothelial cell proliferation and migration, promoting
the formation of new blood vessels to supply oxygen and nutrients
needed for tumor growth and metastasis (3). Increased angiogenesis and elevated
levels of angiogenic factors are associated with poor prognosis in
lung cancer (4-6).
Recently, inhibiting angiogenesis has gained attention as a
therapeutic strategy for lung cancer (2). Drugs targeting vascular endothelial
growth factor (VEGF) and receptor tyrosine kinase can inhibit tumor
growth and spread; however, their efficacy remains limited
(2). Therefore, an improved
understanding of the mechanisms driving angiogenesis is essential
for the development of more effective therapies for lung
cancer.
Angiogenesis is tightly regulated by a balance
between pro-angiogenic and anti-angiogenic factors (7). Among the pro-angiogenic factors, VEGF
serves as a key player in tumor angiogenesis (8). Previously, it was considered that
endothelial cells did not produce VEGF, but the accumulation of
evidence indicates that the cells can produce small amount of VEGF
for vascular homeostasis and VEGF can act as both a paracrine and
an autocrine signaling molecule (9). During tumor growth, hypoxia and
conditions within the TME stimulate VEGF expression in endothelial
cells to initiate angiogenesis (8,10,11).
VEGF binds to its receptors, VEGFR1 and VEGFR2, on endothelial
cells to promote endothelial cell proliferation and survival
(8,12). Activated endothelial cells
subsequently migrate, invade the extracellular matrix and aggregate
with neighboring cells, eventually forming tubular structures that
develop into a functional vascular network (7,13).
Heat shock protein family D member 1 (HSPD1), also
known as HSP60 or chaperonin 60, is a multifunctional protein
implicated in various cancers (14). It has been shown to regulate cancer
cell apoptosis, proliferation and metastasis, while extracellular
HSPD1 functions as an immune modulator involved in tumor immunity
(14,15). Studies highlight its significant
role in lung cancer development and progression (16-19).
Increased HSPD1 expression is associated with poor prognosis in
patients with non-small cell lung cancer (16-18).
HSPD1 promotes lung cancer growth by regulating mitochondrial
functions (17) and plays a role in
modulating the TME (18,19). HSPD1 expression is associated with
the infiltration of various immune cells (18). A previous study demonstrates that
the knockdown of HSPD1 in lung cancer cells alters the composition
of secreted proteins and diminishes its effect on the activation of
cancer-associated fibroblasts (CAFs) (19). Evidence also suggests that HSPD1
regulates endothelial cell function and angiogenesis (20-23).
Recent findings indicate that the long non-coding RNA LINC01503
promotes angiogenesis in colorectal cancer by increasing VEGFA
expression through interactions with microRNA (miR)-342-3p and
HSPD1(24). Collectively, these
findings suggest that HSPD1 may play a pivotal role in regulating
angiogenesis. However, its specific role in lung cancer remains
unclear and needs further elucidation.
The present study aimed to investigate the
involvement of HSPD1 in lung cancer cell-induced angiogenesis using
indirect co-culture experiments. The effects of secretome derived
from HSPD1-knockdown lung cancer cells on the angiogenic properties
of endothelial cells were investigated, in comparison with
secretome from control cells. Angiogenic behaviors, including cell
proliferation, migration, invasion, aggregation and tube formation,
were evaluated in endothelial cells following secretome treatment.
VEGF secretion levels from endothelial cells were measured.
Finally, the correlation of VEGFA expression with HSPD1 expression
and overall survival in patients with lung adenocarcinoma was
analyzed using bioinformatics. The findings of the present study
provided new insights into the role of HSPD1 in promoting tumor
angiogenesis of lung cancer. Targeting HSPD1 may be a potential
therapeutic strategy to inhibit angiogenesis and tumor progression
in lung cancer patients.
Materials and methods
Cell culture
Human lung cancer A549 (CCL-185) and human
endothelial EA.hy926 (CRL-2922) cell lines were obtained from the
American Type Culture Collection (ATCC). Knockdown of HSPD1 in A549
cells was achieved using a shRNA-lentiviral system, as previously
described (19). Stable
HSPD1-knockdown (shHSPD1-A549) and shControl-A549 cells were
maintained in Dulbecco's Modified Eagle's Medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1
µg/ml puromycin. EA.hy926 cells were cultured in DMEM supplemented
with 10% FBS, 100 IU/ml penicillin G and 100 µg/ml streptomycin.
All cells were incubated at 37˚C in a humidified incubator with 5%
CO2.
Establishment of shHSPD1-A549 and
shControl-A549 cells
shHSPD1 with the following oligonucleotide sequence:
5'-GCTAAACTTGTTCAAGATGTTTCAAGAGAACATCTTGAACAAGTTTAGCTTTTTT-3' was
cloned into the pLKO.1-puro plasmid (a gift from Dr Bob Weinberg,
Whitehead Institute for Biomedical Research and Department of
Biology, Massachusetts Institute of Technology, MA, USA; Addgene
plasmid no. 8453). The scrambled shRNA (shControl) with the
oligonucleotide sequence:
5'-CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG-3' inserted
into the pLKO.1-puro plasmid (a gift from Dr David Sabatini,
Whitehead Institute for Biomedical Research and Department of
Biology, Massachusetts Institute of Technology, MA, USA; Addgene
plasmid no. 1864) served as a negative control. Using a
third-generation lentivirus system, lentiviral particles were
produced by co-transfecting 293T cells (CRL-3216; ATCC) with either
pLKO.1-puro-shHSPD1 (6 µg) or pLKO.1-puro-shControl (6 µg), along
with packaging plasmids, including pMDLg/pRRE (a gift from Dr
Didier Trono, Department of Genetics and Microbiology, Faculty of
Medicine, University of Geneva, Switzerland; Addgene plasmid no.
12251), pRSV-Rev (a gift from Dr Didier Trono, Addgene no. 12253),
and pCMV-VSV-G (a gift from Dr Bob Weinberg, Addgene plasmid no.
8454) using jetPRIME transfection reagent (Polyplus) for 12 h at
37˚C. At 24 h post-transfection, the viral supernatant was
harvested and used to transduce A549 cells at a multiplicity of
infection of 10 in the presence of polybrene (4 µg/ml) at 37˚C for
24 h. Stable shHSPD1-A549 and shControl-A549 cells were selected
using puromycin (1 µg/ml) for ~1 week. Knockdown efficiency was
confirmed by western blot analysis.
Western blot analysis for assessment
of knockdown efficacy
Protein was extracted from shHSPD1-A549 and
shControl-A549 cells using RIPA buffer (Abcam). Protein
concentrations were determined by the Bradford assay (Bio-Rad
Laboratories, Inc.). Equal amounts of protein from each sample (20
µg) were separated via 12% SDS-PAGE and transferred onto a
nitrocellulose membrane. The membrane was blocked with 5% (w/v)
skimmed milk in phosphate-buffered saline (PBS) at room temperature
for 1 h. Subsequently, the membrane was incubated overnight at 4˚C
with either rabbit monoclonal anti-HSPD1 (1:1,000; cat. no. 12165;
Cell Signaling Technology, Inc.) or mouse monoclonal anti-GAPDH
(1:5,000; cat. no. ab8245; Abcam). After three washes with PBS
containing 0.05% (v/v) Tween 20 (PBS-T), the membrane was incubated
with horseradish peroxidase-conjugated secondary anti-rabbit IgG
(1:5,000; cat. no. ab6721; Abcam) or anti-mouse IgG (1:5,000; cat.
no. ab205719; Abcam) antibodies at room temperature for 1 h.
Immunoreactive bands were visualized using Clarity Western ECL
Substrate and captured with the ChemiDoc Touch imaging system
(Bio-Rad Laboratories, Inc.).
Secretome treatment
Secretome was collected from shHSPD1-A549 and
shControl-A549 cells according to the protocol described in the
previous study (19). Briefly, an
equal number of shHSPD1-A549 and shControl-A549 cells were seeded
in complete medium overnight. After washing with PBS, the cells
were incubated in serum-free DMEM for 24 h. The collected
supernatants were centrifuged at 1,000 x g for 5 min at 4˚C to
remove debris and used for subsequent experiments. Cell
proliferation, total cell number and VEGF levels in the secretome
of shHSPD1-A549 and shControl-A549 cells were assessed. Total cell
number of shHSPD1-A549 and shControl-A549 cells after incubation in
serum-free DMEM for 24 h was determined by trypan blue staining.
The cells were harvested, resuspended in PBS, and stained with 0.4%
trypan blue solution (Gibco, Thermo Fisher Scientific, USA) at a
1:1 ratio for 2 min at room temperature. The stained cell
suspension was loaded onto a hemocytometer. Total cell number was
counted under a Zeiss Primovert inverted microscope (Zeiss
GmbH).
For EA.hy926 cell treatment, secretome, derived from
an equal number of cells was mixed with serum-free DMEM at a 1:1
(v/v) ratio. EA.hy926 cells were initially plated in complete
medium and incubated at 37˚C overnight. After washing with PBS, the
cells were treated with either shControl or shHSPD1 secretome at
37˚C for 24 h. Cells incubated with serum-free DMEM served as
untreated controls. The morphology of EA.hy926 cells was observed
and captured using a Zeiss Primovert inverted microscope (Zeiss
GmbH).
MTT assay
Cell viability was evaluated using the MTT assay.
Following 24 h of secretome treatment, EA.hy926 cells were exposed
to 0.5 mg/ml MTT solution. After incubation, the solution was
discarded and dimethyl sulfoxide was added to dissolve the
resulting formazan crystals. Absorbance was recorded at 570 nm
using a Tecan Spark multimode microplate reader (Tecan Group,
Ltd.). Cell viability was expressed as a percentage relative to the
untreated control group.
BrdU assay
Cell proliferation was determined using a BrdU Cell
Proliferation ELISA Kit (ab126556; Abcam) according to the
manufacturer's instructions. EA.hy926 cells were seeded in a
96-well plate and treated with the secretome for 24 h. After
incubation with BrdU solution for 2 h, the cells were fixed using a
fixing solution (included in the kit) for 30 min at room
temperature. The fixed cells were then incubated with a primary
anti-BrdU antibody, followed by a horseradish peroxidase-conjugated
secondary antibody. Subsequently, 3,3',5,5'-tetramethylbenzidine
substrate was added and the absorbance at 450 nm was measured using
a Tecan Spark multimode microplate reader (Tecan Group, Ltd.). Cell
proliferation was calculated as a fold change relative to the
untreated control group.
Wound healing-scratch assay
Cell migration was assessed using a wound
healing-scratch assay, as previously described (19). A 200-µl pipette tip was used to
scratch the EA.hy926 cell monolayers and detached cells were
removed by washing with PBS. The remaining cells were treated with
secretome, without FBS supplementation, and images of the scratch
area were captured at various time points (0, 8, 16 and 24 h after
treatment with secretome) using an IX83 inverted microscope
(Olympus Corporation) equipped with an STX stage-top incubator
(Tokai Hit). Image analysis was performed using ImageJ version
1.54d (National Institutes of Health) with the wound healing size
tool plugin (25). The percentage
of wound healing was calculated using the formula: Percentage of
wound healing=100% x (initial wound size - wound size at each time
point)/initial wound size.
Cell invasion assay
Cell invasion was determined using Transwell
invasion assay. Transwell inserts with an 8 µm pore size (Corning
Inc., Corning, NY, USA) were pre-coated with Matrigel Basement
Membrane Matrix (Corning Inc.) at 37˚C overnight, following the
manufacturer's instructions. EA.hy926 cells were seeded into the
upper chamber of each insert in a serum-free medium containing
either shControl or shHSPD1 secretome, while the lower chamber was
filled with DMEM supplemented with 20% FBS. After a 24-h
incubation, cells remaining on the upper surface of the membrane
were removed with a cotton swab. The filters were washed with PBS,
fixed with 4% formaldehyde in PBS for 10 min and stained with 0.5%
crystal violet for 20 min. Images of stained cells were captured
using a Zeiss Primovert inverted microscope (Zeiss GmbH). Crystal
violet bound to the cells was then dissolved in 33% acetic acid and
absorbance was measured at 590 nm using a Tecan Spark multimode
microplate reader (Tecan Group, Ltd.). Cell invasion was expressed
as a fold change relative to the untreated control group.
Cell aggregation assay
Cell aggregation was assessed using a hanging drop
assay, as previously described (26). EA.hy926 cells were detached from the
monolayer culture using trypsinization and then resuspended in a
serum-free medium containing either shControl or shHSPD1 secretome
at a concentration of 5,000 cells/ml. Drops of 20 µl of the cell
suspension were placed on the inner side of the lid of a 100-mm
tissue culture dish. To maintain humidity, 5 ml of PBS was added to
the bottom of the dish and the lid was inverted to cover it. After
24-h incubation, a total of 30 drops per group were collected,
dispersed by gentle pipetting and examined for multicellular
spheroid formation using a Zeiss Primovert inverted microscope
(Zeiss GmbH). The sizes of spheroids were measured from 30 cell
aggregates for each sample using ImageJ version 1.54d (National
Institutes of Health) (27).
Endothelial tube formation assay
Capillary-like tube formation by EA.hy926 cells was
assessed on 96-well plates pre-coated with Matrigel® Basement
Membrane Matrix (Corning Inc.). Each well was coated with 50 µl of
Matrigel and incubated at 37˚C for 30 min to allow for
solidification. Following a 24-h treatment with either shControl or
shHSPD1 secretome, EA.hy926 cells were detached and seeded onto the
solidified Matrigel at a density of 5x104 cells per
well. The cells were then incubated in DMEM supplemented with 10%
FBS for an additional 24 h. Tube formation was visualized using an
IX83 inverted microscope (Olympus) with an STX stage-top incubator
(Tokai Hit). Tube length and area were quantified from 30 tube
formations for each sample using ImageJ version 1.54d (National
Institutes of Health) (27).
ELISA
The concentration of VEGF in the culture supernatant
of EA.hy926 cells following secretome treatment was measured using
a human VEGF ELISA Kit (cat. no. ab222510; Abcam), following the
manufacturer's protocol. VEGF levels were determined from a
standard curve.
Bioinformatic analysis
The correlation between VEGFA and HSPD1 mRNA
expression was analyzed in The Cancer Genome Atlas-Lung
Adenocarcinoma (TCGA-LUAD) cohort (n=515) using the Tumor IMmune
Estimation Resource (TIMER) database (https://cistrome.shinyapps.io/timer/) (28,29).
Spearman's ρ and P-values were calculated by the database. The
association between VEGFA mRNA expression and overall survival in
patients with lung adenocarcinoma was evaluated in a lung
adenocarcinoma cohort (n=1,863) using the Kaplan-Meier Plotter
database (https://www.kmplot.com) (30). Patients were divided into low- and
high-expression groups based on the median VEGFA mRNA expression
levels (Affymetrix ID: 210513_s_at). The hazard ratio and log-rank
P-value were computed by the database.
Statistical analysis
Quantitative data are presented as mean ± SD.
Comparisons between two groups were conducted using an unpaired
t-test, while multiple group comparisons were performed using
one-way analysis of variance (ANOVA) followed by Tukey's post-hoc
test. Statistical analyses were conducted using GraphPad Prism
version 8.0.1 (Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of shHSPD1-A549 and
shControl-A549 secretomes on EA.hy926 cell proliferation
The secretome was derived from an equal number of
shHSPD1-A549 and shControl-A549 cells. At the time of secretome
collection, no significant difference in cell proliferation and
total cell number was observed (Fig.
S1). Additionally, VEGF levels in the shHSPD1-A549 and
shControl-A549 secretomes showed no significant difference
(Fig. S1). These secretomes were
then used to treat EA.hy926 cells. After 24-h of treatment, the
cell morphology of EA.hy926 cells was observed under an inverted
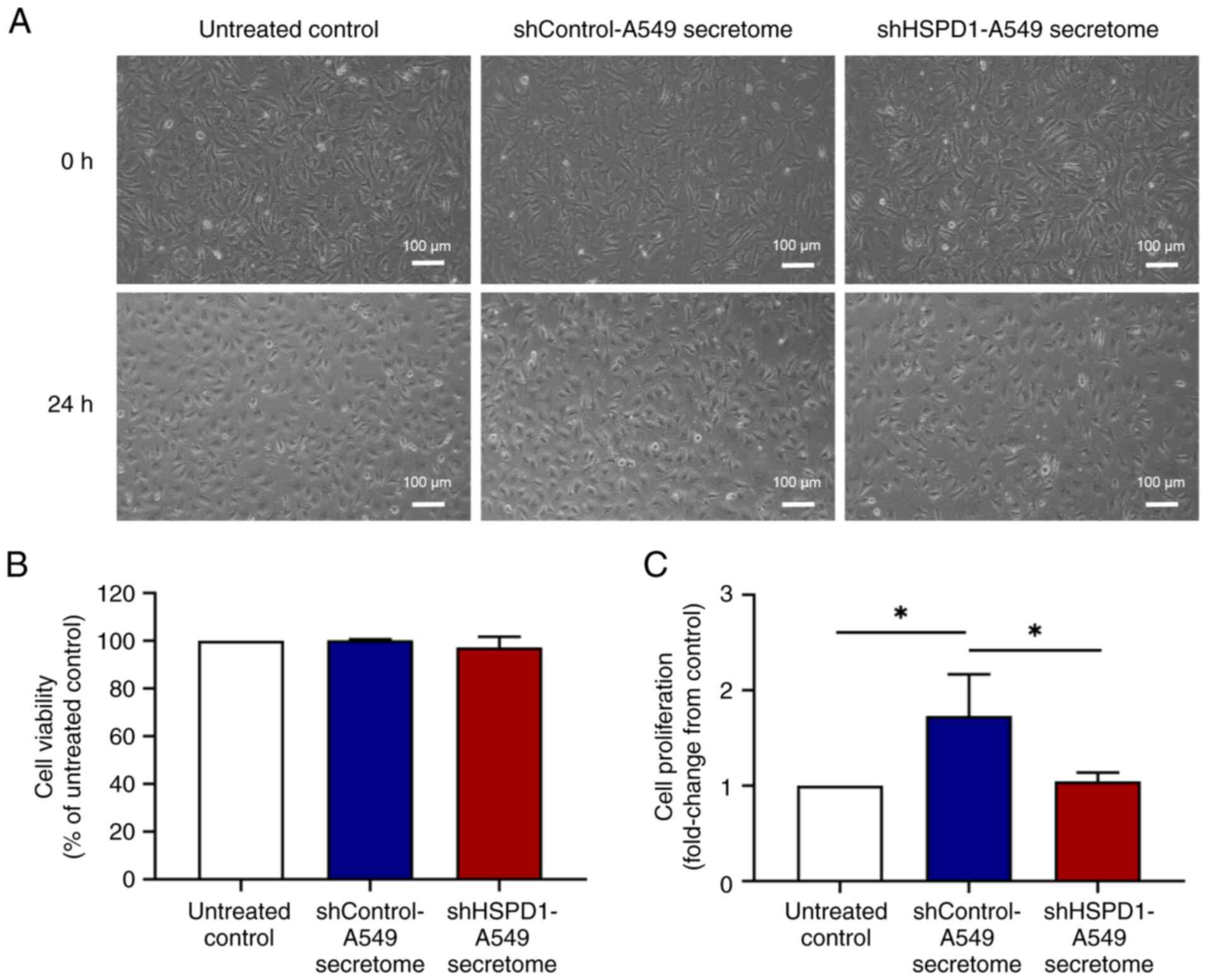
microscope. As shown in Fig. 1A, no
morphological signs of cytotoxicity were observed following
treatment with either the shHSPD1-A549 or shControl-A549
secretomes. Results of MTT assay further confirmed that these
secretomes had no significant cytotoxic effect on EA.hy926 cells
(Fig. 1B). Notably, the results of
BrdU assay demonstrated that the shControl-A549 secretome markedly
promoted cell proliferation, while the shHSPD1-A549 secretome
showed no stimulatory effect (Fig.
1C). These findings suggested that HSPD1 might play a role in
stimulating endothelial cell proliferation.
Effect of shHSPD1-A549 and
shControl-A549 secretomes on EA.hy926 cell migration
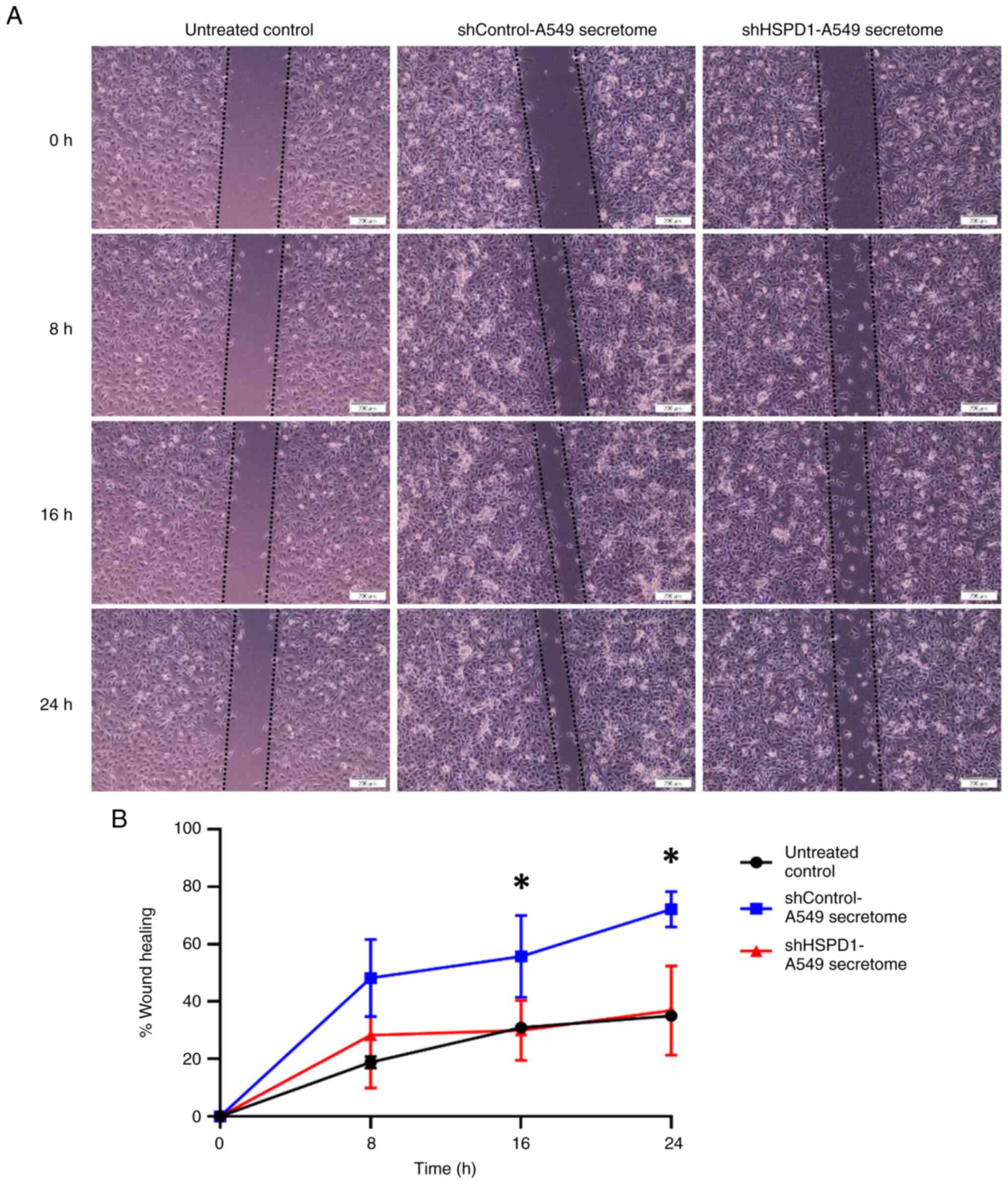
The effect of shHSPD1-A549 and shControl-A549
secretomes on EA.hy926 cell migration was evaluated using a wound
healing-scratch assay. Results indicated that the percentage of
wound healing increased gradually with extended incubation time.
Cells treated with the shControl-A549 secretome showed a markedly
higher percentage of wound healing at the 16- and 24-h time points.
By contrast, no significant difference was observed between cells
treated with the shHSPD1-A549 secretome and the untreated control
group (Fig. 2). These findings
suggested that HSPD1 could enhance migration capability of
endothelial cells.
Effect of shHSPD1-A549 and
shControl-A549 secretomes on EA.hy926 cell invasiveness
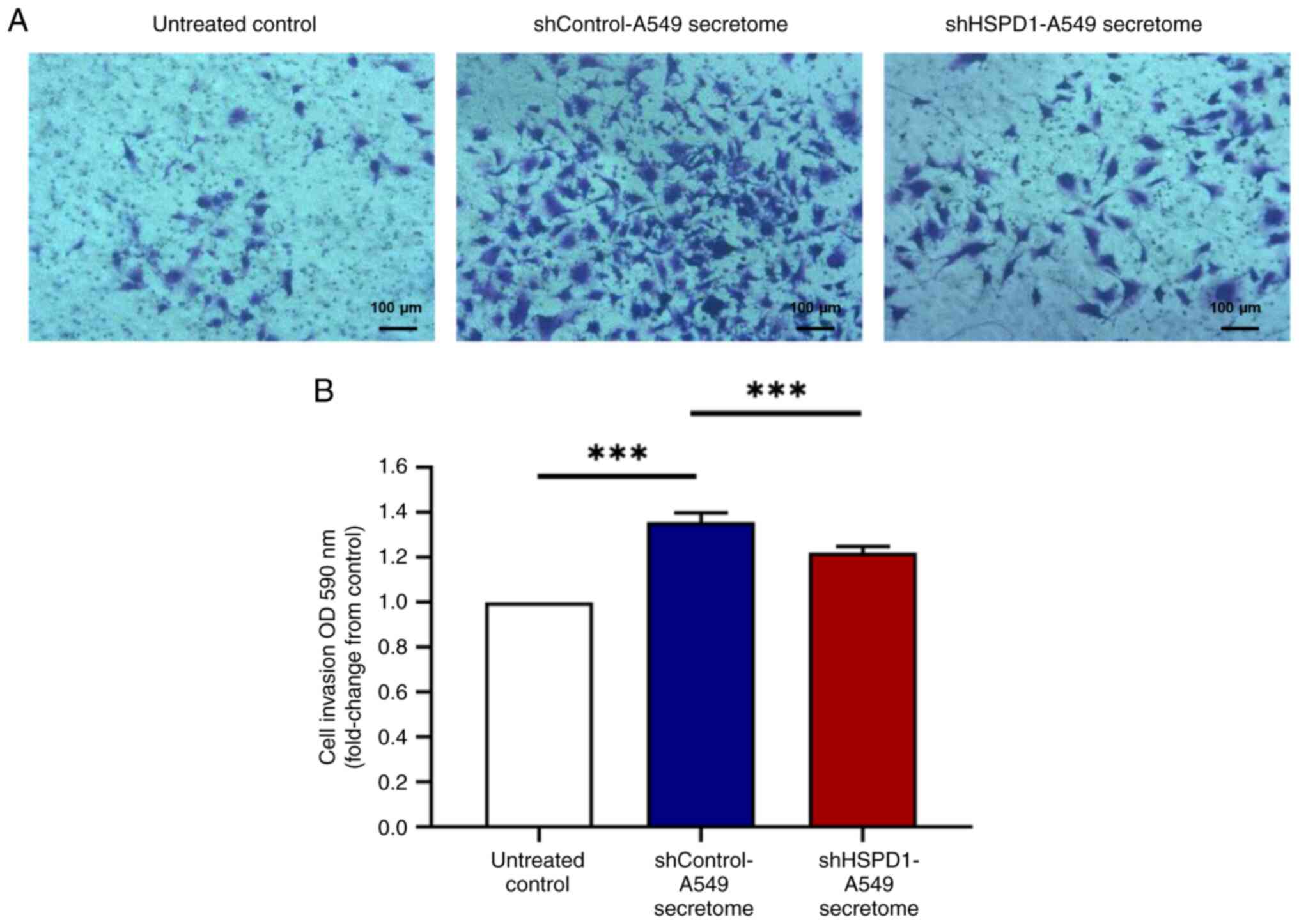
A Transwell invasion assay was performed to assess
the invasiveness of EA.hy926 cells following secretome treatment.
The shControl-A549 secretome markedly enhanced invasion of EA.hy926
cells, while the secretome from HSPD1 knockdown did not increase
cell invasion (Fig. 3). These
findings indicated a crucial role of HSPD1 in promoting invasion of
endothelial cells.
Effect of shHSPD1-A549 and
shControl-A549 secretomes on EA.hy926 cell aggregation
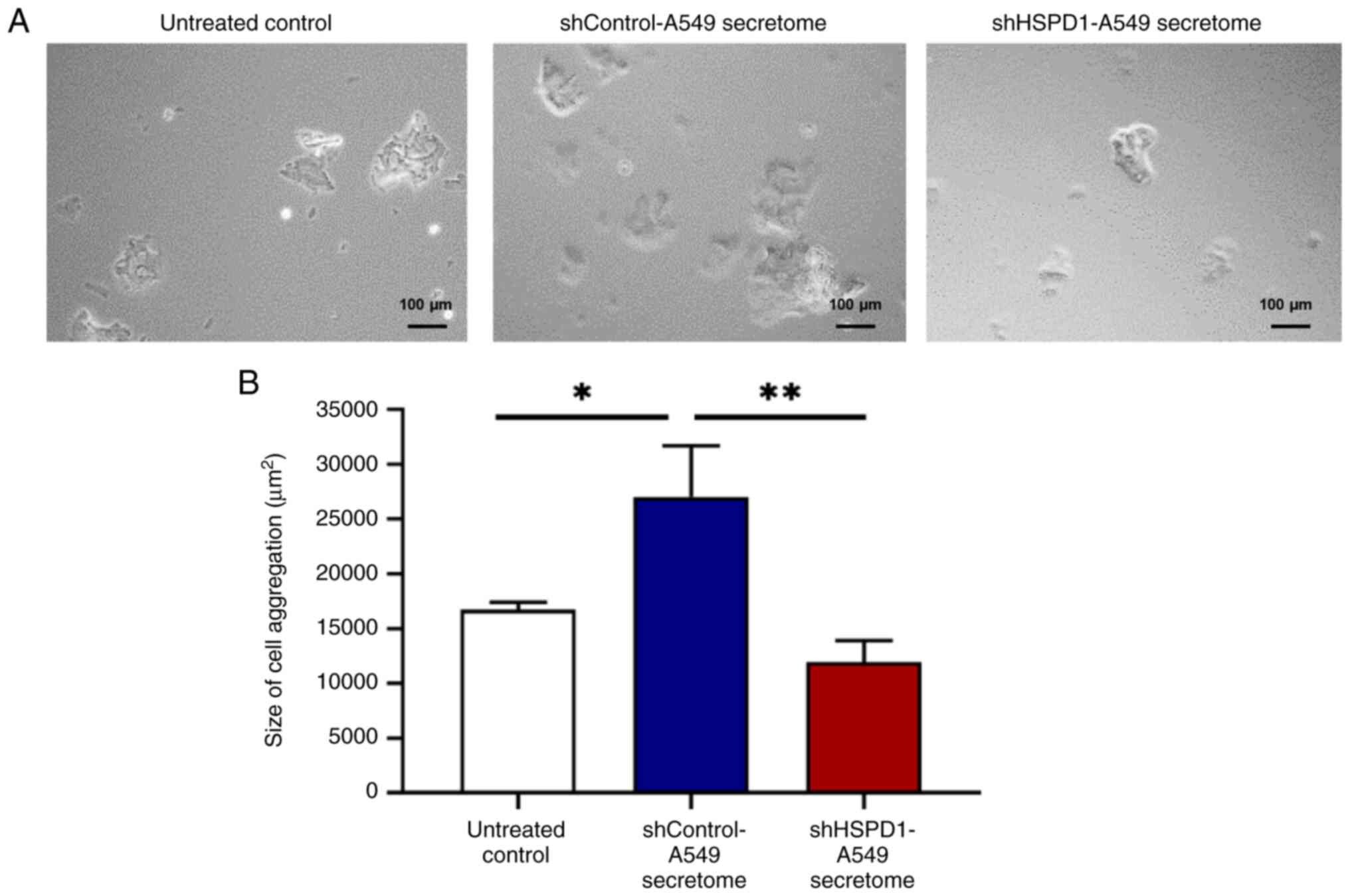
A hanging-drop assay was performed to assess the
effect of shHSPD1-A549 and shControl-A549 secretomes on cell
aggregation in EA.hy926 cells. As shown in Fig. 4, the shControl-A549 secretome
promoted cell aggregation in EA.hy926 cells. The size of cell
aggregates appeared similar between cells treated with the
shHSPD1-A549 secretome and those in the untreated control group,
indicating that HSPD1 may contribute to lung cancer-induced cell
aggregation in EA.hy926 cells.
Effect of shHSPD1-A549 and
shControl-A549 secretomes on endothelial tube formation in EA.hy926
cells
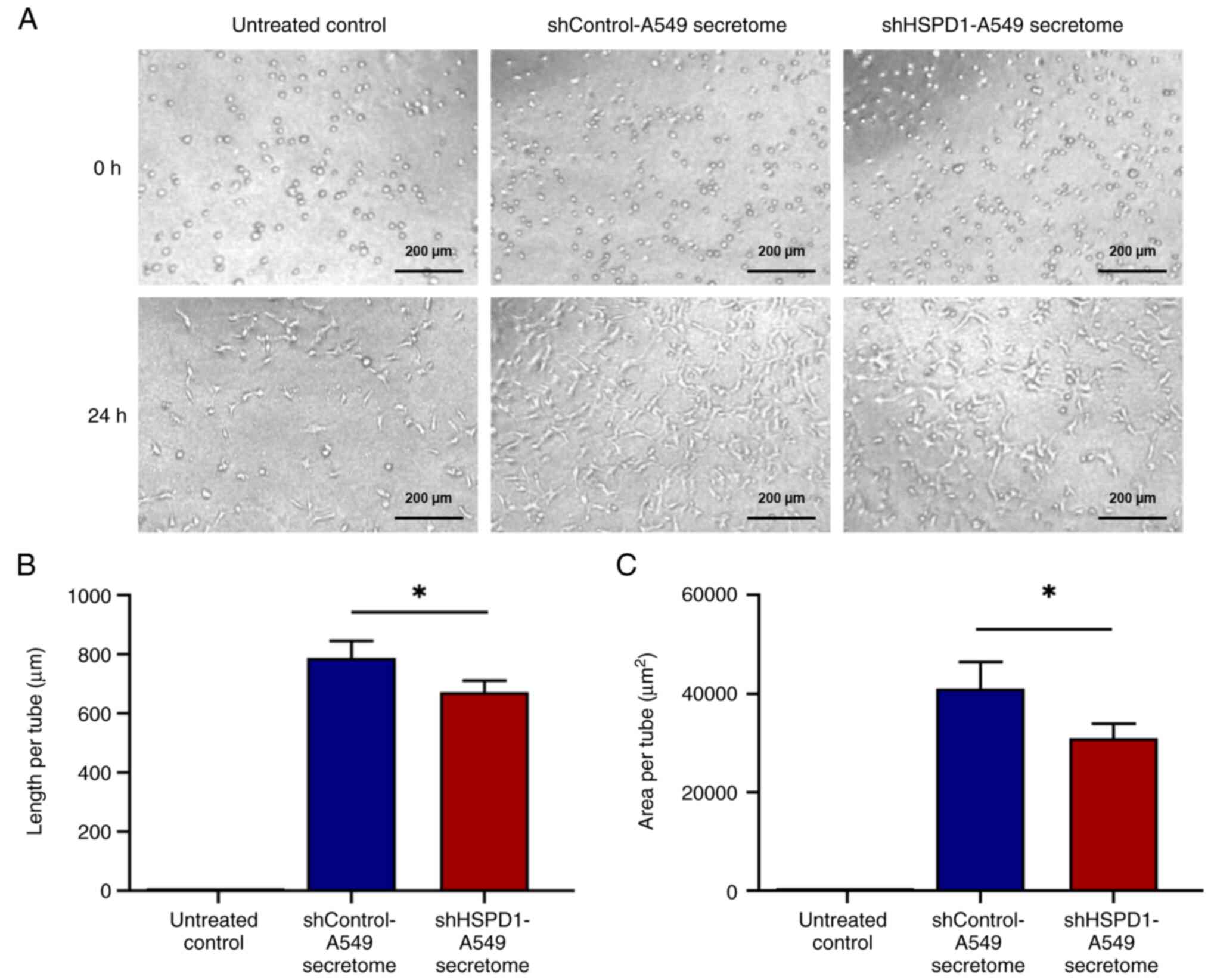
To assess the angiogenic potential of EA.hy926 cells
in response to secretome treatment, a tube formation assay was
conducted. No tube formation was observed in the untreated control
group. By contrast, EA.hy926 cells treated with the shControl-A549
secretome could form a network of tubular structure, which was
markedly longer and larger than the tubular structure of EA.hy926
cells treated with the shHSPD1-A549 secretome (Fig. 5). These findings suggested that
HSPD1 may play a critical role in tube formation of EA.hy926
cells.
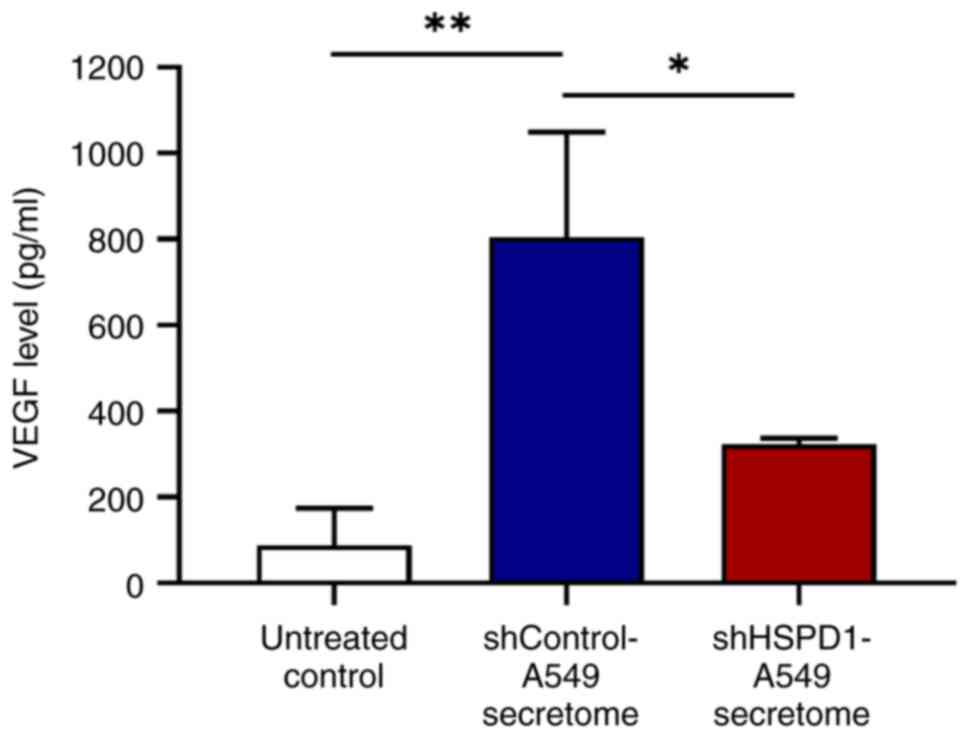
Effect of shHSPD1-A549 and
shControl-A549 secretomes on VEGF secretion from EA.hy926
cells
Given the role of VEGF as a key pro-angiogenic
factor in cancer-induced angiogenesis (8), VEGF levels in the culture supernatant
of EA.hy926 cells treated with shHSPD1-A549 and shControl-A549
secretomes were measured by ELISA. Consistent with its stimulatory
effect on tube formation, the shControl-A549 secretome markedly
increased VEGF secretion. By contrast, cells treated with the
shHSPD1-A549 secretome exhibited lower VEGF levels, suggesting that
HSPD1 may induce VEGF-mediated angiogenesis in lung cancer
(Fig. 6).
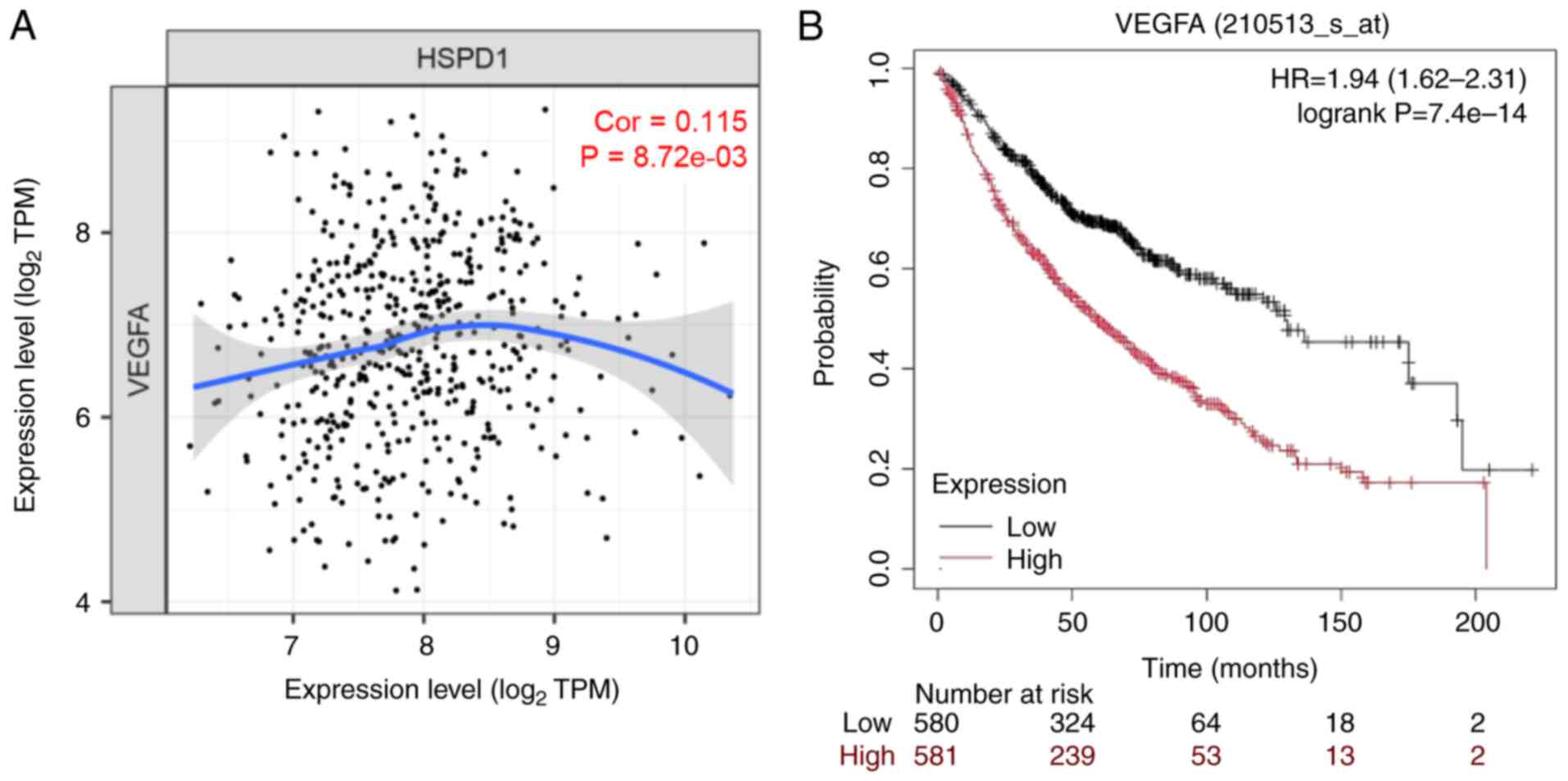
Correlation of VEGFA expression with
HSPD1 expression and overall survival in patients with lung
adenocarcinoma
The TIMER database was used to assess the
correlation between VEGFA and HSPD1 expression in the TCGA-LUAD
cohort. The results showed that VEGFA expression was markedly
associated with HSPD1 expression in lung adenocarcinoma (Fig. 7A). Additionally, the relationship
between VEGFA expression and overall survival in lung
adenocarcinoma patients was evaluated. Data from the KM Plotter
database revealed that patients with high VEGFA expression had
poorer survival outcomes compared to those with low VEGFA
expression (Fig. 7B). These
findings highlighted the clinical relevance of HSPD1 and VEGFA, as
well as the prognostic significance of VEGFA in lung cancer
patients.
Discussion
Angiogenesis is recognized as a hallmark of cancer
that plays a pivotal role in tumor growth and metastasis (7). HSPD1 has been implicated in various
cancers due to its roles in regulating apoptosis, metabolism and
immune responses (14,15). It has been shown to regulate
endothelial cell function and the secretion of pro-angiogenic
factors, such as VEGF (20-24).
Previous studies have demonstrated that HSPD1 not only exerts its
oncogenic effects on lung cancer cells (17), but also modulates the TME to further
support cancer growth and progression (18,19).
Knockdown of HSPD1 in lung cancer cells has been shown to alter
secretory protein composition and reduce their activity in
activating CAFs (19). Notably, the
secretome from shHSPD1-knockdown cells exhibited decreased levels
of HSPD1(19). These findings
suggested that HSPD1 may also play a role in regulating other cells
within the TME, including endothelial cells.
As secreted proteins are present in very limited
amounts in the culture medium, concentration and preparation steps
are required for accurate quantification (31). To minimize variations and ensure
consistency, the secretome in the present study was collected from
an equal number of shHSPD1-A549 and shControl-A549 cells. There was
no significant difference in cell proliferation and cell number at
the time of secretome collection. Therefore, it was considered that
biomolecules were present in comparable amounts in the secretomes
of these two cell groups. Additionally, VEGF levels in the
shHSPD1-A549 and shControl-A549 secretomes showed no significant
difference, suggesting that other secreted proteins may play key
roles in regulating angiogenesis in lung cancer.
Previous studies have demonstrated that secreted
proteins from lung cancer cells promote endothelial cell
proliferation, migration and angiogenesis (32-34).
Consistent with these findings, the present study revealed that
treatment with the secretome from shControl-A549 cells markedly
enhanced endothelial cell proliferation, migration, invasion,
aggregation and tube formation. By contrast, the secretome from
shHSPD1-A549 cells showed no significant effects on these
processes. These results suggested that HSPD1 may serve as a key
regulator of endothelial cell behavior and angiogenesis. However,
the wound healing-scratch and Transwell invasion assays used in the
present study could not distinguish the effects of cell
proliferation from those of migration and invasion (35). Additional experiments using
proliferation inhibitors are needed to more precisely define the
role of the secretome in endothelial cell migration and
invasion.
The mechanism by which HSPD1 promotes angiogenesis
is not fully understood. However, the findings of the present study
suggested that HSPD1 may induce VEGF-mediated angiogenesis. VEGF is
a potent pro-angiogenic factor that stimulates endothelial cell
proliferation, migration and tube formation (8). A recent study demonstrates that HSPD1
enhances the protein stability and secretion of VEGF in colorectal
cancer (24). Additionally, HSPD1
is recognized as a ligand for Toll-like receptor 4 (TLR4) (36,37),
whose signaling plays a critical role in regulating endothelial
cell functions (38). Activation of
TLR4 promotes the secretion of cytokines and pro-angiogenic
factors, including VEGF (39,40).
Therefore, HSPD1 may induce VEGF secretion through mechanisms
involving TLR4 and its downstream signaling pathways, contributing
to angiogenesis in lung cancer. However, further experiments using
recombinant HSPD1 protein are needed to clarify its role in
angiogenesis and the underlying mechanisms.
VEGF exerts its effects by binding to its receptors,
VEGFRs, leading to the activation of various downstream signaling
pathways, such as phospholipase C, phosphoinositide
3-kinase/protein kinase B and mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase signaling pathways,
which are critical for promoting angiogenesis (8,12). In
addition to regulating endothelial cell functions, VEGF modulates
the anti-tumor immune response within the tumor microenvironment
(8,12). VEGF can promote an immunosuppressive
environment by inhibiting the activity of immune cells such as
cytotoxic T cells and dendritic cells (41,42).
These findings suggest that HSPD1 may promote angiogenesis and
contribute to an immunosuppressive environment in lung cancer by
enhancing VEGF secretion from endothelial cells.
In addition, HSPD1 knockdown in lung cancer cells
leads to a significant decrease in other secretory proteins,
including HSP90 and prohibitin-1 (PHB1) (19), both of which are implicated in
angiogenesis (43,44). Secreted HSP90 has been shown to
promote endothelial cell migration and tube formation (43), while PHB1 regulates angiogenesis by
modulating mitochondrial functions (44). These observations suggest that HSPD1
may influence angiogenesis not only directly but also through the
regulation of other pro-angiogenic secreted proteins. Further
studies are required to explore the interplay between HSPD1 and
these proteins in lung cancer-induced angiogenesis.
The present study has several limitations that
should be addressed. First, the precise molecular mechanisms by
which HSPD1 modulates endothelial cell behavior remain unclear.
Second, the use of in vitro models with EA.hy926 cells and
lung cancer cell secretomes may not fully recapitulate the
complexity of the TME in vivo. Third, the contributions of
other secretory proteins, such as HSP90 and PHB1, to angiogenesis
in lung cancer were not independently validated. Additionally, the
immunosuppressive role of VEGF in the TME was not explored.
Finally, the present study did not include experiments using
recombinant HSPD1 protein to directly evaluate its effects on
endothelial cell behavior and underlying mechanisms. Future
research addressing these limitations will provide a deeper
understanding of the role of HSPD1 in lung cancer-induced
angiogenesis and its potential as a therapeutic target.
In conclusion, the present study demonstrated the
role of HSPD1 in promoting cell proliferation, migration, invasion,
aggregation and angiogenesis in endothelial cells, potentially
through VEGF-mediated pathways. Targeting HSPD1 may be a promising
therapeutic strategy for inhibiting tumor angiogenesis and
preventing tumor progression.
Supplementary Material
Levels of HSPD1, cell morphology,
proliferation, total cell number and VEGF concentration in the
culture supernatant of shHSPD1-A549 and shControl-A549 cells at the
time of secretome collection (after 24 h of incubation in
serum-free DMEM). (A) Representative immunoblotting of HSPD1. (B)
Representative images of shHSPD1-A549 and shControl-A549 cells
before (0 h) and after 24 h of incubation with serum-free DMEM,
captured at x200 magnification. (C) Proliferation of shHSPD1-A549
and shControl-A549 cells measured using the BrdU assay. Data are
shown as fold change relative to the shControl-A549 cells. (D)
Total cell number of shHSPD1-A549 and shControl-A549 cells
determined by trypan blue staining. (E) VEGF levels in the culture
supernatant of shHSPD1-A549 and shControl-A549 cells measured by
ELISA. Bar graphs represent the mean ± SD from three independent
experiments. HSPD1, heat shock protein family D member 1; VEGF,
vascular endothelial growth factor; sh, short hairpin; DMEM,
Dulbecco's Modified Eagle's Medium.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Thailand Science
Research and Innovation (TSRI) (grant no. 672A07023) and the TSRI,
Chulabhorn Research Institute (grant no. 49890/4759797).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KS and SA contributed to the conception and design
of the study, performed experiments, analyzed data, created
visualizations and were major contributors to manuscript writing.
KS also managed the project and secured funding. AR, ATM, PH and JN
conducted experiments. ANM and SK contributed to data analysis and
supplied resources. KL, SP and JS contributed to data analysis and
supervised the project. KS and SA confirm the authenticity of all
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (6 Suppl 16):S15–S18.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Y, Lin M, Wang S, Cao B, Li C and Li G:
Novel angiogenic regulators and anti-angiogenesis drugs targeting
angiogenesis signaling pathways: Perspectives for targeting
angiogenesis in lung cancer. Front Oncol. 12(842960)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Altorki NK, Markowitz GJ, Gao D, Port JL,
Saxena A, Stiles B, McGraw T and Mittal V: The lung
microenvironment: an important regulator of tumour growth and
metastasis. Nat Rev Cancer. 19:9–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano
H, Perry C, Hanaoka J, Fukuoka J, Chung JY and Hewitt SM:
Tumor-associated macrophage, angiogenesis and lymphangiogenesis
markers predict prognosis of non-small cell lung cancer patients. J
Transl Med. 18(443)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: The prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gu X, Chu L and Kang Y: Angiogenic
factor-based signature predicts prognosis and immunotherapy
response in non-small-cell lung cancer. Front Genet.
13(894024)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Majidpoor J and Mortezaee K: Angiogenesis
as a hallmark of solid tumors-clinical perspectives. Cell Oncol
(Dordr). 44:715–737. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ghalehbandi S, Yuzugulen J, Pranjol MZI
and Pourgholami MH: The role of VEGF in cancer-induced angiogenesis
and research progress of drugs targeting VEGF. Eur J Pharmacol.
949(175586)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee S, Chen TT, Barber CL, Jordan MC,
Murdock J, Desai S, Ferrara N, Nagy A, Roos KP and Iruela-Arispe
ML: Autocrine VEGF signaling is required for vascular homeostasis.
Cell. 130:691–703. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Y, Cox SR, Morita T and Kourembanas S:
Hypoxia regulates vascular endothelial growth factor gene
expression in endothelial cells. Identification of a 5' enhancer.
Circ Res. 77:638–643. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Du H, Shi H, Chen D, Zhou Y and Che G:
Cross-talk between endothelial and tumor cells via basic fibroblast
growth factor and vascular endothelial growth factor signaling
promotes lung cancer growth and angiogenesis. Oncol Lett.
9:1089–1094. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X,
Chen Y, Li M, Chen M, Li X, et al: VEGF/VEGFR-targeted therapy and
immunotherapy in non-small cell lung cancer: Targeting the tumor
microenvironment. Int J Biol Sci. 18:3845–3858. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vakhrushev IV, Nezhurina EK, Karalkin PA,
Tsvetkova AV, Sergeeva NS, Majouga AG and Yarygin KN: Heterotypic
multicellular spheroids as experimental and preclinical models of
sprouting angiogenesis. Biology (Basel). 11(18)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yun CW, Kim HJ, Lim JH and Lee SH: Heat
shock proteins: Agents of cancer development and therapeutic
targets in anti-cancer therapy. Cells. 9(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Quintana FJ and Cohen IR: The HSP60 immune
system network. Trends Immunol. 32:89–95. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang Y, Yang Y, Luo J, Liu S, Zhan Y, Zang
H, Zheng H, Zhang Y, Feng J, Fan S and Wen Q: Overexpression of
HSP10 correlates with HSP60 and Mcl-1 levels and predicts poor
prognosis in non-small cell lung cancer patients. Cancer Biomark.
30:85–94. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Parma B, Ramesh V, Gollavilli PN, Siddiqui
A, Pinna L, Schwab A, Marschall S, Zhang S, Pilarsky C, Napoli F,
et al: Metabolic impairment of non-small cell lung cancers by
mitochondrial HSPD1 targeting. J Exp Clin Cancer Res.
40(248)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aluksanasuwan S, Somsuan K, Ngoenkam J,
Chutipongtanate S and Pongcharoen S: Potential association of HSPD1
with dysregulations in ribosome biogenesis and immune cell
infiltration in lung adenocarcinoma: An integrated bioinformatic
approach. Cancer Biomark. 39:155–170. 2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aluksanasuwan S, Somsuan K, Ngoenkam J,
Chiangjong W, Rongjumnong A, Morchang A, Chutipongtanate S and
Pongcharoen S: Knockdown of heat shock protein family D member 1
(HSPD1) in lung cancer cell altered secretome profile and
cancer-associated fibroblast induction. Biochim Biophys Acta Mol
Cell Res. 187(119736)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Duan Y, Tang H, Mitchell-Silbaugh K, Fang
X, Han Z and Ouyang K: Heat shock protein 60 in cardiovascular
physiology and diseases. Front Mol Biosci. 7(73)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qiu J, Gao HQ, Liang Y, Yu H and Zhou RH:
Comparative proteomics analysis reveals role of heat shock protein
60 in digoxin-induced toxicity in human endothelial cells. Biochim
Biophys Acta. 1784:1857–1864. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Billack B, Heck DE, Mariano TM, Gardner
CR, Sur R, Laskin DL and Laskin JD: Induction of cyclooxygenase-2
by heat shock protein 60 in macrophages and endothelial cells. Am J
Physiol Cell Physiol. 283:C1267–C1277. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin CS, He PJ, Hsu WT, Wu MS, Wu CJ, Shen
HW, Hwang CH, Lai YK, Tsai NM and Liao KW: Helicobacter
pylori-derived heat shock protein 60 enhances angiogenesis via a
CXCR2-mediated signaling pathway. Biochem Biophys Res Commun.
397:283–289. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng D, Zhang X, Xu J, Chen S, Wang B and
Yuan X: LncRNA LINC01503 promotes angiogenesis in colorectal cancer
by regulating VEGFA expression via miR-342-3p and HSP60 binding. J
Biomed Res: Oct 25, 2024 (Epub ahead of print).
|
|
25
|
Suarez-Arnedo A, Torres Figueroa F,
Clavijo C, Arbeláez P, Cruz JC and Muñoz-Camargo C: An image J
plugin for the high throughput image analysis of in vitro scratch
wound healing assays. PLoS One. 15(e0232565)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Somsuan K, Peerapen P, Boonmark W,
Plumworasawat S, Samol R, Sakulsak N and Thongboonkerd V: ARID1A
knockdown triggers epithelial-mesenchymal transition and
carcinogenesis features of renal cells: Role in renal cell
carcinoma. FASEB J. 33:12226–12239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li B, Severson E, Pignon JC, Zhao H, Li T,
Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al: Comprehensive
analyses of tumor immunity: Implications for cancer immunotherapy.
Genome Biol. 17(174)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Győrffy B: Transcriptome-level discovery
of survival-associated biomarkers and therapy targets in
non-small-cell lung cancer. Br J Pharmacol. 181:362–374.
2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Makridakis M and Vlahou A: Secretome
proteomics for discovery of cancer biomarkers. J Proteomics.
73:2291–2305. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
da Cunha BR, Domingos C, Stefanini ACB,
Henrique T, Polachini GM, Castelo-Branco P and Tajara EH: Cellular
interactions in the tumor microenvironment: The role of secretome.
J Cancer. 10:4574–4587. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cheng HW, Chen YF, Wong JM, Weng CW, Chen
HY, Yu SL, Chen HW, Yuan A and Chen JJ: Cancer cells increase
endothelial cell tube formation and survival by activating the
PI3K/Akt signalling pathway. J Exp Clin Cancer Res.
36(27)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhong L, Roybal J, Chaerkady R, Zhang W,
Choi K, Alvarez CA, Tran H, Creighton CJ, Yan S, Strieter RM, et
al: Identification of secreted proteins that mediate cell-cell
interactions in an in vitro model of the lung cancer
microenvironment. Cancer Res. 68:7237–7245. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kramer N, Walzl A, Unger C, Rosner M,
Krupitza G, Hengstschläger M and Dolznig H: In vitro cell migration
and invasion assays. Mutat Res. 752:10–24. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tian J, Guo X, Liu XM, Liu L, Weng QF,
Dong SJ, Knowlton AA, Yuan WJ and Lin L: Extracellular HSP60
induces inflammation through activating and up-regulating TLRs in
cardiomyocytes. Cardiovasc Res. 98:391–401. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vabulas RM, Ahmad-Nejad P, da Costa C,
Miethke T, Kirschning CJ, Häcker H and Wagner H: Endocytosed HSP60s
use toll-like receptor 2 (TLR2) and TLR4 to activate the
toll/interleukin-1 receptor signaling pathway in innate immune
cells. J Biol Chem. 276:31332–31339. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Stierschneider A and Wiesner C: Shedding
light on the molecular and regulatory mechanisms of TLR4 signaling
in endothelial cells under physiological and inflamed conditions.
Front Immunol. 14(1264889)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pei Z, Lin D, Song X, Li H and Yao H: TLR4
signaling promotes the expression of VEGF and TGFbeta1 in human
prostate epithelial PC3 cells induced by lipopolysaccharide. Cell
Immunol. 254:20–27. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Riddell JR, Maier P, Sass SN, Moser MT,
Foster BA and Gollnick SO: Peroxiredoxin 1 stimulates endothelial
cell expression of VEGF via TLR4 dependent activation of HIF-1α.
PLoS One. 7(e50394)2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Voron T, Colussi O, Marcheteau E, Pernot
S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N,
Tanchot C, et al: VEGF-A modulates expression of inhibitory
checkpoints on CD8+ T cells in tumors. J Exp Med. 212:139–148.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gabrilovich D, Ishida T, Oyama T, Ran S,
Kravtsov V, Nadaf S and Carbone DP: Vascular endothelial growth
factor inhibits the development of dendritic cells and dramatically
affects the differentiation of multiple hematopoietic lineages in
vivo. Blood. 92:4150–4166. 1998.PubMed/NCBI
|
|
43
|
Song X and Luo Y: The regulatory mechanism
of Hsp90alpha secretion from endothelial cells and its role in
angiogenesis during wound healing. Biochem Biophys Res Commun.
398:111–117. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Schleicher M, Shepherd BR, Suarez Y,
Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS and Sessa
WC: Prohibitin-1 maintains the angiogenic capacity of endothelial
cells by regulating mitochondrial function and senescence. J Cell
Biol. 180:101–112. 2008.PubMed/NCBI View Article : Google Scholar
|